Introduction
Bladder cancer is a heterogeneous disease that can
manifest as superficial tumors (70% of patients) or muscle-invasive
tumors (30% of patients) with a high risk of invasive metastasis
(1). The disease is more common in
men, and risk peaks at the ages of 50–70 (2). While high-grade non-invasive tumors
can be treated intravesically, the standard of care for
muscle-invasive tumors is radical cystoprostatectomy or,
alternatively, transurethral tumor resection, radiation and
chemotherapy (1). Environmental
factors, such as cigarette smoking (1), and genetic factors have been linked to
bladder cancer, including oncogenes TP63 (3), epidermal growth factor receptors
(EGFR) (4), tumor suppressor genes
TP53 and RB1 (5), Ras p21 proteins
(6), and cell cycle regulatory
proteins, such as CABLES, Ki67 and cyclin D1 (1). Despite these advances, the morbidity
associated with invasive bladder cancer remains high (7). Thus, a better understanding of the
cell proliferation and differentiation mechanisms in bladder cancer
cells is required before improved therapies can be designed.
RNA interference (RNAi) is an endogenous mechanism
in which RNA molecules inhibit post-transcriptional gene expression
(8). RNAi has been extensively
employed by contemporary researchers to establish experimental
knockdown models of mammalian cells using engineered gene-specific
small interfering RNAs (siRNAs) (9). To better understand tumorigenesis,
RNAi techniques have been used to elucidate the function and full
cellular processes involved in complex signaling pathways, such as
the Notch signaling pathway (10).
The Notch signaling pathway is of particular interest in cancer
research as it is evolutionarily conserved in virtually all
multi-cellular organisms (11),
which generally exhibit 4 types of Notch transmembrane receptors:
Notch1, Notch2, Notch3 and Notch4 (12). As key regulators of intracellular
signaling, these Notch receptors are critical to cellular
differentiation, proliferation and apoptosis (13,14).
Notch signaling pathways have been implicated in
epithelial-mesenchymal transition (EMT) modulation, tumor
angiogenesis processes, and anoikis-resistance of tumor cells that
have been linked with metastatic tumor development in osteosarcoma,
prostate cancer, breast cancer and melanoma (15). Notch signaling was first implicated
in tumorigenesis when the recurrent t(7;9)(q34;q34.3) chromosomal
translocation in the human Notch1 gene was identified in a
subset of cells from human pre-T-cell acute lymphoblastic leukemia
(T-ALL) (16). Since then, Notch
signaling has been recognized as a potential target for therapeutic
intervention in cancer, although available data pertaining to Notch
signaling in bladder cancer is limited.
It has been reported that the Notch cascade directly
regulates the expression of intestinal epithelium Kruppel-like
transcription factors (KLFs) (17),
key regulators of cell proliferation, differentiation and
apoptosis. Members of the KLF family are characterized by
triplicate C-terminus Cys2 His2 zinc fingers separated by highly
conserved H/C links (18).
Downregulation of several of the 15 identified KLFs, particularly
KLF4, has already been linked to growth suppression and
apoptosis induction in bladder cancer (19). While short-chain fatty acids can
increase KLF4 expression, Notch signaling acts to inhibit KLF4
expression (20). Thus, Notch
inhibition indirectly induces KLF4 expression (20), potentially playing a role in
tumorigenesis and metastasis in bladder cancer.
To further characterize the role of Notch-1 in the
bladder cancer cells, RNAi was used to generate Notch-1 knockdown
models of human bladder cancer cell lines (T24 and BIU87) using
siRNA eukaryotic expression vector Notch-1 (psiRNA1). KLF4
expression in bladder cancer cells after transfection was also
detected in order to investigate the relationship between Notch-1
and KLF4 levels in bladder cancer cells. This study provides a
basis for understanding the role of Notch-1 in cell proliferation
and differentiation in bladder cancer.
Materials and methods
Cell cultures
Human bladder cancer cell lines T24 and BIU87 were
provided by our lab. All cells were maintained at 37°C in a
humidified atmosphere of 5% CO2 in RPMI-1640 medium
supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS;
both from Gibco, USA).
Transfection of notch-expressing
vectors
The siRNA eukaryotic expression vector of Notch-1
(psiRNA1) was constructed and identified as previously described
(21). Then, psiRNA1 was
transfected into T24 and BIU87 cells (Notch-1 knockdown cells)
using a Lipofectamine™ 2000 Transfection kit (Invitrogen,
Netherlands), according to the manufacturer’s instructions, using
the siRNA sequence: 5′-TGGCGGGAAGTGTGAAGCG-3′. A vector without
psiRNA1 was also transfected into T24 and BIU87 cells as control
cells. Following transfection, all cells were treated with 800
μg/ml G418 (Sigma, USA) for 3 weeks. Stably transfected cells
(resistant clones) were ring-cloned and cultured for further
analysis. Downregulation of KLF4 expression (KLF4 knockdown cells)
was achieved using siRNA against human KLF4 and a negative control
siRNA was established for these cells.
Cell proliferation assessment by MTT
assay
The proliferation of Notch-1 knockdown and KLF4
knockdown cells was assessed simultaneously by
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium (MTT) assay.
