Introduction
microRNAs are an abundant class of short endogenous
non-coding RNAs that act as important post-transcriptional
regulators of gene expression involved in crucial biological
processes, including development, differentiation, proliferation
and apoptosis. microRNAs inhibit their target gene expression
through imperfect pairing with their target messenger of
protein-coding genes (1–4). Growing evidence suggests that miRNAs
are aberrantly expressed in many human cancers, which may act as
oncogenes or tumor suppressors in human cancer including lung
carcinoma (5–7).
miR-7 has been identified to be dysregulated in some
human cancers and is functionally associated with cancer cell
proliferation, differentiation and apoptosis (8,9).
Recent studies also demonstrated miR-7 to be a potential tumor
suppressor in several human cancers including brain, colon and
liver (8–10). However, the biological function of
miR-7 in lung cancer and its molecular mechanism remain to be
further elucidated. In addition, to the best of our knowledge,
little information is available on the miR-7 expression profiles
and function in the well-defined animal model specimens including
Lewis lung cancer (3LL).
Therefore, in the present study, we described and
characterized the expression and function of miR-7 in the 3LL cell
line originating from mouse lung cancer compared with normal lung
tissues. We showed that the expression of mature miR-7 is
downregulated in 3LL cells compared to normal lung tissues.
Restoration of its expression triggered a considerable decrease in
cell proliferation and induced apoptosis in 3LL cells in
vitro. This type of miR-7 restoration also significantly
reduced tumorigenicity in vivo. With regard to its
mechanism, miR-7 directly targeted the oncogene epidermal growth
factor receptor (EGFR) and murine leukemia viral oncogene
homologue-1 (RAF-1) and downregulated their expression.
Collectively, our results suggest miR-7 to be a tumor suppressor
gene that suppresses the growth of 3LL cells.
Materials and methods
Cell lines and cell culture
The 3LL cell line (murine Lewis lung cancer cell
line) was provided by the Institute of Biochemistry and Cell
Biology of the Chinese Academy of Science, China. 3LL cells were
cultured at 37°C under 5% CO2 in a complete RPMI-1640
(31800089) medium containing 10% heat-inactivated fetal bovine
serum (10100139) and supplemented with 2mM glutamine (21051040)
(all from Gibco, Grand Island, NY, USA), 100 IU/ml penicillin and
100 μg/ml streptomycin sulfate (Shanghai No. 4 Pharmaceutical
Factory, China).
RNA extraction
Total RNA of cultured cells and normal lung tissues
from mice were extracted with TRIzol reagent (15596-026;
Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
protocol. RNAs were quantitated and then stored at −80°C prior to
RT-PCR analysis.
Quantitative RT-PCR (qRT-PCR) for
miRNA
For mature miRNA expression analysis, ~10 ng of
total RNA was converted to cDNA using the PrimerScript RT reagent
kit (DRR037A; Takara, Dalian, China) with miR-7-specific and U6
primers (RiboBio, Guangzhou, China). After reverse transcription,
qRT-PCR was performed using FastStart Universal SYBR-Green Master
kit (04913850001; Roche, Mannheim, Germany) with Bulge-Loop™ miR-7
qRT-PCR primers (RiboBio) on the ABI 7500 Thermocycler (Applied
Biosystems, Foster City, CA, USA) according to the manufacturer’s
protocol. U6 gene was used as a normalization control for all
samples.
miRNA mimics and transfection
The mature miR-7 duplex mimics (miR-7) and negative
control oligonucleotide duplex mimics (miR-NC) were designed and
provided by RiboBio. Confluent 3LL cells (30–50%) were transfected
with miRNAs by Lipofectamine 2000 (11668-019; Invitrogen) according
to the manufacturer’s protocol. Total RNA was extracted 24 h after
transfection, and total cell proteins were extracted 48 or 72 h
after transfection.
Cell proliferation assay
Cell proliferation assay was determined by Cell
Counting Kit-8 assay (CK04–11; Dojindo, Japan), a redox assay
similar to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) according to the manufacturer’s protocol. Cell
proliferation assay was carried out in hexakis.
Apoptotic morphology by DAPI
staining
Cells were stained with 4,6-diamidino-2-phenylindole
(DAPI) (40728ES10; Yeasen, Shanghai, China) and cells with
fragmented or condensed nuclei were defined as apoptotic cells. At
least three visual fields were observed under a fluorescence
microscope for each sample.
qRT-PCR for mRNA expression
Synthesis of cDNA was performed on 1 μg of total RNA
per sample with the PrimerScript RT reagent kit according to the
manufacturer’s manual. qRT-PCR was performed in triplicate for each
sample by FastStart Universal SYBR-Green Master kit (04913850001;
Roche) according to the manufacturer’s instructions.
