Introduction
Glioblastoma is a type of lethal brain tumor with a
median survival of approximately 14 months, even with aggressive
surgery, radiation and chemotherapy (1,2).
Malignant gliomas have a highly tumorigenic subpopulation, known as
cancer stem cells (CSCs), which drives tumor formation and
proliferation (3–5). These cells are defined as glioma stem
cells (GSCs) with higher proliferation and poorer differentiation
than glioblastoma cells around them (6–10).
Although these cells represent only a small fraction of the tumor
bulk, their high self-renewal capacity is thought to sustain tumor
growth (11). Therapies which
induce the differentiation of GSCs to non-stem-like glioblastoma
cells can decrease the growth of tumor and improve the sensitivity
to radiotherapy and chemotherapy (3,11,12).
For this reason, differentiation therapy is regarded as a
therapeutic strategy for GSCs. Inducing the differentiation of GSCs
will be valuable for patients suffering from glioblastoma.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a member of the cap ‘n’ collar family, which has been described
as a central orchestrator of the expression of antioxidant and
detoxifying genes (13,14). Nrf2 escapes Kelch-like
ECH-associating protein 1 (Keap1) and accumulates in the nucleus
where it binds to antioxidant response element (ARE) sequences in
the regulatory regions of its target genes to induce their
expression (14–16).
Recently, the role of Nrf2 was observed in several
types of tumors, such as non-small cell lung cancer, glioma,
bladder carcinoma and hepatocarcinoma (17,18).
Nrf2 protects tumors from chemotherapy and radiotherapy (19,20).
Our previous study found the significant role of Nrf2 in the
self-renewal of GSCs (21).
Furthermore, knockdown of Nrf2 significantly reduced the expression
of stem cell markers Bmi1, Sox2 and Cyclin E (21). Since GSCs were generally at a poor
stage of differentiation and performed high proliferation and low
maturity, we hypothesized that Nrf2 plays an important role in the
differentiation of GSCs, and inhibiting Nrf2 could induce GSC
differentiating to non-stem-like glioblastoma cells, decreasing the
growth of the tumor and improving the sensitivity to radiotherapy
and chemotherapy.
In this study, the GSCs were obtained from the
tissues of patients with glioblastoma and Nrf2 was knocked down by
lentivirus-transported shRNA to investigate the role of Nrf2 in the
differentiation of GSCs both in vitro and in vivo,
providing a possible therapeutic target for GSCs.
Materials and methods
The study was approved by the Ethics Committee of
Jinling Hospital (Nanzi 20120017). Patients who were recruited
provided written informed consent allowing the scientific use of
their samples. Animal experiments were approved by the Animal
Ethics Committee of the Animal Experiment Center at Jinling
Hospital (SCXK 2012–012).
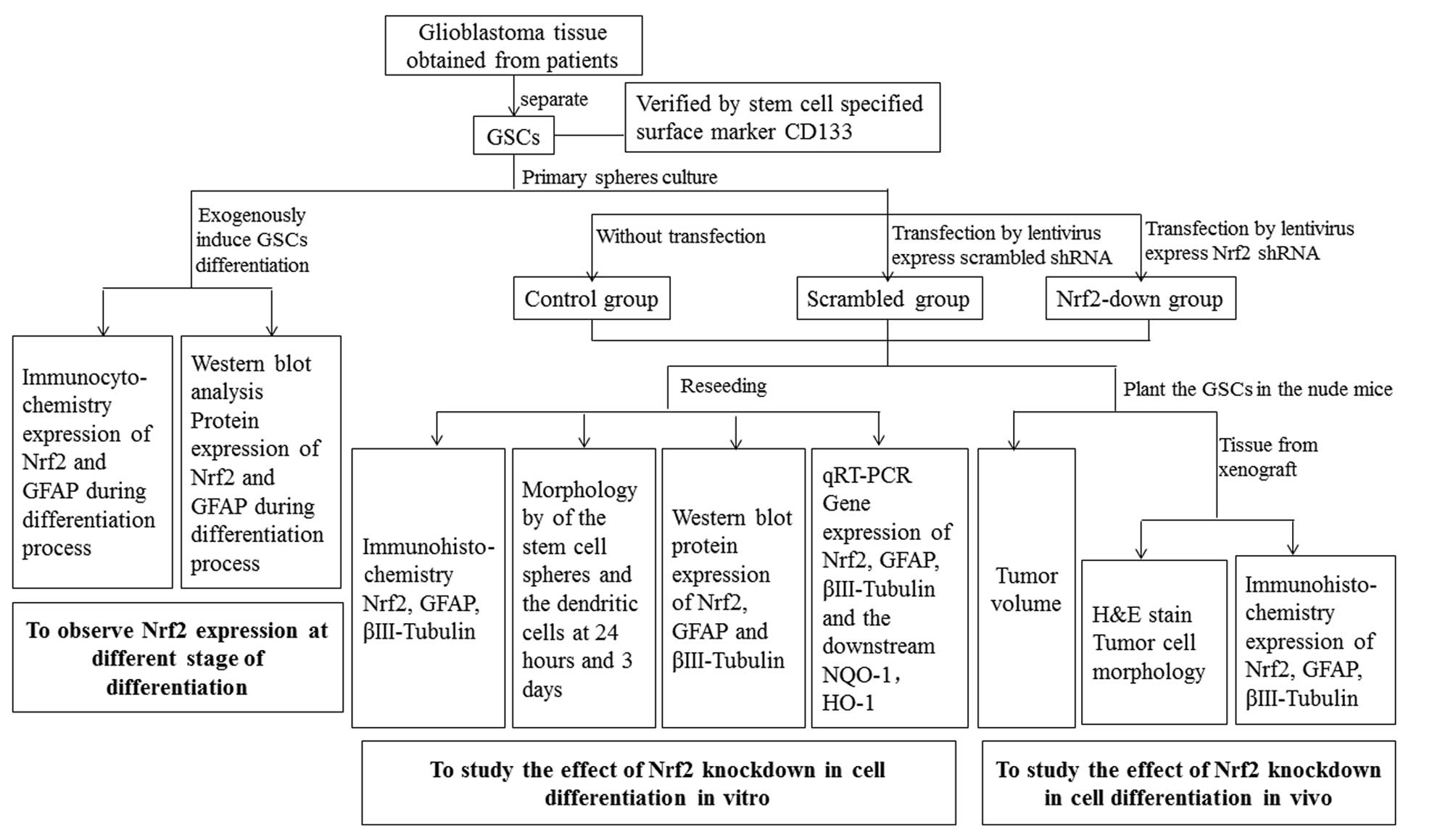
The GSCs used in this study were separated from
glioblastoma tissue obtained from patients. The experiment was
divided into three parts: i) induction of the differentiation of
primary spheres and analysis of the expression of Nrf2 during the
differentiation, ii) knockdown of Nrf2 from GSCs by lentivirus and
examination of the differentiation in vitro, iii) planting
of the GSCs in nude mice and studying the differentiation in
vivo. The experimental process is shown in Fig. 1.
