Introduction
A recent study predicted that given the current
trends in population aging and growth, 22.2 million new cancer
cases and 13.2 million cancer-related mortalities are expected in
2030 (1). Although cancer pain may
be present at any time during the course of the disease, it
generally increases with disease progression. Thus, 75–90% of
patients with metastatic or advanced stage cancer experience
significant pain (2–4). The treatment of bone cancer pain
continues to present a major clinical challenge since all the
available therapies have significant unwanted side effects, and
specific cell and molecular mechanisms, which may involve a
combination of inflammatory and neuropathic pain, remain elusive
(5,6). The development of new analgesic
therapies that are efficacious and have fewer side effects is of
utmost clinical significance since it may improve patient quality
of life and functional status.
NR2B, the most important N-methyl-D-aspartate (NMDA)
receptor subunit, is intensively distributed at the site of
transmission and regulation of pain, such as the spinal cord dorsal
horn (7,8). Central sensitization is an important
mechanism in the development of chronic pain, and the NR2B subunit
is substantially upregulated in the synaptic membrane, which is
involved in the pain hypersensitivity caused by central
sensitization (9–11). It was demonstrated that in mice with
bone cancer pain, the expression of the spinal NR2B subunit was
significantly increased, and it was thought important in the
development of bone cancer pain (12). Prior to exerting its physiological
role, newly synthesized NR2B must be transported into dendrites and
localized to synapses, which is one of the prerequisites for NMDA
receptor activity (13,14).
Kinesin superfamily protein 17 (KIF17), a molecular
motor expressed abundantly in mammalian neurons, has been proposed
to exclusively bind to a protein complex that contains mLin-10
(Mint1/X11α, a synaptic-receptor sorting protein) and the NR2B
subunit from the cell body to the dendrites of neurons (15,16).
Recent studies have focused on the cerebral cortex and the
hippocampus and demonstrated that KIF17-based NR2B transport
mechanisms for maintaining NR2A/2B levels may underlie multiple
phases of memory processes in vivo (17). However, little is known regarding
the precise roles and mechanisms of NR2B subunit transport at the
spinal cord level in the mouse model of bone cancer pain.
In the present study, we determined that KIF17 is
important in the process of bone cancer pain. Intrathecal
administration of KIF17 antisense oligodeoxynucleotide (ODN)
attenuated the pain behaviors in this model of bone cancer pain and
decreased the expression of NR2B in mice. Furthermore, the
expression of mLin-10 was suppressed. The results showed that in
the spinal cord, KIF17 participates in the regulation of NR2B
transshipment, which is involved in the development of bone cancer
pain in mice, and mLin-10 may also be involved.
Materials and methods
Animals, drugs and administration
Animals were obtained from the Model Animal Research
Center of Nanjing University. Sixty 4–6-weeks old adult male
C3H/HeJ mice, weighing 20–25 g, were used in the present study. The
mice were maintained in a temperature-controlled (21±1°C) room with
12-h alternating dark/light cycles. The experimental protocols were
approved by the Animal Care and Use Committee at the Medical School
of Nanjing University. All efforts were made to minimize animal
suffering and to reduce the number of animals used.
The sequences of the sense and antisense KIF17 ODNs
were designed as previously reported (15). The sequences used were: sense,
5′-TTCGGTGGTGAGCCTCTG-3′, and antisense, 5′-CAGAGGCTCACCACCGAA-3′.
The ODNs were modified with phosphorothioate to improve stability
and were purchased from Sangon Biotechnology Co. (Shanghai, China).
The ODNs were dissolved in saline, and 5 μl were injected
intrathecally (i.t.) via lumbar puncture at the intervertebral
space of L4–5 or L5–6. Injections occurred once per day for six
consecutive days starting from day 14 after tumor cell implantation
(TCI). KIF17 sense ODN (5 μg), antisense ODN (5 μg) and control
saline (5 μl) were injected i.t.
Murine model of bone cancer pain
TCI was achieved by injecting the osteosarcoma NCTC
2472, purchased from the American Type Culture Collection
(Manassas, VA, USA; no. 2087787) into the intramedullary space of
the right femur to induce bone cancer in mice. According to the
ATCC recommendations, the tumor cells were cultured in NCTC 135
medium (Sigma-Aldrich, St. Louis, MO, USA) with 10% horse serum
(Gibco, Grand Island, NY, USA). The cell incubator (Thermo Forma,
Inc., Marietta, OH, USA) was kept at 37°C and had an atmosphere of
5% CO2 and 95% air humidity. The cells were passaged
twice per week.
The protocol described by Schwei et al was
used to induce the murine model of bone cancer pain (18). The surgery was performed under
anesthesia with pentobarbital sodium (50 μg/kg, intraperitoneally,
i.p.). Gonarthrotomy was performed, exposing the femur condyles. A
needle was employed to perforate the cortex and a hole was drilled
using a dental bur. Twenty microliters of α-minimal essence medium
(α-MEM), containing 2×105 NCTC 2472 cells, were injected
into the intramedullary space of the right femur to induce bone
cancer pain. α-MEM (20μl) with no cancer cells was injected in the
sham surgery. The injection hole was subsequently plugged with a
dental amalgam to tightly seal the injection hole in the bone and
prevent the escape of tumor cells from the bone. The wound was
irrigated with saline and then closed. The surgical operations were
conducted under aseptic conditions.
