Ovarian cancer is the most lethal cancer of the
female reproductive system, with a high rate of mortality
worldwide. Approximately 70% of ovarian cancers are diagnosed at
advanced stage and only 40% of women with such cancers can expect
to survive 5 years (1). The current
therapy for ovarian cancer is debulking surgery followed by
cisplatin-centered chemotherapy (2). Although cisplatin-centered
chemotherapy, which is the currently preferred treatment modality
in human ovarian cancer, can achieve a complete response rate of
40–60% in advanced ovarian cancer patients, the main obstacle to a
successful treatment for ovarian cancer is the development of drug
resistance to combined chemotherapy, and that finally leads to
mortality (3–5).
Drug resistance, including intrinsic and acquired
resistance, generally develops after the treatments to advanced
stage cancer patients with chemotherapies, and results from a
variety of factors including individual variations in patients and
somatic cell genetic differences in tumors (5,6).
Several molecular mechanisms implicated in the rise of resistance
in cellular models of ovarian cancer include decreased
cell-associated drugs, altered drug inactivation, increased DNA
damage tolerance/repair, increased anti-apoptotic regulator
activity and growth factor receptor deregulation (4,7). In
addition, apoptosis, which is associated with the expression of
specific ‘death’ genes and downregulation of ‘survival’
counterparts, is crucial in determining the response to
chemotherapeutic agents (8,9). However, regardless of the mechanisms,
abnormal expression of drug resistance-related genes often plays
important roles in drug resistance (10).
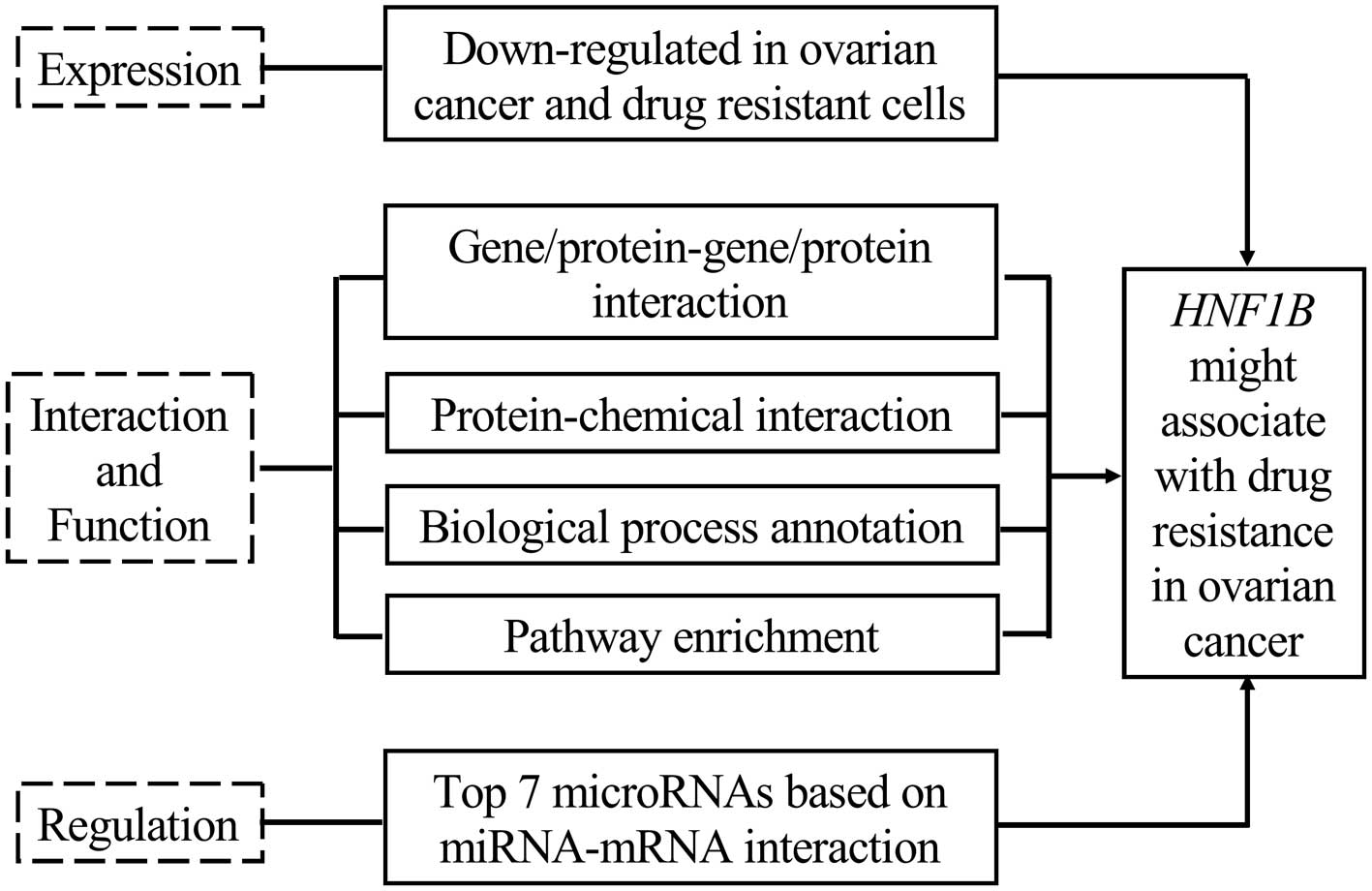
Collectively, the expression of HNF1B is associated
with cancer risk in several tumors, and its decreased expression
play roles in cancer development. However, studies of HNF1B with
ovarian cancer are limited, and its association with drug
resistance in cancer has yet to be reported. In the present study,
we demonstrated that the expression of HNF1B was significantly
decreased in serous cystadenocarcinomas and platinum-resistant
A2780 ovarian cancer cells, according to the microarray data
retrieved from the Oncomine and Gene Expression Omnibus (GEO)
online database, respectively, and it indicated that HNF1B may be
involved in the drug resistance in ovarian cancer. Following this
premise, the present study illustrated that the downregulation of
HNF1B may contribute to drug resistance in ovarian cancer, based on
our comprehensive bioinformatics analyses.
