Introduction
Neuroblastoma is a common childhood malignant tumor
of neural crest origin, arising in the paravertebral sympathetic
ganglia and the adrenal medulla (1). The clinical characteristics of
neuroblastoma include extreme heterogeneity, easy transfer and high
malignant potential (2,3). Neuroblastoma accounts for
approximately 10% of all pediatric cancers and 15% of
cancer-related deaths in children (3–9).
Although conventional therapies that are based on surgery,
chemotherapy and radiotherapy are available, these approaches have
limited efficacy in many cases (8).
Therapy failure is generally caused by acquired chemoresistance or
high toxicity in neuroblastoma patients. The major challenge in
neuroblastoma treatment is the identification and development of
new drugs with specific effects (10).
Natural plant compounds represent a large group of
untapped potential medicines. Various chemical compounds extracted
from natural plants have been reported to have high efficacy and
few side-effects (11). Artemisinin
(ART), also known as Qinghaosu, was isolated from the Chinese herb
Artemisia annua L. (Qinghao), a type of wormwood native to
Asia. It was discovered in the early 1970s by Tu Youyou (12,13).
It has been approved as a potent anti-malarial agent by the Food
and Drug Administration (FDA), and artemisinin is commonly used in
the clinical management of malaria (14,15).
Recently, it was reported that artemisinin or its derivatives have
anticancer activity in many types of cancers, such as in multiple
tumors (16,17), breast cancer (18), hepatocellular carcinoma (19), leukemia (20), prostate cancer (16) and oral cancer (21). Yet, the role of artemisinin in
neuroblastoma has not been well characterized.
In the present study, we investigated the potential
role of artemisinin in neuroblastoma cells. We determined that
artemisinin induces the inhibition of cell proliferation and
induces the apoptosis of the neuroblastoma cells in vitro.
Moreover, we also demonstrated that artemisinin suppressed
clonogenic formation ability and xenograft tumor growth in NOD/SCID
mouse models. As a natural product, artemisinin has been confirmed
to have no or fewer side effects in the treatment of malaria. Thus,
artemisinin could be used as a novel potential drug for the
treatment of neuroblastoma.
Materials and methods
Cell culture
Human neuroblastoma cell lines SK-N-AS, SK-N-DZ and
SHEP1 were grown in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics
penicillin and streptomycin (P/S). BE(2)-C cells were cultured in a
1:1 mixture of DMEM and Ham’s nutrient mixture F12 (DMEM/F12),
supplemented with 10% FBS and 1% antibiotics (P/S). All four cell
lines were purchased from ATCC (Manassas, VA, USA). The growth
media, antibiotics and FBS were purchased from Life Technologies.
All cells were cultured at 37°C in a 5% CO2 humidified
incubator.
Cell growth and proliferation assay
Artemisinin was dissolved in DMSO. The cell growth
curve was detected using the Cell Counting Kit-8 assay (CCK-8;
Beyotime, China). Briefly, approximately 1,000 cells in 200 μl
medium were seeded in 96-well plates and incubated with artemisinin
at concentrations of 100, 200, 300 and 400 μM; DMSO was used as a
control. CCK-8 (20 μl) was added and incubated for 2 h every 2
days, and the absorbance at 450 nm was used to detect metabolically
intact cells according to the manufacturer’s protocol. Cells were
exposed to 300 μM artemisinin or DMSO for 72 h, and cell
morphologic examination was carried out using an inverted
microscope (TS100, Nikon). Then adherent cells were collected and
washed with ice-cold PBS. The sample obtained was analyzed by the
TC10™ Automated Cell Counter (Bio-Rad, Hercules, CA, USA).
Immunofluorescence staining
Cells were grown on coverslips and treated with
either DMSO or 300 μM artemisinin for 72 h. Cells were washed with
PBS, fixed for 20 min in 4% paraformaldehyde (PFA), and
permeabilized with 0.3% Triton X-100 for 5 min. The cells were
blocked with 10% goat serum in PBS for 1 h, incubated with a
primary rat antibody against Ki67 (1:300, 556003; BD) in blocking
buffer for 1 h at room temperature, and then incubated with the
secondary antibody Alexa Fluor 595 goat anti-mouse IgG (H+L)
(1:2,000; A-21422; Invitrogen). DAPI (300 nM) in PBS was used for
nuclear staining. Cells were examined using a Nikon microscope
(80i) with Image-Pro Plus software for image analysis. Ki67 uptake
was calculated in 10 microscopic fields.
Cell cycle assay
Cells were plated in 10-cm plates and treated either
with 300 μM artemisinin or DMSO. After 72 h of treatment, cells
were fixed with 70% cold ethanol, stained with propidium iodide
(PI) and analyzed by flow cytometry (Accuri C6; BD). The data were
analyzed with ModfitLT software.
Western blot analysis
Cells treated with artemisinin for 72 h were
harvested and suspended in RIPA lysis buffer (Beyotime, China).
Protein concentrations were determined with the Enhanced BCA
protein assay kit (Beyotime). Sixty micrograms of protein was
separated on 10% sodium dodecyl sulfate polyacrylamide gels
(SDS-PAGE), transferred to PVDF membranes, and analysis was
performed with the primary antibodies including anti-cyclinD1
(1:500; Santa Cruz), anti-α-tubulin (1:2,000; Sigma-Aldrich),
anti-CDK4 (1:500; Santa Cruz), anti-cyclinE2 (1:500; Santa Cruz),
anti-cyclinB1 (1:500; Santa Cruz). Horseradish
peroxidase-conjugated goat anti-mouse (1:20,000) and rabbit
anti-goat (1:10,000) immunoglobulin G (IgG; KPL, Gaithersburg, MD,
USA) were used as secondary antibodies. Proteins were visualized
with BeyoECL Plus (Beyotime, China).
