Of the 12.7 million new cancer cases that occurred
in 2008 in the world, the population-attributable fraction for
infectious agents was 16.1%, and that of Epstein-Barr virus (EBV)
was 5.4% (1). EBV is a ubiquitous
virus that infects almost all adults throughout the world. EBV is
also known as a causative agent of i) Burkitt’s lymphoma, an
endemic malignant tumor in East African children; ii)
nasopharyngeal carcinoma (NPC), which has a tendency of high
occurrence in the Chinese and iii) various tumors associated with
immuno-suppressive states such as those observed
post-transplantation and in AIDS (2,3).
Although foods, environment, and genetic differences must be
considered, I hypothesized that more human tumors are generated by
EBV infection than has been reported, and to test this hypothesis
EBV transcripts were examined in various human tumors with mRNA
in situ hybridization (mRNA ISH) and immunofluorescence
staining. The results of these procedures showed that EBV infects
and proliferates in macrophages. The neoplasms of
macrophage-related cells were also investigated. Seven types of
cancer other than NPC, four types of lymphoma, and Langerhans cell
histiocytosis (LCH) expressed EBV transcripts, including its
oncogenes. None of the patients examined herein showed an
immunocompromised state, and most of the patients with LCH were
North American. Here, I review the recent reports correlating EBV
transcripts with human cancers, and I discuss the role of
macrophages in the oncogenesis of EBV.
Although breast cancers were not examined, a 1995
study that used polymerase chain reaction (PCR) over a region of
BamHIW found that 21% of the breast cancer specimens
evaluated were positive for EBV DNA (5). Tierney et al reported that the
five-repeat number of BamHIW was most effective for B-cell
transformation (6).
In a previous study, I demonstrated that EBV nuclear
antigen-2 (EBNA2) is a strong oncogene of EBV (11). After a 2–3-week inoculation of a
transfected rat fibroblast cell line with a recombinant EBNA2
expression plasmid, four of seven clones reproducibly formed tumors
in nude mice. All of these tumorigenic clones could be grown in low
serum, and two of the four tumorigenic clones formed colonies in
soft agar. These four tumorigenic clones showed EBNA2 expression,
but the non-tumorigenic clones did not. These results indicate that
the expression of EBNA2 is correlated with tumorigenicity.
The mRNA expression of EBNA2 can be detected with
the mRNA ISH protocol that we established (12), and the EBNA2 protein expression can
be detected with indirect immunofluorescence staining using a
monoclonal antibody.
The transformation of mouse cells by the latent
membrane protein-1 (LMP1) gene of EBV was reported by Baichwal
et al (18). LMP1 was
reported to have a role in the carcinogenesis of NPC (19). Kim et al also showed that the
transformation of canine kidney epithelial cells by LMP1 formed
spheroidal cysts in collagen gel matrix and induced invasive growth
(20). Shair et al compared
the development of papilloma and carcinoma in epithelial cells of
transgenic mice with model carcinogens DMBA and TPA, and they found
that LMP1 was a weak promoter, that it increased papilloma
formation, and that it slightly increased squamous cell carcinoma
(SCC); the development of SCC was significantly increased in LMP2A
double-transgenic animals (21).
No new reports are available.
A rare case of thyroid involvement by malignant
histiocytosis of the Langerhans cell type was reported in 1996
(47), but its association with EBV
is unclear. There are many reports regarding LCH co-existing with
papillary thyroid cancer, but none of the relevant studies examined
EBV. Furthermore, some reports described that numerous
tumor-associated macrophages (TAMs) were distributed in advanced
thyroid cancer and thyroid papillary carcinoma, and that an
increased density of macrophages is associated with decreased
survival, but none of these reports mentioned an association with
EBV (48,49).
There are many reports of EBV association with
uterine cervical carcinoma, but almost all used data obtained by
PCR; the detection of EBV transcripts is very rare. Using nested
PCR, Santos et al examined 66 women with high-grade cervical
intraepithelial neoplasia (CIN) and 14 women with invasive cervical
cancer, and they found that the invasive cancer group showed
significantly higher positivity for EBV (56). Kim et al showed similar
results of EBV positivity, and they noted that p16 methylation was
observed in the cases of EBV-positivity (57). Szostek et al found EBV
integration in 50% of cervical SCCs, but not in CIN (58).
Since the WHO classification system was changed in
2004, cases of anaplastic large-cell lymphoma (ALCL) may not be the
same as those before 2004. Here, I describe the ALCL according to
the criteria of the classification at each period of time.
Park and Ko reported that 5 of 12 cutaneous T-cell
lymphoma (CTCL) cases showed EBER positivity by ISH (70), and Erkek et al showed that 9
of 92 subjects with mycosis fungoides (MF) evidenced EBV DNA
(71). In a study by Novelli et
al, 7 of 71 MF cases were EBV DNA-positive by PCR, but EBER
signals were negative by ISH (72).
Copur et al reported the full clinical recovery of a patient
with EBV-associated CTCL after topical acyclovir treatment
(73).
Although EBV DNA was detected in 3 of 19 LCH
patients by PCR, no signal of EBERs in LCH cells was revealed by
ISH, and thus the experiments did not support the role of EBV in
the pathogenesis of 83 patients with LCH (74). However, there are some reports
supporting EBV pathogenesis of LCH. Csire et al found that
antiviral therapy effectively cleared EBV (75), which I also reported in 2004 with
one patient (37). The development
of LCH was also described with chronic active EBV infection
(76). As shown in Table I, immunofluorescence of BZLF1 was
detected in all LCH cases, which showed lytic infection occurring
in LCH, and thus LCH was sensitive to acyclovir (37,75).
