Introduction
Pituitary adenomas (PAs) are the most common tumors
of the pituitary, frequently asymptomatic and discovered at autopsy
in up to 15% of patients. PAs comprise ~7% of intracranial tumors
(1). Patients present with signs of
mass effect (visual loss), hormone overproduction, or
hypopituitarism. These benign tumors have a low growth rate but
some recur after surgery. Tumor size, invasion and the adequacy of
resection are important risk factors for recurrence or progression
(2).
Previously investigated proliferative markers
include Ki-67, the mitotic index and p53. Ki-67 is a nuclear
antigen expressed in the G1, G2 and synthesis phases of the cell
cycle but not in the quiescent G0 phase. Ki-67 is useful for
predicting recurrence (3). The
recent World Health Organization Tumor Classification of Tumors of
Endocrine Organs defines an atypical pituitary adenoma as a tumor
with a Ki-67 labeling index >3% and extensive p53 positivity
(4). No clear relationship exists
between immunohistochemical p53 overexpression and recurrence or
progression (5). One study reported
that downregulation of the Bax protein is associated with
progression of PA, whereas Bcl overexpression does not play a role
in progression (6).
Predictive markers of recurrence and progression are
yet to be firmly established. Angiogenesis is a fundamental process
during the expansion of solid tumors and is also essential for
progression of PA (7,8). VEGF-A is one of the most important
angiogenic factors stimulating endothelial cell proliferation,
motility and permeability (9).
VEGF-A binds to different tyrosine kinase receptors such as VEGFR-1
and VEGFR-2. These cells also express neuropilin-1 (NRP-1), a
VEGFR-2 co-receptor lacking the intracellular signaling tyrosine
kinase domain that enhances VEGF-A binding to VEGFR-2. VEGF
selectively upregulates its homologous receptor, NRP1. VEGF-induced
angiogenesis is derived not only from the direct effects on
endothelial cells but also from NRP-1 upregulation stimulated by
VEGF itself (10).
Here, we evaluated patients who had PAs with and
without progression in a unique series that included tumor
materials obtained by transsphenoidal surgery (TSS). We
investigated expression of Ki-67, p53, Bax, Bcl, VEGFR-1, VEGRR-2,
VEGF-A and NRP-1 in primary and recurrent specimens to identify a
predictive marker associated with tumor progression.
Materials and methods
Patients and tumor samples
We searched the database of any patients who
underwent TSS for treatment of PAs by the author (Lee S.W.) between
2002 and 2006. Cases that fulfilled the following criteria were
enrolled for the study: pathology reports with a diagnosis of
adenoma; medical records containing imaging reports and at least 5
years of postoperative follow-up; available pathology material.
Recurrent or residual adenomas treated with radiation were
excluded. Cushing’s disease and growth hormone-secreting tumors
were excluded.
Data from clinical notes, surgical reports and
radiological findings were obtained for analyses. Morbidity,
follow-up and outcome results were identified from entries in the
clinical notes. Tumor location, size and relationship to
neighboring anatomic structures were determined using preoperative
computed tomography (CT) and magnetic resonance (MR) imaging. The
invasion of cavernous sinus was determined by preoperative MR image
and intraoperative inspection. The extent of tumor resection was
determined intraoperatively and confirmed by follow-up MR images
taken postoperatively.
Patients with deteriorating vision due to chiasmal
compression by a regrowth of the tumor after an initial
postoperative imaging study underwent re-TSS. Re-TSS was chosen by
patients who wished to avoid the risks of radiation such as
hypopituitarism. Tumor progression was defined as cases treated
with re-TSS within 5 years after the first TSS and an
interoperation interval >1 year (11). This study was approved by the
institutional review board.
Immunohistochemical analysis
The protocol for Ki-67, p53, Bcl-2, Bax, VEGF-A,
VEGFR-1, VEGFR-2 and NRP-1 antibody staining has been described
previously (12,13). The immunostained slides were
examined under light microscopy by one of the authors (Yoo C.Y.)
who was blinded to the patients’ clinical histories. Ki-67 was
recorded as a labeling index by counting the number of
immunopositive nuclei among 100 tumor cells in at least five
representative high power fields across the slide. The results of
p53, Bax, Bcl-2, VEGFR-1 and VEGFR-2 staining were simplified as
positive (at least some stained cells) or negative. The
immunohistochemical results for VEGF-A and NRP-1 were scored
semi-quantitatively using a four-point scale: 0, no immunoreaction;
1+, faint or equivocal immunoreaction in <10% of cells; 2+,
unequivocal, strong immunoreaction in <30% of cells; 3+,
unequivocal, strong immunoreaction in <60% of cells and 4+,
unequivocal, strong immunoreaction in <100% of cells.
Statistical analysis
The distribution of patient characteristics was
compared between the no progression and progression groups (treated
with re-TSS), using the Wilcoxon rank-sum test and the Fisher’s
exact test for continuous and discrete variables, respectively.