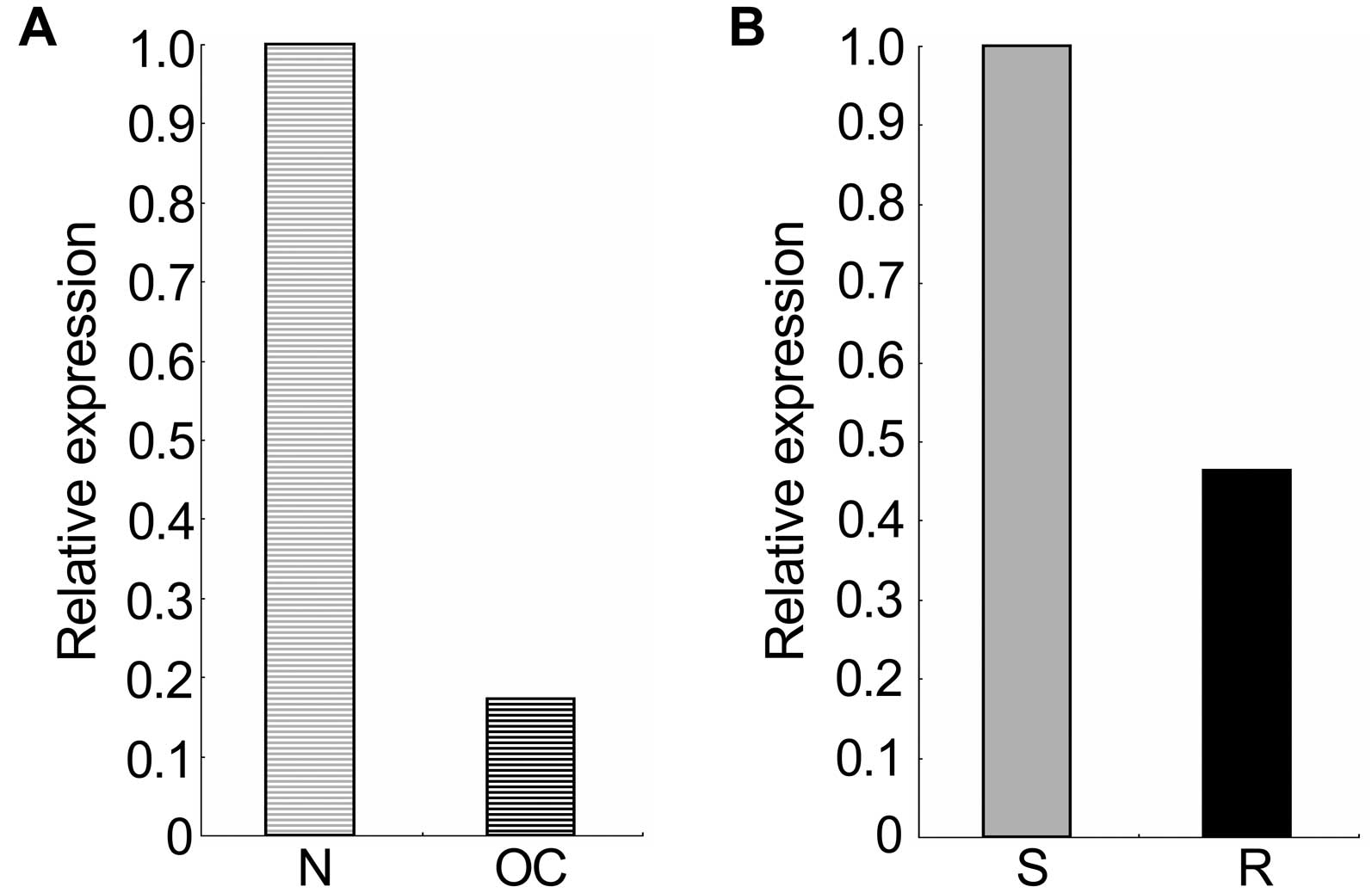
The mRNA expression data of HNF1B in ovarian cancers
and in platinum-resistant A2780 ovarian cancer cells was retrieved
from the Oncomine and GEO profile online database, respectively. As
shown in Fig. 1, the mRNA
expression of HNF1B in 586 ovarian serous cystadenocarcinomas was
significantly decreased compared with the expression in 8 ovaries
used as normal controls (p=7.06E-6; fold-change=−5.776), and its
expression in platinum-resistant A2780 epithelial ovarian cancer
cells was notably decreased compared with the expression in their
sensitive counterpart (with 5 replicates each; fold-change=−2.16).
These results indicated that the decreased expression of HNF1B may
be involved in the development of ovarian serous
cystadenocarcinomas and drug resistance.
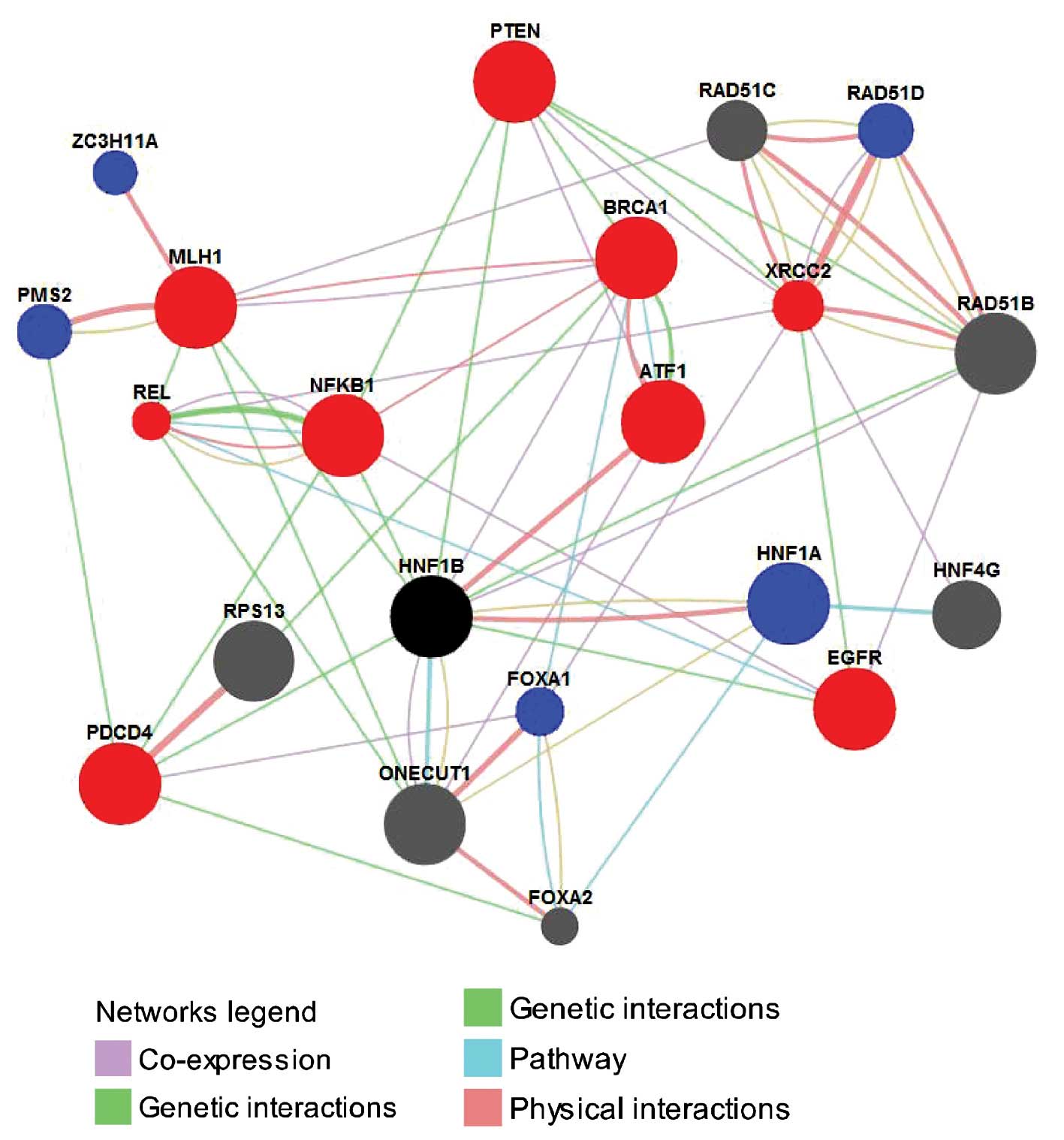
The protein/gene interaction of HNF1B with other
proteins/genes was analyzed using the GeneMANIA online database. As
shown in Fig. 2, HNF1B has direct
interactions with 10 proteins/genes; among these, HNF1B shared
protein domain, shared pathways and co-expressed with ONECUT1, had
genetic interactions with NFKB1, PTEN, EGFR, PDCD4 and MLH1, had
genetic interactions and co-expressed with RAD51B, had physical
interactions and shared the pathway with HNF1A, had physical
interactions with ATF1 and co-expressed with BRCA1. With the
exception of the RAD51B and ONECUT1, the other 8 proteins/genes
have all been proven to be closely associated with drug resistance
in ovarian cancer. For example, BRCA1 is a well-known TSG and its
downregulation contributes to the enhancement of drug resistance in
ovarian cancer (42,43). PTEN also is a TSG, and its
downregulation results in the development of drug resistance in
OVCAR-3 cells and the alterations conferred resistance to cisplatin
through the activation of PI3K/Akt and the inhibition of Bax
translocation (44). Further
research indicated that overexpression of PTEN reverses
chemoresistance to cisplatin in human ovarian cancer cells through
inactivation of the PI3K/AKT cell survival pathway and may serve as
a potential molecular target for the treatment of chemoresistant
ovarian cancer (45). NFKB1
functions as a biphasic regulator, either suppressing or enhancing
the development of ovarian cancer. As a tumor suppressor in ovarian
cancer cell lines, NFKB1 regulates MAPK, while in the aggressive
chemoresistant isogenic variants of these lines it plays a role in
apoptosis (46). In addition, PDCD4
enhances chemosensitivity of ovarian cancer cells by activating
death receptor pathway in vitro and in vivo (47), and the loss of MLH1 mediated by
methylation can lead to the cisplatin-resistance in ovarian cancer
(48,49). In addition, EGFR (50,51),
ATF1 (52,53) and HNF1A (55) are all associated with drug
resistance in ovarian and other types of cancer.
