Introduction
Hepatic carcinoma (HCC) is the fifth most common
malignancy worldwide and the second leading cause of cancer-related
death in Asia generally and in China in particular (1). Currently, surgical resection and liver
transplantation offer the best potential for treating HCC (2–4), but
most HCC patients are diagnosed in advanced stages. At present,
sorafenib, a multikinase inhibitor with antiangiogenic and
antiproliferative effects, currently sets the new standard for
advanced HCC (5,6). However, the survival benefit is only
2.8 months.
Antiangiogenic therapy has been thought to hold
significant potential for the treatment of cancer (7). However, clinical and preclinical
observations indicate that these therapies may have limited
efficacy. Although these agents typically produce inhibition of
primary tumor growth, lasting responses are rare, with only a
moderate increase in progression-free survival and little benefit
in overall survival (8). In
addition, recent reports describe that treatment of tumor-bearing
mice with antiangiogenic drugs leads to increased local tumor cell
invasion and enhanced distant metastasis after prolonged treatment
or after only short-term treatment (9,10).
Notably, sorafenib, the only approved molecular-targeted drug for
HCC, was found to promote invasion and metastasis of HCC by
increased intrahepatic metastasis, lung metastasis, and circulating
tumor cells in tumors with higher expression of HTATIP2 in
xenograft models (11). Therefore,
it is important to clarify the molecular mechanisms of invasion and
metastasis caused by sorafenib from all aspects in HCC.
Epithelial-mesenchymal transition (EMT) plays a key
role in tumor invasion and metastasis. During this process,
epithelial cells lose their epithelial signatures while acquiring
the characteristics of mesenchymal cells including morphology,
cellular structure and biological function (12). Transcription factor Snail has also
been shown to confer survival properties either concomitantly with
induction of EMT or independent of EMT (13–15).
Snail, Slug and Twist transcription factors can act as E-box
repressors and block E-cadherin transcription (16). In addition, Snail transcription
factor can mediate an increase in expression of mesenchymal markers
such as vimentin, fibronectin, matrix metalloproteinases (MMPs) and
RhoA (17–20). The overall effect of Snail is
increased migration and invasion (18,19).
Numerous signaling pathways are involved in the
regulation of EMT. PI3K/Akt signaling is an important
survival/proliferative pathway involving various growth factors,
cytokines and activation of receptors (21). In addition, the PI3K/Akt signaling
pathway plays a key role in the control of cell invasion and
metastasis and the activation of PI3K/AKT is a central feature of
EMT in the development of cancer (22–27).
On the one hand, the PI3K/AKT signaling pathway can increase the
expression of matrix metalloproteinases to induce EMT (28,29).
On the other hand, the PI3K/AKT signaling pathway can upregulate
the expression of transcription factor Snail to induce EMT
(30–32). Notably, activation of the PI3K/Akt
signaling pathway plays a key role in mediating resistance to
sorafenib. The combination of MK-2206, an Akt inhibitor, and
sorafenib overcomes such resistance (33). Yet, little is known concerning the
role of the PI3K/Akt signaling pathway on the invasion and
metastasis induced by sorafenib in HCC.
In the present study, we tested and verified that
sorafenib promotes invasion and metastasis of HCC by inducing EMT.
More importantly, we showed that activation of the
PI3K/Akt/Snail-dependent pathway may play a key role in this
process.
Materials and methods
Reagents and antibodies
Sorafenib was purchased from Bayer Corporation (West
Haven, CT, USA). Antibodies against E-cadherin, N-cadherin,
vimentin, Snail and GAPDH were purchased from Epitomics
(Burlingame, CA, USA); antibodies against p-PI3K and p-AKT were
purchased from Bioworld Technology (Minneapolis, MN, USA).
Cell culture
The human HCC cell lines SMMC7721 and HCCLM3
originated from the American Type Culture Collection (ATCC) and
were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS;
Biochrom, Berlin, Germany) in 5% CO2 at 37°C.
SMMC7721-GFP cells were SMMC7721 cells transfected with green
fluorescence protein (GFP) and were labeled as SMMC7721-GFP
cells.
Cell proliferation, migration and
invasion assays
Cell proliferation analysis was performed as
previously described by us (34).
Briefly, cells were plated at 5,000/well in 96-well microtiter
plates and incubated overnight at 37°C in a humidified incubator
containing 5% CO2. On the following day, various
concentrations of sorafenib were added to the wells, and cultures
were incubated for an additional 24, 48 and 72 h. Cell viability
was determined using a Cell Counting Kit-8 (Dojindo, Gaithersburg,
MD, USA) according to the manufacturer’s instructions. For cell
migration assay, cell migration was assessed using the Transwell
assay (Boyden chambers; Corning, Cambridge, MA, USA). Cells
(5×104) were seeded in serum-free medium in the upper
chamber and allowed to migrate toward the lower chamber that
contained 10% FBS. After 48 h, cells that had traveled through and
adhered to the underside of the membrane were counted at ×200
magnification. The cell invasion assay was carried out similarly,
except that 50 μl Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) diluted 1:6 with serum-free medium was added to each well
overnight before cells (2×105) were seeded onto the
membrane.