Patient glioma samples
Glioma specimens were obtained from the Department
of Neurosurgery in Jinling Hospital. All the patients were
diagnosed with glioma. The tumors were classified as Stage IV by
two pathologists, according to the WHO Classification of central
nervous system tumors.
Primary sphere culture
Tumors were dissociated with 0.25% trypsin and
released by gentle pipetting and filtrated through a 70-μm cell
strainer. Adherent culture of cells was performed by plating the
cells in a gelatin-coated plastic flask in DMEM for 24 h and
washing with phosphate buffered saline (PBS) to remove red blood
cells and cell debris. Then, the tumor cells were collected and
seeded in neural stem cell (NSC) medium (Gibco-BRL, USA) at a
density of 2,000 cells/cm2 to obtain floating
tumor-spheres. Primary GSCs were incubated at 37°C in an atmosphere
containing 5% CO2 for 5–7 days. The medium was
half-renewed every 3 days. After growing >100 μm in diameter,
the spheres were tentatively defined as GSC spheres.
Induction of differentiation of primary
spheres
To induce differentiation, primary spheres were
reseeded into a 24-well cluster at a density of approximately 50
spheres/well. Cells were cultivated in DMEM/F12 (Gibco-BRL) and
supplemented with 10% fetal bovine serum (FBS). Cells were
separated into 6 equal groups, each group containing 4 wells.
Approximately 4 h later, spheres in every well adhered to the
plates and dendritic branches could be found at the base. Twelve
hours, 24 h, 2, 3 and 5 days after induction, we chose 2 wells in
each group to fix with formaldehyde, respectively. We collected one
more well of each group to prepare protein and 1 well to stain for
immunocytochemistry. Seven days later, we separated each group into
2 equal parts: part 1 was observed with microscopy and part 2 was
stained with gentian violet. They were viewed with a microscope
(Carl Zeiss, Germany).
Knockdown of Nrf2 by lentivirus and
observation of the morphology of differentiation in spheres in
vitro
Primary spheres were dissociated with Accutase
(Sigma-Aldrich, USA) for 15 min and equal cells were reseeded into
6-well plates (1×104 cells/well). Lentiviruses for
expression of scrambled shRNA or Nrf2 shRNA were diluted in NSC
medium containing 5 μg/ml polybrene, and the medium was added to
GSCs according to the groups separately. After 72 h, infected cells
rebuilt secondary spheres and were selected for puromycin
resistance by refeeding the cells with fresh NSC medium containing
5 μg/ml puromycin for 24 h.
Then, the spheres were reseeded into 6-well plates.
Twenty-four hours after reseeding, spheres in half of the wells
were collected for protein and mRNA, and the other half were
collected for morphology analysis. At 24 h and 3 days after
reseeding, the sphere-like colonies in the scrambled and Nrf2
shRNA-treated cells were scored in 20 random 4× fields by two
independent scorers who were unaware of the sample designation.
The human Nrf2 short hairpin RNA (shRNA) sequence
was: 5′-GCAGTTCAATGAAGCTCAACT-3′, while the scrambled shRNA
sequence was: 5′-TTCTCCGAACGTGTCACGT-3′. The lentivirus vector was
purchased from GenePharma (Shanghai, China).
Western blotting
Equal amounts of proteins were separated using
SDS-PAGE (8–12% gradient gel). Separated proteins were transferred
onto PVDF membranes (Millipore, Germany) and each membrane was cut
into narrow pieces according to the protein molecular massive
marker (Thermo, USA), blocked with 5% non-fat milk for 1 h at room
temperature and probed with the appropriate antibody, as described
previously. The dilutions of primary and secondary antibody were
made in 3% BSA and Tris-buffered saline (TBST) with 0.1% Tween-20,
respectively. The membranes were incubated in primary antibody
overnight at 4°C, and in secondary antibody for 60 min at room
temperature. The primary antibodies used were against: Nrf2
(1:1,000; Abcam), glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
1:5,000; Bioworld, USA), glial fibrillary acidic protein (GFAP;
1:500; BD, USA), βIII-tubulin (1:10,000; Abcam). The secondary
antibody was the anti-rabbit-IgG-HRP antibody conjugated
(Bioworld), and it was used at a 1:5,000 dilution.
RNA isolation and quantification
GSCs were non-infected or infected with lentiviruses
that expressed either scrambled or Nrf2 shRNA, as described above.
