Introduction
AMP-activated protein kinase (AMPK) primarily
functions as an energy sensor, and is a heterotrimer with a
catalytic subunit (α) and two regulatory subunits (β and γ)
(1). The AMPK catalytic subunit is
phosphorylated by the upstream kinases, liver kinase B1 (LKB1) and
calmodulin-dependent protein kinase kinase β (CaMKKβ) (2). Additionally, tumor-suppressor
proteins, such as LKB1 (3),
tuberous sclerosis complex 2 (TSC2) (4) and p53 (5), and oncoproteins, such as ERK and
cyclooxygenase-2 (COX-2) (6), are
involved in the AMPK signaling network and contribute to the
regulation of cell proliferation and death. Consistent with this
role, AMPK inhibits prosurvival growth pathways, mediates cell
cycle checkpoints and regulates mitotic progression (7). Recent studies have identified a
function for AMPK as a sensor of genomic stability, which is an
important mechanism for the inhibition of tumorigenesis (8). These AMPK activities are essential to
prevent carcinogenesis. AMPK also acts as a regulator of cell
motility. In a previous study, using melanoma cells, it was shown
that berberine-mediated AMPK activation inhibited melanoma cell
migration via inhibition of the ERK and COX-2 signaling pathways
(9). Moreover, the increased
phosphorylation of cytoplasmic linker protein-170, which is an AMPK
substrate, regulates cell migration via dynamic microtubule
assembly, and the LKB1-AMPK-S6K axis inhibits breast cancer cell
migration and invasion (10,11).
Broussonetia kazinoki belongs to the family
Moraceae and is distributed throughout Korea, Japan and
China. The leaves, branches and fruits of this medicinal plant have
been used in Chinese folk medicine as a diuretic, tonic and edema
suppressant. Previous investigations have demonstrated that this
plant exerts various beneficial effects, such as anti-oxidative
(12), cytotoxic (13), anti-hyperglycemic (14) and tyrosinase inhibitory activities
(15). Kazinol C is a 1,3-diphenyl
propane isolated from Broussonetia kazinoki. It enhances
antioxidant effects through Fyn inhibition and prevents arachidonic
acid and iron-induced cell death through the activation of the
LKB1-AMPK pathway (12). However,
the beneficial effects of kazinol C in cancer development are
poorly understood.
In the present study, we investigated whether
kazinol C induces AMPK activation and whether this induction
affects HT-29 colon cancer cell apoptosis and migration. Kazinol C
increases AMPK activity, which is critical in kazinol C-induced
cell apoptosis and inhibition of the metastatic potential of HT-29
colon cancer cells.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM),
glucose-free DMEM, neomycin (G418) and fetal bovine serum (FBS)
were obtained from Gibco-Invitrogen (Carlsbad, CA, USA). Propidium
iodide (PI), 12-O-tetradecanoylphorbol-13-acetate (TPA) and
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
were obtained from Sigma-Aldrich (St. Louis, MO, USA). We obtained
5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) and
antibodies recognizing the phospho-specific forms of
AMPKα-Thr172 and ACC-Ser79 from Cell
Signaling Technology (Boston, MA, USA). Antibodies against
α-actinin, poly (ADP-ribose) polymerase (PARP) and AMPKα were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Compound C was purchased from Calbiochem (San Diego, CA,
USA).
Cell cultures
HT-29 colon cancer cells (ATCC, Manassas, VA, USA)
were maintained in DMEM supplemented with 10% heat-inactivated FBS,
100 U/ml penicillin and 100 mg/ml streptomycin (Gibco-Invitrogen)
at 37°C in a 5% CO2 humidified incubator.
Kazinol C isolation
The air-dried root bark of Broussonetia
kazinoki (voucher specimen no. SPH 07002) (0.6 kg) was
extracted with ethanol. The extract (51 g) was fractionated into
n-hexane, EtOAc, CHCl3 and BuOH soluble fractions. The
EtOAc fraction (31 g) was subject to silica gel column
chromatography followed by eluting with an n-hexane/acetone
gradient system (20:1→1:10) and 11 fractions were collected as
previously described (16).
Fraction 7 was chromatographed using a silica gel column with
CHCl3:MeOH (100:1→10:1) to yield 6 subfractions.
Sub-fraction 3 of fraction 7 was chromatographed on a RP-C18 column
with a gradient MeOH (40→100%) elution to yield kazinol C (260 mg).
The purity was confirmed by HPLC analyses and 1H-NMR
spectrum. The chemical structure of kazinol C was elucidated by
spectroscopic analyses and confirmed by comparison with previous
studies (17).
Cell apoptosis and viability
Cell apoptosis was assessed using a
fluorescence-activated cell sorter (FACS). In brief, the cells were
treated with kazinol C for 24 h. The cells were harvested by
trypsinization and washed with phosphate-buffered saline (PBS).
After fixation in 70% ethanol, the cells were resuspended in PBS
containing 10 μg/ml PI. The fluorescence intensity was determined
using a FACSCanto™ II flow cytometer (BD Biosciences, San Jose, CA,
USA). Cell viability was assessed using the MTT assay. The cells
were treated with 5 μg/ml MTT solution and incubated for 3 h, and
then dissolved in DMSO. The absorbance was measured at 570 nm.
In vitro cell growth and soft agar colony
formation assays
For the in vitro cell growth analysis, the
cells were seeded in a 6-well plate and treated with 0–60 μM of
kazinol C for 24–48 h. The cells were harvested and counted using
the trypan blue exclusion test. To determine anchorage-independent
cell growth, 1×103 HT-29 cells were suspended in 1.5 ml
of the medium containing 0.3% agar, 10% FBS and 15 μM kazinol C,
and the mixture was applied onto pre-solidified 0.5% agar (1.5 ml)
in 6-well plates. After incubation of two weeks, soft agar colonies
were observed under a phase-contrast microscope and
photographed.
Wound healing assay
The cells were seeded in 6-well plates and incubated
for 12 h in starvation medium. The cellular monolayer was wounded
with a sterile 200-μl pipette tip and washed with starvation medium
to remove detached cells from the plates. The cells were pretreated
with 0–15 μM kazinol C for 30 min, and incubated in the presence or
absence of 40 ng/ml TPA for 36 h. The medium was replaced with PBS,
and the cells were photographed using a phase-contrast
microscope.