Animal models and treatments
Six-week-old BALBc nu/nu female mice were obtained
from the Shanghai Institute of Material Medica, Chinese Academy of
Science. All mice were bred in laminar flow cabinets under specific
pathogen-free conditions. We followed internationally recognized
guidelines on animal welfare. The study design was approved by the
Animal Ethics Committee, and the experiments were undertaken in
accordance with the Ethical Principles of Animal Experimentation of
Fudan University. SMMC7721-GFP cells [5×106/0.2 ml
phosphate-buffered saline (PBS)] were subcutaneously inoculated
into the right flanks of 6-week-old BALBc nu/nu female mice. After
4 weeks, non-necrotic tumor tissue was cut into 1 mm3
pieces and orthotopically implanted into the liver. Treatment was
started 2 weeks after orthotopic implantation of the tumors. Mice
were randomly separated into two groups with 6 mice in each group.
Mice in the experimental group received 30 mg/kg/day sorafenib,
whereas the control mice received vehicle alone. Animal weight was
measured twice a week for 4 weeks. At the end of the experiment,
mice were sacrificed, tumors were excised from each mouse, weighed
and snap-frozen for further analysis.
Detection of metastasis
Tumors were excised and their largest (a) and
smallest (b) diameters were measured to calculate tumor volume (V =
ab2/2). The livers were also excised, and green
fluorescent protein-positive metastatic foci were imaged by
Lumazone imaging system (Mag Biosystems, Tucson, AZ, USA).
Hematoxylin and eosin staining (H&E) was further applied to
detect liver metastasis.
Western blot analysis
Cells were washed with cold PBS and lysed in culture
dishes using PhosphoSafe™ Extraction Reagent (Merck, Darmstadt,
