Introduction
Pancreatic neuroendocrine neoplasms (PNENs)
represent 2–4% of all clinically detected pancreatic tumors
(1), with an incidence of 2–3 per
million based on data from the US and Norway (2,3). PNENs
are generally classified according to their tumor-node-metastasis
(TNM) pattern (4), grading as it
emerges from histopathological findings (5,6), and
biologic behavior, which depends on the presence of clinical
symptoms caused by abnormal hormone secretion.
At present, clinical management of patients with
PNEN is largely based on TNM stage and grading. However, the
malignant potential among PNENs at the same TNM stage and of the
same grade may vary considerably. A more precise classification of
PNEN based on the tumors’ pathogenetic characteristics might
predict their inherent aggressiveness better and further knowledge
of the molecular pathology of this disease might serve as a
starting point for novel therapies directed specifically against
the primary event behind tumorigenesis.
PNEN, as cancer in general, is the phenotypic result
of the acquisition of one or more genomic change(s) taking place at
the chromosomal and/or gene level. Thus, screening of the whole
tumor genome is a natural starting point when trying to understand
the pathogenetic mechanisms behind tumor development (7). Although studies of PNEN using
comparative genomic hybridization (CGH) have been performed
(8), the results thus obtained have
not been correlated with karyotyping and cell proliferation
data.
The Mitelman database on chromosome aberrations and
gene fusions in cancer (9) reports
seven PNENs with karyotypic aberrations, but no common chromosomal
abnormalities (10–12). Thus, knowledge regarding the
chromosomal characteristics of this type of cancer is clearly
insufficient. Information on genomic imbalances in nonfunctioning
(i.e. without hormone production) PNEN detected by CGH is limited
to 54 cases (13–16). Common genomic imbalances involved
gains of 7q, 17q, and 20q and losses of 6q, 11p, and 11q. The
available CGH-data on PNENs were obtained on small and
heterogeneous series of tumors and it is therefore difficult to
generalize the findings (8).
In the present study, we sought to gain more
information regarding the genomic rearrangements in PNENs by
performing karyotyping of G-banded chromosomes and high resolution
CGH (HR-CGH) analyses on a series of sporadic PNENs.
Materials and methods
Tumor samples and clinicopathological
data
The tumor samples were obtained from a prospective
series collected between April 2011 and January 2013, at the
Department of Hepato-Pancreato-Biliary Surgery, Rikshospitalet,
Oslo University Hospital. Sixteen specimens from 15 patients were
included in the study (Table I); 15
stemmed from the pancreas, whereas one sample was of concurrent
metastatic tissue in the liver of the same patient (case 15b). The
patients had a median age of 57 years (range, 30–78). The tumors
were diagnosed by an experienced pathologist according to the World
Health Organization 2010 classification (5). Tumors were classified as functioning
if they caused clinical symptoms due to hormone secretion and
immunohistochemistry confirmed hormone overproduction. Cases 10 and
15 had functioning tumors (insulinoma). Thirteen patients had
nonfunctioning tumors, indicating that they did not have clinical
symptoms due to hormonal secretion. In order to allow for
subdivision of the tumor samples analyzed, the following tumor
characteristics were registered: degree of cell proliferation as
indicated by the value of Ki-67 (< or ≥5%), the presence/absence
of synchronous distant metastases, and the size of the primary
tumor (< or ≥3.5 cm). We used a Ki-67 cut-off value of 5% as
this is a known prognostic predictor (17,18).
The cut-off value of the size of the primary tumor was chosen in
such a way that an equal amount of analyzed samples were below and
above this value.
 | Table IClinicopathological characteristics of
15 patients with sporadic PNENs. |
Table I
Clinicopathological characteristics of
15 patients with sporadic PNENs.
| Case no.
(biobank) | Gender/Age
(years) | Tumor location | Tumor diameter
(cm) | Clinical
behavior | Ki-67 (%) | ENETS TNMa |
|---|
| 1 (5) | m/66 | Tail | 4.0 | Nf | 0.8 | T2N0M0 |
| 2 (10) | m/73 | Tail | 3.6 | Nf | 12.7 | T2N1M1 |
| 3 (18) | m/52 | Tail | 10.0 | Nf | 32.6 | T3N1M1 |
| 4 (19) | f/78 | Tail | 2.1 | Nf | 2.3 | T2N0M0 |
| 5 (26) | m/68 | Tail | 0.7 | Nf | 0.8 | T1NxM0 |
| 6 (27) | f/57 | Head | 2.5 | Nf | 1.7 | T2N0M0 |
| 7 (31) | m/57 | Head/body/tail | 10.0 | Nf | 13.0 | T4N0M1 |
| 8 (36) | f/33 | Tail | 1.5 | Nf | 1.0 | T1N0M0 |
| 9 (37) | f/60 | Tail | 8.8 | Nf | 12.4 | T3N0M1 |
| 10 (42) | f/37 | Tail | 1.3 | F (insulinoma) | 6.3 | T1N0M0 |
| 11 (45) | m/40 | Tail | 1.2 | Nf | 1.6 | T1N0M0 |
| 12 (47) | f/67 | Body | 3.5 | Nf | 1.3 | T2N0M0 |
| 13 (54) | f/61 | Tail | 4.0 | Nf | 9.1 | T2N0M1 |
| 14 (56) | f/51 | Head/body/tail | 5.0 | Nf | 2.1 | T3N0M0 |
| 15a (59T) | m/30 | Tail | 1.5 | F (insulinoma) | 9.3 | T1N1M1 |
| 15b (59L) | m/30 | Liver metastasis | 2.5 | F (insulinoma) | 26.0 | NA |
The study was approved by the Regional Ethics
Committee (project number: 2011/497A and 2011/1945D). Informed
consent was obtained from all patients.