Cell death and apoptosis assay
For cell death assay, all cells grown to 60–70%
confluency were treated with 300 μM artemisinin for 72 h, and cells
were treated with DMSO as control. After treatment, adherent and
floating cells were collected by centrifugation, and then the
sample obtained was analyzed using the TC10™ Automated Cell
Counter. Cell counting was carried out using trypan blue dye
(145-0021, Bio-Rad) staining as previously described (22). The apoptotic ratios of the cells
were determined with the Annexin V-FITC apoptosis detection kit
(Sigma). Briefly, after a 72-h treatment with DMSO or 300 μM
artemisinin, the cells were collected and washed twice with cold
PBS buffer, resuspended in 100 μl of binding buffer, and incubated
with 5 μl of Annexin V conjugated to FITC and 10 μl PI for 15 min
at room temperature. Cell were then analyzed by flow cytometry
(Accuri C6; BD). The data were analyzed with Flowjo software.
Soft agar clonogenic assay
Five hundred cells were mixed with 0.3% Noble agar
in growth medium and plated into 6-well plates containing a
solidified bottom layer (0.6% Noble agar in growth medium). After
21 days of cell growth, colonies were stained with 5 mg/ml MTT
(Sigma), photographed and recorded.
In vivo tumorigenic assay
BE(2)-C cells were grown to 70–80% confluency and
trypsinized. Cells (1×106 in 200 μl DMEM) were injected
into the flanks of NOD/SCID mice. After one week of tumor growth,
the mice were administered intraperitoneal injections of
