Introduction
Glioblastoma multiforms (GBMs) are among the most
aggressive types of cancer. They are very invasive, poorly operable
and highly resistant to both conventional chemotherapy and
radiotherapy. Hence, their prognosis remains poor and the survival
rate of patients with GBM is extremely low due to the local
evolution of the tumor. Resistance to anticancer drugs and
radiation therapy (RT) results in large part from the incapacity of
GBM cells to undergo apoptosis in response to these treatments
(1).
Regarding RT, the use of high-linear energy transfer
(LET) radiation instead of the low-LET radiation currently used in
conventional therapy allows for more efficient anticancer treatmens
due to ballistic distribution, inductor of hypoxic cell death or
both (2). On the other hand,
standard chemotherapy of GBM principally consists in the
administration of temozolomide (TMZ), an alkylating agent that is
capable of crossing the hematoencephalic barrier. Moreover,
molecularly targeted drugs are currently being evaluated in
clinical trials, alone or combined with radiation, and the initial
preclinical data are encouraging (3–7). This
is the case of everolimus (RAD001), which acts as a potent
inhibitor of the mechanistic target of rapamycin (mTOR), a key
downstream protein kinase in the phosphatidylinositol 3-kinase
(PI3K/AKT) pathway. The rationale for using RAD001 in cancer
therapy is that mTOR expression is augmented with increasing grade
of malignancy in a number of tumors including brain cancers
(8) and that aberrant mTOR
activation has been linked to cancer progression (9). This mTOR pathway can be hyperactivated
by excessive stimulation via mutated growth factor receptors of ras
(10). Considering their reciprocal
advantages, combining high-LET radiation, classical and
molecular-targeted drugs may thus represent a pleiotropic and
potentially effective approach for the treatment of GBM.
In the present study, we investigated the effect of
combining RAD001, TMZ and low-LET or high-LET radiation on the
growth of U-87, a PTEN-deficient human GBM cell line (10). In our experiments, fast neutrons
provided high-LET radiation. Although these particles are no longer
used in radiation therapy in most countries, they still remain
appropriate models for exploring the biological effects of high-LET
radiation (11). We report here
that a pronounced antiproliferative and cytotoxic effect resulted
from this triple combination. As expected, at the same physical
dose of radiation, fast neutrons were more efficient than γ-rays at
depressing cell growth and at inducing cell death. We further
sought to determine how cell death occurred in U-87 cells submitted
to this triple combination.
Materials and methods
Treatments
RAD001 was provided by Novartis (Basel,
Switzerland). A stock solution was dissolved in dimethylsulfoxide
(DMSO) at 40 mM, stored at −20°C and final dilutions were prepared
in culture medium. TMZ was purchased from Sigma-Aldrich
(Saint-Quentin Fallavier, France) and was also dissolved in DMSO in
a 1 mM stock solution. Both treatments, alone or together, were
added to the cells 24 h before irradiation.
Cell culture
The human glioblastoma U-87 MG cell line was
purchased from the American Type Culture Collection (#HBT-14; ATCC;
Rockville, MD, USA). Cultures were maintained at 37°C in a
humidified atmosphere of 5% CO2. Cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM; PAN Biotech GmbH,
Dominique Dutscher, Brumath, France) supplemented with 10% fetal
bovine serum (FBS; PAN Biotech GmbH), 1 mM sodium pyruvate, 1 mM
non-essential amino acids and 50 μg penicillin-streptomycin (PAN
Biotech GmbH). Disaggregation was carried out by a 10-min
incubation at 37°C with a solution of trypsin-EDTA (PAN Biotech
GmbH).
Irradiation
Cells, in their exponential growth phase, were
exposed at room temperature to low-LET or high-LET radiation, 24 h
after the addition of the treatment to the culture medium. Cells
were contained in 6-well plates or in 96-well flat-bottomed
microplates, filled with 4 ml or 0.2 ml culture medium,
respectively. Low-LET irradiations were carried out with a
137Cs γ-irradiator (Biobeam GM8000; GSM GmbH, Leipzig,
Germany) at the Paul Strauss Center (Strasbourg, France). The dose
rate was 3.4 Gy/min and doses ranged from 2 to 8 Gy. For high-LET
radiation, cells were exposed to a beam of p(65) + Be neutrons
produced by a Cyclone cyclotron at the Cyclotron Resources Center
(CRC) (Louvain-la-Neuve, Belgium). The dose rate was usually 0.2
Gy/min and doses ranged from 2 to 8 Gy. A series of preliminary
experiments with neutrons was conducted at iThemba Labs (Somerset
West, South Africa). Each experiment was carried out at least three
times. Control flasks were sham treated and/or irradiated.
Cell proliferation assay
The effects of the treatments, alone or combined, on
the growth of U-87 cells were investigated using sulforhodamine B
(SRB; Sigma-Aldrich) colorimetric assay. Cells were seeded at a
density of 5×103 cells/well in 100 μl in 96-well
flat-bottomed plates (Falcon 3072). Subsequently, 100 μl of
different dilutions of RAD001 with/without TMZ were added 24 h
later to quintuplicate wells. Cells were then irradiated and
incubated at 37°C for 6 days. Then, they were fixed with 10%
trichloroacetic acid (TCA; Sigma-Aldrich) for 1 h at 4°C, washed
five times with tap water, air dried and stained with 0.4% SRB in
1% acetic acid for 30 min. SRB-stained cells were then dissolved in
200 μl 10 mM Tris-base (pH 10.5), and the absorbance of each well
was measured at 565 nm using a Synergy™ HT microplate reader
(Biotek, Colmar, France). Results are expressed in optical density
(OD), after subtraction of the value for the blank (no cells).