Briefly, cell suspensions (1×103 cells/ml) of Notch-1
knockdown, KLF4 knockdown, and their respective control cells were
transferred to 96-well plates in triplicate. Cells were then
incubated for 12, 24, 36, 48, 72 and 96 h. At 4 h before the end of
each incubation period, 20 μl of MTT solution (5 mg/ml) (Sigma,
USA) was added. Then, cells were centrifuged at 2,200 × g for 5
min, the supernatant was removed, 200 μl of dimethyl sulfoxide
(DMSO) was added, and the optical density (OD) was determined at
492 nm.
Detection of apoptosis by flow
cytometry
For flow cytometry, T24 and BIU87 cells were
resuspended to 1×105 cells/ml in phosphate-buffered
saline (PBS) solution supplemented with 10% FCS. Cells were then
transfected as previously described and incubated for 3 days at
37°C in 5% CO2. Cells were then washed, resuspended in
PBS, dyed with propidium iodide (PI) for 30 min at room
temperature, and analyzed by flow cytometry using a flow cytometer
(BD FACSAria™III, FACSCalibur; BD Biosciences, USA). Annexin
V-FITC/PI double staining assay was used to detect cell apoptosis.
Briefly, after similar transfection and incubation, cells were
collected, centrifuged and washed twice with PBS. Binding buffer
was then added to each tube, cells were resuspended, and then
incubated with 5 μl of Annexin V-FITC and 5 μl of PI for 15 min at
room temperature under dark conditions. Flow cytometry was
conducted within the following 1-h period.
Clonal forming assay
T24 and BIU87 cells were infected with Notch-1
siRNA, cultured for 24 h, and plated in 24-well plates (200
cells/well). Plates were incubated for 14 days in a humidified
incubator at 37°C, and resultant colonies were stained with 0.5 ml
of 0.0005% crystal violet solution for 1 h. Cells in 5
randomly-selected fields from each sample were counted by
microscopy using an Olympus Optical Microscope (CX21; Olympus
Japan) (×100), and data were reported as means ± standard deviation
(SD) of five measurements.
RNA preparation and semi-quantitative
RT-PCR
Total-RNA was extracted from ~5×106 to
1×107 cells using an RNeasy kit (Qiagen, Germany),
according to the manufacturer’s instructions. Resultant total-RNA
was stored at −80°C for use in the following experiments.
Semi-quantitative analysis of Notch-1 mRNA expression was completed
by multiplex reverse transcription-polymerase chain reaction
(RT-PCR), and β-actin was used as a standard. The primer sequences
used were: Notch-1 forward, 5′-CTGCCAGGACCCGGACAA-3′ and reverse,
5′-TACCCGGCAACGTCGTCAATAC-3′; β-actin forward,
5′-CCTGTACGCCAACACAGTGC-3′ and reverse, 5′-ATACTCCTGCTTGCTGATCC-3′.
Extracted total-RNA samples (1 μg) were subjected to reverse
transcriptase reactions to produce cDNA using
SuperScript® II reverse transcriptase (Invitrogen Life
Technologies), primed with oligo(dT). Semi-quantitative PCR was
then performed using synthesized cDNA templates. cDNA templates (1
μl) were amplified with Platinum Taq DNA polymerase (Invitrogen).
PCR products were separated by 1% agarose gel electrophoresis,
stained with ethidium bromide and visualized with an ultraviolet
(UV) transilluminator (WUV-L50; Kor).
Quantitative real-time RT-PCR
KLF4 mRNA expression was assessed using quantitative
real-time PCR (qRT-PCR) with the QuantiTect SYBR-Green RT-PCR
system (Qiagen, Germany), according to the manufacturer-recommended
relative quantitation method. After optimization of each primer
pair, samples were assayed in a 50 μl reaction mixture containing
10 μl of sample RNA and optimal concentrations of each primer.
Thermal profiles consisted of a 30-min RT step at 50°C and 15 min
of Taq polymerase activation at 95°C, followed by 40 cycles of PCR
at 95°C for 20 sec (denaturation), 55°C for 30 sec (annealing) and
72°C for 30 sec (extension). Each experiment was conducted in
duplicate.
Western blotting
Cells were washed with PBS and lysed with lysis
buffer for 40 min on ice. Extracts were then centrifuged at 15,000
× g for 15 min. Equal amounts of protein from the supernatant were
electrophoresed on 8% SDS-polyacrylamide gel and sequentially
electrophoretically transferred to a polyvinylidene difluoride
(PVDF) membrane (Millipore, USA). After blocking with 5% non-fat
milk in PBS containing 0.1% Tween-20 for 2 h at room temperature,
membranes were incubated with monoclonal rabbit anti-Notch-1
antibody (1:5,000), anti-KLF4 antibody (1:1,000) and anti-β-actin
(1:2,000; all by Abcam, USA) at 4°C overnight and washed three
times for 5 min with PBS. Membranes were then incubated with
horseradish peroxidase (HRP)-conjugated secondary goat anti-rabbit
antibody (1:1,000) (Abcam) for 1 h at room temperature and washed
three times for 5 min with PBS. Results were visualized by
chemiluminescence using an enhanced chemiluminescence (ECL)
immunoblotting kit (Cell Signaling Technology, USA) with a digital
luminescent image analyzer LAS-1000 (Fujifilm, Tokyo, Japan). Band
intensities were semi-quantified using densitometric analysis.
