Introduction
Esophageal cancer (EC) is a malignant tumor of the
esophagus. There are two main subtypes of EC: esophageal
adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC).
EAC is common in Western countries, while ESCC is common in East
Asia, particularly in China (1).
Over 600,000 new cases are diagnosed annually worldwide (2). ESCC is often locally advanced by the
time patients are preovided with medical attention and since
surgery is the only curative treatment option, patient survival is
closely associated with the stage of disease (3–6).
Despite improvements in diagnosis and treatment, the 5-year
survival rate for patients with advanced and metastatic EC remains
<20% after surgery (1).
Epigallocatechin-3-gallate (EGCG), a major
polyphenolic constituent of green tea, has been shown to inhibit
cancer growth and induce apoptosis in hepatocellular carcinoma,
breast, and head and neck cancers (7–9). EGCG
has been suggested to contribute to the effect of anticancer drugs
by increasing cell cycle arrest, initiating apoptosis and
down-regulating the pro-angiogenic molecule, vascular endothelial
growth factor receptor-2 (VEGFR-2) (10). Results of recent studies have shown
that EGCG has different effects on the production of reactive
oxygen species (ROS) for its antioxidant and pro-oxidant activities
(10,11). Caspases are key regulators of
apoptosis, with caspase-3 acting as an effector (12). Additionally, caspase-3 was found to
play a role in EGCG-induced apoptosis for cholangiocarcinoma and
laryngeal epidermoid carcinoma (13,14).
In addition, downregulation of the pro-angiogenic factor, VEGF has
been documented to inhibit lung cancer growth (15). Few reports are available on the
effect of EGCG on esophageal squamous cell carcinoma. Therefore, we
examined the mechanism of EGCG by studying apoptosis, ROS
generation, cleaved caspase-3 and VEGF expression in Eca-109 and
Te-1 cell lines.
Materials and methods
Cell culture and reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was purchased from Sigma (St. Louis, MO, USA).
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum
(FBS) were purchased from HyClone (Logan, UT, USA). Antibodies
against cleaved caspase-3 and VEGF were purchased from Cell
Signaling Technology (Danvers, MA, USA) and the Proteintech Group
(Chicago, IL, USA), respectively. Horseradish peroxidase-labeled
secondary antibodies were purchased from Sigma.
The H9c2 rat cardiomyocyte cell line and Eca-109 and
Te-1 human esophageal squamous cell carcinoma cell lines were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Human foreskin fibroblast cells were a kind gift
from Dr Ming Zhang (Department of Cardiovascular Medicine, The
Second Affiliated Hospital of Xi’an Jiaotong University). Cell
lines were cultured in DMEM-H medium supplemented with 10% FBS and
1% (100 U/ml) penicillin/streptomycin (Sigma-Aldrich Co.). The
culture medium was changed every two days. Upon reaching 80–90%
confluency, the cells were passaged and tested for logarithmic
growth.
MTT assay
To assess the effect of EGCG on cell growth, H9c2
rat cardiomyocytes, HFF, Eca-109 and Te-1 cells (1×104
cells/well) were inoculated into a 96-well microtiter plate and
cultured for 24 h. The cells were treated with various
concentrations of EGCG (25, 50, 100, 200 and 400 μM) or vehicle
control (DMSO). After 24 and 48 h of incubation, 20 μl 5 mg/ml MTT
was added to each well for 4 h of incubation. The absorbance was
measured at 490 nm using a multi-channel microplate reader (BD
Biosciences, San Jose, CA, USA). The inhibition rate of cell growth
was determined using the formula: (OD value for control group − OD
value of experimental group)/OD value of the control group × 100%.
The IC50 value for EGCG was determined using GraphPad
Prism software (version 5.0).
Cell cycle analysis
Cell cycle distribution was analyzed by flow
cytometry using propidium iodide (PI) DNA staining as previously
described with modifications (16).
Briefly, Eca-109 and Te-1 were treated with 256 and 162 μM EGCG
(IC50) value, respectively, for 24 h in serum-free DMEM.
The cells were collected, washed with phosphate-buffered saline
(PBS), centrifuged at 1,000 rpm for 5 min, and fixed in 5 ml of 70%
ice-cold ethanol for 24 h at 4°C. After washing, the pellets were
resuspended in 2.5 μl of ribonuclease A solution (final
concentration of 50 μg/ml) and incubated at 37°C for 30 min. Cell
suspensions received 5 μl PI (final concentration of 50 μg/ml),
were protected from light, and incubated at room temperature for 30
min. Flow cytometric analysis of DNA content was carried out using
the FACSCalibur system (BD Biosciences).
Annexin V-FITC/PI staining
To analyze the effect of EGCG on apoptotic cell
death, Annexin V-FITC/PI staining was performed as previously
described and modified (17).
Briefly, the cells were treated with EGCG (IC50) value
for 24 h in serum-free DMEM and gently washed three times in PBS.
The cells were collected, centrifuged at 1,000 rpm for 5 min, and
washed with PBS. A cell density of 5×105 was resuspended
in 500 μl 1X binding buffer and stained with 5 μl Annexin V-FITC
and 10 μl PI staining solution. The cell suspension was covered and
incubated for 15 min at room temperature. The experiments were
performed three times and analyzed using the FACSCalibur
system.
ROS production
Eca-109 and Te-1 cells (2×105 cells/well)
were seeded into a 6-well plate and treated with EGCG
(IC50) value in serum-free DMEM for 24 h. After 24 h,
cells (5×105 cells/ml) were resuspended in serum-free
DMEM and labeled with 1 ml DCFH-DA dye (1 μg/μl) for 20 min with
continuous agitation. FACS (BD Co.) analysis detected the oxidative
burst (hydrogen peroxide).
Western blotting
Immunoblot analysis was performed as described and
modified (18). Eca-109 and Te-1
cells (1×105 cells/ml) were treated with or without EGCG
for 24 h. Cell lysates (20 μg protein) were separated by SDS-PAGE,
transferred to polyvinylidene difluoride membranes (Millipore,
Bedford, MA, USA), and blocked. The following primary antibodies
were applied overnight at 4°C: cleaved caspase-3 (1:1,000) and VEGF
(1:1,000). Anti-rabbit secondary antibodies (1:5,000) were
incubated for 2 h at room temperature.
Mice and xenograft models
Male BALB/c (nu-nu) athymic nude mice (4–5 weeks of
age) were purchased from the Shanghai Silaike Laboratory Animal
Company, Ltd. (Shanghai, China). The mice were maintained at Xi’an
Jiatong University in compliance with the Institutional Animal Care
and Use Committee (IACUC) regulations. For subcutaneous
implantation, Eca-109 cells (2×106) were suspended in
serum-free DMEM mixed with equal parts of Matrigel (BD Biosciences,
San Diego, CA, USA) and subcutaneously injected into the flank of
nude mice (n=30). Engrafted mice received weekly visual inspections
and palpitations until tumors reached 50 mm3 in size.
