Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin’s lymphoma (NHL) worldwide (1). DLBCL represents a heterogeneous group
of tumors with a high variance of genetic abnormalities, clinical
features, response to treatment and prognosis (2). Combinatorial cyclophosphamide,
doxorubicin, vincristine and prednisone (CHOP) chemotherapy has
been a systemic therapy for DLBCL with a cure rate of 40–50%
(3) and is widely used in China.
Although a subset of DLBCL patients is cured with CHOP regimens,
many succumb to chemorefractory disease (4). Resistance to the CHOP
anthracycline-based regimen continues to be a serious challenge in
the cure of DLBCL (3). The
molecular basis for development of the multi-drug chemoresistance
in DLBCL remains unclear.
Annexins are predominantly cytosolic soluble
proteins that can reversibly bind to negatively charged
phospholipids in a Ca2+-mediated manner. Twelve Annexins
common to vertebrates are known as Annexins A1–A11 and A13
(5,6). Annexin A5, also known as placental
anticoagulant protein I, thromboplastin inhibitor V, endonexin II,
calphobindin I and lipocortin V, was first described functionally
as a vascular anticoagulant in 1985 (7,8).
Annexin A5 deregulations were observed as causative phenomena in a
range of physiological and pathological processes. Annexin A5
reportedly promotes tumorigenesis and progression of a variety of
cancers, including hepatocarcinoma, breast, colorectal, pancreatic,
bladder and prostate cancer (9).
Nevertheless, upregulation of Annexin A5 has also been found to be
negatively correlated with the malignancy of thyroid cancer
(9). Previous studies suggested
that Annexin A5 enhances chemoresistance in gastric cancer and
nasopharyngeal carcinoma (10,11).
However, a recent study showed that Annexin A5 was upregulated in
CHOP-sensitive DLBCL tissues, suggesting that Annexin A5 may
inhibit chemoresistance in DLBCL (12).
Our pilot studies suggested that Annexin A5 could
increase chemosensitivity in DLBCL cells. In the present study, we
explored the effects of Annexin A5 on DLBCL cell invasion and
chemoresistance to CHOP.
Materials and methods
Cells lines and reagents
Toledo (CRL-2631) and Pfeiffer (CRL-2632) human
DLBCL cell lines were purchased from the American Type Culture
Collection (Manassas, VA, USA). Annexin A5 shRNA lentiviral
particles (sc-29686-V), control shRNA lentiviral particles-A
(sc-108080), selective phosphatidylinositol 3-kinase (PI3K)
inhibitor BKM120 (sc-364437A), anti-Annexin A5 antibody (sc-74438),
anti-matrix metalloproteinase-9 (MMP-9) antibody (sc-21733), and
anti-Akt (5C10) (sc-81434) and anti-P-Akt (ser473) (sc-101629)
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). The SensoLyte® 520 MMP-9 Assay kit
(71155) was purchased from AnaSpec (Fremont, CA, USA). The QCM
ECMatrix 24-well (8 μM) Fluorimetric Cell Invasion assay kit
(ECM554) was purchased from Chemicon (Millipore, Billerica, MA,
USA). The TiterTACS in situ apoptosis detection kit
(4822-96-K) was purchased from R&D Systems (Minneapolis, MN,
USA). The PI3K Activity ELISA kit (K-1000s) was purchased from
Echelon Biosciences (Salt Lake City, UT, USA). Superfect™
transfection reagent was purchased from Qiagen (Valencia, CA, USA).
The Annexin A5 expression vector (RC205619), in which the
full-length human Annexin A5 cDNA was subcloned into the
pCMV6-entry vector, was purchased from Origene (Beijing, China).
Puromycin, G418, cyclophosphoramide, doxorubicin, vincristine,
prednisone and all chemicals of reagent grade were purchased from
Sigma (St. Louis, MO, USA). Cyclophosphoramide and doxorubicin were
dissolved in Millipore-purified water, vincristine was dissolved in
methanol, and prednisone was dissolved in chloroform/ethanol (1:1).
All CHOP reagents were stored at −80°C.
Transfection and lentiviral
transduction
The Annexin A5 expression vector was transfected
into cells using Superfect™ transfection reagent (Qiagen) according
to the manufacturer’s instructions. Pools of stable transductants
were generated via selection with G418 (600 μg/ml) following the
manufacturer’s protocol. The Annexin A5 shRNA lentiviral particles
contain expression constructs encoding target-specific 19–25 nt
(plus hairpin) shRNA designed to specifically knock down Annexin
A5 gene expression. The control shRNA lentiviral particles
contain a scrambled shRNA sequence that will not lead to specific
degradation of any cellular mRNA, and was used as a negative
control for Annexin A5 shRNA lentiviral particles. Lentiviral
transduction was performed in Toledo and Pfeiffer cells. Pools of
stable transductants were generated via selection with puromycin (4
μg/ml) according to the manufacturer’s protocol (Santa Cruz
Biotechnology).
Cell invasion assay
In vitro cell invasion assays were performed
with the QCM ECMatrix® 24-well (8 μM) Fluorimetric Cell
Invasion assay kit (Chemicon; Millipore) according to the
manufacturer’s instructions (13,14).
The kit used an insert polycarbonate membrane with an 8-μM pore
size. The insert in the invasion kit was coated with a thin layer
of ECMatrix. Cell invasion was determined by fluorescence. Each
experiment was repeated three times in duplicates.
Western blot analysis
Cells dissolved in 250 μl of 2× SDS loading buffer
(62.5 mm TrisHCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol
blue, 5% 2-mercaptoethanol), and incubated at 95°C for 10 min.
Equal amounts of proteins for each sample were separated by 10%
SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride
microporous membrane (Millipore). Membranes were incubated for 1 h
with a 1/500 dilution of anti-MMP-9, anti-P-Akt (ser473) or
anti-Akt antibody, and then washed and revealed using secondary
antibodies with horseradish peroxidase conjugate (1/4000, 1 h).
Peroxidase was revealed with a GE Healthcare ECL kit (Shanghai,
China). Three independent experiments were performed for each
western blot analysis.
MMP-9 activity assay
MMP-9 activity was measured with the SensoLyte 520
MMP-9 assay kit (AnaSpec) according to the manufacturer’s
instructions (15,16). The supernatants were collected and
then incubated with 4-aminophenylmercuric acetate (AMPA) and MMP-9
substrate. The fluorescence intensity at Ex/Em wave lengths of
490/520 nm were used as a measure of MMP-9 activity. Each
experiment was repeated three times in duplicates.
Cell apoptosis assay
Cells were cultured at 9×104 cells/well
in 96-well tissue culture plates and incubated at 37°C for 24 or 48
h under CHOP treatment. The composition of CHOP consisted of
cyclophosphoramide, doxorubicin, vincristine and prednisone at the
clinical ratio of 80/5.5/0.16/11.1 (3), with the combined CHOP concentration
set at 80 ng/ml. Cell apoptosis was measured at 24 and 48 h with a
microplate reader-based TiterTACS in situ apoptosis
detection kit (R&D Systems) as described by the manufacturer.
