Introduction
Osteosarcoma accounts for ~20% of all primary bone
cancers, and is the second highest cause of cancer-associated
mortality in the pediatric age group (1,2).
Although modern surgery and neo-adjuvant chemotherapy have
developed over the past decades, ~35% of patients are likely to
succumb to the disease within 5 years of diagnosis (3). Originating from cells of the
osteoblast lineage, osteosarcoma cells exhibit complex and
unbalanced karyotypes, characterized by numerous recurrent DNA
amplifications as well as chromosomal abnormalities (4).
The high mobility group protein family (HMG)
consists of three subfamilies, including the high mobility group B
(HMGB), high mobility group A (HMGA) and high mobility group N
(HMGN) (5). Each subfamily appears
to exert a single characteristic nuclear function, specifically
binding to nucleosomes and contributing to the diversity of
chromatin function (6). Among them,
HMGN2 is an abundant, highly conserved cell protein, widely known
as a nuclear DNA-binding protein, which stabilizes nucleosome
formation and facilitates gene transcription.
Findings of previous studies demonstrated that HMGN2
was one of the most abundant non-histone nuclear proteins in
vertebrates and invertebrates (6,7).
Subsequently, it was found that HMGN2 was mainly involved in the
growth of tumor vascular endothelia and played an important role in
tumorigenesis (8). The aberrant
expression of HMGN2 was correlated with several tumors (9,10).
In vitro, HMGN2 was released by the stimulation of IL-2 in
human peripheral blood mononuclear leukocytes (8). HMGN2 protein was demonstrated to
inhibit growth and induce apoptosis in the Tca8113 oral squamous
cell carcinoma cell line (11).
In vivo, HMGN2 significantly inhibited growth of the
transplanted tumor in nude mice (12).
Therefore, HMGN2 is a newly identified gene
associated with cancer growth and metastasis, representing a new
therapeutic target for the treatment of cancer. However, the
effects and molecular mechanisms of HMGN2 on osteosarcoma
progression have not yet been comprehensively explored. In the
present study, we examined the endogenous expression of HMGN2 in
osteosarcoma cell lines using RT-PCR and western blot analysis.
HMGN2 lentivirus was used to infect the osteosarcoma SaO2 and U2-OS
cell lines with a relatively low HMGN2 expression to determine the
functional relevance of HMGN2 overexpression in osteosarcoma cell
growth and migration in vitro, and to examine the underlying
signaling pathway involved in the progression of osteosarcoma.
Materials and methods
Materials
MG63, HOS, SaO2 and U2-OS osteosarcoma cell lines
were purchased from the American Type Culture Collection (ATCC,
Vanassas, MA, USA). HMGN2 lentivirus vector, negative control
vector GFP and virion-packaging elements were purchased from
Genechem (Shanghai, China). All the antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Pharmaceuticals and reagents
TRIzol reagent, Lipofectamine 2000, Double stain
apoptosis detection kit (Annexin V-FITC/PI), Dulbecco’s modified
Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased
from Gibco Invitrogen Corporation (Grand Island, NY, USA).
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium
(WST-8) was purchased from Beyotime (Beyotime, Haimen, China) and
SYBR-Green master mixture was purchased from Takara (Otsu,
Japan).
Overexpression of HMGN2
Human-HMGN2 cDNA was prepared by RT-PCR (Fig. 1A) and inserted into the BamHI
and XhoI restriction sites of the pGC-FU-3FLAG vector
plasmid (Addgene, Cambridge, MA, USA). For the stable
overexpression of HMGN2, HEK293T cells were plated in
75-cm2 culture flasks and transfected with 10 μg HMGN2
or GFP (control) lentivirus vectors. The medium was changed and the
viral supernatant was harvested 48 h later. Viral-containing medium
was collected and passed through 0.45-μm syringe filters. SaO2 and
U2-OS cells were incubated with the lentivirus supernatant for 24 h
and selected with 2 μg/ml puromycin (Sigma, St. Louis, MO, USA)
according to the manufacturer’s instructions. The clone in which
the HMGN2 lentivirus vectors transfected was designated as the
HMGN2 group, the negative control GFP transfected vectors was
designated as the GFP group and SaO2 and U2-OS osteosarcoma cells
were designated as the CON group.
RT-qPCR
To quantitatively determine the expression level of
HMGN2 in osteosarcoma cell lines, HMGN2 mRNA expression was
determined by RT-qPCR using SYBR-Premix Ex Taq (Takara, Japan) and
an ABI Prism 7500 sequence detection system (Applied Biosystems,
USA). Total RNA of each clone was extracted with TRIzol according
to the manufacturer’s instructions. The genes were amplified using
specific oligonucleotide primers and human β-actin gene was used as
an endogenous control. The PCR primer sequences used were: HMGN2,
forward: 5′-CCAGCCATCAGCCATGAGGGT-3′ and reverse:
5′-GGAGCCCTTTCTGAATCCGCA-3′); β-actin, forward:
5′-GCGGGAAATCGTGCGTGACATT-3′ and reverse:
5′-GGCAGATGGTCGTTTGGCTGAATA-3′. Data were analyzed using the
comparative Ct method (2−ΔΔCt). Three separate
experiments were performed for each clone.