Statistical analysis
All data were analyzed with SPSS v. 18.0 (SPSS Inc.,
USA), and results are expressed as the means ± standard deviation
(SD). Two group comparisons were analyzed with a t-test, and
P-values were calculated. P<0.05 was considered to indicate
statistically significant differences.
Results
Transfection efficiency of siRNA
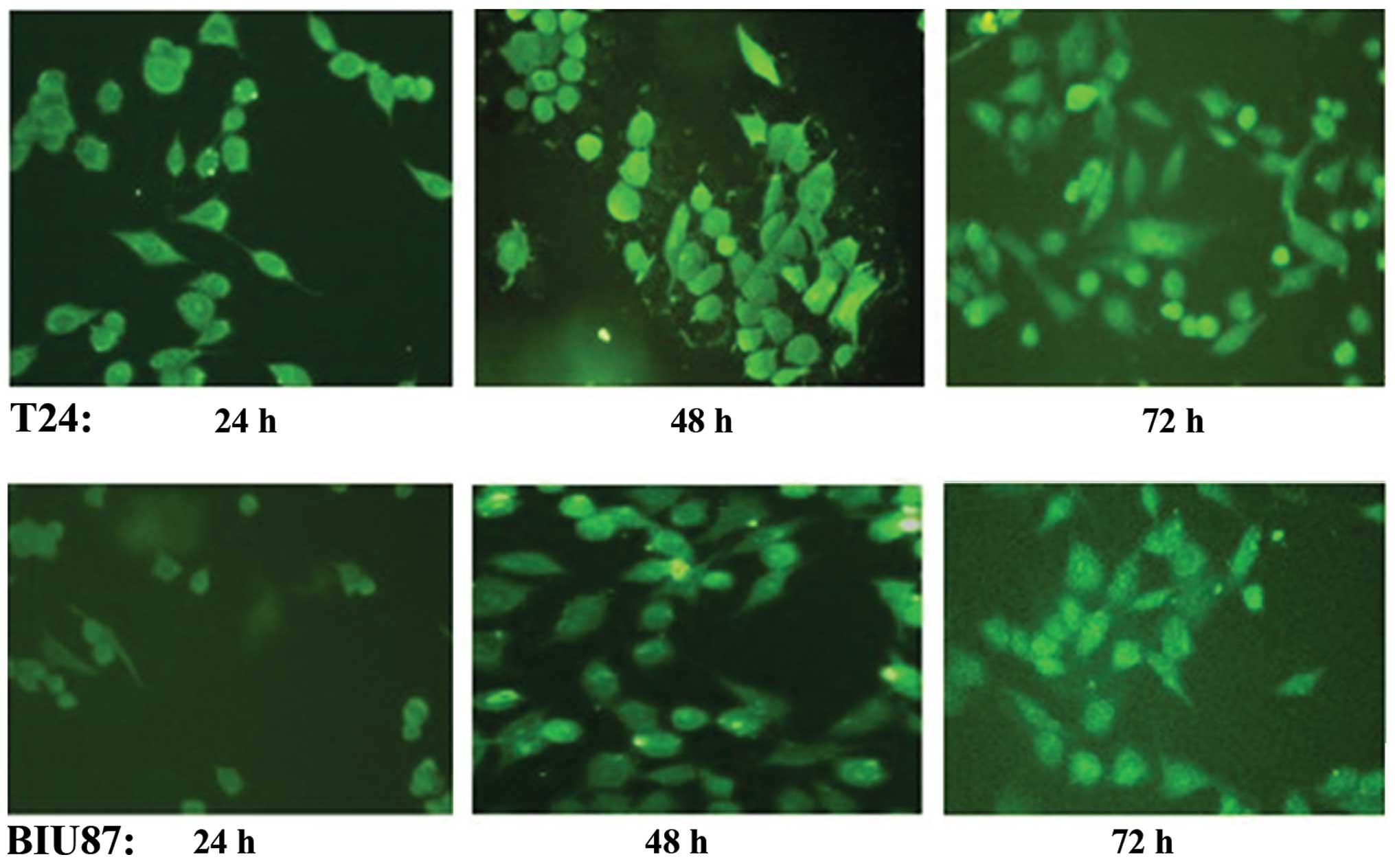
Fluorescence microscopy indicated that the Notch-1
expression plasmid psiRNA1 was successfully transfected into T24
and BIU87 cells. From 24 to 48 h after transfection, high levels of
fluorescence were observed in transfected compared to
non-transfected cells, which decreased by 72 h (Fig. 1). No significant differences in
transfection efficiency were observed between T24 and BIU87 cells
by fluorescence microscopy (P>0.05).