Each experiment was repeated three times in triplicates.
PI3K activity assay
PI3K activity was determined with the PI3K Activity
ELISA kit (Echelon Biosciences) according to the manufacturer’s
instructions (17,18). For direct functional assessment of
PI3K activity, PI3K was isolated by immunoprecipitation using an
anti-PI3K antibody (Millipore, #06-195) to the p85 adapter subunit,
and the ability of the co-precipitated catalytic p110 catalytic
subunit to convert a standard PIP2 to PIP3 in a kinase reaction was
assessed by measuring the generated PIP3 by the ELISA kit. Each
experiment was repeated three times in duplicates.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 10.0. All data values are expressed as means ± SD.
Comparisons of means among multiple groups were performed with
one-way ANOVA followed by post hoc pairwise comparisons
using Tukey’s tests. A two-tailed p<0.05 was considered to
indicate statistically significant differences.
Results
Overexpression and knockdown of Annexin
A5 in human DLBCL cells
We used Toledo and Pfeiffer human DLBCL cells as
cell models in this study. The Toledo and the Pfeiffer cell lines
were established from peripheral blood leukocytes and metastatic
site (pleural effusion) of patients with DLBCL, respectively.
Western blot analyses revealed that Toledo cells had higher
constitutive Annexin A5 expression than Pfeiffer (Fig. 1). We overexpressed and knocked down
Annexin A5 in both cell lines by stable transfection of an Annexin
A5 expression vector and lentiviral transduction of Annexin
A5-shRNA, respectively. As shown in Fig. 1, compared with the controls, Annexin
A5 was overexpressed 3.4- and 2.8-fold in Toledo and Pfeiffer
cells, respectively. On the other hand, the endogenous Annexin A5
level was knocked down 81 and 89% in Toledo and Pfeiffer cells,
respectively (Fig. 1). As our pilot
studies suggested that Annexin A5 would regulate DLBCL cell
invasion and chemosensitivity through a PI3K-dependent mechanism
(data not shown), we included a selective PI3K inhibitor BKM120 (50
μM) in all experiments in this study. As shown in Fig. 1, the PI3K inhibitor had no
significant effect on Annexin A5 expression in both Toledo and
Pfeiffer cells.
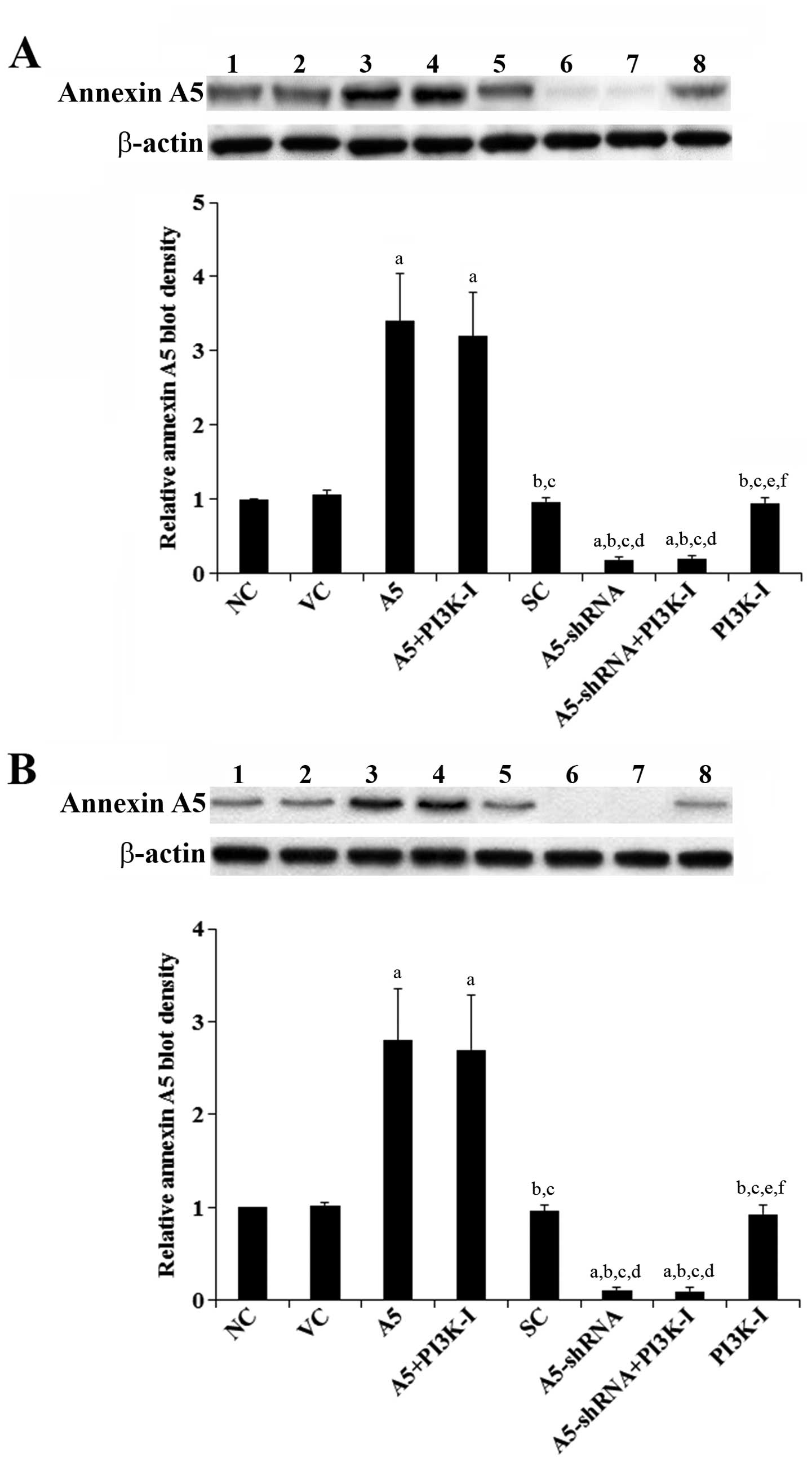 | Figure 1Annexin A5 expression in human diffuse
large B-cell lymphoma (DLBCL) cells with overexpression or
knockdown of Annexin A5. In (A) Toledo and (B) Pfeiffer human DLBCL
cells, expression of Annexin A5 in normal control cells (NC, lane
1), cells stably transfected with the empty pCMV6-entry vector (VC,
lane 2), cells stably transfected with Annexin A5 with or without
phosphatidylinositol 3-kinase (PI3K) inhibitor BKM120 (50 μM)
treatment (A5, lane 3; A5+PI3K-I, lane 4), cells stably transduced
with scramble control shRNA (SC, lane 5), cells stably transduced
with Annexin A5-shRNA with or without BKM120 (50 μM) treatment
(A5-shRNA, lane 6; A5-shRNA+PI3K-I, lane 7), and cells treated with
BKM120 (50 μM) (PI3K-I, lane 8) was analyzed with western blot
analysis. β-actin blotting was used as a loading control. Density
of the Annexin A5 blot was normalized against that of the β-actin
blot to obtain a relative Annexin A5 blot density, which was
expressed as fold changes to that of NC (designated as 1). Three
independent experiments were performed for each western blot
analysis. Data values are expressed as mean ± SD.