G-banding and karyotyping
Representative fresh samples from 10 PNENs were sent
to the Section for Cancer Cytogenetics immediately after the
operation. The samples were disaggregated mechanically and
enzymatically using collagenase Type II (Worthington, Freehold, NJ,
USA). The resulting cells and cell clumps were seeded into tissue
culture flasks and cultured in DMEM/Ham’s F12 medium supplemented
with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1%
ITS+ Premix, 20 ng/ml epidermal growth factor (both from BD
Biosciences, Bedford, MA, USA). After 7–10 days, the cultures were
harvested as described by Mandahl (19). Chromosome preparations were G-banded
using Wright stain and karyotyped according to the ISCN (2009).
High Resolution Comparative Genomic
Hybridization (HR-CGH)
Representative fresh-frozen tissues stored at −80°C
in a biobank were used for molecular cytogenetic analysis. DNA from
16 tumor samples was isolated using the MagAttract DNA Mini M48 kit
(Qiagen, Valencia, CA, USA). HR-CGH was performed according to our
modifications of the standard procedure (20–22).
Chromosomes were karyotyped based on their inverted DAPI appearance
and the relative hybridization signal intensity was determined
along each chromosome. On average, 10–15 metaphases were analyzed.
The description of the CGH copy number changes was based on the
recommendations of the ISCN (2009).
Results
Of the 10 cytogenetically analyzed tumor samples
(Table II), eight showed normal
karyotype whereas two were abnormal (cases 9 and 10). Case 9 had an
extra chromosome 12 as the only clonal aberration in an otherwise
incomplete karyotypic description, while case 10 showed a near
tetraploid genome that could not be further described (Table II).
 | Table IIGenomic alteration detected in the
PNENs examined by karyotyping and HR-CGH. |
Table II
Genomic alteration detected in the
PNENs examined by karyotyping and HR-CGH.
| Case no.
(biobank) | Karyotype | CGH imbalances |
|---|
| 1 (5) | - | No imbalances |
| 2 (10) | - | rev ish
enh(4q13,4q21q24),dim(1p,1q21q32,2p,2q11q22,2q23,2q31q35,2q36q37,
6p12p21,11p11p14,11q12q13,11q22q23,16p,16q12q23) |