artemisinin at 100 mg/kg (mouse body weight) daily or vehicle
control DMSO (1 μl/ml DMEM) (16)
for 12 days. Tumor diameter was measured with digital calipers
every 3 days, and the tumor volume (V) was calculated by the
formula: V = 1/2 (length × width2). After treatment,
mice were sacrificed by CO2, and tumors were observed
and weighed. All animal experiments were pre-approved by the
Institutional Animal Care and Use Committee of Chongqing
University.
Quantification and statistical
analysis
Quantitative data are expressed as the mean ± SD.
The two-tailed Student’s t-test was performed for paired samples. A
minimum of three independent experiments was performed. Differences
were considered statistically significant at P<0.05.
Results
Artemisinin significantly inhibits cell
proliferation in neuroblastoma cells
Neuroblastoma cells were treated with various
concentrations of artemisinin, from 100 μM to 400 μM, for an
indicated period of time. As shown in Fig. 1A, a concentration- and
time-dependent response to artemisinin in neuroblastoma cells was
observed. Artemisinin markedly inhibited cell proliferation in the
neuroblastoma cells. After 72 h of treatment with 300 μM
artemisinin, both morphologic examination and cell counting
revealed that artemisinin significantly reduced cell growth in all
four neuroblastoma cell lines (Fig. 1B
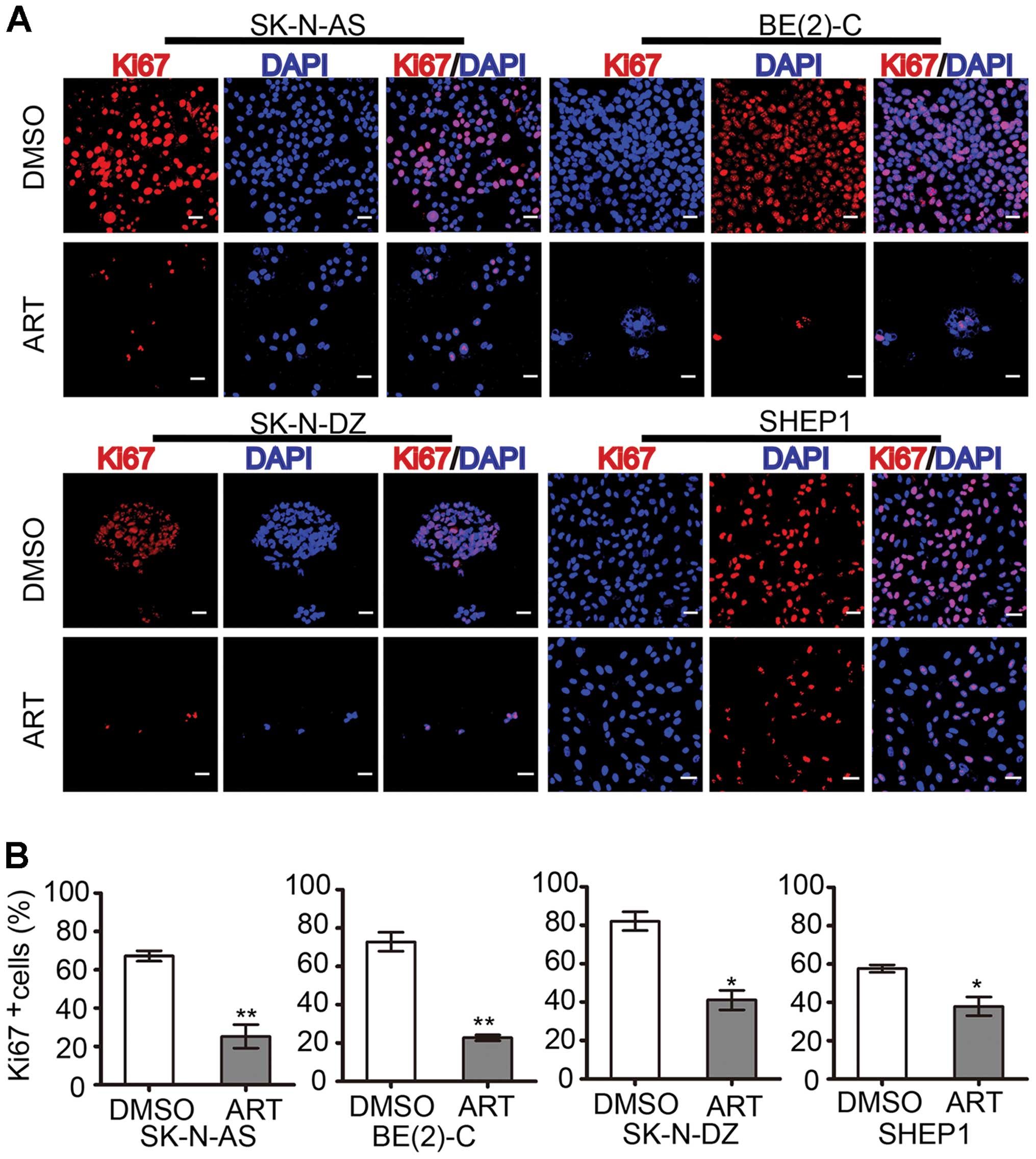
and C). As a proliferation marker, Ki67 staining also confirmed
that artemisinin markedly inhibited cell proliferation (Fig. 2A). The histogram statistics of the