Assessment of bone cancer-related pain
behavior
The mice were randomly divided into the sham (n=20)
and TCI (n=40) groups. On post-operation day 14, after the
pain-associated behaviors were tested, the TCI mice were randomly
divided into the TCI + saline group (n=10), TCI + KIF17 sense ODN
group (n=10) and TCI + KIF17 antisense ODN group (n=10). The number
of spontaneous lifting actions and mechanical allodynia events was
assessed in mice with bone cancer to evaluate bone
cancer-associated pain. The tests were performed during the light
phase; prior to each test, the mice were allowed to acclimatize for
a minimum of 30 min.
Spontaneous lifting behavior
The mice were placed in individual plexiglass
compartments (10×10×15 cm). The behaviors of the mice were
evaluated by measuring the number of spontaneous flinches of the
right hind limb over a 2-min period of observation (19). Five periods were recorded for each
mouse. Positive responses of the right hind limb involved a lifting
movement and were not related to walking or grooming.
Mechanical allodynia test
Mechanical allodynia was measured using von Frey
filaments (Stoelting, Wood Dale, IL, USA) with a protocol similar
to that previously described (20).
Briefly, the animals were individually placed beneath transparent
plexiglass compartments (10×10×15 cm) on a metal-mesh floor
(graticule: 0.5×0.5 cm). The minimum pressure was set to 0.16 g,
and the maximum pressure was set to 2.0 g. The duration of each
stimulus was 6–8 sec, and the interstimulus interval was a minimum
of 15 sec. Brisk withdrawal of the paw and paw flinching were
considered positive responses. The paw withdrawal mechanical
threshold (PWMT) was determined by sequentially increasing and
decreasing the stimulus strength (the ‘up- and -down’ method),
which is the lowest von Frey filament that had three or more
positive responses. Every mouse was tested five times per stimulus
strength.
Western blot analysis
The spinal cord L3–5 segments were immediately
removed from the deeply anesthetized mice (pentobarbital sodium, 50
μg/kg, i.p.) and stored in liquid nitrogen. The tissue samples were
homogenized in lysis buffer containing protease and phosphatase
inhibitor. The samples were centrifuged at 12,000 rpm for 10 min at
4°C, and the supernatant was removed. The BCA method was used to
determine the protein concentration (Kaiji Biotechnology, Nanjing,
China). The samples were boiled at 100°C for 10 min with 1X loading
buffer. Protein lysates (50 μg) were separated using 10% SDS-PAGE
and run for 30 min at 80 V and 90 min at 120 V. The lysates were
then transferred into polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA) at 200 mA for 2 h (depending on the