ap<0.05 compared with NC and VC;
bp<0.05 compared with A5; cp<0.05
compared with A5+PI3K-I; dp<0.05 compared with SC;
ep<0.05 compared with A5-shRNA; fp<0.05
compared with A5-shRNA+PI3K-I. |
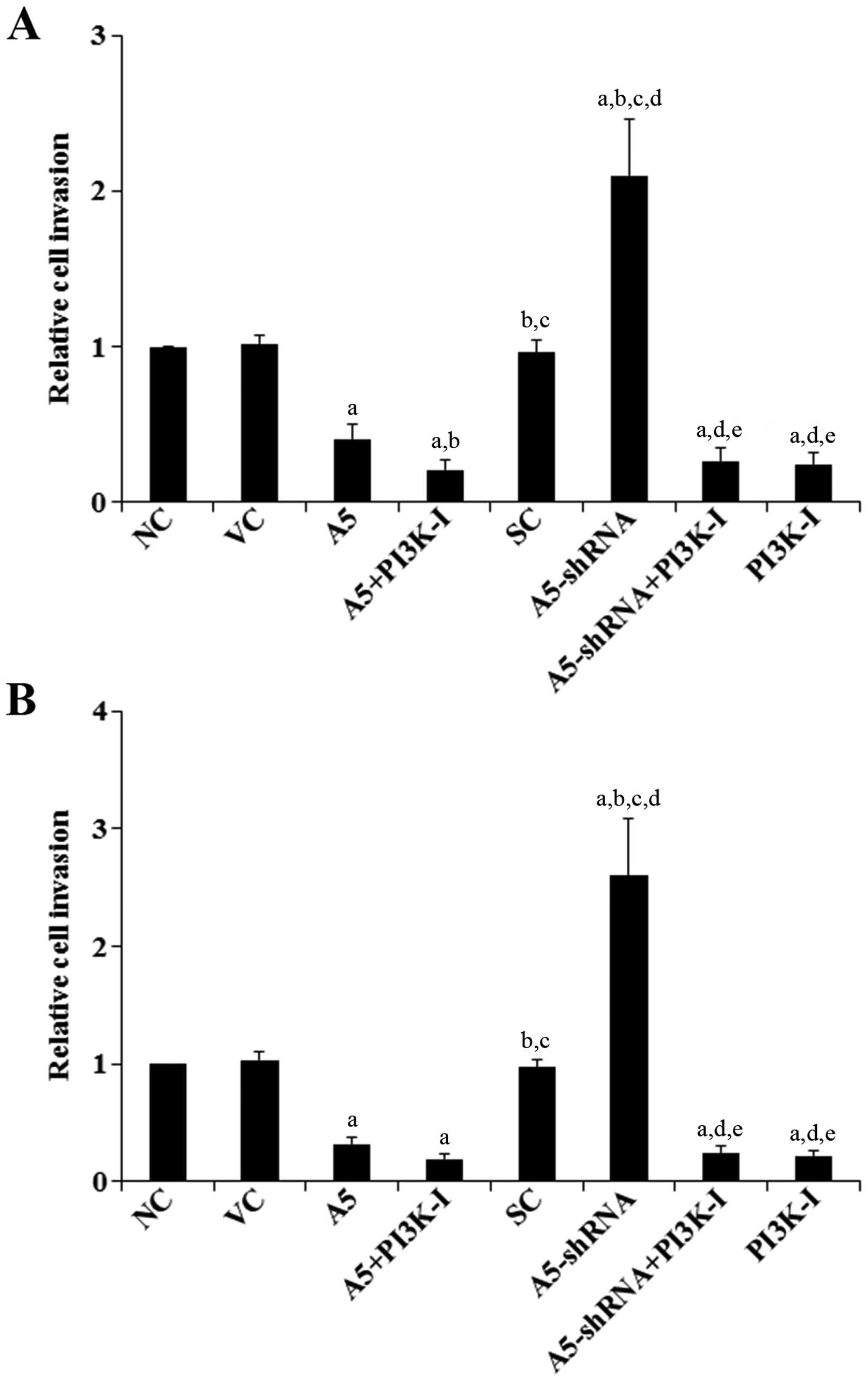
Effects of Annexin A5 on DLBCL cell
invasion and MMP-9 expression/activity
To examine the effect of Annexin A5 on DLBCL cell
invasion, we performed in vitro cell invasion assays.
Compared with the controls, overexpression of Annexin A5 decreased
cell invasion by >60% in both Toledo and Pfeiffer cells
(Fig. 2). On the other hand,
knockdown of Annexin A5 respectively increased cell invasion by
2.1-fold in Toledo cells and by 2.6-fold in Pfeiffer cells, which
was abolished by BKM120 (50 μM) (Fig.
2).