I frequently observed the lytic infection of EBV in
human neoplastic tissues, and my review of the literature revealed
the following. An EBV-positive epithelial cell tumor, gastric
carcinoma, and NPC showed ganciclovir susceptibility, which
indicated lytic infection of the tumors (77). Sporadic lytic viral replication was
observed in breast carcinoma cell lines (78). In NPC cells, EBV lytic infection was
reported to induce interleukin-10 (IL-10) in monocytes (79). The data regarding IL-10, monocytes
and macrophages will be examined further in the ‘Discussion’
section. Viral reactivation from latency was controlled by the
expression of BZLF1 of EBV, and Murata and Tsurumi recently found
that histone H3 lysin 27 trimethylation and H4K20me3 markers are
crucial for the suppression of BZLF1 (80). These results are expected to be
useful in the development of therapy for EBV-positive cancers using
epigenetic disruptors.
Our group reported increased sensitivity of
EBNA2-transformed cells to ionizing radiation (81), and more recent studies have followed
up on this finding. Plasma EBV DNA levels significantly decreased
after radiotherapy (82). Among 270
patients with CTCL, complete response was observed in 255 patients
treated with 700–800 cGy (83).
Although the association with EBV was not examined,
85% of human macrophages expressed the Hodgkin’s cell-associated
antigen Ki-1 (CD30) in vitro, whereas normal human monocytes
did not express the antigen (90).
This finding indicates that the progression from monocyte to
macrophage needs the expression of CD30 in at least 85% of the
cases. EBV is suspected to participate in this progression with the
production of IL-10. These findings will be evaluated in the
‘Discussion’ section.
The inflammatory process has been indicated to be a
cofactor in carcinogenesis, where tumor-associated macrophages
(TAMs) have been found to be a major component of the infiltrate of
tumors (91). TAMs have also been
described to enhance tumor progression and metastasis (92). Interactions between macrophages and
lymphocytes in cancer have been discussed (93,94).
Regarding RCC, 98 RCC patients were examined and correlations were
identified for TAMs, microvessel density and the proliferative
index with a tendency for poor prognosis (95) and the coculture of macrophages with
RCC cells induced TAMs and the activation of signal transducers or
activators (96). Macrophage
recruitment was also suggested to be associated with the disease
progression of Wilms’ tumor (97).
We detected TAMs in many EBV-associated neoplasms
including RCC, uterine carcinoma, oral cancer and others; TAMs,
which were in situ hybridized with EBV antisense RNAs, were
double-stained immunohistochemically with monoclonal anti-CD68
antibody (98). These findings
indicate that TAMs infiltrate into EBV-associated neoplasms that
express EBV transcripts, and they suggest a strong influence on
oncogenesis.
Although the association between EBV and human
neoplasms has been accepted in a restricted number of diseases, I
propose that more attention should be paid to EBV-associated human
cancers. The reasons are as follows. As indicated above, much
epidemiological data have now been accumulated; in tissue cultures,
EBV is one of the most potent transforming viruses (11); EBV persists in circulating memory
cells and therefore will be found in all tissues (99); and in CTCL and LCH, some cases
showed full clinical recovery after acyclovir treatment (37,73,75).
The macrophage involvement of EBV, which is
described above, is also an important problem. IL-10 and viral
IL-10 (v-IL-10) strongly reduce antigen-specific human T-cell