molecular weight of the target protein). The membranes were blocked
in 5% non-fat milk in Tris-buffered saline with Tween-20 (TBST) for
2 h at room temperature and then incubated with primary rabbit
antibodies [anti-KIF17 (1:1,000), anti-NR2B (1:1,000), anti-mLin-10
antibody (1:1,500) all from Abcam, Cambridge, UK, and anti-β-actin
(1:5,000) Cell Signaling Technology, MA, USA] diluted in blocking
buffer. The following day, after washing six times with TBST for 1
h, the membranes were incubated with the secondary goat anti-rabbit
antibody conjugated with horseradish peroxidase (1:5,000; Cell
Signaling Technology) for 2 h at room temperature and then washed
again for 1 h. Immunoblots were detected using the ECL system
(Millipore). The images of western blot analysis products were
collected and analyzed using Quantity One V4.40 (Bio-Rad, Hercules,
CA, USA). Each of the control and treatment groups was first
standardized with β-actin.
Statistical analysis
Data are presented as the means ± SD (standard
deviation). The data were analyzed using repeated measures analysis
of variance. The LSD post-hoc tests were performed to determine the
sources of the differences when significant main effects were
observed. P values of ≤0.05 was considered significant.
Results
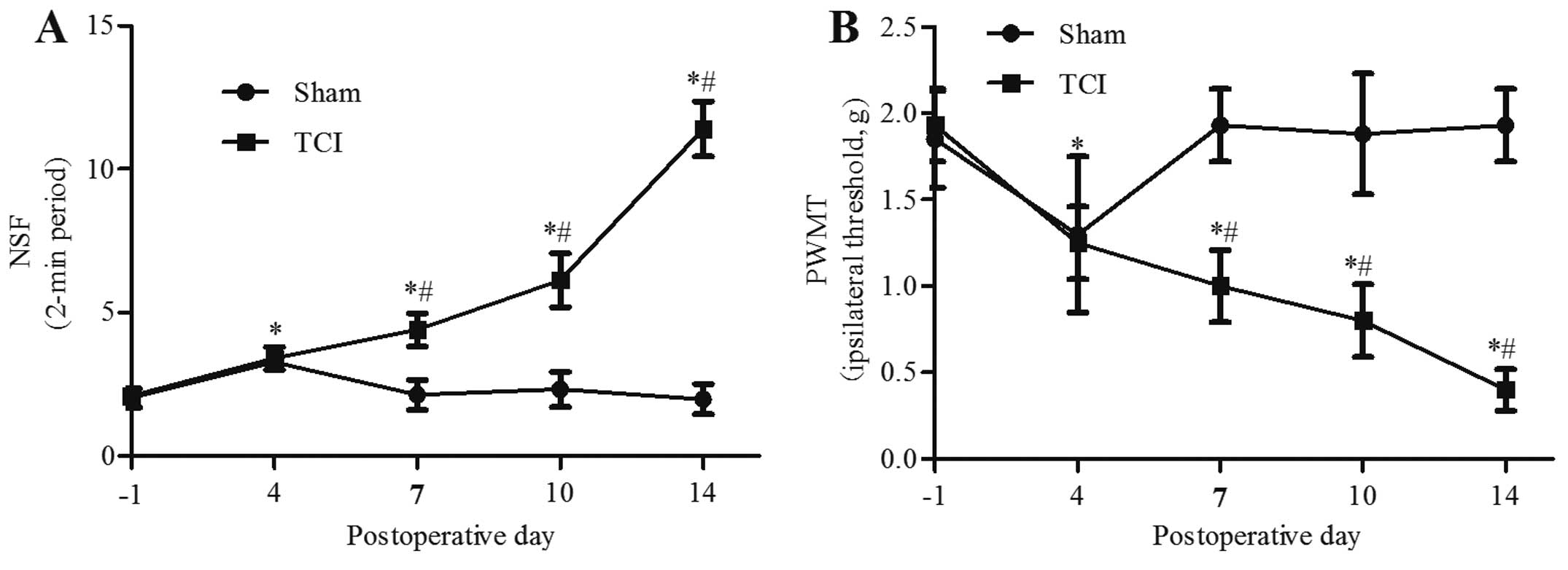
Pain behavior over time
Prior to the operation, there was no significant
difference between groups in the number of spontaneous flinches or
in the PWMT (P>0.05). Compared with the baseline before the
operation, on day 4 after the operation, the spontaneous flinches
increased and the PWMT decreased in the TCI and sham groups
(P<0.05). However, the pain behaviors in the sham group
recovered to the baseline level at day 7. By contrast, TCI induced
an increase of the number of spontaneous flinches at day 7
(4.40±0.58), which gradually increased over time until day 14, when
the P-value was 11.40±0.96 (Fig.
1A). The PWMT decreased by day 7 (1.00±0.21 g), and on day 14,
the P-value was 0.40±0.12 g (P<0.05) (Fig. 1B).
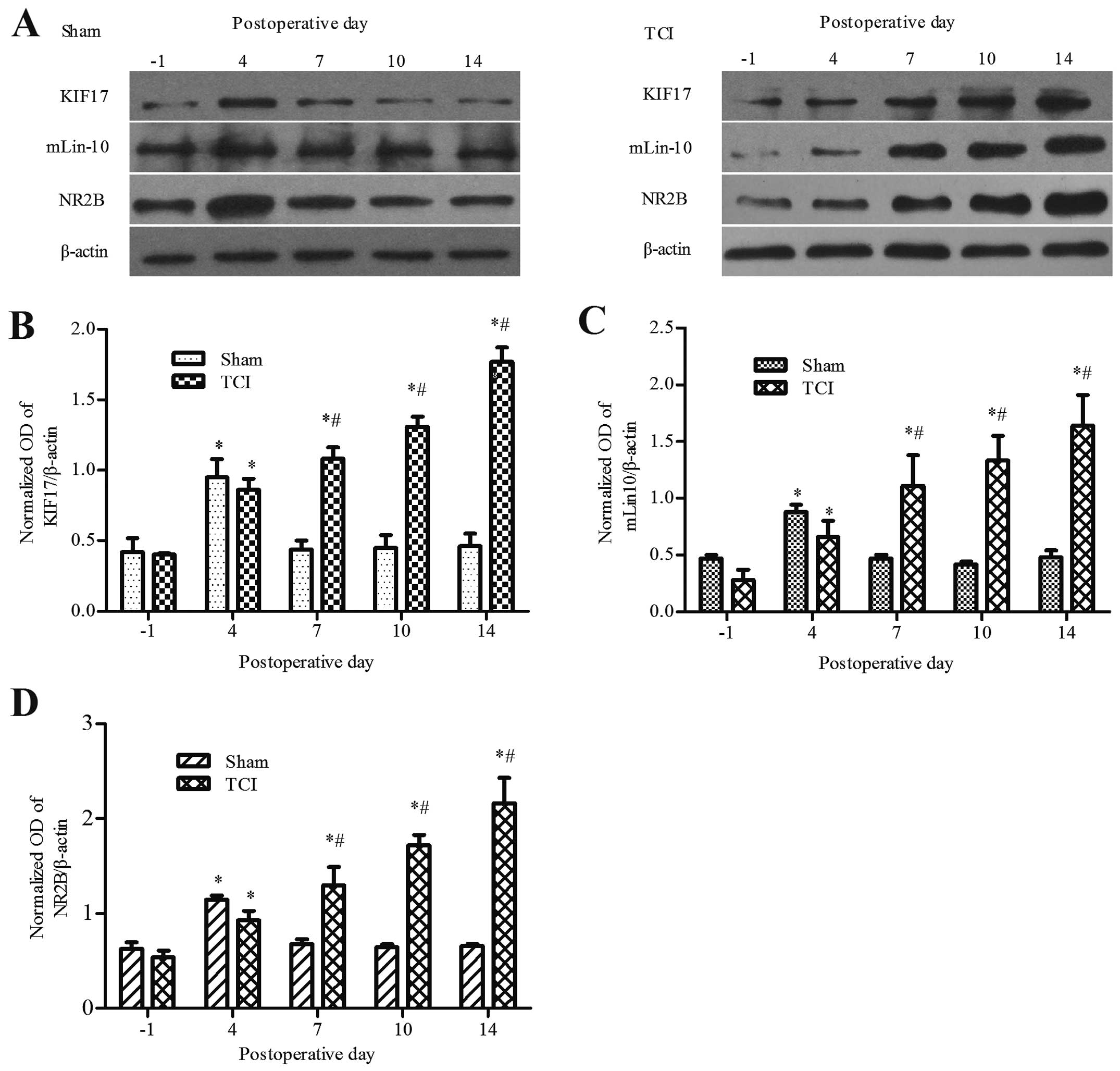
Expression of KIF17, mLin-10 and NR2B in
bone cancer pain
The results confirmed that in the sham and TCI
groups, when compared with the baseline prior to operation, the
expression of KIF17, mLin-10 and NR2B increased beginning on day 4
after the operation (P<0.05). However, the sham group recovered
to the baseline level on days 7–14 (P>0.05). Compared with the
sham group, the expression of KIF17, mLin-10, and NR2B in the TCI
group was increased at day 7 and peaked on day 14 after the
operation (P<0.05). At day 14, the P-values were 1.77±0.10,
1.64±0.27 and 2.16±0.27, respectively (Fig. 2).