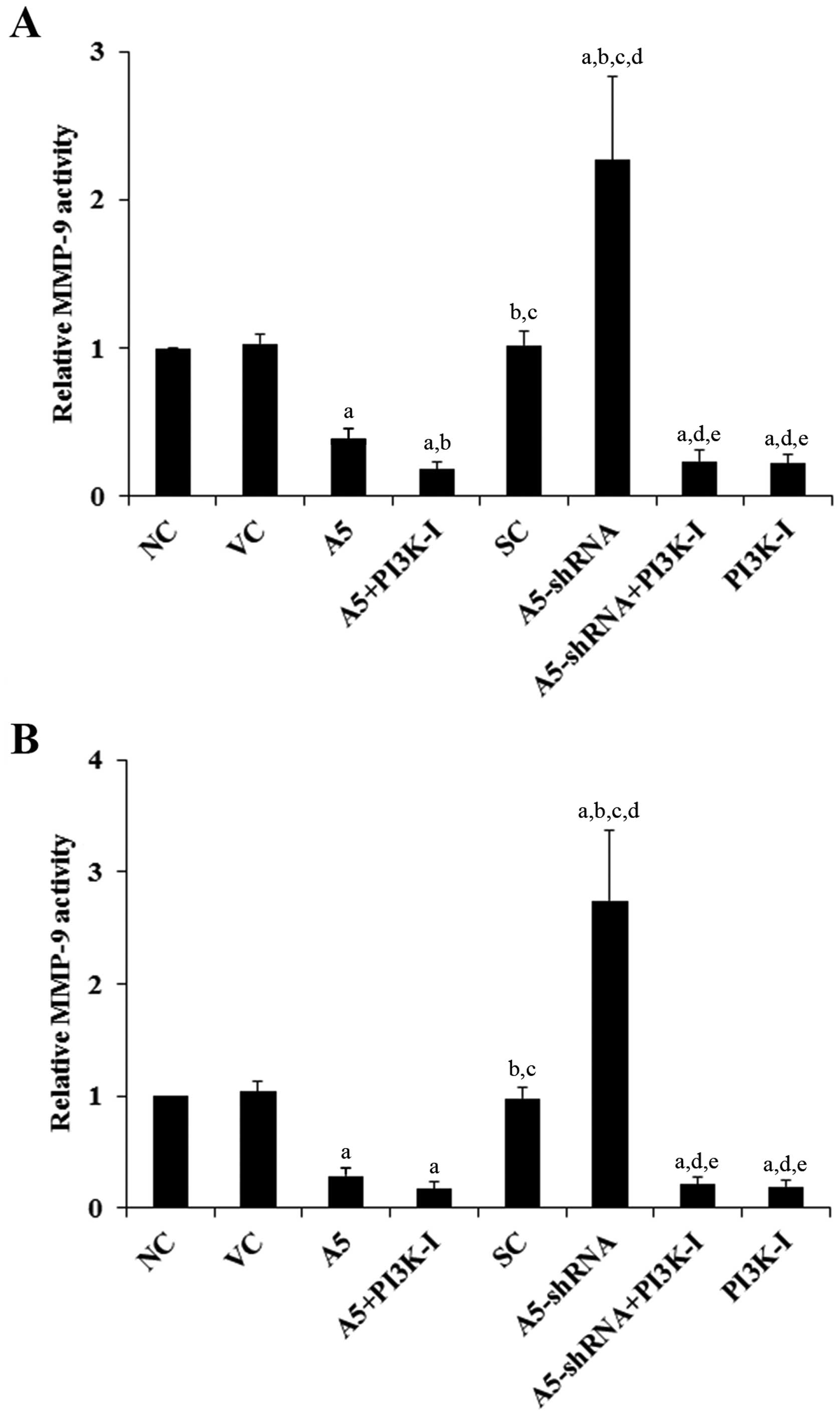
MMPs play a critical role in cancer cell invasion
(19). Among different MMPs tested,
we found that the MMP-9 expression was significantly altered by
Annexin A5 in DLBCL cells. As shown in Fig. 3, compared with the controls,
over-expression of Annexin A5 decreased MMP-9 expression by >70%
in both Toledo and Pfeiffer cells. In contrast, knockdown of
Annexin A5 respectively increased MMP-9 expression by 2.5-fold in
Toledo cells and by 2.9-fold in Pfeiffer cells, which was abolished
by BKM120 (50 μM) (Fig. 3). Similar
data trend was observed with the MMP-9 activity (Fig. 4).
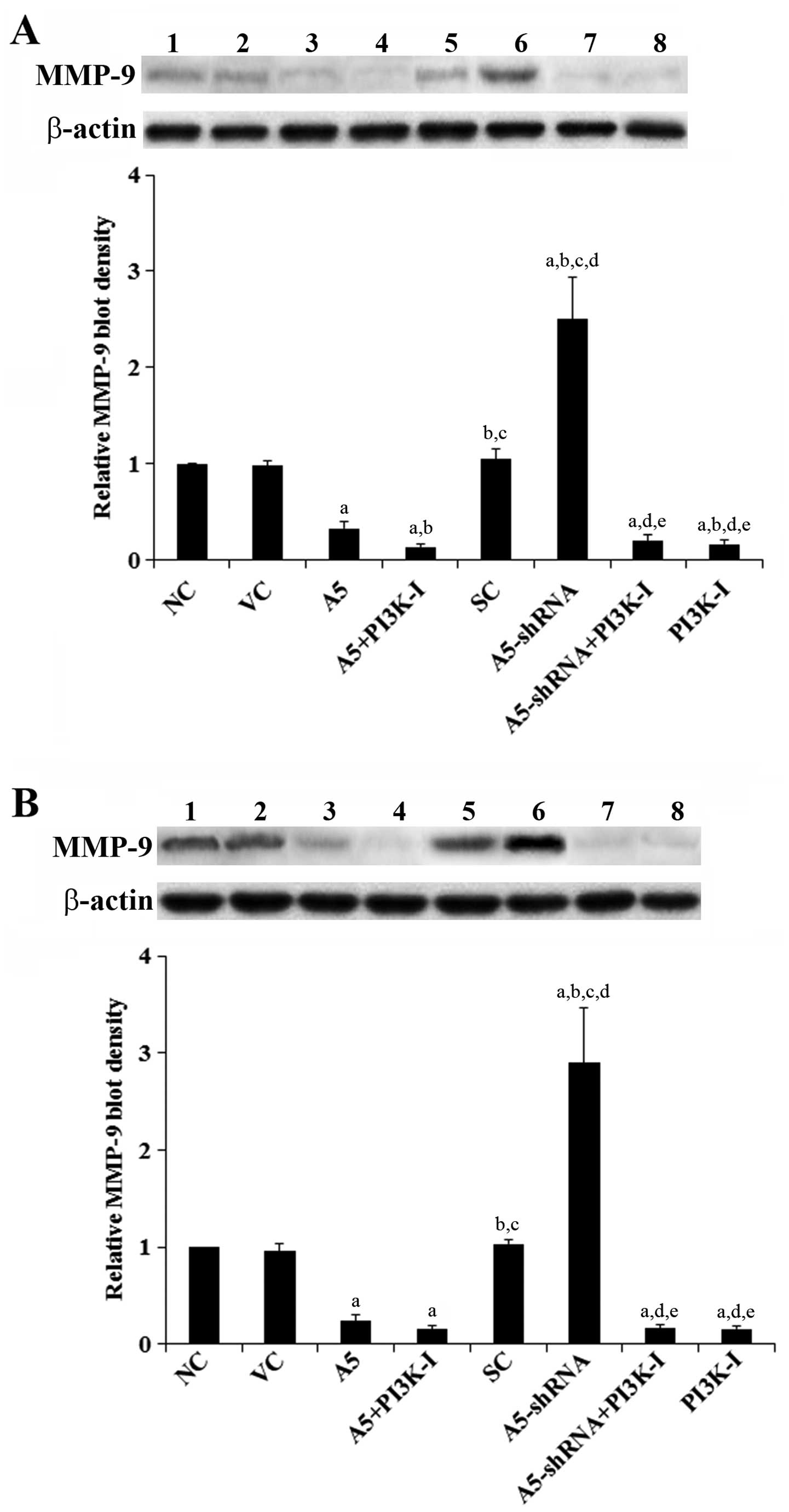 | Figure 3Effects of Annexin A5 on matrix
metalloproteinase-9 (MMP-9) expression in diffuse large B-cell
lymphoma (DLBCL) cells. In (A) Toledo and (B) Pfeiffer DLBCL cells,
expression of MMP-9 in normal control cells (NC, lane 1), cells
stably transfected with the empty pCMV6-entry vector (VC, lane 2),
cells stably transfected with Annexin A5 with or without
phosphatidylinositol 3-kinase (PI3K) inhibitor BKM120 (50 μM)
treatment (A5, lane 3; A5+PI3K-I, lane 4), cells stably transduced
with scramble control shRNA (SC, lane 5), cells stably transduced
with Annexin A5-shRNA with or without BKM120 (50 μM) treatment
(A5-shRNA, lane 6; A5-shRNA+PI3K-I, lane 7), and cells treated with
BKM120 (50 μM) (PI3K-I, lane 8) was analyzed with western blot
analysis. β-actin blotting was used as a loading control. Density
of the MMP-9 blot was normalized against that of the β-actin blot
to obtain a relative MMP-9 blot density, which was expressed as
fold changes to that of NC (designated as 1). Three independent
experiments were performed for each western blot analysis. Data
values are expressed as mean ± SD. ap<0.05 compared
with NC and VC; bp<0.05 compared with A5;
cp<0.05 compared with A5+PI3K-I;
dp<0.05 compared with SC; ep<0.05
compared with A5-shRNA. |
Effects of Annexin A5 on DLBCL cell
chemoresistance to CHOP
To explore the effect of Annexin A5 on DLBCL
chemoresistance, we examined cell apoptosis in DLBCL cells treated
with CHOP in vitro. Overexpression or knockdown of Annexin
A5 did not significantly alter cell apoptosis in either Toledo or
Pfeiffer cells under normal culture conditions (data not shown).
