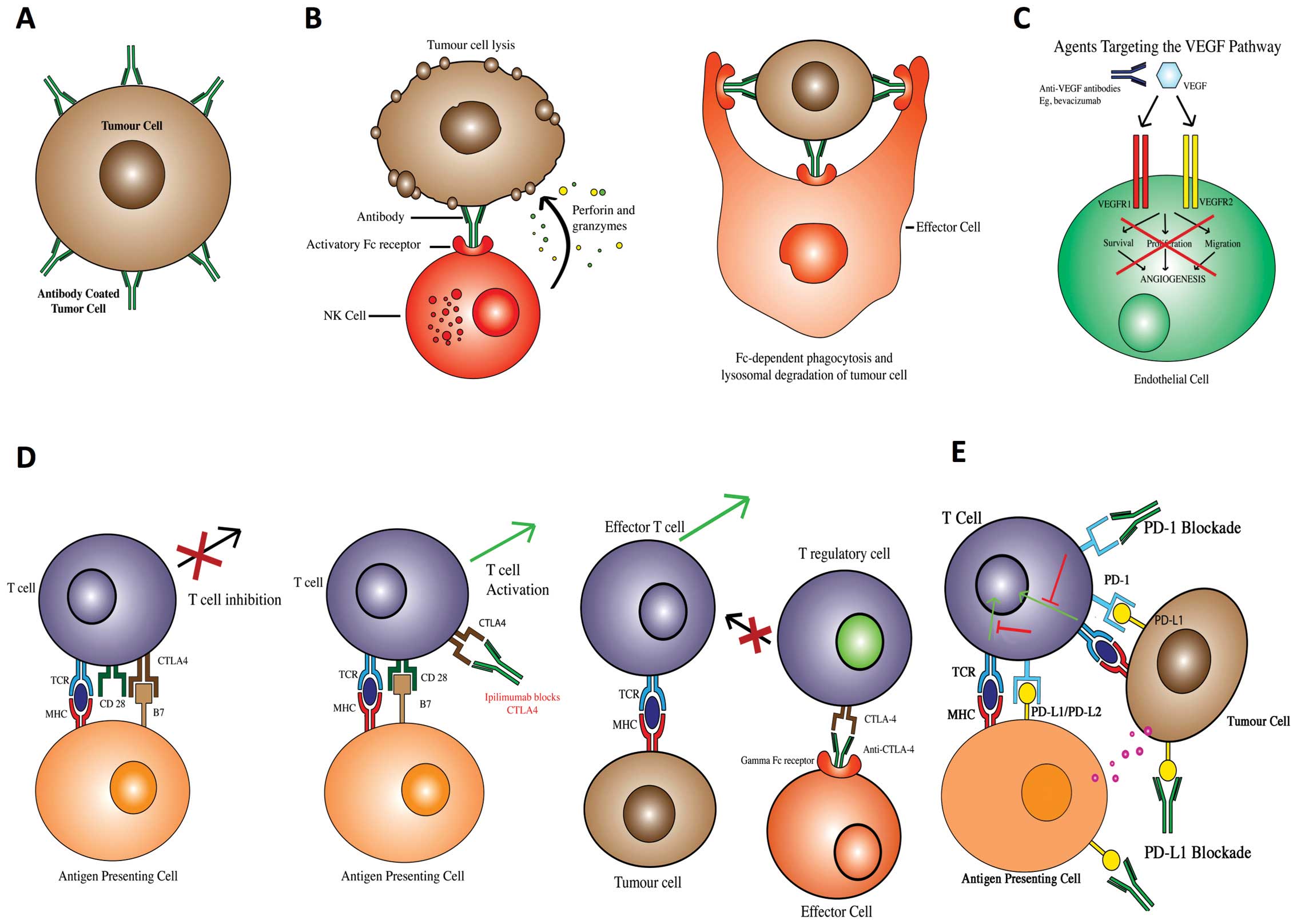
|
1
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final version of 2009 AJCC melanoma staging and classification. J
Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerner RE, Moore GE and Dickey C:
Combination chemotherapy in disseminated melanoma and other solid
tumors in adults. Oncology. 31:22–30. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atkins MB: Cytokine-based therapy and
biochemotherapy for advanced melanoma. Clin Cancer Res.