proliferation by diminishing the antigen-presenting capacity of
monocytes (104), and IL-10
promotes the differentiation of monocytes to mature macrophages and
blocks their differentiation to dendritic cells (105). The transfection of v-IL-10 into
murine cells resulted in tumors after 4 weeks and showed local
immunosuppressive effects which depended on the v-IL-10 dose
(106). The conservation and
mutation of the v-IL-10 gene in various EBV isolates (107) and in human gastric carcinomas and
NPCs were also reported (108).
Although EBV association was not examined, Lee et al showed
that bone morphogenic proteins (BMPs) in RCC promoted tumor
proliferation through an IL-10-dependent M2 polarization of TAMs
with mouse cell lines and 50 samples of human RCC, and they showed
that BMP-6/IL-10/CD68 was associated with a poor prognosis
(109). The interactions between
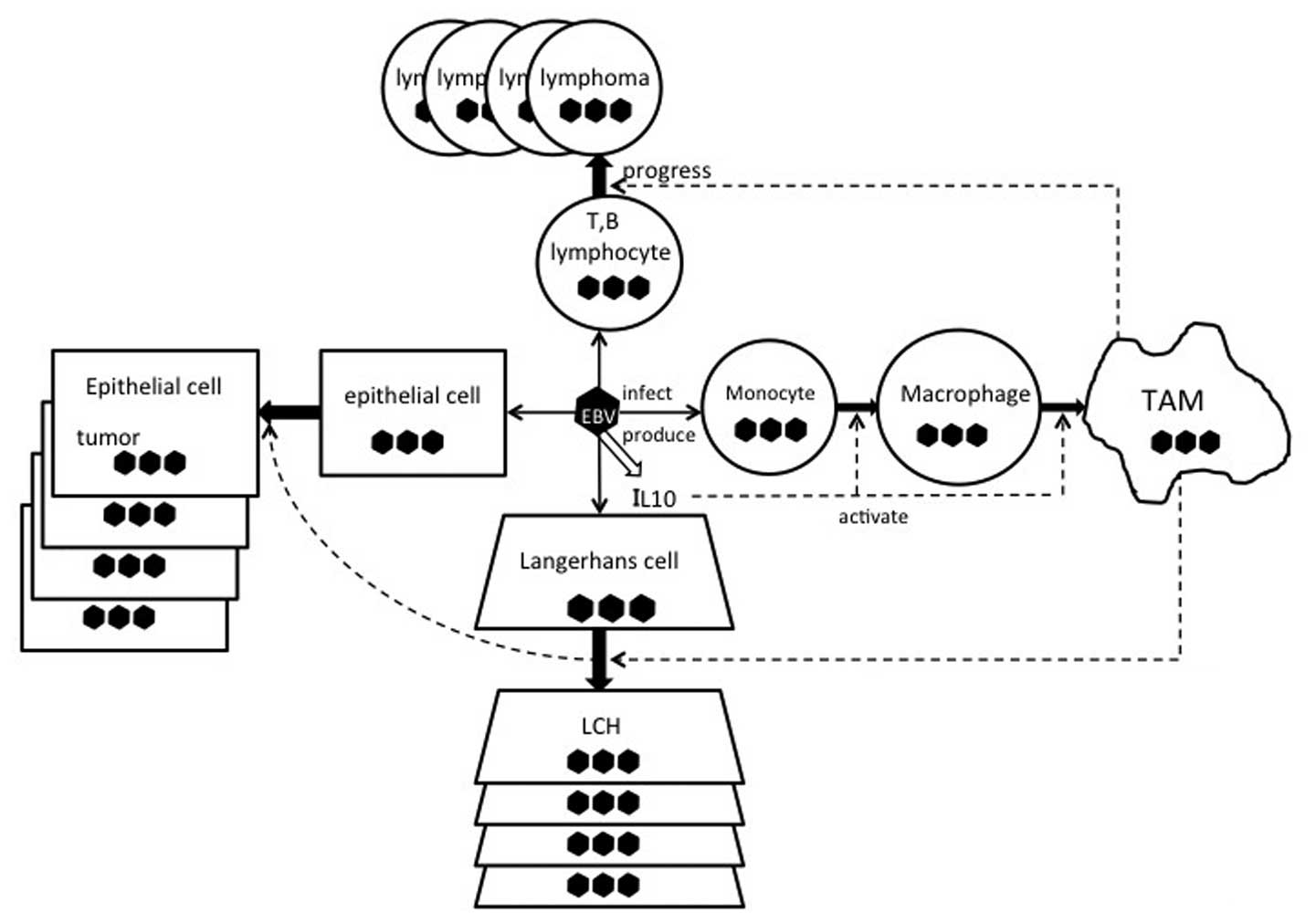
EBV, IL-10, and TAMs are illustrated in Fig. 1.
Epidemiological information takes priority over the
investigation of mechanisms, and the epidemiological evidence must
be therefore accumulated. Virus-directed therapeutics against
EBV-associated malignancies have been reported, which include EBV
lytic phase induction followed by anti-herpesvirus drug mainly for
EBV-positive lymphoid tumors (110), and for EBV-positive epithelial
cell tumors (111), followed by
radiation therapy for EBV-positive epithelial cell tumors (112). Radiation therapy should be
recommended for EBV-associated neoplasms, since EBNA2 is sensitive
to ionizing radiation (81). It is
suspected that infected Langerhans cells serve as a reservoir of
EBV in oral epithelial cells (46),
and following this infection, oral epithelial dysplasia and SCC are
induced (28). The role of
macrophages in the oncogenesis of EBV merits further study. The
effect of acyclovir and radiation for treatment of EBV-associated
neoplasms must be further studied.
|
1
|
de Martel, Ferlay J, Franceschi S, Vignat
J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: a review and synthetic
analysis. Lancet Oncol. 13:607–645. 2012.PubMed/NCBI
|
|
2
|
Young LS and Murray PG: Epstein-Barr virus
and oncogenesis: from latent genes to tumours. Oncogene.
22:5108–5121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young LS and Rickinson AB: Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 4:757–768. 2004.PubMed/NCBI
|
|
4
|
Shimakage M, Oka T, Shinka T, Kurata A,
Sasagawa T and Yutsudo M: Involvement of Epstein-Barr virus
expression in testicular tumors. J Urol. 156:253–257. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Labrecque LG, Barnes DM, Fentiman IS and
Griffin BE: Epstein-Barr virus in epithelial cell tumors: A breast
cancer study. Cancer Res. 55:39–45. 1995.PubMed/NCBI
|
|
6
|
Tierney R, Kao K-U, Nagra JK and Rickinson
AB: Epstein-Barr virus BamHIW repeat number limits
EBNA2/EBNA-LP coexpression in newly infected B cells and the
efficiency of B-cell transformation: a rationale for multiple W
repeats in wild-type virus strains. J Virol. 85:12363–12375.
2011.
|
|
7
|
Allan GJ, Inman GJ, Parker BD, Rowe DT and
Farrell PJ: Cell growth effects of Epstein-Barr virus leader
protein. J Gen Virol. 73:1547–1551. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng RS, Moses SC, Tan J, Kremmer E and
Ling PD: The Epstein-Barr virus EBNA-LP protein preferentially
coactivates EBNA2-mediated stimulation of latent membrane proteins
expressed from the viral divergent promoter. J Virol. 79:4492–4505.
2005. View Article : Google Scholar
|
|
9
|
Shimakage M, Harada S, Kawahara K, Oka T,
Yanoma S, Horii K and Sasagawa T: Detection of Epstein-Barr virus
nuclear antigen leader protein expression in various human cancers.