Notch-1 knockdown inhibits cell growth
and induces apoptosis
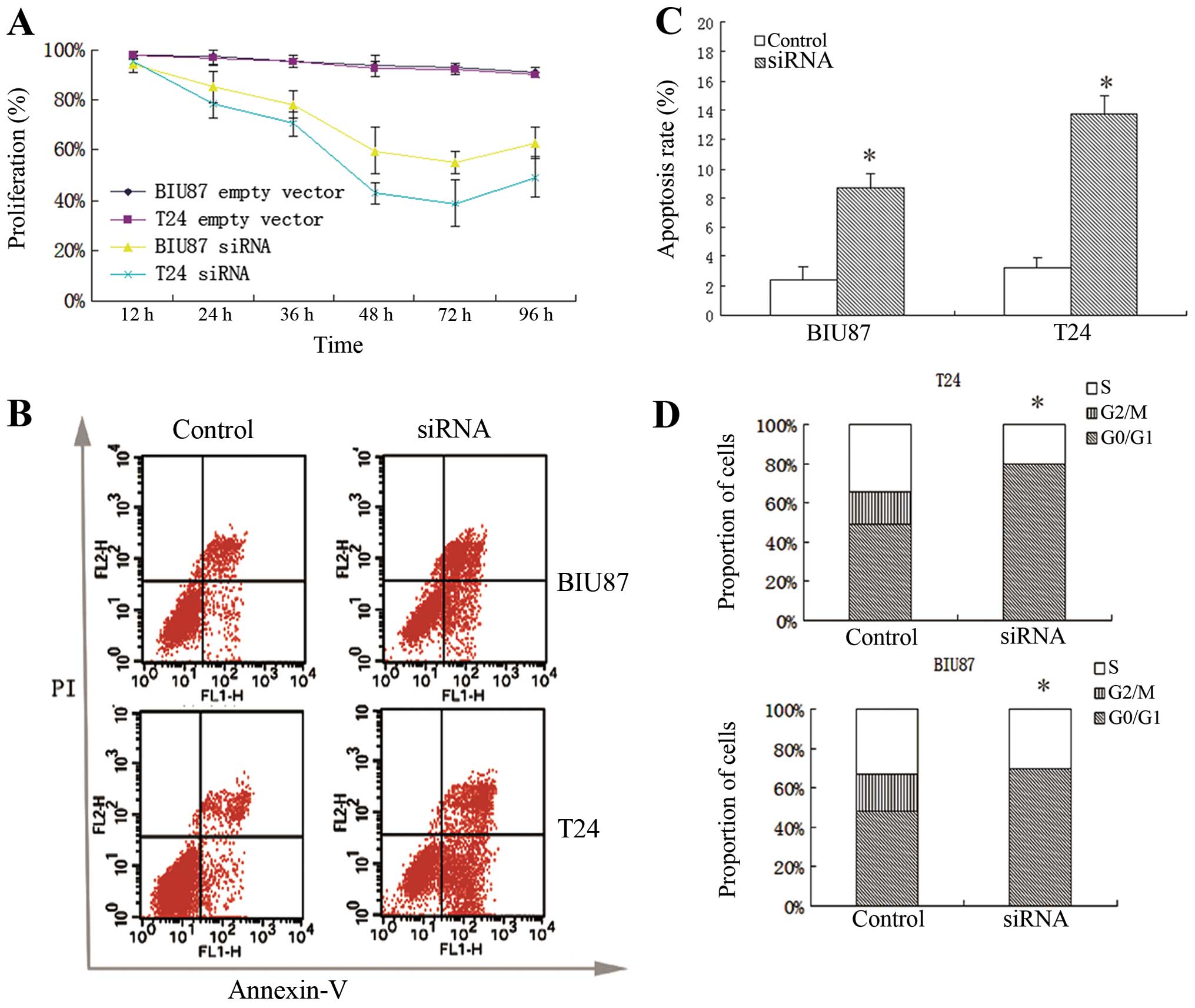
MTT assay revealed that Notch-1 knockdown cells
exhibited significantly lower proliferation rates at 24, 26, 48, 72
and 96 h compared to control cells (P<0.05) (Fig. 2A). No significant differences in
growth rate were observed between Notch-1 knockdown T24 cells and
Notch-1 knockdown BIU87 cells (P>0.05). Cell cycle status and
apoptosis were detected at 72 h in T24 and BIU87 indicating mean
apoptosis percentages of 13.75±1.23 and 8.72±1.01, respectively
(Fig. 2B and C). Notch-1 knockdown
significantly increased apoptosis in both cell lines (P<0.05).
Furthermore, the proportion of cells in the G0/G1 phase (G0/G1
arrest) significantly increased after Notch-1 knockdown compared to
those observed in the control groups (P<0.05; Fig. 2D).