12:2353s–2358s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ballantyne AD and Garnock-Jones KP:
Dabrafenib: first global approval. Drugs. 73:1367–1376. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bollag G, Tsai J, Zhang J, et al:
Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat
Rev Drug Discov. 11:873–886. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright CJ and McCormack PL: Trametinib:
first global approval. Drugs. 73:1245–1254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sznol M: Advances in the treatment of
metastatic melanoma: new immunomodulatory agents. Semin Oncol.
39:192–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oble DA, Loewe R, Yu P and Mihm MC Jr:
Focus on TILs: prognostic significance of tumor infiltrating
lymphocytes in human melanoma. Cancer Immun. 9:32009.PubMed/NCBI
|
|
9
|
Shimanovsky A, Jethava A and Dasanu CA:
Immune alterations in malignant melanoma and current immunotherapy
concepts. Expert Opin Biol Ther. 13:1413–1427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cipponi A, Mercier M, Seremet T, et al:
Neogenesis of lymphoid structures and antibody responses occur in
human melanoma metastases. Cancer Res. 72:3997–4007. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilbert AE, Karagiannis P, Dodev T, et al:
Monitoring the systemic human memory B cell compartment of melanoma
patients for anti-tumor IgG antibodies. PLoS One. 6:e193302011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lacy KE, Karagiannis SN and Nestle FO:
Advances in the treatment of melanoma. Clin Med. 12:168–171. 2012.
View Article : Google Scholar
|
|
13
|
Fujimura T, Ring S, Umansky V, Mahnke K
and Enk AH: Regulatory T cells stimulate B7-H1 expression in
myeloid-derived suppressor cells in ret melanomas. J Invest
Dermatol. 132:1239–1246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karagiannis P, Gilbert AE, Nestle FO and
Karagiannis SN: IgG4 antibodies and cancer-associated inflammation:
insights into a novel mechanism of immune escape. Oncoimmunology.
2:e248892013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karagiannis P, Gilbert AE, Josephs DH, et
al: IgG4 subclass antibodies impair antitumor immunity in melanoma.
J Clin Invest. 123:1457–1474. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang T, Ge Y, Xiao M, et al:
Melanoma-derived conditioned media efficiently induce the
differentiation of monocytes to macrophages that display a highly
invasive gene signature. Pigment Cell Melanoma Res. 25:493–505.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atkins MB, Lotze MT, Dutcher JP, et al:
High-dose recombinant interleukin 2 therapy for patients with
metastatic melanoma: analysis of 270 patients treated between 1985
and 1993. J Clin Oncol. 17:2105–2116. 1999.PubMed/NCBI
|
|
18
|
Hauschild A: Adjuvant interferon alfa for
melanoma: new evidence-based treatment recommendations? Curr Oncol.
16:3–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petrella T, Quirt I, Verma S, Haynes AE,
Charette M, Bak K, et al: Single-agent interleukin-2 in the
treatment of metastatic melanoma: a systematic review. Cancer Treat
Rev. 33:484–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasse AD, Sasse EC, Clark LG, Ulloa L and
Clark OA: Chemoimmunotherapy versus chemotherapy for metastatic
malignant melanoma. Cochrane Database Syst Rev.
CD0054132007.PubMed/NCBI
|
|
21
|
Borden EC: Interferons: pleiotropic
cellular modulators. Clin Immunol Immunopathol. 62:S18–24. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bart RS, Porzio NR, Kopf AW, Vilcek JT,
Cheng EH and Farcet Y: Inhibition of growth of B16 murine malignant
melanoma by exogenous interferon. Cancer Res. 40:614–619.