In addition to the direct interactions, there were
another 10 proteins/genes in network which indirectly interacted
with HNF1B; among those, 6 proteins/genes including REL (55,56),
CRCC2 (57), PMS2 (58), ZC3H11A (10), FOXA1 (59) and RAD51D (60) have been proven to be associated with
drug resistance in ovarian and other cancers. For example, REL
contributes directly to elevated uPA gene expression in human
ovarian cancer cells (55), thereby
promoting the multiple functions of uPA during tumor growth and
metastasis, including drug resistance (56). Similarly, a naturally occurring
genetic variant of human XRCC2 confers increased resistance to
cisplatin-induced DNA damage in ovarian cancer (57).
Collectively, among the total 20 proteins/genes that
interacted with HNF1B, 14 were associated with drug resistance in
cancers, of which 9 were associated with drug resistance in ovarian
cancer. Thus, given the strong interactions of HNF1B with those
proteins/genes, we concluded that HNF1B may be involved in the drug
resistance in cancer, particularly in ovarian cancer.
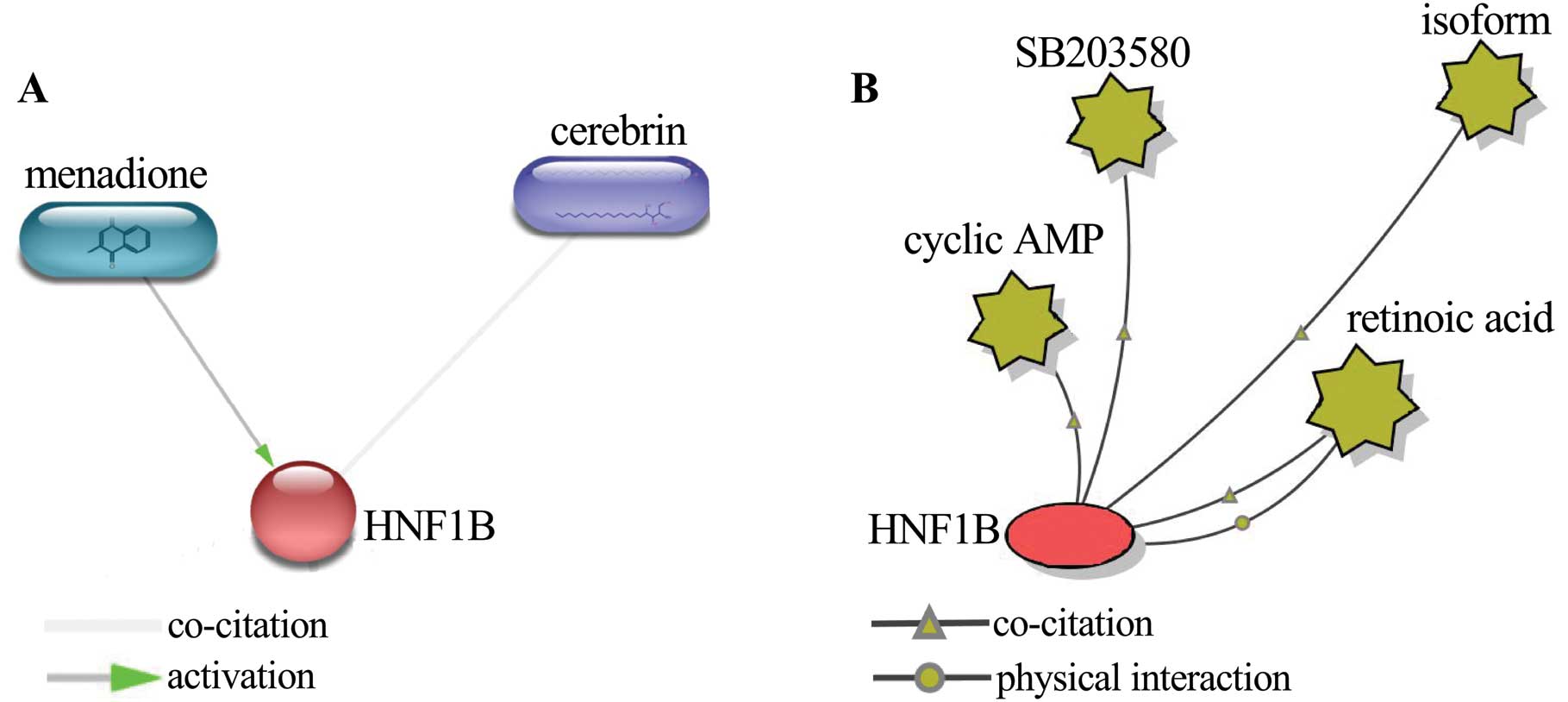
Protein-small molecule/chemical interaction analysis
was performed using STITCH 4.0 beta and BiologicalNetworks2 to
further elucidate the associations of HNF1B with drug resistance in
ovarian cancer. A total of 6 small molecules/chemicals were
identified to interact with HNF1B. Among these, 4 chemicals,
including menadione, SB203580, retinoic acid and cyclic AMP, have
been proven to be closely related to drug resistance in ovarian
cancer. Menadione is identified as a substrate of P-gp, which,
presumably, acts as the mechanism for the chemosensitizing effect
(61). The treatment of ovarian
cancer cells with ascorbate:menadione resulted in the degradation
of nuclear and DNA, which finally led to the cell death (62,63).
Thus, menadione is considered to be a promising chemotherapeutic
enhancer by its ability to circumvent drug resistance, in addition
to its own anticancer activity (61). Menadione activated HNF1B according
to protein-chemical interaction (Fig.
3A), suggesting that the expression of HNF1B sensitizes the
cancer cells to the anticancer drug; this, in turn, indicated that
the decreased expression of HNF1B would contribute to drug
resistance. Furthermore, HNF1B had physical interaction and
co-citation with retinoic acid. Retinoic acid is identified as a
suppressor of ovarian carcinoma cell growth (64); it sensitizes cancer cells to
paclitaxel in part through survivin downregulation and the
promotion of aberrant mitotic progression results in apoptosis
(65). Retinoic acid also
potentiates the chemotherapeutic effect of cisplatin by inducing
differentiation of tumor initiating cells (66). In addition, HNF1B interacts with
SB203580 and cyclic AMP. SB203580 is an inhibitor of p38MAPK, which
is related to paclitaxel resistance of ovarian carcinoma, and
blockade of the p38MAPK pathway can promote the apoptosis of the
drug-resistant cells and reverse the drug resistance (67). The cyclic AMP can reduce the
induction of AP-1 binding, which is required for the activation of
IL8 by paclitaxel (68). The
presence of IL8 in paclitaxel-treated ovarian cancer cells
contributed to the development of paclitaxel resistance (69). These results indicated that the
cyclic AMP is also involved in the development of drug resistance
in ovarian cancer. Collectively, of the 6 small molecules/chemicals
that interacted with HNF1B, 4 were associated with drug resistance
in ovarian cancer, suggesting that HNF1B may contribute to the
development of drug resistance in ovarian cancer.