Xenografts were randomly divided into the EGCG- and PBS-treated
groups. Experimental mice received intraperitoneal injections of
EGCG (10 mg/kg) once a day for two weeks while control mice were
injected with 200 μl PBS (19). The
tumor volume was measured using a caliper and calculated using the
formula: (width in mm)2 × (length in mm)/2. The mice
were sacrificed by cervical dislocation. Subcutaneous tumors,
liver, lung and kidney tissues were harvested, fixed in 4%
paraformaldehyde, and embedded in paraffin. Paraffin sections
(4-μm) were histopathologically evaluated.
Immunohistochemical and histological
analyses
Immunohistochemistry was performed as described and
modified (20). Antigen retrieval
of serial sections was conducted with a 3-min microwave treatment
in 10 mM sodium citrate buffer (pH 6.0). The sections were
incubated with primary antibodies against cleaved caspase-3 (1:100)
and VEGF (1:100). The sections were then incubated with
biotinylated anti-rabbit antibodies (1:100) at 37°C for 30 min,
followed by a 30-min exposure to streptavidin-horseradish
peroxidase (1:200), and counterstained with hematoxylin. Optical
density was analyzed with Image-Pro Plus software (version 6.0).
Hematoxylin and eosin (H&E) staining was used to evaluate
normal liver, lung and kidney tissues.
Statistical analysis
A one-way ANOVA and Dunnett’s t-test were used to
analyze the significant differences between treatment groups.
Statistical analysis was performed using SPSS (version 17.0). The
results are expressed as means ± SD. P<0.05 was considered to
indicate a statistically significant result.
Results
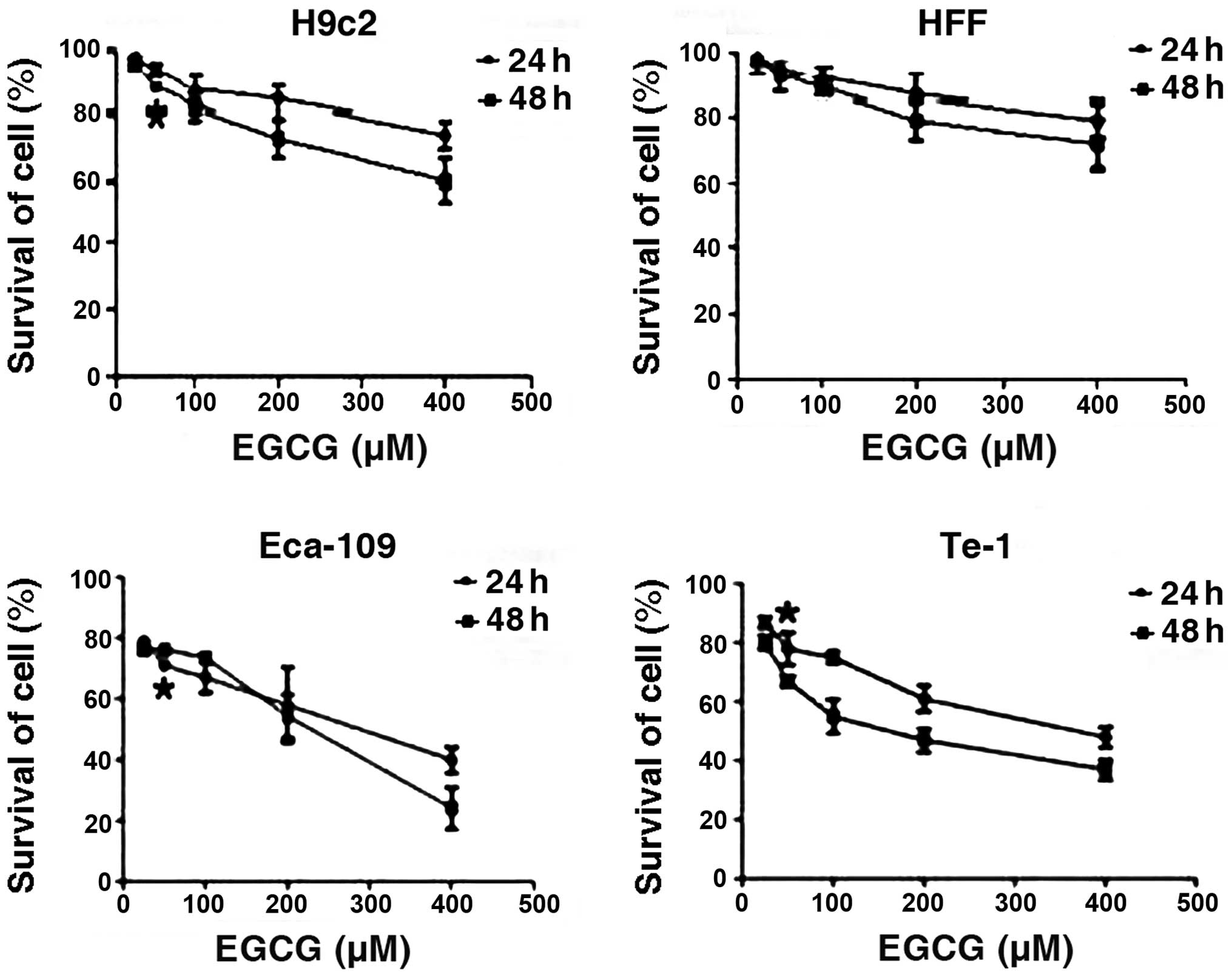
EGCG inhibits cell growth in a time- and
dose-dependent manner
In order to ascertain the capability of EGCG to
inhibit cell growth, we studied the effect of EGCG treatment on
Eca-109 and Te-1 cells compared with normal rat cardiomyocytes and
HFF cells. The results revealed that, 24 and 48 h treatment with
EGCG selectively inhibited the growth of the Eca-109 and Te-1 cells
in a time- and dose-dependent manner when the concentration was
>25 μM. For Te-1 cells EGCG inhibition of cell growth was more
effective at 48 h post-treatment than that at 24 h when the
concentration remained unchanged. For Eca-109 cells it appeared
that the effect of EGCG was more effective at 48 h post-treatment
when the concentration was >200 μM. The estimated
IC50 value for rat H9c2 cardiomyocytes, HFF, Eca-109 and
Te-1 cell lines was 995, 1,584, 256 and 162 μM, respectively
(Fig. 1).
