Introduction
Gastric cancer (GC) is one of the most common
malignancies worldwide. In particular, scirrhous type GC, composed
mainly of a diffusely infiltrating type of poorly differentiated GC
cells, forms a Borrmann type 4 lesion and is characterized by
highly metastatic potential and rapid proliferation (1–3).
Histologically, scirrhous type GC shows diffuse infiltration into
the gastric wall with extreme stromal fibrosis (1–3).
Transforming growth factor-β (TGF-β), produced by cancer cells,
activates stromal fibroblasts to produce various growth factors and
stimulates collagen synthesis in scirrhous type GC (4–5).
Growth-promoting factors from peri-tumoral fibroblasts also
contribute to the progression of scirrhous type GC (3). Furthermore, increasing matrix rigidity
may cause proliferation, and interstitial pressure created by
fibrosis in the cancer stroma may interfere with drug delivery to
cancer cells (6–9). Reflecting such characteristics,
scirrhous type GC carries an extremely poor patient prognosis in
comparison with other types of GC. Therefore, enhanced knowledge of
the pathological and biological basis of scirrhous type GC may lead
to improved diagnosis and treatment.
MicroRNAs (miRNAs) are small non-coding RNAs of
19–25 nucleotides in length that play important regulatory roles in
post-transcriptional repression (10,11).
Through inhibition of target gene translation, miRNAs are involved
in a wide range of cellular processes. Aberrant miRNA expression is
found in a variety of cancers, suggesting novel roles as oncogenes
or tumor-suppressor genes depending on the function of the target
genes (12). Previously, we
performed miRNA microarray analysis and identified several miRNAs
that are significantly upregulated in scirrhous type GC (13). Among them, we reported that miR-143
regulates collagen type III expression to contribute to
interstitial fibrosis and the poor prognosis of scirrhous type GC
(13). It has been shown that
miR-143 and miR-145 have a number of common features (14–16).
It is well known that their expression is mediated by the same
promoter and is induced by TGF-β signaling (15), and they work cooperatively in the
regulation of vascular smooth muscle cell differentiation (16). miR-145 was picked up as the second
highest miRNA after miR-143 in our miRNA microarray analysis of
scirrhous type GC (13). However,
no reports, to our knowledge, have focused on the role of miR-145
in scirrhous type GC.
In the present study, we studied miR-145 expression
in scirrhous type GC and the associations between miR-145
expression and clinicopathological factors including prognosis were
investigated using quantitative RT-PCR (qRT-PCR) of formalin-fixed
paraffin-embedded (FFPE) samples. Furthermore, we studied the
function of miR-145 in stromal fibroblast in scirrhous type GC,
particularly in the expression of α-smooth muscle actin (α-SMA) by
stromal fibroblasts.
Materials and methods
Tissue samples
In total, 138 primary gastric tumors and 30
corresponding non-neoplastic mucosa specimens were collected from
patients diagnosed with GC. Patients were treated at Miyoshi
Central Hospital or Hiroshima University Hospital. For miRNA
microarray analysis, frozen GC tissue samples including 5 scirrhous
type and 15 non-scirrhous type GCs were used. For qRT-PCR analysis,
frozen GC tissue samples from 20 patients and archival FFPE tissue
from 98 GC patients and 30 corresponding non-neoplastic mucosa
samples were used. For immunohistochemical analysis and in
situ hybridization, archival FFPE tissues from scirrhous type
GC cases with high miR-145 expression, as measured by qRT-PCR, were
used. Since written informed consent was not obtained, for strict
privacy protection, identifying information for all samples was
removed before analysis. This procedure was in accordance with the
Ethical Guidelines for Human Genome/Gene Research of the Japanese
Government.
Cell cultures
Nine cell lines derived from human GC were used. The
TMK-1 cell line was established in our laboratory (17). The HSC-39, HSC-44PE and HSC-57 cell
lines were established by one of the authors (Yanagihara et
al) (18,19). Four GC cell lines of the MKN series
were kindly provided by Dr Toshimitsu Suzuki, and the KATO-III cell
line was kindly provided by Dr Morimasa Sekiguchi. Four human
normal gastric fibroblasts (NFs), NF-33, 34, 35 and 38, and four
cancer-associated fibroblasts (CaFs), CaF-33, 34, 35 and 38, were
established by one of the authors (M.Y.) and as previously
described (13). NF-33, 38 and
CaF-33, 38 were derived from scirrhous type GC patients, and NF-34,
35 and CaF-34, 35 were derived from patients with poorly
differentiated gastric adenocarcinoma. CaFs had been established
from the primary tumor site of GC tissue and NF cell lines from the
‘normal’ non-neoplastic counterpart (as matched pairs). These cell
lines were maintained as previously described (13,20,21).