| 3 (18) | - | rev ish
enh(1p12p31,1q,2p,5p,5q11q31,6,7,8,10,12,14,16,17,19p13,20p,21),
dim(Xp22,1p31pter,3,9p,11,18) |
| 4 (19) | - | rev ish
dim(11,18) |
| 5 (26) | 46,XY[20] | rev ish
dim(11) |
| 6 (27) | 46,XX[15] | rev ish dim(3) |
| 7 (31) | 46,XY[10] | rev ish
enh(Xp22,Xq26q28,4p13p15,4q,5p13p14,5q,7,9p13p21,9q21qter,12p11p13,
12q,13,14q12q24,14q31q32,17q,18q,19q13),dim(1p36,2p11p12,3p13p21,8q24,11p1
4p15, 11q23,16p11p12,16q12q13) |
| 8 (36) | 46,XX[15] | rev ish
enh(4,5,9,12q21,20),dim(11p11) |
| 9 (37) | 47~48,XX,+12,inc
[cp 5]/46,XX[3] | rev ish
enh(4p13pter,4q,5p13pter,7,9q21qter,12p11pter,12q,13,14,15,17,18p11,18q,
20p11pter,20q11qter),
dim(Xp11,Xq12,1p36,1p12p13,1q21q22,1q42,2p24,2p21,2p16,
2p11,2q11,2q21q22,6p23,10p13p15,11p15,11p11,16p11p13,16q12q23,22q12q13) |
| 10 (42) |
81~87,inc[3]/46,XX[22] | rev ish
enh(5,9,20) |
| 11 (45) | 46,XY[25] | No imbalances |
| 12 (47) | 46,XX[25] | rev ish
enh(4,5,8,9,10p12pter,10q,12,13,20p,20q11q13),dim(11p,11q14qter,16p12p13,
16q12q13,22q13) |
| 13 (54) | 46,XX[25] | rev ish
enh(4p,4q12q31,5p,5q,7p,9p,9q13q22,13q,18q12,18q22q23) |
| 14 (56) | 46,XX[25] | rev ish
enh(5,9,15),dim(11) |
| 15a/59T | - | No imbalances |
| 15b/59L | - | rev ish
enh(2q22q24,4q13,13q),dim(1p,3,9q13q34) |
The HR-CGH analysis provided informative results in
all 16 tumors analyzed. In 13 tumors, DNA copy number changes were
detected, whereas three tumors (case 1, 11 and 15) showed a
balanced genome (Table II). Of the
16 tumor samples, 13 were nonfunctioning tumors. In general, gains
were more frequent than losses in these PNENs, but no amplification
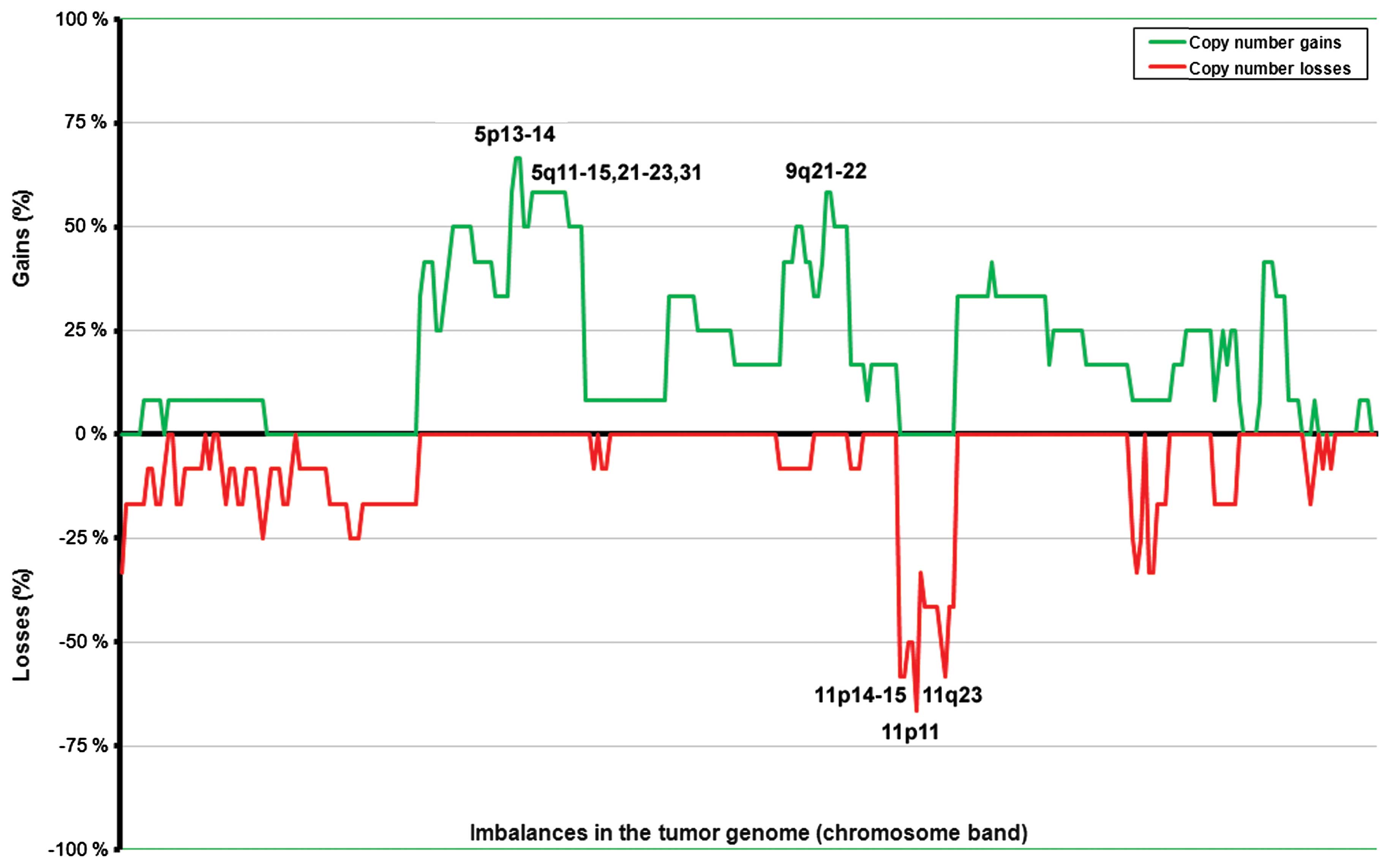
was scored. Commonly gained regions in the nonfunctioning tumors
were found at 5p12–13 (in seven out of 11 tumors with
abnormalities; 64%) and at 4q13–24, 5p15, 5q11–31, and 9q21–22 (in
six tumors; 55%; Fig. 1). Losses
were scored at 11p11 (in eight tumors out of 11 with imbalances;
73%) followed by 11p14–15 and 11q23 (seven tumors; 64%), and
11p12–13 and 11q22 (six tumors; 55%). The average number of copy
aberrations (ANCA index) was 12 for the 13 nonfunctioning primary
tumors.