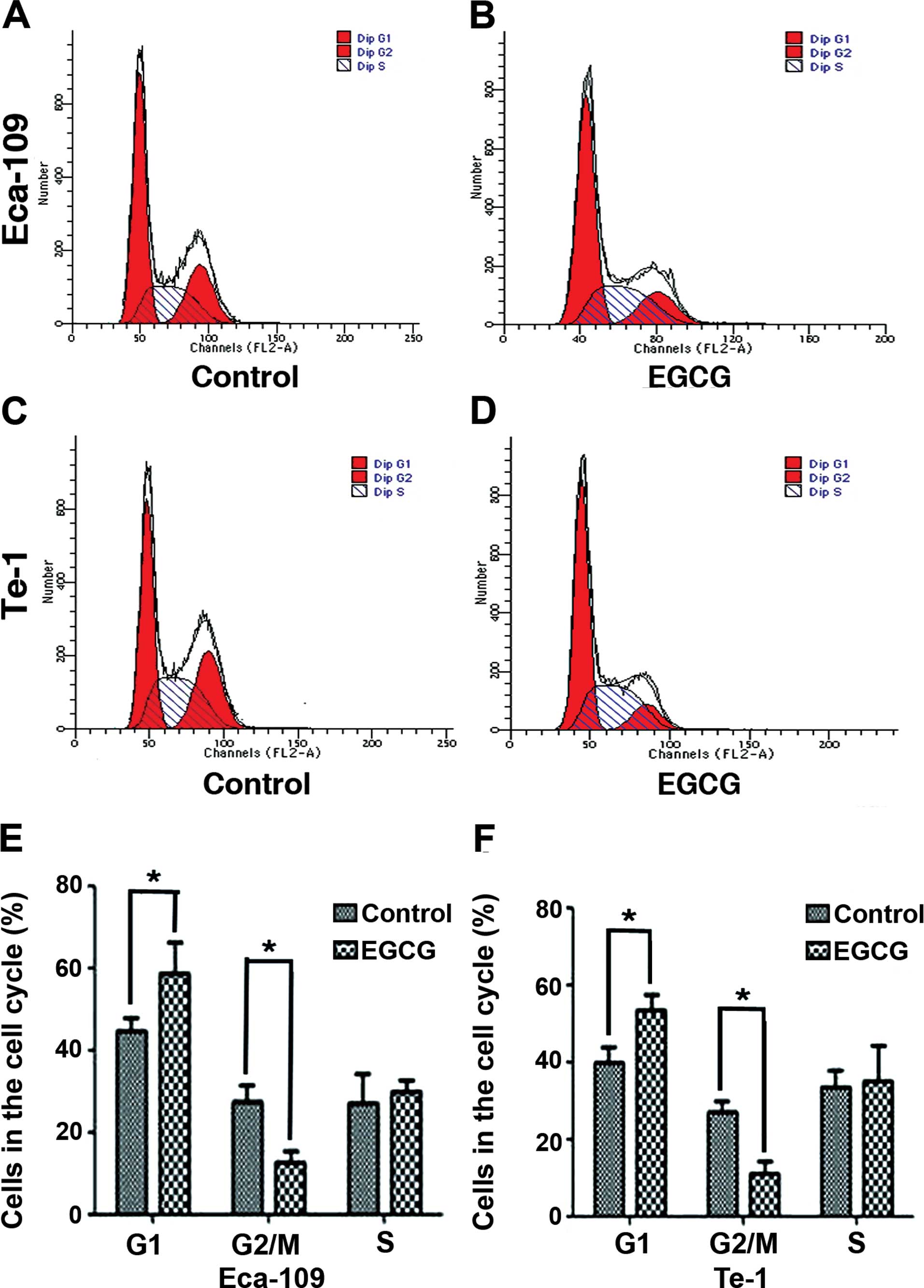
EGCG arrests Eca-109 and Te-1 cells in
the G1 phase
We analyzed the DNA content of Eca-109 and Te-1
cells treated with the EGCG IC50 value for 24 h. FACS
analysis showed a significant increase in the percentage of Eca-109
(from 44.49±3.32 to 58.45±7.78%) and Te-1 cells (from 39.69±4.23 to
53.66±3.87%) in the G1 phase compared to control-treated
cells. Furthermore, there was a decrease in the percentage of
Eca-109 cells (from 27.29±4.21 to 12.73±2.89%) and Te-1 cells (from
27±2.67 to 11.14±3.06%) in the G2/M phase. These results
demonstrated that EGCG can arrest Eca-109 and Te-1 cells at the
G1 checkpoint of the cell cycle (Fig. 2).
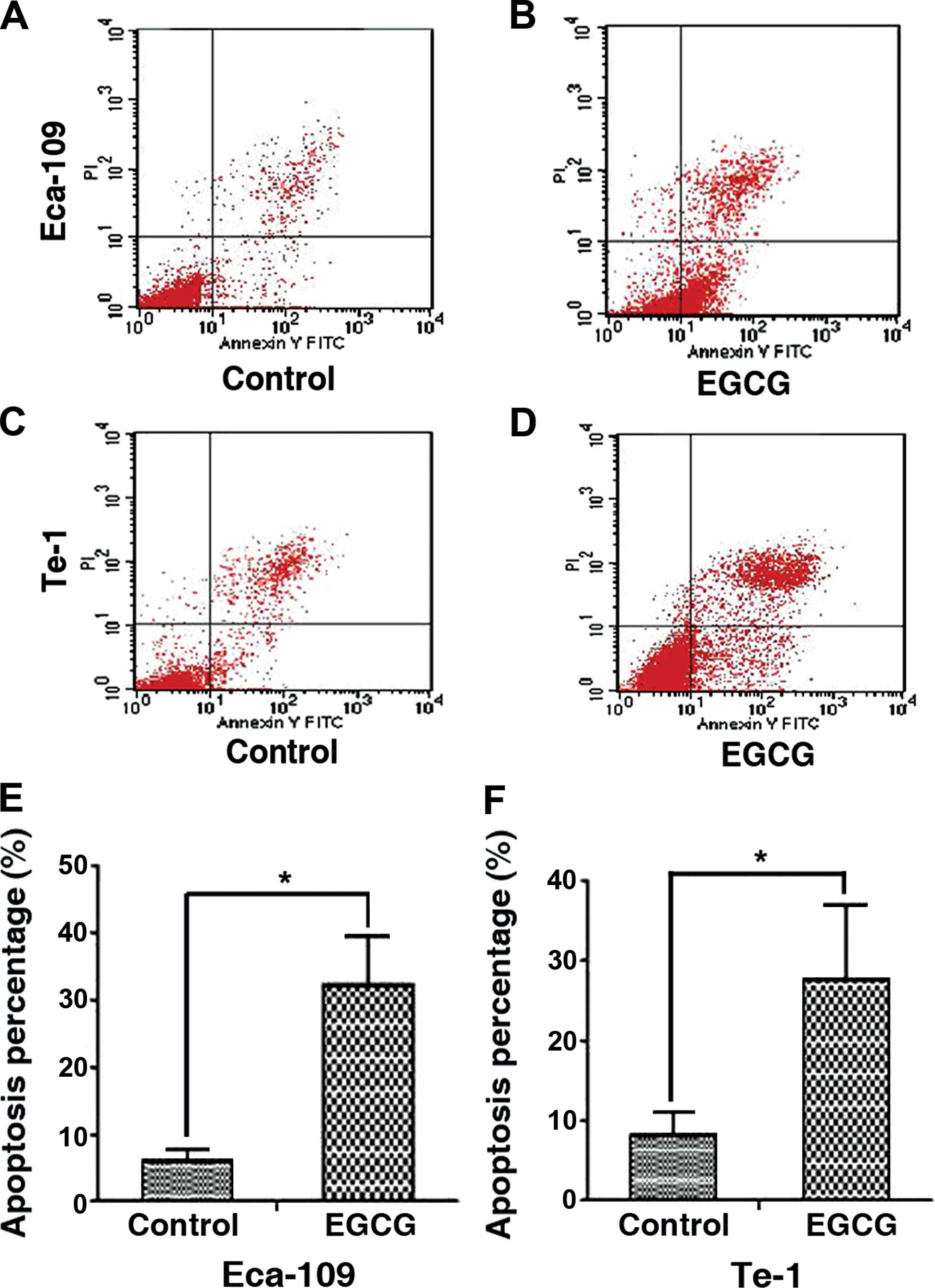
EGCG induces apoptosis in Eca-109 and
Te-1 cells
Annexin V-FITC/PI-labeled cells were treated with
EGCG and analyzed by FACS to determine the apoptotic rate.