New Developments in Epstein-Barr Virus Research. Umar S: Nova
Science; New York: pp. 261–276. 2006
|
|
10
|
Portal D, Zhou H, Zhao B, Kharchenko PV,
Lowry E, Wong L, Quackenbush J, Holloway D, Jiang S, Lu Y and Kieff
E: Epstein-Barr virus nuclear antigen leader protein localizes to
promoters and enhancers with cell transcription factors and EBNA2.
Proc Natl Acad Sci USA. 110:18537–18542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimakage M, Kurata A, Inoue H, Okamoto Y
and Yutsudo M: Tumorigenicity of EBNA2-transfected cells. FEBS
Lett. 371:245–248. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasagawa T, Shimakage M, Nakamura M,
Sakaike J, Ishikawa H and Inoue M: Epstein-Barr virus (EBV) genes
expression in cervical intra-epithelial neoplasia and invasive
cervical cancer: A comparative study with human papillomavirus
(HPV) infection. Hum Pathol. 31:318–326. 2000. View Article : Google Scholar
|
|
13
|
Pan SH, Tai CC, Lin CS, Hsu WB, Chou SF,
Lai CC, Chen JY, Tien HF, Lee FY and Wang WB: Epstein-Barr virus
nuclear antigen 2 disrupts mitotic checkpoint and causes
chromosomal instability. Carcinogenesis. 30:366–375. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sano H, Nagata K, Kato K, Kanai K,
Yamamoto K, Okuno K, Kuwamoto S, Higashi-Mori H, Sugihara H, Kato
M, Murakami I, Kanzaki S and Hayashi K: EBNA-2-deleted Epstein-Barr
virus from P3HR1 can infect rabbits with lower efficiency than
prototype Epstein-Barr virus from B95–8. Intervirology. 56:114–121.
2013.PubMed/NCBI
|
|
15
|
Wu Y, Maruo S, Yajima M, Kanda T and
Takada K: Epstein-Barr virus (EBV)-encoded RNA2 (EBER2) but not
EBER1 plays a critical role in EBV-induced B-cell growth
transformation. J Virol. 81:11236–11245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosato P, Anastasiadou E, Garg N, Lenze D,
Boccellato F, Vincenti S, Severa M, Coccia EM, Bigi R, Cirone M,
Ferretti E, Campese AF, et al: Differential regulation of miR-21
and miR-146a by Epstein-Barr virus-encoded EBNA2. Leukemia.
26:2343–2352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pfeffer S, Zavolan M, Grasser A, Chien M,
Russo J, Ju J, John B, Enright AJ, Marks D, Sander C and Tuschi T:
Identification of virus-encoded microRNAs. Science. 304:734–736.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baichwal VR and Sugden B: Transformation
of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus.
Oncogene. 5:461–467. 1988.PubMed/NCBI
|
|
19
|
Zheng H, Li L, Hu D, Deng X and Cao Y:
Role of Epstein-Barr virus encoded latent membrane protein 1 in the
carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol.
4:185–196. 2007.PubMed/NCBI
|
|
20
|
Kim K-R, Yoshizaki T, Miyamori H, Hasegawa
K, Horikawa T, Furukawa M, Harada S, Seiki M and Sato H:
Transformation of Madin-Darby canine kidney (MDCK) epithelial cells
by Epstein-Barr virus LMP1 induces expression of Ets 1 and invasive
growth. Oncogene. 19:1764–1771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shair KH, Bendt KM, Edwards RH, Nielsen
JN, Moore DT and Raab-Traub N: Epstein-Barr virus-encoded latent
membrane protein 1 (LMP1) and LMP2A function cooperatively to
promote carcinoma development in a mouse carcinogenesis model. J
Virol. 86:5352–5365. 2012. View Article : Google Scholar
|
|
22
|
Swaminathan S, Tomkinson B and Kieff E:
Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted
transforms lymphocytes and replicates in vitro. Proc Natl
Acad Sci USA. 88:1546–1550. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komano J, Maruo S, Kurozumi K, Oda T and
Takada K: Oncogenic role of Epstein-Barr virus-encoded RNAs in
Burkitt’s lymphoma cell line Akata. J Virol. 73:9827–9831.
1999.
|
|
24
|
Houmani JL, Davis CI and Ruf IK:
Growth-promoting properties of Epstein-Barr virus EBER-1 RNA
correlates with ribosomal protein L22 binding. J Virol.
83:9844–9853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiss LM and Chen YY: EBER in situ
hybridization for Epstein-Barr virus. Meth Mol Biol. 999:223–230.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimakage M, Sasagawa T, Yoshino K,
Yutsudo M, Kimura M, Yamamoto N and Yanoma S: Expression of
Epstein-Barr virus in mesopharyngeal and hypopharyngeal carcinoma.
Hum Pathol. 30:3071–3076. 1999. View Article : Google Scholar
|
|
27
|
Chatani M, Teshima T, Inoue T, Yoshino K,
Ikegami N, Hirai K and Shimakage M: Antibody response against the
Epstein-Barr virus-coded nuclear antigen 2 (EBNA2) in
nasopharyngeal carcinoma. Laryngoscope. 101:626–629. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimakage M, Horii K, Tempaku A, Kakudo K,
Shirasaka T and Sasagawa T: Association of Epstein-Barr virus with
oral cancers. Hum Pathol. 33:608–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimakage M, Kawahara K, Sasagawa T, Inoue
H, Yutsudo M, Yoshida A and Yanoma S: Expression of Epstein-Barr
virus in thyroid carcinoma correlates with tumor progression. Hum
Pathol. 34:1170–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimakage M, Kawahara K, Harada S,
Sasagawa T, Shinka T and Oka T: Expression of Epstein-Barr virus in
renal cell carcinoma. Oncol Rep. 18:41–46. 2007.PubMed/NCBI
|
|
31
|
Shimakage M and Sasagawa T: Detection of
Epstein-Barr virus-determined nuclear antigen-2 mRNA by in
situ hybridization. J Virol Meth. 93:23–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimakage M, Dezawa T, Tamura S, Tabata T,
Aoyagi N, Koike M, Inoue H, Yutsudo M, Hakura A and Ikegami N: A
Ki-1-positive cell line expressing Epstein-Barr virus antigens,
established from child with Ki-1-positive lymphoma. Intervirology.