Western blot assay
Treated cells were collected and extracted using an
assay kit (Beyotime, China) and the concentration was determined by
using a Bio-Rad protein assay (Hercules, CA, USA). Equal amounts of
cell extracts were separated on SDS-PAGE gels according to the
molecular weight of the tested proteins. Separated protein bands
were transferred onto polyvinylidene fluoride membranes and blocked
in 5% skimmed milk powder. The primary antibodies against HMGN2,
cyclin E, cyclin D1, PCNA, and Ki-67 (all from Santa Cruz
Biotechnology) were diluted according to the instructions and
incubated overnight at 4°C. Horseradish peroxidase-linked secondary
antibodies were added at a dilution ratio of 1:1,000, and incubated
at room temperature for 2 h. The membranes were washed with
phosphate-buffered saline three times and the immunoreactive bands
were visualized using an ECL-Plus kit according to the
manufacturer’s instructions [Multi Sciences (Lianke) Biotech Co.,
Ltd., Hangzhou, China]. The relative protein level in different
cell lines was normalized to β-actin concentration. Three separate
experiments were performed for each clone.
Cell proliferation assay
Cell proliferation was analyzed with the cell count
assay using WST-8 kits. Briefly, cells infected with HMGN2 were
incubated in 96-well plates at a density of 1×105
cells/well with DMEM with 10% FBS. The cells were treated with 10
μl WST at 6, 12 and 24 h. The color reaction was measured at 570 nm
with enzyme immunoassay analyzer (Bio-Rad Laboratories, Hercules,
CA, USA). The proliferative activities were calculated for each
clone.
Cell cycle analysis
For the cell cycle analysis, harvested cells were
centrifuged at 1,000 × g for 5 min, washed with phosphate buffered
saline and fixed in 70% ethanol overnight. The cells were then
treated with 100 μg/ml propidium iodide plus RNase (10 μg/ml) for
30 min. The cell-cycle phase distribution was determined by