However, overexpression of Annexin A5 significantly increased
CHOP-induced DLBCL cell apoptosis compared with the controls
(Fig. 5). On the other hand,
knockdown of Annexin A5 markedly decreased DLBCL cell apoptosis
during CHOP treatment, which was abolished by BKM120 (50 μM)
(Fig. 5).
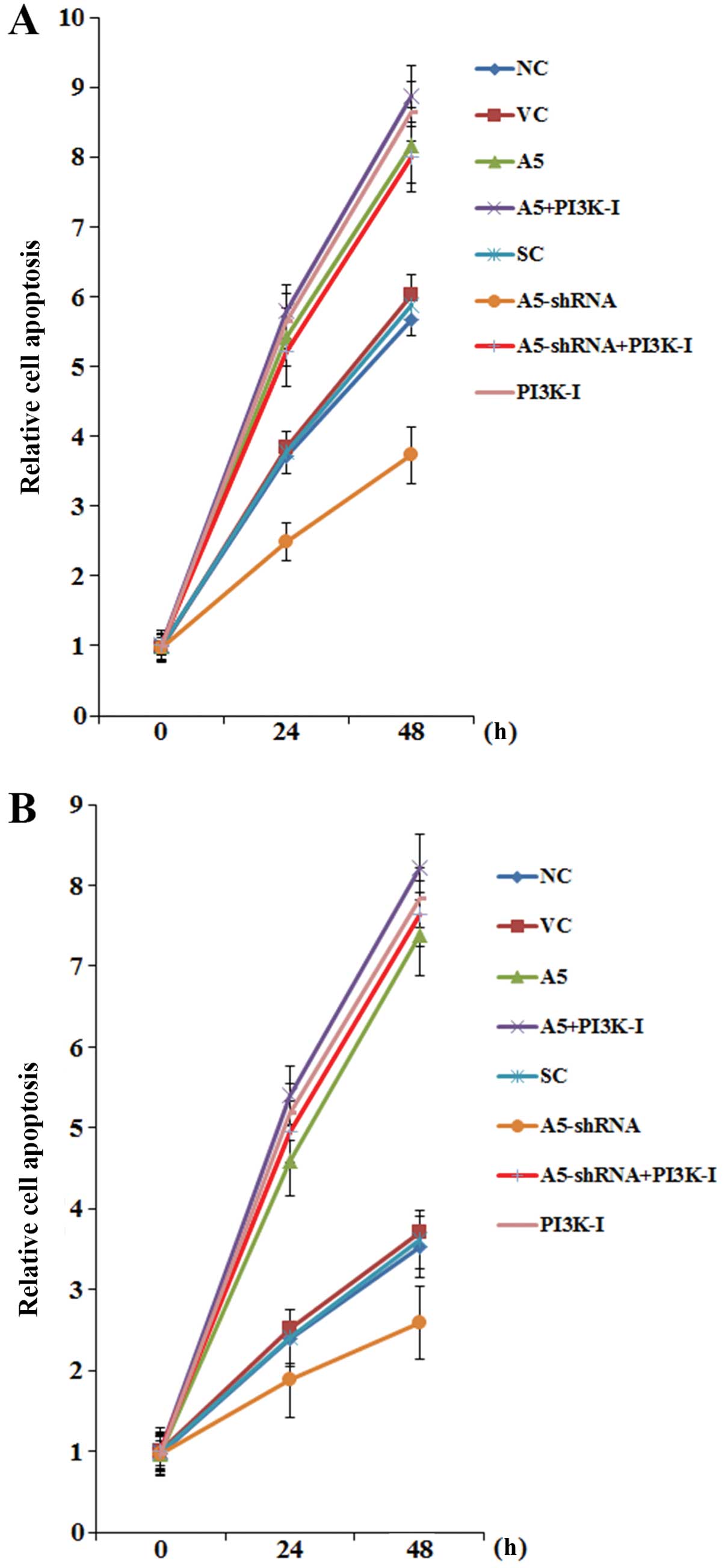 | Figure 5Effects of Annexin A5 on
cyclophosphamide, doxorubicin, vin-cristine and prednisone
(CHOP)-induced apoptosis in diffuse large B-cell lymphoma (DLBCL)
cells. (A) Toledo and (B) Pfeiffer DLBCL cells were treated with
CHOP for 24 and 48 h. The composition of CHOP consisted of
cyclophosphoramide, doxorubicin, vincristine and prednisone at the
clinical ratio of 80/5.5/0.16/11.1, with the combined CHOP
concentration set at 80 ng/ml. Apoptosis was measured with a
microplate reader-based TiterTACS in situ apoptosis
detection kit (R&D Systems) in normal control cells (NC), cells
stably transfected with the empty pCMV6-entry vector (VC), cells
stably transfected with Annexin A5 with or without
phospha-tidylinositol 3-kinase (PI3K) inhibitor BKM120 (50 μM)
treatment (A5; A5+PI3K-I), cells stably transduced with scramble
control shRNA (SC), cells stably transduced with Annexin A5-shRNA
with or without BKM120 (50 μM) treatment (A5-shRNA;
A5-shRNA+PI3K-I), and cells treated with BKM120 (50 μM) (PI3K-I).