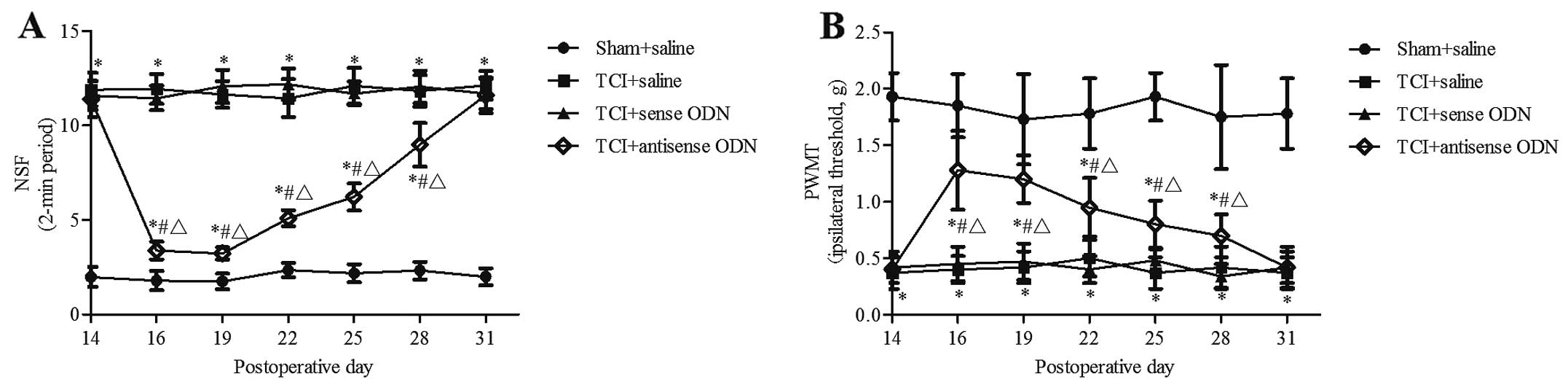
Effect of the intrathecal administration
of KIF17 antisense ODN on pain behavior in bone cancer pain
In the TCI group injected with saline and KIF17
sense ODN and the sham group injected with saline, the pain
behaviors did not change, but remained at a regular level
(P>0.05). Compared with the pre-lesion P-value prior to dosing
and the TCI group injected with saline or KIF17 sense ODN, from 16
to 28 days after the operation, the spontaneous flinches were
decreased and the PWMT was significantly increased in the TCI +
KIF17 antisense ODN group (P<0.05). At day 16, the P-values were
3.38±0.47 and 1.28±0.35 g. At day 28, the P-values were 8.98±1.15
and 0.70±0.19 g, respectively. However, at day 31, there was no
significant difference between TCI groups in terms of the number of
spontaneous flinches or the PWMT (P>0.05). Although injected
KIF17 antisense ODN produced a significant transient inhibition of
the spontaneous flinches and PWMT, compared with the sham + saline
group, the difference remained significant (P<0.05) (Fig. 3).