qRT-PCR and western blot analyses
Quantification of levels of α-SMA and β-actin was
performed using real-time fluorescence detection as previously
described (22). α-SMA primer
sequences were: 5′-AGCCAAGCACTGTCAGGAATC-3′ and
5′-GAGCCCAGAGCCATTGTCAC-3′. β-actin primer sequences were:
5′-TCACCGAGCGCGGCT-3′ and 5′-TAATGTCACGCACGATTTCCC-3′. qRT-PCR was
performed with a SYBR-Green PCR Core Reagents kit (Applied
Biosystems, Foster City, CA, USA). For analysis of miR-145 and U6B
expression levels from frozen samples, total RNA was extracted with
a miRVana™ Isolation kit (Ambion, Austin, TX, USA) according to the
manufacturer’s instructions. Total RNA was isolated from FFPE
tissue samples using the RecoverAll™ Total Nucleic Acid Isolation
kit (Ambion) as previously described (23). Expression levels of miR-145 were
normalized by RNU6B expression and calculated using the ΔΔCt
method.
For western blot analysis, cells were lysed as
previously described (24). The
lysates (30 μg) were solubilized in Laemmli sample buffer by
boiling and then subjected to 12% SDS-polyacrylamide gel
electrophoresis followed by electrotransfer onto a nitrocellulose
membrane. The membrane was incubated with primary anti-α-SMA
antibody (Dako, Carpinteria, CA, USA) and anti-β-actin antibody
(Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunocomplexes
were visualized with an ECL Prime Western Blot Detection System (GE
Healthcare, Little Chalfont, Buckinghamshire, UK ).
Cell transfection and TGF-β1
treatment
Transfection of cells was performed with
Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Briefly, the cells
were seeded at 50% confluence the day before transfection.
Pre-miR-145, miR-145 inhibitor, and negative control miRNA (Ambion)
were used for each transfection at a final concentration of 100 nM.
Fibroblasts were incubated in DMEM containing 10 ng/ml TGF-β1
(R&D Systems, Minneapolis, MN, USA). After 0–48 h of
incubation, collagen type III or miR-145 expression of fibroblasts
was examined by qRT-PCR as described above.
Statistical analysis
The Mann-Whitney U test was used to calculate the
significance of differences between two samples. Statistical
differences between miRNA expression levels in GC samples and
non-neoplastic mucosa samples were evaluated using the Wilcoxon
matched pair test. The correlation between expression levels of
miR-145 and clinicopathological parameters was analyzed with
Fisher’s exact test. A log-rank test and Kaplan-Meier plots were
constructed for the miR-145 high and low groups, based on one third
of the miR-145 expression level. Univariate and multivariate
analyses of factors influencing survival were carried out using the
Cox proportional hazards model. Parameters for multivariate
analysis were selected by the stepwise method. A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
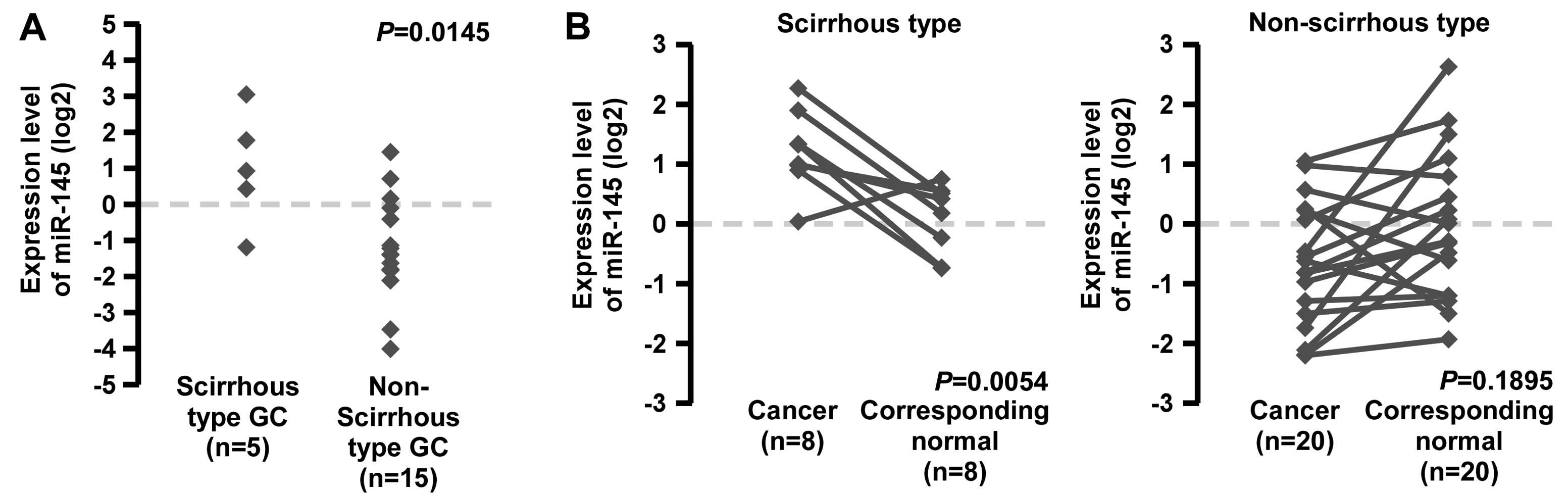
Expression levels of miR-145 are greater
in scirrhous type GC than in non-scirrhous type GC
Although miR-145 expression in GC has been discussed
by several authors (25–28), there is no unified view of the
altered expression of miR-145 in scirrhous type GC. To confirm
miR-145 expression in GC tissue, we performed qRT-PCR in 20 frozen
GC tissue samples. Scirrhous type GC cases showed significantly
higher miR-145 expression, compared with non-scirrhous type GC
cases (Fig. 1A).