analytical DNA flow cytometry (FACSCalibur; BD Biosciences,
Becton-Dickinson, San Jose, CA, USA) as described by Evans et
al (13).
Wound-healing assay
SaO2 and U2-OS cells were plated in each well of a
6-well culture plate and allowed to grow to 90% confluence.
Treatment with HMGN2 lentivirus was then performed. The following
day, a wound was created using a 10 μl micropipette tip. The
migration of cells towards the wound was monitored daily, and
images were captured at time intervals of 24 h.
In vivo tumor xenograft studies
Three mice were injected subcutaneously with
1×108 SaO2 and U2-OS cells in 50 ml of PBS pre-mixed
with an equal volume of Matrigel matrix (Becton Dickinson). When
the tumor size reached ~5 mm in length, sections were surgically
removed, cut to 1-mm3, and re-seeded individually into
other mice. When the tumor size reached ~5 mm in length, the mice
were randomly assigned to the GFP and HMGN2 groups, in which 15 ml
of lentivirus was injected into subcutaneous tumors using a
multi-site injection format. The injections were repeated on the
third day after initial treatment. The tumor volume was measured
every three days with a caliper, using the formula volume = (length
× width)2/2.
Statistical analysis
The results obtained were expressed as the mean ±
standard error values from at least three independent experiments.
One-way analysis of variance (ANOVA) was used to analyze the
differences between groups. The LSD method of multiple comparisons
was used when the probability for ANOVA was statistically
significant. P<0.05 was considered to indicate statistical
significance.
Results
Endogenous expression of HMGN2 in
osteosarcoma cell lines
The endogenous expression of HMGN2 in human
osteosarcoma SaO2, MG-63, U2-OS and HOS cell lines was evaluated
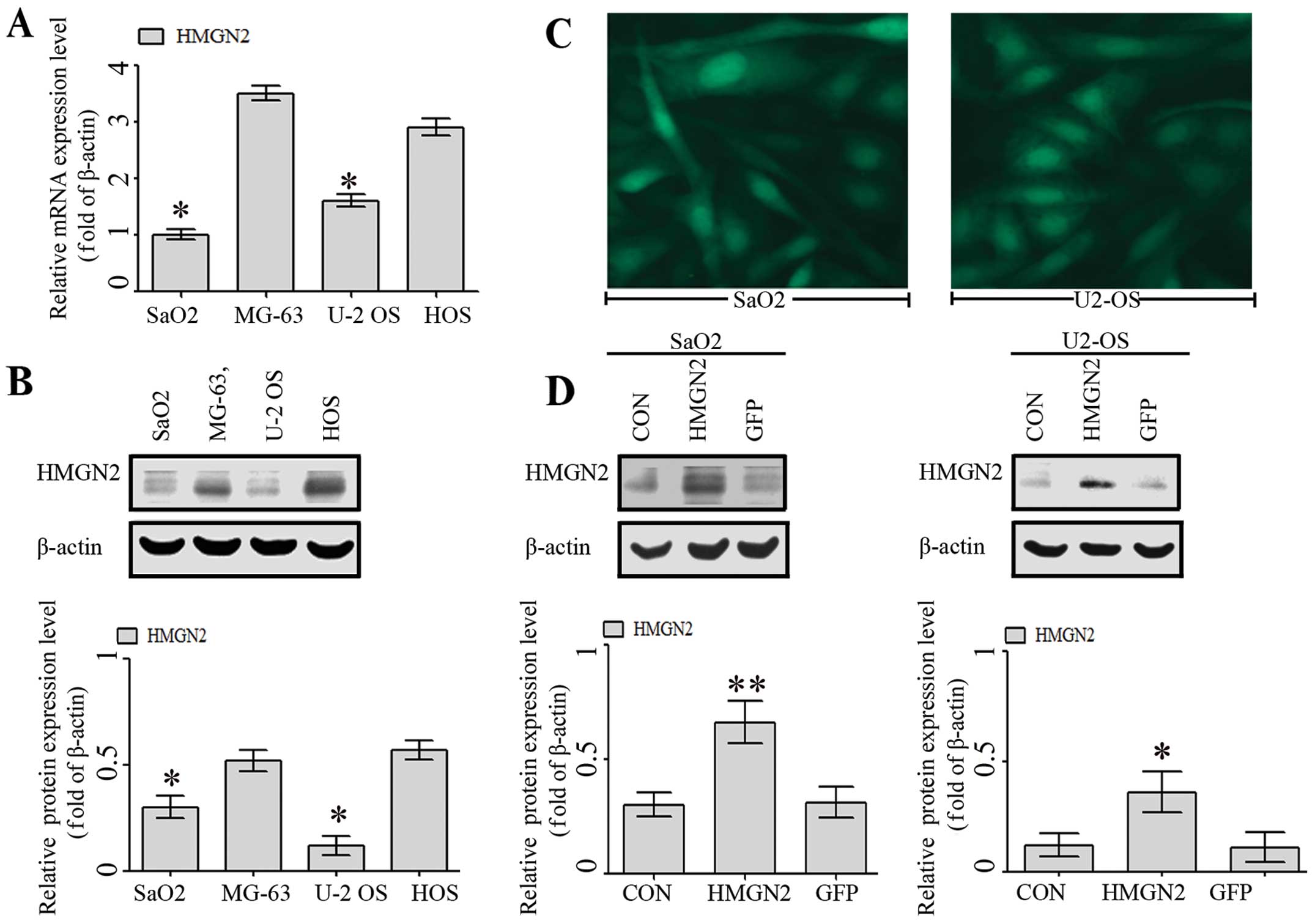
using RT-qPCR and western blot analysis. As shown in Fig. 1A and B, there were different levels