Oligonucleotides were designed by the PrimerExpress software 2.0
and synthesized by Sangon Biotech (Shanghai, China). GAPDH was used
as a housekeeping gene for normalization. The sequences of primers
in this section were: (EGFR forward, 5′-CCAACTATGGGACAAACAGAA-3′
and reverse, 5′-ATCGCACAGCACCAATCA-3′; RAF-1 forward,
5′-AGTCACGCTGGAGTGGTTCT-3′ and reverse, 5′-GTCTCGGTTGTTGATGTGGG-3′;
GAPDH forward, 5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GCATGGACTGTGGTCATGAG-3′.
Western blot analysis
Proteins extracted from cells were immunoblotted
with different antibodies following a published protocol (11,12).
The primary antibodies used were EGFR and RAF-1 (1:3,000
dilutions), GAPDH (1:10,000 dilutions) (Cell Signaling, Danvers,
MA, USA).
Tumorigenicity assay in mice
C57/BL/6 mice (4–6 weeks old) were purchased from
Shanghai Experimental Center, Chinese Academy of Science. All
animal experiments were carried out in compliance with the Guide
for the Care and Use of Laboratory Animals published by the US
National Institutes of Health (NIH publication no. 85-23, revised
1996) and the Guidelines of Anhui Medical Laboratory Animal Care
and Use Committee. miR-7- and miR-NC-transfected 3LL tumor cells
(5×104) per mouse were injected subcutaneously (s.c.)
into either side of the posterior flank of the same C57/BL/6 female
mouse. After 7 days, the tumor volume was measured with a vernier
caliper at weekly intervals. The tumor volume = W2×L/2,
where W is the short diameter and L is the long diameter.
Statistical analysis
Data are expressed as the means + SEM of three
independent experiments. For all statistical tests, PRISM 5.0
(GraphPad Software Inc., San Diego, CA, USA) was used. miR-7 data
between two groups was calculated using t-test analysis. ANOVA
analysis was used for the multigroup comparison while the Student’s
t-test was applied for the comparison between two groups. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
miR-7 is significantly downregulated in
3LL cells
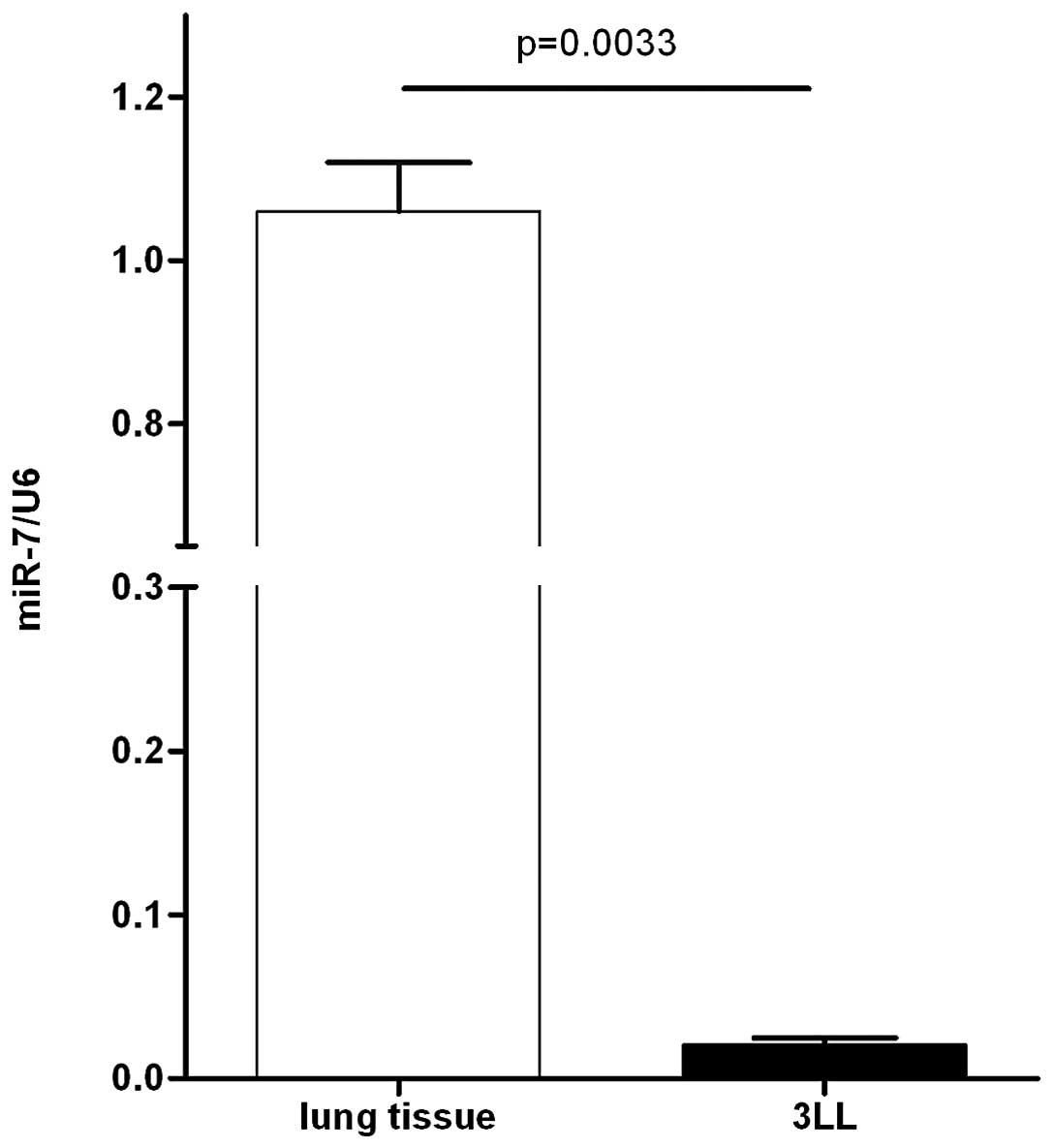
To assess the biological role of miR-7 in the 3LL
model, we first examined the expression of miR-7 by quantitative
real-time PCR in 3LL cells and mouse lung normal tissues. As a
result, miR-7 showed significantly lower expression in 3LL cells
than in normal lung tissues (Fig.