Differences in immunohistochemical results between groups were
analyzed using the Chi-square test. A P-value <0.05 was
considered significant.
Cell cultures and shRNA lentivirus
production
The MMQ cell line was purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA). MMQ cells were
grown in F-12K, 15% horse serum, 2.5% FBS and 1% penicillin-
streptomycin at 37°C in humidified 5% CO2/95% air.
The human U6 promoter containing the shRNA
lentiviral vector was constructed as follows: a human U6 promoter
was amplified from the pLKO.1 vector (Sigma-Aldrich, St. Louis, MO,
USA) by polymerase chain reaction; this fragment was placed into
the CMV promoter site of the pCDH-MCS vector (#CD513-B; System
Biosciences, Palo Alto, California, CA, USA). The shRNA sequences
were as follows: NRP1 shRNA sense,
5′-GCCCGAATGTTCTCAGAACTATCAAGAGTA GTTCTGAGAACATTCGGGCTTTTTT-3′;
NRP1 shRNA antisense, 5′-AAAAAAGCCCGAATGTTCTCAGAACTA
CTCTTGATAGTTCTGAGAACATTCGGGC-3′; control shRNA (#TR30019, OriGene)
sense, 5′-GCACTACCAGAG CTAACTCAGATAGTACTTCAAGAGAGTACTATCTGAG
TTAGCTCTGGTAGTGCTTTTTT-3′; control l shRNA antisense,
5′-AAAAAAGCACTACCAGAGCTAACTCAG
ATAGTACTCTCTTGAAGTACTATCTGAGTTAGCTCTG GTAGTGC-3′.
The shRNA lentiviruses were produced by transfecting
Lenti-X 293T cells (Clontech, Palo Alto, CA, USA) with the shRNA
lentiviral expression plasmids and the packaging plasmids (Addgene,
Cambridge, MA, USA), which were pCMV-VSV-G, pMDLg/pRRE and
pRSV-Rev, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
reagent. Infectious shRNA lentiviruses were harvested at 48 h
post-transfection and then used to infect MMQ cells with 8 μg/ml
polybrene (Sigma-Aldrich). Oligonucleotide sequences used for
real-time polymerase chain reaction are summarized in Table I.
 | Table IOligonucleotide sequences used for
real-time polymerase chain reaction. |
Table I
Oligonucleotide sequences used for
real-time polymerase chain reaction.
| Gene name | Primer sequence
(5′→3′) |
|---|
| NRP1 | GGAGCTACTGGGCTGTGAAG,
CCTCCTGTGAGCTGGAAGTC |
| VEGF | TATCTTCAAGCCGTCCTGTG,
GATCCGCATGATCTGCATAG |
| p53 |
GACAGCCAAGTCTGTTATGTGCAC,
GACTTCTTGTAGATGGCCATGGC |
| p21 | CCTGGTGATGTCCGACCTG,
CCATGAGCGCATCGCAATC |
| Bad | AAGTCCGATCCCGGAATCC,
GCTCACTCGGCTC AAACTCT |
| Bax | CCCGAGCTGATCAGAACCAT,
TTGGATCCAGACAAGCAGCC |
| Bid |
GAGCCAGATTCTGAAAGTCAGGA,
GCCTTGTCGTTCTCCATGTC |
| Bcl2 |
ATGCCTTTGTGGAACTATATGGC,
GGTATGCACCCAGAGTGATGC |
| Bcl2l1 |
AGCGTAGACAAGGAGATGCAG,
CCAAGGCTCTAGGTGGTCATTC |
| 18s | GTAACCCGTTGAACCCCATT,
CCATCCAATCGGTAGTAGC |
Results
One hundred and forty-seven patients underwent TSS
for the treatment of PAs in our institution between 2002 and 2006.
We selected 28 consecutive patients who met the inclusion criteria.
Nineteen patients had no progression for >5-years of follow-up.
Nine patients had tumor progression within 5 years of their first
TSS and underwent re-TSS for treatment of adenoma progression. The
patient characteristics are summarized in Table II. Tumor size was larger in the
progression group than that in the no progression group (P=0.035).
Involvement of the cavernous sinus was more frequent in the
progression group than that in the no progression group (P=0.035).