We further subdivided the nonfunctioning tumors
according to the degree of cell proliferation as indicated by the
value of Ki-67 (< or ≥5%), the presence/absence of synchronous
distant metastases, and the size of the primary tumor (< or ≥3.5
cm).
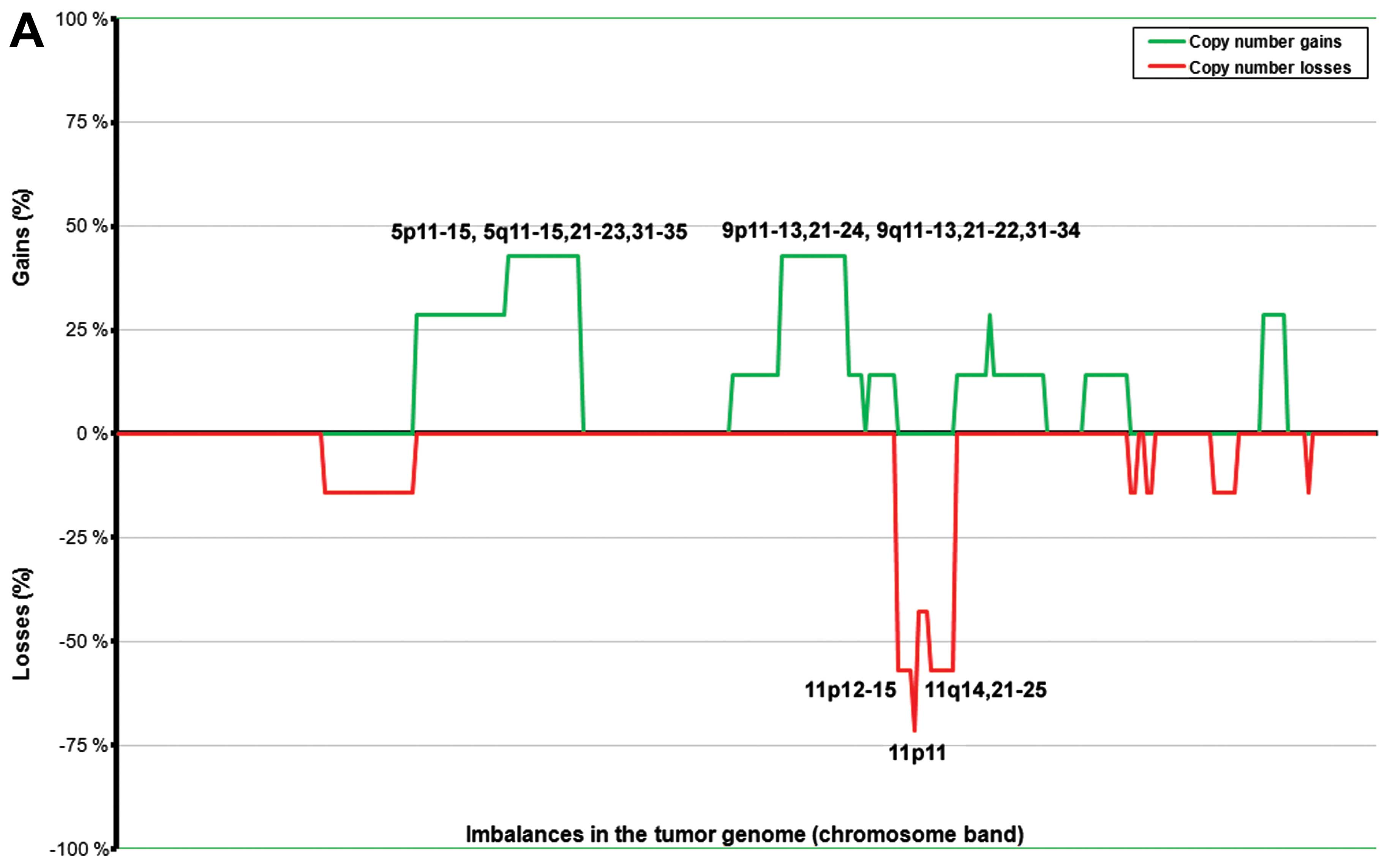
The ANCA index for the tumors with low Ki-67 was
4.83, whereas the tumors with high Ki-67 had an ANCA index of 21.2.
An overview of the genomic imbalances and the specific values of
the parameters used is presented in Tables I and II. The tumors with high Ki-67 values
(≥5%) showed a more complex picture of imbalances compared to those
with low Ki-67 (Fig. 2), which
showed fewer imbalances or a balanced genome (two cases). The
subgroup with high Ki-67 showed frequent gains at 4q13–24, 5p13–14,
and 7p11–22 (in four out of five tumors with imbalances; 80%),
followed by 4p13–15, 4q25–31, 5q11–31, 7q11–36, 9q21–22, 12p, 12q,
13q, 14q12–33, 17q, 18q12, and 18q22–23 (in three tumors; 60%).