Clonogenic survival assay
Cells were trypsinized and collected 24 h after
irradiation in the presence or absence of treatment and enumerated
using a Countess® Cell Counter (Countess; Invitrogen,
Carlsbad, CA, USA). Then, they were seeded at an appropriate number
and plated at two different dilutions into 6-well plates. The
treatments were performed in triplicate and the experiments were
repeated three times. Fifteen days later, the clones were stained
with 0.05% crystal violet (Sigma-Adrich) in a 5% ethanol solution,
and positive colonies (>50 cells) were scored. The plating
efficiency was calculated by dividing the number of positive
colonies that grew in the absence of treatment and irradiation by
the number of cells that were seeded.
Apoptotic detection assay
Apoptotic cells were quantified 6 days after
irradiation according to Riccardi and Nicoletti (12). Briefly, cells (5×105)
were fixed in cold 70% ethanol for at least 1 h, then they were
washed in phosphate buffered saline [PBS; (pH 7.2)] and resuspended
in 100 μl of PBS containing 25 μg of RNase A, 2 mM EDTA and 10 μg
of propidium iodide (PI). After incubation in the dark at 37°C for
30 min, the fluorescence of 10,000 cells was analyzed using a
FACScan flow cytometer and Cell Quest software (both from Becton
Dickinson, San Jose, CA, USA). Cells with a sub-diploid DNA content
were recorded as being apoptotic.
Autophagic detection assay
For autophagy determination 6 days after
irradiation, we used the Cyto-ID™ Autophagy detection kit (Enzo
Life Sciences, Plymouth Meeting, PA, USA) according to the
manufacturer’s instructions. This test measures autophagic vacuoles
and monitors autophagic flux in live cells using a fluorescent
cationic amphiphilic dye that selectively labels autophagic
vacuoles. Briefly, cells (5×105) were washed in PBS (pH
7.2), and resuspended in 500 μl of freshly diluted
Cyto-ID® Green Detection Reagent to a final volume of 2
ml with PBS, according to the manufacturer’s instructions. The
fluorescence of 10,000 cells was analyzed using a FACScan flow
cytometer and Cell Quest software (Beckton Dickinson).
Determination of γ-H2AX foci
Cells were grown on microscopic glass slides placed
in 6-well plates. Twenty-four hours after irradiation, the culture
medium was removed and the slides were washed once with PBS.
Fixation and permeabilization were carried out using 4%
formaldehyde and 0.5% Triton, according to a standard procedure.
Labeling was performed using a monoclonal mouse anti-γ-H2AX
polyclonal antibody (clone JBW301; Upstate, Lake Placid, NY, USA).
Coverslips were mounted in 4′-6-diamidino-2-phenylindole
(DAPI)-stained Vectashield (Abcys, Paris, France). The formation of
γ-H2AX foci in nuclei were monitored by immunofluorescence
microscopic imaging using an Olympus BH-2 fluorescence microscope
equipped with a digital camera. Foci were counted in 50 cells in
each condition.
Statistical analysis
All experiments were repeated at least three times
independently. Statistical analyses were performed using the Prism
5 statistical software. Differences between the subgroups in term
of cell counts, proportion of apoptotic and autophagic cells and
foci number were evaluated using an ANOVA, together with a
Student-Newman-Keuls test of all pairwise possible comparisons.
Differences between subgroups were considered statistically
significant at P<0.05.