No difference was found in regards to gender, age, suprasellar
extension, or extent of resection.
 | Table IIClinical characteristics of the
pituitary adenoma cases with and without progression within 5 years
of the initial surgery. |
Table II
Clinical characteristics of the
pituitary adenoma cases with and without progression within 5 years
of the initial surgery.
| No. of patients
(%) | |
|---|
|
| |
|---|
| Characteristics | No progression group
(n=19) | Progression group
(n=9) | P-value |
|---|
| Gender | | | 0.064 |
| Male | 2 (10.5) | 4 (44.4) | |
| Female | 17 (89.5) | 5 (55.6) | |
| Age (years) | | | 0.369 |
| Median (range) | 33 (20–68) | 35 (24–57) | |
| Tumor size | | | |
| <10 mm | 7 (36.8) | 0 (0.0) | 0.035 |
| 10–25 mm | 9 (47.4) | 4 (44.4) | |
| >25 mm | 3 (15.8) | 5 (55.6) | |
| Cavernous sinus
involvement | | | 0.035 |
| + | 4 (21.1) | 6 (66.7) | |
| − | 15 (78.9) | 3 (33.3) | |
| Suprasellar
extension | | | 0.228 |
| + | 7 (36.8) | 6 (66.7) | |
| − | 12 (63.2) | 3 (33.3) | |
| Extent of
resection | | | 0.409 |
| Total | 13 (68.4) | 4 (44.4) | |
| Subtotal | 6 (31.6) | 5 (55.6) | |
| Hormonal
status | | | >0.05 |
|
Non-functioning | 8 (42.1) | 4 (44.4) | |
| Prolactinoma | 11 (57.9) | 5 (55.6) | |
Table III shows
the distribution of the progression and control groups according to
molecular marker expression. A strong association was observed
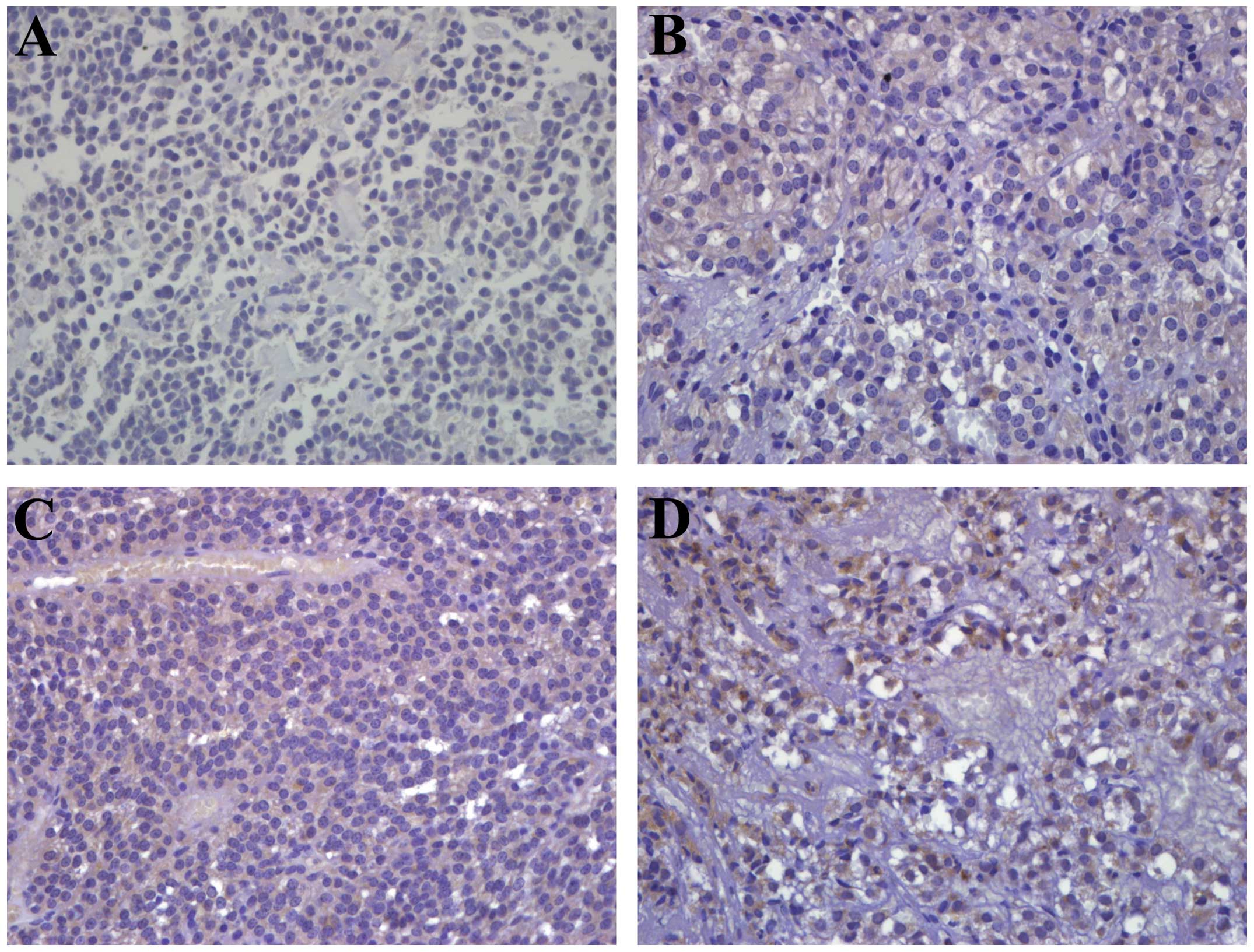
between NRP-1 expression and tumor progression. NRP-1
immunoreactivity 3+ and 1+ were observed in three (33.3%) and one
patient (11.1%), respectively, in the progression group. Thirteen
patients (68.4%) showed negative immunoreactivity to NRP-1 in the
control group (Fig. 1). No
significant risk for developing tumor progression was associated
with Ki-67, p53, Bax, Bcl-2, VEGFR-1, VEGFR-2 or VEGF-A
expression.