Losses were present at 1p36 (five tumors with imbalances; 80%)
followed by 2p11, 11p14–15, 11p11, 11q23, 16p11–12, and 16q12–13
(four tumors; 60%). The subgroup with low Ki-67 showed gains of one
copy of each of chromosomes 5 and 9 in three tumors out of six with
imbalances (50%). Losses were scored at 11p11 (83%), 11p12–15 and
11q14–25 (67%), and 11q11–13 (50%).
The tumors with synchronous distant metastases were
the same as those with Ki-67 ≥5%. Therefore, the imbalances as well
as the ANCA index are the same in the two subgroups.
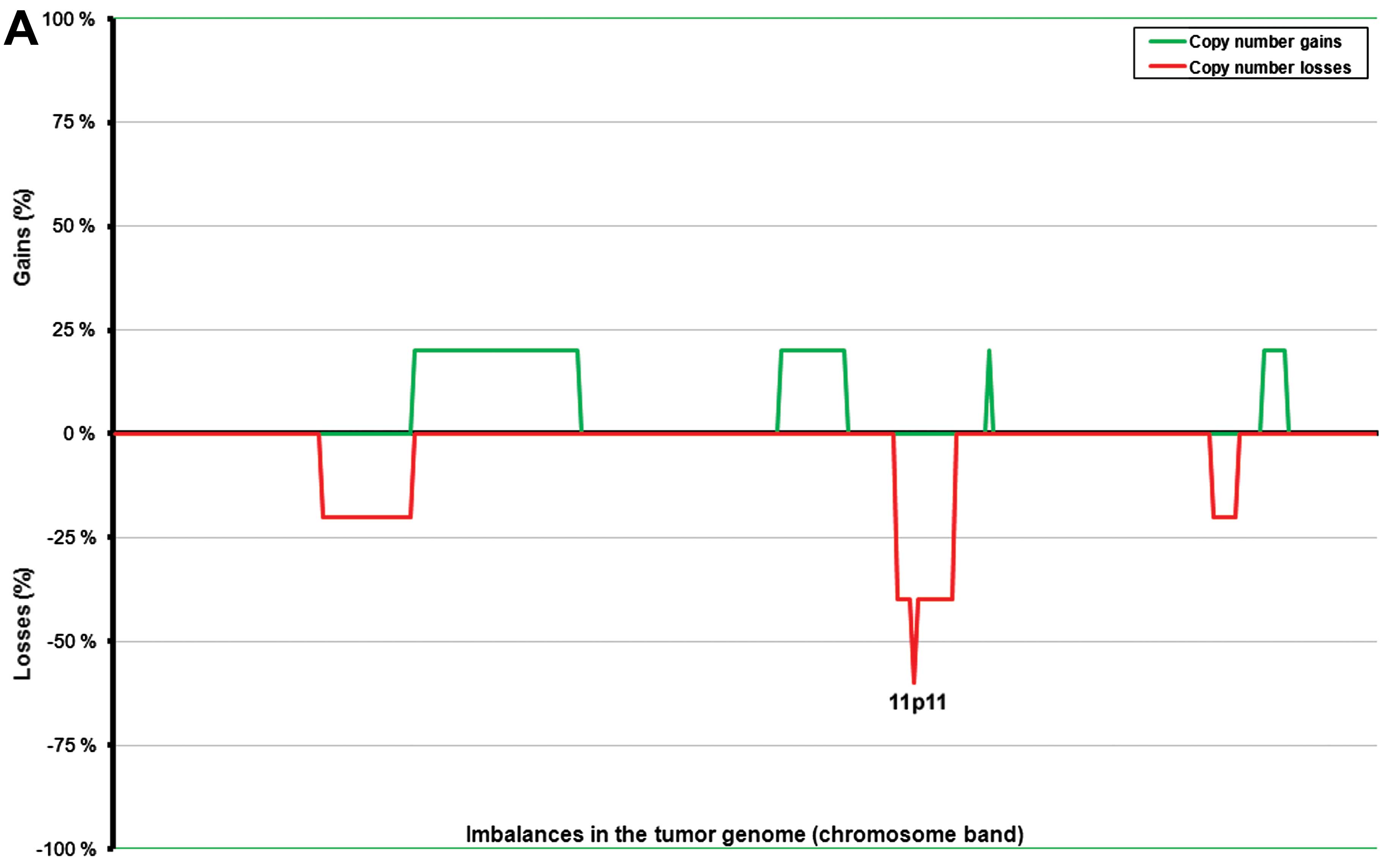
In general, the tumors with a diameter <3.5 cm
(n=5, range 0.7–2.5 cm) showed fewer imbalances than those with a
diameter ≥3.5 cm (n=8, range 3.5–10.0 cm) (Fig. 3). The ANCA index for the first
subgroup of tumors (size <3.5 cm) was 2.5, whereas for the
latter (size ≥3.5 cm) it was 17.85. To some extent, the imbalances
also affected different genomic regions in the two subgroups. More
precisely, the larger tumors showed common gains of 5p13–14 (in six
out of seven tumors with imbalances; 86%) followed by 4q13–24,
5p15, 5q11–31, and 9q21–22 (five tumors; 71%), and 4p13–15, 4q12,
4q25–31, 5p12, 5q32–35, 7p, 9p13–21, 9q31–34, 12p, 12q, and 13q
(57%). Losses were scored more frequently at 11p14–15, 11p11, and
11q23 (five out of seven tumors with imbalances; 71%) followed by
1p36, 11p12–13, 11q22, 11q24, 16p12, and 16q12–13 (57% of tumors
with imbalances) (Fig. 2A). The
smaller tumors showed losses from chromosome 11 as the only common
imbalance; more precisely, 11p11 was lost in three out of four
tumors with imbalances (75%), whereas chromosomal regions 11p12–15
and 11q were lost in 50% of the tumors with imbalances.