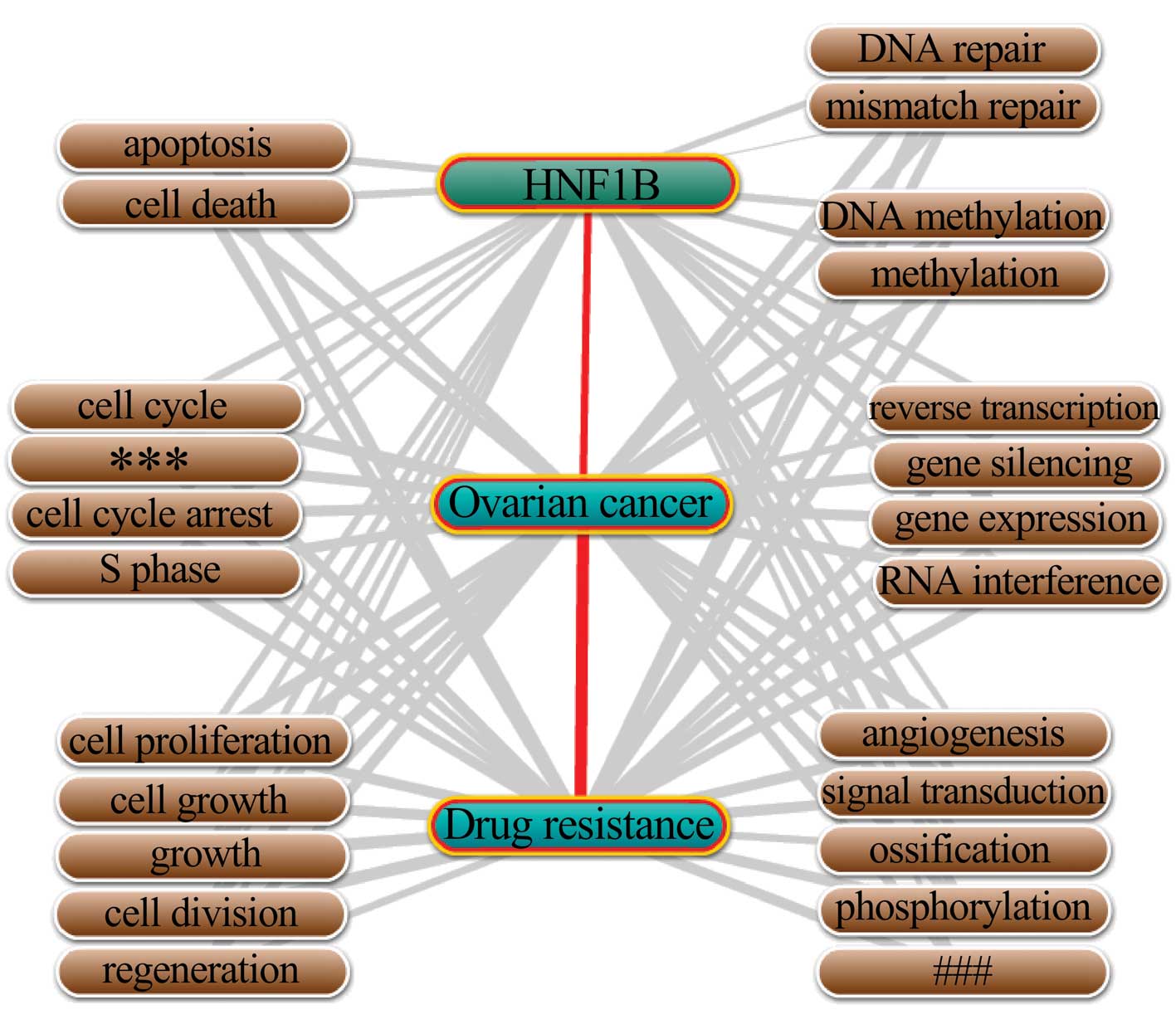
The biological process annotation was performed
using Coremine Medical online database/tool. As shown in Fig. 4, a total of 24 biological processes
were annotated with HNF1B, ovarian cancer and drug resistance
(p<0.01). Given the close relationships of HNF1B with the 24
processes, and the close relationships of the 24 processes with
ovarian cancer and drug resistance, we concluded that HNF1B may
contribute to the drug resistance in ovarian cancer via its effects
on these biological processes. The 24 biological processes which
annotated with HNF1B, ovarian cancer and drug resistance were
varied, while it could still be sub-grouped. As shown in Fig. 4, cell growth related biological
processes (covered 5 processes including cell proliferation, cell
growth, growth, cell division and regeneration), cell cycle-related
(covered 4 processes including cell cycle, regulation of cell
cycle, cell cycle arrest and S phase) and gene expression
regulation-related (covered 4 processes including gene expression,
gene silencing, RNA interference and reverse transcription) may be
the main processes by which HNF1B performs its drug
resistance-related functions in ovarian cancer.
A total of 36 genes (TP53, BCL2, JUN, INS, MYC,
H3F3AP6, TCEAL1, MUC16, ERBB2, WT1, ABCC2, EGFR, SYCE1L, KLHL1,
CDKN1A, AFP, CCND1, PTEN, BCL2L1, BRCA1, CDX2, TYMS, TOP2A, AKT1,
MSLN, EPCAM, KRT20, FOXL2, CASP3, HIF1A, COL11A2, PARP1, YBX1,
PIK3CA, ERBB3 and CDH1) which notably co-occurred with HNF1B,
ovarian cancer and drug resistance, were annotated (p<0.01)
based on the analysis by Coremine Medical online tool, and provided
a strong potentiality that HNF1B would perform its drug
resistance-related functions in ovarian cancer through the
interactions with those genes. The pathway enrichment analysis of
the 36 genes was performed by DAVID online database to enrich the
potential pathways with which HNF1B may be involved in the
regulation of drug resistance. As shown in Table I, in addition to the pathways in
cancers, 4 pathways including ErbB signaling pathway, focal
adhesion, apoptosis and p53 signaling pathway were enriched,
suggesting that HNF1B may be associated with drug resistance
through its regulation on the 4 pathways.
Among the transcriptional targets of HNF1B were 426
microRNAs as predicted through miRWalk, which is made up of ten
miRNA-mRNA prediction tools. Seven microRNAs, i.e., those yielding
the highest score for HNF1B, were selected for subsequent analysis
(Table II). As shown in Table II, among the top 7 microRNAs, 5 of
them, including miR-24, -194, -367, -25 and -375 that targeted
HNF1B influenced drug resistance in ovarian and other types of
cancers. For example, miR-367 is specifically involved in the drug
resistance in paclitaxel-sensitive ovarian cancer cells (70). Similarly, miR-375 is associated with
drug resistance in ovarian (71)
and cervical cancer (72). Although
no study has reported the role of miR-32 and -217 in drug
resistance, it is associated with drug resistance-related processes
such as cell proliferation, invasion and migration (73–75).
Collectively, among the 7 microRNAs most strongly targeting HNF1B,
the majority were involved in drug resistance in ovarian and other
types of cancers, suggesting that the gene also mediates drug
resistance.
The increasing number of sequenced genomes makes it
important to develop methods that can assign functions to newly
discovered genes in a timely and cost-effective manner.
Experimental determination of protein functions is not only
expensive but also time-consuming. Thus, computational approaches
that utilize diverse biological datasets to generate automated
predictions are useful, as they can guide laboratory experiments
and facilitate more rapid annotation of genomes (81,82).
The computational approaches to gene function prediction have
relied on a variety of genomic and proteomic data, at least
including usage of microarray expression data (83), protein-protein interaction networks
(84), protein-small
molecule/chemical interactions (33–35),
and the annotation of gene with biological processes (81). Thus, on the basis of many
large-scale databases and networks, gene function prediction based
on bioinformatics analysis is a potential, feasible and valuable
way for gene function prediction (82). Using the comprehensive
bioinformatics analyses, Yin et al (10) performed an integrated analysis of
tumor suppressor genes with drug resistance in ovarian cancer, and
two genes CCL21 and SPARCL1 associated with drug resistance were
identified (85). Using similar
bioinformatics analysis, upregulation of NEK2 was identified to be
associated with drug resistance in ovarian cancer (86), and upregulation of E2F3 was
identified to be associated with poor prognosis in HCC (87).