Germany) containing 1% protease inhibitor cocktail (EDTA-Free;
Thermo, San Jose, CA, USA). Protein concentrations were then
determined using Bio-Rad detergent compatible protein assays
(Bio-Rad, Hercules, CA, USA). A total of 30 μg protein from each of
the control and treated cell lysates was loaded on 8–12% gradient
NuPAGE gels (Novex, San Diego, CA, USA), electrophoresed under
reducing conditions, and transferred onto polyvinylidene difluoride
membranes (0.22 Å; Millipore). Western blot analysis was carried
out as previously described (34).
Immunohistochemistry
Procedures for the immunohistochemistry were
previously described (35).
Briefly, the tumor sections were stained with rabbit anti-p-Akt,
and rabbit antip-PI3K at 4°C overnight. Goat anti-rabbit
IgG/horseradish peroxidase was applied as the secondary antibody
according to the standard protocols provided by the manufacturer.
For negative controls, primary antibodies were replaced with PBS.
The procedures were performed by two independent investigators,
both of whom were blinded to the model/treatment type for the
series of experiments.
Real-time polymerase chain reaction
RT-PCR analysis was performed as previously
described by us (36). The
following primers for amplification of human genes were used:
E-cadherin forward, 5′-AGCCCCGCCTTATGATTCTCTG-3′ and reverse,
5′-TGCCCCATTCGTTCAAGTAGTCAT-3′; N-cadherin forward,
5′-CCACGCCGAGCCCCAGTAT-3′ and reverse,
5′-GGCCCCCAGTCGTTCAGGTAAT-3′; vimentin forward,
5′-CCTTGACATTGAGATTGCCACCTA-3′ and reverse,
5′-TCATCGTGATGCTGAGAAGTTTCG-3′; Snail forward,
5′-CAGCCTGGGTGCCCTCAAGAT-3′ and reverse,
5′-GCACACGCCTGGCACTGGTA-3′.
Statistical analysis
All analyses of the results were performed using the