To assess the values scored for each subgroup, we
used the non-parametric Mann-Whitney U test which showed a
significant difference within all subgroups. Specifically, we found
a statistically significant difference in the amount of copy number
changes in the group with low Ki-67 value compared to the group
with high Ki-67 value (P=0.017); the same statistically significant
difference was found for the number of aberrations found in the
tumors with synchronous distant metastases and the group without.
Furthermore, the subgroups of tumors smaller and larger than 3.5 cm
also showed a statistically significant difference in the number of
changes (P=0.018) (Table
III).
 | Table IIIAverage number of copy aberrations
(ANCA index) in subgroups of cell proliferation (Ki-67), status of
metastatic disease and tumor size in 13 patients with sporadic
nonfunctioning PNEN. |
Table III
Average number of copy aberrations
(ANCA index) in subgroups of cell proliferation (Ki-67), status of
metastatic disease and tumor size in 13 patients with sporadic
nonfunctioning PNEN.
| Ki-67 | Distant
metastasis | Primary tumor
diameter (cm) |
|---|
|
|
|
|
|---|
| <5% | ≥5% | yes | no | <3.5 | ≥3.5 |
|---|
| ANCA index | 4.83 | 21.2 | 4.83 | 21.2 | 2.5 | 17.85 |
| U-testa (P-value) | 0.017 | 0.018 | 0.018 |
The two cases of primary insulinoma, the only
functioning tumors in our series, showed gains of the entire
chromosomes 5, 9, and 20 (case 10) and a balanced genome (case
15a). The liver metastasis from the latter case (15b) showed gains
at 2q22–24,4q13, and 13q and losses at 1p, 3, and 9q.
Discussion
Pronounced variability from case to case has made
research on pancreatic neuroendocrine neoplasms (PNENs) demanding.
In order to improve our understanding of the relationship between
clinical behavior, histopathology and the acquired genetics of this
tumor entity, systematic analysis of defined series of PNENs is
required.
Only two of the ten tumors sent for cytogenetic
analysis showed clonal chromosome abnormalities. In both cases, the
karyotypic description was incomplete and the number of abnormal
cells constituted a minority. However, case 9 showed an extra
chromosome 12 by karyotyping, which was also found gained, among
other imbalances, by HR-CGH, indicating that both techniques
identified the same, or at least related, abnormal clones.
Karyotyping of tumors requires culturing of neoplastic parenchyma
cells in vitro. It appears that pancreatic neuroendocrine
tumor cells do not divide well under laboratory conditions and this
may account for the severely limited cytogenetic information of
PNENs hitherto reported in the literature with only seven
karyotypical abnormal cases in three studies (10–12).
Investigations of genomic imbalances by means of CGH
are easier in this regard inasmuch as it does not require the
neoplastic cells to enter mitosis. Most of the available data from
CGH studies of PNENs were obtained on small and heterogeneous
series. Moreover, different tumor classifications were used by the
investigators, making a comparative analysis of different PNEN
subtypes difficult. We used HR-CGH to screen the genomes of 16 PNEN
samples for imbalances. All tumors yielded informative results with
13 showing imbalances. The use of dynamic standard reference
intervals (D-SRI), which represent a ‘normal’ ratio profile that
takes into account the amount of variation detected in negative
controls for each chromosome band, provided more objective and
sensitive scoring criteria than fixed thresholds and, consequently,
a higher resolution. In our series, most patients presented with
nonfunctioning tumors (n=13). In these, the most frequent imbalance
(loss) was scored for chromosome 11. Loss of chromosome 11 has been
previously reported (13,23). In our study, 11p11 was found lost in
73% of the nonfunctioning tumors with imbalances, followed by
11p14–15 and 11q23 (64% of the tumors), and 11p12–13 and 11q22 in
55%. Further studies at the gene level may provide information as
to the possible presence of tumor suppressor genes of importance in
PNEN tumorigenesis and/or progression at this location. The most
frequently gained region was 5p12–13 in the nonfunctioning tumors,
and overall gains were present in 64% of the nonfunctioning tumors
with imbalances. Previous studies have shown frequent gain of
chromosome 5 (13,23); however, this is the first time that
a more detailed map of specifically gained bands was identified.