Results
Proliferation study
We first evaluated the ability of different
concentrations of TMZ to decrease cell growth either alone, or
combined with RAD001 and radiation using SRB assays. TMZ was added
at increasing concentrations, ranging from 10 to 50 μM. Based on a
previous study (13) RAD001 was
used at a concentration of 10 nM. Cells were left unirradiated or
irradiated at 2 Gy with both types of radiation. At this dose,
γ-rays as well as neutrons alone failed to significantly reduce the
growth of U-87 cells, consistent with the known relative
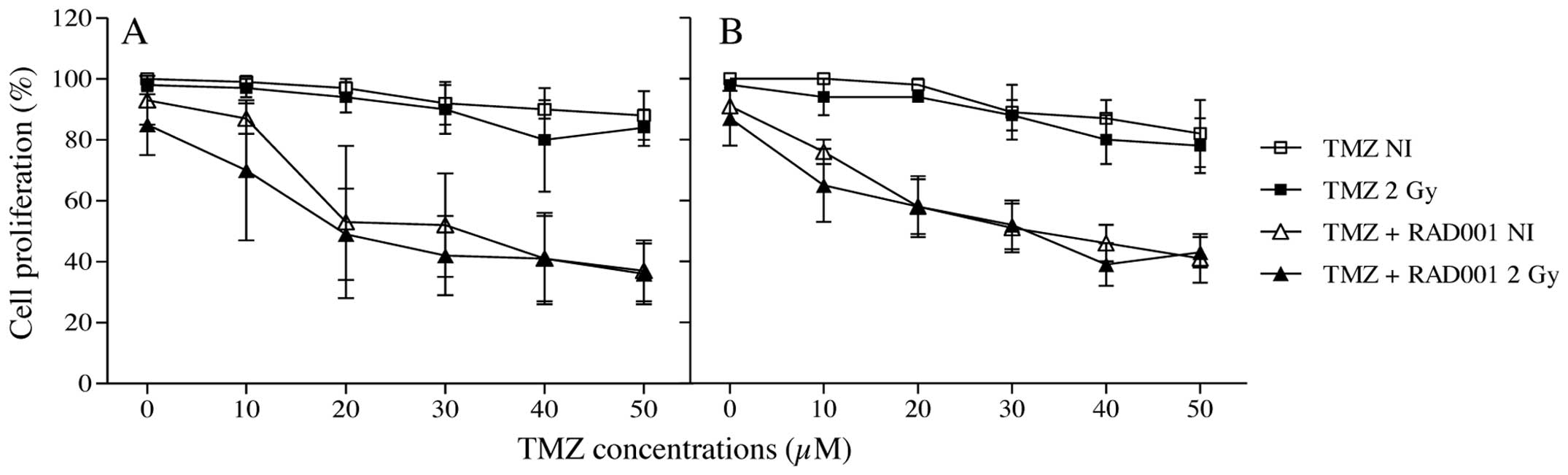
radioresistance of this cell line. As shown in Fig. 1, in the unirradiated cells, a slight
decrease in the viable cell number was noted at higher
concentrations of TMZ. Contrarily, in RAD001/TMZ co-treated
unirradiated cells, a marked decrease in growth was noted. In
γ-irradiated cells, a marked reduction in cell growth was obtained
in the RAD001 co-treated cells, as a function of TMZ concentration
(Fig. 1A), confirming previous
results (13). Nevertheless, no
significant differences were observed between both treatments with
or without irradiation. Similar results were obtained in the
neutron-irradiated cells (Fig.
1B).
In subsequent experiments, a unique TMZ
concentration (30 μM) was retained and cells treated with RAD001,
TMZ and the combination RAD001/TMZ were irradiated at increasing
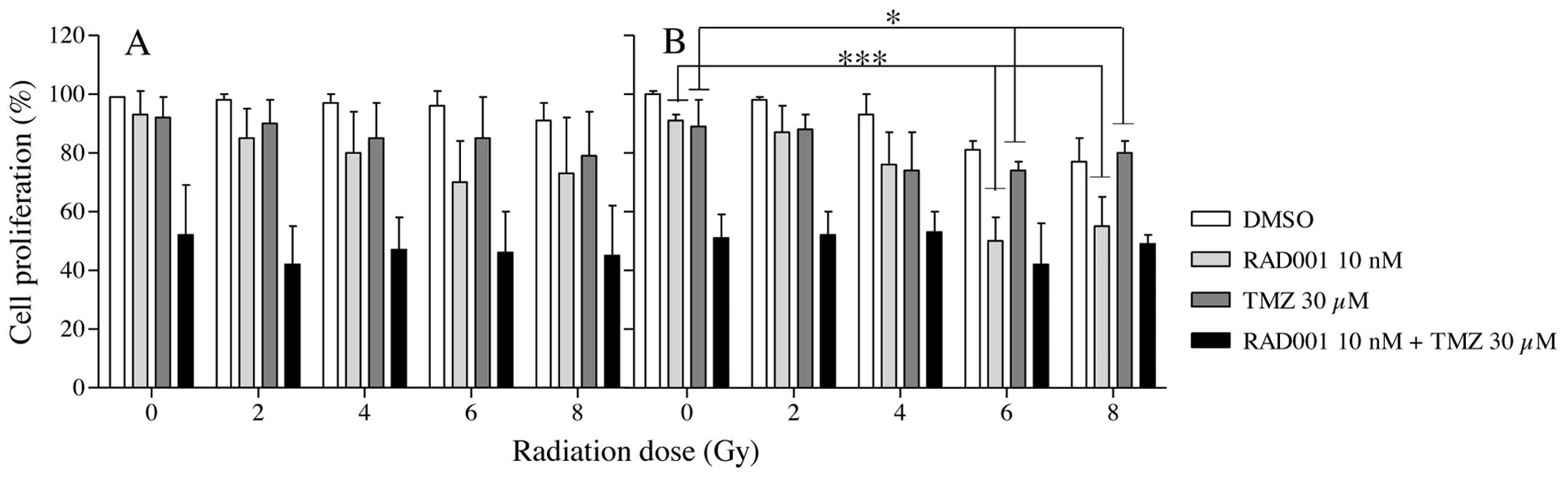
doses of either γ-rays or neutrons. As shown in Fig. 2A, γ radiation alone had no
significant effect on cell growth. The identical outcome was
obtained with RAD001- or TMZ-treated U-87 cells whatever the
irradiation dose. In contrast, in the RAD001/TMZ co-treated cells,
a significant reduction in cell growth was obtained compared with
the control (DMSO), RAD001 or TMZ independently from the delivered
dose and the radiation type. In neutron-irradiated cells (Fig. 2B), no decrease in cell growth was
observed in the treated cells, according to the radiation dose. In
the RAD001-treated cells, a marked decrease in proliferation was
recorded from 2 to 6 Gy. At the same dose range, in TMZ-treated
cells, a slight decrease was observed. The most important effect in
cell proliferation was recorded in the RAD001/TMZ co-treated cells
without a difference from unirradiated to 8 Gy as obtained with
γ-irradiated cells. To conclude, with or without exposure to either
type of radiation, the combination RAD001/TMZ strongly depressed
the proliferation of U-87 GBM cells.