Ki67-positive rates also demonstrated that artemisinin was an
effective agent for inhibiting the proliferation of neuroblastoma
cells (Fig. 2B). The percentage of
Ki67-positive SK-N-AS cells was reduced from 67.2±2.76 to
25.2±6.11%. The percentage of Ki67-positive BE(2)-C cells was
reduced from 72.8±4.94 to 22.7±1.51%. The percentage of
Ki67-positive SK-N-DZ cells was reduced from 82.1±4.89 to
41.1±5.08%, and the percentage of Ki67-positive SHEP1 cells was
reduced from 57.6±1.88 to 37.9±4.85%.
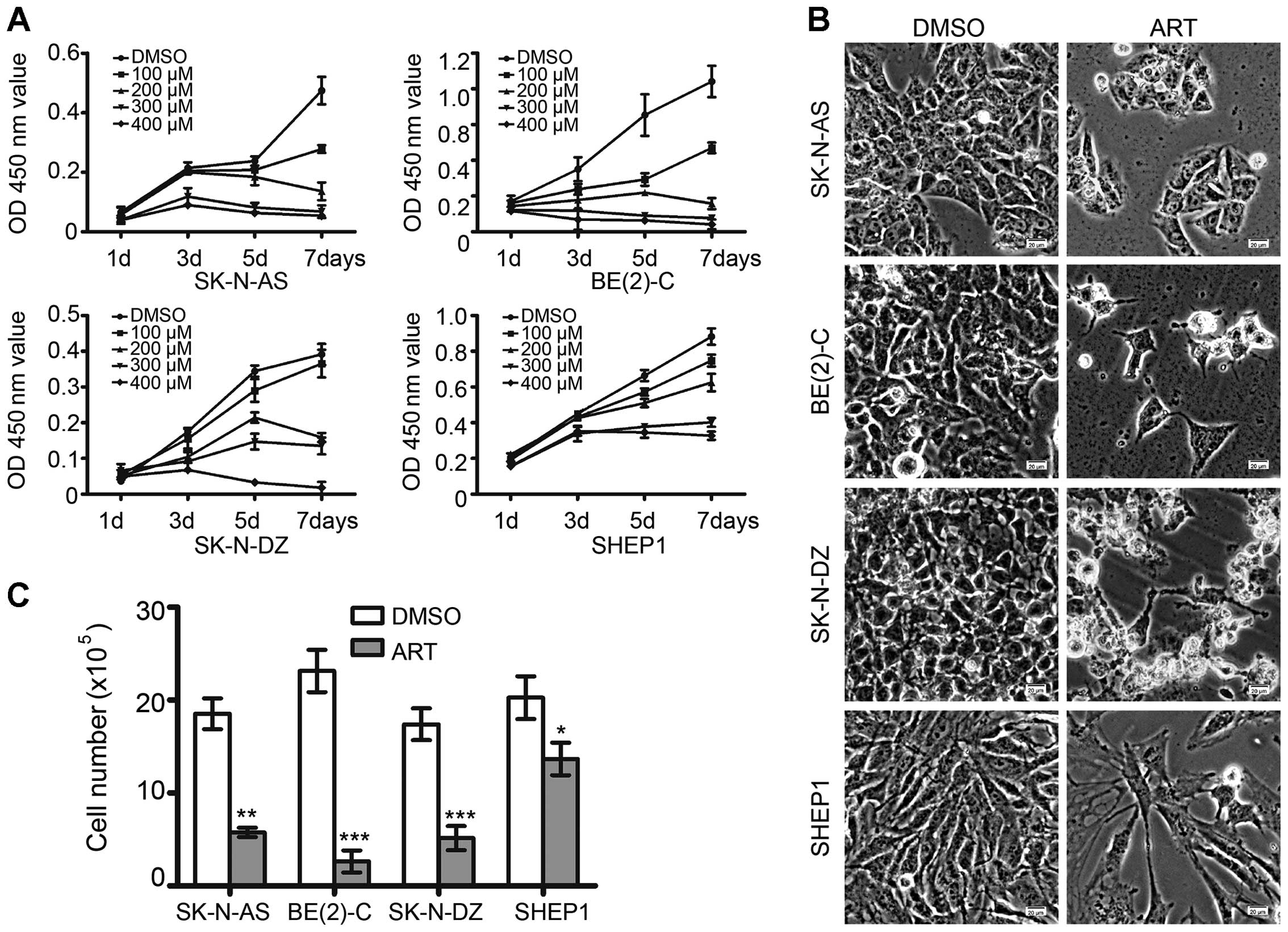 | Figure 1Artemisinin inhibits the cell growth
of neuroblastoma cells. (A) SK-N-AS, BE(2)-C, SK-N-DZ and SHEP1
cells were treated with 100, 200, 300 and 400 μM artemisinin; DMSO
was used as a control. Cell growth was assessed by the CCK-8 assay
every 2 days. (B) Morphologic examination of neuroblastoma cell
growth after treated with 300 μM artemisinin or DMSO for 72 h
respectively. Scale bar, 20 μm. (C) The cell number of SK-N-AS,
BE(2)-C, SK-N-DZ and SHEP1 cells was determined after artemisinin
treatment at 300 μM for 72 h; DMSO was used as a control. In A and
C, data represent the average ± SD of at least three independent
experiments. *P<0.05, **P<0.001,
***P<0.001. ART, artemisinin. |
Artemisinin treatment induces G1 phase
cell cycle arrest
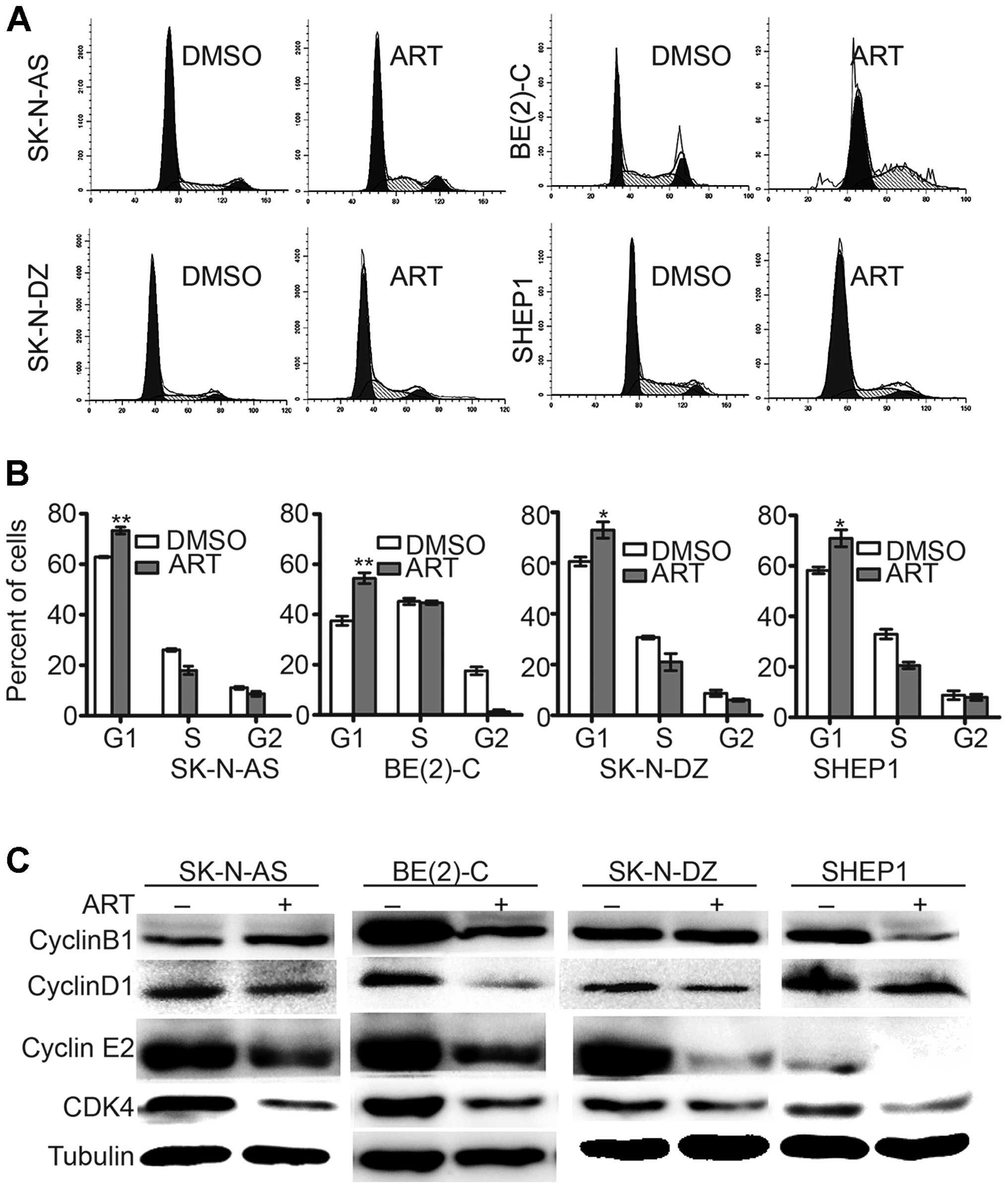
Furthermore, to gain insight into
artemisinin-induced inhibition of cell proliferation, we examined
the cell cycle distribution of the four neuroblastoma cell lines.