36:215–224. 1993.PubMed/NCBI
|
|
33
|
Shimakage M, Nakamine H, Tamura S,
Takenaka T, Yutsudo M and Hakura A: Detection of Epstein-Barr virus
transcripts in anaplastic large-cell lymphoma by mRNA in
situ hybridization. Hum Pathol. 28:1415–1419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimakage M, Sasagawa T, Kawahara K,
Yutsudo M, Kusuoka H and Kozuka T: Expression of Epstein-Barr virus
in cutaneous T-cell lymphoma including mycosis fungoides. Int J
Cancer. 92:226–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakajima H, Shimakage M, Takeda Y,
Furutama D, Sugino M, Kimura F, Shibayama Y and Hanafusa T:
Epstein-Barr virus-associated primary leptomeningeal lymphoma. Eur
J Neurol. 13:e4–e6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimakage M, Sakamaoto H, Harada S,
Sasagawa T and Kodama K: Expression of the Epstein-Barr virus in
lymphoproliferative diseases of the lung. Oncol Rep. 17:1347–1352.
2007.PubMed/NCBI
|
|
37
|
Shimakage M, Sasagawa T, Kimura M,
Shimakage T, Seto S, Kodama K and Sakamaoto H: Expression of
Epstein-Barr virus in Langerhans’ cell histiocytosis. Hum Pathol.
35:862–868. 2004.
|
|
38
|
Shamaa A, Zyada M, Wagner M, Awad S, Osman
MM and Azeem AA: The significance of Epstein-Barr virus (EBV) &
DNA topoisomerase II alpha (DNA-topo II alpha) immunoreactivity in
normal oral mucosa, oral epithelial dysplasia (OED) and oral
squamous cell carcinoma (OSCC). Diagnostic Pathol. 3:45–57.
2008.
|
|
39
|
Slots J, Saygun I, Sabeti M and Kubar A:
Epstein-Barr virus in oral diseases. J Periodontal Res. 41:235–244.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jalouli J, Lalouli MM, Sapkota D, Ibrahim
SO, Larsson PA and Sand L: Human papilloma virus, herpes simplex
virus and Epstein-Barr virus in oral squamous cell carcinoma from
eight different countries. Anticancer Res. 32:571–580. 2012.
|
|
41
|
Szkaradbiewicz A, Kruk-Zagajewska A, Wal
M, Jopek M, Wierzbicka A and Kuch A: Epstein-Barr virus and human
papillomavirus infections and oropharyngeal squamous cell
carcinoma. Clin Exp Med. 2:137–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gonzalez-Moles MA, Gutierrez J, Rodriquez
MJ, Ruiz-Avila I and Rodriquez-Archilla A: Epstein-Barr virus
latent membrane protein-1 (LMP-1) expression in oral squamous cell
carcinoma. Laryngoscope. 112:482–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kobayashi I, Shima K, Saito I, Kiyoshima
T, Matsuo K, Ozeki S, Onishi M and Sakai H: Prevalence of
Epstein-Barr virus in oral squamous cell carcinoma. J Pathol.
189:34–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herrmann K, Frangou P, Middeldorp J and
Niedobitek G: Epstein-Barr virus replication in tongue epithelial
cells. J Gen Virol. 83:2995–2998. 2002.PubMed/NCBI
|
|
45
|
Frangou P, Buettner M and Niedobitek G:
Epstein-Barr virus (EBV) infection in epithelial cells in vivo:
rare detection of EBV replication in tongue mucosa but not in
salivary glands. J Infect Dis. 191:238–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Walling DM, Ray AJ, Nichols JE, Flaitz CM
and Nichols CM: Epstein-Barr virus infection of Langerhans cell
precursors as a mechanism of oral epithelial entry, persistence,
and reactivation. J Virol. 81:7249–7268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kitahara S, Iitaka M, Shimizu T, Serizawa
N, Fukasawa N, Miura S, Kawasaki S, Yamanaka K, Kawakami Y,
Murakami S, Ishii J and Katayama S: Thyroid involvement by
malignant histiocytosis of Langerhans’ cell type. Clin Endocrinol.
45:357–363. 1996.
|
|
48
|
Ryder M, Ghossein RA, Ricarte-Filho JCM,
Knauf JA and Fagin JA: Increased density of tumor associated
macrophages is associated with decreased survival in advanced
thyroid cancer. Endocr Relat Cancer. 15:1069–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liotti F, Visciano C and Melillo RM:
Inflammation in thyroid oncogenes. Am J Cancer Res. 2:286–297.
2012.
|
|
50
|
Kim KH, Han EM, Lee ES, Park HS, Kim I and
Kim YS: Epstein-Barr virus infection in sarcomatoid renal cell
carcinoma tissues. BJU Int. 96:547–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Betge J, Pollheimer MJ, Schelemmer A,
Hoefler G and Langner C: Gastric cancer and concomitant renal
cancer: A systematic immunohistochemical and molecular analysis.
Oncol Rep. 26:567–575. 2011.PubMed/NCBI
|
|
52
|
Rajpert-De Meyts E, Hørding U, Nielsen HW
and Skakkebaek NE: Human papillomavirus and Epstein-Barr virus in
the etiology of testicular germ cell tumours. APMIS. 102:38–42.