Determination of in vitro tumor cell
migration
The in vitro migration and invasion assays
were performed using a 24-well Transwell unit with polycarbonate
filters with a 6.5 mm diameter and 8.0-μm pore size (Corning
Costar, Cambridge, MA, USA). For the invasion assay, the lower
chamber of the Transwell was filled with DMEM plus 10% FBS as a
chemoattractant and the Transwell was assembled to serve as the
intervening invasive barrier in a 24-well plate. The cells
(5×104) were suspended in serum-free DMEM with 15 μM
kazinol C, added to the upper chamber of the Transwell and
incubated for 48 h at 37°C. The cells that attached to the upper
surface of the filter were completely removed by wiping with a
cotton swab, and the filters were stained with a 0.2% crystal
violet/20% methanol (w/v) solution.
Plasmid transfection
pcDNA3-AMPK dominant-negative (DN) form was prepared
as previously described (18). The
plasmid was transfected into HT-29 cells using PolyFect
Transfection Reagent (Qiagen, Valencia, CA, USA) according to the
manufacturer's instructions. Transfected cells were selected using
complete medium containing 1 mg/ml neomycin.
Western blotting
For the whole-cell lysate preparation, the treated
cells were lysed on ice in the PRO-PREP™ protein extraction
solution (iNtRON Biotechnology, Seoul, Korea) for 30 min at 4°C.
The supernatant fractions were recovered by centrifugation (14,000
× g for 20 min at 4°C), and the protein concentration was
determined using the Bradford protein assay. The samples were
prepared with 2-mercaptoethanol and denatured by heating at 95°C
for 3 min. The proteins were separated on 8–12% polyacrylamide gels
and transferred to nitrocellulose membranes. The membranes were
blocked with 1% bovine serum albumin or 5% skim milk and hybridized
with the primary antibody. Following hybridization with the
HRP-conjugated secondary antibody, the protein bands were
visualized using a chemiluminescence detection kit (Amersham
Pharmacia Biotech, Piscataway, NJ, USA) and a LAS-3000 or LAS-4000
imaging system (Fujifilm Corporation, Tokyo, Japan). The western
blotting band intensity was analyzed using Quantity One software
(Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
The results are presented as the means ± SD. The
data were analyzed for statistical significance using GraphPad
Prism 5 software (GraphPad Software, La Jolla, CA, USA).
Significant differences were analyzed using one-way ANOVA followed
by the Newman-Keuls multiple comparison tests >3 groups or the
unpaired t-test for two groups. P<0.05 was considered to
indicate a statistically significant result.
Results
Kazinol C induces apoptosis in HT-29
colon cancer cells
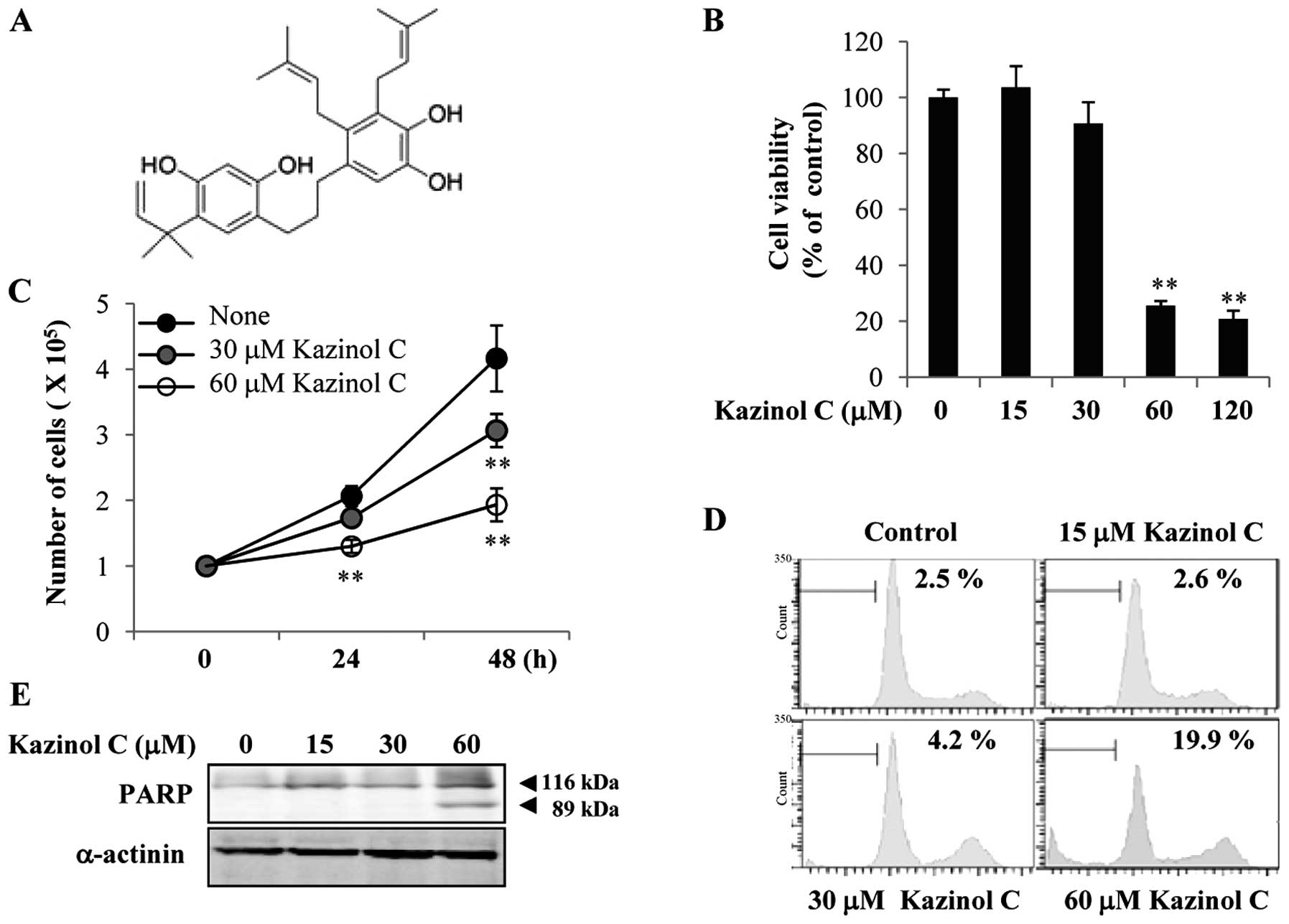
Kazinol C is an active component of Broussonetia
kazinoki. The kazinol C chemical structure used in the present
study is shown in Fig. 1A. Kazinol
C reportedly prevents cellular injury from reactive oxygen species
(12). However, the anticancer
effects of kazinol C remain unclear. In the present study, we first
examined the effects of kazinol C on HT-29 colon cancer cell death.