GraphPad Prism software version 5.0 (GraphPad Software, San Diego,
CA, USA) and the SPSS 19.0 software package (SPSS, Inc., Chicago,
IL, USA). Statistical analyses were performed using the Student’s
t-test and analysis of variance (ANOVA) models. Differences were
considered statistically significant at P<0.05.
Results
Sorafenib promotes invasion and migration
in vivo
To elucidate the effects of sorafenib on HCC
invasion and migration, mice were orthopically implanted with
SMMC7721-GFP cells and treated with 30 mg/kg/day sorafenib. Tumor
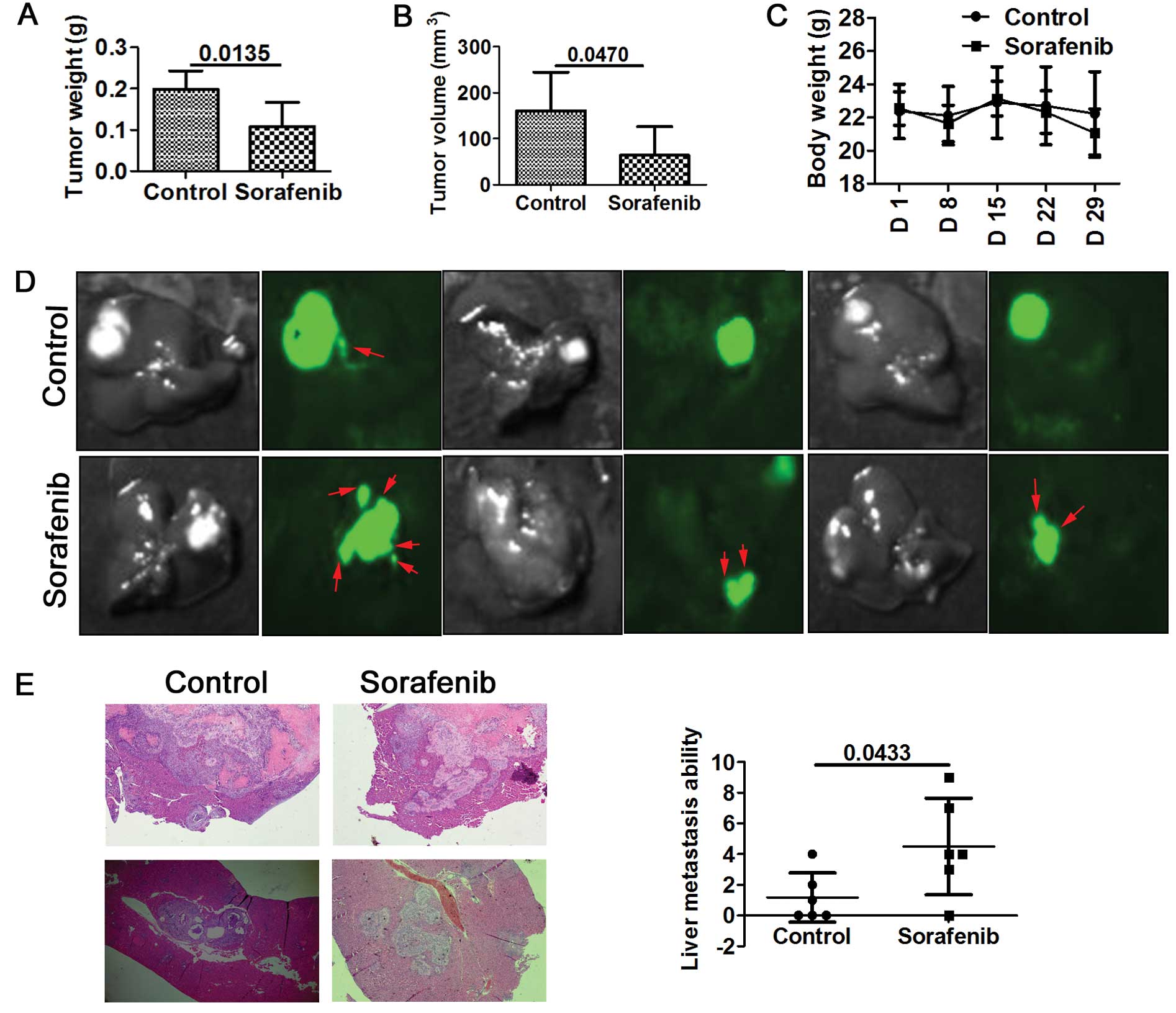
growth and metastasis were monitored. Our results showed that
sorafenib substantially reduced the primary tumor growth compared
with the control tumors. Tumor weight and volume were reduced in
the sorafenib-treated mice (Fig. 1A and
B). Additionally, sorafenib was well tolerated by the mice as
no apparent weight loss was noted (Fig.
1C). Unfortunately, sorafenib-treated mice developed more
intrahepatic metastatic lesions and exhibited irregular tumor
margins as detected by green fluorescence imaging (Fig. 1D). To further explore the effect of
sorafenib on the invasion and metastasis of HCC, liver metastatic
nodules were evaluated by H&E staining as observed under a
microscope. The number of metastatic nodules was then statistically
analyzed. A higher number of intrahepatic metastatic nodules was
detected in the sorafenib-treated mice (Fig. 1E).
Sorafenib promotes invasion and migration
of HCC cells
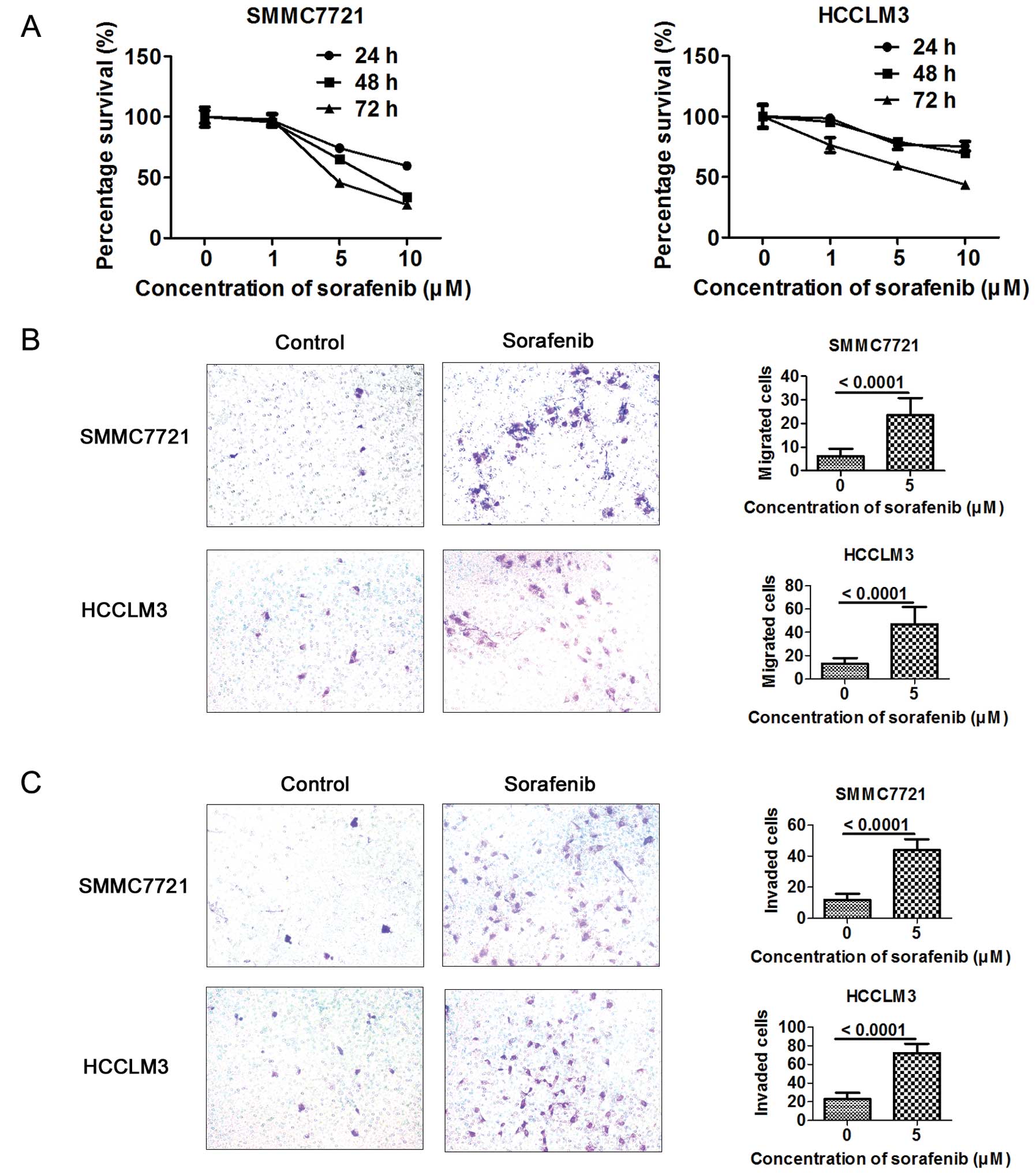
As sorafenib promoted invasion and migration in
vivo, we wanted to further validate whether sorafenib could
promote the invasion and migration in vitro. Cell
