Introduction
Centella asiatica (C. asiatica) is used as a
traditional medicine in Asia (1).
Four classes of triterpenoids are present in C. asiatica
extract: asiatic acid, madecassic acid, asiaticoside, and
madecassoside (2). Asiaticoside,
the most abundant triterpene glycoside in the extract, is converted
to asiatic acid by hydrolysis in vivo (3). Asiatic acid functions as an
antioxidant and antitoxic agent in normal cells, and as an
anticancer agent in cancer cells (4–8).
Several studies have shown that pretreatment with asiatic acid has
a protective effect against H2O2-induced
cytotoxic damage in neuronal cells and against
D-galactosamine/lipopolysaccharide-induced toxicity in hepatocytes
(4,5). However, various reports have also
shown that asiatic acid mediates a variety of anticancer effects as
a result of growth inhibition and apoptosis in several types of
cancer cells, such as HepG2 human hepatoma, HT-29 human colon
adenocarcinoma, and MCF7 human breast cancer cells (6–8).
Although the role of asiatic acid in cancer cell death has been
widely studied, asiatic-mediated cellular mechanisms are less well
known.
Apoptosis, or programmed cell death, is
characterized by several morphological and biochemical changes,
including reduced cell volume, condensation of nuclear chromatin,
DNA fragmentation, and an increased proportion of sub-G1 phase
cells (9). Initiation of apoptotic
processes in cells induces activation of proapoptotic proteins, as
well as inactivation of antiapoptotic proteins (10). B cell CLL/lymphoma 2 (BCL2) family
proteins are involved in the regulation of apoptosis either as
death antagonists or death agonists (11). Of these, BCL2 is a critical
regulator of cell growth and antiapoptotic processes. Under normal
physiological conditions, BCL2 binds to the proapoptotic proteins
BAX and BAK to prevent mitochondrial outer membrane
permeabilization and cytochrome c release into the cytosol.
Many tumor cells overexpress antiapoptotic BCL2 and become
resistant to chemotherapy and radiotherapy (11). Recently, asiatic acid was reported
to induce apoptosis in several types of cancer cell, and to affect
the level of expression of BCL2 and BCL-XL (7,8).
However, the mechanisms underlying asiatic acid-induced
downregulation of BCL2 are largely unknown.
MicroRNAs (miRNAs) are small RNAs, 19–25 nucleotides
in length, that play important roles in growth, differentiation and
cell death (12). Various oncogenic
miRNAs (oncomiRs) and tumor suppressor miRNAs have been identified.
For example, miR-34a inhibits oncogenic cellular transformation by
suppressing oncogenes, such as proto-oncogene c-Met,
cyclin-dependent kinase 4 (CDK4), and BCL2 (13–15).
miR-21, which is a well-studied oncomiR, also targets many key
tumor suppressor genes, such as phosphatase and tensin homolog
(PTEN), programmed cell death 4 (PDCD4), and TP63 (16–19).
Recently, miR-21 was implicated in the phenomenon of cancer cell
addiction to key oncogenes (20).
This finding strongly indicates that an examination of miRNA-based
cellular mechanisms is critical to an understanding of
tumorigenesis and anticancer processes, and certain miRNAs may be
critical targets for anticancer therapy. We previously reported
that treatment of human cells with C. asiatica extract
altered miRNA expression profiles (21,22),
indicating that the effects of the extract on cells might involve
miRNA-specific mechanisms.
In the present study, using miRNA microarray, we
showed that asiatic acid alters expression profiles of specific
miRNAs in A549 non-small cell lung carcinoma (NSCLC) cells. We also
found that miR-1290 is the most extensively upregulated miRNA, and
that asiatic acid-induced anticancer activity is dependent on the
expression of this miRNA. We further demonstrated that miR-1290
directly targets BCL2 mRNA in the cell.
Materials and methods
Cell lines, RNA oligonucleotide and
chemicals
A549 NSCLC cells (American Type Culture Collection,
Manassas, VA, USA) were grown in RPMI-1640 (Gibco; Life
Technologies, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml
penicillin, and 100 μg/ml streptomycin. Asiatic acid
(Sigma-Aldrich) was dissolved in DMSO and stock in 25, 50, 75, and
100 mM. To evaluate the cytotoxicity of asiatic acid, A549 cells
were seeded in 96-well plates at a density of 4×104
cells/well. miR-1290 mimic, antagomiR-1290, and negative control
miRNA were purchased from Qiagen (Hilden, Germany). All miRNAs were
resuspended in nuclease-free water (USB; Affymetrix, Santa Clara,
CA, USA) to a final stock concentration of 100 μM. miRNA
transfection was performed in serum-free medium with Lipofectamine
RNAiMAX (Invitrogen; Life Technologies) for 6 h. Medium was
replaced with fresh medium, and the cells were incubated for 24
h.
Cell viability assay
The cytotoxicity of asiatic acid in A549 cells was
evaluated using a water-soluble tetrazolium salt (WST-1) assay
(EZ-Cytox Cell Viability Assay Kit; Itsbio, Seoul, Korea). WST-1
solution was added to cultures at a volume 10% that of the culture
medium, and cells were incubated at 37°C for 0.5 h. Cell viability
was evaluated by measuring the absorbance at 450 nM using an iMark
microplate reader (Bio-Rad, Hercules, CA, USA). All results are
presented as the mean percentage ± standard deviation (SD) of three
independent experiments. A P-value of <0.005 (or <0.01), as
determined by the Student’s t-test, was considered to indicate a
statistically significant difference.
Analysis of cell cycle by flow
cytometry
Cells were collected and fixed in cold 70% ethanol
at 4°C for 1 h. Fixed cells were then stained by incubation with
propidium iodide (PI) staining solution [50 μg/ml PI, 0.5% Triton
X-100 (both from Sigma-Aldrich), and 100 μg/ml RNase] at 37°C for 1
h. Changes in cell cycle were determined based on evaluation of the
intensity of fluorescent PI staining of 10,000 cells using the
FL2-H channel of a FACSCalibur (BD Biosciences, San Jose, CA,
USA).