At 72 h after transduction, RNA was isolated from cells. RNA
isolation and cDNA was synthesized using Strand cDNA synthesis Kit
(Takara, Japan). RNA was isolated from three independent cell
culture preparations. Levels of transcripts for specific genes were
determined by SYBR-Green quantitative reverse transcription-PCR
(qRT-PCR), using gene-specific primers for human transcripts
encoding Nrf2, NAD(P)H:quinone oxidoreductase-1 (NQO-1), heme
oxygenase-1 (HO-1), cyclin E, βIII-tubulin, GFAP. Sequences of
primers for the remaining human genes are shown in Table I.
 | Table ISequences of primers for the remaining
human genes. |
Table I
Sequences of primers for the remaining
human genes.
| Gene | Primer sequence |
|---|
| Nrf2 | F:
5′-TCAGCGACGGAAAGAGTATGA-3′
R: 5′-CCACTGGTTTCTGACTGGATGT-3′ |
| GAPDH | F:
5′-GAAATCCCATCACCATCTTC-3′
R: 5′-CCACTGGTTTCTGACTGGATGT-3′ |
| βIII-tubulin | F:
5′-CAAGATGTCGTCCACCTTCAT-3′
R: 5′-CTCAGACACCAGGTCGTTCAT-3′ |
| Cyclin E | F:
5′-ACCAGTTTGCGTATGTGA-3′
R: 5′-TGTGGGTCTGTATGTTGTG-3′ |
| GFAP | F:
5′-AGGGACAATCTGGCACAGG-3′
R: 5′-CGGTAGTCGTTGGCTTCG-3′ |
| HO-1 | F:
5′-TCTCCGATGGGTCCTTACACTC-3′
R: 5′-GGCATAAAGCCCTACAGCAACT-3′ |
| NQO-1 | F:
5′-ATGGTCGGCAGAAGAGC-3′
R: 5′-GGAAATGATGGGATTGAAGT-3′ |
Immunocytochemistry and
immunofluorescence
At 24 h after seeding, cells were fixed with 4%
formaldehyde (Sigma-Aldrich, USA) in PBS for 20 min at room
temperature, and permeabilized in 0.1% Triton X-100 in PBS for 10
min at room temperature. Then, cells were blocked using 5% bovine
serum albumin (BSA; Sigma-Aldrich) in PBS for 1 h at room
temperature. Following blocking, cells were incubated in Nrf2, GFAP
and α-tubulin primary antibody overnight, at 4°C on a rocking
platform. Cells were washed 3 times in PBS. The appropriate
secondary antibody was then added and allowed to incubate for 1 h
in the dark at room temperature. Images of the cells were captured
with a fluorescence microscope (Carl Zeiss). Primary antibodies
used were against: Nrf2 (1:100; rabbit polyclone; Abcam), GFAP
(1:100; rabbit monoclone; BD), βIII-tubulin (1:1,000; rabbit
monoclone; Abcam), CD133 (1:100; rabbit polyclone; Biobyte).
Secondary antibodies were against mouse-IgG-FITC (Sigma-Aldrich),
rabbit-IgG-Cy3 (F0382; Sigma-Aldrich). Nuclei were visualized using
4′,6-diamidino-2-phenylindole (DAPI) staining (D9542;
Sigma-Aldrich) at 1:10,000 in PBS.
Knockdown of Nrf2 by lentivirus and