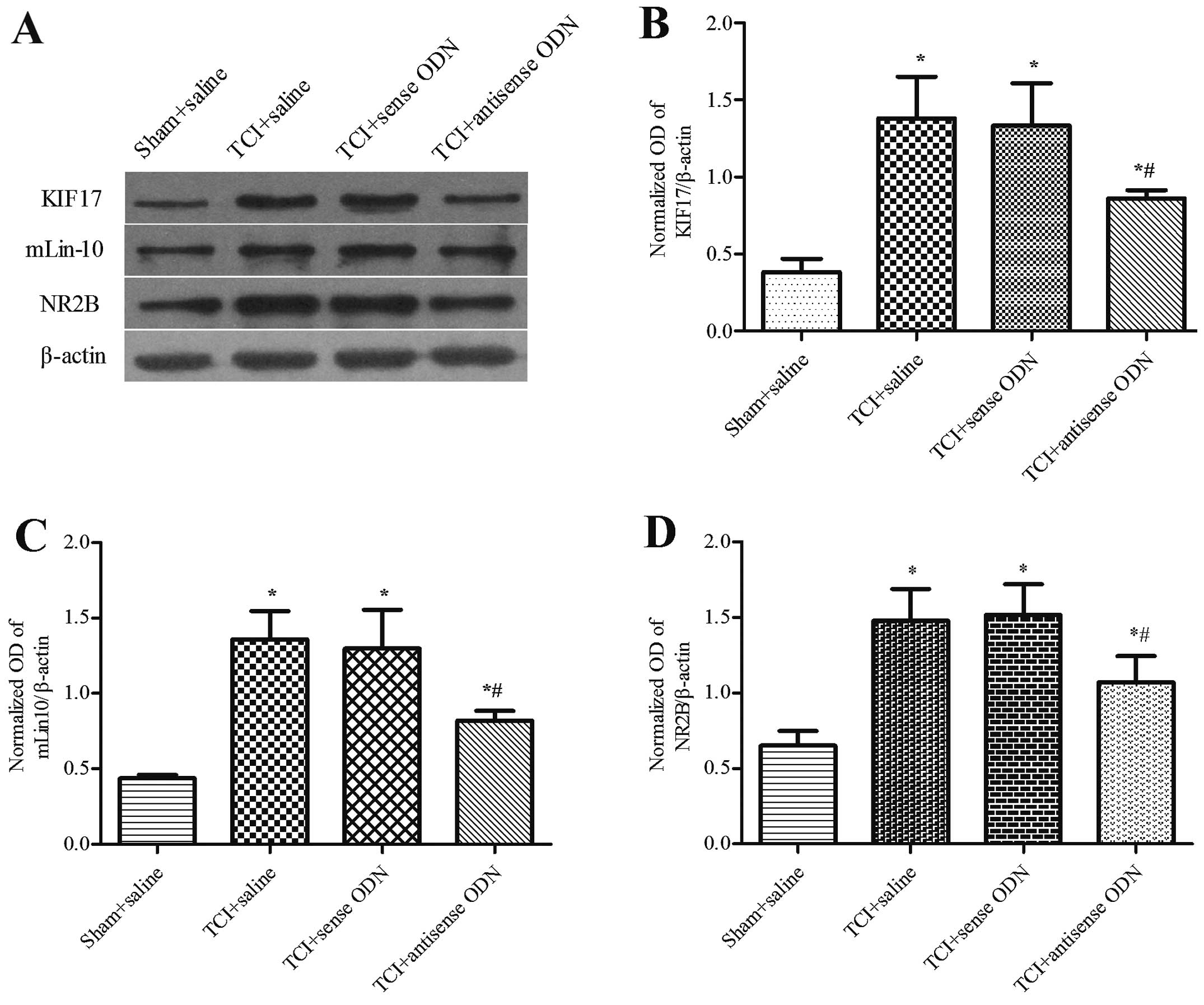
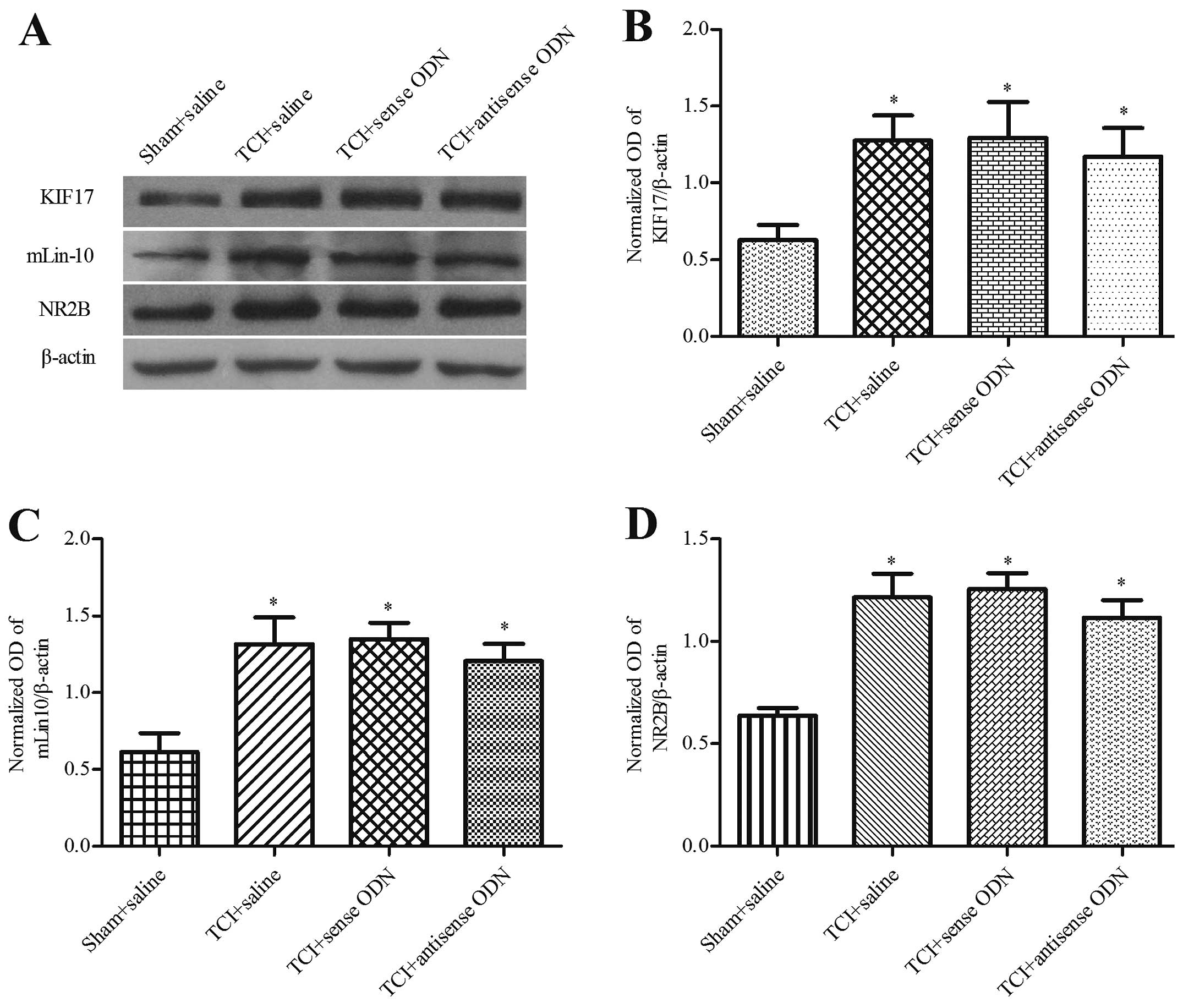
Effect of the intrathecal administration
of KIF17 antisense ODN on mLin-10 and NR2B expression in bone
cancer pain
We examined whether the effect of KIF17 antisense
ODN during the development of bone cancer pain caused the reduction
of NR2B protein within the spinal cord. Western blot analysis
demonstrated that compared with the other TCI groups, the
expression levels of KIF17, mLin-10 and NR2B in the spinal cord
were significantly reduced following the application of KIF17
antisense ODN on day 19 and for the 6 days after intrathecal
administration (P<0.05). Compared with the sham + saline group,
the expression of KIF17, mLin-10 and NR2B remained upregulated
(P<0.05). The P-values were 0.86±0.05, 0.82±0.07 and 1.07±0.17,
respectively. No significant difference was identified between the
saline group and the KIF17 sense ODN group for TCI (P>0.05)
(Fig. 4). However, at 31 days after
the operation, there was no significant difference among the three
TCI groups in terms of the expression levels of KIF17, mLin-10 and
NR2B in the spinal cord (P>0.05). The P-values were 1.17±0.19,
1.21±0.11 and 1.12±0.09, respectively (Fig. 5).
Discussion
KIF17 is a microtubule-based molecular motor that
converts the chemical energy of ATP hydrolysis into a mechanical
force used to transport cargo through microtubules (21). It has a pair of head motor domains
that bind to microtubules, a coiled-coil stalk, and a tail domain
that binds cargos (22,23). In neurons, KIF17 participated in the
transport of the NMDA receptor NR2B subunit (15) along microtubules from the cell body
to dendrites, which is considered to be the most important
physiological effect of KIF17 and is associated with synaptic
transmission, learning, memory and other functions.
The present study revealed the critical role of
KIF17 in the process of bone cancer pain in mice. On day 4 after
the operation, the pain behaviors and the expression of KIF17 and
NR2B were altered in the TCI and sham groups, however, they were
all recovered to the baseline level on day 7 in the sham group.
These findings suggest that the pain behaviors and protein
expression upregulation on day 4 were associated with the surgery
and wound healing. In the TCI group, the number of spontaneous
flinches was increased and the PWMT continuously decreased on days
7, 10 and 14. Simultaneously, the expression of KIF17 and NR2B in