Twenty-four hours of treatment with EGCG significantly increased
the percentage of apoptotic Eca-109 cells (6.13±1.65 to
32.23±7.23%) and Te-1 cells (8.22±2.78 to 27.7±9.35%). The results
indicated that EGCG had an obvious effect on inducing apoptosis
(Fig. 3).
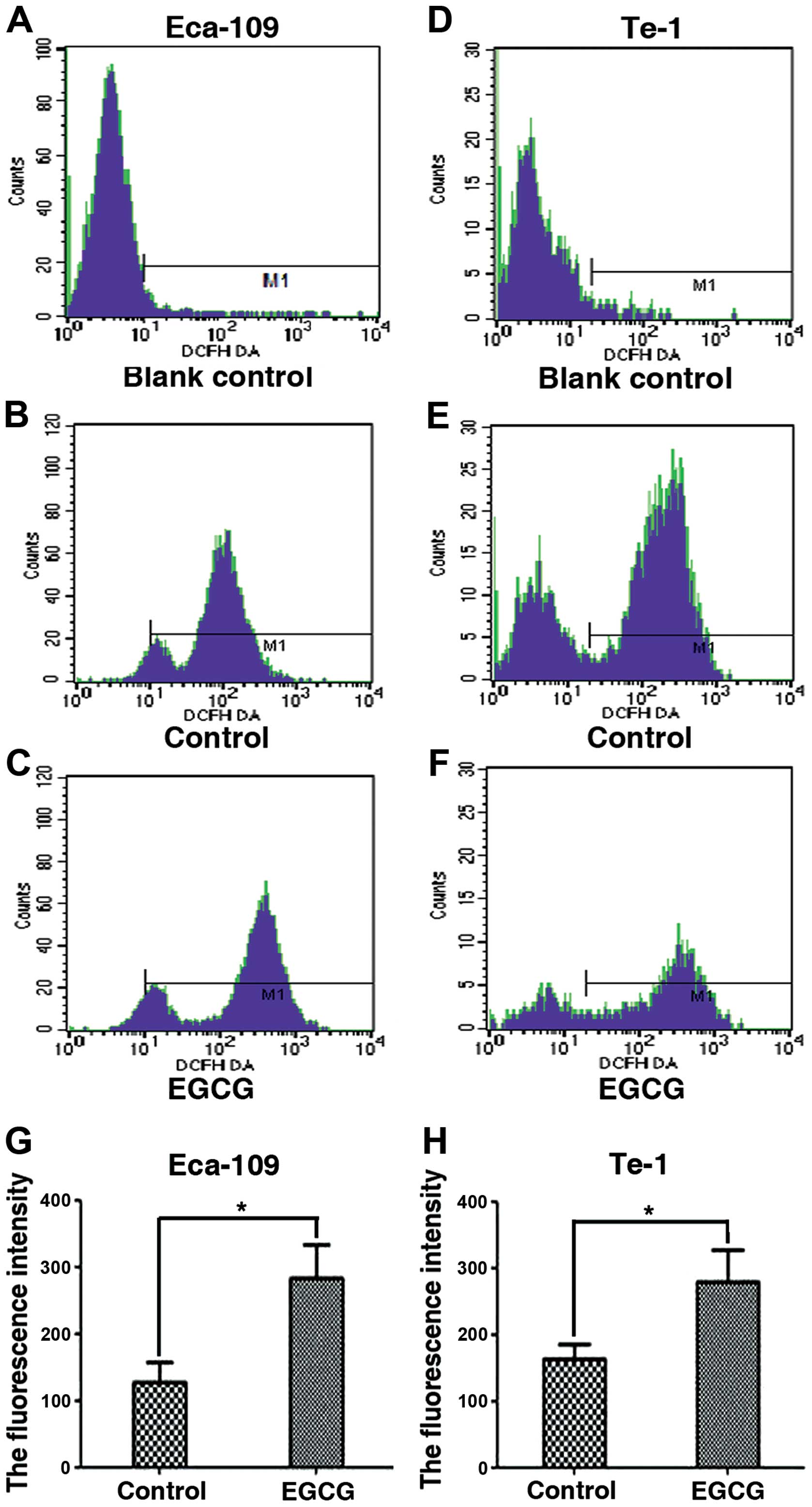
EGCG induces ROS production in Eca-109
and Te-1 cells
Since we observed that EGCG induces apoptosis, we
examined the production of ROS in Eca-109 and Te-1 cells treated
with an EGCG IC50 value for 24 h. Using the
ROS-sensitive probe, DCFH-DA, we found that the fluorescent
intensity was significantly increased in Eca-109 and Te-1 cells
compared to the control-treated cells (Fig. 4).