Clonogenic survival study
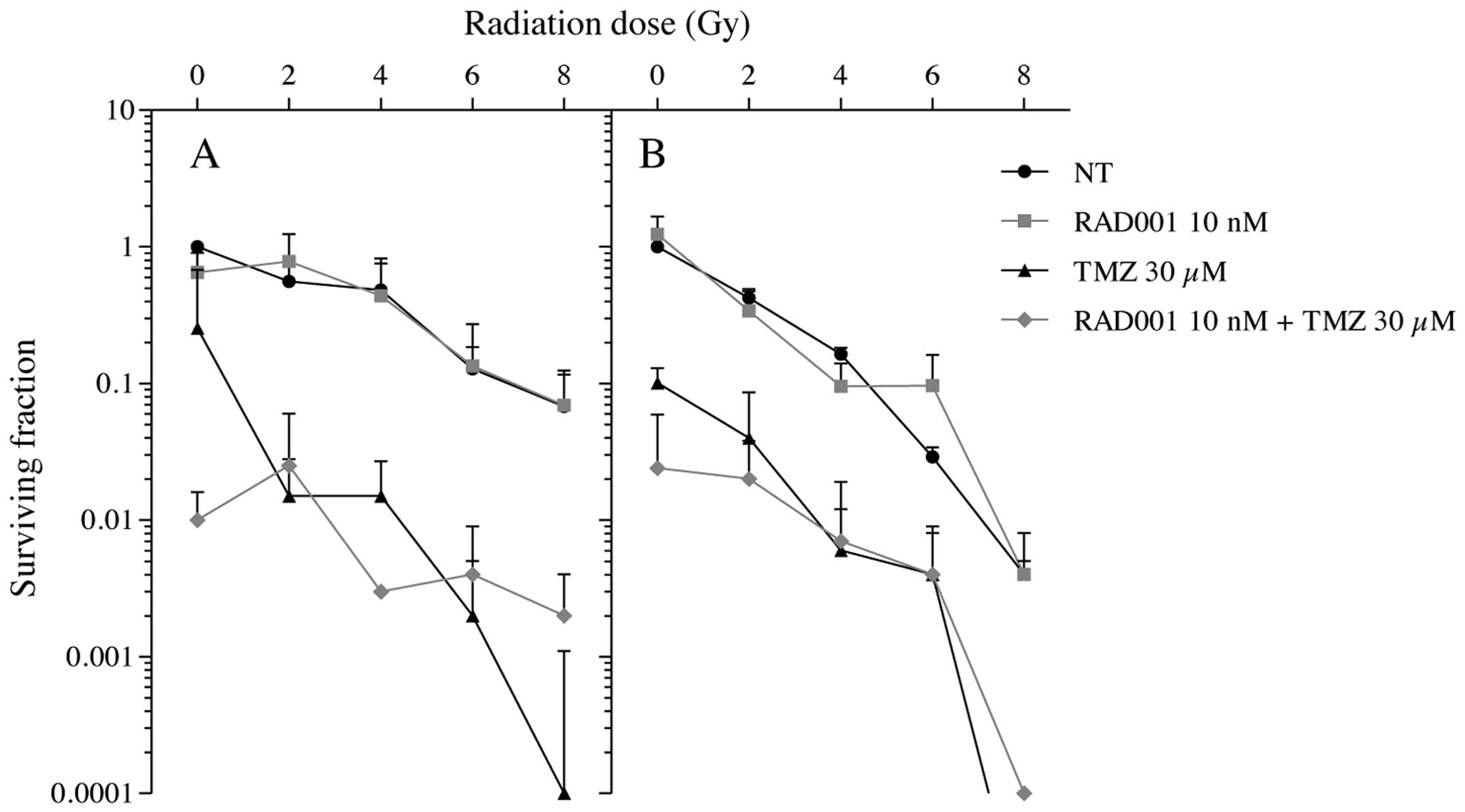
In order to extend these results, colony-formation
assays were carried out. With photons alone, we obtained a typical
shoulder of the survival curve (Fig.
3A) although with neutrons alone, a sharp decrease in the
number of colonies was observed (Fig.
3B) reaching a more efficient decrease in cell survival than
with photons. Once combined with RAD001, no significant changes of
this effect could be measured with both types of radiation. In
contrast, in the TMZ-treated cells, a drastic decrease in the
clonogenic survival was obtained as a function of the radiation
dose whatever the radiation type. In irradiations with neutrons,
the combination RAD001/TMZ was found to be much more efficient at
reducing the clonogenic survival than with γ-rays.