the spinal cord was significantly increased. Consistent with
previous studies (?), the pain behaviors were most clear and stable
at 14 days after the inoculation of bone cancer pain in mice. Our
intrathecal administration was initiated on day 14 after the
operation and occurred once per day for six consecutive days.
During the period of continuous injection of antisense KIF17 ODN,
the pain behaviors were clearly attenuated. Following the
continuous dosing (at 19 days after the operation), the expressions
of KIF17 and NR2B protein within the spinal cord was decreased.
Thirty-one days after the operation, the analgesic effects
disappeared, and KIF17 and NR2B expression levels were again
upregulated.
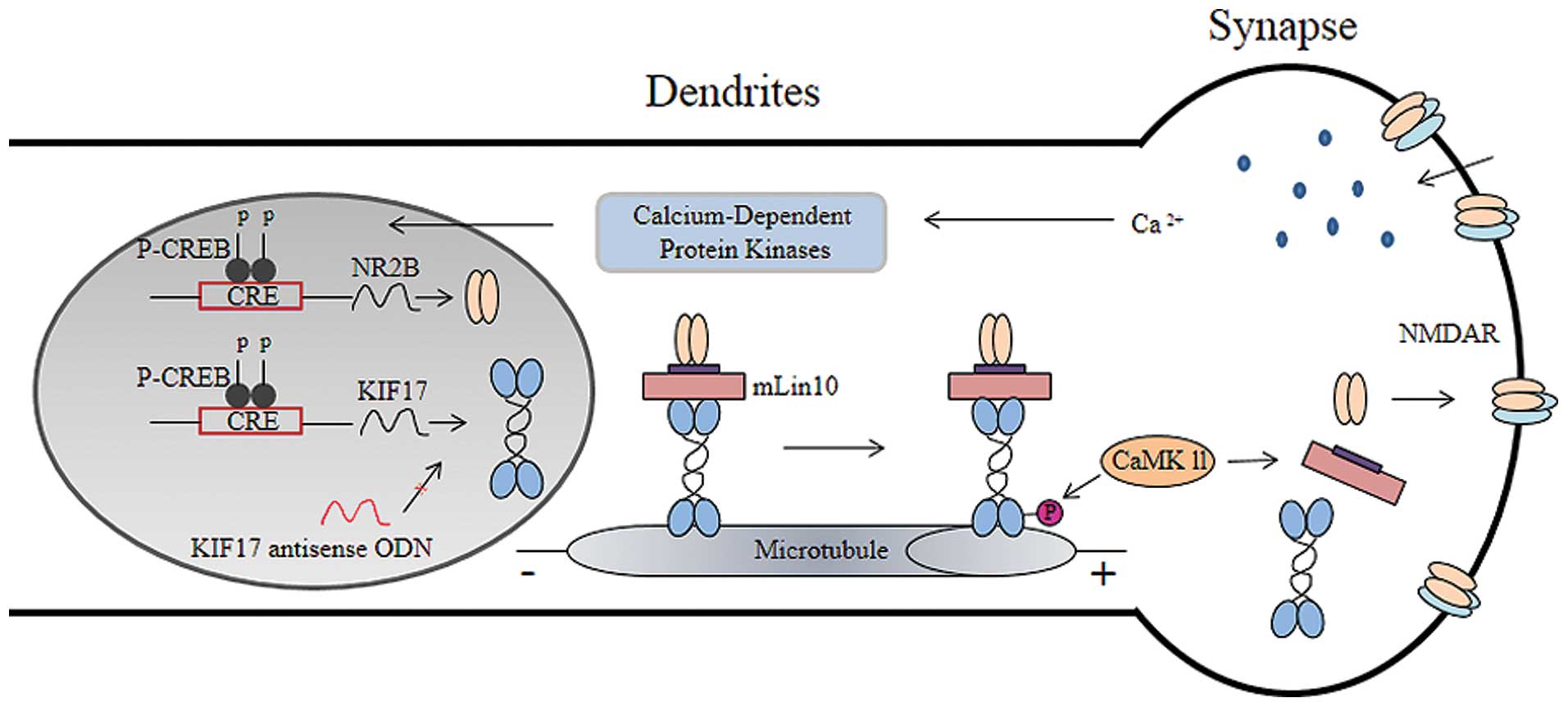
The present study suggests that KIF17 transport of
NR2B in the spinal cord may contribute to the development of bone
cancer pain. When bone cancer pain occurs, the peripheral
nociceptive receptors are activated by the noxious stimulation
receptors, and the pain signals are then transferred to the spinal
cord dorsal horn. This transmission results in increased excitatory
neurotransmitter release on the presynaptic membrane, which results
in the activation of calcium ion influx and calcium-dependent
protein kinases. The kinases may also activate downstream of the
substrate and ultimately increase the level of cAMP response
element-binding protein (CREB). The KIF17 and NR2B gene promoter
regions have the same CRE (TGACGTCA) sequence (24). Thus, the expression of KIF17 and
NR2B increased after TCI. Then, NR2B was transported from the cell
body. CaMKII-dependent phosphorylation of Ser 1029 is the molecular
switch that triggers the release of NR2B from KIF17, thereby
unloading NR2B to the dendrites (25), which forms the NMDA receptor and
participates in the occurrence of bone cancer pain. Following the
injection of antisense KIF17 ODN, the expression of KIF17 was
decreased and the inhibition of KIF17 transported NR2B. The NR2B on
the dendritic membrane decreased, and the pain behaviors of the
mice were suppressed.
Previous studies considered that the transshipment
of NR2B was accomplished via a direct interaction of the KIF17 tail
domain with a PDZ domain of mLin-10 (Mint1/X11α) (26,27).
mLin-10 is a shared component of the polarized protein localization
pathway in neurons and epithelia and is detectable only in neurons.
It comprises of a variable N-terminal region and a constant