of mRNA and protein expression of HMGN2 in SaO2, MG-63, U-2 OS, and
HOS cell lines, while the expression levels of HMGN2 were
significantly reduced in the SaO2 and U-2 OS cell lines than those
in the MG-63 and HOS cell lines.
Since HMGN2 exhibited a low expression in SaO2 and
U-2 OS cell lines, the cell lines were selected as the infective
objects of HMGN2 lentivirus. The infection efficiencies of HMGN2
(at a multiplicity of infection =50) in SaO2 and U-2 OS cell lines
were >80% under fluorescence microscopy (Fig. 2C). To confirm that HMGN2
successfully transfected into SaO2 and U-2 OS cells, western blot
analysis was applied. After 48 h following HMGN2 or GFP lentivirus
infection and the expression of HMGN2 protein was significantly
increased in the HMGN2 group (Fig.
1D).
HMGN2 inhibits osteosarcoma cell
growth
Deregulated cell proliferation is a hallmark of
cancer (16). To determine the
effect of HMGN2 overexpression on SaO2 and U-2 OS cell growth, we
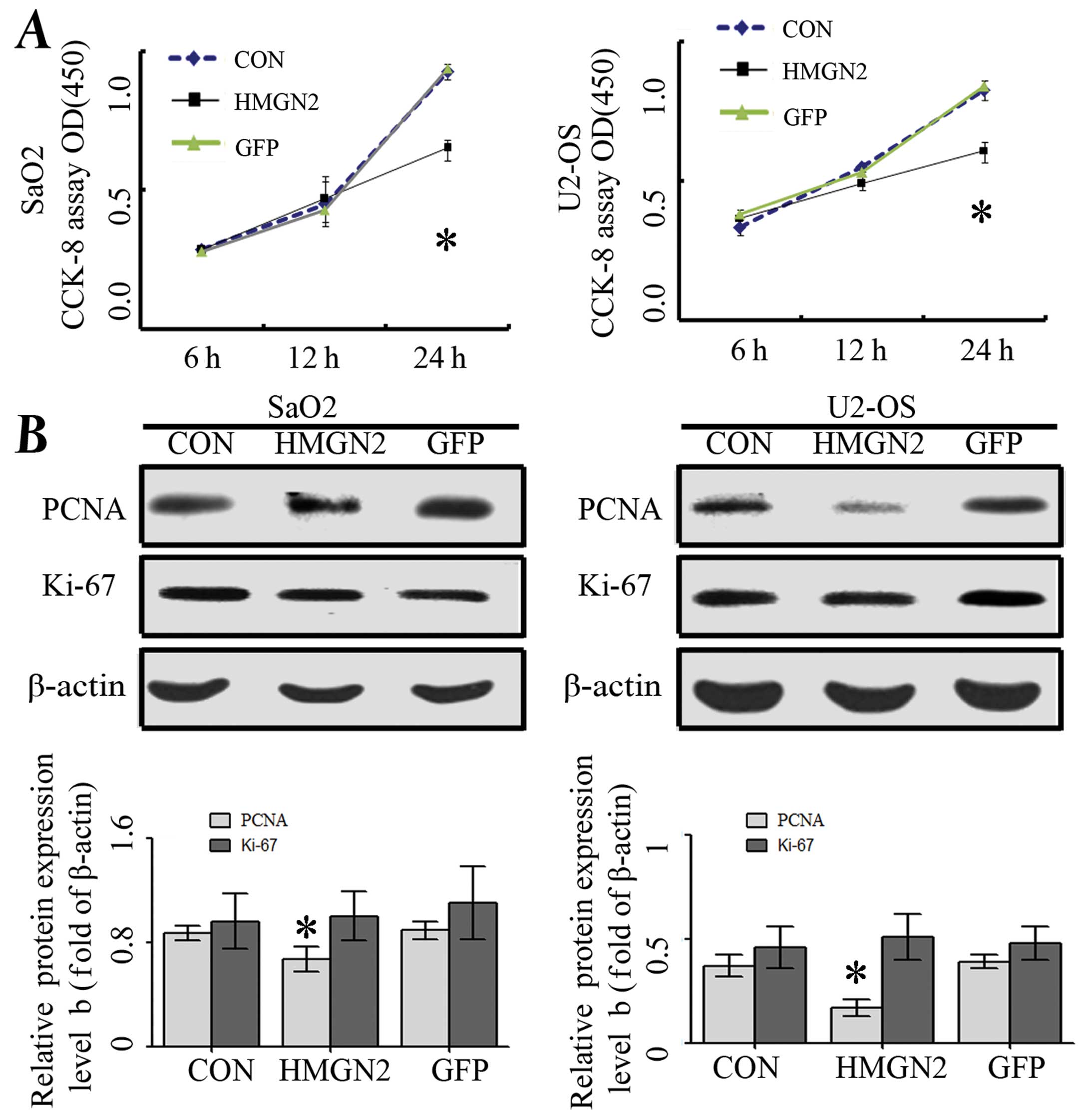
investigated the proliferative activities by WST assay.
Overexpression of HMGN2 significantly reduced the proliferative
activities of SaO2 and U-2 OS cells compared with the GFP and CON
groups (Fig. 2A). In addition, PCNA
and Ki-67, which were indicators for cell proliferation, were
examined by western blot assay to determine whether HMGN2
overexpression suppressed cell growth through translational
repression. The expression of PCNA protein was significantly
decreased in Lenti-HMGN2 group compared with the CON and GFP groups
(P<0.01) while Ki-67 did not alter among these groups (Fig. 2B). These data suggested that the
overexpression of HMGN2 may inhibit osteosarcoma cell proliferation
through the downregulation of PCNA expression.
Effect of HMGN2 on osteosarcoma cycle
distribution
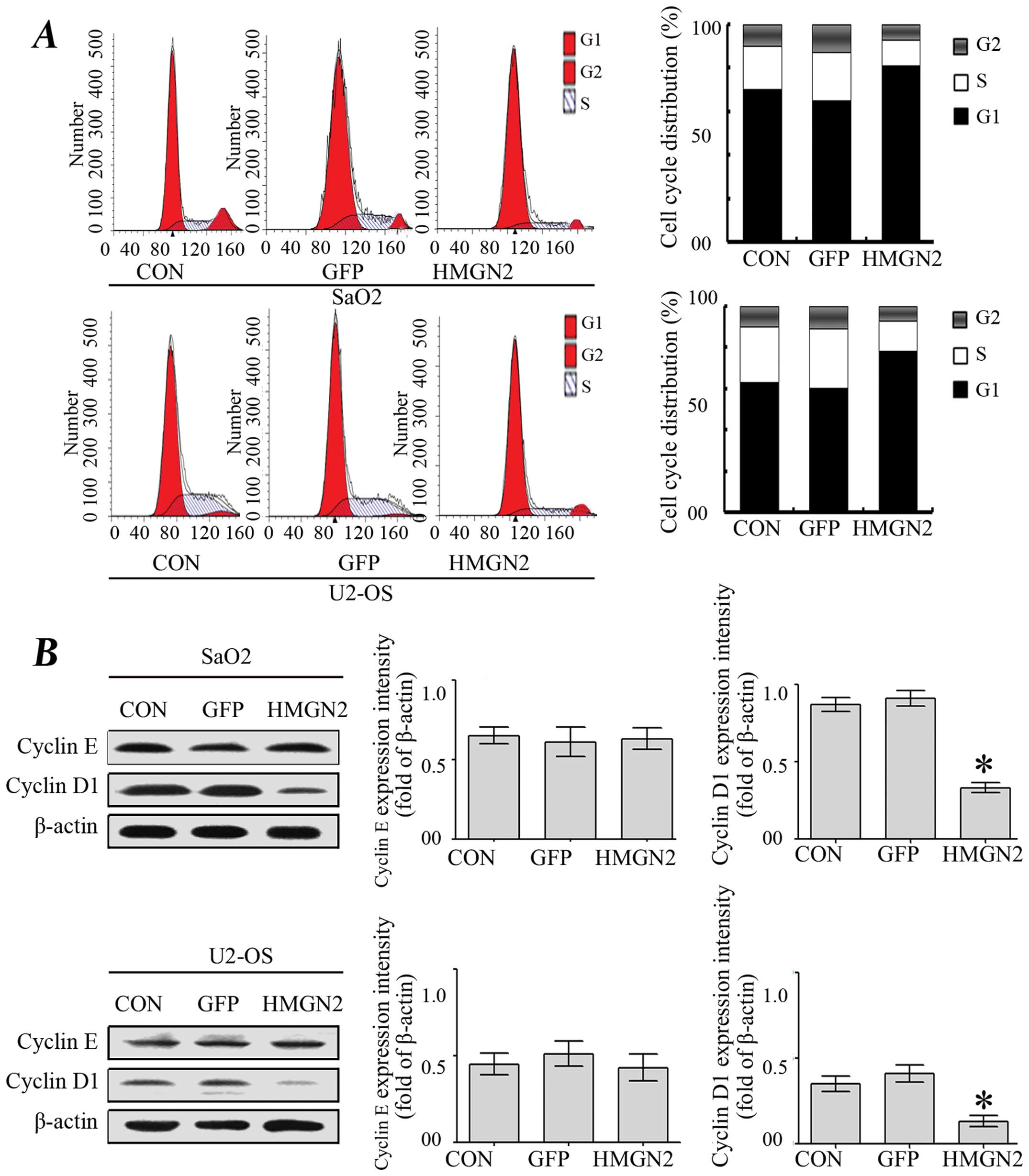
The cycle distribution of SaO2 and U-2 OS cells was
also analyzed. As shown in Fig. 3A,