The cells maintained in complete medium were exposed to kazinol C
for 24 h. Cells exposed to low concentrations (<30 μM) of
kazinol C for 24 h exhibited no substantial increase in cell death,
as assessed by the MTT assay (Fig.
1B). However, high concentrations (60–120 μM) of kazinol C
significantly increased HT-29 cell death. Additionally, treatment
with 30–60 μM kazinol C for 48 h significantly reduced HT-29 cell
growth; however, cell growth was not affected by treatment with 30
μM kazinol C for 24 h (Fig. 1C). To
verify whether kazinol C induces apoptosis, we performed FACS
analysis of the sub-G1 DNA content. Low concentrations (<30 μM)
of kazinol C did not significantly affect HT-29 cell apoptosis at
24 h, whereas 60 μM kazinol C significantly induced apoptosis
(Fig. 1D). This result was
supported by PARP cleavage following treatment with 60 μM kazinol C
(Fig. 1E). Collectively, these
results showed that kazinol C effectively induces HT-29 cell
apoptosis.
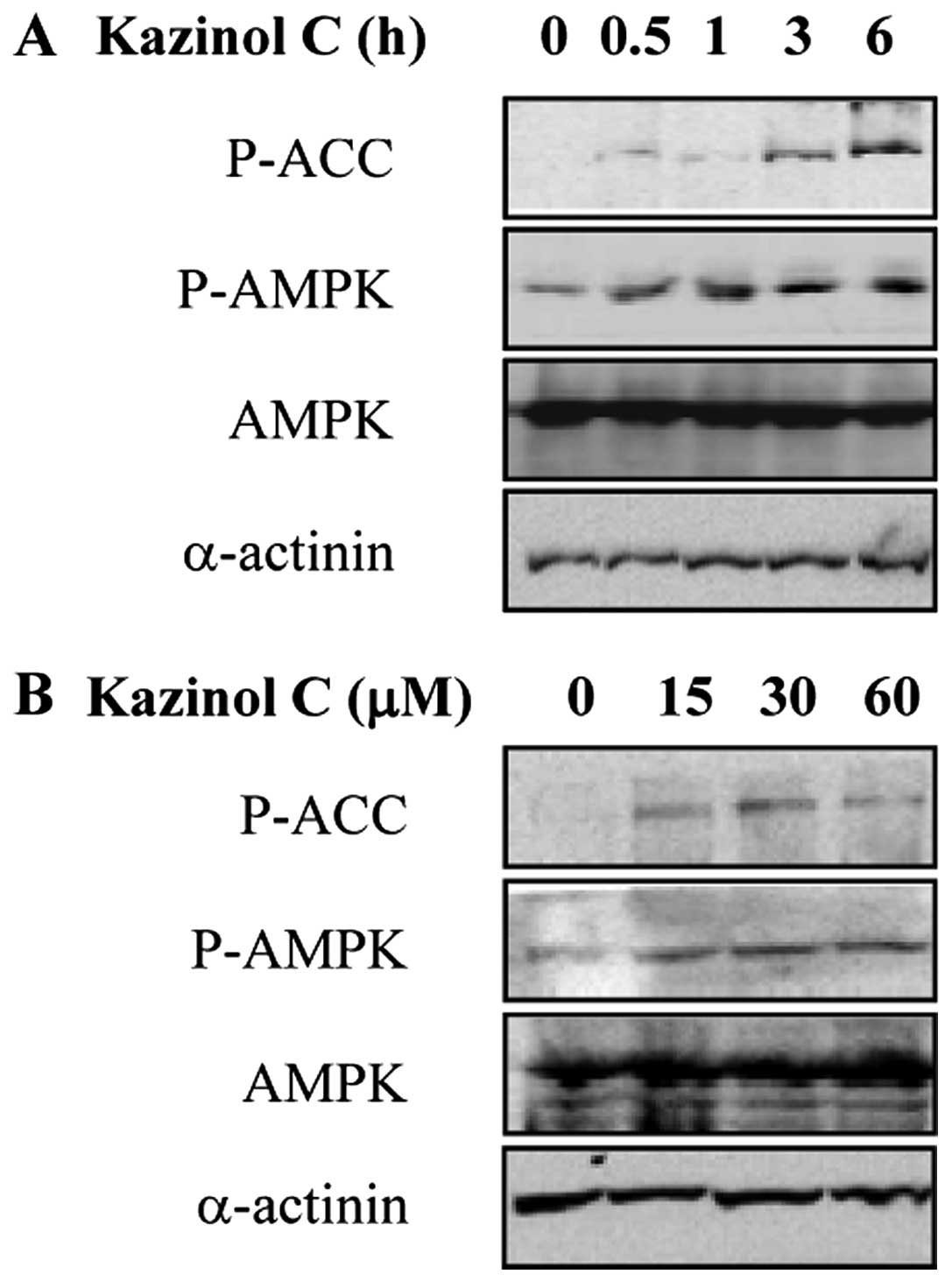
Kazinol C increases AMPK activity in
HT-29 colon cancer cells
AMPK activation results in apoptosis induction by
modulating signaling pathways involved in cell proliferation and
apoptosis (19). In our previous
screening for natural compounds that act as AMPK activators,
kazinol C was selected as a potent AMPK activator (data not shown).