1994.PubMed/NCBI
|
|
53
|
Fend F, Hittmair A, Rogatsch H, Gredler E,
Obrist P and Mikuz G: Seminomas positive for Epstein-Barr virus by
the polymerase chain reaction: viral RNA transcripts
(Epstein-Barr-encoded small RNAs) are present in intratumoral
lymphocytes but absent from the neoplastic cells. Mod Pathol.
8:622–625. 1995.
|
|
54
|
Arke O, Lipworth L, Tretli S, Linde A,
Engstrand L, Adami HO, Melbye M, Andersen A and Ekbom A:
Epstein-Barr virus and cytomegalovirus in relation to
testicular-cancer risk: a nested case-control study. Int J Cancer.
82:1–5. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Holl K, Surcel HM, Koskela P, Dillner J,
Hallmans G, Wadell G, Kaasila M, Olafsdottir GH, Ogmundsdottir HM,
Pukkala E, Stattino P and Lehtinen M: Maternal Epstein-Barr virus
and cytomegalovirus infections and risk of testicular cancer in the
offspring: a nested case-control study. APMIS. 116:816–822. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santos NBM, Villanova FE, Andrade PM,
Ribalta J, Focchi J, Otsuka AY and Silva ID: Epstein-Barr virus
detection in invasive and pre-invasive lesion of the uterine
cervix. Oncol Rep. 21:403–405. 2009.PubMed/NCBI
|
|
57
|
Kim NR, Lin Z, Kim KR, Cho HY and Kim I:
Epstein-Barr virus and p16INK4A methylation in squamous
cell carcinoma and precancerous lesions of the cervix uteri. J
Korean Med Sci. 20:636–642. 2005.PubMed/NCBI
|
|
58
|
Szostek S, Zawilinska B, Kopec J and
Kosz-Vnenchak M: Herpesviruses as possible cofactors in
HPV-16-related oncogenesis. Acta Biochimica Pol. 56:337–342.
2009.PubMed/NCBI
|
|
59
|
Kuze T, Nakamura N, Hashimoto Y, Abe M and
Wakasa H: Clinicopathological, immunological and genetic studies of
CD30+ anaplastic large cell lymphoma of B-cell type;
association with Epstein-Barr virus in Japanese population. J
Pathol. 180:236–242. 1996.PubMed/NCBI
|
|
60
|
Tazzari PL, de Totero D, Bolognesi A,
Testoni N, Pileri S, Roncella S, Reato G, Stein H, Gobbi M and
Stirpe F: An Epstein-Barr virus-infected lymphoblastoid cell line
(D430B) that grows in SCID-mice with the morphologic features of a
CD30+ anaplastic large cell lymphoma, and is sensitive
to anti-CD30 immunotoxins. Haematologica. 84:988–995.
1999.PubMed/NCBI
|
|
61
|
Agarwal S, Ramanathan U and Naresh KN:
Epstein-Barr virus association and ALK gene expression in
anaplastic large-cell lymphoma. Hum Pathol. 33:146–152. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Noorali S, Pervez S, Yaqoob N, Moatter T,
Nasir MI, Haroon S, Hodges E and Smith JL: Prevalence and
characterization of anaplastic large cell lymphoma and its
association with Epstein-Barr virus in Pakistani patients. Pathol
Res Pract. 200:669–679. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim YC, Yang WI, Lee MG, Kim SN, Cho KH,
Lee SL, Lee MW and Koh JK: Epstein-Barr virus on CD30 anaplastic
large cell lymphoma involving the skin and lymphomatoid papulosis
in South Korea. Int J Dermatol. 45:1312–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sasikala PS, Nirmala K, Sundersingh S,
Mahli U and Rajkumar T: Frequency and distribution of Epstein-Barr
virus infection and its association with p53 expression in a series
of primary nodal non-Hodgkin lymphoma patients from South India.
Int J Lab Hematol. 32:56–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ma L, Katz Y, Sharan KP, Scwarting R and
Kim AS: Epstein-Barr virus positive anaplastic large cell lymphoma:
myth or reality? Int J Clin Exp Pathol. 4:100–110. 2011.PubMed/NCBI
|
|
66
|
Herling M, Rassidakis GZ, Jones D,
Schmitt-Graeff A, Sarris AH and Medeiros LJ: Absence of
Epstein-Barr virus in anaplastic large cell lymphoma: a study of 64
cases classified according to World Health Organization criteria.
Hum Pathol. 35:455–459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kasai K, Kon S, Kikuchi K, Sato Y and
Kameya T: Expression of carbohydrate antigens, p80NPM/ALK,
cytotoxic cell-associated antigens, and Epstein-Barr virus gene
products in anaplastic large cell lymphomas. Pathol Int.
48:171–178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lopategui JR, Gaffey MJ, Chan JK, Fierson
HF, Sun LH, Bellafiore FJ, Chang KL and Weiss LM: Infrequent
association of Epstein-Barr virus with CD30-positive anaplastic
large cell lymphomas from American and Asian patients. Am J Surg
Pathol. 19:42–49. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hellier I, Dereure O, Segondy M, Guillot
B, Baldet P and Guilhou JJ: Unlikely role of Epstein-Barr virus in
the pathogenesis of primary cutaneous CD30+ anaplastic
large cell lymphoma. Eur J Dermatol. 11:203–208. 2001.PubMed/NCBI
|
|
70
|
Park CK and Ko YH: Detection of EBER
nuclear RNA in T-cell lymphoma involving the skin - an in situ
hybridization study. Br J Dermatol. 134:488–493. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Erkek E, Sahin S, Atakan N, Kokagoz T,
Olut A and Goköz A: Examination of mycosis fungoides for the
presence of Epstein-Barr virus and human herpesvirus-6 by
polymerase chain reaction. J Eur Acad Dermatol Venereol.