Construction of 3′-UTR reporter plasmid
and luciferase assay
To construct luciferase reporter plasmids, target
fragments were ligated into the XbaI site downstream of the
luciferase gene in the pGL3-promoter vector (Promega, Madison, WI,
USA). The region (+2776 to +3286) in the human BCL2 gene containing
miR-1290 recognition site was amplified by PCR using the following
primers adapted to the XbaI site:
5′-CTAGTCTAGACTAGGTGACAGTTATATCTGTTG TCC-3′ and
5′-CTAGTCTAGACTAGCCACGTGGAGCATA CTGC-3′. DNA sequence analyses
confirmed the nucleotides sequence of the constructed plasmid
(pGL3-BCL2-3′-UTR). For the luciferase assays, pGL3 and
pGL3-BCL2-3′-UTR plasmids were transiently transfected with
negative control miRNA, miR-1290 or antagomiR-1290 mimics and the
pSV-β-galactosidase (pSV-β-gal) plasmid into the cells. Then,
luciferase activity and β-galactosidase activity were assayed as
described previously (36).
Briefly, after transfection, the cells were re-suspended in Passive
Lysis Buffer (Promega Corp., Madison, WI, USA), and the luciferase
activity was measured with a Veritas Luminometer (Turnur Designs,
Sunnyvale, CA, USA). β-galactosidase activity was measured using
Luminescent β-galactosidase Detection Kit II (Clontech
Laboratories, Inc., Mountain View, CA, USA) according to the
manufacturer’s protocol. The relative luciferase activity was
normalized to β-galactosidase activity. The results are the
averages of three independent experiments.
Isolation of total RNA and quantitative
real-time PCR analysis
Total RNA was isolated using TRIzol reagent (Life
Technologies) according to the manufacturer’s protocol. The purity
and concentration of the RNA was evaluated based on the OD at 260
nm and the OD 260/230 and 260/280 ratios using
MaestroNano®, a micro-volume spectrophotometer
(Maestrogen, Las Vegas, NV, USA). The recommended parameters of RNA
quality for cDNA synthesis are OD 260/230 >1.8 and OD 260/280
ratio in the range of 1.8–2.0. cDNAs for sensitive and specific
miRNA detection were synthesized using the miScript II RT Kit
(Qiagen) according to the manufacturer’s protocol. Quantitative
real-time PCR was performed using the miR-1290-specific primer,
Hs_miR-1290_1 miScript Primer Assay (Qiagen), and U6 snRNA-specific
primer, Hs_RNU6-2_11 miScript Primer Assay (Qiagen). PCR was
performed using the miScript SYBR-Green PCR Kit (Qiagen) with
Line-Gene K software (Bioer Technology Co. Ltd., Hangzhou, China).
The CT value for miR-1290 was normalized to U6
snRNA. The 2−ΔΔCt method was used to calculate relative
expression level of miRNAs.
miRNA microarray analysis
Microarray analysis was performed using SurePrint G3
Human V16 miRNA 8×60K (Agilent, Santa Clara, CA, USA), according to
the manufacturer’s protocol. Briefly, 50 ng total purified RNA was
treated with calf intestine alkaline phosphatase and labeled with
cyanine 3-cytidine bisphosphate using T4 RNA ligase. The labeled
RNAs were hybridized to the probes on the microarray. The
microarray data were analyzed using GeneSpring GX software version
11.5 (Agilent). The raw data were filtered using FLAG and t-test,
and applied to the fold-change analysis. The fold-change analysis
was conducted based on a factor of 2.0-fold between two groups,