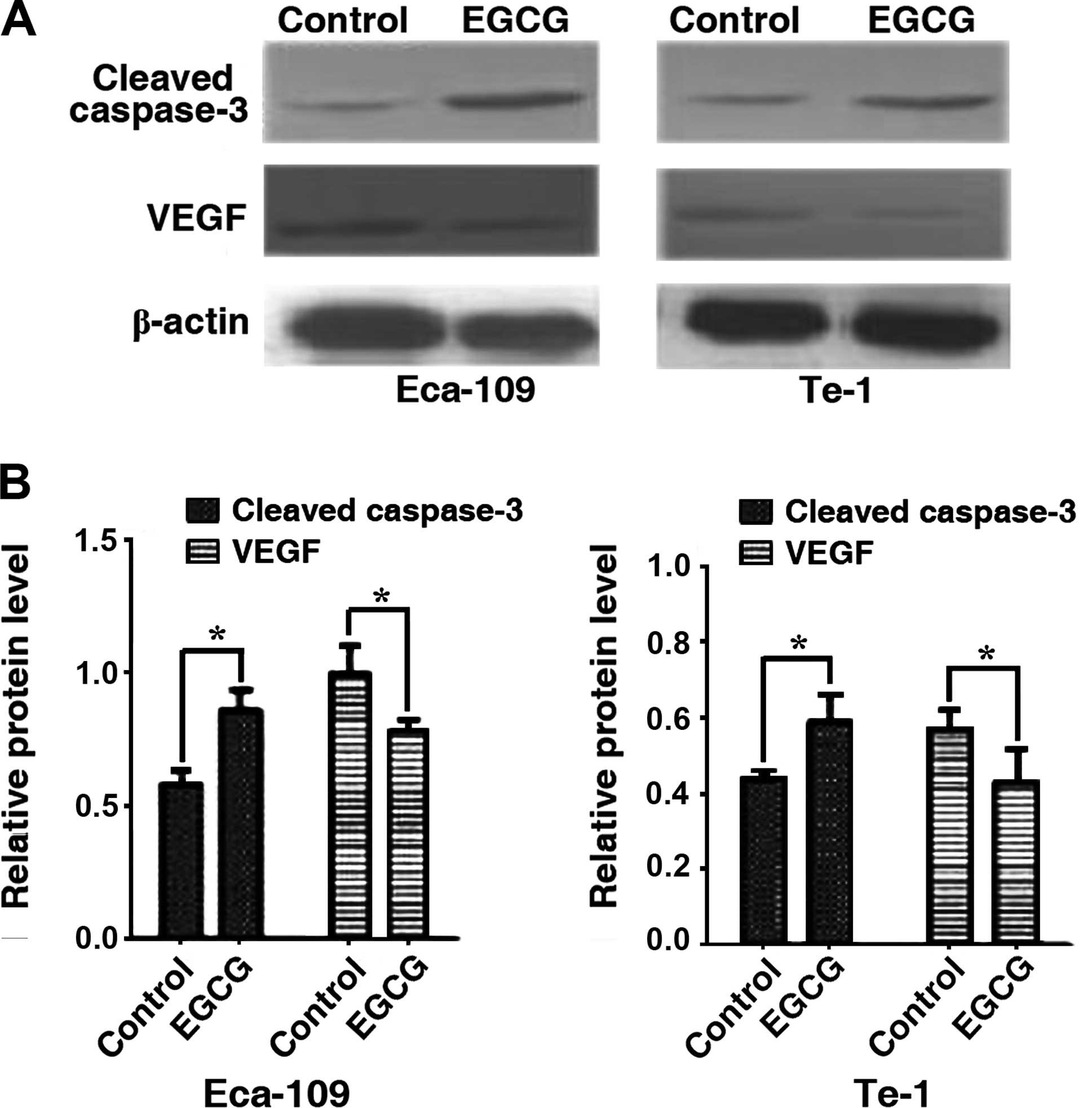
EGCG exhibits differing effects on
cleaved caspase-3 and VEGF expression
We assessed the effects of EGCG on the expression of
cleaved caspase-3 and VEGF by western blotting. Cleaved caspase-3
levels were significantly increased in Eca-109 and Te-1 cells after
24 h of treatment with the EGCG IC50 value while VEGF
expression was decreased significantly (Fig. 5). The results suggested that EGCG
can simultaneously increase cleaved caspase-3 and decrease VEGF
protein levels.
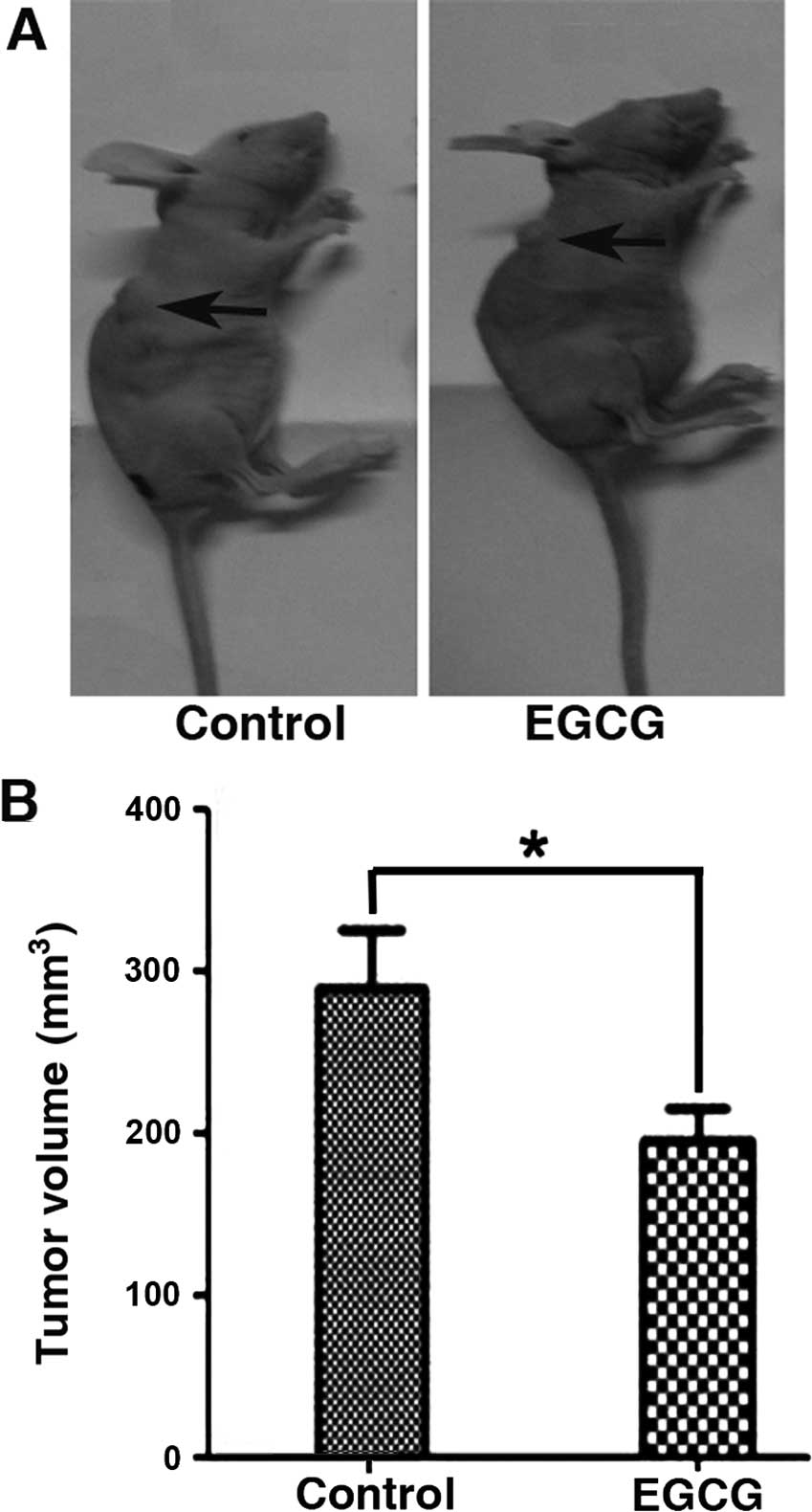
EGCG inhibits tumor growth in vivo