Cell death induction
We next sought to determine what type of cell death
was caused by treatment with TMZ, RAD001 and radiation, applied
separately or in association. Percentages of cells undergoing
apoptotic and autophagic cell death were determined at day 6 after
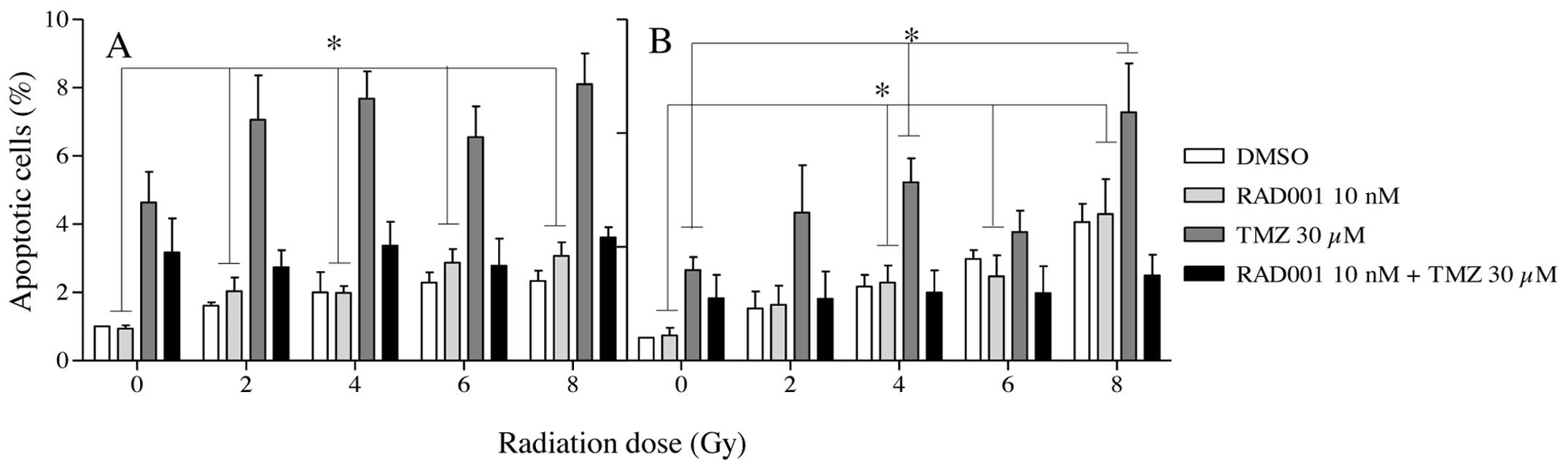
irradiation. As shown in Fig. 4A and
B, apoptosis levels were very low in all groups of treated
cells, independently of the radiation type and dose. The highest
values of apoptosis were recorded in the TMZ-treated cells, but
apoptosis never exceeded 8%. In cells submitted to the treatment
RAD001/TMZ, these values were in fact decreased compared to those
treated with TMZ alone.
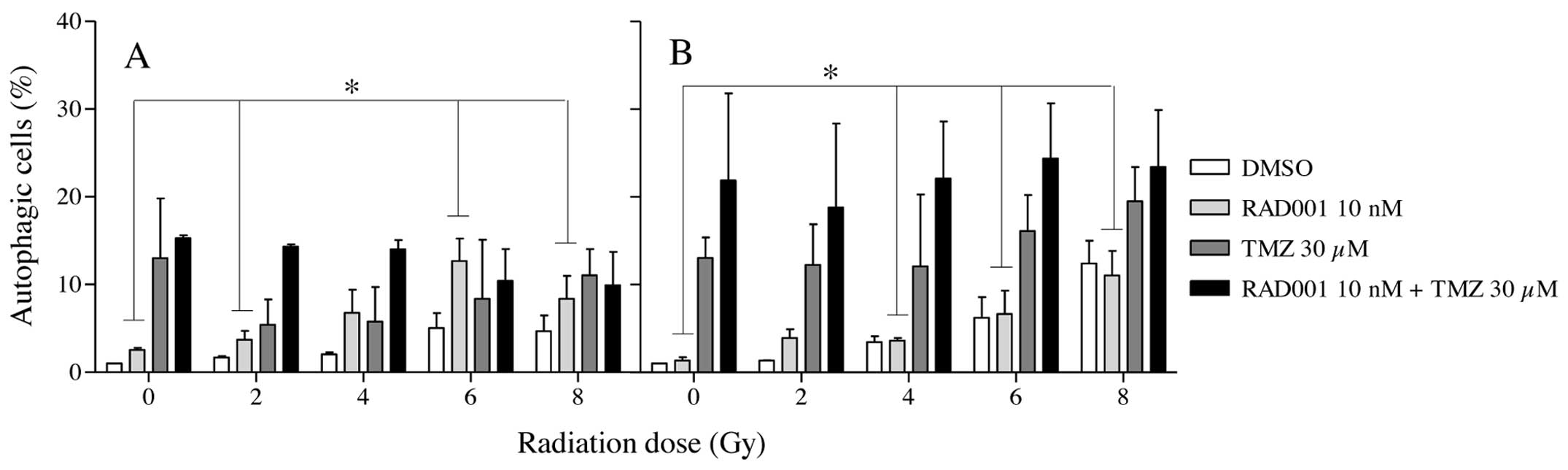
Comparatively with apoptosis, autophagy levels were
found to be higher in TMZ and RAD001/TMZ co-treated cells but
again, irradiation failed to significantly increase the numbers of
autophagic cells (Fig. 5A and B).