DMSO-treated control cells and asiatic acid (50 μM)-treated
cells.
Western blotting
Cells were collected and washed with cold
phosphate-buffered saline (PBS). The cell pellets were lysed using
modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris,
pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS)
containing protease inhibitors (complete Tablets, Mini, EDTA-free,
EASYpack; Roche Applied Science, Mannheim, Germany) at 4°C for 20
min. The lysates were then centrifuged at 12,000 × g for 30 min.
The supernatant was decanted and saved. The concentration of total
protein was determined using Bradford assay (Bio-Rad). Fifty
micrograms of total cellular protein were solubilized in SDS sample
buffer and resolved by SDS-PAGE. Anti-BCL2 and anti-β-actin primary
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA) and Sigma-Aldrich, respectively.
Results
Characterization of asiatic acid-induced
toxicity in A549 NSCLC cells
We first sought to determine whether asiatic acid, a
triterpenoid derived from C. asiatica, affects the viability
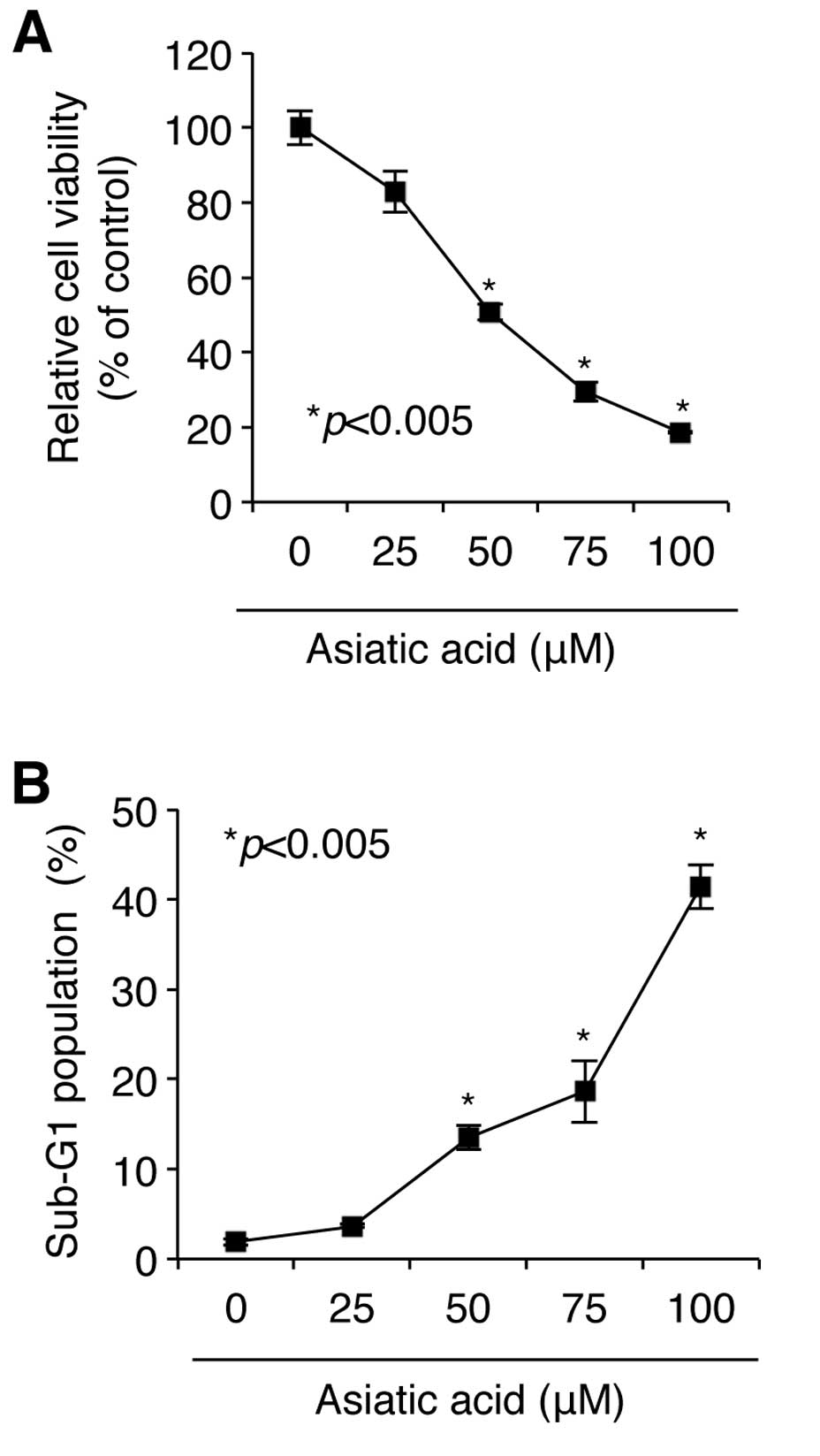
of A549 NSCLC cells. As shown in Fig.
1A, exposure of A549 cell cultures to concentrations of asiatic
acid ranging from 0–100 μM for 24 h induced cytotoxicity in a
concentration-dependent manner, as determined by WST-1 assay.
Maximal toxicity was obtained at a concentration of 100 μM, at
which concentration cell viability was reduced to 18.63±0.13% of
the control value (n=3). The IC50 (the concentration at
which half maximal toxicity is observed) of asiatic acid was 50 μM;
at this concentration, cell viability was reduced to 50.81±2.13% of
control value (Fig. 1A).
We then examined the mechanism underlying asiatic
acid-induced loss of viability in NSCLC A549 cells. Cells were
treated with asiatic acid ranging from 0–100 μM for 24 h,
stained with PI, and DNA content was analyzed by flow cytometry. As
shown in Fig. 1B, asiatic acid
caused a concentration-dependent increase in the proportion of
cells in the sub-G1 phase, indicating that the effect of asiatic
acid in NSCLC A549 cells is cell death-dependent. Although 25
μM asiatic acid increased the proportion of cells in sub-G1
by only 3.67±0.16%, concentrations of 50 and 100 μM asiatic acid
were associated with increases of 13.50±1.31% and 41.46±2.45%,
respectively. These results demonstrate that asiatic acid induces
cell death-mediated loss of cell viability in NSCLC A549 cells.
Identification of miRNAs altered by
asiatic acid treatment in NSCLC A549 cells
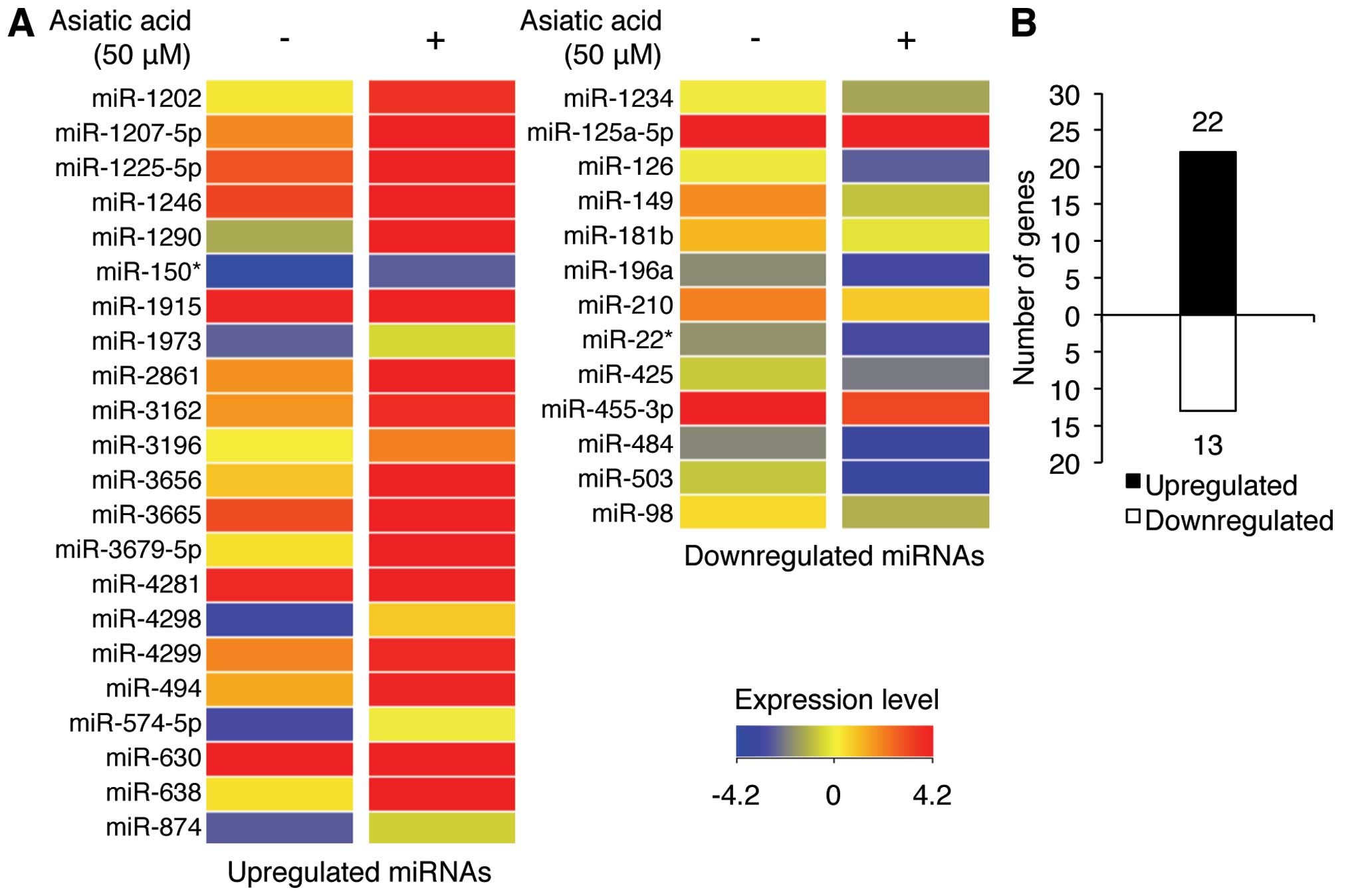
We used miRNA microarray analysis to investigate the
effect of asiatic acid treatment of A549 cells on miRNA expression
profiles. Exposure of A549 cells to 50 μM asiatic acid
increased or decreased (P<0.05) the expression levels of certain
miRNAs by >1.5-fold (Fig. 2A and
Table I). Of these, 22 miRNAs were
upregulated and 13 were downregulated (Fig. 2B). Notably, miR-1290, miR-1246, and
miR-630 were significantly increased by 44.56-, 20.35-, and
9.11-fold, respectively, whereas expression of miR-503, miR-149-5p,
and miR-126 were significantly downregulated by 2.53-, 2.47-, and
2.33-fold, respectively (Table I).