To expand the in vitro studies, we tested the
effects of EGCG on tumor growth in vivo. Eca-109 EC cells
were subcutaneously implanted in the flank of nude mice for two
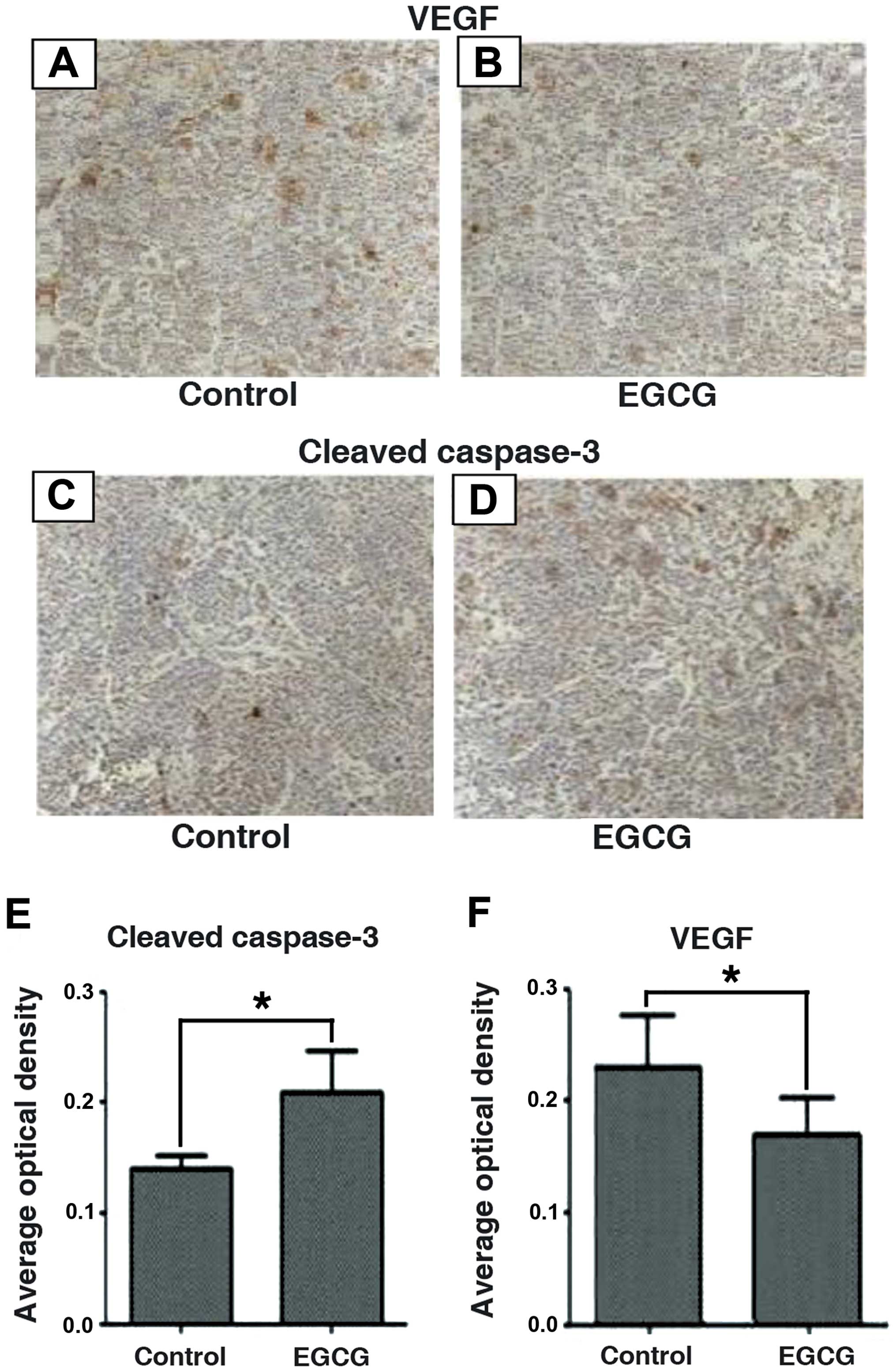
weeks (Fig. 6). Tumor tissues were
processed and analyzed by immunohistochemistry for cleaved
caspase-3 and VEGF proteins (Fig.
7). Consistent with the in vitro data, we confirmed that
EGCG significantly inhibited tumor growth by increasing caspase-3
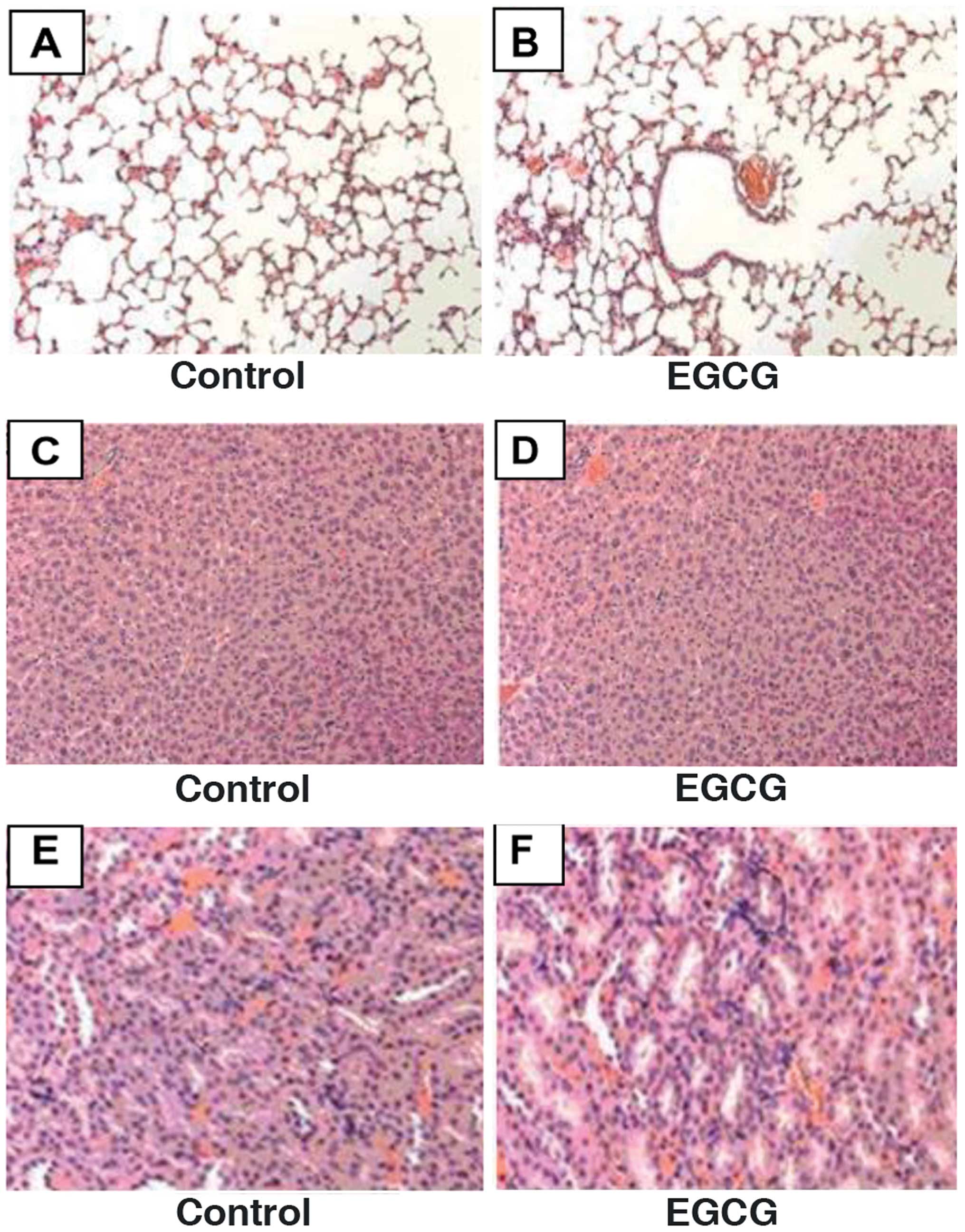
cleavage and decreasing VEGF protein expression. Furthermore,
H&E staining showed that EGCG was non-toxic to normal tissues
(Fig. 8).