the percentage of untransfected SaO2 and U-2 OS cells in S phase
was 52.1 and 58%, respectively, whereas the percentage of SaO2 and
U-2 OS cells transfected with HMGN2 in S phase was 35.5 and 33%,
respectively. In addition, 10 and 15% of untreated SaO2 and U-2 OS
cells were in the G2/M phase compared with 5 and 8% of cells
transfected with HMGN2 lentivirus. These results indicated that
cell cycle was arrested in G0/G1 phase in HMGN2 group compared with
the CON and GFP groups. Subsequently, cyclin D1 and cyclin E, two
regulators of cell cycle progression from G1 to S phase, were
assessed by western blotting (17).
The results showed that only HMGN2 led to a decreased level of
cyclin D1, whereas no significant changes were identified in the
levels of cyclin E (Fig. 3B),
suggesting that HMGN2 modulates the cell cycle through the
regulation of cyclin D1.
Effect of HMGN2 on osteosarcoma cell
invasion and metastasis
To determine the effect of HMGN2 on osteosarcoma
cell invasion and metastasis, Transwell assay and wound-healing
assay were carried out. As was shown in Fig. 4A, the migrative ability of SaO2 and
U-2 OS cells in the HMGN2 group was lower than that in the CON and
GFP groups. However, there were no significant differences between
the CON and GFP groups. Furthermore, a Transwell assay was
performed to determine the ability of cells to invade a matrix
barrier. The representative micrographs of Transwell filters are
shown in Fig. 4B. The invasive cell
count demonstrated that invasive potential was significantly
reduced in the HMGN2 group relative to the CON and GFP groups.
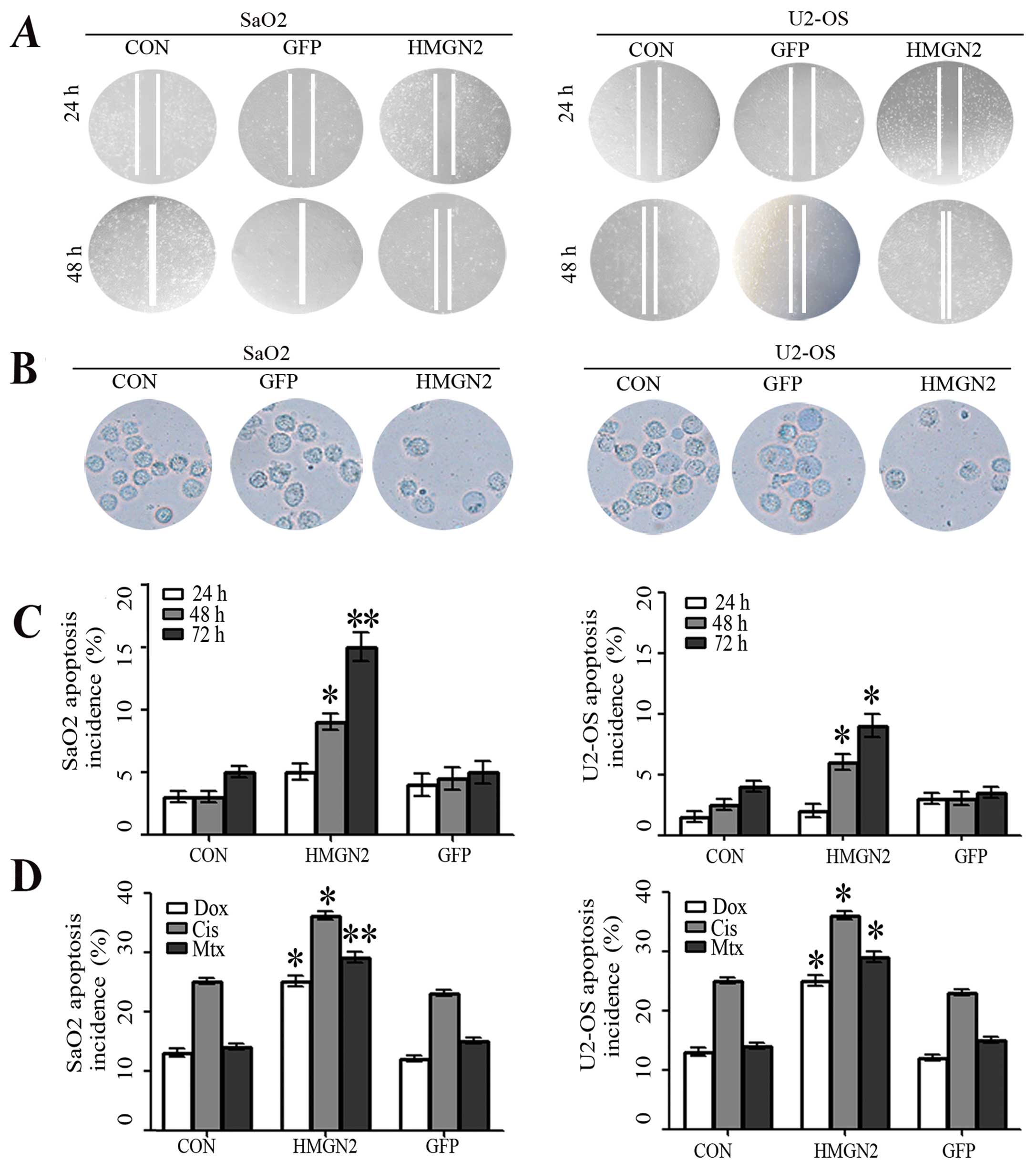 | Figure 4Inhibition of osteosarcoma cell
migration and invasiveness by HMGN2. (A) Wound-healing assay showed
that the migration capacity of SaO2 and U2-OS cells in the HMGN2
group were markedly lower than those in the GFP and CON groups. (B)
Transwell invasion assay for the transmembrane ability of each