These data suggest that the mechanism underlying asiatic
acid-induced cell death is likely mediated by specific miRNAs.
Notably, miR-1290 showed the greatest increase in expression with
asiatic acid treatment. We, therefore, hypothesized that asiatic
acid-induced cell death in NSCLS A549 cells may be related to the
altered level of miR-1290 expression.
 | Table ImiRNAs altered in response to asiatic
acid treatment in A549 cells. |
Table I
miRNAs altered in response to asiatic
acid treatment in A549 cells.
| miRNAa | FCb | Chromosome |
|---|
| hsa-miR-1202 | 3.10 | 6 |
|
hsa-miR-1207-5p | 2.51 | 8 |
|
hsa-miR-1225-5p | 2.43 | 16 |
| hsa-miR-1246 | 20.35 | 2 |
| hsa-miR-1290 | 44.56 | 1 |
| hsa-miR-150-3p | 2.20 | 19 |
|
hsa-miR-1915-3p | 2.23 | 10 |
| hsa-miR-1973 | 1.96 | 4 |
| hsa-miR-2861 | 3.36 | 9 |
|
hsa-miR-3162-5p | 1.96 | 11 |
| hsa-miR-3196 | 1.91 | 20 |
| hsa-miR-3656 | 3.23 | 11 |
| hsa-miR-3665 | 1.79 | 13 |
|
hsa-miR-3679-5p | 4.08 | 2 |
| hsa-miR-4281 | 2.36 | 5 |
| hsa-miR-4298 | 3.79 | 11 |
| hsa-miR-4299 | 1.88 | 11 |
| hsa-miR-494 | 2.44 | 14 |
| hsa-miR-574-5p | 2.72 | 4 |
| hsa-miR-630 | 9.11 | 15 |
| hsa-miR-638 | 3.63 | 19 |
| hsa-miR-874 | 1.95 | 5 |
| hsa-miR-1234 | −1.57 | 8 |
|
hsa-miR-125a-5p | −1.53 | 19 |
| hsa-miR-126-3p | −2.33 | 9 |
| hsa-miR-149-5p | −2.47 | 2 |
|
hsa-miR-181b-5p | −1.58 | 1 |
|
hsa-miR-196a-5p | −1.52 | 12 |
| hsa-miR-210 | −1.52 | 11 |
| hsa-miR-22-5p | −1.53 | 17 |
| hsa-miR-425-5p | −1.59 | 3 |
| hsa-miR-455-3p | −1.52 | 9 |
| hsa-miR-484 | −1.56 | 16 |
| hsa-miR-503 | −2.53 | X |
| hsa-miR-98 | −1.77 | X |
Upregulation of miR-1290 expression is
associated with asiatic acid-induced cell death in NSCLC A549
cells
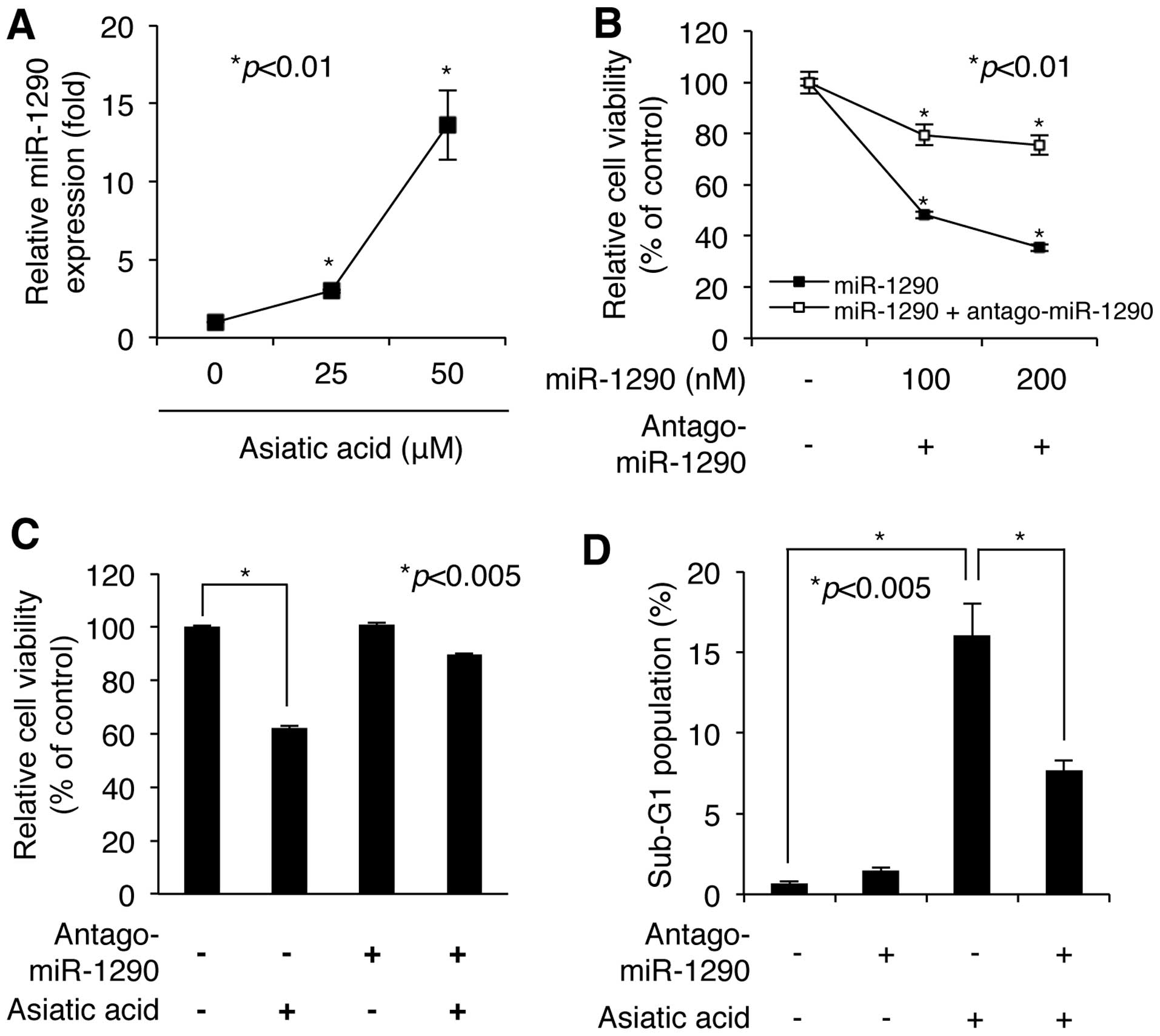
We next confirmed the asiatic acid-induced increase
in miR-1290 level using quantitative RT-PCR with primers specific
for the mature miR-1290 sequence. As shown in Fig. 3A, exposure of A549 NSCLC cells to 25
and 50 μM asiatic acid for 24 h induced large increases of
2.99-fold and 13.61-fold, respectively, in expression level of
mature miR-1290. We then investigated the biological significance
of miR-1290 upregulation in A549 cells in transient transfection
experiments. As shown in Fig. 3B,
A549 cells transfected with 100 and 200 nM miR-1290 mimic underwent
loss of viability in a dose-dependent manner, as assessed by WST-1
assay. Maximal loss of cell viability was obtained at a
concentration of 200 nM miR-1290 mimic, which decreased cell
viability to 35.35±1.2% of the control value (n=3) (Fig. 3B). However, inhibition of miR-1290
activity by antagomiR-1290 blocked most of the miR-1290-induced
decrease in cell viability (Fig.
3B). Notably, cotransfection of 200 nM miR-1290 mimic and an
equal amount of its antagomiR showed 75.49±3.76% (n=3) of control
cell viability, whereas transfection with miR-1290 mimic alone
showed 35.35±1.2% of control viability (Fig. 3B). These results indicate that
miR-1290 upregulation could be involved in cell growth defect.
Next, we further evaluated whether asiatic
acid-induced loss of cell viability is dependent on miR-1290