Notch-1 knockdown inhibits cell
viability
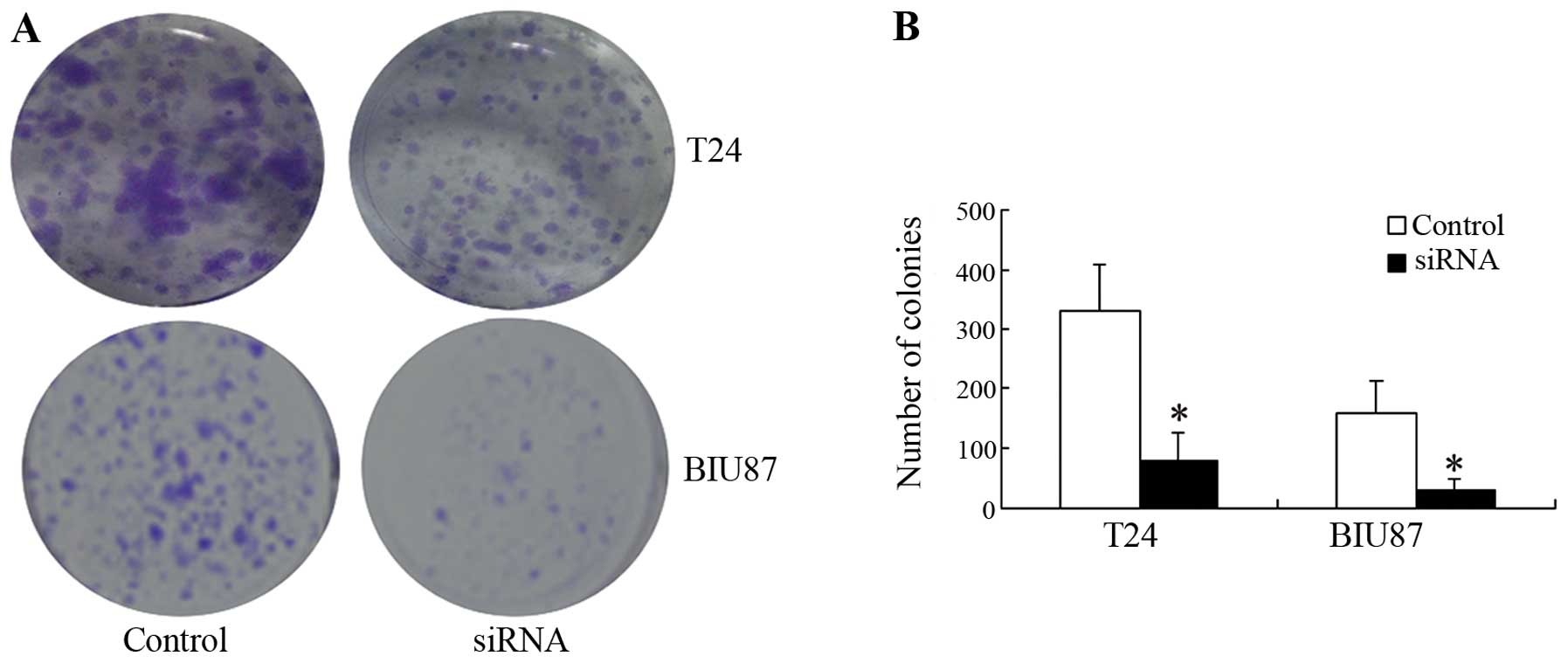
Knockdown of Notch-1 inhibited cell viability in
both cell lines, producing significantly fewer cancer cell colonies
than observed in the control groups (P<0.05; Fig. 3).
Notch-1 mRNA expression in Notch-1
knockdown cells
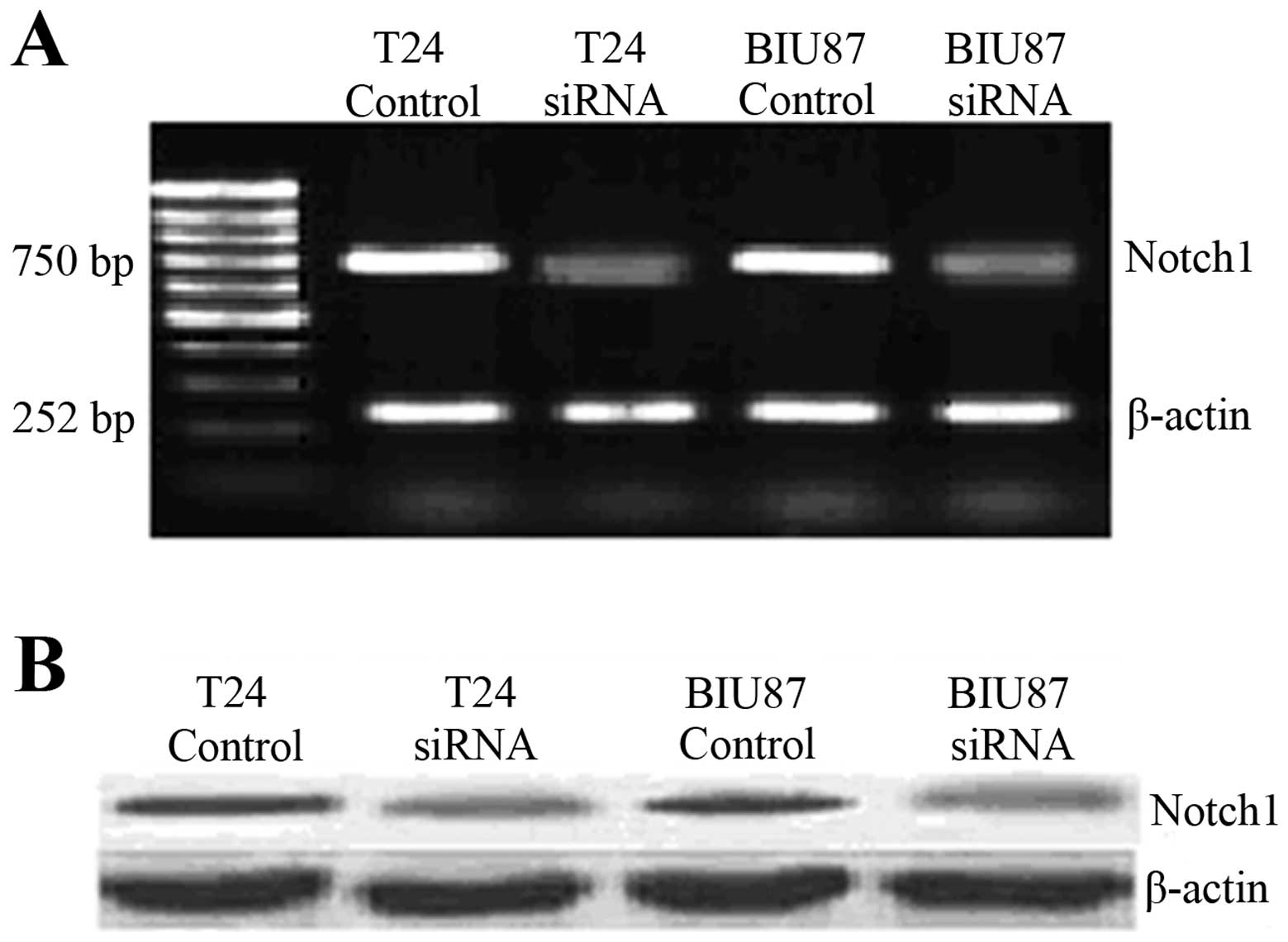
At 72 h, Notch-1 knockdown cells exhibited