 | Table IIIComparison of molecular marker
expression between the pituitary adenoma cases with and without
progression within 5 years of the initial surgery. |
Table III
Comparison of molecular marker
expression between the pituitary adenoma cases with and without
progression within 5 years of the initial surgery.
| No. of patients
(%) | |
|---|
|
| |
|---|
|
Characteristics | No progression
group (n=19) | Progression group
(n=9) | P-value |
|---|
| Ki-67 | | | >0.05 |
| Negative | 18 (94.7) | 9 (100) | |
| 1–2% | 1 (5.3) | 0 (0.0) | |
| p53 | | | 0.530 |
| Negative | 16 (84.2) | 9 (100) | |
| Positive | 3 (15.8) | 0 (0.0) | |
| Bax | | | 0.670 |
| Negative | 12 (63.2) | 7 (77.8) | |
| Positive | 7 (36.8) | 2 (22.2) | |
| Bcl-2 | | | >0.05 |
| Negative | 18 (94.7) | 8 (88.9) | |
| Positive | 1 (5.3) | 1 (11.1) | |
| VEGFR-1 | | | >0.05 |
| Negative | 18 (94.7) | 8 (88.9) | |
| Positive | 1 (5.3) | 1 (11.1) | |
| VEGFR-2 | | | 0.530 |
| Negative | 16 (84.2) | 9 (100) | |
| Positive | 3 (15.8) | 0 (0.0) | |
| VEGF-A | | | 0.228 |
| − | 1 (5.3) | 0 (0.0) | |
| + | 0 (0) | 1 (11.1) | |
| ++ | 1 (5.3) | 2 (22.2) | |
| +++ | 7 (36.8) | 4 (44.5) | |
| ++++ | 10 (52.6) | 2 (22.2) | |
| NRP-1 | | | 0.034 |
| − | 13 (68.4) | 5 (55.6) | |
| + | 6 (31.6) | 1 (11.1) | |
| ++ | 0 (0) | 0 (0.0) | |
| +++ | 0 (0) | 3 (33.3) | |
| ++++ | 0 (0) | 0 (0.0) | |
Table IV shows a
comparison of molecular marker expression in the specimens obtained
during the first and second TSS in the progression group. No
significant expression changes in Ki-67, p53, Bax, Bcl-2, VEGFR-1
or VEGFR-2 were observed between the initial adenomas and
progressive adenomas. Five patients showed stronger VEGF-A
expression in progressive adenomas, compared with that in the
initial adenomas and four patients showed the same degree of
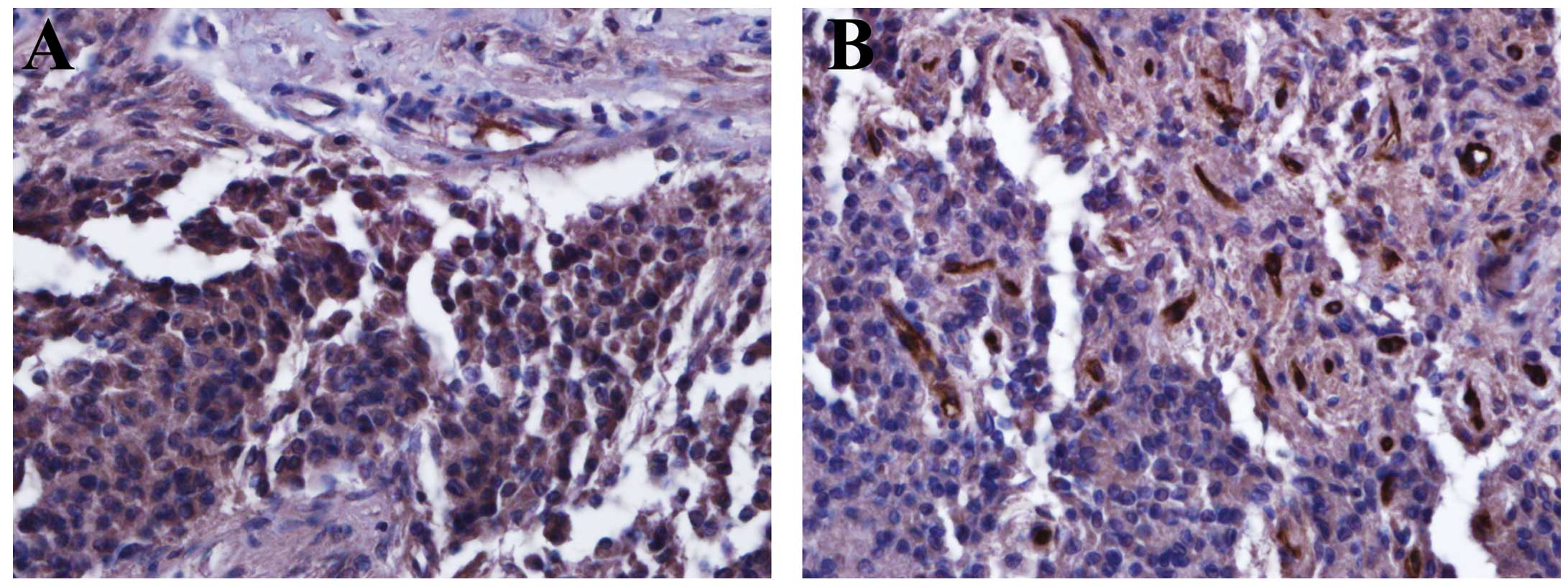
immunoreactivity. Notablu, NRP-1 expression was converted from
negative immunoreactivity in the initial specimens to strong
positivity in the progressive specimens of five patients. One
patient showed 4+ NRP-1 expression in the progressive specimen,
although the initial specimen showed 1+ NRP-1 expression. Three