1), indicating that the downregulation of miR-7 may be involved
in mouse 3LL carcinogenesis.
Restoration of miR-7 decreases the
proliferation and induces apoptosis of 3LL cells in vitro
Since miR-7 was significantly downregulated in 3LL
cells compared with normal lung tissues, miR-7 may serve as a tumor
suppressor. Therefore, we next investigated the effect of miR-7 on
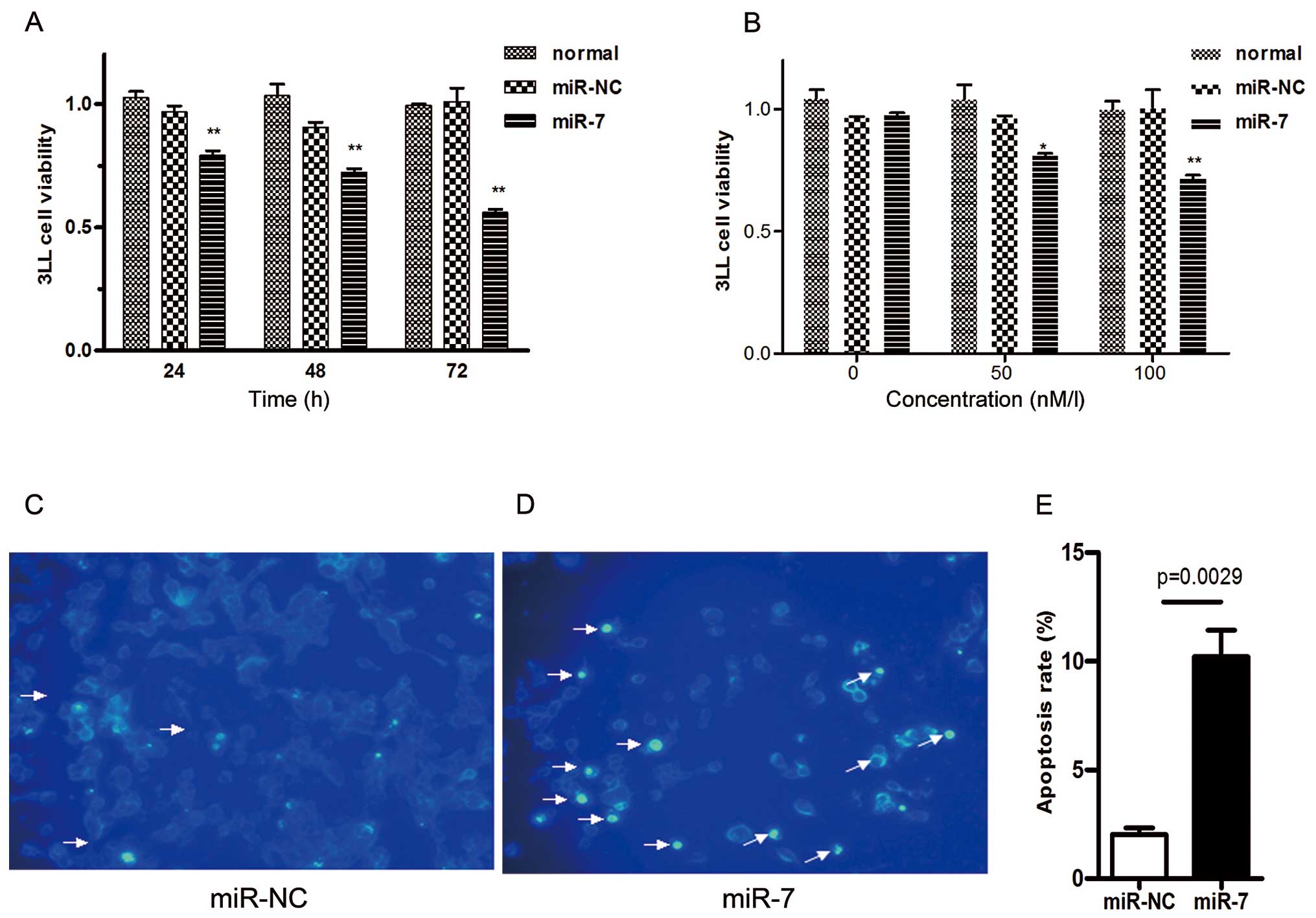
phenotypes of 3LL cells. 3LL cells were transiently transfected
with mature miR-7 mimic (miR-7). Control cells were also
transfected with negative control mimic (miR-NC) without
specifically targeting any mouse gene products. After 48 h, cells
transfected with miR-7 grew more slowly than the negative control
and normal group in CCK-8 proliferation assay. 3LL cells also
exhibited reduced cell proliferation in the presence of miR-7 in a
time- (Fig. 2A) and dose-dependent
manner (Fig. 2B).