To investigate the effect of kazinol C on AMPK activity in HT-29
cells, we treated the cells with kazinol C in a time- and
dose-dependent manner. Kazinol C markedly increased
Thr172 phosphorylation in the catalytic subunit of AMPK
and Ser79 phosphorylation in acetyl-CoA carboxylase
(ACC), which is a well-characterized AMPK cellular substrate
(Fig. 2A and B). These results
suggested that kazinol C activates AMPK.
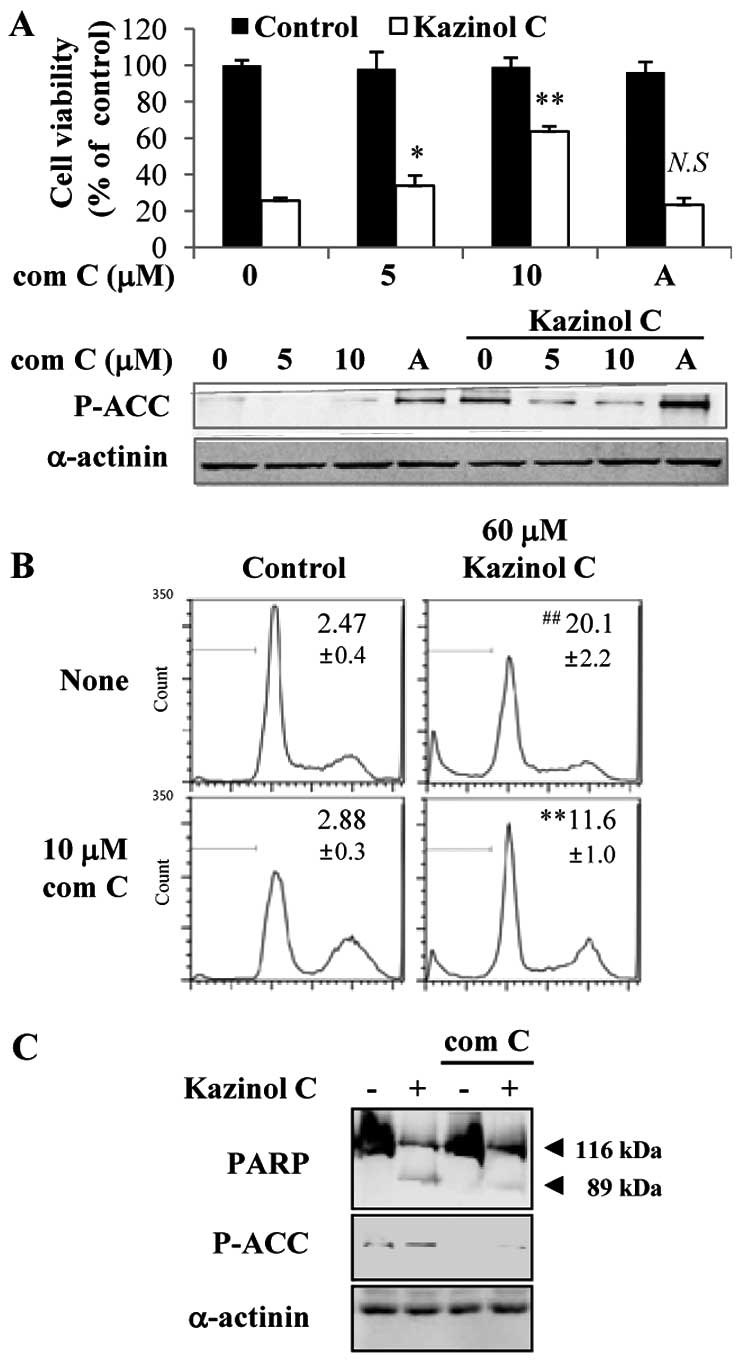
AMPK activation is critical for kazinol
C-induced HT-29 colon cancer cell apoptosis
To examine the causal relationship between AMPK
activation and kazinol C-induced cell death, the MTT assay was
performed after HT-29 cells were pretreated with the AMPK inhibitor
compound C or the AMPK activator AICAR. Compound C-mediated
inhibition of AMPK activity markedly abrogated the kazinol
C-induced cell death in a dose-dependent manner (Fig. 3A). By contrast, the AMPK activator
AICAR did not significantly affect kazinol C-induced cell death. Of
note, although the AICAR concentration did not induce cell death,
AICAR increased AMPK activity at this concentration (Fig. 3A). Moreover, compound C inhibited
kazinol C-induced apoptosis, as assessed by FACS analysis of the
sub-G1 DNA content (Fig. 3B) or
PARP cleavage (Fig. 3C). To confirm
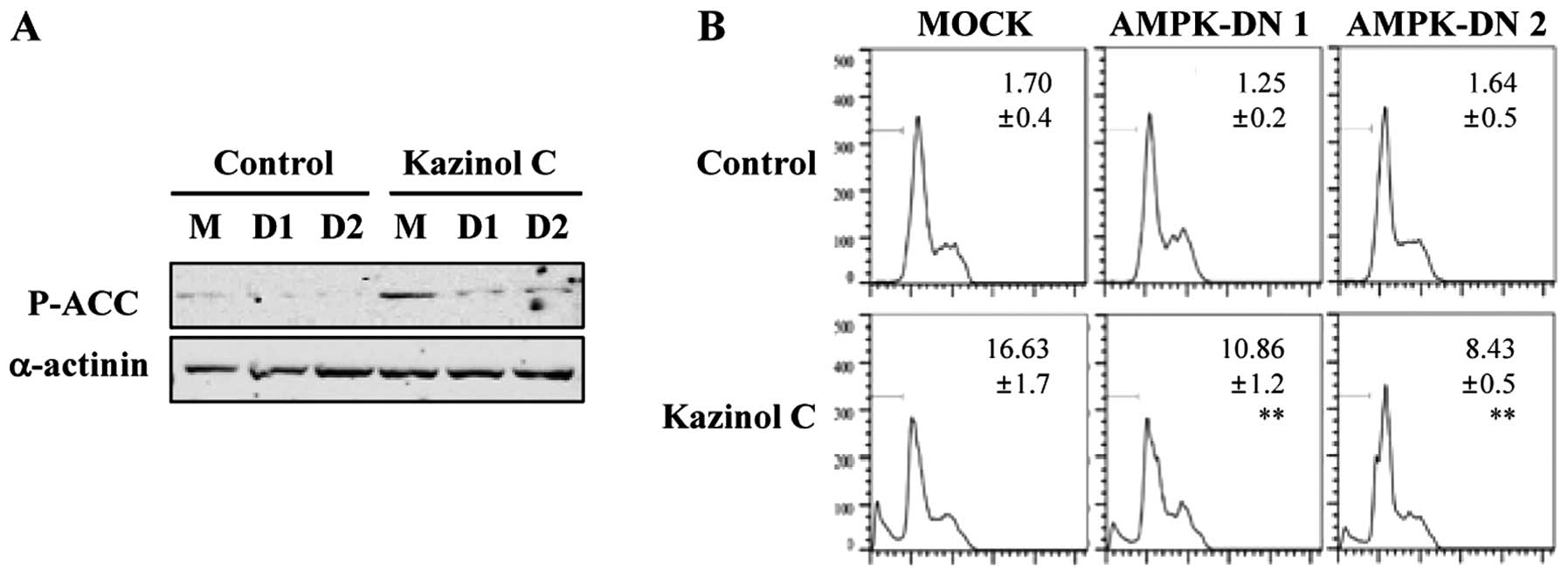
these results, we used a molecular approach to regulate AMPK
activity. Stable clones of HT-29 cells following transfection with
pcDNA3 or AMPK-DN plasmids were established. We confirmed that AMPK
activity was attenuated by the stable expression of the DN form of
AMPK and that kazinol C-induced AMPK activation was markedly
reduced by AMPK-DN expression (Fig.