After artemisinin treatment at 300 μM for 72 h, the proportion of
cells in the G1 phase was significantly increased in all four cell
lines (Fig. 3A). The cell cycle
analysis of artemisinin-treated BE(2)-C cells revealed a
significant increase in the proportion of cells in the G0/G1 phase
(54.46±2.14%), compared with the vehicle-treated cells (G0/G1,
37.53±1.82%). Similar results were obtained in the SK-N-AS, SK-N-DZ
and SHEP1 cell lines (Fig. 3B).
These data demonstrated that artemisinin induced cell cycle arrest
in the G1 phase. Western blot analysis also showed that artemisinin
led to a marked downregulation of cyclinD1, CDK4 and cyclinE2
(Fig. 3C), which are collectively
required for cell cycle progression through the G1 to S phases
(23,24). These data suggest that artemisinin
inhibits cell proliferation through cell cycle arrest in
neuroblastoma cells.
Artemisinin accelerates apoptosis in
neuroblastoma cells
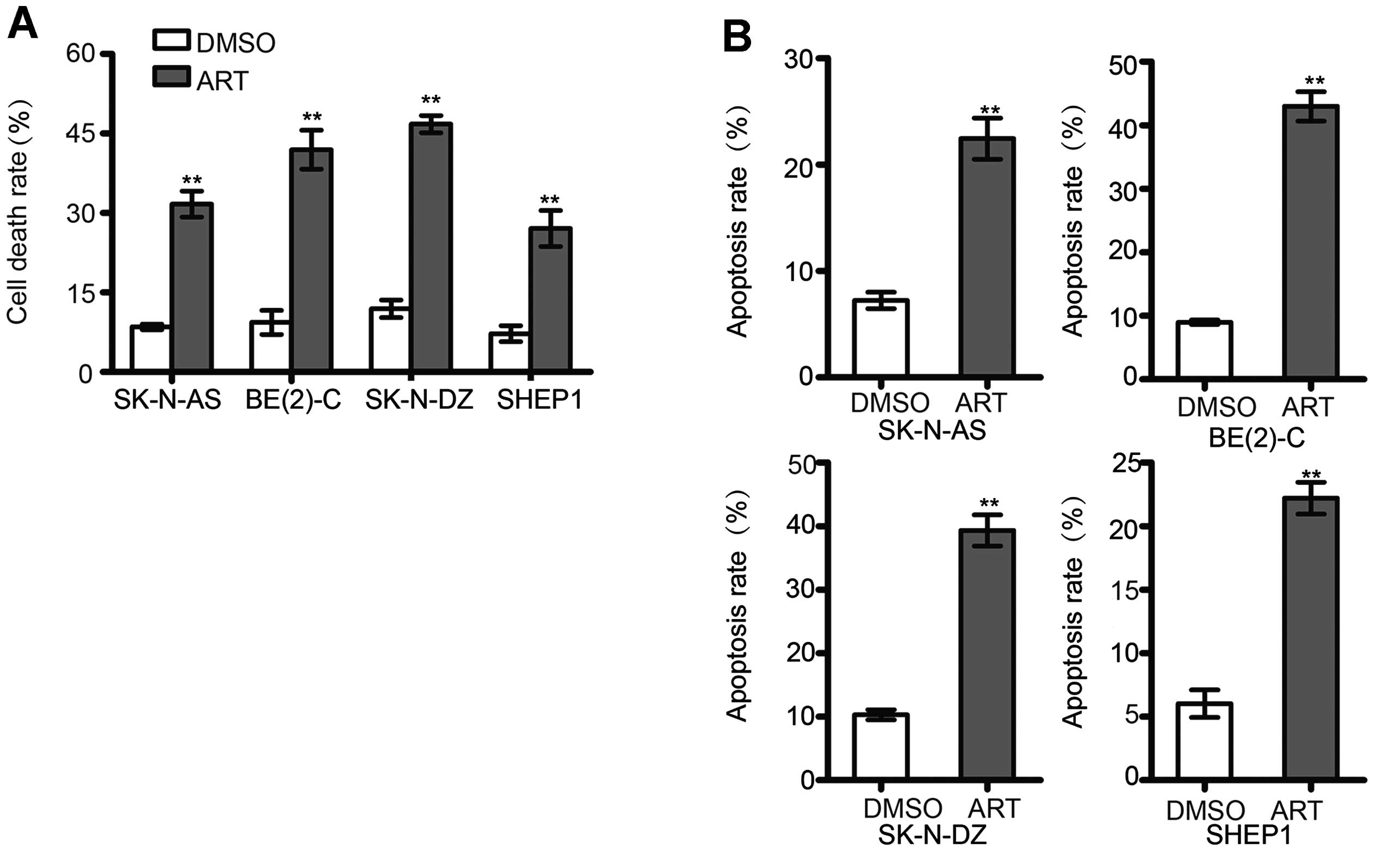
To investigate whether artemisinin induces apoptosis
in neuroblastoma cells, SK-N-AS, BE(2)-C, SK-N-DZ, and SHEP1 cell
lines were incubated with 300 μM artemisinin for 72 h. Trypan blue
assay indicated that the cell death was increased after artemisinin
incubation when compared with the control (Fig. 4A). Notably, treatment with
artemisinin resulted in a higher proportion of cells with positive
Annexin V and/or PI staining. Artemisinin markedly induced
apoptosis in all four neuroblastoma cell lines (Fig. 4B), which indicated that artemisinin
induced cell death through apoptosis in neuroblastoma cells.