The association of HNF1B with drug resistance in
ovarian and other cancers has yet to be reported. In the present
study, a comprehensive bioinformatics analysis was performed to
illustrate the associations of HNF1B with drug resistance in
ovarian cancer, including array data retrieving, protein/gene
interaction, protein-small molecule/chemical interaction,
biological process annotation, gene co-occurrence and pathway
enrichment analysis, and microRNA-mRNA interaction. The
database/tool/software used in this analysis including Oncomine
online database (27,28), GEO profiles (28,29),
GeneMANIA online tool (30–32), STITCH 4.0 beta (33–35),
BiologicalNetworks2 (36,37), Coremine Medical (38), DAVID online tool (39,40)
and miRWalk (41), which are all
regularly used and reliable databases/tools. For example, GeneMANIA
is a web-based database and a tool for prediction of gene functions
on the basis of multiple networks derived from different genomic or
proteomic data/sources (30). Seven
organisms including Homo sapiens are currently supported,
and hundreds of data sets have been collected from GEO, BioGRID,
IRefIndex and I2D, as well as organism-specific functional genomics
data sets (32). With a query gene,
GeneMANIA could find a small set of genes that are most likely to
share function with that gene based on their interactions with it,
and with a query gene list, GeneMANIA could extend the list with
functionally similar genes that it identifies using available
genomics and proteomics data (32).
Several studies indicated that HNF1B is a downstream
transcription activator of Wnt signaling pathway, and performs its
functions via the interaction with the signaling (88–90).
However, the pathway with which HNF1B is involved in cancer
development is less understood. In the present study, pathway
enrichment analysis of 36 genes which co-occurred with HNF1B,
ovarian cancer and drug resistance, was performed. In addition to
the pathways in cancer and pathways related to specified cancers
(such as prostate and colorectal cancer), 4 pathways including ErbB
signaling, focal adhesion, apoptosis and p53 signaling were
enriched, suggesting that HNF1B may contribute to drug resistance
in ovarian cancer via those pathways. ErbB signaling (91), focal adhesion (92,93),
apoptosis (94,95) and p53 signaling (96,97)
have been reported to associate with drug resistance in ovarian
cancer. For example, miR-21 regulates drug resistance via apoptosis
and cellular survival pathways (94), and loss of DOK2 induces carboplatin
resistance in ovarian cancer via suppression of apoptosis (98).
In summary, on the basis of comprehensive
bioinformatics analysis, for the first time, we illustrated that
the downregulation of HNF1B may be associated with drug resistance
in ovarian cancer. The present study may set the stage for further
investigation of the drug resistance-related functions of HNF1B in
ovarian cancer.
This study was sponsored by the Shandong Province
Science and Technology Development Program (2012GSF11821).
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel
E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM,
Sood AK, Stronach EA, Walczak H, Bowtell DD and Balkwill FR:
Rethinking ovarian cancer: recommendations for improving outcomes.
Nat Rev Cancer. 11:719–725. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
4
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson SW, Ozols RF and Hamilton TC:
Mechanisms of drug resistance in ovarian cancer. Cancer. 71(Suppl
2): S644–S649. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng JQ, Jiang X, Fraser M, Li M, Dan HC,
Sun M and Tsang BK: Role of X-linked inhibitor of apoptosis protein
in chemoresistance in ovarian cancer: possible involvement of the
phosphoinositide-3 kinase/Akt pathway. Drug Resist Updat.
5:131–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fraser M, Leung BM, Yan X, Dan HC, Cheng
JQ and Tsang BK: p53 is a determinant of X-linked inhibitor of
apoptosis protein/Akt-mediated chemoresistance in human ovarian
cancer cells. Cancer Res. 63:7081–7088. 2003.PubMed/NCBI
|
|
10
|
Yin F, Liu X, Li D, Wang Q, Zhang W and Li
L: Tumor suppressor genes associated with drug resistance in
ovarian cancer (Review). Oncol Rep. 30:3–10. 2013.PubMed/NCBI
|
|
11
|
Bach I and Yaniv M: More potent
transcriptional activators or a transdominant inhibitor of the HNF1
homeoprotein family are generated by alternative RNA processing.
EMBO J. 12:4229–4242. 1993.PubMed/NCBI
|
|
12
|
Edghill EL, Bingham C, Slingerland AS,
Minton JA, Noordam C, Ellard S and Hattersley AT: Hepatocyte
nuclear factor-1 beta mutations cause neonatal diabetes and
intrauterine growth retardation: support for a critical role of
HNF-1β in human pancreatic development. Diabet Med. 23:1301–1306.
2006.PubMed/NCBI
|
|
13
|
Wu G, Bohn S and Ryffel GU: The HNF1β
transcription factor has several domains involved in nephrogenesis
and partially rescues Pax8/lim1-induced kidney malformations. Eur J
Biochem. 271:3715–3728. 2004.
|
|
14
|
Shao DD, Tsherniak A, Gopal S, Weir BA,
Tamayo P, Stransky N, Schumacher SE, Zack TI, Beroukhim R, Garraway
LA, Margolin AA, Root DE, Hahn WC and Mesirov JP: ATARiS:
computational quantification of gene suppression phenotypes from
multisample RNAi screens. Genome Res. 23:665–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rebouissou S, Vasiliu V, Thomas C,
Bellanné-Chantelot C, Bui H, Chrétien Y, Timsit J, Rosty C,
Laurent-Puig P, Chauveau D and Zucman-Rossi J: Germline hepatocyte
nuclear factor 1α and 1β mutations in renal cell carcinomas. Hum
Mol Genet. 14:603–614. 2005.
|
|
16
|
Terasawa K, Toyota M, Sagae S, Ogi K,
Suzuki H, Sonoda T, Akino K, Maruyama R, Nishikawa N, Imai K,
Shinomura Y, Saito T and Tokino T: Epigenetic inactivation of
TCF2 in ovarian cancer and various cancer cell lines. Br J
Cancer. 94:914–921. 2006.
|
|
17
|
Silva TD, Vidigal VM, Felipe AV, DE Lima
JM, Neto RA, Saad SS and Forones NM: DNA methylation as an
epigenetic biomarker in colorectal cancer. Oncol Lett. 6:1687–1692.
2013.PubMed/NCBI
|
|
18
|
Grisanzio C, Werner L, Takeda D, Awoyemi
BC, Pomerantz MM, Yamada H, Sooriakumaran P, Robinson BD, Leung R,
Schinzel AC, Mills I, Ross-Adams H, Neal DE, Kido M, Yamamoto T,
Petrozziello G, Stack EC, Lis R, Kantoff PW, Loda M, Sartor O,
Egawa S, Tewari AK, Hahn WC and Freedman ML: Genetic and functional
analyses implicate the NUDT11, HNF1B, and
SLC22A3 genes in prostate cancer pathogenesis. Proc Natl
Acad Sci USA. 109:11252–11257. 2012.PubMed/NCBI
|
|
19
|
Gudmundsson J, Sulem P, Steinthorsdottir
V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T,
Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A,
Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason
A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B,
Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G,
Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber
CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T,
Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F,
Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW,
Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge
ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer
CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R,
Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA,
Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A and
Stefansson K: Two variants on chromosome 17 confer prostate cancer
risk, and the one in TCF2 protects against type 2 diabetes.
Nat Genet. 39:977–983. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun J, Zheng SL, Wiklund F, Isaacs SD,
Purcell LD, Gao Z, Hsu FC, Kim ST, Liu W, Zhu Y, Stattin P, Adami
HO, Wiley KE, Dimitrov L, Li T, Turner AR, Adams TS, Adolfsson J,
Johansson JE, Lowey J, Trock BJ, Partin AW, Walsh PC, Trent JM,
Duggan D, Carpten J, Chang BL, Grönberg H, Isaacs WB and Xu J:
Evidence for two independent prostate cancer risk-associated loci
in the HNF1B gene at 17q12. Nat Genet. 40:1153–1155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas G, Jacobs KB, Yeager M, Kraft P,
Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A,
Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J,
Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ,
Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR,
Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL,
Crawford ED, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover R, Hayes
RB, Hunter DJ and Chanock SJ: Multiple loci identified in a
genome-wide association study of prostate cancer. Nat Genet.