patients showed strong NRP-1 immunoreactivity in both the initial
and progressive specimens (3+). NRP-1 immunoreactivity was noted in
PA cells (Fig. 2). The median
interval between the first and second TSS was 28 months.
 | Table IVComparison of molecular marker
expression in pituitary adenomas obtained following the first and
second TSS for treatment of progression within 5 years of the
initial surgery. |
Table IV
Comparison of molecular marker
expression in pituitary adenomas obtained following the first and
second TSS for treatment of progression within 5 years of the
initial surgery.
| Case no. | Surgery | Ki-67 | p53 | Bax | Bcl | VEGFR-1 | VEGFR-2 | VEGF-A | NRP-1 | Interval between
the first and second TSS (in months) |
|---|
| 1 | First | − | − | − | − | − | − | ++ | − | 25 |
| Second | − | − | − | − | − | − | ++++ | +++ | |
| 2 | First | − | − | − | − | + | − | +++ | + | 44 |
| Second | − | − | − | − | − | − | ++++ | ++++ | |
| 3 | First | − | − | + | − | − | − | +++ | − | 18 |
| Second | − | − | + | − | − | − | ++++ | ++ | |
| 4 | First | − | − | − | − | − | − | ++++ | − | 55 |
| Second | − | − | + | + | − | − | ++++ | ++ | |
| 5 | First | − | − | + | + | − | − | ++++ | +++ | 38 |
| Second | − | + | + | + | − | − | ++++ | +++ | |
| 6 | First | − | − | − | − | − | − | +++ | − | 26 |
| Second | − | − | − | − | − | − | ++++ | ++ | |
| 7 | First | − | − | − | − | − | − | + | +++ | 30 |
| Second | − | − | − | − | − | − | ++ | +++ | |
| 8 | First | − | − | − | − | − | − | ++ | +++ | 24 |
| Second | − | − | + | − | − | − | ++ | +++ | |
| 9 | First | − | − | − | − | − | − | +++ | − | 34 |
| Second | − | + | − | − | − | − | +++ | +++ | |
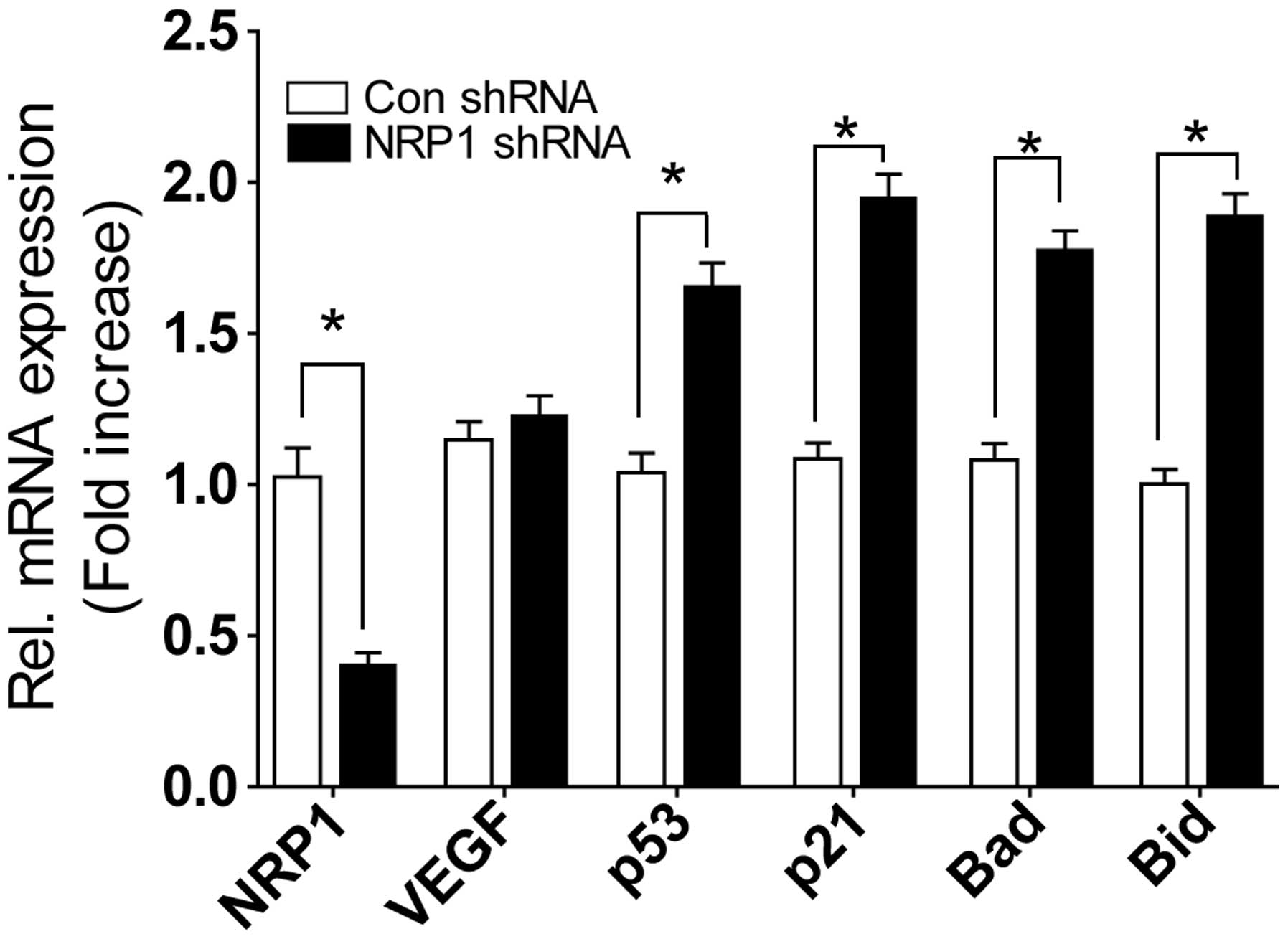
NRP-1 shRNA lentivirus significantly suppressed mRNA
of NRP-1. Knockdown of NRP-1 in MMQ cells did not decrease VEGF
expression, but increased the expression of p53, p21, Bad and Bid
genes (Fig. 3).
Discussion
No acceptable markers are available to predict tumor
recurrence and progression and thus aid the clinician in assessing
the need for adjuvant therapy or determining further follow-up for