15:422–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Novelli M, Merlino C, Ponti R, Bergallo M,
Quaglino P, Cambien I, Comessatti A, Sidoti F, Costa C, Corino D,
Cavallo R, Ponzi AN, Fierro MT and Bernengo MG: Epstein-Barr virus
in cutaneous T-cell lymphomas: evaluation of the viral presence and
significance in skin and peripheral blood. J Invest Dermatol.
129:1556–1561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Copur MS, Deshpande A, Mleczko K, Norvell
M, Hernicek GJ, Woodward S, Frankforter S, Mandolfo N, Fu K and
Chan WC: Full clinical recovery after topical treatment of
Epstein-Barr virus associated cutaneous B-cell lymphoma in patient
with mycosis fungoides. Croat Med J. 46:458–462. 2005.PubMed/NCBI
|
|
74
|
Jeziorski E, Senechal B, Molina TJ, Devez
F, Leruez-Ville M, Morand P, Glorion C, Mansuy L, Gaudelus J, Debre
M, Jaubert F, Seigneurin J-M, Thomas C, Joab I, Donadieu J and
Geissmann F: Herpesvirus infection in patients with Langerhans cell
histiocytosis: a case-controlled sero-epidemiological study, and in
situ analysis. PLos One. 3:e32622008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Csire M, Mikala G, Jako J, Masszi T,
Janosi J, Dolgos J, Fule J, Tordai A, Berencsi G and Valyi-Nagy I:
Persistent long-term human herpesvirus 6 (HHV-6) infection in a
patient with Langerhans cell histiocytosis. Pathol Oncol Res.
13:157–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sakata N, Toguchi N, Kimura M, Nakayama M,
Kawa K and Takemura T: Development of Langerhans cell histiocytosis
associated with chronic active Epstein-Barr virus infection.
Pediatr Blood Cancer. 50:924–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Feng WH, Bruce I, Raab-Traub N, Busson P
and Kenney SC: Chemotherapy induces lytic EBV replication and
confers ganciclovir susceptibility to EBV-positive epithelial cell
tumors. Cancer Res. 62:1920–1926. 2002.PubMed/NCBI
|
|
78
|
Huang J, Chen H, Hutt-Fletcher L, Ambinder
F and Hayward SD: Lytic viral replication as a contributor to the
detection of Epstein-Barr virus in breast cancer. J Virol.
77:13267–13274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee CH, Yeh TH, Lai HC, Wu SY, Su IJ,
Takada K and Chang Y: Epstein-Barr virus Zta-induced
immunomodulators from nasopharyngeal carcinoma cells upregulate
interleukin-10 production from monocytes. J Virol. 85:7333–7342.
2011. View Article : Google Scholar
|
|
80
|
Murata T and Tsurumi T: Epigenetic
modification of the Epstein-Barr virus BZLF1 promoter regulates
viral reactivation from latency. Front Genet. 4:532013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shimakage M, Miyata Y, Inoue H, Yutsudo M
and Hakura A: Increased sensitivity of EBNA2-transformed rat
fibroblasts to ionizing radiation. Int J Cancer. 68:612–615. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang ZY, Liu QF, Wang H, Jin J, Wang WH,
Wang SL, Song YW, Liu YP, Fang H, Ren H, Wu RY, Chen B, Zhang XM,
Lu NN, Zhou LQ and Li YX: Clinical implications of plasma
Epstein-Barr virus DNA in early-stage extranodal nasal-type
NK/T-cell lymphoma patients receiving primary radiotherapy. Blood.
10:2003–2010. 2012. View Article : Google Scholar
|
|
83
|
Thomas TO, Agrawal P, Guitart J, Rosen ST,
Rademaker AW, Querfeld C, Heyes JP, Kuzal TM and Mittal BB: Outcome
of patients treated with a single-fraction dose of palliative
radiation for cutaneous T-cell lymphoma. Int J Radiat Oncol Biol
Phys. 85:747–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shimakage M, Kimura M, Yanoma S, Ibe M,
Yokota S, Tsujino G, Kozuka T, Dezawa T, Tamura S, Ohshima A,
Yutsudo M and Hakura A: Expression of latent and
replicative-infection genes of Epstein-Barr virus in macrophages.
Arch Virol. 144:157–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Masy E, Adriaenssens E, Montepellier C,
Crepieux P, Mougel A, Quatannens B, Goormachtigh G, Faumont N,
Meggetto F, Auriault C, Groux H and Coll J: Human monocytic cell
lines transformed in vitro by Epstein-Barr virus type II latency
and LMP-1-dependent proliferation. J Virol. 76:6460–6472. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Salek-Ardakani S, Lyons SA and Arrand JR:
Epstein-Barr virus promotes human monocyte survival and mutation
through a paracrine induction of IFN-alpha. J Immunol. 173:321–331.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tugizov S, Herrara R, Veluppillai P,
Greenspan D and Palefsky JM: Epstein-Barr virus (EBV)-infected
monocytes facilitate dissemination of EBV within the oral mucosal
epithelium. J Virol. 81:5484–5496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li H, Ikuta K, Sixbey JW and Tibbetts SA:
A replication-defective gammaherpesvirus efficiency establishes
long-term latency in macrophages but not in B cells in vivo. J
Virol. 82:8500–8508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hwang KK, Chen X, Kozink DM, Gustilo M,
Marshall DJ, Whitesides JF, Liao XH, Catera R, Chu CC, Yan XJ,
Luftig MA, Haynes GF and Chiorazzi N: Enhanced outgrowth of
EBV-transformed chronic lymphocytic leukemia B cells mediated by
coculture with macrophage feeder cells. Blood. 119:e35–e44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Andereesen R, Grugger W, Lohr GW and Bross
KJ: Human macrophages can express the Hodgkin’s cell-associated
antigen Ki-1 (CD30). Am J Pathol. 134:187–192. 1989.