It must be noted that in the neutron experiments, autophagy was
always found to be higher in the TMZ unirradiated cells than in the
γ-irradiated ones. Consequently, neither apoptosis nor autophagy
could entirely account for the loss of survival in cells submitted
to the triple combination.
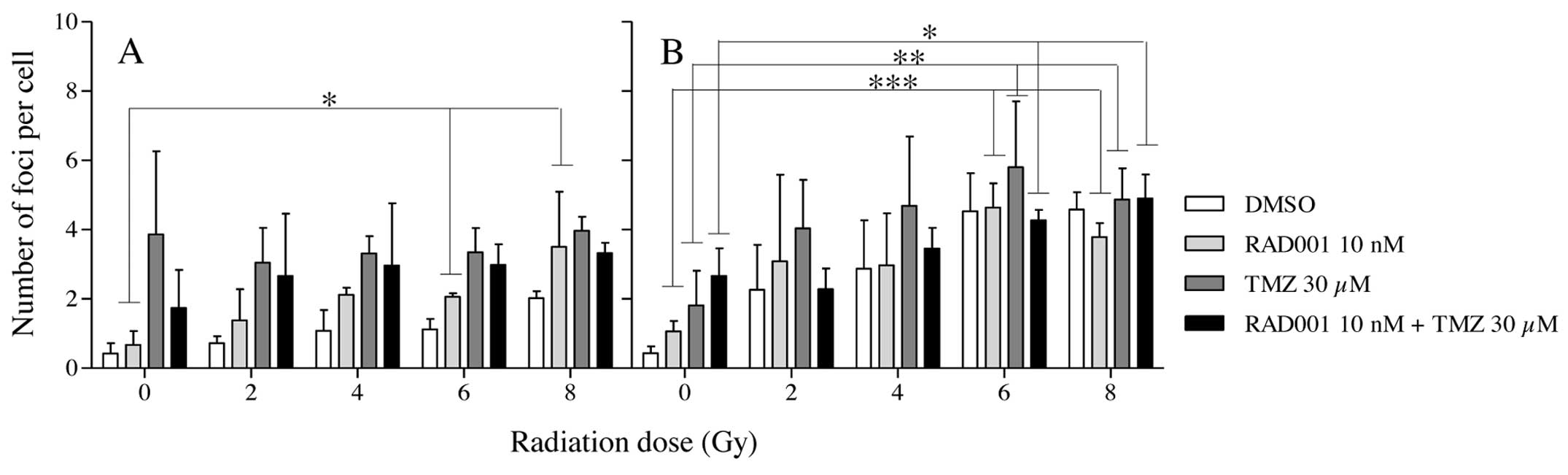
γ-H2AX activation and persistence
Since severe and irreparable damages to DNA, such as
double-strand breaks (DSBs), are generally at the origin of cell
death, foci corresponding to the persistent activation of γ-H2AX in
control and treated cells were recorded 24 h after the exposure to
both types of radiation. As shown in Fig. 6A, in γ-irradiated cells, the foci
number remained at a low level in all groups. Rather surprisingly,
the radiation dose did not significantly increase their number. In
contrast, foci numbers were slightly increased in the
neutron-irradiated cells according to dose (Fig. 6B). TMZ alone or combined with RAD001
did not significantly affect their occurrence.
Discussion
We report the ability of a triple treatment
associating RAD001, TMZ and irradiation with low-LET or high-LET
radiation to reduce the growth and the survival of glioblastoma
cells in vitro. Our results indicate that cell death
occurred partly by autophagy, independently of the irradiation, and
that apoptosis was not involved in cell destruction, consistent
with our previous findings in the hepatocellular carcinoma cell
line, SK-Hep-1 (14). These data
suggest that other modes of cell death may contribute to the
effectiveness of the combined treatment with RAD001, TMZ and
irradiation. In addition, they showed that the radiation LET does
not influence the outcome of this triple association treatment,
since γ-radiation as well as fast neutrons gave similar results.
Together, the present findings clearly emphasize the interest of
this trimodal therapeutic approach to circumvent the resistance of
glioblastoma cells to conventional treatments. Molecular mechanisms
underlying the efficacy of such a combination remain to be
analyzed.
An important feature of our investigations is the
comparative effect resulting from the combination of RAD001 with
high-LET particles or low-LET radiations. In radiation therapy, the
former offers two main advantages over the latter for the treatment
of some types of cancers (15).
First, high-LET particles can be delivered with a high accuracy for
normal tissue volume, due to the Braggs distribution in depth of
the radiation dose. Second, unlike sparsely ionizing low-LET
radiation, high-LET particles provoke clustered severe and
irreparable damage in DNA (16,17).
As a consequence, they kill or inactivate malignant cells more
effectively than conventional, photon-based RT.