upregulation. Prior to exposure of NSCLC A549 cells to asiatic acid
(50 μM), cells were transfected with antagomiR-1290 (200 nM)
for 24 h. As shown in Fig. 3C,
cells transfected with the antagomiR showed resistance to asiatic
acid-induced cytotoxicity. Flow cytometric analysis after staining
with PI also showed that asiatic acid-induced cell death was
significantly attenuated in the presence of antagomiR-1290
(Fig. 3D). Although exposure of
control cells to asiatic acid (50 μM) showed a 16.05±1.99%
increase in the proportion of the cell population in sub-G1 phase,
exposure of antagomiR-1290-transfected cells to asiatic acid showed
only a 7.69±0.59 increase. These results demonstrate that asiatic
acid-induced cell death is mediated by accumulation of miR-1290 in
NSCLC A549 cells.
miR-1290 upregulated by asiatic acid
targets BCL2 mRNA in NSCLC A549 cells
Previous studies showed that asiatic acid induced
cell growth inhibition by decreasing the level of BCL2 protein in
MCF7 human breast cells and HT-29 human colon adenocarcinoma cells
(7,8). We also evaluated the effect of asiatic
acid on the levels of BCL2 in A549 cells. Asiatic acid strongly
decreased intracellular levels of BCL2 protein, as revealed by
western blotting (Fig. 4A). We next
investigated whether there was a correlation between the reduced
level of BCL2 and increased miR-1290 expression in asiatic
acid-treated A549 cells. We first used bioinformatics analysis to
analyze the possibility that miR-1290 targets BCL2 mRNA. As
shown in Fig. 4B, miR-1290 contains
a sequence complementary sequence to the 3′-UTR of human
BCL2 mRNA. The predicted target site of miR-1290 is encoded
by nucleotides 2898–2916 in the 3′-UTR (Fig. 4B). We therefore examined whether
miR-1290 reduces BCL2 protein levels in NSCLC A549 cells. As shown
in Fig. 4C, exposure of cells to
miR-1290 mimic (50, 100, and 150 nM) significantly decreased BCL2
level in a dose-dependent manner. We also found that the decrease
in BCL2 was largely blocked by antagomiR-1290 (Fig. 4D). In addition, we sought to
determine whether the downregulation of BCL2 by asiatic acid
treatment was affected by inhibition of miR-1290 activity. Notably,
the asiatic acid-induced decrease in BCL2 decrease was largely
rescued in antagomiR-1290-transfected cells (Fig. 4E). To assess whether BCL2 is a
direct target of miR-1290, we constructed recombinant luciferase
plasmids in which the 3-UTR of BCL2 is cloned downstream of the
firefly luciferase reporter gene in pGL3 vectors. The reporter
plasmid was cotransfected with either the miR-1290 mimic or its
antagomiR in NSCLC A549 cells, and luciferase activity was measured
24 h after transfection. As shown in Fig. 4F, overexpression of miR-1290
significantly reduced the luciferase activity of pGL3-BCL2-3-UTR to
62.71±6.72% (n=3). Conversely, this downregulation was rescued by
cotransfection with antagomiR-1290 (Fig. 4F). These results indicate that
miR-1290 directly targets the 3′-UTR of BCL2 mRNA, and
negatively regulates BCL2 expression post-transcriptionally.