1980.PubMed/NCBI
|
|
23
|
Kirkwood JM, Strawderman MH, Ernstoff MS,
Smith TJ, Borden EC and Blum RH: Interferon alfa-2b adjuvant
therapy of high-risk resected cutaneous melanoma: the Eastern
Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 14:7–17.
1996.PubMed/NCBI
|
|
24
|
Wheatley K, Ives N, Hancock B, Gore M,
Eggermont A and Suciu S: Does adjuvant interferon-alpha for
high-risk melanoma provide a worthwhile benefit? A meta-analysis of
the randomised trials. Cancer Treat Rev. 29:241–252. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hillner BE: Cost-effectiveness assessment
of interferon alfa-2b as adjuvant therapy of high-risk resected
cutaneous melanoma. Eur J Cancer. 34:S18–S21. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eggermont AM, Suciu S, Santinami M, et al:
Adjuvant therapy with pegylated interferon alfa-2b versus
observation alone in resected stage III melanoma: final results of
EORTC 18991, a randomised phase III trial. Lancet. 372:117–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: the first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zikich D, Schachter J and Besser MJ:
Immunotherapy for the management of advanced melanoma: the next
steps. Am J Clin Dermatol. 14:261–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dannull J, Haley NR, Archer G, et al:
Melanoma immunotherapy using mature DCs expressing the constitutive
proteasome. J Clin Invest. 123:3135–3145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan J, Ku GY, Gallardo HF, et al: Safety
and immunogenicity of a human and mouse gp100 DNA vaccine in a
phase I trial of patients with melanoma. Cancer Immun.
9:52009.PubMed/NCBI
|
|
31
|
Reichert JM and Dhimolea E: The future of
antibodies as cancer drugs. Drug Discov Today. 17:954–963. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Azijli K, Stelloo E, Peters GJ and van den
Eertwegh AJ: New developments in the treatment of metastatic
melanoma: immune checkpoint inhibitors and targeted therapies.
Anticancer Res. 34:1493–1505. 2014.PubMed/NCBI
|
|
33
|
Price MA, Colvin Wanshura LE, Yang J, et
al: CSPG4, a potential therapeutic target, facilitates malignant
progression of melanoma. Pigment Cell Melanoma Res. 24:1148–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Osada T, Wang Y, et al: CSPG4
protein as a new target for the antibody-based immunotherapy of
triple-negative breast cancer. J Natl Cancer Inst. 102:1496–1512.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Price MA, Neudauer CL, et al:
Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK
activation by distinct mechanisms. J Cell Biol. 165:881–891. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J, Price MA, Li GY, et al: Melanoma
proteoglycan modifies gene expression to stimulate tumor cell
motility, growth, and epithelial-to-mesenchymal transition. Cancer
Res. 69:7538–7547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chekenya M, Krakstad C, Svendsen A, et al:
The progenitor cell marker NG2/MPG promotes chemoresistance by
activation of integrin-dependent PI3K/Akt signaling. Oncogene.
27:5182–5194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maciag PC, Seavey MM, Pan ZK, Ferrone S
and Paterson Y: Cancer immunotherapy targeting the high molecular
weight melanoma-associated antigen protein results in a broad
antitumor response and reduction of pericytes in the tumor
vasculature. Cancer Res. 68:8066–8075. 2008. View Article : Google Scholar
|
|
39
|
Kusama M, Kageshita T, Chen ZJ and Ferrone
S: Characterization of syngeneic antiidiotypic monoclonal
antibodies to murine anti-human high molecular weight
melanoma-associated antigen monoclonal antibodies. J Immunol.
143:3844–3852. 1989.
|
|
40
|
Chen ZJ, Yang H, Kageshita T and Ferrone
S: Human high-molecular-weight melanoma-associated antigen mimicry
by mouse antiidiotypic monoclonal antibody TK7-371. Cancer Res.