It was reported that miR-145 expression is decreased
in several tumor tissues relative to normal tissue (29–31).
To assess miR-145 expression between tumor tissues and
corresponding non-neoplastic tissues, we performed qRT-PCR of
miR-145 using 28 FFPE GC tissue samples and corresponding normal
gastric mucosa (Fig. 1B). Although
miR-145 expression was significantly lower in non-scirrhous type GC
tissues than in corresponding normal gastric mucosa, the expression
levels in scirrhous type GC were sustained or higher than those in
the corresponding normal gastric mucosa (Fig. 1B). These data suggest that miR-145
expression is downregulated in GCs but is sustained or increased in
scirrhous type GC.
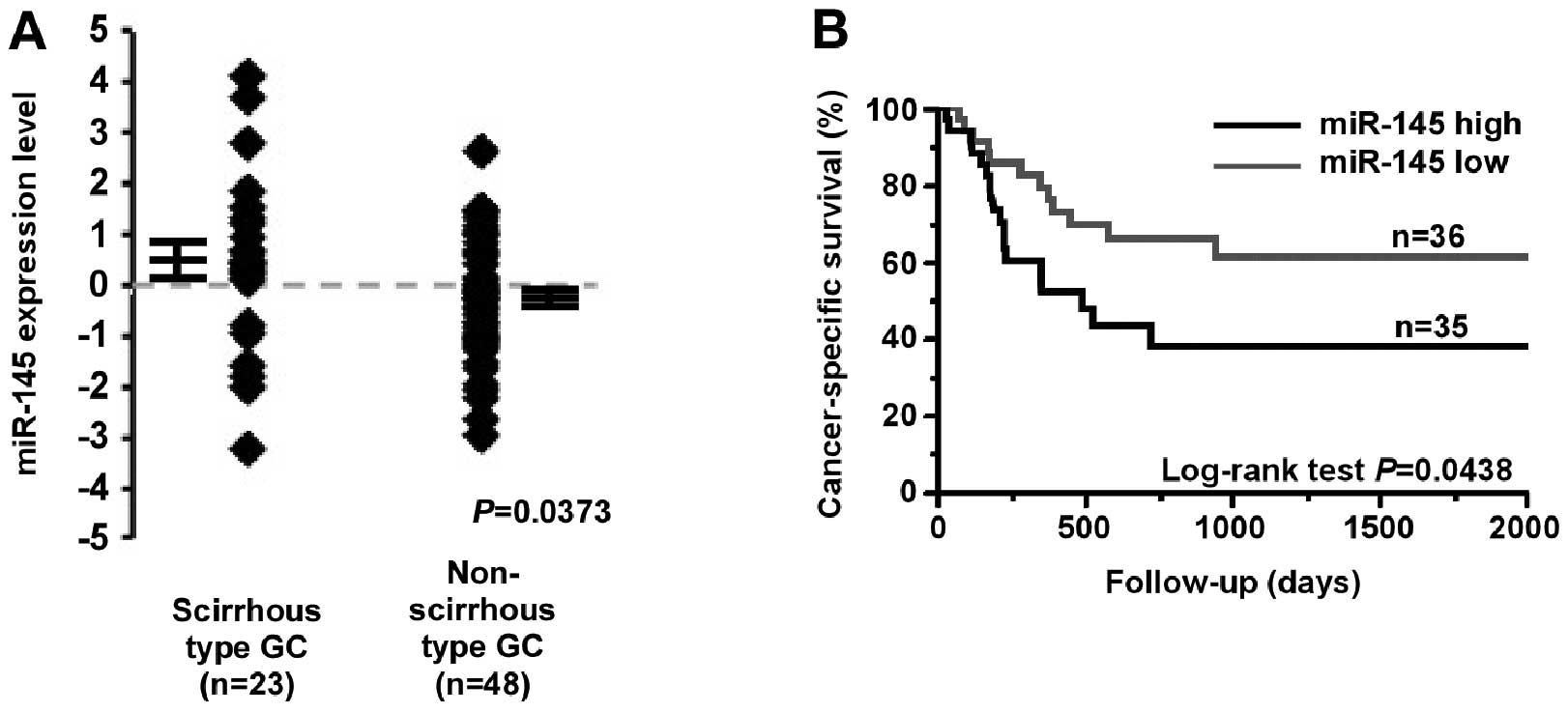
Association between miR-145 expression
and clinicopathological characteristics
To evaluate the correlation between miR-145
expression and clinicopathological factors, miR-145 expression was
examined in 71 FFPE GC tissue samples using qRT-PCR. In this sample
set, miR-145 expression was also significantly higher in scirrhous
type GC cases than in non-scirrhous type GC cases (P=0.0373;
Fig. 2A). miR-145 expression was
significantly associated with tumor stage (P=0.0156) and scirrhous
type GC cases (P=0.0054; Table
I).