Another aspect of the present study concerns the
mode of cell death triggered by the triple combined treatment. It
is known that in glioblastoma cells, several forms of cell death,
including apoptosis (18),
autophagy (19), necroptosis
(20) and senescence (10) could underlie the cytotoxic response
to radiation. In the present case, our results clearly indicate
that apoptosis participates very little in the efficacy of the
co-treatment. In contrast, in RAD001/TMZ co-treated cells,
autophagy was significantly increased, but the irradiation did not
reinforce its occurrence. In fact, in a previous study (13), we reported that the combination TMZ
with RAD001 was able to synergize for reducing U-87 cell growth and
to induce autophagy of these cells. The importance of autophagy, an
important catabolic process of degradation of cellular components
for eukaryotic cells in physiological as well as in pathological
situations (21,22) still remains debated. In radiation
therapy, it is not yet established whether autophagy plays a
protective role toward radiation or a cell death inductor function
(23). The induction of autophagy
has also been suggested to influence the response of glioma cells
to radiation by promoting their differentiation and their
radiosensitivity (24). On the
other hand, whether the mechanisms underlying radiosensitization by
RAD001 or more recent mechanistic target of rapamycin (mTOR)
inhibitors are linked to the induction of autophagy remains
questionable. In fact, many pathways involved in mTORC1 and mTORC2,
could be potentially affected by radiation (25). Actually, in a number of tumor cell
lines, the inhibition of mTORC1 has been reported to decrease
rather than increase the radiosensitivity of tumor cells in
vitro. This was shown in HeLa, a cervical adenocarcinoma cell
line following pretreatment with rapamycin followed by irradiation.
However, the increased radioresistance was observed only when
rapamycin was added to the cells before irradiation, indicating
that mTOR inhibition may sometimes promote radioprotection
(26). The balance between various
factors involved in cell death, in the activation of cell survival
pathways and in DNA damage repair processes could account for these
differences. The latter point appears to be the most critical for
determining the cell fate. Interestingly, rapamycin has been
reported to suppress DSB repair (27). Thus, one may assume that when
combined together, RAD001, TMZ and radiation could mutually
reinforce their reciprocal action by converging towards the
induction of irreversible DNA damage.
In conclusion, our results highlight the potential
therapeutic value of associating a conventional chemotherapeutic
agent, a molecular-targeted drug and radiation, to substantially
suppress GBM cell growth. They also indicate that in the context of
this triple combination, low-LET radiations are almost as efficient
as high-LET radiations in producing such a growth-suppressing
effect. Thus, the benefit of using high-LET radiation rather than
conventional low-LET radiations may be questioned, all the more so
since linear accelerators of the latest generation that are now
increasingly used in anticancer centers allow for much higher
precision in focusing on the tumor mass than earlier systems.
Therefore, high doses of low-LET radiation, such as 6 Gy or higher,
could be allocated in one fraction along with TMZ and RAD001
without severely affecting healthy surrounding tissues.
Nevertheless, it would be hazardous to conclude from the present
results that high-LET radiation has no role to play in the
treatment of GBM. Indeed, when administered alone or associated
with TMZ, high-LET radiation is more capable of suppressing
malignant cell growth than low-LET radiation. Moreover, apart from
their targeting advantage, they differ from low-LET radiation in
their capacity to produce much more lethal damage in the critical
structure of cells. Given this unique specificity, one may assume
that molecular-targeted drugs other than mTOR inhibitors could be
more efficiently combined with high-LET radiation. Finally,
determining the types of cell death and particularly the role of
autophagy induced by this trimodal treatment represents another
challenge for researchers. We are currently further exploring these
points.
Acknowledgements
The present study was supported in part by funds
from the University of Strasbourg, France and from the Cyclotron
Resources Center of the Catholic University of Louvain, Belgium. We
thank Dr Francis J. Dumont for reviewing the manuscript.
References
|
1
|
Emdad L, Qadeer ZA, Bederson LB, Kothari
HP, Uzzaman M and Germano IM: Is there a common upstream link for
autophagic and apoptotic cell death in human high-grade gliomas?
Neuro Oncol. 13:725–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maucort-Boulch D, Baron MH, Pommier P,
Weber DC, Mizoe JE, Rochat J, Boissel JP, Balosso J, Tsuji H and
Amsallem E: Rationale for carbon ion therapy in high-grade glioma
based on a review and a meta-analysis of neutron beam trials.
Cancer Radiother. 14:34–41. 2010. View Article : Google Scholar
|
|
3
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scaringi C, Enrici RM and Minniti G:
Combining molecular targeted agents and radiation therapy for
malignant gliomas. Onco Targets Ther. 6:1079–1095. 2013.
|
|
5
|
Chinnaiyan P, Won M, Wen PY, Rojiani AM,
Wendland M, Dipetrillo TA, Com BW and Mehta MP: RTOG 0913: a phase
1 study of daily everolimus (RAD001) in combination with radiation
therapy and temozolomide in patients with newly diagnosed
glioblastoma. Int J Radiat Oncol Biol Phys. 86:880–884. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang M, Herion TW, Timke C, Han N, Hauser
K, Weber KJ, Peschke P, Wirkner U, Lahn M and Huber PE: Trimodal
glioblastoma treatment consisting of concurrent radiotherapy,
temozolomide and the novel TGF-β receptor I kinase inhibitor
LY2109761. Neoplasia. 13:537–549. 2011.PubMed/NCBI
|
|
7
|
Sarkaria JN, Galanis E, Wu W, Peller PJ,
Giannini C, Brown PD, Uhm JH, McGraw S, Jaeckle KA and Buckner JC:
North central cancer treatment group phase I trial N057K of
everolimus (RAD001) and temozolomide in combination with radiation
therapy in patients with newly diagnosed glioblastoma multiforme.