significantly lower Notch-1 mRNA expression in both T24 and BIU87
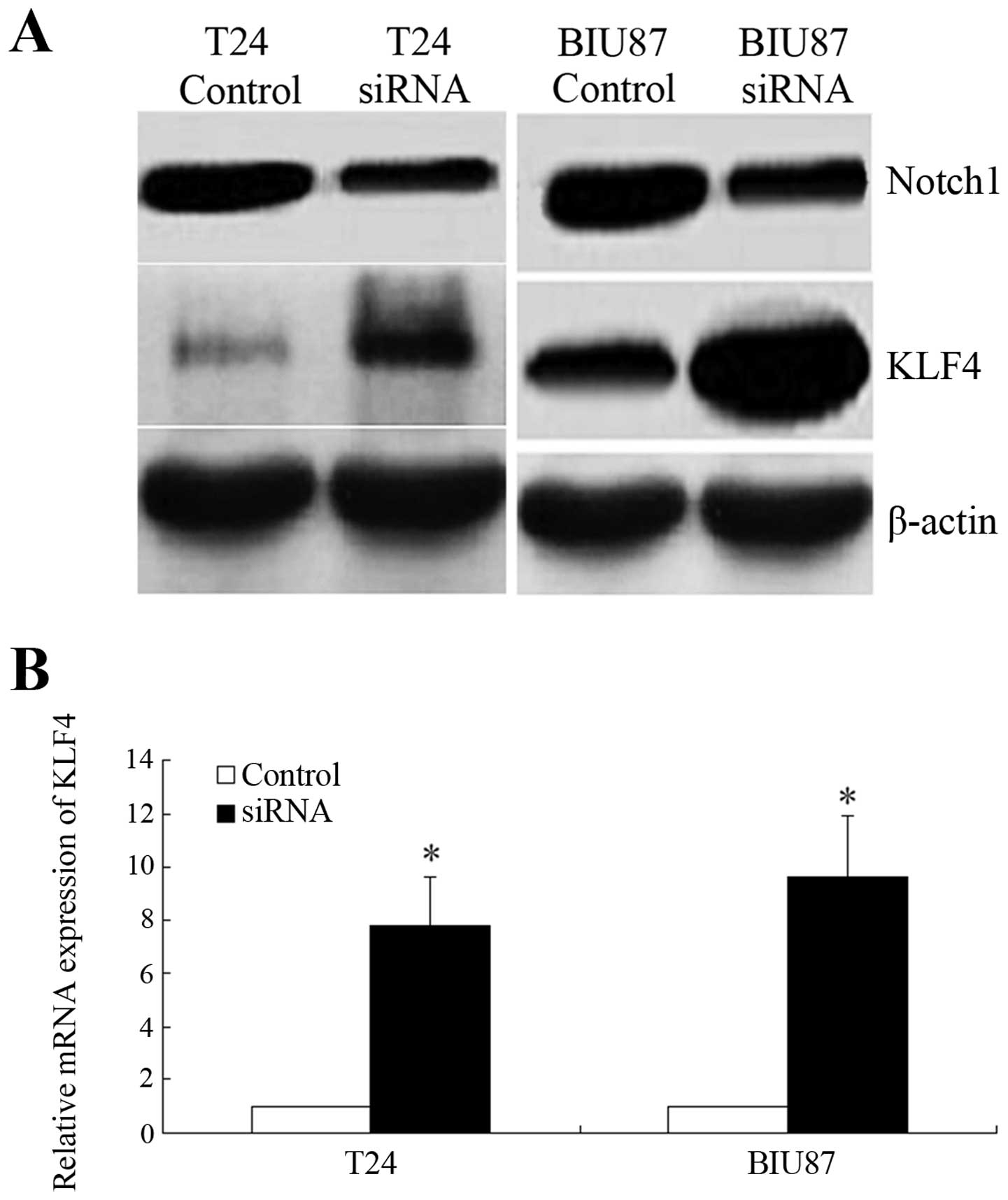
cells according to RT-PCR (P<0.001; Fig. 4A). Western blotting further
confirmed that the expression of Notch-1 was significantly
decreased by 72 h in both cell lines (Fig. 4B). KLF4 expression was significantly
upregulated by Notch-1 knockdown (P<0.05; Fig. 5).