Discussion
In the present study, we have demonstrated that EGCG
is able to inhibit the growth of malignant Eca-109 and Te-1
esophageal cancer cells in vitro and in vivo. Eca-109
and Te-1 cells were arrested in the G1 phase of the cell
cycle and underwent apoptotic cell death following exposure to
EGCG. ROS production was increased in Eca-109 and Te-1 cells in
vitro. The same effect has been reported in in vitro
studies on human cervical cancer and hepatocellular carcinoma
(21,22). EGCG-treated xenograft models
demonstrated reduced tumor growth, an increased expression of
cleaved caspase-3, and decreased VEGF protein levels. The results
suggest that EGCG effectively inhibits esophageal squamous cell
carcinoma by inducing apoptosis and caspase-3 expression and
suppressing VEGF expression.
Conflicting results have been reported regarding
cell cycle arrest by EGCG. Findings of a recent study showed that
EGCG induced cell cycle arrest in G2/M phase in the
epithelioid malignant mesothelioma-derived REN cells (18). Ma et al (23) reported that poorly differentiated
AGS gastric cancer cells were arrested at S phase by EGCG. Thakur
et al (24) demonstrated
that HCT116 colon cancer cells were arrested at G1
phase. In the present study, we observed that Eca-109 and Te-1
cells were arrested at G1 phase.
EGCG acts as an antioxidant (25), and possesses significant pro-oxidant
activity (26). Reactive oxygen
species (ROS) were reported to be responsible for EGCG-induced
apoptosis in mesothelioma (27) and
EGCG-induced ROS production in endometrial carcinoma cells
(28). However, ROS were not
involved in EGCG-induced apoptosis in human laryngeal epidermoid
carcinoma (Hep2) cells (14),
although EGCG reduced deoxynivalenol-induced ROS in HT-29 cells
(29). In the present study, ROS
were found to contribute to EGCG-induced apoptosis in Eca-109 and
Te-1 cells.
In the present study, cleaved caspase-3 expression
was increased by EGCG in vitro and in vivo. EGCG was
reported to sensitize hepatocellular carcinoma HepG2
cells to apoptosis by increasing caspase-3 activity (7). Mitochondria play a crucial role in
many apoptotic responses (30).
Stress signals cause mitochondria to release cytochrome c,
leading to activated caspase-3 as the critical executioner of
apoptosis (31). In endometrial
carcinoma, high levels of ROS caused oxidative stress and EGCG was
demonstrated to induce apoptosis by ROS generation and increase
caspase-3 (28). Consistent with
the findings of the aforementioned studies, the present study
reports that EGCG-induced apoptosis in Eca-109 and Te-1 cell lines
by increasing caspase-3 activity and ROS generation. Due to cross
signaling of intrinsic and extrinsic apoptotic pathways, more
studies are required to elucidate the molecular mechanism
involved.
The VEGF signaling pathway has been identified in
the angiogenic process (32,33).
This process is required for the growth of normal and tumor tissues
such as esophageal carcinomas (34). Consistent with in vitro and
in vivo results from the present study, EGCG has been shown
to inhibit tumor growth by downregulating VEGF expression (35,36).
Although EGCG shows a cytoprotective effect at low
concentrations (10–20 μM) (37), it
has been reported that consumption of green tea-derived supplements
at a high dose (120 mg/kg) can produce toxic effects in rodents
(38). However, in the present
study we found that EGCG showed no obvious toxicity in normal rat
cardiomyocytes, human foreskin fibroblast, liver, spleen or kidney
tissues of xenograft mice. The results may be due to a selective
effect on certain types of cancer (39). Thus, additional investigations into
EGCG toxicity are necessary.
In conclusion, our in vitro and in
vivo studies confirmed the growth inhibition of human
esophageal carcinoma cell lines, Eca-109 and Te-1 in xenograft
models. EGCG arrested the growth of cancer cells in the
G1 phase, induced apoptosis and ROS generation,
decreased VEGF levels, and activated caspase-3 without affecting
normal tissues.
Acknowledgements
We would like to thank Professor Chen Huang at Xi’an
Jiaotong University for providing the platform to conduct
experiments and giving expert advice, and Dr Ming Zhang for sharing
the human foreskin fibroblast cell line.
References
|
1
|
Demeester SR: Epidemiology and biology of
esophageal cancer. Gastrointest Cancer Res. 3(Suppl 1): S2–S5.
2009.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chowdhury FU, Bradley KM and Gleeson FV:
The role of 18F-FDG PET/CT in the evaluation of
oesophageal carcinoma. Clin Radiol. 63:1297–1309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi J, Kim SG, Kim JS, Jung HC and Song
IS: Comparison of endoscopic ultrasonography (EUS), positron
emission tomography (PET), and computed tomography (CT) in the
preoperative locoregional staging of resectable esophageal cancer.
Surg Endosc. 24:1380–1386. 2010. View Article : Google Scholar
|
|
5
|
Salahudeen HM, Balan A, Naik K, Mirsadraee
S and Scarsbrook AF: Impact of the introduction of integrated
PET-CT into the preoperative staging pathway of patients with
potentially operable oesophageal carcinoma. Clin Radiol.
63:765–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noble F and Bailey D; SWCIS Upper
Gastrointestinal Tumour Panel. Tung K and Byrne JP: Impact of
integrated PET/CT in the staging of oesophageal cancer: a UK
population-based cohort study. Clin Radiol. 64:699–705. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abou El Naga RN, Azab SS, El-Demerdash E,
Shaarawy S, El-Merzabani M and Ammar el-SM: Sensitization of
TRAIL-induced apoptosis in human hepatocellular carcinoma HepG2
cells by phytochemicals. Life Sci. 92:555–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farabegoli F, Papi A, Bartolini G, Ostan R
and Orlandi M: (−)-Epigallocatechin-3- gallate downregulates Pg-P
and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine.
17:356–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Nam HJ, Kang HJ, Kwon HW and Lim
YC: Epigallocatechin-3-gallate attenuates head and neck cancer stem
cell traits through suppression of Notch pathway. Eur J Cancer.
49:3210–3218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lecumberri E, Dupertuis YM, Miralbell R
and Pichard C: Green tea polyphenol epigallocatechin-3-gallate
(EGCG) as adjuvant in cancer therapy. Clin Nutr. 32:894–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian Y, Guan T, Huang M, et al:
Neuroprotection by the soy isoflavone, genistein, via inhibition of
mitochondria-dependent apoptosis pathways and reactive oxygen
induced-NF-κB activation in a cerebral ischemia mouse model.