group of cells. The ability in HMGN2 group was markedly decreased
as compared with the GFP and CON groups. (C) The apoptosis of SaO2
and U2-OS cells was analyzed by flow cytometry (Annexin V-FITC/PI)
at the end of the incubation period for 24, 48 and 72 h. The
apoptosis incidence increased in a time-dependent manner and HMGN2
resulted in a further increase (*P<0.05,
**P<0.01), although no difference was found between
the GFP and CON groups (P>0.05). (D) Annexin V-FITC/PI staining
for apoptosis induced by chemotherapy agents. SaO2/CON, SaO2/GFP,
SaO2/HMGN2, U2-OS/CON, U2-OS/GFP, and U2-OS/HMGN2 cells were
treated with Dox (0.2 mg/ml), Cis (20 mmol/l), and Mtx (50 mmol/l)
for 24 h and the apoptosis incidence was quantified by flow
cytometer. Overexpression of HMGN2 resulted in a further increase
of apoptosis induced by Dox, Cis and Mtx. (*P<0.05,
**P<0.01 vs. CON group). |
HMGN2 increases apoptosis and sensitivity
to chemotherapy
The action mechanism of many anticancer factors is
based on their ability to induce apoptosis. Consequently, SaO2 and
U-2 OS osteosarcoma cells treated with HMGN2 lentivirus underwent
apoptosis as their mode of cell death. At the end of the incubation
period for 24, 48 and 72 h, the osteosarcoma cells were stained
using an Annexin V-FITC/PI detection kit. As shown in Fig. 4C, the number of SaO2 and U-2 OS
apoptotic cells in the HMGN2 group significantly increased compared
with that in the GFP and CON groups at various time points. To
further investigate whether HMGN2 affected the sensitivity to
chemotherapy, the apoptosis in HMGN2-overexpressed osteosarcoma
cells was induced by chemotherapy. A significant increase of
apoptosis incidence was detected in cells treated with Dox, Cis,
and Mtx for 24 h (Fig. 4D).
Overexpression of HMGN2 resulted in a further increase of apoptosis
induced by these anticancer agents, suggesting that HMGN2 increased
the sensitivity to chemotherapy.