observation of the differentiation in vivo
Lentiviruses for expression of scrambled shRNA or
Nrf2 shRNA were diluted in NSC medium containing 5 μg/ml polybrene,
and the medium was added to GSCs according to the groups
separately. After 72 h, infected cells were selected for puromycin
resistance by refeeding the cells with fresh NSC medium containing
5 μg/ml puromycin for 24 h.
Spheres were then dissociated with Accutase
(Sigma-Aldrich) for 15 min and equal cells (2×104) were
dissociated in 50 μl PBS and implanted subcutaneously into the
flanks of 4-week-old male nude mice (6 mice in each group, a total
of 3 groups, mice were randomly selected). Five weeks later,
animals were sacrificed and xenograft tumors were measured and
subjected to immunofluorescent analysis. Tumor growth was measured
using an external caliper every week and tumor volume was
calculated as (length/2) × width2. After sacrifice, the
tumors were removed for paraffin sectioning. The sections were
stained by hematoxylin and eosin (H&E), and treated with
polyclonal rabbit antibodies to Nrf2, GFAP, βIII-tubulin. For
xenografts of nude mice, secondary antibody was the
anti-rabbit-IgG-HRP antibody conjugated (1:5,000 dilution;
Bioworld) for 1 h, and stained with 3,3′-diaminobenzidine
tetrahydrochloride (DAB).
Statistical analysis
All statistical analyses were performed using SPSS
10.0 software (SPSS Inc, USA). Data are shown as means ± SD. Data
were statistically analyzed using one-way analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant difference.
Results
The expression of Nrf2 decreases during
the differentiation of primary GSCs and the GFAP increases
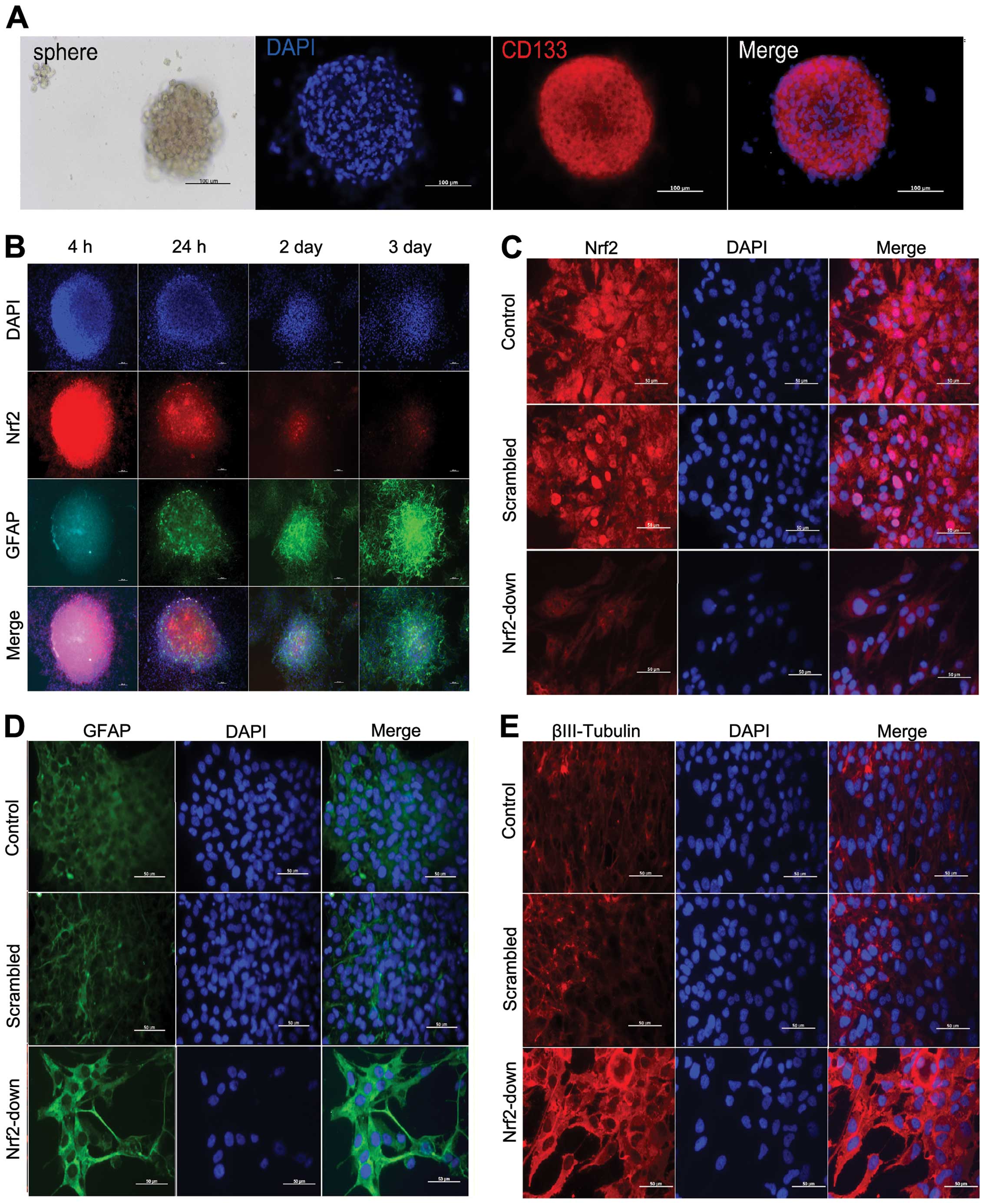
After reseeding, the primary GSC spheres were
observed under microscopy (Fig.
2A). The primary spheres were identified with CD133 in
immunocytochemistry and the high expression of CD133 in the cells
was viewed easily (Fig. 2A).
After 24 h of differentiation induction, spheres in
every well adhered to the plates and dendritic branches were found
in the basement (Fig. 2B). The
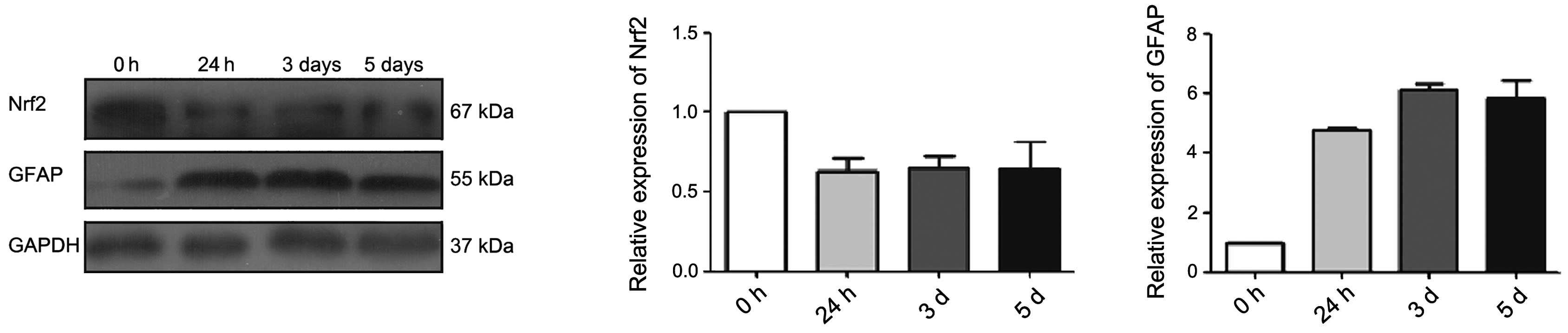
expression level of differentiation marker GFAP was substantially
increased after FBS inducing differentiation. The expression level
of Nrf2 was high in the base, and it decreased as cell
differentiation increased (Fig. 3).
These observations demonstrate the function of Nrf2 in the
differentiation of glioma stem-like cells.