To further explore whether the reduced cancer cell
number was due to cell apoptosis, we performed DAPI staining assay.
3LL cells were transfected with miR-7 mimic (50 nM) or negative
control mimic (50 nM) in low-serum (3%) RPMI-1640 medium for 48 h
and subsequently analyzed by DAPI staining assay. We observed
considerably more apoptotic cells in miR-7-transfected 3LL cells
(Fig. 2D) than in negative control
cells (Fig. 2C). The percentage of
cells with apoptotic nuclei significantly increased in
miR-7-transfected 3LL cells compared to the control cells in DAPI
staining (Fig. 2E). Taken together,
these results indicate that the inhibition of cell proliferation by
miR-7 is associated with the increased 3LL cell apoptosis.
miR-7 inhibits tumor growth of 3LL cells
in vivo
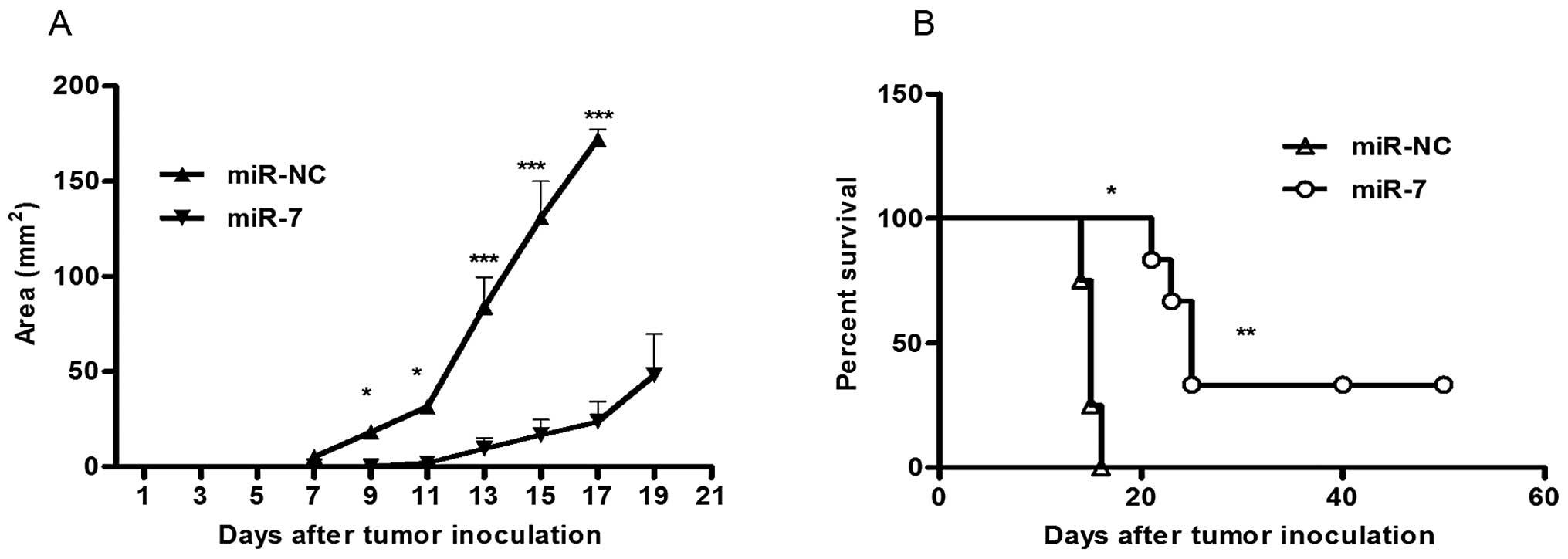
Considering the inhibition of 3LL cell proliferation
by miR-7 in vitro, we further evaluated the effect of miR-7
on tumor growth of 3LL cells in vivo. 3LL cells were
transfected either with miR-7 or miR-NC. After 6 h, they were
implanted subcutaneously into either posterior flank of the same
C57BL/6J mice (5×104 cells/injection site). 3LL cells
transfected with miR-NC formed tumors 7 days after implantation.