|
|
91
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mantovani A: B cells and macrophages in
cancer: yin and yang. Nat Med. 17:285–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hamada I, Kato M, Yamasaki T, Iwabuchi K,
Watanabe T, Yamada T, Itoyama S, Ito H and Okada K: Clinical
effects of tumor-associated macrophages and dendritic cells on
renal cell carcinoma. Anticancer Res. 22:4281–4284. 2002.PubMed/NCBI
|
|
96
|
Komohara Y, Hasita H, Ohnishi K, Fujiwara
Y, Suzu S, Eto M and Takeya M: Macrophage infiltration and its
prognostic relevance in clear cell renal cell carcinoma. Cancer
Sci. 102:1424–1431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liou P, Bader L, Wang A, Yamashiro D and
Kandel J: Correlation of tumor-associated macrophages and
clinicopathological factors in Wilms tumor. Vasc Cell. 5:52013.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shimakage M and Sakamoto H: Macrophage
involvement in Epstein-Barr virus-related tumors. Exp Ther Med.
1:285–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Thorley-Lawson DA: EBV the prototypical
human tumor virus - just how bad is it? J Allergy Clin Immunol.
116:251–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Vieira P, de Waal-Malefyt R, Dang MN,
Johnson KE, Kastelein R, Fiorentino DF, DeVries JE, Roncarolo MG,
Mosmann TR and Moore KW: Isolation and expression of human cytokine
synthesis inhibitory factor cDNA clones: homology to Epstein-Barr
virus open reading frame BCRF1. Proc Natl Acad Sci USA.
88:1172–1176. 1991. View Article : Google Scholar
|
|
101
|
Miyazakai I, Cheung RK and Dosch H-M:
Viral interleukin 10 is critical for the induction of B cell growth
transformation by Epstein-Barr virus. J Exp Med. 178:439–447. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hsu DH, de Waal Malefyt R, Fierentino DF,
Dang MN, Vieira P, DeVries V, Spits H, Mosmann TR and Moore KW:
Expression of interkeukin-10 activity by Epstein-Barr virus protein
BCRF1. Science. 250:830–832. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jochum S, Moosmann A, Lang S,
Hammerschmidt W and Zeidler R: The EBV immunoevasins vIL-10 and
BNLF2a protect newly infected B cells from immune recognition and
elimination. PLoS Pathog. 5:e10027042012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
de Waal Malefyt R, Haanen J, Spits H,
Roncarolo MG, te Velde A, Figdor C, Johnson K, Yssel H and de Vries
JE: Interleukin 10 (IL-10) and viral IL-10 strongly reduce
antigen-specific human T cell proliferation by diminishing the
antigen-presenting capacity of monocytes via downregulation of
class II major histocompatibility complex expression. J Exp Med.
174:915–924. 1991.
|
|
105
|
Sica A, Allavena P and Mantovani A: Cancer
related inflammation: The macrophage connection. Cancer Lett.
264:204–215. 2008. View Article : Google Scholar
|
|
106
|
Suzuki T, Tahara H, Narula S, Moore KW,
Robbins P and Lotze MT: Viral interleukin 10 (IL-10), the human
herpesvirus 4 cellular IL-10 homologue, induces local anergy to
allogeneic and syngeneic tumors. J Exp Med. 182:477–486. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kanai K, Satoh Y, Yamanaka H, Kawaguchi A,
Horie K, Hoshikawa Y, Sata T and Sairenji T: The vIL-10 gene of the
Epstein-Barr virus (EBV) is conserved in a stable manner except for
a few point mutations in various EBV isolates. Virus Genes.
35:563–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chao Y, Jing Y, Jia Y, Wang Y, Zhao C and
Luo B: Conservation and mutation of viral interleukin-10 gene in
gastric carcinomas and nasopharyngeal carcinomas. J Med Virol.
83:644–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ,
Kim WJ and Kim IY: BMP-6 in renal cell carcinoma promotes tumor
proliferation through IL-10-dependent M2 polarization of
tumor-associated macrophages. Cancer Res. 73:3604–3614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ghosh SK, Perrine S and Faller DV:
Advances in virus-directed therapeutics against Epstein-Barr
virus-associated malignancies. Adv Virol. Mar 5–2012.(Epub ahead of
print). View Article : Google Scholar
|
|
111
|
Feng WH, Israel B, Raab-Traub N, Busson P
and Kenney SC: Chemotherapy induces lytic EBV replication and
confers ganciclovir susceptibility to EBV-positive epithelial cell
tumors. Cancer Res. 62:1920–1926. 2002.PubMed/NCBI
|
|
112
|
Fu DX, Tanhehco Y, Chen J, Foss CA, Fox
JJ, Chong JM, Hobbs RF, Fukayama M, Sgouros G, Kowalski J, Pomper
MG and Ambinder RF: Bortezomib-induced enzyme-targeted radiation
therapy in herpesvirus-associated tumors. Nat Med. 14:1118–1122.
2008. View Article : Google Scholar : PubMed/NCBI
|