40:310–315. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spurdle AB1, Thompson DJ, Ahmed S,
Ferguson K, Healey CS, O’Mara T, Walker LC, Montgomery SB and
Dermitzakis ET; Australian National Endometrial Cancer Study Group.
Fahey P, Montgomery GW, Webb PM, Fasching PA, Beckmann MW, Ekici
AB, Hein A, Lambrechts D, Coenegrachts L, Vergote I, Amant F,
Salvesen HB, Trovik J, Njolstad TS, Helland H, Scott RJ, Ashton K,
Proietto T and Otton G; National Study of Endometrial Cancer
Genetics Group. Tomlinson I, Gorman M, Howarth K, Hodgson S,
Garcia-Closas M, Wentzensen N, Yang H, Chanock S, Hall P, Czene K,
Liu J, Li J, Shu XO, Zheng W, Long J, Xiang YB, Shah M, Morrison J,
Michailidou K, Pharoah PD, Dunning AM and Easton DF: Genome-wide
association study identifies a common variant associated with risk
of endometrial cancer. Nat Genet. 43:451–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elliott KS, Zeggini E, McCarthy MI,
Gudmundsson J, Sulem P, Stacey SN, Thorlacius S, Amundadottir L,
Gronberg H, Xu J, Gaborieau V, Eeles RA, Neal DE, Donovan JL, Hamdy
FC, Muir K, Hwang SJ, Spitz MR, Zanke B, Carvajal-Carmona L, Brown
KM, Hayward NK, Macgregor S, Tomlinson IP, Lemire M, Amos CI,
Murabito JM, Isaacs WB, Easton DF, Brennan P, Barkardottir RB,
Gudbjartsson DF, Rafnar T, Hunter DJ, Chanock SJ, Stefansson K and
Ioannidis JP: Evaluation of association of HNF1B variants
with diverse cancers: collaborative analysis of data from 19
genome-wide association studies. PLoS One. 5:e108582010.PubMed/NCBI
|
|
24
|
Shen H, Fridley BL, Song H, Lawrenson K,
Cunningham JM, Ramus SJ, Cicek MS, Tyrer J, Stram D, Larson MC and
Köbel M; PRACTICAL Consortium. Ziogas A, Zheng W, Yang HP, Wu AH,
Wozniak EL, Woo YL, Winterhoff B, Wik E, Whittemore AS, Wentzensen
N, Weber RP, Vitonis AF, Vincent D, Vierkant RA, Vergote I, Van Den
Berg D, Van Altena AM, Tworoger SS, Thompson PJ, Tessier DC, Terry
KL, Teo SH, Templeman C, Stram DO, Southey MC, Sieh W, Siddiqui N,
Shvetsov YB, Shu XO, Shridhar V, Wang-Gohrke S, Severi G, Schwaab
I, Salvesen HB, Rzepecka IK, Runnebaum IB, Rossing MA,
Rodriguez-Rodriguez L, Risch HA, Renner SP, Poole EM, Pike MC,
Phelan CM, Pelttari LM, Pejovic T, Paul J, Orlow I, Omar SZ, Olson
SH, Odunsi K, Nickels S, Nevanlinna H, Ness RB, Narod SA, Nakanishi
T, Moysich KB, Monteiro AN, Moes-Sosnowska J, Modugno F, Menon U,
McLaughlin JR, McGuire V, Matsuo K, Adenan NA, Massuger LF, Lurie
G, Lundvall L, Lubiński J, Lissowska J, Levine DA, Leminen A, Lee
AW, Le ND, Lambrechts S, Lambrechts D, Kupryjanczyk J, Krakstad C,
Konecny GE, Kjaer SK, Kiemeney LA, Kelemen LE, Keeney GL, Karlan
BY, Karevan R, Kalli KR, Kajiyama H, Ji BT, Jensen A, Jakubowska A,
Iversen E, Hosono S, Høgdall CK, Høgdall E, Hoatlin M, Hillemanns
P, Heitz F, Hein R, Harter P, Halle MK, Hall P, Gronwald J, Gore M,
Goodman MT, Giles GG, Gentry-Maharaj A, Garcia-Closas M, Flanagan
JM, Fasching PA, Ekici AB, Edwards R, Eccles D, Easton DF, Dürst M,
du Bois A, Dörk T, Doherty JA, Despierre E, Dansonka-Mieszkowska A,
Cybulski C, Cramer DW, Cook LS, Chen X, Charbonneau B, Chang-Claude
J, Campbell I, Butzow R, Bunker CH, Brueggmann D, Brown R,
Brooks-Wilson A, Brinton LA, Bogdanova N, Block MS, Benjamin E,
Beesley J, Beckmann MW, Bandera EV, Baglietto L, Bacot F, Armasu
SMK and Hildebrandt MA; Australian Ovarian Cancer Study Group;
Australian Cancer Study. Schildkraut JM, Sellers TA, Huntsman D,
Berchuck A, Chenevix-Trench G, Gayther SA, Pharoah PD, Laird PW,
Goode EL and Pearce CL: Epigenetic analysis leads to identification
of HNF1B as a subtype-specific susceptibility gene for
ovarian cancer. Nat Commun. 4:16282013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anglesio MS, Wiegand KC, Melnyk N, Chow C,
Salamanca C, Prentice LM, Senz J, Yang W, Spillman MA, Cochrane DR,
Shumansky K, Shah SP, Kalloger SE and Huntsman DG: Type-specific
cell line models for type-specific ovarian cancer research. PLoS
One. 8:e721622013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalloger SE, Köbel M, Leung S, Mehl E, Gao
D, Marcon KM, Chow C, Clarke BA, Huntsman DG and Gilks CB:
Calculator for ovarian carcinoma subtype prediction. Mod Pathol.
24:512–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: a cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barrett T and Edgar R: Mining microarray
data at NCBI’s Gene Expression Omnibus (GEO)*. Methods Mol Biol.
338:175–190. 2006.
|
|
30
|
Mostafavi S, Ray D, Warde-Farley D,
Grouios C and Morris Q: GeneMANIA: a real-time multiple association
network integration algorithm for predicting gene function. Genome
Biol. 9(Suppl 1): S42008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD and
Morris Q: The GeneMANIA prediction server: biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38:W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuberi K, Franz M, Rodriguez H, Montojo J,
Lopes CT, Bader GD and Morris Q: GeneMANIA prediction server 2013
update. Nucleic Acids Res. 41:W115–W122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuhn M, Szklarczyk D, Franceschini A, von
Mering C, Jensen LJ and Bork P: STITCH 3: zooming in on
protein-chemical interactions. Nucleic Acids Res. 40:D876–D880.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuhn M, Szklarczyk D, Franceschini A,
Campillos M, von Mering C, Jensen LJ, Beyer A and Bork P: STITCH 2:
an interaction network database for small molecules and proteins.
Nucleic Acids Res. 38:D552–D556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuhn M, von Mering C, Campillos M, Jensen
LJ and Bork P: STITCH: interaction networks of chemicals and
proteins. Nucleic Acids Res. 36:D684–D688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baitaluk M, Sedova M, Ray A and Gupta A:
BiologicalNetworks: visualization and analysis tool for systems
biology. Nucleic Acids Res. 34:W466–W471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kozhenkov S, Dubinina Y, Sedova M, Gupta
A, Ponomarenko J and Baitaluk M: BiologicalNetworks 2.0 - an
integrative view of genome biology data. BMC Bioinformatics.
11:6102010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Leeuw N1, Dijkhuizen T, Hehir-Kwa JY,
Carter NP, Feuk L, Firth HV, Kuhn RM, Ledbetter DH, Martin CL, van
Ravenswaaij-Arts CM, Scherer SW, Shams S, Van Vooren S, Sijmons R,
Swertz M and Hastings R: Diagnostic interpretation of array data
using public databases and internet sources. Hum Mutat. 33:930–940.