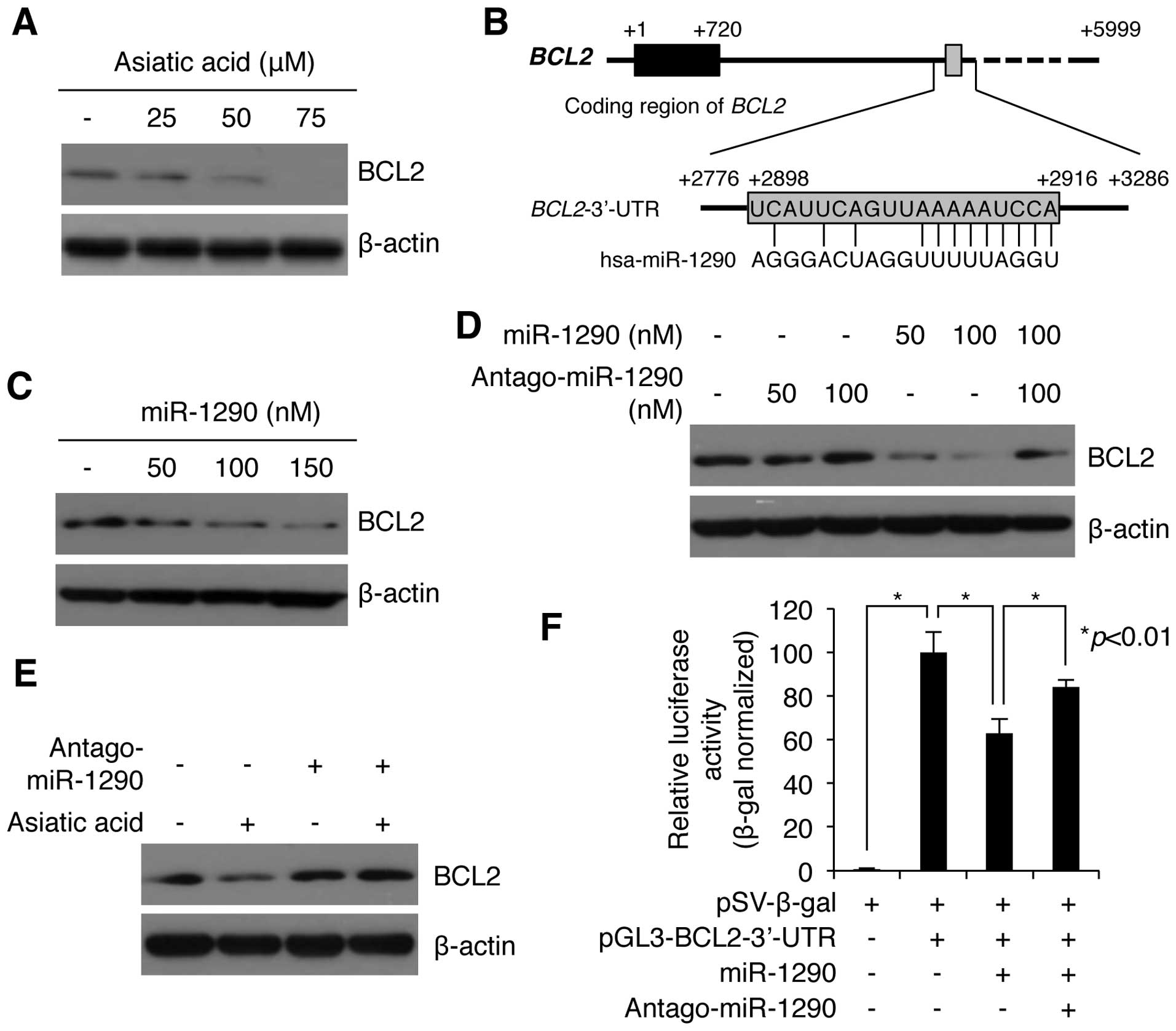 | Figure 4miR-1290 negatively regulates BCL2
expression in asiatic acid-treated A549 cells. (A) Western blot
analysis of BCL2 expression in A549 cells treated with the
indicated amounts of asiatic acid. β-actin served as internal
control. A549 cells were treated with different doses of asiatic
acid (0, 25 and 50 μM) for 24 h. Cells were collected and subjected
to western blotting using anti-BCL2 and anti-β-actin antibodies.
Anti-β-actin antibody was used as a loading control in experiments.
(B) In silico analysis of BCL2 mRNA and the predicted
target sequence of miR-1290. ATG start codon is indicated by +1.
The 3′ end of BCL2 coding region is indicated by +720. The
sequences of the 3-′UTR containing miR-1290 recognition sequences
are located from +2776 to +3286 in human BCL2 mRNA,
respectively. Gray box (+2886 to +2916) indicates target-specific
binding site of miR-1290 in 3′-UTR of BCL2 mRNA. (C) Western
blot analysis of BCL2 expression in A549 cells transfected with the
indicated amounts of miR-1290. β-actin served as internal control.
Different concentrations of miR-1290 mimic (0, 50, 100 and 150 nM)
were transfected into A549 cells. After 24 h of incubation, the
levels of BCL2 proteins were analyzed by western blotting with
anti-BCL2-specific antibody. (D) Western blot analysis of BCL2
expression in A549 cells transfected with the indicated amounts of
miR-1290 and its antagomiR. β-actin served as internal control.
AntagomiR-1290 inhibited miR-1290-mediated BCL2 downregulation.
A549 cells were transfected with miR-1290 and/or antagomiR-1290 as
indicated above. After 24 h of incubation, the cells were collected
and subjected to western blotting using anti-BCL2 and anti-β-actin
antibody. (E) Western blot analysis of BCL2 expression in A549
cells transfected with miR-1290 or control miRNA and incubated in
the presence or absence of asiatic acid. β-actin served as internal
control. AntagomiR-1290 impeded asiatic acid-induced BCL2 decrease.