Antitumor effect of lenti-HMGN2 in the
osteosarcoma xenograft model
The in vitro experiments confirmed that HMGN2
efficiently inhibited the growth and migration of U2-OS and SaO2
cells. However, whether HMGN2 has the same inhibitory effect on
in vivo osteosarcoma remains to be determined. We
investigated the antitumor effect of HMGN2 in vivo using
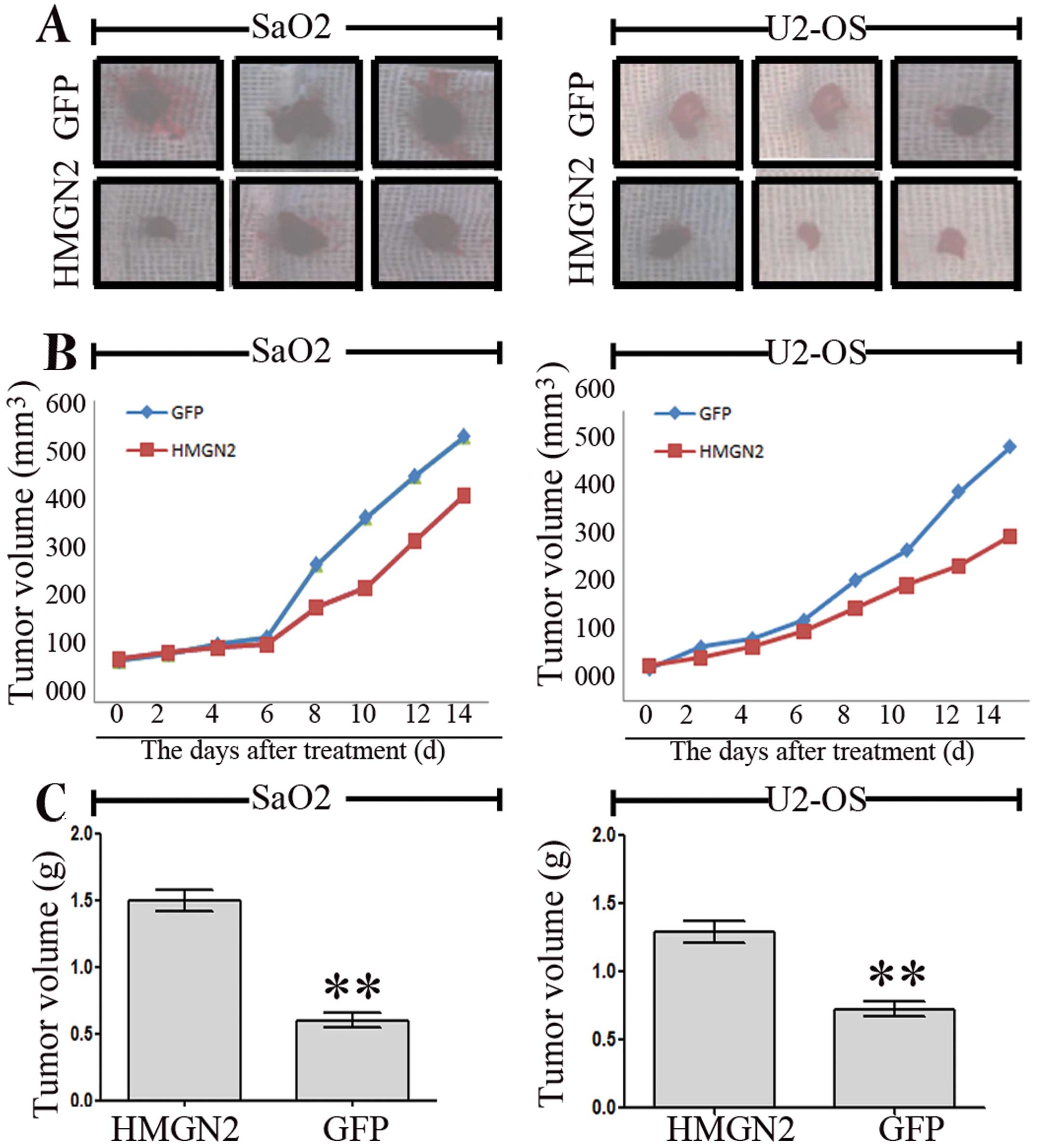
SaO2 and U2-OS xenograft models. The mean volumes of SaO2 and U2-OS
xenograft tumors were 52.3±10.5 and 35.5±8.6 mm3 in the
experimental mice prior to treatment. On day 14, the average
volumes of SaO2 and U2-OS xenograft tumors were significantly
smaller in the HMGN2 group than those in the CON and GFP groups
(Fig. 5A). During the whole tumor
growth period, the tumor growth activity was measured. Tumors
treated with HMGN2 lentivirus grew substantially slower than the
GFP group (Fig. 5B). When the
tumors were harvested, the average weights of SaO2 and U-2 OS
xenograft tumors in HMGN2 group were significantly lighter than
those in the GFP group (**P<0.01; Fig. 5C). These results in vivo
indicated that overexpression of HMGN2 was able to inhibit SaO2 and
U-2 OS cell growth.
Discussion
HMGNs were initially regarded as transcription
coregulators, however, their roles in DNA repair and cancer
progression were determined using HMGN1 knockout mice (14). In addition to HMGN1, the expression
of HMGN5 (formerly NSBP1) was found to be one of the significant
factors in the prognosis of bone tumor (15), breast cancer (16) and prostate cancer (17). Collectively, results of those
studies suggested that HMGNs were involved in osetosarcoma cell
progression and exhibited characteristics of a tumor-suppressor
gene. However, whether HMGN2 was expressed in osteosarcoma and the
role of HMGN2 was not previously reported. The present study
investigated the activity of HMGN2 in the MG-63, HOS, SaO2 and
U2-OS osteosarcoma cell lines. To the best of our knowledge, the
present study documented for the first time the endogenous
expression of HMGN2 in osteosarcoma cell lines, and demonstrated
that the expression levels of HMGN2 were significantly lower in
SaO2 and U2-OS cell lines than those in MG-63 and HOS cell lines,
which were selected as the infective objects of HMGN2 lentivirus.
In pilot studies, the infectious efficiency of HMGN2 in SaO2 and
U2-OS cell lines was extremely high, and a marked increase of HMGN2
expression was observed in the HMGN2 group compared with the GFP
and CON groups. HMGN2 is mainly expressed in vertebrates and
invertebrates (6,7) and functions as a modifier of the
nucleosomal organization. In addition to the above function, the
findings in our study demonstrated that overexpression of HMGN2
significantly reduced the proliferative activities of SaO2 and
U2-OS cell lines in a time dependent manner. As shown in our
findings, the possible underlying mechanism is that HMGN2, via
downregulation of PCNA and cyclin D1 expression, inhibits
osteosarcoma cell proliferation.