However, 3LL cells transfected with miR-7 partially failed to grow
7 days after injection. Furthermore, cells transfected with miR-7
exhibited a marked reduction in tumor size (Fig. 3A) and an increase in survival rate
(Fig. 3B) compared to the control
group. These data indicate that elevated miR-7 level in 3LL cells
markedly reduce their ability to form tumors.
miR-7 suppresses its target gene
expression of EGFR and RAF-1 in 3LL cells
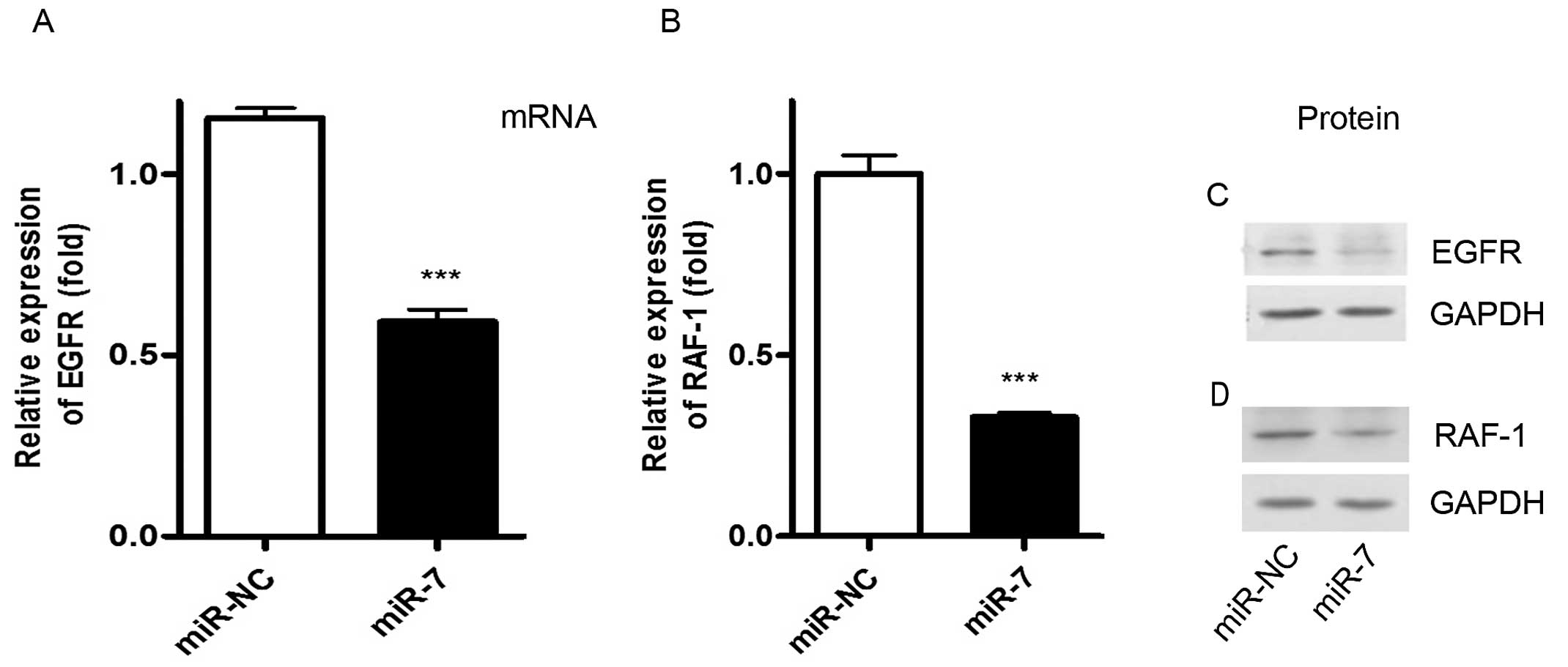
We next investigated the molecular mechanism through
which miR-7 inhibits cancer cell proliferation and induces cell
apoptosis. Using bioinformatics analysis, we found the potential
biding sites of EGFR and RAF-1 (data not shown). To further assess
whether miR-7 had a functional role in regulating these two targets
(EGFR and RAF-1) in 3LL, 3LL cells were transfected with miR-7
mimic for 24 or 72 h and we then analyzed the EGFR and RAF-1
expression by real-time RT-PCR and western blot analysis. As a
result, overexpression of miR-7 markedly reduced the expression of
EGFR and RAF-1 at mRNA (Fig. 4A and
B) and protein level (Fig. 4C and
D) compared with the control groups. Taken together, these
results suggest that miR-7 decreases expression of EGFR and RAF-1
through its direct negative regulation in 3LL cells originating
from mice.