51:4790–4797. 1991.PubMed/NCBI
|
|
41
|
Mittelman A, Chen ZJ, Yang H, Wong GY and
Ferrone S: Human high molecular weight melanoma-associated antigen
(HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody
MK2-23: induction of humoral anti-HMW-MAA immunity and prolongation
of survival in patients with stage IV melanoma. Proc Natl Acad Sci
USA. 89:466–470. 1992. View Article : Google Scholar
|
|
42
|
Mittelman A, Chen GZ, Wong GY, Liu C,
Hirai S and Ferrone S: Human high molecular weight-melanoma
associated antigen mimicry by mouse anti-idiotypic monoclonal
antibody MK2-23: modulation of the immunogenicity in patients with
malignant melanoma. Clin Cancer Res. 1:705–713. 1995.PubMed/NCBI
|
|
43
|
Mittelman A, Wang X, Matsumoto K and
Ferrone S: Antiantiidiotypic response and clinical course of the
disease in patients with malignant melanoma immunized with mouse
antiidiotypic monoclonal antibody MK2-23. Hybridoma. 14:175–181.
1995. View Article : Google Scholar
|
|
44
|
Murray JL, Gillogly M, Kawano K, et al:
Fine specificity of high molecular weight-melanoma-associated
antigen-specific cytotoxic T lymphocytes elicited by anti-idiotypic
monoclonal antibodies in patients with melanoma. Cancer Res.
64:5481–5488. 2004. View Article : Google Scholar
|
|
45
|
Torisu-Itakura H, Schoellhammer HF, Sim
MS, et al: Redirected lysis of human melanoma cells by a
MCSP/CD3-bispecific BiTE antibody that engages patient-derived T
cells. J Immunother. 34:597–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rybczynska AA, Dierckx RA, Ishiwata K,
Elsinga PH and van Waarde A: Cytotoxicity of sigma-receptor ligands
is associated with major changes of cellular metabolism and
complete occupancy of the sigma-2 subpopulation. J Nucl Med.
49:2049–2056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Bruyn M, Rybczynska AA, Wei Y, et al:
Melanoma-associated Chondroitin Sulfate Proteoglycan
(MCSP)-targeted delivery of soluble TRAIL potently inhibits
melanoma outgrowth in vitro and in vivo. Mol Cancer. 9:3012010.
|
|
48
|
Geldres C, Savoldo B, Hoyos V, et al: T
lymphocytes redirected against the chondroitin sulfate
proteoglycan-4 control the growth of multiple solid tumors both in
vitro and in vivo. Clin Cancer Res. 20:962–971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mehnert JM, McCarthy MM, Jilaveanu L, et
al: Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3
in melanoma tissue microarrays. Hum Pathol. 41:375–384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Spinella F, Caprara V, Cianfrocca R, et
al: The interplay between hypoxia, endothelial and melanoma cells
regulates vascularization and cell motility through endothelin-1
and vascular endothelial growth factor. Carcinogenesis. 35:840–848.
2014. View Article : Google Scholar
|
|
51
|
Hsu JY and Wakelee HA: Monoclonal
antibodies targeting vascular endothelial growth factor: current
status and future challenges in cancer therapy. BioDrugs.
23:289–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Molhoek KR, Griesemann H, Shu J,
Gershenwald JE, Brautigan DL and Slingluff CL Jr: Human melanoma
cytolysis by combined inhibition of mammalian target of rapamycin
and vascular endothelial growth factor/vascular endothelial growth
factor receptor-2. Cancer Res. 68:4392–4397. 2008. View Article : Google Scholar
|
|
53
|
Del Vecchio M, Mortarini R, Canova S, et
al: Bevacizumab plus fotemustine as first-line treatment in
metastatic melanoma patients: clinical activity and modulation of
angiogenesis and lymphangiogenesis factors. Clin Cancer Res.