proliferation assay was applied to assess the proliferative effect
on hepatoma cells after sorafenib treatment. The antiproliferative
effect of sorafenib on HCC cells was dose- and time-dependent at a
concentration of 1–10 μM in the SMMC7721 and HCCLM3 cells (Fig. 2A). Sorafenib at a concentration of 5
μM, with little effect on cell proliferation, was applied to assess
the effect of sorafenib on the migration and invasion of HCC cells.
Cells (5×104 or 2×105) were seeded in the
upper chamber. A higher number of metastatic and invasive cells
were detected in the sorafenib-treated HCC cells as assessed by
Transwell assay (Fig. 2B and
C).
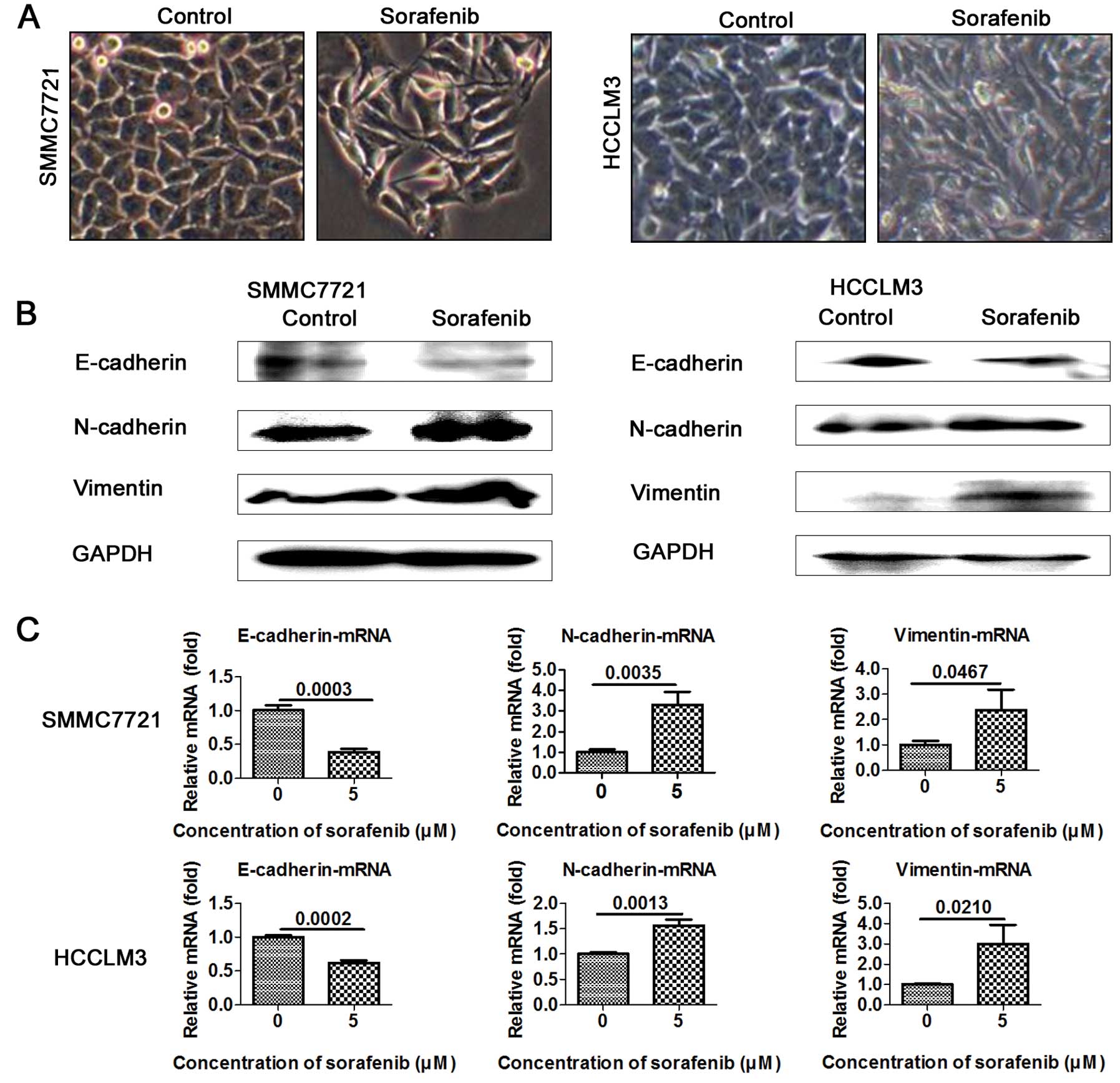
Sorafenib promotes EMT in HCC cells
EMT is well known to closely correlate with cancer
metastasis. To test and verify whether 5 μM sorafenib promotes the
EMT process, we evaluated the expression of EMT markers in the
sorafenib-treated and the control cells. As expected, SMMC7721 and
HCCLM3 cells treated with 5 μM sorafenib underwent significant
morphological changes and displayed the mesenchymal phenotype
(Fig. 3A). Importantly, epithelial
marker E-cadherin was downregulated and mesenchymal markers
N-cadherin and vimentin were upregulated in the sorafenib-treated
cells (Fig. 3B). RT-PCR assay
further confirmed the decreased levels of epithelial marker
E-cadherin and the increased levels of mesenchymal markers
N-cadherin and vimentin in the SMMC7721 and HCCLM3 cells (Fig. 3C).
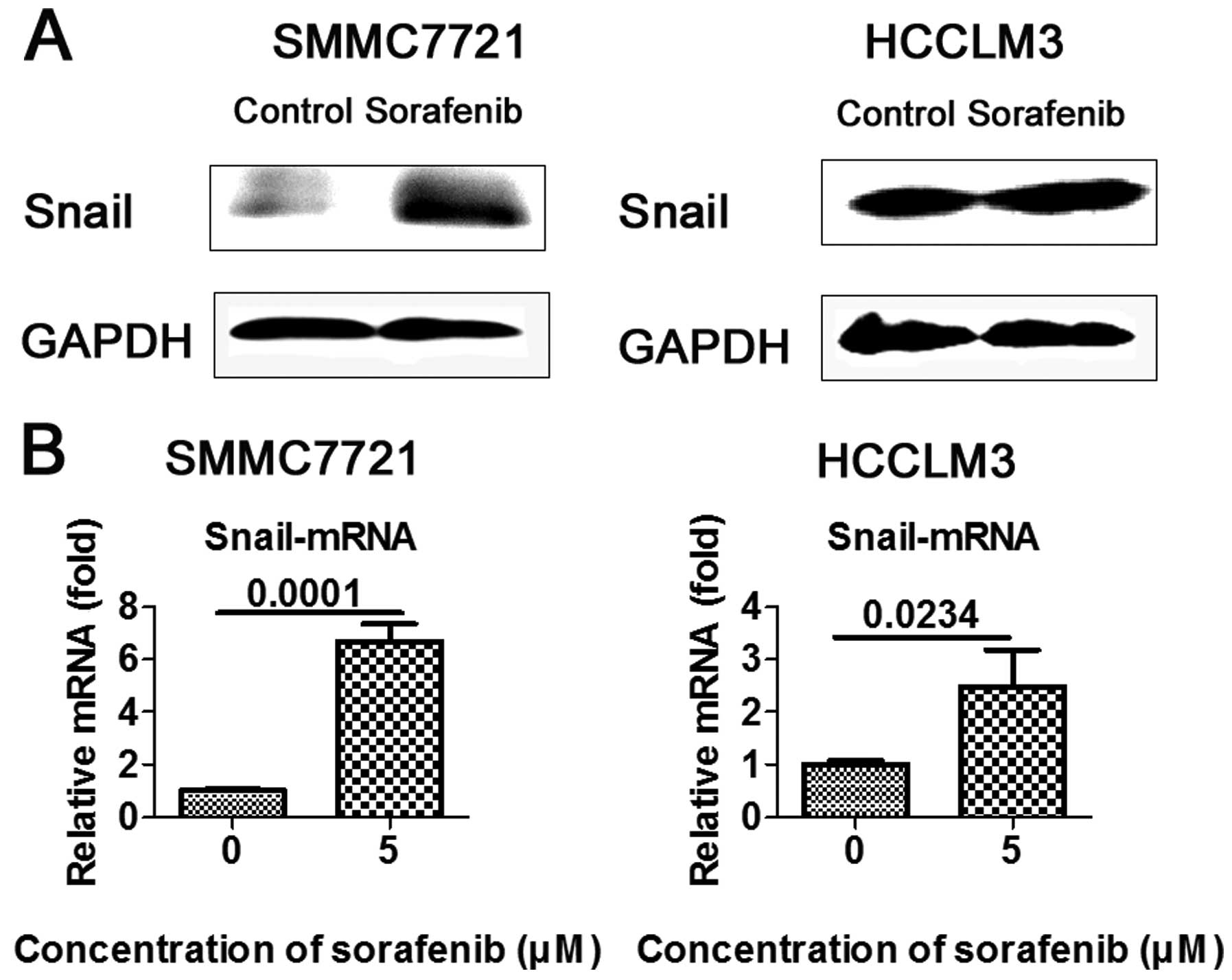
Sorafenib upregulates the expression of
Snail in vitro
As zinc-finger transcriptional repressor Snail plays
a key role in EMT-mediated tumor invasion and metastasis, we
ascertained whether Snail is involved in sorafenib-mediated EMT.