pituitary adenomas (PAs) in the postoperative setting. In our
study, a strong association was observed between NRP-1 expression
and PA progression in the immunohistochemical analysis. The
involvement of the cavernous sinus was associated with tumor
progression. NRP-1 expression was associated with the invasion of
cavernous sinus (P=0.024). NRP-1 could have a role in mediating the
invasiveness of PAs.
NRPs are a family of transmembrane glycoprotein
receptors that interact with members of the VEGF ligand family.
NRP-1 is expressed in various human cancers, including prostate
cancer, breast cancer, melanoma and pancreatic adenocarcinoma but
not in corresponding normal epithelial tissues (14,15).
Those studies focused on the ability of NRP-1 to act as a
co-receptor to increase the affinity of a ligand (e.g., VEGF) for a
tyrosine kinase receptor (e.g., VEGF-2). Normal pituitary tissue
(from normal controls) seems to display more vascularity than
tissue from adenoma samples (16,17).
The vessels of normal tissue may be larger than those from adenomas
(18). Several studies have been
unable to show a relationship between VEGF expression and tumor
recurrence (19,20), although VEGF overexpression is
associated with extrasellar growth (21). No correlation was observed between
the degree of VEGF and its receptors and tumor progression
parameters, such as grade, proliferation index and vessel density
(22). In our series, VEGFR-1,
VEGFR-2 and VEGF-A expression were not different between the
progression and no progression groups in initial specimens obtained
during the first TSS. Negative immunoreactivity of VEGFR-1 and
VEGFR-2 was predominant in both groups. Therefore, it is suggested
that the VEGF/VEGFR-1,2 system could not be directly involved in
tumor progression. There was a tendency for VEGF-A overexpression
in both the first and second specimens obtained during repeat TSS
in the progression group. VEGF contributes to adequate tumoral
vascular supply through complex mechanisms, other than tumor cell
proliferation (23). Other authors
have suggested that VEGF may prolong cell survival by inducing
expression of the antiapoptotic protein Bcl-2 in PAs (24,25).