16:5862–5872. 2010.PubMed/NCBI
|
|
54
|
Varker KA, Biber JE, Kefauver C, et al: A
randomized phase 2 trial of bevacizumab with or without daily
low-dose interferon alfa-2b in metastatic malignant melanoma. Ann
Surg Oncol. 14:2367–2376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guenterberg KD, Grignol VP, Relekar KV, et
al: A pilot study of bevacizumab and interferon-alpha2b in ocular
melanoma. Am J Clin Oncol. 34:87–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Corrie PG, Marshall A, Dunn JA, et al:
Adjuvant bevacizumab in patients with melanoma at high risk of
recurrence (AVAST-M): preplanned interim results from a
multicentre, open-label, randomised controlled phase 3 study.
Lancet Oncol. 15:620–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shrimali RK, Yu Z, Theoret MR, Chinnasamy
D, Restifo NP and Rosenberg SA: Antiangiogenic agents can increase
lymphocyte infiltration into tumor and enhance the effectiveness of
adoptive immunotherapy of cancer. Cancer Res. 70:6171–6180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Perez DG, Suman VJ, Fitch TR, et al: Phase
2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab
in patients with unresectable stage IV melanoma: a North Central
Cancer Treatment Group study, N047A. Cancer. 115:119–127. 2009.
View Article : Google Scholar
|
|
59
|
Perez EA, Hillman DW, Dentchev T, et al:
North Central Cancer Treatment Group (NCCTG) N0432: phase II trial
of docetaxel with capecitabine and bevacizumab as first-line
chemotherapy for patients with metastatic breast cancer. Ann Oncol.
21:269–274. 2010. View Article : Google Scholar
|
|
60
|
Allison JP, Chambers C, Hurwitz A, et al:
A role for CTLA-4 mediated inhibitory signals in peripheral T cell
tolerance? Novartis Found Symp. 215:92–98. 1998.PubMed/NCBI
|
|
61
|
Linsley PS, Brady W, Urnes M, Grosmaire
LS, Damle NK and Ledbetter JA: CTLA-4 is a second receptor for the
B cell activation antigen B7. J Exp Med. 174:561–569. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Simpson TR, Li F, Montalvo-Ortiz W, et al:
Fc-dependent depletion of tumor-infiltrating regulatory T cells
co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J
Exp Med. 210:1695–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bulliard Y, Jolicoeur R, Windman M, et al:
Activating Fc γ receptors contribute to the antitumor activities of
immunoregulatory receptor-targeting antibodies. J Exp Med.
210:1685–1693. 2013.
|
|
64
|
Friedline RH, Brown DS, Nguyen H, et al:
CD4+regulatory T cells require CTLA-4 for the
maintenance of systemic tolerance. J Exp Med. 206:421–434.
2009.PubMed/NCBI
|
|
65
|
Phan GQ, Yang JC, Sherry RM, et al: Cancer
regression and autoimmunity induced by cytotoxic T
lymphocyte-associated antigen 4 blockade in patients with
metastatic melanoma. Proc Natl Acad Sci USA. 100:8372–8377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Weber JS, O’Day S, Urba W, et al: Phase
I/II study of ipilimumab for patients with metastatic melanoma. J
Clin Oncol. 26:5950–5956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ribas A, Camacho LH, Lopez-Berestein G, et
al: Antitumor activity in melanoma and anti-self responses in a
phase I trial with the anti-cytotoxic T lymphocyte-associated
antigen 4 monoclonal antibody CP-675,206. J Clin Oncol.
23:8968–8977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hersh EM, O’Day SJ, Powderly J, et al: A
phase II multicenter study of ipilimumab with or without
dacarbazine in chemotherapy-naive patients with advanced melanoma.