C-terminal region that contains two PDZ domains. One domain binds
to KIF17, and the binding tail is necessary for this function of
KIF17 (26). The binding was very
specific between the KIF17 tail domain and the first PDZ domain of
mLin-10. The disruption of the first PDZ domain decreased the
binding with KIF17, whereas the disruption of the second PDZ domain
or the deletion of the phospholipid interaction (PI) domain did not
alter the interaction (26).
Similarly, it has been demonstrated that the intrathecal
administration of selected peptide Myr-RC-13 perturbs the spinal
KIF17/mLin-10 interaction and attenuates bone cancer pain (28).
The expression of mLin-10 in the spinal cord in bone
cancer pain was assessed. We determined that the expression of
mLin-10 is consistent with KIF17 and NR2B and was upregulated after
TCI, and intrathecal administration of KIF17 antisense ODN
suppressed the increase of mLin-10. When the analgesic effects
disappeared, mLin-10 expression was again upregulated. This finding
is consistent with previous studies which showed that: i) in the
forebrain, overexpression of KIF17 increased the expression of
mLin-10 and NR2B (24); ii) when
KIF17 expression was blocked in hippocampal neurons, the
expressions of mLin-10 and NR2B was also decreased (15). These results suggest KIF17, mLin-10
and NR2B may be a common adjustment, although we do not understand
the specific mechanism involved in mLin-10 expression. However,
these findings demonstrate that cargoes that contain NR2B are
transported by KIF17, and mLin-10 is necessary for this function.
Thus, in the spinal cord, KIF17 participated in NR2B transshipment
through mLin-10 and plays an important role in the process of bone
cancer pain in mice. KIF17 and mLin-10 may constitute a transfer
complex protein (Fig. 6). In a
subsequent study, we aim to show the specific mechanism of mLin-10
expression and whether other proteins exist that are involved in
NR2B transport.
The present study contributes to the understanding
of the mechanisms of bone cancer pain. In consideration of the
finding that newly synthesized NR2B must be transported into
dendrites and localized to synapses, the results may provide a new
perspective on NR2B transport for clinical treatment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81070892, 81171048, 81171047) and
the Key Subject of Anesthesiology in the Jiangsu Province of China
(XK201140).