Cell apoptosis was shown as fold changes to that of NC at 0 h
(designated as 1). Each experiment was repeated three times in
triplicates. Data values are expressed as mean ± SD. |
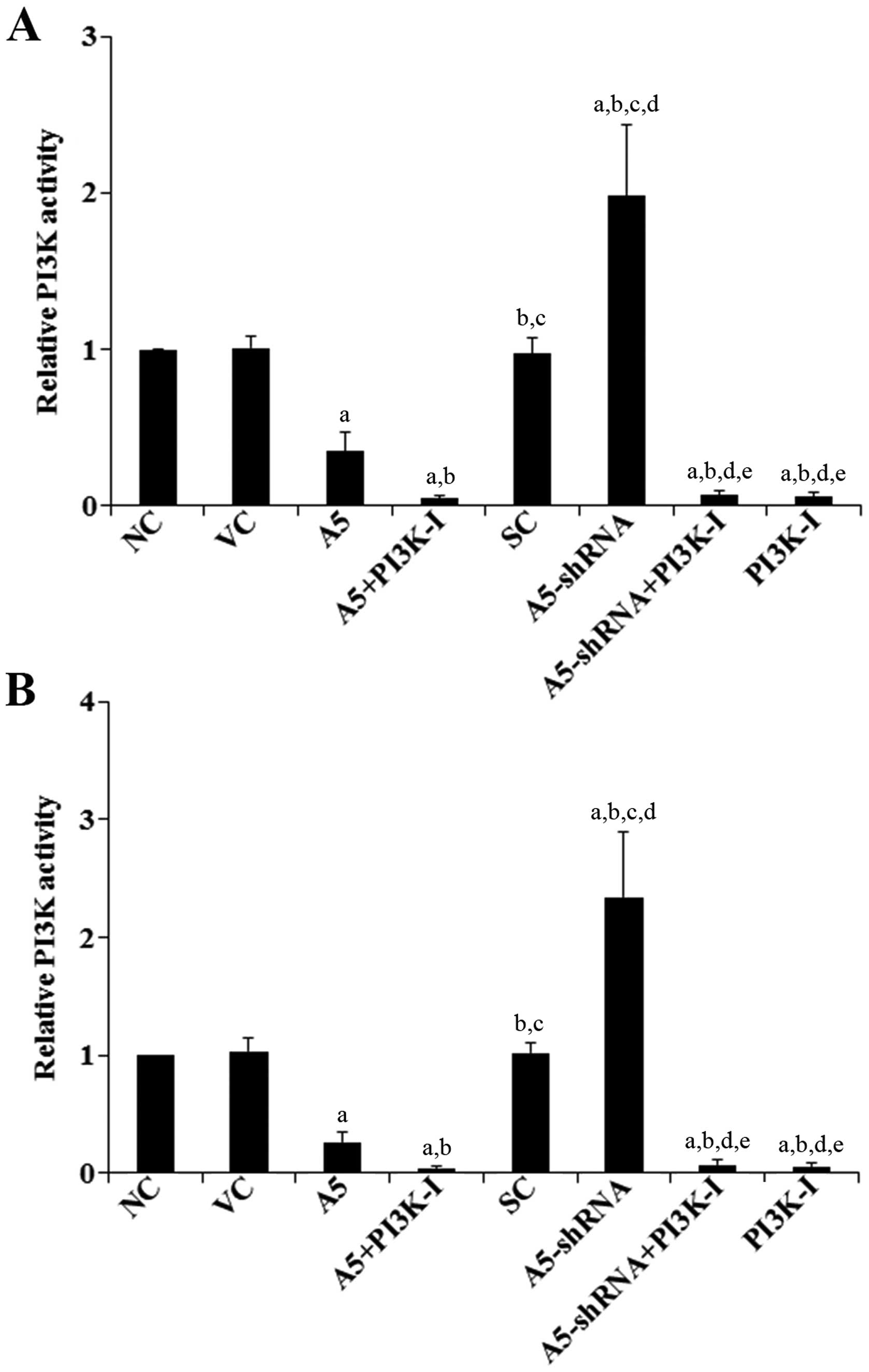
Effects of Annexin A5 on PI3K activity
and phosphorylation of Akt in DLBCL cells
The above results suggested that Annexin A5
inhibited DLBCL cell chemoresistance to CHOP through a
PI3K-dependent mechanism. Indeed, the PI3K/ Akt pathway is
reportedly important for cancer cell chemoresistance (20–23).
Thus, we next examined the effects of Annexin A5 on the PI3K
activity and phosphorylation of Akt in DLBCL cells. As shown in
Fig. 6, compared with the controls,
overexpression of Annexin A5 decreased the PI3K activity by ~65% in
Toledo cells and by 75% in Pfeiffer cells, respectively. In
contrast, knockdown of Annexin A5 respectively increased the PI3K
activity by ~2-fold in Toledo cells and by 2.3-fold in Pfeiffer
cells, which was abolished by BKM120 (50 μM) (Fig. 6). Similar data trend was observed
with phosphorylation at serine 473 (ser473) of Akt (Fig. 7), which is required for full
activation of Akt by PI3K.