Knockdown of Nrf2 by lentivirus improves
the differentiation of glioma stem-like cells in morphology
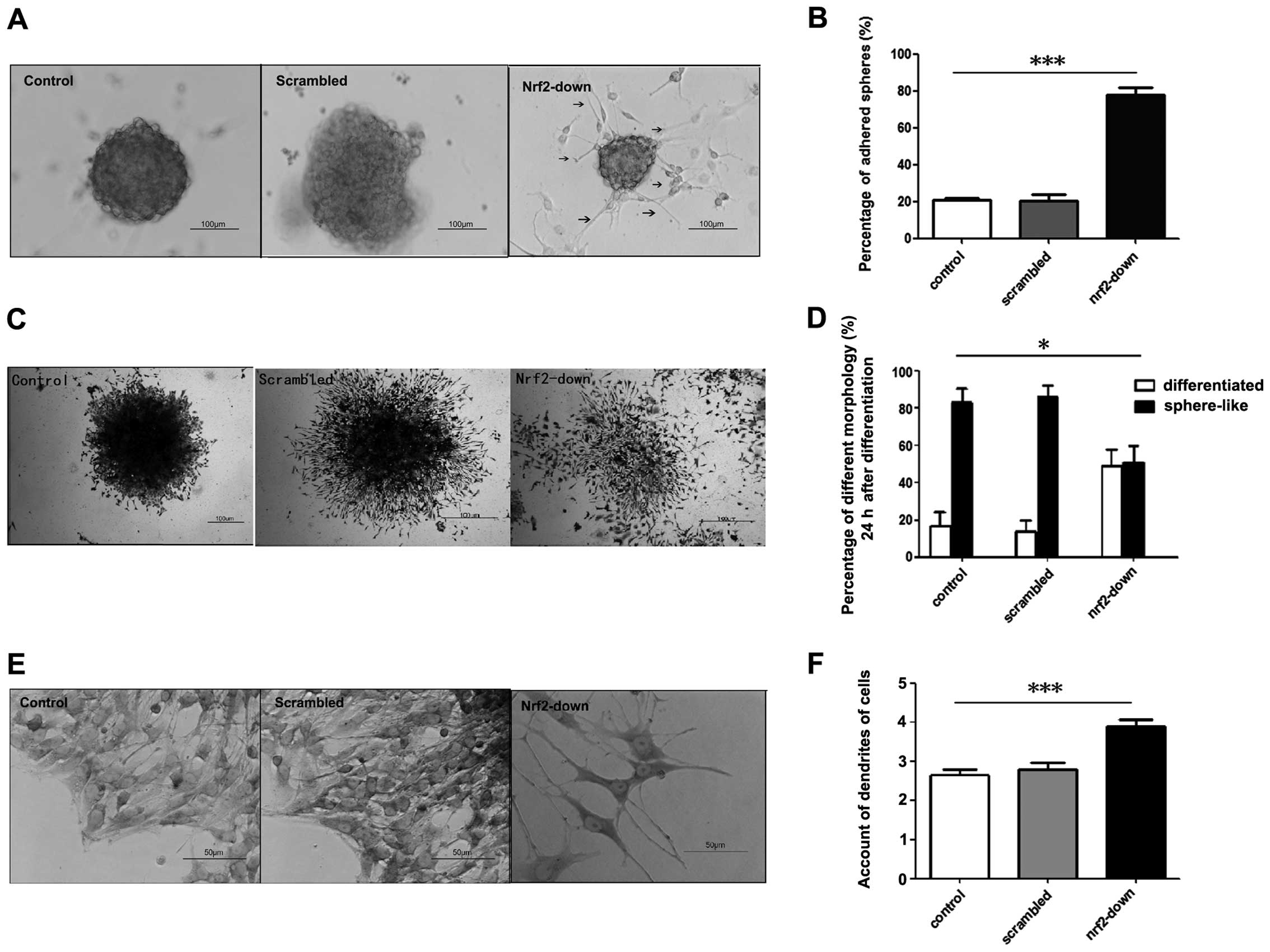
Twenty-four hours after reseeding, there were two
types of sphere-like colonies in each group: i) the suspended
spheres which floated in the medium and ii) the adhered spheres, in
the basement of which were the dendrites of differential cells.
Nrf2 knockdown increased the adhesion of spheres in GSCs (Fig. 4A). The proportions of adhered
spheres in the control and scrambled group were 20.7±1.36 and
20.5±3.41% respectively. However, the proportion of adhered spheres
was 77.7±4.19% in the Nrf2-down group (Fig. 4B). The percentage of adhered spheres
was higher in the Nrf2-down group (P=0.001).
After 3 days, the majority of the GSCs
differentiated in the Nrf2-down group. In the other groups, GSCs
maintained the sphere-like and low differentiated colonies
(Fig. 4C). GSCs in the control
group and the group infected with the scrambled shRNA vector
exhibited 17.6±2.69% and 13.9±2.23% ratios of differentiated
colonies, whereas the ratio was 48.6±3.40% in the Nrf2-down group
(Fig. 4D). There was also a higher
number of differentiated colonies in the Nrf2-down group
(P=0.018).
At the base of the spheres, there were many
dendrites in adhered cells, which were the morphological markers of
differentiation. In the Nrf2-down group, there were considerably
more dendrites than in the control and scrambled group, and the
dendrites were much longer (Fig.
4E). The average dendrites in the control and scrambled group
were 2.65±0.67 and 2.8±0.70, and the dendrites in the Nrf2-down
group were 3.9±0.72 (Fig. 4F). The
Nrf2-down group differentiated faster than the other groups in
morphology (P<0.001).
Knockdown of Nrf2 by lentivirus induces
the differentiation-associated markers
In western blot analysis and immunocytochemistry,
Nrf2 expression was lower in the Nrf2-down group than in the other
two groups (Fig. 5A and C),
confirming that the Nrf2 was downregulated by lentivirus.
Furthermore, the expression levels of differentiation marker GFAP
and βIII-tubulin protein increased after Nrf2 knockdown as
demonstrated both by western blotting (Fig. 5A) and immunocytochemistry analysis
(Fig. 2D and E).