Invest New Drugs. 29:489–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. New Engl J Med. 363:711–723. 2010. View Article : Google Scholar
|
|
71
|
Callahan MK, Postow MA and Wolchok JD:
Immunomodulatory therapy for melanoma: ipilimumab and beyond. Clin
Dermatol. 31:191–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Postow MA, Callahan MK and Wolchok JD: The
antitumor immunity of ipilimumab: (T-cell) memories to last a
lifetime? Clin Cancer Res. 18:1821–1823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Della Vittoria Scarpati G, Fusciello C,
Perri F, et al: Ipilimumab in the treatment of metastatic melanoma:
management of adverse events. Onco Targets Ther. 7:203–209.
2014.PubMed/NCBI
|
|
74
|
Ribas A, Kefford R, Marshall MA, et al:
Phase III randomized clinical trial comparing tremelimumab with
standard-of-care chemotherapy in patients with advanced melanoma. J
Clin Oncol. 31:616–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hino R, Kabashima K, Kato Y, et al: Tumor
cell expression of programmed cell death-1 ligand 1 is a prognostic
factor for malignant melanoma. Cancer. 116:1757–1766. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ahmadzadeh M, Johnson LA, Heemskerk B, et
al: Tumor antigen-specific CD8 T cells infiltrating the tumor
express high levels of PD-1 and are functionally impaired. Blood.
114:1537–1544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brahmer JR, Drake CG, Wollner I, et al:
Phase I study of single-agent anti-programmed death-1 (MDX-1106) in
refractory solid tumors: safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010. View Article : Google Scholar
|
|
79
|
Topalian SL, Sznol M, McDermott DF, et al:
Survival, durable tumor remission, and long-term safety in patients
with advanced melanoma receiving nivolumab. J Clin Oncol.
32:1020–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lipson EJ, Sharfman WH, Drake CG, et al:
Durable cancer regression off-treatment and effective reinduction
therapy with an anti-PD-1 antibody. Clin Cancer Res. 19:462–468.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hamid O, Robert C, Daud A, et al: Safety
and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New
Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. New Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yao X, Ahmadzadeh M, Lu YC, et al: Levels
of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively
associated with clinical response to adoptive immunotherapy of
human cancer. Blood. 119:5688–5696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
De Panfilis G, Campanini N, Santini M, et
al: Phase- and stage-related proportions of T cells bearing the
transcription factor FOXP3 infiltrate primary melanoma. J Invest
Dermatol. 128:676–684. 2008.PubMed/NCBI
|
|
85
|
Miracco C, Mourmouras V, Biagioli M, et
al: Utility of tumour-infiltrating
CD25+FOXP3+regulatory T cell evaluation in
predicting local recurrence in vertical growth phase cutaneous
melanoma. Oncol Rep. 18:1115–1122. 2007.PubMed/NCBI
|
|
86
|
Agius E, Lacy KE, Vukmanovic-Stejic M, et
al: Decreased TNF-alpha synthesis by macrophages restricts
cutaneous immunosurveillance by memory CD4+T cells
during aging. J Exp Med. 206:1929–1940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jacobs JF, Punt CJ, Lesterhuis WJ, et al:
Dendritic cell vaccination in combination with anti-CD25 monoclonal
antibody treatment: a phase III study in metastatic melanoma
patients. Clin Cancer Res. 16:5067–5078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wolchok JD, Kluger H, Callahan MK, et al:
Nivolumab plus ipilimumab in advanced melanoma. New Engl J Med.
369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ly LV, Sluijter M, van der Burg SH, Jager
MJ and van Hall T: Effective cooperation of monoclonal antibody and
peptide vaccine for the treatment of mouse melanoma. J Immunol.
190:489–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Alderson KL, Luangrath M, Elsenheimer MM,
et al: Enhancement of the anti-melanoma response of Hu14.18K322A by
αCD40 + CpG. Cancer Immunol Immunother. 62:665–675. 2013.PubMed/NCBI
|
|
92
|
Flaherty KT: Dividing and conquering:
controlling advanced melanoma by targeting oncogene-defined
subsets. Clin Exp Metastasis. 29:841–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chapman PB, Hauschild A, Robert C, et al:
Updated overall survival (OS) results for BRIM-3, a phase III
randomized, open-label, multicenter trial comparing BRAF inhibitor
vemurafenib (vem) with dacarbazine (DTIC) in previously untreated
patients with BRAFV600E-mutated melanoma. J Clin Oncol.