 | Table IRelationship between miR-145
expression and clinicopathological characteristics in 71 patients
with GC. |
Table I
Relationship between miR-145
expression and clinicopathological characteristics in 71 patients
with GC.
| miR-145
expression | |
|---|
|
| |
|---|
| High (%) | Low | P-valuea |
|---|
| Age (years) |
| <60 | 16 (59.3) | 11 | 0.2265 |
| ≥60 | 19 (43.2) | 25 | |
| Gender |
| Male | 11 (50.0) | 11 | 1.0000 |
| Female | 24 (49.0) | 25 | |
| Stage |
| I/II | 9 (31.0) | 20 | 0.0156 |
| III/IV | 26 (61.9) | 16 | |
| Histological
classification |
| Well
differentiated | 12 (38.7) | 19 | 0.1528 |
| Poorly
differentiated | 23 (57.5) | 17 | |
| Non-scirrhous | 18 (37.5) | 30 | 0.0054 |
| Scirrhous | 17 (73.9) | 6 | |
We also examined the relationship between survival
and miR-145 expression in 71 GC patients. Kaplan-Meier analysis
showed that high miR-145 expression in GC patients correlated
significantly with poorer cancer-specific mortality (P=0.0438,
log-rank test; Fig. 2B). When
scirrhous type GC cases were compared with non-scirrhous type GC
cases, miR-145 expression had no prognostic impact (data not
shown). To determine the potential for miR-145 expression as a
prognostic predictor in patients with GC, univariate and
multivariate Cox proportional hazards analyses were used to further
evaluate the relationship between miR-145 expression and
cancer-specific mortality (Table
II). In the univariate analysis, high miR-145 expression and
tumor stage were associated with poor survival rate (P=0.0457), and
scirrhous type histology was also moderately associated with
survival (P=0.0606). In the multivariate analysis, including
miR-145 expression and tumor stage, miR-145 expression was not
found to be an independent prognostic indicator of cancer-specific
mortality (P=0.3595; Table
II).
 | Table IIUnivariate and multivariate analysis
of factors influencing survival in 71 patients with GC. |
Table II
Univariate and multivariate analysis
of factors influencing survival in 71 patients with GC.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
| <60 | 1 (Ref.) | | | |
| ≥60 | 1.14
(0.55–2.48) | 0.7311 | | |
| Gender |
| Female | 1 (Ref.) | | | |
| Male | 0.75
(0.36–1.65) | 0.4715 | | |
| Stage |
| I/II | 1 (Ref.) | | 1 (Ref.) | |
| III/IV | 9.12