Neurochem Int. 60:759–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forbes-Hernández TY, Giampieri F,
Gasparrini M, Mazzoni L, Quiles JL, Alvarez-Suarez JM and Battino
M: The effects of bioactive compounds from plant foods on
mitochondrial function: a focus on apoptotic mechanisms. Food Chem
Toxicol. 68:154–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwak TW, Kim do H, Chung CW, Lee HM, Kim
CH, Jeong YI and Kang DH: Synergistic anticancer effects of
vorinostat and epigallocatechin-3-gallate against HuCC-T1 human
cholangiocarcinoma cells. Evid Based Complement Alternat Med.
2013:1851582013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH, Jeong YJ, Lee SW, et al: EGCG
induces apoptosis in human laryngeal epidermoid carcinoma Hep2
cells via mitochondria with the release of apoptosis-inducing
factor and endonuclease G. Cancer Lett. 290:68–75. 2010. View Article : Google Scholar
|
|
15
|
Sakamoto Y, Terashita N, Muraguchi T,
Fukusato T and Kubota S: Effects of epigallocatechin-3-gallate
(EGCG) on A549 lung cancer tumor growth and angiogenesis. Biosci
Biotechnol Biochem. 77:1799–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spagnuolo P, Rasini E, Luini A, et al:
Isoflavone content and estrogenic activity of different batches of
red clover (Trifolium pratense L.) extracts: an in vitro study in
MCF-7 cells. Fitoterapia. 94:62–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eskandani M, Hamishehkar H and Ezzati
Nazhad Dolatabadi J: Cytotoxicity and DNA damage properties of
tert-butylhydroquinone (TBHQ) food additive. Food Chem.
153:315–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valenti D, de Bari L, Manente GA, Rossi L,
Mutti L, Moro L and Vacca RA: Negative modulation of mitochondrial
oxidative phosphorylation by epigallocatechin-3 gallate leads to
growth arrest and apoptosis in human malignant pleural mesothelioma
cells. Biochim Biophys Acta. 1832:2085–2096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tran PL, Kim SA, Choi HS, Yoon JH and Ahn
SG: Epigallo-catechin-3-gallate suppresses the expression of HSP70
and HSP90 and exhibits anti-tumor activity in vitro and in vivo.
BMC Cancer. 10:2762010. View Article : Google Scholar
|
|
20
|
Hwang YS, Park KK and Chung WY:
Epigallocatechin-3 gallate inhibits cancer invasion by repressing
functional invadopodia formation in oral squamous cell carcinoma.
Eur J Pharmacol. 715:286–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh M, Singh R, Bhui K, Tyagi S, Mahmood
Z and Shukla Y: Tea polyphenols induce apoptosis through
mitochondrial pathway and by inhibiting nuclear factor-κB and Akt
activation in human cervical cancer cells. Oncol Res. 19:245–257.
2011. View Article : Google Scholar
|
|
22
|
Shan X, Li Y, Meng X, Wang P, Jiang P and
Feng Q: Curcumin and (−)-epigallocatechin-3-gallate attenuate
acrylamide-induced proliferation in HepG2 cells. Food Chem Toxicol.
66:194–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Shi M, Li G, et al: Regulation of
Id1 expression by epigallocatechin-3-gallate and its effect on the
proliferation and apoptosis of poorly differentiated AGS gastric
cancer cells. Int J Oncol. 43:1052–1058. 2013.PubMed/NCBI
|
|
24
|
Thakur VS, Ruhul Amin AR, Paul RK, et al:
p53-Dependent p21-mediated growth arrest pre-empts and protects
HCT116 cells from PUMA-mediated apoptosis induced by EGCG. Cancer
Lett. 296:225–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai CF, Hsu YW, Ting HC, Huang CF and Yen
CC: The in vivo antioxidant and antifibrotic properties of green
tea (Camellia sinensis, Theaceae). Food Chem. 136:1337–1344. 2013.
View Article : Google Scholar
|
|
26
|
Kim HS, Quon MJ and Kim JA: New insights
into the mechanisms of polyphenols beyond antioxidant properties;
lessons from the green tea polyphenol, epigallocatechin 3-gallate.
Redox Biol. 2:187–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Satoh M, Takemura Y, Hamada H, Sekido Y
and Kubota S: EGCG induces human mesothelioma cell death by
inducing reactive oxygen species and autophagy. Cancer Cell Int.
13:192013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manohar M, Fatima I, Saxena R, Chandra V,
Sankhwar PL and Dwivedi A: (−)-Epigallocatechin-3-gallate induces
apoptosis in human endometrial adenocarcinoma cells via ROS
generation and p38 MAP kinase activation. J Nutr Biochem.
24:940–947. 2013. View Article : Google Scholar
|
|
29
|
Kalaiselvi P, Rajashree K, Bharathi Priya
L and Padma VV: Cytoprotective effect of epigallocatechin-3-gallate
against deoxynivalenol-induced toxicity through anti-oxidative and
anti-inflammatory mechanisms in HT-29 cells. Food Chem Toxicol.
56:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Susin SA, Lorenzo HK, Zamzami N, et al:
Molecular characterization of mitochondrial apoptosis-inducing
factor. Nature. 397:441–446. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao Y, Li W, Jia L, Li B, Chen YC and Tu
Y: Enhancement of (−)-epigallocatechin-3-gallate and
theaflavin-3-3′-digallate induced apoptosis by ascorbic acid in
human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma
Eca-109 cells via MAPK pathways. Biochem Biophys Res Commun.
438:370–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deindl E: Mechanistic insights into the
functional role of vascular endothelial growth factor and its
signalling partner brain-derived neurotrophic factor in angiogenic
tube formation. Acta Physiol. 211:268–270. 2014. View Article : Google Scholar
|
|
33
|
Gorman JL, Liu ST, Slopack D, et al:
Angiotensin II evokes angiogenic signals within skeletal muscle
through co-ordinated effects on skeletal myocytes and endothelial
cells. PLoS One. 9:e855372014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takala H, Saarnio J, Wiik H, Ohtonen P and
Soini Y: HIF-1α and VEGF are associated with disease progression in
esophageal carcinoma. J Surg Res. 167:41–48. 2011. View Article : Google Scholar
|
|
35
|
Hsieh DS, Wang H, Tan SW, Huang YH, Tsai
CY, Yeh MK and Wu CJ: The treatment of bladder cancer in a mouse
model by epigallocatechin-3-gallate-gold nanoparticles.
Biomaterials. 32:7633–7640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimizu M, Shirakami Y, Sakai H, et al:
(−)-Epigallocatechin gallate inhibits growth and activation of the
VEGF/VEGFR axis in human colorectal cancer cells. Chem Biol
Interact. 185:247–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Valenti D, De Rasmo D, Signorile A, et al:
Epigallocatechin-3-gallate prevents oxidative phosphorylation
deficit and promotes mitochondrial biogenesis in human cells from
subject with Down’s syndrome. Biochim Biophys Acta. 1832:542–552.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galati G, Lin A, Sultan AM and O’Brien PJ:
Cellular and in vivo hepatotoxicity caused by green tea phenolic
acids and catechins. Free Radic Biol Med. 40:570–580. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Min NY, Kim JH, Choi JH, et al: Selective
death of cancer cells by preferential induction of reactive oxygen
species in response to (−)-epigallocatechin-3-gallate. Biochem
Biophys Res Commun. 421:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|