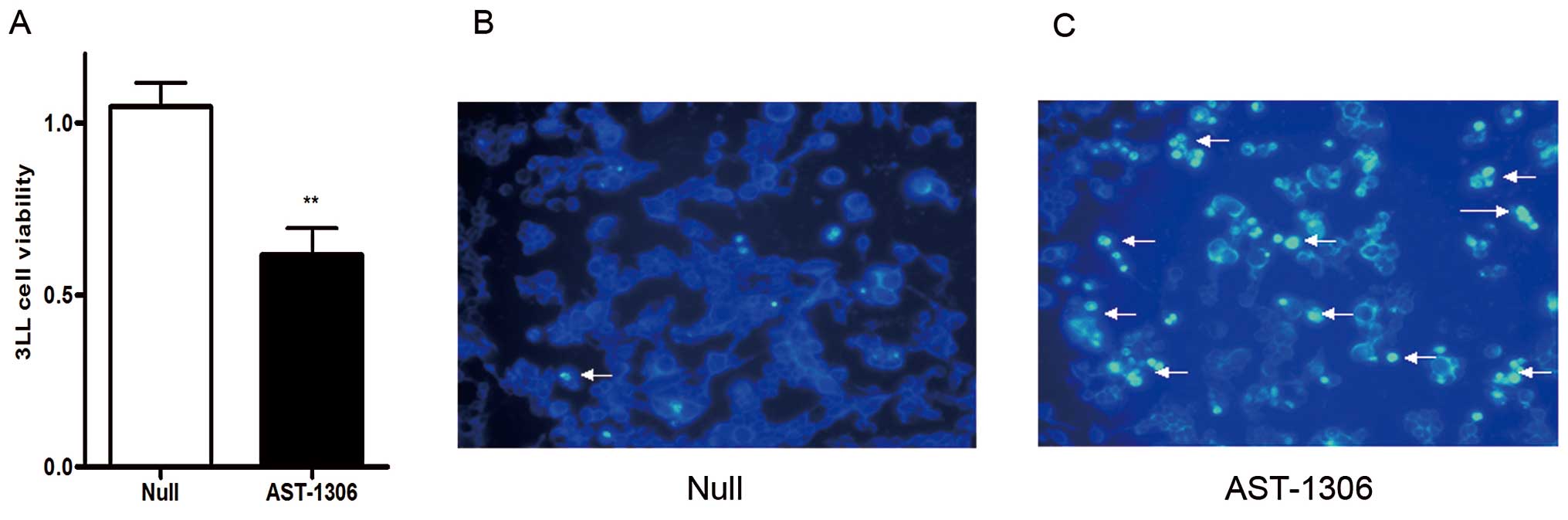
Inhibition of EGFR suppresses the
proliferation and induces apoptosis of 3LL cells in vitro
The above data showed that miR-7 downregulated the
expression of EGFR and its downstream RAF-1. We next examined
whether such type of inhibition is involved in the effect of miR-7
on 3LL cells. As shown in Fig. 5
blocking EGFR with its inhibitor AST-1306 (Selleck, USA) (3 nM)
significantly decreased the cell viability of 3LL cells in CCK-8
assay (Fig. 5A), and also induced
3LL cell apoptosis in DAPI staining assay (Fig. 5B). These results were consistent
with the effect of miR-7 on 3LL cells (Fig. 2A, B and D), providing further
evidence that miR-7 mediates cell proliferation suppression and
induces apoptosis by downregulation of EGFR in 3LL cells.
Discussion
Increasing evidence suggests that miRNAs play a key
regulatory role in cancer-related biological effectors (13,14),
and their abnormal expression is closely related to various
cancers, including lung cancer (1,15,16).
miRNAs are directly involved in cancer initiation and progression
by directly regulating the expression of important cancer-related
genes, thereby functioning as tumor suppressors or oncogenes
(17,18). For example, miR-21, which is highly
expressed in pancreatic and breast cancer, promotes tumor cell
growth by inhibiting the tumor suppressor PTEN (19,20).
On the contrary, miR-7, which has low expression in human brain,
liver, breast and lung cancer, often acts as a tumor suppressor
(9,21–23).
To date, however, in a mouse cancer model, the expression and
function of miR-7 has not been fully elucidated. In this study, the
expression of miR-7 in 3LL cells from murine Lewis lung cancer was
significantly reduced. 3LL cell proliferation was markedly
suppressed and cell apoptosis was also induced in vitro
following administration of miR-7. Markedly, miR-7 could also
decrease the tumorigenicity in C57BL/6J in vivo. Therefore,
our data suggested miR-7 to be a tumor suppressor in 3LL cell
lines, which is consistent with that of the human A549 cell lines
in our previous study (23,24). However, another study also showed
that miR-7 promoted, rather than inhibited, cell growth and tumor
formation in human lung cancer line CL1–5 (25). Therefore, the role of miR-7 may vary
in different cancer types according to the biological background of
the tumor cells themselves and the main target genes involved in
this process (13,14).