Discussion
By transfecting bladder cancer cell line T24 and
BIU87 cultures with psiRNA1, the effects of Notch-1 knockdown and
KLF4 knockdown were observed both independently and together,
indicating that Notch-1 inhibits growth and apoptosis of bladder
cancer cells. Both expression of Notch-1 mRNA and protein in T24
and BIU87 cells were decreased significantly following
transfection, associated with Notch-1 downregulation. Furthermore,
the mechanism of action was shown to be dependent on KLF4,
suggesting that these two proteins are both important in bladder
cancer tumorigenesis and metastasis. Thus, Notch-1 downregulation
may provide a noteworthy target for inhibition of bladder cancer
cell proliferation, with potential clinical implications.
The Notch signaling pathways have been implicated in
the oncogenic processes involved in tumorigenesis, although
conflicting data exist regarding the oncogenic and tumor
suppressing role of Notch-1 (22).
It is, however, possible that Notch-1 plays both roles, depending
on specific cellular context and crosstalk with other
signal-transduction pathways (23,24).
This may potentially explain the overexpression of Notch pathway
components observed in human solid tumors, including those of
uterine cervix cancer, endometrial cancer, head and neck squamous
cell carcinomas, lung adenocarcinoma, renal cell carcinomas and
neuroblastomas (24–29). In bladder cancer, Notch family
expression patterns in papillary bladder transitional cell
carcinoma have been reported to be very different from those
observed in invasive bladder transitional cell carcinoma,
suggesting that Notch-1 activity is a context-specific marker of
prognosis and survival in these patients (30). Thus, aberrant Notch signaling in
bladder cancer acts through complex and interdependent mechanisms
that require further biochemical and clinical exploration.
Relationships between Notch-1 signaling and KLF4
have been previously reported. In colon cancer, inhibition of
Notch-1 signaling was reported to reduce proliferation and tumor
formation by increasing KLF4 expression (17,31).
Since the human Notch-1 promoter is bound by KLF4, suppression of
KLF4 in human breast cancer cells resulted in attenuation of
Notch-1 expression (32).
Furthermore, Ohnishi et al (19) reported that KLF4 expression was
downregulated in bladder cancer cells and that expression of KLF4
inhibited cell growth and induced apoptosis (19). In the present study, consistent
results were found, indicating that the expression of KLF4 mRNA and
protein were significantly upregulated in T24 and BIU87 cells after
Notch-1 knockdown. Additionally, proliferation of cells transfected
with Notch-1 siRNA was significantly lower than cells downregulated
of both Notch-1 and KLF4. Cumulatively, these findings suggest that
Notch-1 regulates cell proliferation and differentiation in bladder
cancer by directly inhibiting KLF4.
It is, however, important to consider the
limitations of this model and the relationships that it suggests.
While the upregulation and downregulation of KLF4 and Notch-1 may
be indicative of a direct relationship, it is also possible that
Notch-1 may signal through a distinct pathway in cells with
increased KLF4 activity, as previously suggested (32). Further study is required to
crosstalk between these pathways, which may both be important in
bladder cell tumorigenesis and metastasis. Additionally, future
research should examine the anticancer effects of Notch-1
downregulation in vivo.
Notch-1 is important in bladder cancer cell
proliferation and survival. Notch-1 may act as an oncogene,
regulating the proliferation and differentiation of bladder cancer
cells by inhibiting KLF4. Transfecting bladder cancer cells with
psiRNA1 exerted significant effects on apoptosis and cell
viability, and proliferation was inhibited by Notch-1 knockdown.
Furthermore, the relationship between KLF4 and Notch-1 merits
further consideration. While further study is required to elucidate
the full mechanism of these pathways, this study provides a
preliminary basis for the design of preclinical studies for
possible therapeutic agents targeting the Notch-1 signaling
pathway.
References
|
1
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
3
|
Urist MJ, Di Como CJ, Lu ML, et al: Loss
of p63 expression is associated with tumor progression in bladder
cancer. Am J Pathol. 161:1199–1206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Junttila TT, Laato M, Vahlberg T, et al:
Identification of patients with transitional cell carcinoma of the
bladder overexpressing ErbB2, ErbB3, or specific ErbB4 isoforms:
real-time reverse transcription-PCR analysis in estimation of ErbB
receptor status from cancer patients. Clin Cancer Res. 9:5346–5357.