However, our results show little association between tumor
progression and the expression of Bax and Bcl-2.
Increased NRP-1 expression has been correlated with
tumor growth and vacularization in vivo and with
invasiveness in human cancer (26).
VEGF and NRP-1 actively participate in the modulation of tumor
angiogenesis and the development of PAs in rats (27). In our series, NRP-1 expression was
significantly associated with tumor progression. All specimens in
the progression group showed negative immunoreactivity to VEGFR-2,
the NRP-1 co-receptor, suggesting a VEGF-independent function for
NRP-1 in NFPA progression. Experimental evidence indicates that
NRP-1 may have functions independent of VEGFR-2, potentially
through a NRP interacting protein (28). Five patients in the progression
group showed negative NRP-1 immunoreactivity in their initial
specimens. Interestingly, specimens obtained at the reoperation
showed positive immunoreactivity. Similarly, NRP-1 is
constitutively expressed in sinusoidal endothelial cells in normal
liver, but its expression increases following resection of liver
for sinusoidal remodeling (29).
NRP-1 expression can be stimulated in response to tissue injury or
hypoxic conditions (30,31). We suggest that expression of NRP-1
is not static and it is affected by the tumor microenvironment.
PA transplants are effectively inhibited by
administration of anti-VEGF-A monoclonal antibody (32). Anti-VEGF (bevacizumab) treatment
stabilizes aggressive PAs in patients (33). However, our results showed that
tumor progression was dependent on NRP-1 expression rather than
that of VEGF. Antitumor activity of monoclonal antibody to NRP-1 is
currently evaluated in cancers (34). We generated an shRNA lentivirus
against NRP-1 to determine the effects of in vitro NRP-1
knockdown. Knockdown of NRP-1 increased the expression of apoptotic
genes such as p53, p21, Bad and Bid. This indicates that
downregulation of NRP-1 can induce the apoptosis of PA cells,
independently of VEGF expression. Taken together, we suggest that
NRP-1 is considered as a novel therapeutic target for PAs.
Acknowledgements
The authors wish to acknowledge the financial
support of the St. Vincent’s Hospital, Research Institute of
Medical Science Foundation in 2014.
References
|
1
|
Ezzat S, Asa SL, Couldwell WT, et al: The
prevalence of pituitary adenomas: a systematic review. Cancer.
101:613–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu DW, Hakim F, Biller BM, et al:
Significance of proliferating cell nuclear antigen index in
predicting pituitary adenoma recurrence. J Neurosurg. 78:753–761.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gejman R, Swearingen B and Hedley-Whyte
ET: Role of Ki-67 proliferation index and p53 expression in
predicting progression of pituitary adenomas. Hum Pathol.
39:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lloyd RV: Pituitary tumors. World Health
Organization Classification of Tumors, Pathology and Genetics of
Tumors of Endocrine Organs. DeLellis A: IARC Press; Lyon: pp.
10–13. 2004
|
|
5
|
Hentschel SJ, McCutcheon lE, Moore W and
Durity FA: P53 and MIB-1 immunohistochemistry as predictors of the
clinical behavior of nonfunctioning pituitary adenomas. Can J
Neurol Sci. 30:215–219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sambaziotis D, Kapranos N and Kontogeorgos
G: Correlation of bcl-2 and bax with apoptosis in human pituitary
adenomas. Pituitary. 6:127–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iuchi T, Saeki N, Osato K and Yamaura A:
Proliferation, vascular endothelial growth factor expression and
cavernous sinus invasion in growth hormone secreting pituitary
adenomas. Acta Neurochir. 142:1345–1351. 2000. View Article : Google Scholar
|
|
8
|
Cohen AB and Lessell S: Angiogenesis and
pituitary tumors. Semin Ophthalmol. 24:185–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tammela T, Enholm B, Alitalo K and
Paavonen K: The biology of vascular endothelial growth factors.
Cardiovasc Res. 65:550–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oh H, Takagi H, Otani A, et al: Selective
induction of neuropilin-1 by vascular endothelial growth factor
(VEGF): a mechanism contributing to VEGF-induced angiogenesis. Proc
Natl Acad Sci USA. 99:383–388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benveniste RJ, King WA, Walsh J, et al:
Repeated transsphenoidal surgery to treat recurrent or residual
pituitary adenoma. J Neurosurg. 102:1004–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JS, Yoon A, Kalapurakal SK, et al:
Expression of p53 oncoprotein in non-small-cell lung cancer: a
favorable prognostic factor. J Clin Oncol. 13:1893–1903.