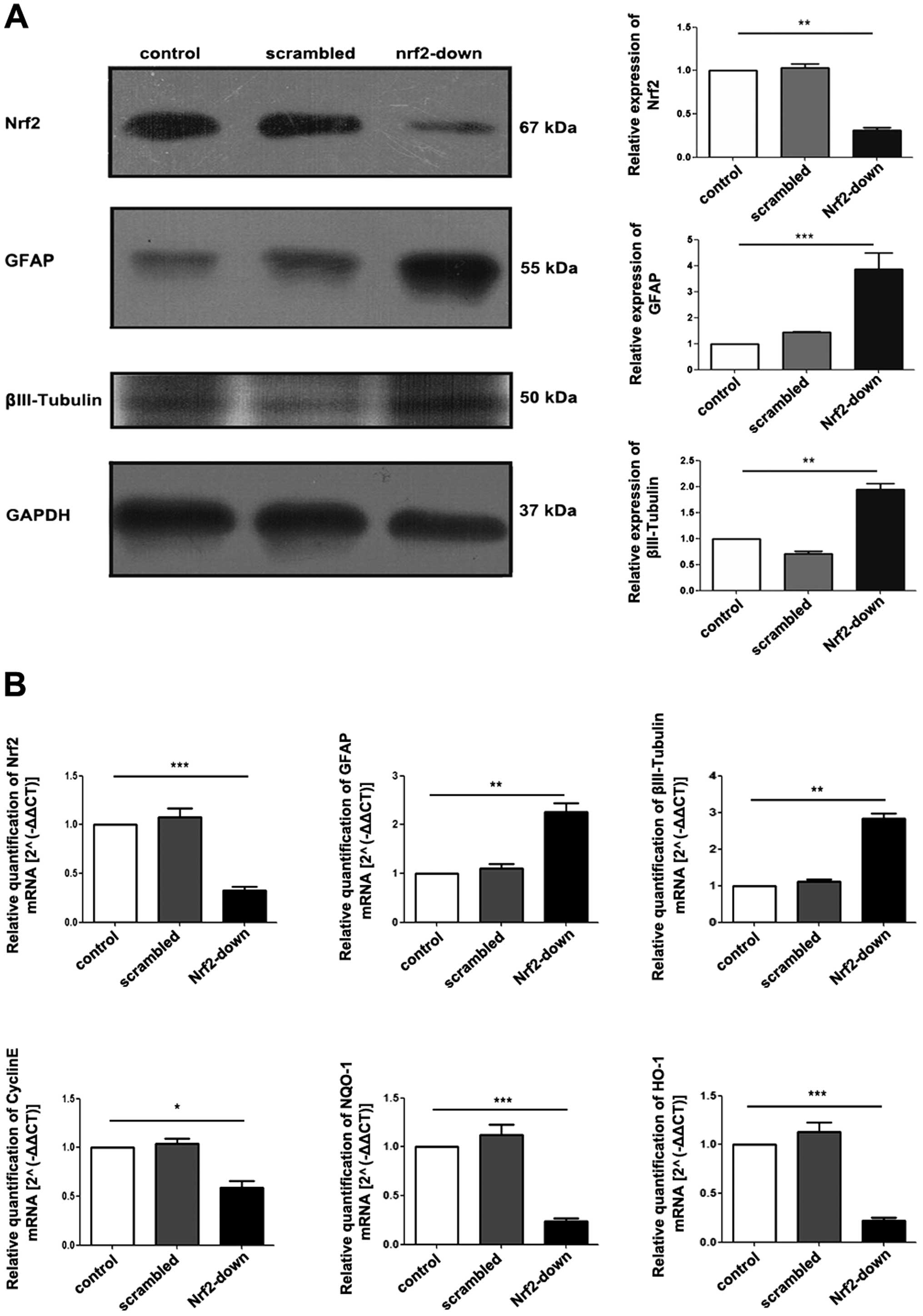 | Figure 5Protein and gene expression of Nrf2
and differentiation-related markers among different groups. Western
blot analysis of proteins isolated from GSCs, protein expression of
GFAP and βIII-tubulin increased in the Nrf2-down group. (B) RT-qPCR
of RNA isolated from GSCs after transduction. GFAP and βIII-tubulin
increased substantially with the knockdown of Nrf2, while Cyclin E,
NQO-1 and HO-1 decreased. Data are shown as means ± SD.
*P<0.05; **P<0.01;
***P<0.005. Nrf2, nuclear factor erythroid 2-related
factor 2; GFAP, glial fibrillary acidic protein; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; NQO-1, NAD(P)H:quinone
oxidoreductase-1; HO-1, heme oxygenase-1; GSCs, glioblastoma stem
cells; qRT-PCR, quantitative reverse transcription-PCR. |
Nrf2 knockdown in GSCs induces the
expression of differentiation-associated genes
qRT-PCR demonstrated that there was a significant
decrease in the gene expression of stem cell marker cyclin E after
Nrf2 knockdown (P<0.001; Fig.
5B). In contrast, gene expression of astrocyte marker GFAP
(P=0.008) and neural surface marker βIII-tubulin (P=0.004) was
significantly increased in the Nrf2-down group, which could be
attributed to spontaneous differentiation within the cell
population (Fig. 5B). Furthermore,
the expression levels of downstream gene HO-1 (P=0.002) and NQO-1
(P=0.002) which were regulated by Nrf2, were also decreased
(Fig. 5B).
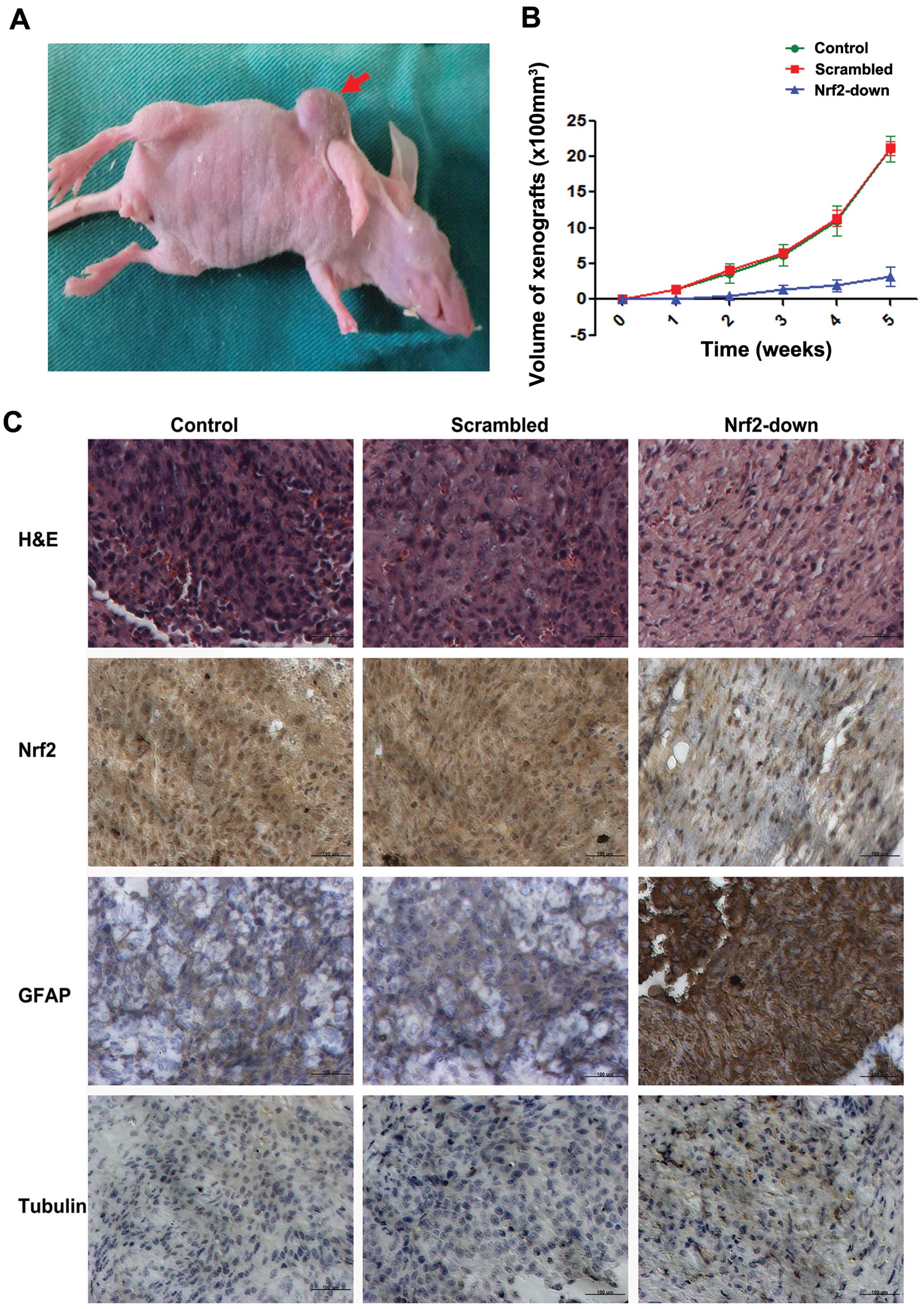
Knockdown of Nrf2 induced the differentiation of
GSCs in vivo. GSCs from the control, scrambled and
Nrf2-knockdown groups were inoculated subcutaneously to nude mice
separately to observe the growth rate of GSCs in vivo
(Fig. 6A). The tumor volume of GSCs
from the Nrf2-down group was significantly smaller than the other
two groups (2,100±74.5 vs. 2,117±104.6 vs. 316±54.3 mm3,
P<0.001) (Fig. 6B).
Further H&E staining showed the lower nuclear
density and higher cytoplasm proportion in the Nrf2 downregulated
group (Fig. 6C).
Immunohistochemistry staining of xenograft tumor tissues
demonstrated increased GFAP positive cells in the Nrf2
downregulated group, while βIII-tubulin was not significantly
increased (Fig. 6C).
Discussion
Glioblastoma is the most prevalent and malignant
primary central nervous system tumor (1). Standard therapy includes surgical
incision, chemotherapy and radiotherapy. In spite of aggressive
treatment, prognosis remains poor and many patients manifest
resistance to traditional chemotherapy and radiotherapy. Studies
have shown that the therapeutic resistance may be due to a crucial
subpopulation of glioma stem cells (GSCs) (22,23).
GSCs present low status of differentiation and display high
proliferation and high rates of self-renewal. Previous studies
showed GSCs in glioblastoma conferred tumorigenic potential and
resistance to chemotherapy and radiotherapy (11,12,24).
As a result, finding an effective method to change the
differentiation status of GSCs, inducing it to differentiate into