The finding indicates the location of possible oncogenes active in
this tumor type. The second most frequently gained regions were
4q13–14 and 9q21–22, both found altered in 55% of the abnormal
nonfunctioning tumors. Previous reports have suggested frequent
gains of chromosome arms 4q and 9q (13,16,23),
and we were able to restrict the commonly gained areas to two
chromosomal bands.
We further subdivided the nonfunctioning tumors with
regard to the degree of cell proliferation as measured by the value
of Ki-67, the presence of synchronous distant metastases, and the
size of the primary tumor. The number of copy number changes,
indicated by the ANCA index, was 4.8 for tumors with low Ki-67
(≥5%) and 21.2 for the group with high Ki-67 (<5%). The same
values were also found for tumors with and without synchronous
distant metastases, and 2.5 for small tumors (<3.5 cm) and 17.8
for larger tumors (≥3.5 cm).
Notably, the three subgroups of nonfunctioning
tumors with low values of Ki-67, no distant metastasis and small
size, all had in common not only the fact that they showed few
aberrations, but also frequent loss of material from chromosomal
band 11p11 (in 83, 83, and 75% of the abnormal tumors,
respectively). This suggests that loss of 11p11 may be a
particularly important, possibly primary, event in the
tumorigenesis of sporadic nonfunctioning PNENs. The large tumors
with high Ki-67 and distant metastases all showed additional
imbalances in their genome probably acquired during tumor
progression. To know the primary event of tumorigenesis, the
conditio sine qua non for the tumor to develop, is
fundamental for the identification of potential tailor-made
treatment of these patients that targets only the abnormal cells.
Furthermore, knowing the specific aberration is also a prerequisite
for a correct pathogenetic classification of the tumor. The study
of the above parameters is a step in the pathogenetic
classification, but clearly we are still far from being able to
establish what constitutes the primary molecular events during
tumorigenesis.
The only functioning tumor (an insulinoma) with
imbalances in our series (case 10) showed gains of one copy of
chromosomes 5, 9 and 20. Gain of chromosome 9q is already known as
a frequent aberration in both benign and malignant insulinomas
(24), and gain of chromosomal band
9q34 has previously been described as an early event in insulinomas
(14). The other abnormal
insulinoma sample was a liver metastasis (case 15b) that showed
gains at 2q22–24,4q13, and 13q and losses at 1p, 3 and 9q13–34
while the primary tumor (case 15a) showed a normal, balanced
profile. Thus, it seems that chromosomal instability increased from
the primary to the metastatic lesion. Jonkers et al found
that chromosome 6q losses and 12q, 14q and 17pq gains are strongly
associated with metastatic insulinoma (24). Floridia et al found that
allelic loss of 22q correlated with distant metastasis (13). Our case did not confirm these
findings. However, data from only one patient are too scarce to
draw any conclusions in this regard.
Acknowledgements
This study was supported by Oslo University
Hospital; Carcinor, the Norwegian patient advocacy association for
neuroendocrine cancer; the Norwegian Society of Gastroenterology
and the Henrik Homan foundation. The authors thank Thu Hong Thy
Nguyen and Lisa Yuen for their support in biobanking the surgical
specimens needed to perform this study.
References
|
1
|
Fendrich V and Bartsch DK: Surgical
treatment of gastrointestinal neuroendocrine tumors. Langenbecks
Arch Surg. 396:299–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hauso O, Gustafsson BI, Kidd M, Waldum HL,
Drozdov I, Chan AK and Modlin IM: Neuroendocrine tumor
epidemiology: contrasting Norway and North America. Cancer.
113:2655–2664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008.
|
|
4
|
Rindi G, Falconi M, Klersy C, Albarello L,
Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A,
Doglioni C, Delle FG, Fischer L, Fusai G, de Herder WW, Jann H,
Komminoth P, de Krijger RR, La RS, Luong TV, Pape U, Perren A,
Ruszniewski P, Scarpa A, Schmitt A, Solcia E and Wiedenmann B: TNM
staging of neoplasms of the endocrine pancreas: results from a
large international cohort study. J Natl Cancer Inst. 104:764–777.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
3. 4th edition. International Agency for Research on Cancer (IARC);
Lyon: 2010
|
|
6
|
Rindi G and Wiedenmann B: Neuroendocrine
neoplasms of the gut and pancreas: new insights. Nat Rev
Endocrinol. 8:54–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heim S and Mitelman F: Cancer
Cytogenetics. 3rd edition. Wiley-Blackwell; NJ: 2009
|
|
8
|
Capurso G, Festa S, Valente R, Piciucchi
M, Panzuto F, Jensen RT and Delle Fave G: Molecular pathology and
genetics of pancreatic endocrine tumours. J Mol Endocrinol.