Artemisinin suppresses the tumorigenicity
in neuroblastoma cells
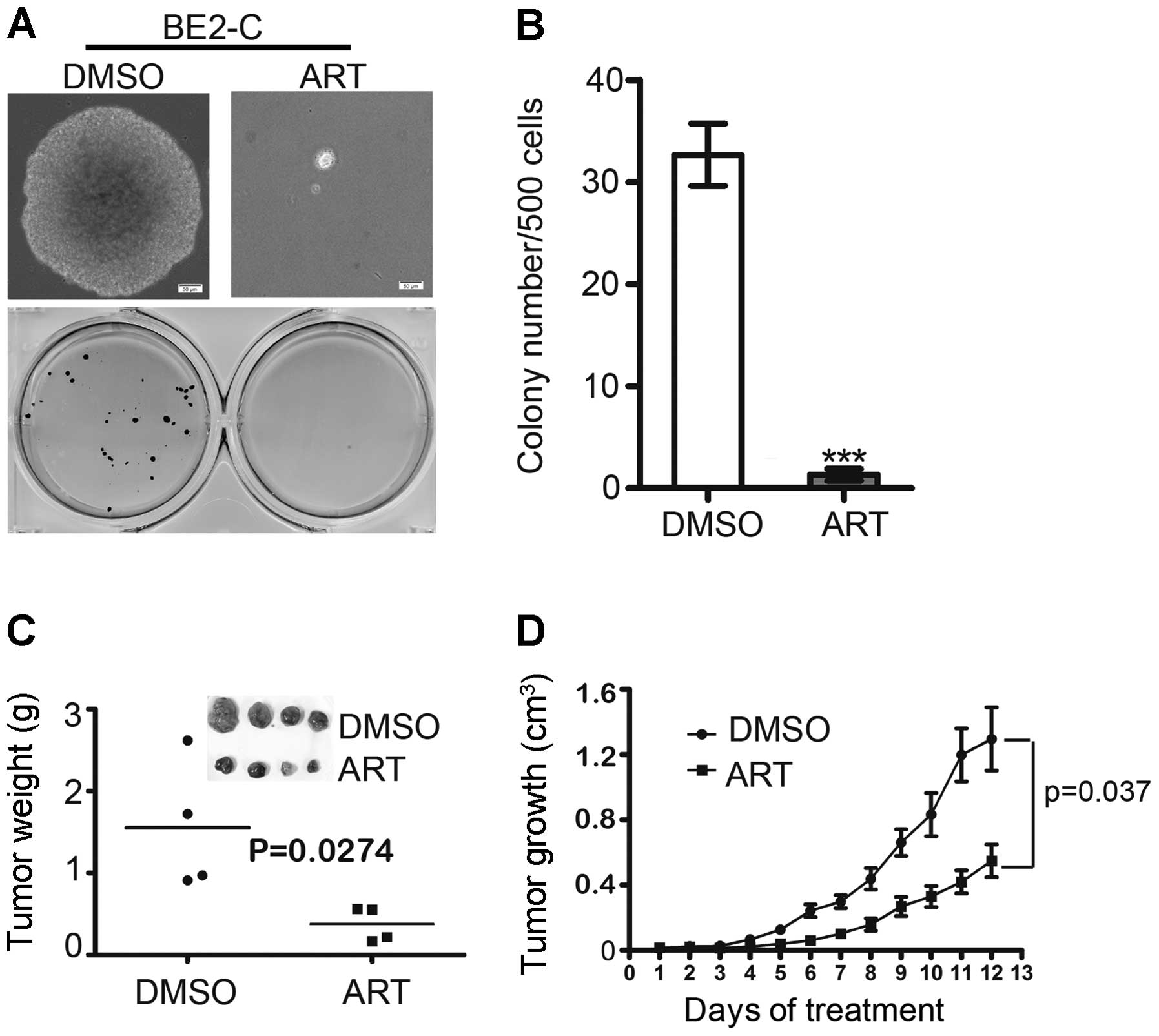
Soft agar is used to test the ability of single
cancer cells to proliferate and form colonies, and is also used as
a ‘human tumor stem-cell assay’ (25). In the present study, BE(2)-C cells
treated with 300 μM ART were observed to give rise to small and
scant colonies in soft agar compared with the cells treated with
DMSO (Fig. 5A and B). To determine
the effect of artemisinin on tumorigenicity in neuroblastoma cells,
we carried out a xenograft study in NOD/SCID mice. BE(2)-C cells
were implanted subcutaneously into the flanks of NOD/SCID mice. One
week after tumor injection, mice were treated intraperitoneally
with either DMSO or artemisinin at 100 mg/kg daily for 12 days. The
volume and weight of the xenograft tumors in the artemisinin
treatment group were much smaller and lighter than those in the
DMSO group (Fig. 5C and D). These
data indicate that artemisinin significantly suppresses the
tumorigenicity of neuroblastoma cells.