(3.21–38.26) | <0.0001 | 8.39
(2.77–36.28) | <0.0001 |
| Histological
type |
| Well
differentiated | 1 (Ref.) | | | |
| Poorly
differentiated | 1.20
(0.58–2.55) | 0.6273 | | |
| Non-scirrhous | 1 (Ref.) | | | |
| Scirrhous | 2.01
(0.96–4.14) | 0.0606 | | |
| Expression of
miR-145 |
| Low | 1 (Ref.) | | 1 (Ref.) | |
| High | 2.10
(1.01–4.50) | 0.0457 | 1.41
(0.67–3.06 | 0.3595 |
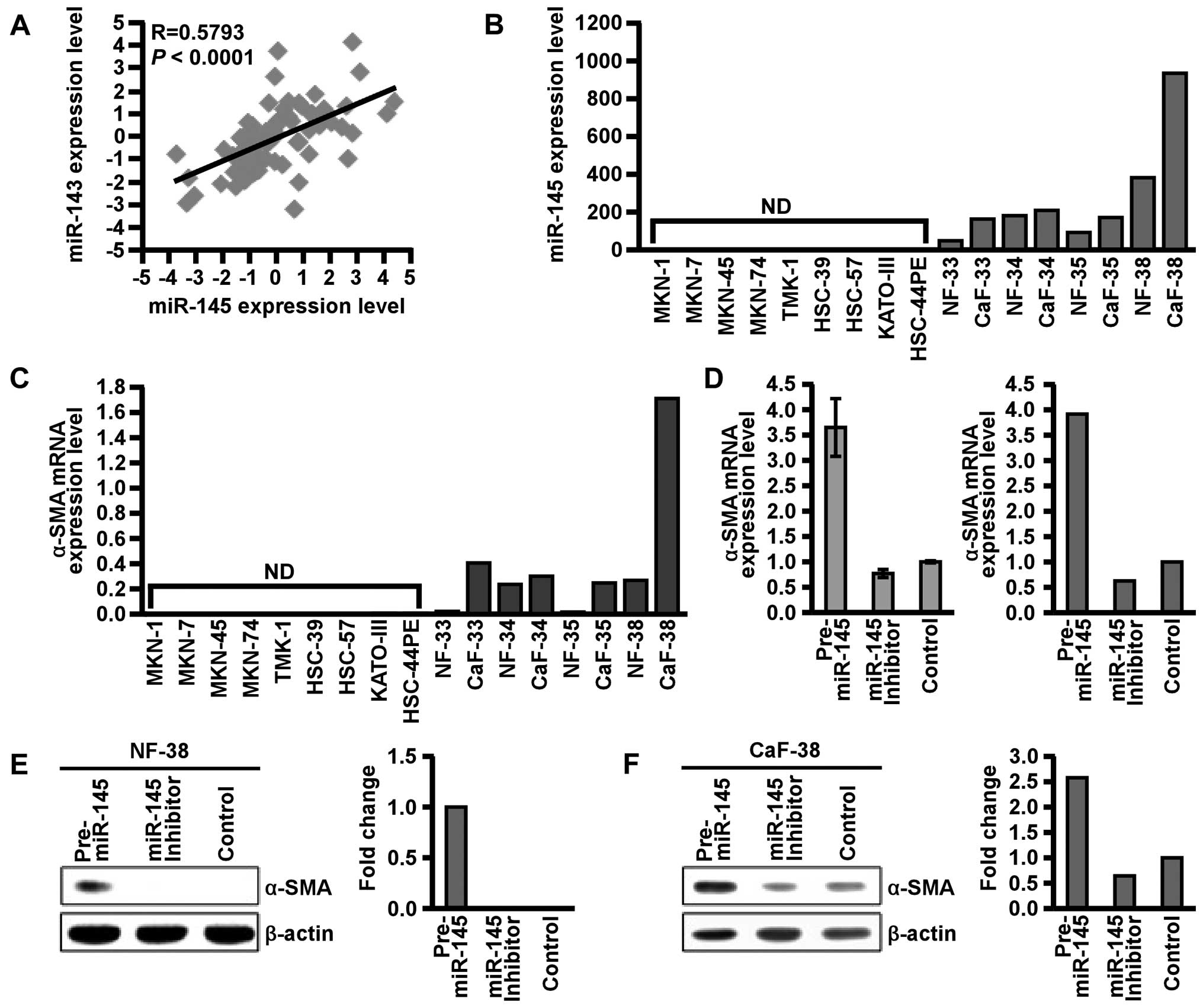
miR-145 regulates α-SMA expression in
stromal fibroblasts of scirrhous type GC
It was previously reported that miR-145 is
co-expressed as a cluster with miR-143 (15). We therefore investigated the
correlation between miR-145 and miR-143 expression in 71 FFPE GC
cases. As expected, expression levels of miR-143 and miR-145 were
significantly correlated (P<0.0001 R=0.5793; Fig. 3A). Since we previously reported that
miR-143 expression was localized in stromal fibroblasts in
scirrhous type GC (13), we next
performed qRT-PCR and confirmed the expression of miR-145 in GC
cell lines and stromal fibroblasts of scirrhous type GC. Expression
of miR-145 was detected in NFs and CaFs but not in GC cells, as
shown in Fig. 3B. Furthermore,
miR-145 expression was higher in CaFs than in NFs. These data
suggest that in scirrhous type GC, miR-145 expression is localized
in surrounding stromal fibroblasts but not in cancer cells.
Cancer-stromal interaction plays an important role
in the progression of scirrhous type GC (3). We focused on the function of miR-145
in stromal fibroblasts. α-SMA, a marker of myofibroblasts (32), was also expressed in CaFs and its
expression was regulated by miR-145 (33). To determine whether miR-145
regulates α-SMA expression in NFs and CaFs, we assessed the
relationship between α-SMA and miR-145. NF-38 and CaF-38 were
selected since they were established from the same patient with
scirrhous type GC. We treated NF-38 and CaF-38 with pre-miR-145 or
miR-145 inhibitor, and sequential changes in α-SMA expression were
examined by qRT-PCR and western blotting. Transfection of miR-145
precursor markedly increased α-SMA expression, whereas transfection
of miR-145 inhibitor sustained or suppressed α-SMA expression
(Fig. 3C–E). These data indicated
that miR-145 positively regulates α-SMA expression in stromal
fibroblasts of scirrhous type GC.