Using bioinformatics analysis, we also found the
potential target genes including EGFR and RAF-1 in murine species
due to the high conservatism of microRNA. EGFR is a trans-membrane
glycoprotein that plays important roles in cancer growth, including
non-small cell lung cancer (NSCLC). Its ligands, such as epidermal
growth factor (EGF) or transforming growth factor-α (TGF-α), cause
EGFR to undergo a conformational change leading to
autophosphorylation of EGFR and activation of the EGFR growth
factor pathway (26). The elevated
expression and activity of EGFR in cancer increase cell
proliferation and angiogenesis and inhibit programmed cell death.
EGFR is expressed in 40–80% of NSCLC and is associated with
development, progression and treatment resistance (27). Previous studies demonstrated that
miR-7 downregulates EGFR expression in a range of cancer cell types
via its specific interaction with the EGFR mRNA 3′-untranslated
region (3′-UTR) (21). These
results are further supported by the present finding that miR-7
downregulated EGFR expression and reduced cell growth in
vitro and in vivo (28,29).
Notably, a previous study indicated that EGFR could also upregulate
miR-7 expression (28). Therefore,
the interaction of miR-7 and EGFR appears as a feedback balance,
and the outcome of the balance among the target gene network
determine the initiation and development of tumor.
The serine/threonine kinase RAF-1 is a downstream
effecter of EGFR signaling. It is commonly activated by mutations
and is involved in the regulation of tumor cell proliferation,
survival and migration. RAF-1 is overexpressed in human cancers
including lung carcinoma, therefore it is emerging as a promising
target for cancer therapy (30–33).
In the present study, we showed that the potential target RAF-1 is
significantly downregulated by enforcement of miR-7 expressing in
3LL cell lines. These results are similar to a study by Webster
et al (33). Thus, miR-7
decreases the expression of both EGFR and its downstream target
RAF-1 and then inhibits 3LL cell growth. Furthermore, blocking EGFR
exhibited similar effects with miR-7 in 3LL cells. Therefore,
identification of both EGFR and its downstream target RAF-1 as the
miR-7 target gene maybe explain, at least in part, the molecular
mechanism of the tumor suppressor miR-7 in murine 3LL.
In conclusion, through analysis of the expression
and function of miR-7 in a mouse cancer model, we found for the
first time that miR-7 has a tumor-suppressive function through
inhibition of cell proliferation and induction of apoptosis in
mouse 3LL cell lines. We also identified both EGFR and its
downstream target RAF-1 as two targets possibly involved in
miR-7-mediated growth suppression and apoptosis induction of 3LL
cells. These findings may provide a basic rationale for the use of
miR-7 in the treatment of lung caner.
Acknowledgements
The authors thank Dr Ronghua Liu (Department of
Immunology, Fudan University, Shanghai, China) for their technical
help and scientific discussions. This study was supported by the
National Science Foundation of China (81272259).
References
|
1
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011.PubMed/NCBI
|
|
2
|
Ryan BM, Robles AI and Harris CC: Genetic
variation in microRNA networks: the implications for cancer
research. Nat Rev Cancer. 10:389–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aguda BD, Kim Y, Piper-Hunter MG, Friedman
A and Marsh CB: MicroRNA regulation of a cancer network:
consequences of the feedback loops involving miR-17–92, E2F, and
Myc. Proc Natl Acad Sci USA. 105:19678–19683. 2008.PubMed/NCBI
|
|
4
|
Xu TP, Zhu CH, Zhang J, et al:
MicroRNA-155 expression has prognostic value in patients with
non-small cell lung cancer and digestive system carcinomas. Asian
Pac J Cancer Prev. 14:7085–7090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang S, Li Y, Gao J, et al: MicroRNA-34
suppresses breast cancer invasion and metastasis by directly
targeting Fra-1. Oncogene. 32:4294–4303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hulf T, Sibbritt T, Wiklund ED, et al:
Epigenetic-induced repression of microRNA-205 is associated with
MED1 activation and a poorer prognosis in localized prostate
cancer. Oncogene. 32:2891–2899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Chen X, Lian H, et al:
MicroRNA-503 acts as a tumor suppressor in glioblastoma for
multiple antitumor effects by targeting IGF-1R. Oncol Rep.
31:1445–1452. 2014.PubMed/NCBI
|
|
8
|
Zhang N, Li X, Wu CW, et al: microRNA-7 is
a novel inhibitor of YY1 contributing to colorectal tumorigenesis.
Oncogene. 32:5078–5088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saydam O, Senol O, Würdinger T, et al:
miRNA-7 attenuation in Schwannoma tumors stimulates growth by
upregulating three oncogenic signaling pathways. Cancer Res.
71:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong SD, Yu K, Liu XH, et al:
Ribosome-inactivating proteins isolated from dietary bitter melon
induce apoptosis and inhibit histone deacetylase-1 selectively in
premalignant and malignant prostate cancer cells. Int J Cancer.
125:774–782. 2009. View Article : Google Scholar
|
|
12
|
Zheng Y, Xiong S, Jiang P, et al:
Glucocorticoids inhibit lipopoly-saccharide-mediated inflammatory
response by downregulating microRNA-155: a novel anti-inflammation
mechanism. Free Radic Biol Med. 52:1307–1317. 2012. View Article : Google Scholar
|
|
13
|
Qiu ZX, Wang L, Han J, et al: Prognostic
impact of Raf-1 and p-Raf-1 expressions for poor survival rate in
non-small cell lung cancer. Cancer Sci. 103:1774–1779. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukherjee R, Bartlett JM, Krishna NS,
Underwood MA and Edwards J: Raf-1 expression may influence
progression to androgen insensitive prostate cancer. Prostate.
64:101–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aurora AB, Mahmoud AI, Luo X, et al:
MicroRNA-214 protects the mouse heart from ischemic injury by
controlling Ca2+ overload and cell death. J Clin Invest.
122:1222–1232. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khoshnaw SM, Green AR, Powe DG and Ellis
IO: MicroRNA involvement in the pathogenesis and management of
breast cancer. J Clin Pathol. 62:422–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andorfer CA, Necela BM, Thompson EA and
Perez EA: MicroRNA signatures: clinical biomarkers for the
diagnosis and treatment of breast cancer. Trends Mol Med.
17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong C, Yao Y, Wang Y, et al:
Up-regulation of miR-21 mediates resistance to trastuzumab therapy
for breast cancer. J Biol Chem. 286:19127–19137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan LW, Wang FF and Cho WC: Genomic
sequence analysis of EGFR regulation by microRNAs in lung cancer.
Curr Top Med Chem. 12:920–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kefas B, Godlewski J, Comeau L, et al:
microRNA-7 inhibits the epidermal growth factor receptor and the
Akt pathway and is down-regulated in glioblastoma. Cancer Res.
68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reddy SD, Ohshiro K, Rayala SK and Kumar
R: MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase
1 and regulates its functions. Cancer Res. 68:8195–8200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong S, Zheng Y, Jiang P, et al:
PA28gamma emerges as a novel functional target of tumour suppressor
microRNA-7 in non-small-cell lung cancer. Br J Cancer. 110:353–362.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chou YT, Lin HH, Lien YC, et al: EGFR
promotes lung tumorigenesis by activating miR-7 through a
Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor
ERF. Cancer Res. 70:8822–8831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Navolanic PM, Steelman LS and McCubrey JA:
EGFR family signaling and its association with breast cancer
development and resistance to chemotherapy (Review). Int J Oncol.
22:237–252. 2003.PubMed/NCBI
|
|
27
|
Sonnweber B, Dlaska M, Skvortsov S,
Dirnhofer S, Schmid T and Hilbe W: High predictive value of
epidermal growth factor receptor phosphorylation but not of
EGFRvIII mutation in resected stage I non-small cell lung cancer
(NSCLC). J Clin Pathol. 59:255–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukohara T, Kudoh S, Yamauchi S, et al:
Expression of epidermal growth factor receptor (EGFR) and
downstream-activated peptides in surgically excised non-small-cell
lung cancer (NSCLC). Lung Cancer. 41:123–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bozzetti C, Tiseo M, Lagrasta C, et al:
Comparison between epidermal growth factor receptor (EGFR) gene
expression in primary non-small cell lung cancer (NSCLC) and in
fine-needle aspirates from distant metastatic sites. J Thorac
Oncol. 3:18–22. 2008. View Article : Google Scholar
|
|
30
|
Lee KM, Choi EJ and Kim IA: microRNA-7
increases radiosensitivity of human cancer cells with activated
EGFR-associated signaling. Radiother Oncol. 101:171–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalinowski FC, Giles KM, Candy PA, et al:
Regulation of epidermal growth factor receptor signaling and
erlotinib sensitivity in head and neck cancer cells by miR-7. PLoS
One. 7:e470672012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duex JE, Comeau L, Sorkin A, Purow B and
Kefas B: Usp18 regulates epidermal growth factor (EGF) receptor
expression and cancer cell survival via microRNA-7. J Biol Chem.
286:25377–25386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Webster RJ, Giles KM, Price KJ, Zhang PM,
Mattick JS and Leedman PJ: Regulation of epidermal growth factor
receptor signaling in human cancer cells by microRNA-7. J Biol
Chem. 284:5731–5741. 2009. View Article : Google Scholar : PubMed/NCBI
|