4A). As demonstrated via FACS analysis of the sub-G1 DNA
content, kazinol C effectively induced cell apoptosis and
expression of the AMPK-DN form significantly abrogated kazinol
C-induced apoptosis (Fig. 4B).
These results suggested that kazinol C markedly increases HT-29
cell apoptosis through the activation of AMPK, which plays an
important role in apoptosis induction.
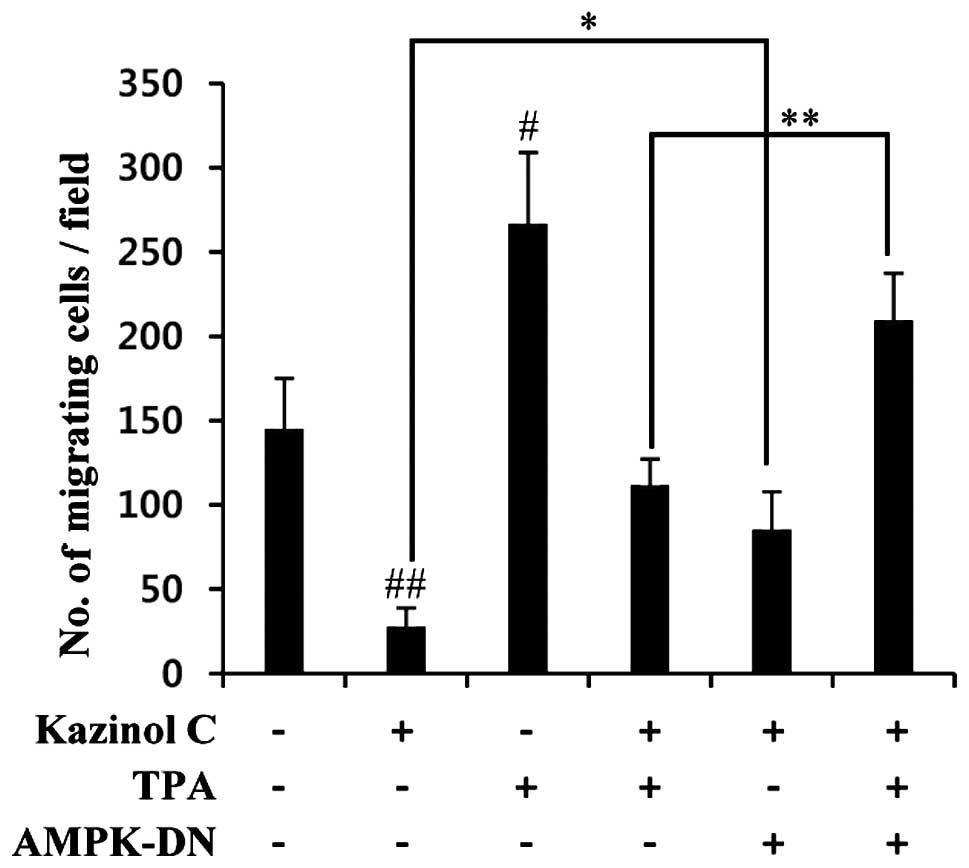
Kazinol C inhibits HT-29 cell migration
and anchorage-independent growth
Tumor metastasis is a multi-step process that
includes migration, invasion, adhesion, proliferation and
angiogenesis. To evaluate the effect of kazinol C on tumor
metastatic activity, we performed in vitro migration and
wound healing assays. As shown in Fig.
5A, 7.5–30 μM kazinol C did not significantly affect HT-29 cell
growth at 24 h. In HT-29 cells, kazinol C-treatment for 24 h
significantly reduced wound healing (Fig. 5B). Similar results were observed
with an in vitro migration assay (Fig. 5C). Given that TPA treatment
typically stimulates colon cancer cell migration, we determined the
effects of TPA on the kazinol C-induced inhibition of cell
migration. Notably, TPA treatment increased wound healing and HT-29
cell migration, whereas kazinol C markedly abrogated TPA-induced
wound healing and migration (Fig. 5B
and C). We tested the ability of cells to form colonies in a
semisolid medium as an indicator of metastatic potential. Untreated
and TPA-treated HT-29 cells formed sizable colonies and increased
proliferation in soft agar (Fig.
5D). However, kazinol C treatment significantly decreased basal
and TPA-induced cell proliferation in soft agar. To examine the
causal relationship between AMPK and kazinol C-induced inhibition
of migration, we used a molecular approach to inhibit AMPK
activity. AMPK inhibition via stable transfection with the AMPK-DN
form significantly abrogated kazinol C-mediated inhibition of
cancer cell migration (Fig. 6).
Collectively, these results suggested that kazinol C inhibits the
migration and anchorage-independent growth of HT-29 cells through
AMPK activation.