Discussion
Neuroblastoma is a malignant pediatric tumor with a
wide range of stages, requiring a wide range of therapeutic
options. However, successful therapeutic options remain limited.
Therefore, it is urgently necessary to identify additional
chemotherapeutic agents to target this disease. It has been
reported that artemisinin has multiple anti-proliferative activity,
including cell growth suppression (26,27),
apoptosis induction (28),
angiogenesis inhibition, cell migration disruption (29–31),
and modulation of nuclear receptor responsiveness (32,33).
Yet, the effect of artemisinin on neuroblastoma remains unclear.
The results presented in the present study demonstrated that
artemisinin led to significantly decreased cell growth and cell
proliferation, and increased apoptosis in neuroblastoma cells. We
first demonstrated that artemisinin treatment suppressed the
ability of colony formation in vitro and tumorigenicity of
neuroblastoma cells in vivo.
Artemisinin has been suggested to promote cytostasis
by G0/G1-phase arrest and to decrease the expression level of
cyclinB1, CDK2 and CDC25A in colon cancer (34), and to inhibit the promoter activity
of CDK4 in prostate cancer (16).
Artemisinin also induced G2/M phase arrest in osteosarcoma cells
(35). Our data indicated that
artemisinin induced cell cycle arrest at the G1 phase, together
with a decrease in the expression levels of cyclinD1, CDK4 and
cyclinE2 in all four neuroblastoma cell lines. cyclinB1 was
downregulated only in the BE(2)-C and SHEP1 cells, but no
significant difference was noted in the SK-N-AS and SK-N-DZ
cells.
Previous studies have shown that artemisinin induced
apoptosis in pancreatic tumor cells (36), lung adenocarcinoma cells (37), liver cancer cells (38), and non-small cell lung cancer cells
(39). In the present study, we
first demonstrated that artemisinin induced the cell death and
apoptosis of neuroblastoma cells at a lower dose than that for
clinical usage. Next, we investigated the mechanism and the
expression of apoptotic relevant proteins.
Collectively, our results revealed that artemisinin
inhibited cell proliferation and tumor growth, with cell cycle
arrest and apoptosis induction in neuroblastoma cells. Since
artemisinin has been used for the treatment of malaria for an
extensive period of time, a large body of data regarding clinical
tests and adverse drug reactions in patients are available.
Therfore, artemisinin may serve as a potential new therapeutic
agent for the treatment of neuroblastoma.
Acknowledgements
This research was supported by the National Basic
Research Program of China (no. 2012cb114603), the National Natural
Science Foundation of China (no. 31200223), the Research Fund for
the Doctoral Program of Higher Education of China (no.
20130182110003) and the Natural Science Foundation of Chongqing
(cstc2013jcyjys0007).
References
|
1
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beckwith JB and Martin RF: Observations on
the histopathology of neuroblastomas. J Pediatr Surg. 3:106–110.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu S, Yan X, Xiang Z, Ding HF and Cui H:
Leflunomide reduces proliferation and induces apoptosis in
neuroblastoma cells in vitro and in vivo. PLoS One. 8:e715552013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li T, Cui ZB, Ke XX, et al: Essential role
for p53 and caspase-9 in DNA damaging drug-induced apoptosis in
neuroblastoma IMR32 cells. DNA Cell Biol. 30:1045–1050. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Wang L, Ke XX, et al: DNA-damaging
drug-induced apoptosis sensitized by N-myc in neuroblastoma cells.
Cell Biol Int. 36:331–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Askin FB and Perlman EJ: Neuroblastoma and
peripheral neuroectodermal tumors. Am J Clin Pathol. 109:S23–S30.
1998.PubMed/NCBI
|
|
7
|
Shah S and Ravindranath Y: Neuroblastoma.
Indian J Pediat. 65:691–705. 1998. View Article : Google Scholar
|
|
8
|
Bessho F: Incidence of neuroblastoma.
Lancet. 353:701999. View Article : Google Scholar
|
|
9
|
Sridhar S, Al-Moallem B, Kamal H, Terrile
M and Stallings RL: New insights into the genetics of
neuroblastoma. Mol Diagn Ther. 17:63–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: from a well-known
antimalarial agent to a potential anti-cancer drug. J Biomed
Biotechnol. 2012:2475972012.PubMed/NCBI
|
|
11
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007.PubMed/NCBI
|
|
12
|
Zhang YJ, Gallis B, Taya M, Wang S, Ho RJ
and Sasaki T: pH-responsive artemisinin derivatives and lipid
nanoparticle formulations inhibit growth of breast cancer cells in
vitro and induce down-regulation of HER family members. PloS One.
8:e590862013. View Article : Google Scholar
|
|
13
|
Miller LH and Su X: Artemisinin: discovery
from the Chinese herbal garden. Cell. 146:855–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sundar SN, Marconett CN, Doan VB,
Willoughby JA Sr and Firestone GL: Artemisinin selectively
decreases functional levels of estrogen receptor-alpha and ablates
estrogen-induced proliferation in human breast cancer cells.