A549 cells were transfected with antagomiR-1290 (150 nM) for 24 h.
Then, asiatic acid (50 μM) was treated into the cells. After 24 h
of incubation, the levels of BCL2 proteins were evaluated by
western blotting using anti-BCL2 antibody. (F) BCL2 is a putative
target of miR-1290. Relative luciferase activity in cells
transfected with reporter construct containing the miR-1290
recognition sequences of the BCL2 gene (pGL-BCL2-3′-UTR), miR-1290
mimic, antagomiR-1290, and pSV-β-galactosidase normalization
vector, as indicated. After 24 h of incubation, cells were
collected and subjected to luciferase assay. Values are mean ± SD
of three independent experiments. *P<0.01 compared
with the control. |
Discussion
The present study examined the role of miR-1290 in
the negative regulation of BCL2 expression in NSCLC A549 cells. The
expression level of miR-1290 was significantly increased by asiatic
acid, as revealed by miRNA microarray and quantitative RT-PCR
analyses. Although we did not examine whether asiatic acid
increases transcription of miR 1290 or enhances its stability, this
increase may be mediated at the transcriptional level. First, the
microarray data indicated alterations in the expression of specific
miRNAs. Second, the level of up- or downregulation of these miRNAs
varied significantly. Third, although several miRNAs, including
miR-382 and miR-154, show differential stability, most miRNAs
appear to be stable in human cells (23). Additionally, our microarray data
showed that only miR-1290 was significantly elevated (by 44-fold),
whereas other miRNAs showed a trend toward increased or decreased
levels, without reaching a similar level of fold change.
We found that the interplay between asiatic acid and
cell death depends on the expression level of miR-1290, which
tightly regulates asiatic acid-induced cell death. Transient
transfection of A549 cells with miR-1290 mimics downregulated BCL2
expression and increased cell growth inhibition, as well as cell
death. MiR-1290-dependent cell death was also investigated using
ectopic expression of antagomiR-1290 oligonucleotides. WST-1 assay
and FACS analysis revealed that asiatic acid-induced cell death was
largely blocked by inhibition of miR-1290 activity. Furthermore,
antagomiR-1290 inhibited asiatic acid-induced BCL2 downregulation.
Collectively, our data indicate that asiatic acid-induced cell
death is miR-1290 upregulation-dependent.
Currently, the effect of miR-1290 on growth
inhibition and cell death is a matter of controversy. Some authors
have suggested that miR-1290 is significantly upregulated in
clinical colon cancer tissues and in the serum of patients with
pancreatic cancer, compared to healthy controls (24,25).
However, others argue that miR-1290 is strongly downregulated in
clear renal cell carcinoma tissue and hypermethylated in cervical
cancer cell lines (26,27). Similarly, our previous reports
showed that miR-1290 is significantly upregulated by 2-fold in
response to ginsenoside Rh2, which induces cell death in NSCLC A549
cells (28). Also our unpublished
data show that asiatic acid induced cell death in MCF7 human breast
cancer and AGS human gastric cancer cells, and upregulated
expression of miR-1290 by 2.58- and 7.01-fold, respectively.
Recently, introduction of miR-1290 into estrogen receptor
α-positive breast cancer cells and neuroblastoma cells decreased
expression of NAT and FOXA1 and led to a slowing down of the cell
cycle (29,30). Collectively, these results indicate
that miR-1290 functions as a putative tumor suppressor in breast,
lung and gastric cancer by inducing cell cycle arrest and
apoptosis; however, it has a oncogenic function in colon and
pancreatic cancer. The data indicate that miR-1290 has a dual role
as tumor suppressor and oncogene in a cell-type-specific manner,
although further investigation is needed to elucidate the precise
mechanisms underlying these functions.
BCL2, a well-known pro-survival protein localized in
mitochondria, has been reported to have antiapoptotic properties
(31,32). In particular, the antiapoptotic
function of BCL2 is based on its ability to inhibit an apoptotic
mechanism induced by several anticancer agents, such as paclitaxel
and cisplatin (33,34), and BCL2 downregulation has been
observed in almost all apoptotic processes (35). Recent studies have shown that
asiatic acid induces apoptosis and cell cycle arrest through BCL2
downregulation in human breast cancer cells and human colon
adenocarcinoma cells (7,8). Also, asiatic acid-induced BCL2
downregulation was attenuated by pretreatment with ERK1/2 inhibitor
U0216 in breast cancer cells (8).
However, the mechanism underlying BCL2 downregulation induced by
asiatic acid treatment of cells remains unknown. Here, we showed
that asiatic acid-induced BCL2 downregulation was due to
miR-1290-mediated posttranscriptional inhibition of BCL2
mRNA. In silico analysis showed that the 3′-UTR of
BCL2 mRNA contains a complementary binding site for
miR-1290, and luciferase assay confirmed the implication of
BCL2 mRNA as a direct target of miR-1290. Endo et al
showed other putative target mRNAs of miR-1290 (29). They demonstrated that NAT and FOXA1
proteins are downregulated by miR-1290 overexpression; however, it
remains unknown whether these genes are direct targets of miR-1290
(29). Therefore, BCL2 is
the first confirmed target mRNA of miR-1290 in cells.
In summary, we determined that asiatic acid induced
growth inhibition and death in A549 NSCLC cells. We also showed
that asiatic acid induces considerable changes in the miRNA
expression profile of the cells. Notably, miR-1290 is the most
extensively upregulated miRNA, and it tightly regulates asiatic
acid-induced cell death. Further experiments demonstrated that
miR-1290 directly regulates BCL2 synthesis. Our results may provide
a useful approach to understanding cellular responses to asiatic
acid, although the clinical significance of miR-1290 and BCL2
warrant further investigation.
Acknowledgements
We would like to thank all members of our laboratory
for their support and advice during this study. This study resulted
from the Konkuk University research support program.
References
|
1
|
Howes MJ and Houghton PJ: Plants used in
Chinese and Indian traditional medicine for improvement of memory
and cognitive function. Pharmacol Biochem Behav. 75:513–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausen BM: Centella asiatica
(Indian pennywort), an effective therapeutic but a weak sensitizer.