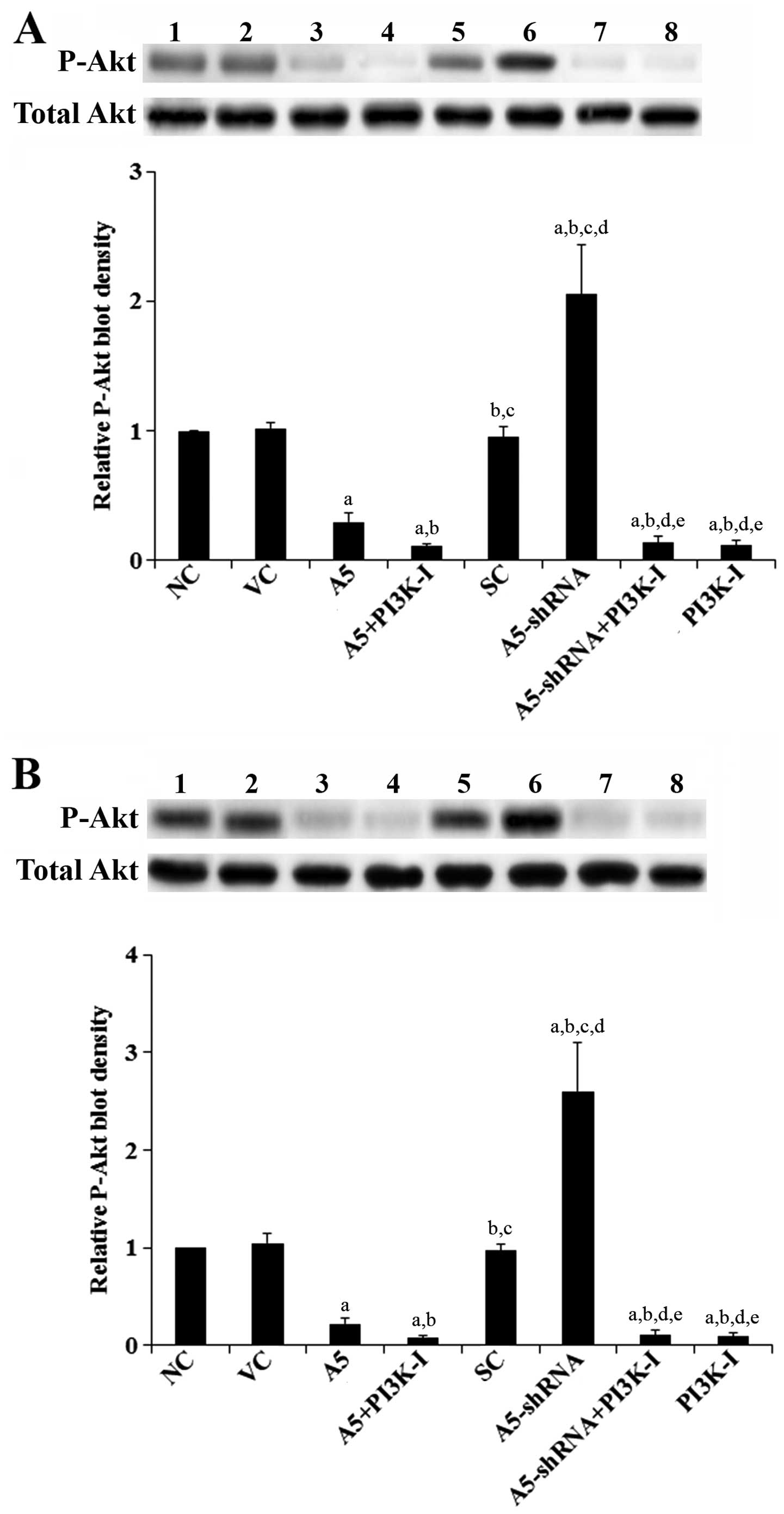 | Figure 7Effects of Annexin A5 on
phosphorylated Akt (P-Akt) level in diffuse large B-cell lymphoma
(DLBCL) cells. In (A) Toledo and (B) Pfeiffer DLBCL cells, levels
of total Akt and P-Akt at serine 473 (ser473) were determined by
western blot analyses in normal control cells (NC, lane 1), cells
stably transfected with the empty pCMV6-entry vector (VC, lane 2),
cells stably transfected with Annexin A5 with or without
phosphatidylino-sitol 3-kinase (PI3K) inhibitor BKM120 (50 μM)
treatment (A5, lane 3; A5+PI3K-I, lane 4), cells stably transduced
with scramble control shRNA (SC, lane 5), cells stably transduced
with Annexin A5-shRNA with or without BKM120 (50 μM) treatment
(A5-shRNA, lane 6; A5-shRNA+PI3K-I, lane 7), and cells treated with
BKM120 (50 μM) (PI3K-I, lane 8). The total Akt level was not
significantly altered by Annexin A5 in both Toledo and Pfeiffer
cells. Density of the P-Akt (ser473) blot was normalized against
that of total Akt to obtain a relative P-Akt blot density, which
was expressed as fold changes to that of NC (designated as 1).
Three independent experiments were performed for each western blot
analysis. Data values are expressed as mean ± SD.
ap<0.05 compared with NC and VC;
bp<0.05 compared with A5; cp<0.05
compared with A5+PI3K-I; dp<0.05 compared with SC;
ep<0.05 compared with A5-shRNA. |
Discussion
CHOP is widely used in China to treat DLBCL.
Although patient outcomes have significantly improved to a >40%
cure rate by CHOP chemotherapy, resistance to the CHOP regimen
continues to pose a challenge in managing or curing DLBCL (3). Understanding the molecular basis for
development of multi-drug chemoresistance in DLBCL may serve as a
basis for identification of novel therapeutic targets and
biomarkers involved in DLBCL resistance to CHOP. Previous studies
have suggested that Annexin A5, a calcium-dependent
phospholipid-binding protein, while promoting the malignancy and
chemoresistance in certain types of cancer, is negatively
correlated with those in other cancers, including DLBCL (9–12). In
the present study, we demonstrated an important inhibitory role for
Annexin A5 in DLBCL cell invasion and chemoresistance to CHOP.
DLBCL is a heterogeneous group of tumors with a high
variance of genetic abnormalities, clinical features, response to
treatment and prognosis (2). Thus,
we used two DLBCL cell lines with considerable background
differences (respectively established from peripheral blood
leukocytes and metastatic site of DLBCL patients) as cell models in
this study to demonstrate a generalizable role of Annexin A5 in
DLBCL cell invasion and chemoresistance to CHOP. In both cell
lines, while overexpression of Annexin A5 significantly decreased
cell invasion and cell survival against CHOP-induced apoptosis,
knockdown of Annexin A5 markedly increased cell invasion and
CHOP-induced apoptosis, which was abolished by a selective PI3K
inhibitor. The results suggest that Annexin A5 inhibited DLBCL cell
invasion and chemoresistance to CHOP through a PI3K-depedent
mechanism. This was corroborated by the results that knockdown of
Annexin A5 increased the PI3K activity and Akt phosphorylation in
DLBCL cells. The findings are also in line with previous studies
that the PI3K/Akt pathway plays a critical role in cancer cell
invasiveness and chemoresistance (20–23).
MMPs play a critical role in cancer cell invasion
(19). Among different MMPs tested,
we found that the MMP-9 expression/activity was significantly
altered by Annexin A5, which showed similar data trend as that in
DLBCL cell invasion. Previous studies have shown that PI3K
signaling can stimulate MMP-9 expression (24,25).
In our study, over-expression of Annexin A5 in DLBCL cells
significantly decreased the PI3K activity, MMP-9
expression/activity, and cell invasion. On the other hand,
knockdown of Annexin A5 markedly increased the PI3K activity as
well as MMP-9 expression/activity and cell invasion, which was
abolished by a selective PI3K inhibitor. The findings suggest that
Annexin A5 could inhibit DLBCL cell invasion by downregulating the
MMP-9 expression/activity through inhibiting PI3K
activity/signaling.
It has been reported that Annexin A5 dysexpression
may lead to deregulated activation of PKC and abnormality of
cellular signal transduction, which may be involved in
carcinogenesis (9). In our study,
however, altered expression of Annexin A5 in DLBCL cells only
showed major regulatory effects on PI3K activity/signaling, which
subsequently led to significant changes in cell invasion and
chemoresistance to CHOP. Future studies are required to examine the
mechanism by which Annexin A5 inhibits PI3K activity/signaling in
DLBCL cells.