1995.PubMed/NCBI
|
|
13
|
Thunnissen FB, Schuurbiers OC and den
Bakker MA: A critical appraisal of prognostic and predictive
factors for common lung cancers. Histopathology. 48:779–786. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bielenberg DR, Pettaway CA, Takashima S
and Klagsbrun M: Neuropilins in neoplasms: expression, regulation,
and function. Exp Cell Res. 312:584–593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guttmann-Raviv N, Kessler O, Shraga-Heled
N, et al: The neuropilins and their role in tumorigenesis and tumor
progression. Cancer Lett. 231:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turner HE, Nagy Z, Gatter KC, et al:
Angiogenesis in pituitary adenomas and the normal pituitary gland.
J Clin Endocrinol Metab. 85:1159–1162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Ieva A, Grizzi F, Ceva-Grimaldi G, et
al: Fractal dimension as a quantitator of the microvasculature of
normal and adenomatous pituitary tissue. J Anat. 211:673–680.
2007.PubMed/NCBI
|
|
18
|
Takada K, Yamada S and Teramoto A:
Correlation between tumor vascularity and clinical findings in
patients with pituitary adenomas. Endocr Pathol. 15:131–139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukui S, Nawashiro H, Otani N, et al:
Vascular endothelial growth factor expression in pituitary
adenomas. Acta Neurochir Suppl. 86:519–521. 2003.PubMed/NCBI
|
|
20
|
Fukui S, Otani N, Nawashiro H, et al: The
association of the expression of vascular endothelial growth factor
with the cystic component and haemorrhage in pituitary adenoma. J
Clin Neurosci. 10:320–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sánchez-Ortiga R, Sánchez-Tejada L,
Moreno-Perez O, et al: Over-expression of vascular endothelial
growth factor in pituitary adenomas is associated with extrasellar
growth and recurrence. Pituitary. 16:370–377. 2013.PubMed/NCBI
|
|
22
|
Onofri C, Theodoropoulou M, Losa M, et al:
Localization of vascular endothelial growth factor (VEGF) receptors
in normal and adenomatous pituitaries: detection of a
non-endothelial function of VEGF in pituitary tumours. J
Endocrinol. 191:249–261. 2006. View Article : Google Scholar
|
|
23
|
Cristina C, Perez-Millan MI, Luque G, et
al: VEGF and CD31 association in pituitary adenomas. Endocr Pathol.
21:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nör JE, Christensen J, Mooney DJ and
Polverini PJ: Vascular endothelial growth factor (VEGF)-mediated
angiogenesis is associated with enhanced endothelial cell survival
and induction of Bcl-2 expression. Am J Pathol. 154:375–384.
1999.PubMed/NCBI
|
|
25
|
Turner HE, Nagy Z, Gatter KC, Esiri MM, et
al: Proliferation, bcl-2 expression and angiogenesis in pituitary
adenomas: relationship to tumour behaviour. Br J Cancer.
82:1441–1445. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan Q, Chanthery Y, Liang WC, et al:
Blocking neuropilin-1 function has an additive effect with
anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Banerjee SK, Zoubine MN, Tran TM, et al:
Overexpression of vascular endothelial growth factor164 and its
co-receptor neuropilin-1 in estrogen-induced rat pituitary tumors
and GH3 rat pituitary tumor cells. Int J Oncol. 16:253–260.
2000.PubMed/NCBI
|
|
28
|
Pan Q, Chathery Y, Wu Y, et al:
Neuropilin-1 binds to VEGF121 and regulates endothelial cell
migration and sprouting. J Biol Chem. 282:24049–24056. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu L, Kitamura T, Iwabuchi K, Ichinose S,
et al: Interplay of neuropilin-1 and semaphorin 3A after partial
hepatectomy in rats. World J Gastroenterol. 18:5034–5041. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matthies AM, Low QE, Lingen MW and
DiPietro LA: Neuropilin-1 participates in wound angiogenesis. Am J
Pathol. 160:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brusselmans K, Bono F, Collen D, et al: A
novel role for vascular endothelial growth factor as an autocrine
survival factor for embryonic stem cells during hypoxia. J Biol
Chem. 280:3493–3499. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Korsisaari N, Ross J, Wu X, et al:
Blocking vascular endothelial growth factor-A inhibits the growth
of pituitary adenomas and lowers serum prolactin level in a mouse
model of multiple endocrine neoplasia type 1. Clin Cancer Res.
14:249–258. 2008. View Article : Google Scholar
|
|
33
|
Ortiz LD, Syro LV, Scheithauer BW, et al:
Anti-VEGF therapy in pituitary carcinoma. Pituitary. 15:445–449.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jubb AM, Strickland LA, Liu SD, et al:
Neuropilin-1 expression in cancer and development. J Pathol.
226:50–60. 2012. View Article : Google Scholar : PubMed/NCBI
|