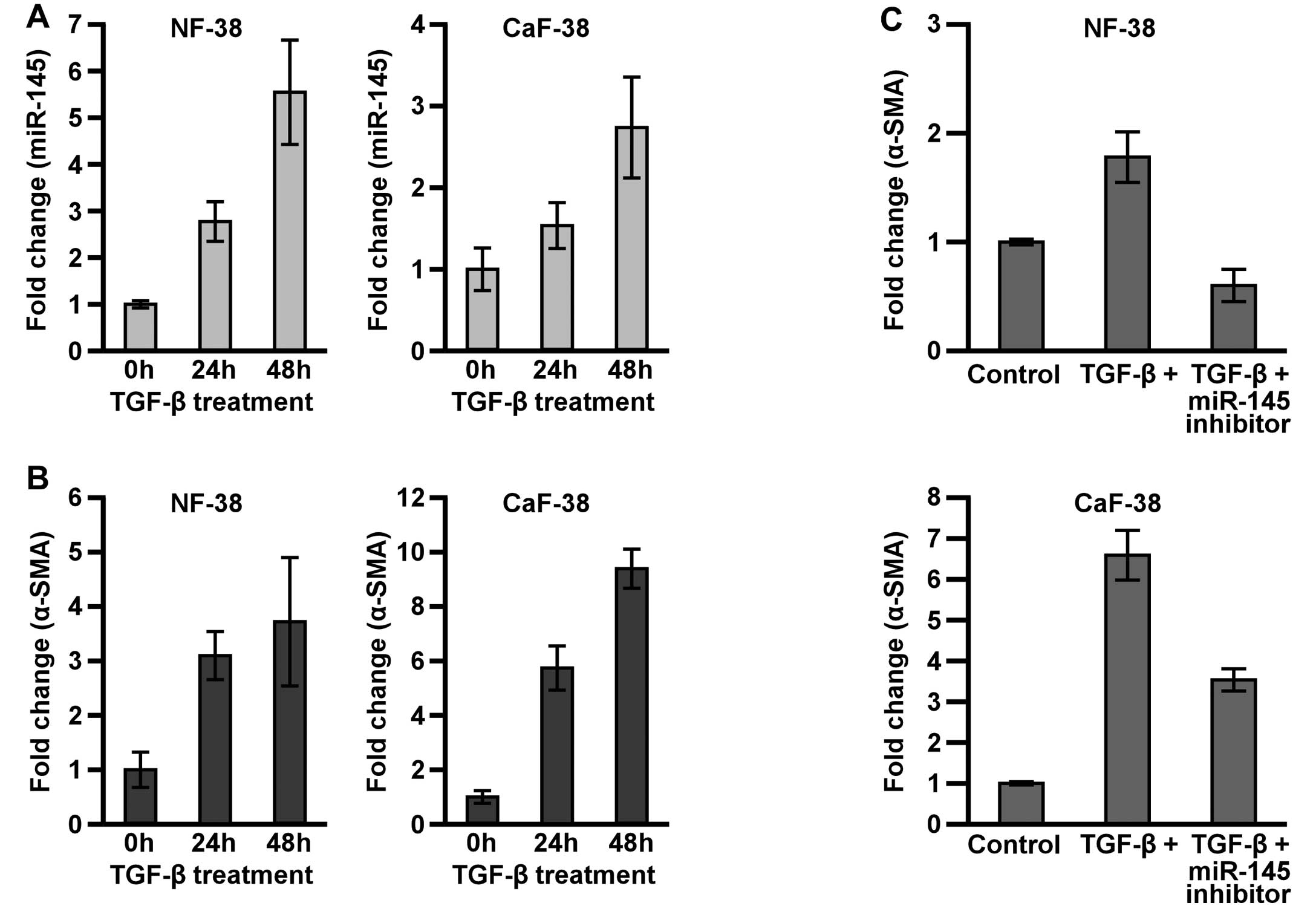
TGF-β regulates α-SMA expression via
miR-145
Scirrhous type GC secretes a larger amount of the
active form of TGF-β than non-scirrhous type GC (34), and TGF-β has important pathological
and biological roles in scirrhous type GC (3,5,35,36).
To investigate the effects of TGF-β1 on miR-145 and α-SMA
expression, NF-38 and CaF-38 were treated with TGF-β1, and their
expression levels of miR-145 and α-SMA were monitored by qRT-PCR.
Treatment with TGF-β1 resulted in strong induction of miR-145 and
α-SMA mRNA expression within 48 h in both NF-38 and CaF-38
(Fig. 4A and B). However, the
induction of α-SMA mRNA by TGF-β1 was significantly suppressed by
pre-treatment with miR-145 inhibitor (Fig. 4C). These data indicate that miR-145
is deeply involved in the regulation of α-SMA expression in NF and
CaF.
Discussion
In the present study, we reported that the
expression of miR-145 was increased in scirrhous type GC tissues
and was significantly associated with advanced cancer stage and
poor clinical survival from GC. However, several lines of evidence
suggest the miR-145 acts as a tumor suppressor gene, and its
upregulation is correlated with better outcome (26–31).