2003.PubMed/NCBI
|
|
5
|
Habuchi T, Marberger M, Droller MJ, et al:
Prognostic markers for bladder cancer: International Consensus
Panel on bladder tumor markers. Urology. 66:64–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shinohara N and Koyanagi T: Ras signal
transduction in carcinogenesis and progression of bladder cancer:
molecular target for treatment? Urol Res. 30:273–281. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fletcher A, Choudhury A and Alam N:
Metastatic bladder cancer: a review of current management. ISRN
Urol. 2011:5452412011.PubMed/NCBI
|
|
8
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paddison PJ and Hannon GJ: RNA
interference: the new somatic cell genetics? Cancer Cell. 2:17–23.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mummery-Widmer JL, Yamazaki M, Stoeger T,
et al: Genome-wide analysis of Notch signalling in
Drosophila by transgenic RNAi. Nature. 458:987–992. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krebs LT, Xue Y, Norton CR, et al: Notch
signaling is essential for vascular morphogenesis in mice. Genes
Dev. 14:1343–1352. 2000.PubMed/NCBI
|
|
12
|
Brou C, Logeat F, Gupta N, et al: A novel
proteolytic cleavage involved in Notch signaling: the role of the
disintegrin-metalloprotease TACE. Mol Cell. 5:207–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miele L and Osborne B: Arbiter of
differentiation and death: Notch signaling meets apoptosis. J Cell
Physiol. 181:393–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu YY, Zheng MH, Zhang R, Liang YM and Han
H: Notch signaling pathway and cancer metastasis. Adv Exp Med Biol.
727:186–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allenspach EJ, Maillard I, Aster JC and
Pear WS: Notch signaling in cancer. Cancer Biol Ther. 1:466–476.
2002. View Article : Google Scholar
|
|
17
|
Ghaleb AM, Aggarwal G, Bialkowska AB,
Nandan MO and Yang VW: Notch inhibits expression of the
Krüppel-like factor 4 tumor suppressor in the intestinal
epithelium. Mol Cancer Res. 6:1920–1927. 2008.PubMed/NCBI
|
|
18
|
Bieker JJ: Krüppel-like factors: three
fingers in many pies. J Biol Chem. 276:34355–34358. 2001.
|
|
19
|
Ohnishi S, Ohnami S, Laub F, et al:
Downregulation and growth inhibitory effect of epithelial-type
Krüppel-like transcription factor KLF4, but not KLF5, in bladder
cancer. Biochem Biophys Res Commun. 308:251–256. 2003.
|
|
20
|
McConnell BB and Yang VW: Mammalian
Krüppel-like factors in health and diseases. Physiol Rev.
90:1337–1381. 2010.
|
|
21
|
Ai X, Ju HZ and Wu ZH: The construction
and identification of siRNA eukaryotic expression vector targeting
Notch1 gene. J Clin Urol. 5:391–394. 2008.
|
|
22
|
Nicolas M, Wolfer A, Raj K, et al: Notch1
functions as a tumor suppressor in mouse skin. Nat Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ehebauer M, Hayward P and Arias AM: Notch,
a universal arbiter of cell fate decisions. Science. 314:1414–1415.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Qin H, Chen B, Xin X, Li J and Han
H: Overexpressed active Notch1 induces cell growth arrest of HeLa
cervical carcinoma cells. Int J Gynecol Cancer. 17:1283–1292. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki T, Aoki D, Susumu N, Udagawa Y and
Nozawa S: Imbalanced expression of TAN-1 and human
Notch4 in endometrial cancers. Int J Oncol. 17:1131–1139.
2000.
|
|
27
|
Leethanakul C, Patel V, Gillespie J, et
al: Distinct pattern of expression of differentiation and
growth-related genes in squamous cell carcinomas of the head and
neck revealed by the use of laser capture microdissection and cDNA
arrays. Oncogene. 19:3220–3224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, De Marco MA, Graziani I, et al:
Oxygen concentration determines the biological effects of Notch-1
signaling in adenocarcinoma of the lung. Cancer Res. 67:7954–7959.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rae FK, Stephenson SA, Nicol DL and
Clements JA: Novel association of a diverse range of genes with
renal cell carcinoma as identified by differential display. Int J
Cancer. 88:726–732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Limpt V, Chan A, Caron H, et al: SAGE
analysis of neuroblastoma reveals a high expression of the human
homologue of the Drosophila Delta gene. Med Pediatr Oncol.
35:554–558. 2000.PubMed/NCBI
|
|
31
|
Shi TP, Xu H, Wei JF, et al: Association
of low expression of notch-1 and jagged-1 in human papillary
bladder cancer and shorter survival. J Urol. 180:361–366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng H, Pritchard DM, Yang X, et al: KLF4
gene expression is inhibited by the notch signaling pathway that
controls goblet cell differentiation in mouse gastrointestinal
tract. Am J Physiol Gastrointest Liver Physiol. 296:G490–G498.
2009. View Article : Google Scholar : PubMed/NCBI
|