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008–2030): a population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Costantini M, Ripamonti C, Beccaro M, et
al: Prevalence, distress, management, and relief of pain during the
last 3 months of cancer patients’ life. Results of an italian
mortality follow-back survey. Ann Oncol. 20:729–735. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van den Beuken-van Everdingen MH, de Rijke
JM, Kessels AG, Schouten HC, van Kleef M and Patijn J: Prevalence
of pain in patients with cancer: a systematic review of the past 40
years. Ann Oncol. 18:1437–1449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantyh P: Bone cancer pain: causes,
consequences, and therapeutic opportunities. Pain. 154:S54–S62.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goblirsch M, Zwolak P and Clohisy DR:
Advances in understanding bone cancer pain. J Cell Biochem.
96:682–688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laurie DJ, Bartke I, Schoepfer R, Naujoks
K and Seeburg PH: Regional, developmental and interspecies
expression of the four NMDAR2 subunits, examined using monoclonal
antibodies. Brain Res Mol Brain Res. 51:23–32. 1997. View Article : Google Scholar
|
|
8
|
Nagy GG, Watanabe M, Fukaya M and Todd AJ:
Synaptic distribution of the NR1, NR2A and NR2B subunits of the
N-methyl-d-aspartate receptor in the rat lumbar spinal cord
revealed with an antigen-unmasking technique. Eur J Neurosci.
20:3301–3312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein T, Magerl W, Hanschmann A, Althaus M
and Treede RD: Antihyperalgesic and analgesic properties of the
N-methyl-D-aspartate (NMDA) receptor antagonist neramexane in a
human surrogate model of neurogenic hyperalgesia. Eur J Pain.
12:17–29. 2008. View Article : Google Scholar
|
|
10
|
Qu XX, Cai J, Li MJ, et al: Role of the
spinal cord NR2B-containing NMDA receptors in the development of
neuropathic pain. Exp Neurol. 215:298–307. 2009. View Article : Google Scholar
|
|
11
|
Woolf CJ: Central sensitization:
uncovering the relation between pain and plasticity.
Anesthesiology. 106:864–867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu X, Zhang J, Ma Z, et al: The role of
N-methyl-D-aspartate receptor subunit NR2B in spinal cord in cancer
pain. Eur J Pain. 14:496–502. 2010. View Article : Google Scholar
|
|
13
|
Paoletti P and Neyton J: NMDA receptor
subunits: function and pharmacology. Curr Opin Pharmacol. 7:39–47.
2007. View Article : Google Scholar
|
|
14
|
Lau CG and Zukin RS: NMDA receptor
trafficking in synaptic plasticity and neuropsychiatric disorders.
Nat Rev Neurosci. 8:413–426. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guillaud L, Setou M and Hirokawa N: KIF17
dynamics and regulation of NR2B trafficking in hippocampal neurons.
J Neurosci. 23:131–140. 2003.PubMed/NCBI
|
|
16
|
Hirokawa N, Niwa S and Tanaka Y: Molecular
motors in neurons: transport mechanisms and roles in brain
function, development, and disease. Neuron. 68:610–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin X, Takei Y, Kido MA and Hirokawa N:
Molecular motor KIF17 is fundamental for memory and learning via
differential support of synaptic NR2A/2B levels. Neuron.
70:310–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwei MJ, Honore P, Rogers SD, et al:
Neurochemical and cellular reorganization of the spinal cord in a
murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999.PubMed/NCBI
|
|
19
|
Luger NM, Sabino MA, Schwei MJ, et al:
Efficacy of systemic morphine suggests a fundamental difference in
the mechanisms that generate bone cancer vs inflammatory pain.
Pain. 99:397–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong-Riley MT and Besharse JC: The kinesin
superfamily protein KIF17: one protein with many functions. Biomol
Concepts. 3:267–282. 2012. View Article : Google Scholar
|
|
22
|
Hirokawa N: Kinesin and dynein superfamily
proteins and the mechanism of organelle transport. Science.
279:519–526. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miki H, Okada Y and Hirokawa N: Analysis
of the kinesin superfamily: insights into structure and function.
Trends Cell Biol. 15:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong RW, Setou M, Teng J, Takei Y and
Hirokawa N: Overexpression of motor protein KIF17 enhances spatial
and working memory in transgenic mice. Proc Natl Acad Sci USA.
99:14500–14505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guillaud L, Wong R and Hirokawa N:
Disruption of KIF17-Mint1 interaction by CaMKII-dependent
phosphorylation: a molecular model of kinesin-cargo release. Nat
Cell Biol. 10:19–29. 2008. View
Article : Google Scholar
|
|
26
|
Setou M: Kinesin superfamily motor protein
KIF17 and mLin-10 in NMDA receptor-containing vesicle transport.
Science. 288:1796–1802. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho A, Morishita W, Hammer RE, Malenka RC
and Sudhof TC: A role for Mints in transmitter release: Mint 1
knockout mice exhibit impaired GABAergic synaptic transmission.
Proc Natl Acad Sci USA. 100:1409–1414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni K, Zhou Y, Sun YE, Liu Y, Gu XP and Ma
ZL: Intrathecal injection of selected peptide Myr-RC-13 attenuates
bone cancer pain by inhibiting KIF17 and NR2B expression. Pharmacol
Biochem Behav. 122:228–233. 2014. View Article : Google Scholar : PubMed/NCBI
|