Int J Radiat Oncol Biol Phys. 81:468–475. 2011. View Article : Google Scholar
|
|
8
|
Annovazzi L, Mellai M, Caldera V, Valente
G, Tessitore L and Schiffer D: mTOR, S6 and AKT expression in
relation to proliferation and apoptosis/autophagy in glioma.
Anticancer Res. 29:3087–3094. 2009.PubMed/NCBI
|
|
9
|
Dancey J: mTOR signaling and drug
development in cancer. Nat Rev Clin Oncol. 7:209–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JJ, Kim BC, Park MJ, Lee YS, Kim YN,
Lee BL and Lee JS: PTEN status switches cell fate between premature
senescence and apoptosis in glioma exposed to ionizing radiation.
Cell Death Differ. 18:666–677. 2011. View Article : Google Scholar :
|
|
11
|
Gueulette J, Slabbert JP, Bischoff P,
Denis JM, Wambersie A and Jones D: Fast neutrons: Inexpensive and
reliable tool to investigate high-LET particle radiobiology. Radiat
Meas. 45:1414–1416. 2010. View Article : Google Scholar
|
|
12
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar
|
|
13
|
Josset E, Burckel H, Noël G and Bischoff
P: The mTOR inhibitor RAD001 potentiates autophagic cell death
induced by temozolomide in a glioblastome cell line. Anticancer
Res. 33:1845–1851. 2013.PubMed/NCBI
|
|
14
|
Altmeyer A, Josset E, Denis JM, Gueulette
J, Slabbert J, Mutter D, Noël G and Bischoff P: The mTOR inhibitor
RAD001 augments radiation-induced growth inhibition in a
hepatocellular carcinoma cell line by increasing autophagy. Int J
Oncol. 41:1381–1386. 2012.PubMed/NCBI
|
|
15
|
Rodriguez-Lafrasse C and Balosso J: From
the carbon track to therapeutic efficiency of hadrontherapy. Cancer
Radiother. 16:16–24. 2012.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hada M and Georgakilas AG: Formation of
clustered DNA damage after high-LET irradiation: a review. J Radiat
Res. 49:203–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamada N, Imaoka T, Masunaga S, Ogata T,
Okayasu R, Takahashi A, Kato TA, Kobayashi Y, Ohnishi T, Ono K,
Shimada Y and Teshima T: Recent advances in the biology of
heavy-ion cancer therapy. J Radiat Res. 51:365–383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuboi K, Moritake T, Tsuchida Y, Tokuuye
K, Matsumura A and Ando K: Cell cycle checkpoint and apoptosis
induction in glioblastoma cells and fibroblasts irradiated with
carbon beam. J Radiat Res. 48:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benzina S, Altmeyer A, Malek F, Dufour P,
Denis JM, Gueulette J and Bischoff P: High-LET radiation combined
with oxaliplatin induce autophagy in U-87 glioblastoma cells.
Cancer Lett. 264:63–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang YG, Peng Y and Koussougbo KS:
Necroptosis: a novel therapeutic target for glioblastoma. Med
Hypotheses. 76:350–352. 2011. View Article : Google Scholar
|
|
21
|
Todde V, Veenhuis M and van der Klei IJ:
Autophagy: principles and significance in health and disease.
Biochim Biophys Acta. 1792:3–13. 2009. View Article : Google Scholar
|
|
22
|
Hönscheid P, Datta K and Muders MH:
Autophagy: detection, regulation and its role in cancer and therapy
response. Int J Radiat Biol. Jun 25–2014.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zois CE and Koukourakis MI:
Radiation-induced autophagy in normal and cancer cells: towards
novel cytoprotection and radio-sensitization policies? Autophagy.
5:442–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhuang W, Li B, Long L, Chen L, Huang Q
and Liang Z: Induction of autophagy promotes differentiation of
glioma-initiating cells and their radiosensitivity. Int J Cancer.
129:2720–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dumont FJ and Bischoff P: Disrupting the
mTOR signaling network as a potential strategy for the enhancement
of cancer radiotherapy. Curr Cancer Drug Targets. 12:899–924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bandhakavi S, Kim YM, Ro SH, Xie H,
Onsongo G, Jun CB, Kim DH and Griffin TJ: Quantitative nuclear
proteomics identifies mTOR regulation of DNA damage response. Mol
Cell Proteomics. 9:403–414. 2010. View Article : Google Scholar :
|
|
27
|
Chen H, Ma Z, Vanderwaal RP, Feng Z,
Gonzales-Suarez I, Wang J, Roti Roti JL, Gonzalo S and Zhang J: The
mTOR inhibitor rapamycin suppresses DNA double-strand break repair.
Radiat Res. 175:214–224. 2011. View
Article : Google Scholar : PubMed/NCBI
|