49:R37–R50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitelman F, Johansson B and Mertens F:
Mitelman database of chromosome aberrations and gene fusion in
cancer. http://cgap.nci.nih.gov/Chromosomes/Mitelman.
2014
|
|
10
|
Bugalho MJ, Roque L, Sobrinho LG, Hoog A,
Nunes JF, Almeida JM, Leitão CN, Santos JR, Pereira MC and Santos
MA: Calcitonin-producing insulinoma: clinical, immunocytochemical
and cytogenetical study. Clin Endocrinol (Oxf). 41:257–260. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Long PP, Hruban RH, Lo R, Yeo CJ,
Morsberger LA and Griffin CA: Chromosome analysis of nine endocrine
neoplasms of the pancreas. Cancer Genet Cytogenet. 77:55–59. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scappaticci S, Brandi ML, Capra E,
Cortinovis M, Maraschio P and Fraccaro M: Cytogenetics of multiple
endocrine neoplasia syndrome. II. Chromosome abnormalities in an
insulinoma and a glucagonoma from two subjects with MEN1. Cancer
Genet Cytogenet. 63:17–21. 1992.PubMed/NCBI
|
|
13
|
Floridia G, Grilli G, Salvatore M,
Pescucci C, Moore PS, Scarpa A and Taruscio D: Chromosomal
alterations detected by comparative genomic hybridization in
nonfunctioning endocrine pancreatic tumors. Cancer Genet Cytogenet.
156:23–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Speel EJ, Scheidweiler AF, Zhao J, Matter
C, Saremaslani P, Roth J, Heitz PU and Komminoth P: Genetic
evidence for early divergence of small functioning and
nonfunctioning endocrine pancreatic tumors: gain of 9Q34 is an
early event in insulinomas. Cancer Res. 61:5186–5192.
2001.PubMed/NCBI
|
|
15
|
Terris B and Bernheim A: Comparative
genomic hybridization (CGH): application to the study of
neuroendocrine tumors. Ann Pathol. 19(Suppl 5): S9–S11. 1999.(In
French).
|
|
16
|
Zhao J, Moch H, Scheidweiler AF, Baer A,
Schaffer AA, Speel EJ, Roth J, Heitz PU and Komminoth P: Genomic
imbalances in the progression of endocrine pancreatic tumors. Genes
Chromosomes Cancer. 32:364–372. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boninsegna L, Panzuto F, Partelli S,
Capelli P, Delle FG, Bettini R, Pederzoli P, Scarpa A and Falconi
M: Malignant pancreatic neuroendocrine tumour: lymph node ratio and
Ki67 are predictors of recurrence after curative resections. Eur J
Cancer. 48:1608–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haugvik SP, Marangos IP, Røsok BI,
Pomianowska E, Gladhaug IP, Mathisen O and Edwin B: Long-term
outcome of laparoscopic surgery for pancreatic neuroendocrine
tumors. World J Surg. 37:582–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mandahl N: Methods in solid tumors. Human
Cytogenetics: A Practical Approach. Vol II: Malignancy and Acquired
Abnormalities. Rooney DE and Czepulkowski BH: IRL Press, Oxford
University Press; UK: pp. 155–187. 1992
|
|
20
|
Kallioniemi A, Kallioniemi OP, Sudar D,
Rutovitz D, Gray JW, Waldman F and Pinkel D: Comparative genomic
hybridization for molecular cytogenetic analysis of solid tumors.
Science. 258:818–821. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Micci F, Teixeira MR, Haugom L, Kristensen
G, Abeler VM and Heim S: Genomic aberrations in carcinomas of the
uterine corpus. Genes Chromosomes Cancer. 40:229–246. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Micci F, Haugom L, Ahlquist T, Andersen
HK, Abeler VM, Davidson B, Trope CG, Lothe RA and Heim S: Genomic
aberrations in borderline ovarian tumors. J Transl Med. 8:212010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Speel EJ, Richter J, Moch H, Egenter C,
Saremaslani P, Rutimann K, Zhao J, Barghorn A, Roth J, Heitz PU and
Komminoth P: Genetic differences in endocrine pancreatic tumor
subtypes detected by comparative genomic hybridization. Am J
Pathol. 155:1787–1794. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jonkers YM, Claessen SM, Perren A, Schmid
S, Komminoth P, Verhofstad AA, Hofland LJ, de Krijger RR, Slootweg
PJ, Ramaekers FC and Speel EJ: Chromosomal instability predicts
metastatic disease in patients with insulinomas. Endocr Relat
Cancer. 12:435–447. 2005. View Article : Google Scholar : PubMed/NCBI
|