The apparent discrepancy between the data obtained from scirrhous
type GC and other malignancies is worth consideration. It should be
noted that these previous reports focused on cancer cells or normal
stromal cells and not on peri-tumoral fibroblasts (26–31).
We previously found that the expression of miR-143, co-regulated
with miR-145, was localized in fibroblasts of scirrhous type GC and
was associated with poor prognosis in the patients with GC
(13). In the present study, we
confirmed that miR-145 expression correlated strongly with miR-143
expression and was also detected in fibroblasts of scirrhous type
GC. Collectively, the overexpression of miR-145 in cancer stroma
may affect the progression of GC.
TGF-β signaling plays an important role in promoting
scirrhous type GC progression (3).
TGF-β also stimulates the proliferation of fibroblasts and
regulates α-SMA expression (3),
which is a marker for activated fibroblasts and the contractility
of fibroblasts (32,33). The contractile force of fibroblasts
is closely associated with TGF-β activation and the viability of
fibroblasts (33). Moreover, such
fibroblasts expressing α-SMA contributed to the proliferation and
motility of scirrhous type GC cells (20). Here, we showed the expression of
miR-145 and α-SMA in NFs and CaFs was induced by TGF-β treatment,
and miR-145 was involved in the induction of α-SMA expression by
TGF-β. These data suggest that miR-145 in peri-tumoral fibroblasts
may support the progression of scirrhous type GC through the
regulation of α-SMA expression.
It was reported that α-SMA expression was regulated
by miR-145 through inhibition of Krüppel-like factor 4 (KLF4) gene
translation (33). Thus, it could
be hypothesized that miR-145 also targets the translation of KLF4
and indirectly regulates α-SMA expression in fibroblasts of
scirrhous type GC. We performed qRT-PCR and western blot analysis
of KLF4 to determine whether miR-145 regulates KLF4 gene
expression. However, we failed to detect the inhibition of KLF4
expression by miR-145 in NF-38 and CaF-38 (data not shown). The
effects of KLF4 and other target genes of miR-145 on α-SMA
regulation were not investigated in the present study. Further
investigations should be performed to clarify the underlying
mechanism of α-SMA regulation by miR-145 in NFs and CaFs of
scirrhous type GC.
In conclusion, miR-145 expression in stromal
fibroblasts of scirrhous type GC may be involved in cancer
progression and lead to poor clinical outcome. Full elucidation of
the molecular mechanisms of miR-145 in stromal fibroblasts
surrounding cancer cells may improve our understanding of tumor
progression in scirrhous type GC.
Acknowledgements
The authors thank Mr. Shinichi Norimura for his
technical assistance. This study was carried out with the kind
cooperation of the Research Center for Molecular Medicine, Faculty
of Medicine, Hiroshima University. We thank the Analysis Center of
Life Science, Hiroshima University, for the use of their
facilities. This study was supported by Grants-in-Aid for Research
from the Ministry of Education, Culture, Science, Sports, and
Technology of Japan, and, in part, by a Grant-in-Aid for the Third
Comprehensive 10-Year Strategy for Cancer Control and for Cancer
Research from the Ministry of Health, Labour and Welfare of Japan,
and for The National Institute of Biomedical Innovation (Program
for Promotion of Fundamental Studies in Health Sciences). This
study was also supported in part by a Research Fellowship of the
Japan Society for the Promotion of Science and the National Cancer
Center Research and Development Fund (23-A-9).
References
|
1
|
Otsuji E, Kuriu Y, Okamoto K, et al:
Outcome of surgical treatment for patients with scirrhous carcinoma
of the stomach. Am J Surg. 188:327–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeguchi M, Miyake T, Matsunaga T, et al:
Recent results of therapy for scirrhous gastric cancer. Surg Today.
39:290–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yashiro M and Hirakawa K: Cancer-stromal
interactions in scirrhous gastric carcinoma. Cancer Microenviron.
3:127–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto M, Sumiyoshi H, Nakagami K,
Taniyama K and Tahara E: Distribution of collagen types I and III
and basal lamina in human gastric carcinoma: an immunohistochemical
and electron microscopic study. Virchows Arch A Pathol Anat
Histopathol. 403:313–322. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida K, Yokozaki H, Niimoto M, Ito H,
Ito M and Tahara E: Expression of TGF-β and procollagen type I and
type III in human gastric carcinomas. Int J Cancer. 44:394–398.
1989.
|
|
6
|
Jain RK: Barriers to drug delivery in
solid tumors. Sci Am. 271:58–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heldin CH, Rubin K, Pietras K and Ostman
A: High interstitial fluid pressure - an obstacle in cancer
therapy. Nat Rev Cancer. 4:806–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakajima TE, Yanagihara K, Takigahira M,
et al: Antitumor effect of SN-38-releasing polymeric micelles,
NK012, on spontaneous peritoneal metastases from orthotopic gastric
cancer in mice compared with irinotecan. Cancer Res. 68:9318–9322.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Worthley DL, Giraud AS and Wang TC: The
extracellular matrix in digestive cancer. Cancer Microenviron.