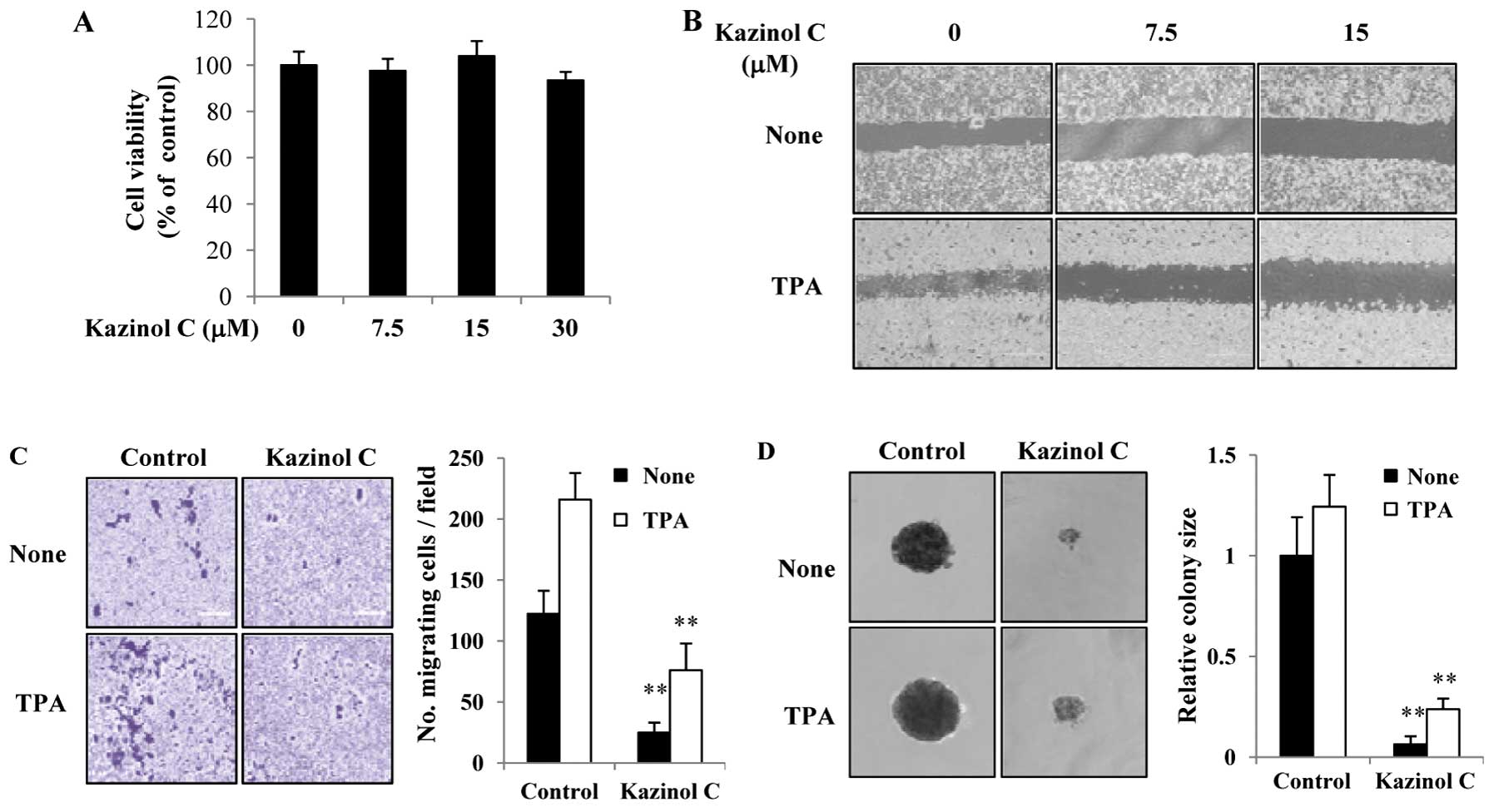 | Figure 5Kazinol C inhibits HT-29 cell
migration and anchorage-independent growth. (A) HT-29 cells were
treated with the indicated concentrations of kazinol C for 24 h,
and cell viability was estimated using the MTT assay. Data from
three experiments are presented as the means ± SD. (B) Cells were
grown to confluence, and the plate was scratched with a pipette
tip. The cells were pretreated with indicated concentrations of
kazinol C for 30 min and then treated with 40 μg/ml TPA. After
incubation for 24 h, the plates were observed using a
phase-contrast microscope. (C) A fixed number of cells were
suspended in serum-free medium with 15 μM kazinol C and added to
the upper chamber of the Transwell, whereas the lower chamber was
filled with medium containing 10% FBS. After incubation for 48 h,
the migrating cells were counted, and the results were presented as
the mean number of migratory cells ± SD/microscopic field. (D)
HT-29 cells (1×103) were suspended in 1.5 ml of medium
containing 0.3% agar, 10% FBS and 15 μM kazinol C or 20 mg/ml TPA,
and the mixture was applied onto pre-solidified 0.5% agar (15 ml)
with kazinol C or TPA in a 6-well culture plate. After incubation
for 2 weeks, the diameter of 5–10 representative colonies was
measured. TPA, 12-O-tetradecanoylphorbol-13-acetate. |
Discussion
Colorectal cancer is the most common cancer and
second leading cause of cancer-related mortality in the USA.
Therefore, an attempt has been made to identify natural active
compounds for the treatment of colorectal cancer. Compounds that
induce apoptosis may have chemotherapeutic value given that a
primary cancer characteristic is an imbalance of proliferation and
apoptosis. Kazinol C is an active component derived from
Broussonetia kazinoki that exerts beneficial effects in
oxidative stress-related diseases, such as cancer (12). However, the anticancer effects of
kazinol C are poorly understood. The aim of this study was to
establish a potential rationale for the clinical application of
kazinol C, thus, the anticancer effect of kazinol C in HT-29 colon
cancer cells was investigated. The present study focused on AMPK,
which is the main molecule of the intracellular energy-sensing
system. We found that kazinol C strongly induces AMPK
phosphorylation and significantly attenuates cancer cell growth and
viability. To examine the relationship between kazinol C-induced
AMPK activation and cancer cell apoptosis, we suppressed AMPK
activity using compound C or stable transfection of the AMPK-DN
form. The inhibition of AMPK activity effectively blocks kazinol
C-induced cell apoptosis. Kazinol C also inhibits cell migration,
which regulates tumor metastasis and the anchorage-independent
growth of HT-29 cells through increased AMPK activity. Thus, our
data clearly demonstrate that kazinol C functions as an effective
anticancer molecule in HT-29 cells.