HCC cells were treated with 5 μM sorafenib and western blot
analysis and RT-PCR were carried out to measure Snail expression.
As anticipated, transcription factor Snail was upregulated in the
SMMC7721 and HCCLM3 cells, when compared to the controls (Fig. 4A and B).
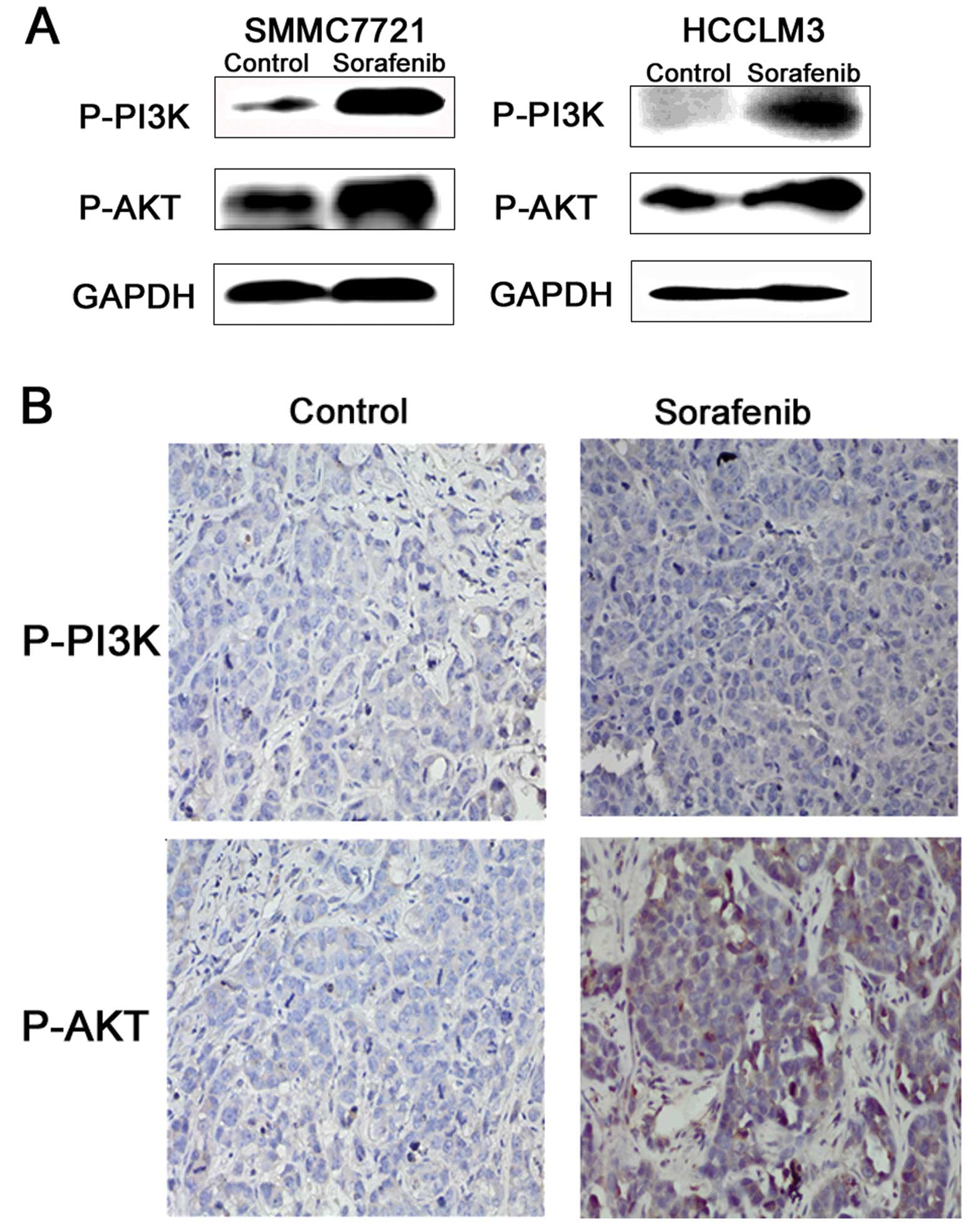
Sorafenib activates the PI3K/AKT
signaling pathway in vivo and vitro
As a highly conserved cellular program, EMT has been
documented to involve several important pathways. Accumulating
research suggests that PI3K/Akt activation plays a pivotal role in
tumor progression via induction of EMT. The
PI3K/Akt/GSK-3β/Snail-dependent signaling pathway is involved in
HCC. Thus, we detected the activity of PI3K/AKT in the
sorafenib-induced invasion and metastasis of HCC. The results
showed that the PI3K/AKT signaling pathway was activated in the HCC
cells treated with 5 μM sorafenib (Fig.
5A). In addition, the marginal tissues of the xenografts were
analyzed by immunohistochemical staining as described earlier.
PI3K/AKT phosphorylation levels were also upregulated (Fig. 5B).
Discussion
As a result of the SHARP and ORIENTAL trials,
sorafenib has become the new standard therapy for patients with
advanced hepatic carcinoma (HCC) (5,6).