3:177–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naito Y, Sakamoto N, Oue N, et al:
MicroRNA-143 regulates collagen type III expression in stromal
fibroblasts of scirrhous type gastric cancer. Cancer Sci.
105:228–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cordes KR, Sheehy NT, White MP, et al:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009.PubMed/NCBI
|
|
15
|
Iio A, Nakagawa Y, Hirata I, Naoe T and
Akao Y: Identification of non-coding RNAs embracing
microRNA-143/145 cluster. Mol Cancer. 9:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long X and Miano JM: Transforming growth
factor-β1 (TGF-β1) utilized distinct pathways for the
transcriptional activation of microRNA 143/145 in human coronary
artery smooth muscle cells. J Biol Chem. 286:30119–30129. 2011.
|
|
17
|
Ochiai A, Yasui W, Kameda T, Takanashi A,
Takekura N and Tahara E: The effect of phorbol esters on cell
growth and epidermal growth factor receptor modulation in a human
gastric carcinoma cell line TMK-1. Hiroshima J Med Sci. 38:191–195.
1989.PubMed/NCBI
|
|
18
|
Yanagihara K, Seyama T, Tsumuraya M,
Kamada N and Yokoro K: Establishment and characterization of human
signet ring cell gastric carcinoma cell lines with amplification of
the c-myc oncogene. Cancer Res. 51:381–386. 1991.PubMed/NCBI
|
|
19
|
Yanagihara K, Tanaka H, Takigahira M, et
al: Establishment of two cell lines from human gastric scirrhous
carcinoma that possess the potential to metastasize spontaneously
in nude mice. Cancer Sci. 95:575–582. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuyuhiro Y, Yashiro M, Noda S, et al:
Cancer-associated orthotopic myofibroblasts stimulates the motility
of gastric carcinoma cells. Cancer Sci. 103:797–805. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naito Y, Oue N, Hinoi T, et al: Reg IV is
a direct target of intestinal transcriptional factor CDX2 in
gastric cancer. PLoS One. 7:e475452012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kondo T, Oue N, Yoshida K, et al:
Expression of POT1 is associated with tumor stage and
telomere length in gastric carcinoma. Cancer Res. 64:523–529.
2004.
|
|
23
|
Shinmei S, Sakamoto N, Goto K, et al:
MicroRNA-155 is a predictive marker for survival in patients with
clear cell renal cell carcinoma. Int J Urol. 20:468–477. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yasui W, Ayhan A, Kitadai Y, et al:
Increased expression of p34cdc2 and its kinase activity in human
gastric and colonic carcinomas. Int J Cancer. 53:36–41. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda T, Volinia S, Okumura H, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: a microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Chen Q, Lai R, et al: Elevated
expression of mature miR-21 and miR-155 in cancerous gastric
tissues from Chinese patients with gastric cancer. J Biomed Res.
24:187–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao P, Xing AX, Zhou GY, et al: The
molecular mechanism of microRNA-145 to suppress invasion-metastasis
cascade in gastric cancer. Oncogene. 32:491–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng L, Pu J, Qi T, et al: miRNA-145
targets v-ets erythroblastosis virus E26 oncogene homolog 1 to
suppress the invasion, metastasis, and angiogenesis of gastric
cancer cells. Mol Cancer Res. 11:182–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ostenfeid MS, Bramsen JB, Lamy P, et al:
miR-145 induces caspase-dependent and -independent cell death in
urothelial cancer cell lines with targeting of an expression
signature present in Ta bladder tumors. Oncogene. 29:1073–1084.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamano R, Miyata H, Yamasaki M, et al:
Overexpression of miR-200c induces chemoresistance in esophageal
cancers mediated through activation of the akt signaling pathway.
Clin Cancer Res. 17:3029–3038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villadsen SB, Bramsen JB, Ostenfeld MS, et
al: The miR-143/−145 cluster regulates plasminogen activator
inhibitor-1 in bladder cancer. Br J Cancer. 106:366–374. 2012.
|
|
32
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
33
|
Yang S, Cui H, Xie N, et al: miR-145
regulates myofibroblast differentiation and lung fibrosis. FASEB J.
27:2382–2391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mahara K, Kato J, Terui T, et al:
Transforming growth factor beta 1 secreted from scirrhous gastric
cancer cells is associated with excess collagen deposition in the
tissue. Br J Cancer. 69:777–783. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawajiri H, Yashiro M, Shinto O, et al: A
novel transforming growth factor β receptor kinase inhibitor, A-77,
prevents the peritoneal dissemination of scirrhous gastric
carcinoma. Clin Cancer Res. 14:2850–2860. 2008.
|
|
36
|
Shinto O, Yashiro M, Kawajiri H, et al:
Inhibitory effect of a TGFβ receptor type-I inhibitor,
Ki26894, on invasiveness of scirrhous gastric cancer cells. Br J
Cancer. 102:844–851. 2010.
|