Contact Dermatitis. 29:175–179. 1993. View Article : Google Scholar
|
|
3
|
Rush WR, Murray GR and Graham DJ: The
comparative steadystate bioavailability of the active ingredients
of Madecassol. Eur J Drug Metab Pharmacokinet. 18:323–326. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong Y, Ding H, Xu M and Gao J:
Protective effects of asiatic acid on rotenone- or
H2O2-induced injury in SH-SY5Y cells.
Neurochem Res. 34:746–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma K, Zhang Y, Zhu D and Lou Y: Protective
effects of asiatic acid against
D-galactosamine/lipopolysaccharide-induced hepatotoxicity in
hepatocytes and kupffer cells co-cultured system via
redox-regulated leukotriene C4 synthase expression pathway. Eur J
Pharmacol. 603:98–107. 2009. View Article : Google Scholar
|
|
6
|
Lee YS, Jin DQ, Kwon EJ, et al: Asiatic
acid, a triterpene, induces apoptosis through intracellular
Ca2+ release and enhanced expression of p53 in HepG2
human hepatoma cells. Cancer Lett. 186:83–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bunpo P, Kataoka K, Arimochi H, et al:
Inhibitory effects of asiatic acid and CPT-11 on growth of HT-29
cells. J Med Invest. 52:65–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu YL, Kuo PL, Lin LT and Lin CC: Asiatic
acid, a triterpene, induces apoptosis and cell cycle arrest through
activation of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase pathways in human breast cancer
cells. J Pharmacol Exp Ther. 313:333–344. 2005. View Article : Google Scholar
|
|
9
|
Sastry PS and Rao KS: Apoptosis and the
nervous system. J Neurochem. 74:1–20. 2000. View Article : Google Scholar
|
|
10
|
Nakano R: Apoptosis: gene-directed cell
death. An overview Horm Res. 48:2–4. 1997. View Article : Google Scholar
|
|
11
|
Moldoveanu T, Follis AV, Kriwacki RW and
Green DR: Many players in BCL-2 family affairs. Trends Biochem Sci.
39:101–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He L, He X, Lim LP, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bommer GT, Gerin I, Feng Y, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christoffersen NR, Shalgi R, Frankel LB,
et al: p53-independent upregulation of miR-34a during
oncogene-induced senescence represses MYC. Cell Death Differ.
17:236–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu C, Yu J, Yu S, et al: MicroRNA-21 acts
as an oncomir through multiple targets in human hepatocellular
carcinoma. J Hepatol. 53:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Kumar M, Choudhury SN, et al: Loss
of the miR-21 allele elevates the expression of its target genes
and reduces tumorigenesis. Proc Natl Acad Sci USA. 108:10144–10149.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papagiannakopoulos T, Shapiro A and Kosik
KS: MicroRNA-21 targets a network of key tumor-suppressive pathways
in glioblastoma cells. Cancer Res. 68:8164–8172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao Q, Cao S, Li C, Mengesha A, Kong B and
Wei M: Micro-RNA-21 regulates TGF-β-induced myofibroblast
differentiation by targeting PDCD4 in tumor-stroma interaction. Int
J Cancer. 128:1783–1792. 2011.PubMed/NCBI
|
|
20
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
An IS, An S, Kang SM, et al: Titrated
extract of Centella asiatica provides a UVB protective
effect by altering microRNA expression profiles in human dermal
fibroblasts. Int J Mol Med. 30:1194–1202. 2012.
|
|
22
|
An IS, An S, Choe TB, et al: Centella
asiatica protects against UVB-induced HaCaT keratinocyte damage
through microRNA expression changes. Int J Mol Med. 30:1349–1356.
2012.
|
|
23
|
Bail S, Swerdel M, Liu H, et al:
Differential regulation of microRNA stability. RNA. 16:1032–1039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu J, Ji X, Zhu L, et al: Up-regulation of
microRNA-1290 impairs cytokinesis and affects the reprogramming of
colon cancer cells. Cancer Lett. 329:155–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li A, Yu J, Kim H, et al: MicroRNA array
analysis finds elevated serum miR-1290 accurately distinguishes
patients with low-stage pancreatic cancer from healthy and disease
controls. Clin Cancer Res. 19:3600–3610. 2013. View Article : Google Scholar
|
|
26
|
White NM, Bao TT, Grigull J, et al: miRNA
profiling for clear cell renal cell carcinoma: biomarker discovery
and identification of potential controls and consequences of miRNA
dysregulation. J Urol. 186:1077–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao T, Rao Q, Liu L, et al: Exploration of
tumor-suppressive microRNAs silenced by DNA hypermethylation in
cervical cancer. Virol J. 10:1752013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An IS, An S, Kwon KJ, Kim YJ and Bae S:
Ginsenoside Rh2 mediates changes in the microRNA expression profile
of human non-small cell lung cancer A549 cells. Oncol Rep.
29:523–528. 2013.PubMed/NCBI
|
|
29
|
Endo Y, Toyama T, Takahashi S, et al:
miR-1290 and its potential targets are associated with
characteristics of estrogen receptor α-positive breast cancer.
Endocr Relat Cancer. 20:91–102. 2013.
|
|
30
|
Yelamanchili SV, Morsey B, Harrison EB, et
al: The evolutionary young miR-1290 favors mitotic exit and
differentiation of human neural progenitors through altering the
cell cycle proteins. Cell Death Dis. 5:e9822014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria:
a primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997.PubMed/NCBI
|
|
32
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gazitt Y, Rothenberg ML, Hilsenbeck SG,
Fey V, Thomas C and Montegomrey W: Bcl-2 overexpression is
associated with resistance to paclitaxel, but not gemcitabine, in
multiple myeloma cells. Int J Oncol. 13:839–848. 1998.PubMed/NCBI
|
|
34
|
Cho HJ, Kim JK, Kim KD, et al:
Upregulation of Bcl-2 is associated with cisplatin-resistance via
inhibition of Bax translocation in human bladder cancer cells.
Cancer Lett. 237:56–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi YM, An S, Lee EM, et al: CYP1A1 is a
target of miR-892a-mediated post-transcriptional repression. Int J
Oncol. 41:331–336. 2012.PubMed/NCBI
|