more mature ones, is key in eliminating the GSCs and enhancing the
therapeutic sensitivity to chemotherapy and radiotherapy, thereby
improving the prognosis of patients with glioblastoma.
Nrf2 is described as a central orchestrator of
antioxidant and detoxifying genes involved in phase II detoxication
enzymes and antioxidant stress enzymes (13,25).
The role of Nrf2 was found in several types of
tumors, such as non-small cell lung cancer, glioma, bladder
carcinoma and hepatocarcinoma (26–28).
Nrf2 can protect tumors from chemotherapy and radiotherapy
(19,20). In general, Nrf2 is highly expressed
in cells with low status of differentiation. In our previous study,
GSCs expressed much higher Nrf2 than glioblastoma and displayed a
lower status of differentiation (29,30).
Pathological research demonstrated the expression of Nrf2 decreases
with the differentiation increase in glioblastoma (30). This evidence indicates that Nrf2 may
play an important role in regulating the differentiation of
GSCs.
In this study, the GSCs separated from glioblastoma
had a low status of differentiation, and expressed a high level of
Nrf2. When we exogenously induced the differentiation of GSCs, the
expression of Nrf2 decreased with the differentiation process. This
indicates that Nrf2 is important in retaining the stem cell
characteristics and maintaining the low status of differentiation
in GSCs.
Furthermore, we knocked down Nrf2 in GSCs by shRNA
transformed by lentivirus. Following Nrf2 knockdown, sphere-like
growing was inhibited in the early stage of culture. Over time, the
GSCs transformed to mature cells, and the astrocytoma surface
marker GFAP and the neuronal surface marker βIII-tubulin expression
increased. In vivo study showed similar results. Following
Nrf2 knockdown, the growth rate of GSCs in vivo was
significantly reduced, and the differentiation of xenografted tumor
cells was improved. These results suggest that Nrf2 is a key factor
inhibiting the differentiation of GSCs, and knockdown of Nrf2 can
promote the differentiation process both in vitro and in
vivo.
In addition, the expression of NQO-1 and HO-1 in
GSCs was decreased after Nrf2 knockdown. Both NQO-1 and HO-1 are
important in eliminating the reactive oxygen species (ROS) in cells
(31–33). In previous studies, ROS was shown to
play a key role in many pathological processes in cancer (34). The decrease of NQO-1 and HO-1 in
this study indicates that Nrf2 may keep the low status of
differentiation by inhibiting the ROS in GSCs. However, the
molecular mechanism of the relationship between ROS and Nrf2 in the
differentiation of GSCs requires further investigation.
Collectively, Nrf2 plays a critical role in
maintaining the low status of differentiation of GSCs, and
inhibition of Nrf2 can induce the differentiation, thereby
providing a valuable therapeutic target for the elimination of
GSCs. Efficient therapy blocking molecular target of Nrf2 may
benefit patients with glioblastoma.