Annexin A5 is negatively correlated with the
malignancy and chemoresistance in some cancers, while it enhances
those in other cancers (9–12). Our study demonstrated an inhibitory
effect of Annexin A5 on DLBCL chemoresistance, while previous
studies have shown that Annexin A5 enhances chemoresistance in
gastric cancer and nasopharyngeal carcinoma (10,11).
Thus, Annexin A5 likely plays a dual role in cancer cell malignancy
and chemoresistance, depending on tissue specificity. The
functional role of Annexin A5 in DLBCL invasiveness and
chemoresistance will be verified in more DLBCL cell lines and in
animal models in our future studies.
Twelve Annexins common to vertebrates are known as
Annexins A1–A11 and A13 (1,2). Annexin A1, A2, A4 and A5 play
important roles in breast, pancreatic and laryngeal carcinoma,
alone and/or synergistically, which has made them potential
therapeutic targets for malignant tumors (26). Based on our findings in the present
study, it is noteworthy to investigate whether other Annexin family
members play a role, alone or in combination with Annexin A5, in
DLBCL cell invasion and chemoresistance.
In conclusion, our study provides the first in
vitro evidence that Annexin A5 inhibits DLBCL cell invasion,
MMP-9 expression/activity, and chemoresistance to CHOP through a
PI3K-dependent mechanism. It provides new insight not only into the
biological function of Annexin A5, but also into the molecular
mechanisms underlying DLBCL progression and chemoresistance.
References
|
1
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: WHO classification of tumours of
hematopoietic and lymphoid tissues. Blood. 117:5019–5032. 2011.
|
|
2
|
Lossos IS and Morgensztern D: Prognostic
biomarkers in diffuse large B-cell lymphoma. J Clin Oncol.
24:995–1007. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maxwell SA, Li Z, Jaya D, Ballard S,
Ferrell J and Fu H: 14-3-3zeta mediates resistance of diffuse large
B cell lymphoma to an anthracycline-based chemotherapeutic regimen.
J Biol Chem. 284:22379–22389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shipp MA, Ross KN, Tamayo P, et al:
Diffuse large B-cell lymphoma outcome prediction by gene-expression
profiling and supervised machine learning. Nat Med. 8:68–74. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moss SE and Morgan RO: The annexins.
Genome Biol. 5:2192004. View Article : Google Scholar
|
|
6
|
Laohavisit A and Davies JM:
Multifunctional annexins. Plant Sci. 177:532–539. 2009. View Article : Google Scholar
|
|
7
|
Mussunoor S and Murray GI: The role of
annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakao H, Watanabe M and Maki M: A new
function of calphobind I (annexin V). Eur J Biochem. 223:901–908.
1994.PubMed/NCBI
|
|
9
|
Peng B, Guo C, Guan H, Liu S and Sun MZ:
Annexin A5 as a potential marker in tumors. Clin Chim Acta.
427:42–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu X, Tang Y, Huang W, et al:
Identification of proteins interacting with multidrug resistance
protein in gastric cancer. World J Gastroenterol. 19:3568–3573.
2011.
|
|
11
|
Tang S, Huang W, Zhong M, et al:
Identification keratin 1 as a cDDP-resistant protein in
nasopharyngeal carcinoma cell lines. J Proteomics. 75:2352–2360.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Zeng L, Zhang S, Zeng S, Huang J,
Tang Y and Zhong M: Identification of differentially expressed
proteins in chemotherapy-sensitive and chemotherapy-resistant
diffuse large B cell lymphoma by proteomic methods. Med Oncol.
30:5282013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Feng P, Xiao Z and Ren EC: LIM and
SH3 protein 1 (Lasp1) is a novel p53 transcriptional target
involved in hepato-cellular carcinoma. J Hepatol. 50:528–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng Y, Hu J, Ma J, et al: RNAi-mediated
silencing of VEGF-C inhibits non-small cell lung cancer progression
by simultaneously down-regulating the CXCR4, CCR7, VEGFR-2 and
VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling
pathways. Eur J Cancer. 47:2353–2363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jo YK, Park SJ, Shin JH, Kim Y, Hwang JJ,
Cho DH and Kim JC: ARP101, a selective MMP-2 inhibitor, induces
autophagy-associated cell death in cancer cells. Biochem Biophys
Res Commun. 404:1039–1043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qazi H, Shi ZD and Tarbell JM: Fluid shear
stress regulates the invasive potential of glioma cells via
modulation of migratory activity and matrix metalloproteinase
expression. PLoS One. 6:e203482011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao CM, Zhang Y, Weisleder N, et al: MG53
constitutes a primary determinant of cardiac ischemic
preconditioning. Circulation. 121:2565–2574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fos C, Salles A, Lang V, et al: ICOS
ligation recruits the p50alpha PI3K regulatory subunit to the
immunological synapse. J Immunol. 181:1969–1977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Liao Q, Li K, Zhong D, Weng X and Mi
M: Knockdown of endothelin A receptor expression inhibits
osteosarcoma pulmonary metastasis in an orthotopic xenograft mouse
model. Mol Med Rep. 5:1391–1395. 2012.
|
|
20
|
Li B, Yang Y, Jiang S, Ni B, Chen K and
Jiang L: Adenovirus-mediated overexpression of BMP-9 inhibits human
osteosarcoma cell growth and migration through downregulation of
the PI3K/ AKT pathway. Int J Oncol. 41:1809–1819. 2012.PubMed/NCBI
|
|
21
|
Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y,
Long XH and Huang SH: Inhibition of fatty acid synthase suppresses
osteo-sarcoma cell invasion and migration via downregulation of the
PI3K/Akt signaling pathway in vitro. Mol Med Rep. 7:608–612.
2013.PubMed/NCBI
|
|
22
|
Zhao G, Cai C, Yang T, et al: MicroRNA-221
induces cell survival and cisplatin resistance through PI3K/Akt
pathway in human osteosarcoma. PLoS One. 8:e539062013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang TF, Wang H, Peng AF, et al:
Inhibition of fatty acid synthase suppresses U-2 OS cell invasion
and migration via downregulating the activity of HER2/PI3K/AKT
signaling pathway in vitro. Biochem Biophys Res Commun.
440:229–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin J, Tang J, Jiao L, et al: A
diterpenoid compound, excisanin A, inhibits the invasive behavior
of breast cancer cells by modulating the integrin
β1/FAK/PI3K/AKT/β-catenin signaling. Life Sci. 93:655–663.
2013.PubMed/NCBI
|
|
25
|
Lin G, Sun L, Wang R, Guo Y and Xie C:
Overexpression of muscarinic receptor 3 promotes metastasis and
predicts poor prognosis in non-small-cell lung cancer. J Thorac
Oncol. 9:170–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng S, Wang J, Hou L, et al: Annexin A1,
A2, A4 and A5 play important roles in breast cancer, pancreatic
cancer and laryngeal carcinoma, alone and/or synergistically. Oncol
Lett. 5:107–112. 2013.PubMed/NCBI
|