More importantly, HMGN2 has been confirmed to
prevent migration and invasiveness and induce apoptosis, which
suggesting that HMGN2 is a tumor suppressor and apoptosis regulator
in osteosarcoma. However, the response to HMGN2 is not concordant
among all types of cancer. The biological response of cancer cells
to HMGN2 may depend, not only on the particular cell type, but also
on the presence of other factors that remain to be defined. Our
gain-of-function studies in vitro and in vivo using
HMGN2 lentivirus revealed a significant decrease in growth and
migration and an increase in apoptosis in SaO2 and U2-OS cells,
suggesting that HMGN2 may function as a tumor suppressor in
osteosarcoma.
To the best of our knowledge, this is the first
study to provide data demonstrating that HMGN2 has an inhibitory
effect on growth and migration of osteosarcoma cells. However,
limited evidence was obtained regarding the use of osteosarcoma
cells in the two cell lines. Investigations using more cell lines
and primary tumor are therefore crucial to confirm the findings of
this study. In conclusion, the present results have shown that the
enhanced expression of HMGN2 in osteosarcoma cells by HMGN2
lentivirus exerts inhibitory effects on growth and migration of
osteosarcoma cells. HMGN2 as a tumor suppressor may provide a novel
approach to human osteosarcoma treatment.
References
|
1
|
Huang J, Ni J, Liu K, et al: HMGB1
promotes drug resistance in osteosarcoma. Cancer Res. 72:230–238.
2012. View Article : Google Scholar
|
|
2
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar
|
|
3
|
Meyers PA, Schwartz CL, Krailo MD, et al:
Osteosarcoma: the addition of muramyl tripeptide to chemotherapy
improves overall survival - a report from the Children’s Oncology
Group. J Clin Oncol. 26:633–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau CC, Harris CP, Lu XY, et al: Frequent
amplification and rearrangement of chromosomal bands 6p12-p21 and
17p11.2 in osteosarcoma. Genes Chromosomes Cancer. 39:11–21. 2004.
View Article : Google Scholar
|
|
5
|
Musselman CA and Kutateladze TG: Methyl
fingerprinting of the nucleosome reveals the molecular mechanism of
high-mobility group nucleosomal-2 (HMGN2) association. Proc Natl
Acad Sci USA. 108:12189–12190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bustin M: Regulation of DNA-dependent
activities by the functional motifs of the high-mobility-group
chromosomal proteins. Mol Cell Biol. 19:5237–5246. 1999.PubMed/NCBI
|
|
7
|
Postnikov YV, Herrera JE, Hock R, Scheer U
and Bustin M: Clusters of nucleosomes containing chromosomal
protein HMG-17 in chromatin. J Mol Biol. 274:454–465. 1997.
View Article : Google Scholar
|
|
8
|
Porkka K, Laakkonen P, Hoffman JA,
Bernasconi M and Ruoslahti E: A fragment of the HMGN2 protein homes
to the nuclei of tumor cells and tumor endothelial cells in vivo.
Proc Natl Acad Sci USA. 99:7444–7449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srikantha T, Landsman D and Bustin M:
Retropseudogenes for human chromosomal protein HMG-17. J Mol Biol.
197:405–413. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spieker N, Beitsma M, van Sluis P, et al:
An integrated 5-Mb physical, genetic, and radiation hybrid map of a
1p36.1 region implicated in neuroblastoma pathogenesis. Genes
Chromosomes Cancer. 27:143–152. 2000. View Article : Google Scholar
|
|
11
|
Hu A, Dong X, Liu X, et al:
Nucleosome-binding protein HMGN2 exhibits antitumor activity in
oral squamous cell carcinoma. Oncol Lett. 7:115–120. 2014.
|
|
12
|
Liu X, Dong X, Zhang Y, et al: Inhibitory
effects of high mobility group chromosomal protein N2 on human
tongue carcinoma transplanted in nude mice. Hua Xi Kou Qiang Yi Xue
Za Zhi. 32:5–8. 2014.(In Chinese). PubMed/NCBI
|
|
13
|
Evans DL, Bishop GR and Jaso-Friedmann L:
Methods for cell cycle analysis and detection of apoptosis of
teleost cells. Methods Cell Sci. 22:225–231. 2000. View Article : Google Scholar
|
|
14
|
Birger Y, Catez F, Furusawa T, et al:
Increased tumorigenicity and sensitivity to ionizing radiation upon
loss of chromosomal protein HMGN1. Cancer Res. 65:6711–6718. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Yuan B, Yuan W, Wang C, Gao R and
Wang J: The expression and clinical significance of high mobility
group nucleosome binding domain 5 in human osteosarcoma. Tumour
Biol. 35:6539–6547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li DQ, Hou YF, Wu J, et al: Gene
expression profile analysis of an isogenic tumour metastasis model
reveals a functional role for oncogene AF1Q in breast cancer
metastasis. Eur J Cancer. 42:3274–3286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang C, Zhou LQ and Song G: Effect of
nucleosomal binding protein 1 in androgen-independent prostatic
carcinoma. Zhonghua Yi Xue Za Zhi. 88:657–660. 2008.(In Chinese).
PubMed/NCBI
|