However, the survival benefit is only a few months. Furthermore,
tumors may progress during sorafenib treatment (9–11). In
the present study, we demonstrated that sorafenib exerted an
antitumor effect and inhibited tumor growth in mouse models of
cancer. However, sorafenib also promoted invasion and metastasis of
HCC in this tumor model by inducing EMT. Similar observations were
reported by other authors. Importantly, we found that sorafenib
upregulated the expression of transcription factor Snail and
activated the PI3K/Akt signaling pathway.
In a previous study, increased local invasion and
distant metastasis during or after treatment with sorafenib were
observed (11). EMT plays a key
role in tumor invasion and metastasis. EMT is also reported to be
involved in the progression of HCC and is correlated with the
prognosis of patients (37). In the
present study, more metastatic lesions in the livers of nude mice
were detected in the sorafenib treatment group. In addition, HCC
cell lines, including SMMC7721 and HCCLM3, were treated with 5 μM
sorafenib, with little effect on cell proliferation as confirmed by
Cell Counting Kit-8. Surprisingly, morphology of the cells
underwent significant changes and presented a mesenchymal phenotype
after treatment for 72 h. Then EMT-related markers were analyzed.
As anticipated, mesenchymal markers were significantly upregulated
and epithelial markers were markedly decreased in the
sorafenib-treated cells. Transwell assay was also used to analyze
the ability of hepatoma cell invasion and migration. Invasion and
migration capacity of the HCC cell lines was enhanced. Therefore,
these data indicate that sorafenib may promote HCC invasion and
metastasis by the induction of EMT, consistent with other
reports.
The Snail transcription factor, a member of the
Snail superfamily, is a zinc finger protein that can mediate EMT
through downregulation of cell adhesion molecules such as
E-cadherin by binding several E-boxes located in the promotor
region (16). Snail has also been
shown to confer survival properties either concomitantly with
induction of EMT or independent of EMT. Snail plays an important
role in inducing EMT in HCC cells (38). In cancer patients, an EMT-phenotype
transcriptome profile, with increased Snail expression correlates
with invasive tumors. Phosphorylation and subsequent degradation of
Snail is controlled by GSK-3β, which is predominantly regulated by
PI3K/Akt (39). The
PI3K/Akt/GSK-3β/Snail-dependent signaling pathway can mediate
invasion and metastasis of HCC (40,41).
Increasing evidence also demonstrates that activation of the
PI3K/Akt pathway plays a central role in the EMT process and
correlates with an aggressive phenotype in several types of
malignancies (22–27). Several signaling pathways that
induce EMT and metastasis often converge at or activate PI3K/Akt,
which itself is frequently activated during tumor progression.
Hyperactivation of Akt is closely associated with elevated invasion
and metastasis, resulting in a poor prognosis and a greater
probability of relapse in many different cancer types (42–46).
The PI3K/Akt signaling pathway plays a key role in invasion and
metastasis of HCC. It was therefore of significance to investigate
whether the PI3K/Akt/Snail-dependent signaling pathway participates
in sorafenib-induced EMT. The PI3K/Akt signaling pathway was
analyzed in the human HCC SMMC7721 and HCCLM3 cells. Notably, we
found that 5 μM sorafenib activated the PI3K/Akt signaling pathway
and upregulated the expression of transcription factor Snail.
Immunohistochemical staining was then applied to the xenograft
marginal tissues. As anticipated, these results were further
confirmed in vivo.
In conclusion, the present study showed that
sorafenib upregulated the expression of transcription factor Snail
and activated the PI3K/AKT signaling pathway. Importantly, these
may be associated with sorafenib-induced invasion and metastasis of
HCC. Therefore, inhibition of the expression of transcription
factor Snail or combined with PI3K/AKT signaling pathway inhibitors
may enhance the effectiveness of sorafenib treatment of HCC.
Currently, relevant studies are being carried out. The present
study may provide the theoretical basis for the combined treatment
of sorafenib and PI3K/AKT signaling pathway inhibitors to treat
HCC.
Acknowledgements
We thank Te Liu (Shanghai Geriatric Institute of
Chinese Medicine, Longhua Hospital, Shanghai University of
Traditional Chinese Medicine, Shanghai) and Ning Zhang (Liver
Cancer Institute and Zhongshan Hospital, Fudan University,
Shanghai) for the experimental technical assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Gao JJ, Song PP, Tamura S, et al:
Standardization of perioperative management on
hepato-biliary-pancreatic surgery. Drug Discov Ther. 6:108–111.
2012.PubMed/NCBI
|
|
3
|
Belghiti J and Fuks D: Liver resection and
transplantation in hepatocellular carcinoma. Liver Cancer. 1:71–82.
2012. View Article : Google Scholar
|
|
4
|
Lee Cheah Y and Chow KHP: Liver
transplantation for hepatocellular carcinoma: an appraisal of
current controversies. Liver Cancer. 1:183–189. 2012.PubMed/NCBI
|
|
5
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pàez-Ribes M, Allen E, Hudock J, et al:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009.PubMed/NCBI
|
|
11
|
Zhang W, Sun HC, Wang WQ, et al: Sorafenib
down-regulates expression of HTATIP2 to promote invasiveness and
metastasis of orthotopic hepatocellular carcinoma tumors in mice.