AMPK is a novel therapeutic cancer target given that
it serves as a pivotal role between cell survival and apoptosis
(19). In previous studies, AMPK
was strongly activated during the early stages of primary brain
tumor formation in rat and glucose deprivation- or hypoxia-induced
AMPK activation was shown to induce cell survival in various cell
types (20–22). However, pharmacological studies have
suggested the tumor-suppressor functions of AMPK. For example,
metformin is a potent AMPK activator. Diabetes patients
administered metformin exhibit a lower risk of colon cancer
development compared with non-metformin users (23). In addition, aspirin, a synthetic
non-steroidal anti-inflammatory drug (NSAID), has also been shown
to reduce colon cancer development through the direct activation of
AMPK (24,25). Inflammation is a key factor involved
in colorectal tumor progression as supported by increasing data,
and AMPK can function as an anti-inflammatory molecule via COX-2 or
NF-κB inhibition (6,26). Furthermore, quercetin, berberine and
resveratrol reportedly exert anticancer effects via AMPK activation
(27–29). Although the role of AMPK in cancer
cells is controversial, pharmacological studies have indicated that
pharmacological induction of AMPK activity results in anticancer
effects. Consequently, the identification of AMPK activators is a
promising strategy for developing novel therapeutic drugs for the
treatment of colon cancer.
AMPK also regulates cell motility. For example,
CaMKKβ-AMPK activation by lysophosphatidic acid or androgen
increases ovarian and prostate cancer cell migration, respectively
(30,31). AMPK activation also contributes to
transendothelial lymphocyte and endothelial cell migration
(32,33). By contrast, berberine-mediated AMPK
activation inhibits melanoma and colon cancer cell migration
(9,34). Adiponectininduced LKB1-AMPK activity
inhibits breast cancer cell migration through inhibition of the
mTor-S6K pathway (11). Moreover,
compound C inhibits vascular smooth muscle cell (SMC) migration in
an AMPK-independent manner and AICAR also inhibits SMC migration
(35). Accordingly, the role of
AMPK in cell migration may be dependent on the type of stimulus or
cell line. In the present study, kazinol C rapidly increased AMPK
phosphorylation and inhibits TPA-induced cell migration. The
inhibition of AMPK activity via transfection of the AMPK-DN form
abrogates TPA-induced cell migration. Therefore, we hypothesize
that kazinol C-mediated AMPK activation is a negative regulator of
HT-29 cell migration. In addition, cancer cell growth in semisolid
medium is an indicator of metastatic potential. Kazinol C
significantly inhibits anchorage-independent HT-29 cell growth, and
this result supports our hypothesis that kazinol C-induced AMPK
activity may be associated with antimetastatic effects.
In conclusion, our data have identified a
significant role of kazinol C-induced AMPK activation in the
induction of HT-29 cell apoptosis. In addition, kazinol C inhibits
migration and anchorage-independent growth of HT-29 cells. AMPK
inhibition via stable transfection with the AMPK-DN form
significantly abrogates the kazinol C-induced inhibition of cancer
cell migration. Furthermore, the present study suggests that AMPK
is a critical and novel regulator of kazinol C-induced cancer cell
apoptosis and inhibition of migration, suggesting that AMPK is a
prime cancer target.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grants (Nos. 2011-0030074 and
2011-0011011) funded by the Korean government (Ministry of Science,
ICT and Future Planning).
References
|
1
|
Hardie DG, Carling D and Carlson M: The
AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of
the eukaryotic cell? Annu Rev Biochem. 67:821–855. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hardie DG and Alessi DR: LKB1 and AMPK and
the cancer-metabolism link - ten years after. BMC Biol. 11:362013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang
Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He
X, MacDougald OA, You M, Williams BO and Guan KL: TSC2 integrates
Wnt and energy signals via a coordinated phosphorylation by AMPK
and GSK3 to regulate cell growth. Cell. 126:955–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones RG, Plas DR, Kubek S, Buzzai M, Mu
J, Xu Y, Birnbaum MJ and Thompson CB: AMP-activated protein kinase
induces a p53-dependent metabolic checkpoint. Mol Cell. 18:283–293.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang JT, Kim YM, Surh YJ, Baik HW, Lee
SK, Ha J and Park OJ: Selenium regulates cyclooxygenase-2 and
extracellular signal-regulated kinase signaling pathways by
activating AMP-activated protein kinase in colon cancer cells.
Cancer Res. 66:10057–10063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanli T, Steinberg GR, Singh G and
Tsakiridis T: AMP-activated protein kinase (AMPK) beyond
metabolism: a novel genomic stress sensor participating in the DNA
damage response pathway. Cancer Biol Ther. 15:156–169. 2014.
View Article : Google Scholar :
|
|
9
|
Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS and
Lim JS: Berberine-induced AMPK activation inhibits the metastatic
potential of melanoma cells via reduction of ERK activity and COX-2
protein expression. Biochem Pharmacol. 83:385–394. 2012. View Article : Google Scholar
|
|
10
|
Nakano A, Kato H, Watanabe T, Min KD,
Yamazaki S, Asano Y, Seguchi O, Higo S, Shintani Y, Asanuma H,
Asakura M, Minamino T, Kaibuchi K, Mochizuki N, Kitakaze M and
Takashima S: AMPK controls the speed of microtubule polymerization
and directional cell migration through CLIP-170 phosphorylation.
Nat Cell Biol. 12:583–590. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taliaferro-Smith L, Nagalingam A, Zhong D,
Zhou W, Saxena NK and Sharma D: LKB1 is required for
adiponectin-mediated modulation of AMPK-S6K axis and inhibition of
migration and invasion of breast cancer cells. Oncogene.