2012.PubMed/NCBI
|
|
39
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2008.PubMed/NCBI
|
|
40
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009.PubMed/NCBI
|
|
41
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk - database: prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011.
|
|
42
|
Zhou C, Smith JL and Liu J: Role of BRCA1
in cellular resistance to paclitaxel and ionizing radiation in an
ovarian cancer cell line carrying a defective BRCA1. Oncogene.
22:2396–2404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang D, Khan S, Sun Y, Hess K, Shmulevich
I, Sood AK and Zhang W: Association of BRCA1 and
BRCA2 mutations with survival, chemotherapy sensitivity, and
gene mutator phenotype in patients with ovarian cancer. JAMA.
306:1557–1565. 2011.
|
|
44
|
Lee S, Choi EJ, Jin C and Kim DH:
Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA
mRNA amplification contributes to cisplatin resistance in an
ovarian cancer cell line. Gynecol Oncol. 97:26–34. 2005.
|
|
45
|
Wu H, Cao Y, Weng D, Xing H, Song X, Zhou
J, Xu G, Lu Y, Wang S and Ma D: Effect of tumor suppressor gene
PTEN on the resistance to cisplatin in human ovarian cancer cell
lines and related mechanisms. Cancer Lett. 271:260–271. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang G, Xiao X, Rosen DG, Cheng X, Wu X,
Chang B, Liu G, Xue F, Mercado-Uribe I, Chiao P, Du X and Liu J:
The biphasic role of NF-κB in progression and chemoresistance of
ovarian cancer. Clin Cancer Res. 17:2181–2194. 2011.
|
|
47
|
Zhang X, Wang X, Song X, Liu C, Shi Y,
Wang Y, Afonja O, Ma C, Chen YH and Zhang L: Programmed cell death
4 enhances chemosensitivity of ovarian cancer cells by activating
death receptor pathway in vitro and in vivo. Cancer Sci.
101:2163–2170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Strathdee G, MacKean MJ, Illand M and
Brown R: A role for methylation of the hMLH1 promoter in
loss of hMLH1 expression and drug resistance in ovarian cancer.
Oncogene. 18:2335–2341. 1999.PubMed/NCBI
|
|
49
|
Plumb JA, Strathdee G, Sludden J, Kaye SB
and Brown R: Reversal of drug resistance in human tumor xenografts
by 2′-deoxy-5-azacytidine-induced demethylation of the hMLH1
gene promoter. Cancer Res. 60:6039–6044. 2000.
|
|
50
|
Qiu L, Di W, Jiang Q, Scheffler E, Derby
S, Yang J, Kouttab N, Wanebo H, Yan B and Wan Y: Targeted
inhibition of transient activation of the EGFR-mediated cell
survival pathway enhances paclitaxel-induced ovarian cancer cell
death. Int J Oncol. 27:1441–1448. 2005.PubMed/NCBI
|
|
51
|
Skirnisdóttir I, Sorbe B and Seidal T: The
growth factor receptors HER-2/neu and EGFR, their relationship, and
their effects on the prognosis in early stage (FIGO I–II)
epithelial ovarian carcinoma. Int J Gynecol Cancer. 11:119–129.
2001.PubMed/NCBI
|
|
52
|
Houvras Y, Benezra M, Zhang H, Manfredi
JJ, Weber BL and Licht JD: BRCA1 physically and functionally
interacts with ATF1. J Biol Chem. 275:36230–36237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Balch C, Naegeli K, Nam S, Ballard B,
Hyslop A, Melki C, Reilly E, Hur MW and Nephew KP: A unique histone
deacetylase inhibitor alters microRNA expression and signal
transduction in chemoresistant ovarian cancer cells. Cancer Biol
Ther. 13:681–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Belanger AS, Tojcic J, Harvey M and
Guillemette C: Regulation of UGT1A1 and HNF1
transcription factor gene expression by DNA methylation in colon
cancer cells. BMC Mol Biol. 11:92010.
|
|
55
|
Reuning U, Guerrini L, Nishiguchi T, Page
S, Seibold H, Magdolen V, Graeff H and Schmitt M: Rel transcription
factors contribute to elevated urokinase expression in human
ovarian carcinoma cells. Eur J Biochem. 259:143–148. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen H, Hao J, Wang L and Li Y:
Coexpression of invasive markers (uPA, CD44) and multiple
drug-resistance proteins (MDR1, MRP2) is correlated with epithelial
ovarian cancer progression. Br J Cancer. 101:432–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Danoy P, Sonoda E, Lathrop M, Takeda S and
Matsuda F: A naturally occurring genetic variant of human
XRCC2 (R188H) confers increased resistance to
cisplatin-induced DNA damage. Biochem Biophys Res Commun.
352:763–768. 2007.PubMed/NCBI
|
|
58
|
Fink D, Nebel S, Aebi S, Nehme A and
Howell S: Loss of DNA mismatch repair due to knockout of MSH2 or
PMS2 results in resistance to cisplatin and carboplatin. Int J
Oncol. 11:539–542. 1997.PubMed/NCBI
|
|
59
|
Gerhardt J, Montani M, Wild P, Beer M,
Huber F, Hermanns T, Muntener M and Kristiansen G: FOXA1 promotes
tumor progression in prostate cancer and represents a novel
hallmark of castration-resistant prostate cancer. Am J Pathol.
180:848–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nadkarni A, Furda A, Rajesh C, McInnes C,
Ruch RJ and Pittman DL: Functional characterization of the RAD51D
E233G genetic variant. Pharmacogenet Genomics. 19:153–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Oh SJ, Han HK, Kang KW, Lee YJ and Lee MY:
Menadione serves as a substrate for P-glycoprotein: implication in
chemosensitizing activity. Arch Pharm Res. 36:509–516. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gilloteaux J, Jamison JM, Lorimer HE,
Jarjoura D, Taper HS, Calderon PB, Neal DR and Summers JL:
Autoschizis: a new form of cell death for human ovarian carcinoma
cells following ascorbate:menadione treatment. Nuclear and DNA
degradation. Tissue Cell. 36:197–209. 2004. View Article : Google Scholar
|
|
63
|
Gilloteaux J, Jamison JM, Arnold D,
Jarjoura D, Von Greuningen V and Summers JL: Autoschizis of human
ovarian carcinoma cells: scanning electron and light microscopy of
a new cell death induced by sodium ascorbate: menadione treatment.
Scanning. 25:137–149. 2003. View Article : Google Scholar
|
|
64
|
Zhang D, Holmes WF, Wu S, Soprano DR and
Soprano KJ: Retinoids and ovarian cancer. J Cell Physiol. 185:1–20.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pratt MA, Niu MY and Renart LI: Regulation
of survivin by retinoic acid and its role in paclitaxel-mediated
cytotoxicity in MCF-7 breast cancer cells. Apoptosis. 11:589–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Y, Guan DX, Shi J, Gao H, Li JJ,
Zhao JS, Qiu L, Liu J, Li N, Guo WX, Xue J, Zhou FG, Wu MC, Wang
HY, Xie D and Cheng SQ: All-trans retinoic acid potentiates the
chemotherapeutic effect of cisplatin by inducing differentiation of
tumor initiating cells in liver cancer. J Hepatol. 59:1255–1263.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu M, Xiao L and Li Z: The relationship
between p38MAPK and apoptosis during paclitaxel resistance of
ovarian cancer cells. J Huazhong Univ Sci Technolog Med Sci.
27:725–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lee LF, Haskill JS, Mukaida N, Matsushima
K and Ting JP: Identification of tumor-specific paclitaxel
(Taxol)-responsive regulatory elements in the interleukin-8
promoter. Mol Cell Biol. 17:5097–5105. 1997.PubMed/NCBI
|
|
69
|
Duan Z, Feller AJ, Penson RT, Chabner BA
and Seiden MV: Discovery of differentially expressed genes
associated with paclitaxel resistance using cDNA array technology:
analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic
protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res.