Gastroenterology. 143:1641–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martínez-Alvarez C, Blanco MJ, Pérez R, et
al: Snail family members and cell survival in physiological and
pathological cleft palates. Dev Biol. 265:207–218. 2004.PubMed/NCBI
|
|
15
|
Emadi Baygi M, Soheili ZS, Schmitz I,
Sameie S and Schulz WA: Snail regulates cell survival and inhibits
cellular senescence in human metastatic prostate cancer cell lines.
Cell Biol Toxicol. 26:553–567. 2010.PubMed/NCBI
|
|
16
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang AL, Wang QS, Zhong YH, et al: Effect
of transcriptional factor snail on epithelial-mesenchymal
transition and tumor metastasis. Ai Zheng. 24:1301–1305. 2005.(In
Chinese).
|
|
19
|
Jordà M, Olmeda D, Vinyals A, et al:
Upregulation of MMP-9 in MDCK epithelial cell line in response to
expression of the Snail transcription factor. J Cell Sci.
118:3371–3385. 2005.PubMed/NCBI
|
|
20
|
Yokoyama K, Kamata N, Fujimoto R, et al:
Increased invasion and matrix metalloproteinase-2 expression by
Snail-induced mesenchymal transition in squamous cell carcinomas.
Int J Oncol. 22:891–898. 2003.PubMed/NCBI
|
|
21
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor β-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000.
|
|
23
|
Grille SJ, Bellacosa A, Upson J, et al:
The protein kinase Akt induces epithelial mesenchymal transition
and promotes enhanced motility and invasiveness of squamous cell
carcinoma lines. Cancer Res. 63:2172–2178. 2003.PubMed/NCBI
|
|
24
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.
|
|
26
|
Wang H, Quah SY, Dong JM, Manser E, Tang
JP and Zeng Q: PRL-3 down-regulates PTEN expression and signals
through PI3K to promote epithelial-mesenchymal transition. Cancer
Res. 67:2922–2926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song LB, Li J, Liao WT, et al: The
polycomb group protein Bmi-1 represses the tumor suppressor PTEN
and induces epithelial-mesenchymal transition in human
nasopharyngeal epithelial cells. J Clin Invest. 119:3626–3636.
2009. View
Article : Google Scholar
|
|
28
|
Yoo YA, Kang MH, Lee HJ, et al: Sonic
hedgehog pathway promotes metastasis via activation of AKT, EMT,
and MMP-9 in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuo JH, Zhu W, Li MY, et al: Activation of
EGFR promotes squamous carcinoma SCC10A cell migration and invasion
via inducing EMT-like phenotype change and MMP-9-mediated
degradation of E-cadherin. J Cell Biochem. 112:2508–2517. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar
|
|
31
|
Emadi Baygi M, Soheili ZS, Essmann F, et
al: Slug/SNAI2 regulates cell proliferation and invasiveness of
metastatic prostate cancer cell lines. Tumour Biol. 31:297–307.
2010.PubMed/NCBI
|
|
32
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: a comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003.
|
|
33
|
Chen KF, Chen HL, Tai WT, et al:
Activation of phosphatidylinositol 3-kinase/Akt signaling pathway
mediates acquired resistance to sorafenib in hepatocellular
carcinoma cells. J Pharmacol Exp Ther. 337:155–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Li HX, Xu LT, et al: Bufalin
enhances the anti-proliferative effect of sorafenib on human
hepatocellular carcinoma cells through downregulation of ERK. Mol
Biol Rep. 39:1683–1689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang P, Chen Z, Meng ZQ, et al: Dual role
of Ski in pancreatic cancer cells: tumor-promoting versus
metastasis-suppressive function. Carcinogenesis. 30:1497–1506.
2009.
|
|
36
|
Li H, Wang P, Gao Y, et al:
Na+/K+-ATPase α3 mediates sensitivity of
hepatocellular carcinoma cells to bufalin. Oncol Rep. 25:825–830.
2011.
|
|
37
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of twist and snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zucchini-Pascal N, Peyre L and Rahmani R:
Crosstalk between beta-catenin and snail in the induction of
epithelial to mesenchymal transition in hepatocarcinoma: role of
the ERK1/2 pathway. Int J Mol Sci. 14:20768–20792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou BP, Deng J, Xia W, et al: Dual
regulation of Snail by GSK-3β-mediated phosphorylation in control
of epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940.
2004.PubMed/NCBI
|
|
40
|
Wen W, Ding J, Sun W, et al: Cyclin
G1-mediated epithelial-mesenchymal transition via phosphoinositide
3-kinase/Akt signaling facilitates liver cancer progression.
Hepatology. 55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi GM, Ke AW, Zhou J, et al: CD151
modulates expression of matrix metalloproteinase 9 and promotes
neoangiogenesis and progression of hepatocellular carcinoma.
Hepatology. 52:183–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:13–42. 2006. View
Article : Google Scholar
|
|
43
|
Pérez-Tenorio G and Stål O; Southeast
Sweden Breast Cancer Group. Activation of AKT/PKB in breast cancer
predicts a worse outcome among endocrine treated patients. Br J
Cancer. 86:540–545. 2002.PubMed/NCBI
|
|
44
|
Scheid MP and Woodgett JR: PKB/AKT:
functional insights from genetic models. Nat Rev Mol Cell Biol.
2:760–768. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xue G, Restuccia DF, Lan Q, et al:
Akt/PKB-mediated phosphorylation of Twist1 promotes tumor
metastasis via mediating cross-talk between PI3K/Akt and TGF-β
signaling axes. Cancer Discov. 2:248–259. 2012.PubMed/NCBI
|