28:2621–2633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim AY, Lee CG, Lee da Y, Li H, Jeon R,
Ryu JH and Kim SG: Enhanced antioxidant effect of prenylated
polyphenols as Fyn inhibitor. Free Radic Biol Med. 53:1198–1208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ko HH, Yen MH, Wu RR, Won SJ and Lin CN:
Cytotoxic isoprenylated flavans of Broussonetia kazinoki. J Nat
Prod. 62:164–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cha JY, Kim YT, Kim HS and Cho YS:
Antihyperglycemic effect of stem bark powder from paper mulberry
(Broussonetia kazinoki Sieb.) in type 2 diabetic Otsuka Long-Evans
Tokushima fatty rats. J Med Food. 11:499–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek YS, Ryu YB, Curtis-Long MJ, Ha TJ,
Rengasamy R, Yang MS and Park KH: Tyrosinase inhibitory effects of
1,3-diphenylpropanes from Broussonetia kazinoki. Bioorg Med Chem.
17:35–41. 2009. View Article : Google Scholar
|
|
16
|
Lee DY, Kim DH, Lee HJ, Lee Y, Ryu KH,
Jung BI, Song YS and Ryu JH: New estrogenic compounds isolated from
Broussonetia kazinoki. Bioorg Med Chem Lett. 20:3764–3767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikuta J, Hano Y, Nomura T, Kawakami Y and
Sato T: Components of Broussonetia kazinoki SIEB. I Structures of
two new isoprenylated flavans and five new isoprenylated
1,3-diphenylpropane derivatives. Chem Pharm Bull. 34:1968–1979.
1986. View Article : Google Scholar
|
|
18
|
Woods A, Azzout-Marniche D, Foretz M,
Stein SC, Lemarchand P, Ferré P, Foufelle F and Carling D:
Characterization of the role of AMP-activated protein kinase in the
regulation of glucose-activated gene expression using
constitutively active and dominant negative forms of the kinase.
Mol Cell Biol. 20:6704–6711. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W and Guan KL: AMP-activated protein
kinase and cancer. Acta Physiol. 196:55–63. 2009. View Article : Google Scholar
|
|
20
|
Jang T, Calaoagan JM, Kwon E, Samuelsson
S, Recht L and Laderoute KR: 5′-AMP-activated protein kinase
activity is elevated early during primary brain tumor development
in the rat. Int J Cancer. 128:2230–2239. 2011. View Article : Google Scholar
|
|
21
|
Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe
W and Ha J: AMP kinase signaling determines whether c-Jun
N-terminal kinase promotes survival or apoptosis during glucose
deprivation. Carcinogenesis. 30:529–537. 2009. View Article : Google Scholar
|
|
22
|
Kim HS, Wannatung T, Lee S, Yang WK, Chung
SH, Lim JS, Choe W, Kang I, Kim SS and Ha J: Quercetin enhances
hypoxia-mediated apoptosis via direct inhibition of AMPK activity
in HCT116 colon cancer. Apoptosis. 17:938–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng CH: Diabetes, metformin use, and
colon cancer: a population-based cohort study in Taiwan. Eur J
Endocrinol. 167:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activated protein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–1515.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hawley SA, Fullerton MD, Ross FA,
Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green
KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR and Hardie DG:
The ancient drug salicylate directly activates AMP-activated
protein kinase. Science. 336:918–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Neill LA and Hardie DG: Metabolism of
inflammation limited by AMPK and pseudo-starvation. Nature.
493:346–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee YK and Park OJ: Regulation of mutual
inhibitory activities between AMPK and Akt with quercetin in MCF-7
breast cancer cells. Oncol Rep. 24:1493–1497. 2010.PubMed/NCBI
|
|
28
|
Yang X and Huang N: Berberine induces
selective apoptosis through the AMPK-mediated mitochondrial/caspase
pathway in hepatocellular carcinoma. Mol Med Rep. 8:505–510.
2013.PubMed/NCBI
|
|
29
|
Puissant A, Robert G, Fenouille N, Luciano
F, Cassuto JP, Raynaud S and Auberger P: Resveratrol promotes
autophagic cell death in chronic myelogenous leukemia cells via
JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res.
70:1042–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EK, Park JM, Lim S, Choi JW, Kim HS,
Seok H, Seo JK, Oh K, Lee DS, Kim KT, Ryu SH and Suh PG: Activation
of AMP-activated protein kinase is essential for lysophosphatidic
acid-induced cell migration in ovarian cancer cells. J Biol Chem.
286:24036–24045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frigo DE, Howe MK, Wittmann BM, Brunner
AM, Cushman I, Wang Q, Brown M, Means AR and McDonnell DP: CaM
kinase kinase β-mediated activation of the growth regulatory kinase
AMPK is required for androgen-dependent migration of prostate
cancer cells. Cancer Res. 71:528–537. 2011. View Article : Google Scholar :
|
|
32
|
Ouchi N, Kobayashi H, Kihara S, Kumada M,
Sato K, Inoue T, Funahashi T and Walsh K: Adiponectin stimulates
angiogenesis by promoting cross-talk between AMP-activated protein
kinase and Akt signaling in endothelial cells. J Biol Chem.
279:1304–1309. 2004. View Article : Google Scholar
|
|
33
|
Martinelli R, Gegg M, Longbottom R,
Adamson P, Turowski P and Greenwood J: ICAM-1-mediated endothelial
nitric oxide synthase activation via calcium and AMP-activated
protein kinase is required for transendothelial lymphocyte
migration. Mol Biol Cell. 20:995–1005. 2009. View Article : Google Scholar :
|
|
34
|
Park JJ, Seo SM, Kim EJ, Lee YJ, Ko YG, Ha
J and Lee M: Berberine inhibits human colon cancer cell migration
via AMP-activated protein kinase-mediated downregulation of
integrin β1 signaling. Biochem Biophys Res Commun. 426:461–467.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peyton KJ, Yu Y, Yates B, Shebib AR, Liu
XM, Wang H and Durante W: Compound C inhibits vascular smooth
muscle cell proliferation and migration in an AMP-activated protein
kinase-independent fashion. J Pharmacol Exp Ther. 338:476–484.
2011. View Article : Google Scholar : PubMed/NCBI
|