5:3445–3453. 1999.
|
|
70
|
Chen N, Chon HS, Xiong Y, Marchion DC,
Judson PL, Hakam A, Gonzalez-Bosquet J, Permuth-Wey J, Wenham RM,
Apte SM, Cheng JQ, Sellers TA and Lancaster JM: Human cancer cell
line microRNAs associated with in vitro sensitivity to
paclitaxel. Oncol Rep. 31:376–383. 2014.PubMed/NCBI
|
|
71
|
Shao X, Mei W, Weng W, Qin J, Zhou J, Liu
J and Cheng J: Mir-375 enhances ruthenium-derived compound Rawq01
induced cell death in human ovarian cancer. Int J Clin Exp Pathol.
6:1095–1102. 2013.PubMed/NCBI
|
|
72
|
Shen Y, Wang P, Li Y, Ye F, Wang F, Wan X,
Cheng X, Lu W and Xie X: miR-375 is upregulated in acquired
paclitaxel resistance in cervical cancer. Br J Cancer. 109:92–99.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang J, Kuai X, Song M, Chen X, Yu Z,
Zhang H and Mao Z: microRNA-32 inhibits the proliferation and
invasion of the SGC-7901 gastric cancer cell line in vitro.
Oncol Lett. 7:270–274. 2014.PubMed/NCBI
|
|
74
|
Wu W, Yang J, Feng X, Wang H, Ye S, Yang
P, Tan W, Wei G and Zhou Y: MicroRNA-32 (miR-32) regulates
phosphatase and tensin homologue (PTEN) expression and promotes
growth, migration, and invasion in colorectal carcinoma cells. Mol
Cancer. 12:302013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li H, Zhao J, Zhang JW, Huang QY, Huang
JZ, Chi LS, Tang HJ, Liu GQ, Zhu DJ and Ma WM: MicroRNA-217,
down-regulated in clear cell renal cell carcinoma and associated
with lower survival, suppresses cell proliferation and migration.
Neoplasma. 60:511–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Husted S, Søkilde R, Rask L, Cirera S,
Busk PK, Eriksen J and Litman T: MicroRNA expression profiles
associated with development of drug resistance in Ehrlich ascites
tumor cells. Mol Pharm. 8:2055–2062. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Singh R and Saini N: Downregulation of
BCL2 by miRNAs augments drug-induced apoptosis - a combined
computational and experimental approach. J Cell Sci. 125:1568–1578.
2012.
|
|
78
|
Srivastava N, Manvati S, Srivastava A, Pal
R, Kalaiarasan P, Chattopadhyay S, Gochhait S, Dua R and Bamezai
RN: miR-24–2 controls H2AFX expression regardless of gene
copy number alteration and induces apoptosis by targeting
antiapoptotic gene BCL-2: a potential for therapeutic
intervention. Breast Cancer Res. 13:R392011.
|
|
79
|
Dong P, Kaneuchi M, Watari H, Hamada J,
Sudo S, Ju J and Sakuragi N: MicroRNA-194 inhibits epithelial to
mesenchymal transition of endometrial cancer cells by targeting
oncogene BMI-1. Mol Cancer. 10:992011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhou Y, Hu Y, Yang M, Jat P, Li K,
Lombardo Y, Xiong D, Coombes RC, Raguz S and Yagüe E: The
miR-106b~25 cluster promotes bypass of doxorubicin-induced
senescence and increase in motility and invasion by targeting the
E-cadherin transcriptional activator EP300. Cell Death Differ.
21:462–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Phuong T and Nhung N: Predicting gene
function using similarity learning. BMC Genomics. 14(Suppl 4):
S42013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sharan R, Ulitsky I and Shamir R:
Network-based prediction of protein function. Mol Syst Biol.
3:882007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Stuart JM, Segal E, Koller D and Kim SK: A
gene-coexpression network for global discovery of conserved genetic
modules. Science. 302:249–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Uetz P, Giot L, Cagney G, Mansfield TA,
Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart
P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T,
Vijayadamodar G, Yang M, Johnston M, Fields S and Rothberg JM: A
comprehensive analysis of protein-protein interactions in
Saccharomyces cerevisiae. Nature. 403:623–627. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yin F, Liu X, Li D, Wang Q, Zhang W and Li
L: Bioinformatic analysis of chemokine (C-C motif) ligand 21 and
SPARC-like protein 1 revealing their associations with drug
resistance in ovarian cancer. Int J Oncol. 42:1305–1316.
2013.PubMed/NCBI
|
|
86
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Upregulation of NEK2 is associated with drug resistance in
ovarian cancer. Oncol Rep. 31:745–754. 2014.PubMed/NCBI
|
|
87
|
Zeng X, Yin F, Liu X, Xu J, Xu Y, Huang J,
Nan Y and Qiu X: Upregulation of E2F transcription factor 3 is
associated with poor prognosis in hepatocellular carcinoma. Oncol
Rep. 31:1139–1146. 2014.PubMed/NCBI
|
|
88
|
Roose J and Clevers H: TCF transcription
factors: molecular switches in carcinogenesis. Biochim Biophys
Acta. 1424:M23–M37. 1999.PubMed/NCBI
|
|
89
|
Lancman JJ, Zvenigorodsky N, Gates KP,
Zhang D, Solomon K, Humphrey RK, Kuo T, Setiawan L, Verkade H, Chi
YI, Jhala US, Wright CV, Stainier DY and Dong PD: Specification of
hepatopancreas progenitors in zebrafish by hnf1ba and
wnt2bb. Development. 140:2669–2679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Welters HJ, Oknianska A, Erdmann KS,
Ryffel GU and Morgan NG: The protein tyrosine phosphatase-BL,
modulates pancreatic β-cell proliferation by interaction with the
Wnt signalling pathway. J Endocrinol. 197:543–552. 2008.PubMed/NCBI
|
|
91
|
Manandhar S, Choi BH, Jung KA, Ryoo IG,
Song M, Kang SJ, Choi HG, Kim JA, Park PH and Kwak MK: NRF2
inhibition represses ErbB2 signaling in ovarian carcinoma cells:
implications for tumor growth retardation and docetaxel
sensitivity. Free Radic Biol Med. 52:1773–1785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kang Y, Hu W, Ivan C, Dalton HJ, Miyake T,
Pecot CV, Zand B, Liu T, Huang J, Jennings NB, Rupaimoole R, Taylor
M, Pradeep S, Wu SY, Lu C, Wen Y, Liu J and Sood AK: Role of focal
adhesion kinase in regulating YB-1-mediated paclitaxel resistance
in ovarian cancer. J Natl Cancer Inst. 105:1485–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Halder J, Landen CN Jr, Lutgendorf SK, Li
Y, Jennings NB, Fan D, Nelkin GM, Schmandt R, Schaller MD and Sood
AK: Focal adhesion kinase silencing augments docetaxel-mediated
apoptosis in ovarian cancer cells. Clin Cancer Res. 11:8829–8836.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chan JK, Blansit K, Kiet T, Sherman A,
Wong G, Earle C and Bourguignon LY: The inhibition of miR-21
promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol
Oncol. 132:739–744. 2014.
|
|
95
|
Farrand L, Byun S, Kim JY, Im-Aram A, Lee
J, Lim S, Lee KW, Suh JY, Lee HJ and Tsang BK: Piceatannol enhances
cisplatin sensitivity in ovarian cancer via modulation of p53,
X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial
fission. J Biol Chem. 288:23740–23750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Benoit DS, Henry SM, Shubin AD, Hoffman AS
and Stayton PS: pH-responsive polymeric sirna carriers sensitize
multidrug resistant ovarian cancer cells to doxorubicin via
knockdown of polo-like kinase 1. Mol Pharm. 7:442–455. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yan X, Fraser M, Qiu Q and Tsang BK:
Over-expression of PTEN sensitizes human ovarian cancer cells to
cisplatin-induced apoptosis in a p53-dependent manner. Gynecol
Oncol. 102:348–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lum E, Vigliotti M, Banerjee N, Cutter N,
Wrzeszczynski KO, Khan S, Kamalakaran S, Levine DA, Dimitrova N and
Lucito R: Loss of DOK2 induces carboplatin resistance in
ovarian cancer via suppression of apoptosis. Gynecol Oncol.
130:369–376. 2013.
|