Carcinogenesis. 29:2252–2258. 2008. View Article : Google Scholar
|
|
15
|
Duffy PE and Mutabingwa TK: Drug
combinations for malaria: time to ACT? Lancet. 363:3–4. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willoughby JA Sr, Sundar SN, Cheung M, Tin
AS, Modiano J and Firestone GL: Artemisinin blocks prostate cancer
growth and cell cycle progression by disrupting Sp1 interactions
with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting
CDK4 gene expression. J Biol Chem. 284:2203–2213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai HC, Singh NP and Sasaki T: Development
of artemisinin compounds for cancer treatment. Invest New Drugs.
31:230–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tin AS, Sundar SN, Tran KQ, Park AH,
Poindexter KM and Firestone GL: Antiproliferative effects of
artemisinin on human breast cancer cells requires the downregulated
expression of the E2F1 transcription factor and loss of E2F1-target
cell cycle genes. Anticancer Drugs. 23:370–379. 2012. View Article : Google Scholar
|
|
19
|
Weifeng T, Feng S, Xiangji L, et al:
Artemisinin inhibits in vitro and in vivo invasion and metastasis
of human hepatocellular carcinoma cells. Phytomedicine. 18:158–162.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reungpatthanaphong P and Mankhetkorn S:
Modulation of multidrug resistance by artemisinin, artesunate and
dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines.
Biol Pharm Bull. 25:1555–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamachika E, Habte T and Oda D:
Artemisinin: an alternative treatment for oral squamous cell
carcinoma. Anticancer Res. 24:2153–2160. 2004.PubMed/NCBI
|
|
22
|
Cui H, Schroering A and Ding HF: p53
mediates DNA damaging drug-induced apoptosis through a
caspase-9-dependent pathway in SH-SY5Y neuroblastoma cells. Mol
Cancer Ther. 1:679–686. 2002.PubMed/NCBI
|
|
23
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee IH and Finkel T: Metabolic regulation
of the cell cycle. Curr Opin Cell Biol. 25:724–729. 2013.
View Article : Google Scholar
|
|
25
|
Agrez M, Kovach J and Lieber M: Cell
aggregates in the soft agar ‘human tumour stem-cell assay’. Br J
Cancer. 46:8801982.
|
|
26
|
Youns M, Efferth T, Reichling J,
Fellenberg K, Bauer A and Hoheisel JD: Gene expression profiling
identifies novel key players involved in the cytotoxic effect of
Artesunate on pancreatic cancer cells. Biochem Pharmacol.
78:273–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Xue F, Cheng Z, et al: Effect of
artesunate on inhibiting proliferation and inducing apoptosis of
SP2/0 myeloma cells through affecting NFkappaB p65. Int J Hematol.
90:513–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng Y, Ni X, Meng WT, Wen Q and Jia YQ:
Inhibitive effect of artesunate on human lymphoblastic
leukemia/lymphoma cells. Sichuan Da Xue Xue Bao Yi Xue Ban.
40:1038–1043. 2009.(In Chinese).
|
|
29
|
Zhou HJ, Wang WQ, Wu GD, Lee J and Li A:
Artesunate inhibits angiogenesis and downregulates vascular
endothelial growth factor expression in chronic myeloid leukemia
K562 cells. Vascul Pharmacol. 47:131–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li LN, Zhang HD, Yuan SJ, Yang DX, Wang L
and Sun ZX: Differential sensitivity of colorectal cancer cell
lines to artesunate is associated with expression of beta-catenin
and E-cadherin. Eur J Pharmacol. 588:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rasheed SAK, Efferth T, Asangani IA and
Allgayer H: First evidence that the antimalarial drug artesunate
inhibits invasion and in vivo metastasis in lung cancer by
targeting essential extracellular proteases. Int J Cancer.
127:1475–1485. 2010. View Article : Google Scholar
|
|
32
|
Chaturvedi D, Goswami A, Saikia PP, Barua
NC and Rao PG: Artemisinin and its derivatives: a novel class of
anti-malarial and anticancer agents. Chem Soc Rev. 39:435–454.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Firestone GL and Sundar SN: Anticancer
activities of artemisinin and its bioactive derivatives. Expert Rev
Mol Med. 11:e322009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Efferth T, Sauerbrey A, Olbrich A, et al:
Molecular modes of action of artesunate in tumor cell lines. Mol
Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Q, Li ZX, Peng HQ, et al: Artesunate
inhibits growth and induces apoptosis in human osteosarcoma HOS
cell line in vitro and in vivo. J Zhejiang Univ Sci B. 12:247–255.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Noori S, Hassan ZM and Farsam V:
Artemisinin as a Chinese medicine, selectively induces apoptosis in
pancreatic tumor cell line. Chin J Integr Med. Jun 15–2013.(Epub
ahead of print).
|
|
37
|
Xiao F, Gao W, Wang X and Chen T:
Amplification activation loop between caspase-8 and -9 dominates
artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis.
17:600–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou JM, Wang DS, Zhang RW and Wang H:
Experimental therapy of hepatoma with artemisinin and its
derivatives: In vitro and in vivo activity, chemosensitization, and
mechanisms of action. Clin Cancer Res. 14:5519–5530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao W, Xiao F, Wang X and Chen T:
Artemisinin induces A549 cell apoptosis dominantly via a reactive
oxygen species-mediated amplification activation loop among
caspase-9, -8 and -3. Apoptosis. 18:1201–1213. 2013. View Article : Google Scholar : PubMed/NCBI
|