85022012.
|
|
94
|
Hauschild A, Grob JJ, Demidov LV, et al:
Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre,
open-label, phase 3 randomised controlled trial. Lancet.
380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Knight DA, Ngiow SF, Li M, et al: Host
immunity contributes to the anti-melanoma activity of BRAF
inhibitors. J Clin Invest. 123:1371–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ngiow SF, Knight DA, Ribas A, McArthur GA
and Smyth MJ: BRAF-targeted therapy and immune responses to
melanoma. Oncoimmunology. 2:e244622013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ascierto PA, Simeone E, Giannarelli D,
Grimaldi AM, Romano A and Mozzillo N: Sequencing of BRAF inhibitors
and ipilimumab in patients with metastatic melanoma: a possible
algorithm for clinical use. J Transl Med. 10:1072012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ribas A, Hodi FS, Callahan M, Konto C and
Wolchok J: Hepatotoxicity with combination of vemurafenib and
ipilimumab. N Engl J Med. 368:1365–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Culos KA and Cuellar S: Novel targets in
the treatment of advanced melanoma: new first-line treatment
options. Ann Pharmacother. 47:519–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Woof JM: Insights from Fc receptor
biology: a route to improved antibody reagents. MAbs. 4:291–293.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bossi G, Buisson S, Oates J, Jakobsen BK
and Hassan NJ: ImmTAC-redirected tumour cell killing induces and
potentiates antigen cross-presentation by dendritic cells. Cancer
Immunol Immunother. 63:437–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
McCormack E, Adams KJ, Hassan NJ, et al:
Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1-
and LAGE-1-positive tumors. Cancer Immunol Immunother. 62:773–785.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Karagiannis SN, Josephs DH, Karagiannis P,
et al: Recombinant IgE antibodies for passive immunotherapy of
solid tumours: from concept towards clinical application. Cancer
Immunol Immunother. 61:1547–1564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Josephs DH, Spicer JF, Karagiannis P,
Gould HJ and Karagiannis SN: IgE immunotherapy: a novel concept
with promise for the treatment of cancer. MAbs. 6:54–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Boross P, Lohse S, Nederend M, et al: IgA
EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med.
5:1213–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lohse S, Brunke C, Derer S, et al:
Characterization of a mutated IgA2 antibody of the m(1) allotype
against the epidermal growth factor receptor for the recruitment of
monocytes and macrophages. J Biol Chem. 287:25139–25150. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lohse S, Derer S, Beyer T, et al:
Recombinant dimeric IgA antibodies against the epidermal growth
factor receptor mediate effective tumor cell killing. J Immunol.
186:3770–3778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Peggs KS, Quezada SA, Chambers CA, Korman
AJ and Allison JP: Blockade of CTLA-4 on both effector and
regulatory T cell compartments contributes to the antitumor
activity of anti-CTLA-4 antibodies. J Exp Med. 206:1717–1725. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Silina K, Rulle U, Kalnina Z and Line A:
Manipulation of tumour-infiltrating B cells and tertiary lymphoid
structures: a novel anti-cancer treatment avenue? Cancer Immunol
Immunother. Apr 3–2014.(Epub ahead of print).
|
|
110
|
Cipponi A, Wieers G, van Baren N and
Coulie PG: Tumor-infiltrating lymphocytes: apparently good for
melanoma patients. But why? Cancer Immunol Immunother.
60:1153–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tsoka S, Ainali C, Karagiannis P, et al:
Toward prediction of immune mechanisms and design of
immunotherapies in melanoma. Crit Rev Biomed Eng. 40:279–294. 2012.
View Article : Google Scholar : PubMed/NCBI
|