Acknowledgements
The authors thank Dr Feng Genbao and Dr He Jin for
the technical assistance. This study was supported by Grants from
the National Natural Science Foundation of China (Nos. 81070974 and
81271377), the Jiangsu Provincial Key Subject (no. X4200722) and
the Jinling Hospital of Nanjing, China (no. 2010Q017).
References
|
1
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu H, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: the avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI
|
|
2
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman1 IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
5
|
Fan X, Salford LG and Widegren B: Glioma
stem cells: evidence and limitation. Semin Cancer Biol. 17:214–218.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin X, Jin X, Jung JE, Beck S and Kim H:
Cell surface Nestin is a biomarker for glioma stem cells. Biochem
Biophys Res Commun. 433:496–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He J, Shan Z, Li L, Liu F, Liu Z, Song M
and Zhu H: Expression of glioma stem cell marker CD133 and
O6-methylguanine-DNA methyltransferase is associated with
resistance to radiotherapy in gliomas. Oncol Rep. 26:1305–1313.
2011.PubMed/NCBI
|
|
8
|
Park D, Xiang AP, Mao FF, et al: Nestin is
required for the proper self-renewal of neural stem cells. Stem
Cells. 28:2162–2171. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park DM and Rich JN: Biology of glioma
cancer stem cells. Mol Cells. 28:7–12. 2009. View Article : Google Scholar
|
|
10
|
Shmelkov SV, St Clair R, Lyden D and Rafii
S: AC133/CD133/ Prominin-1. Int J Biochem Cell Biol. 37:715–719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
12
|
Johannessen TC, Bjerkvig R and Tysnes BB:
DNA repair and cancer stem-like cells - potential partners in
glioma drug resistance. Cancer Treat Rev. 34:558–567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alam J and Stewart D: Nrf2, a Cap’n’Collar
transcription factor, regulates induction of the heme oxygenase-1
gene. J Biol Chem. 274:26071–26078. 1999.
|
|
14
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HJ, Zheng M, Kim SK, Cho JJ, Shin CH,
Joe Y and Chung HT: CO/HO-1 induces NQO-1 expression via Nrf2
activation. Immune Netw. 11:376–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piao MS, Choi JY, Lee DH, Yun SJ, Lee JB
and Lee SC: Differentiation-dependent expression of NADP(H):quinone
oxidoreductase-1 via NF-E2 related factor-2 activation in human
epidermal keratinocytes. J Dermatol Sci. 62:147–153. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji L, Li H, Gao P, Shang G, Zhang DD,
Zhang N and Jiang T: Nrf2 pathway regulates
multidrug-resistance-associated protein 1 in small cell lung
cancer. PLoS One. 8:e634042013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai JJ, Dudakov JA, Takahashi K, et al:
Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol.
15:309–316. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XJ, Sun Z, Villeneuve NF, et al: Nrf2
enhances resistance of cancer cells to chemotherapeutic drugs, the
dark side of Nrf2. Carcinogenesis. 29:1235–1243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau A, Villeneuve NF, Sun Z, Wong PK and
Zhang DD: Dual roles of Nrf2 in cancer. Pharmacol Res. 58:262–270.
2008. View Article : Google Scholar
|
|
21
|
Zhu J, Wang H, Sun Q, et al: Nrf2 is
required to maintain the self-renewal of glioma stem cells. BMC
Cancer. 13:3802013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar
|
|
23
|
Venere M, Fine HA, Dirks PB and Rich JN:
Cancer stem cells in gliomas: identifying and understanding the
apex cell in cancer’s hierarchy. Glia. 59:1148–1154.
2011.PubMed/NCBI
|
|
24
|
Stiles CD and Rowitch DH: Glioma stem
cells: a midterm exam. Neuron. 58:832–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cullinan SB, Zhang D, Hannink M, et al:
Nrf2 is a direct PERK substrate and effector of PERK-dependent cell
survival. Mol Cell Biol. 23:7198–7209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iida K, Itoh K, Kumagai Y, et al: Nrf2 is
essential for the chemopreventive efficacy of oltipraz against
urinary bladder carcinogenesis. Cancer Res. 64:6424–6431. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh A, Misra V, Thimmulappa RK, et al:
Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer.
PLoS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ikeda H, Nishi S and Sakai M:
Transcription factor Nrf2/MafK regulates rat placental glutathione
S-transferase gene during hepatocarcinogenesis. Biochem J.
380:515–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan H, Wang H, Zhu L, Wang X, Cong Z, Sun
K and Fan Y: The involvement of Nrf2-ARE pathway in regulation of
apoptosis in human glioblastoma cell U251. Neurol Res. 35:71–78.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji XJ, Chen SH, Zhu L, et al: Knockdown of
NF-E2-related factor 2 inhibits the proliferation and growth of
U251MG human glioma cells in a mouse xenograft model. Oncol Rep.
30:157–164. 2013.PubMed/NCBI
|
|
31
|
Park EJ, Lim JH, Nam SI, Park JW and Kwon
TK: Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through
reactive oxygen species (ROS) dependent and PKC delta-independent
pathway in human colon cancer HT29 cells. Biochimie. 92:110–115.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JE, Kang YJ, Lee KY and Choi HC:
Isoproterenol inhibits angiotensin II-stimulated proliferation and
reactive oxygen species production in vascular smooth muscle cells
through heme oxygenase-1. Biol Pharm Bull. 32:1047–1052. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsieh HL, Wang HH, Wu CY and Yang CM:
Reactive oxygen species-dependent c-Fos/activator protein 1
induction upregulates heme oxygenase-1 expression by bradykinin in
brain astrocytes. Antioxid Redox Signal. 13:1829–1844. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
DeNicola GM, Karreth FA, Humpton TJ, et
al: Oncogene-induced Nrf2 transcription promotes ROS detoxification
and tumorigenesis. Nature. 475:106–109. 2011. View Article : Google Scholar : PubMed/NCBI
|