Introduction
Oral submucous fibrosis (OSF) is a potentially
malignant disease predominantly found in Asian people (1). OSF is characterized by submucosal
fibrosis that affects most of the parts of the oral cavity and
pharynx. OSF has been associated with oral squamous cell carcinoma,
particularly in Taiwan and South-Central China where up to 80% of
oral squamous cell carcinoma cases are associated with betel quid
chewing (2). Possible etiological
factors that have been implicated in this disease, include the
areca nut, capsaicin in chillies, micronutrient deficiencies of
iron, zinc and essential vitamins (3).
Arecoline, one of the areca alkaloids, is the main
agent of the areca nut responsible for fibroblast proliferation
(4). When influenced by slaked
lime, arecoline is hydrolyzed to arecadine, which has pronounced
effects on fibroblasts (5). It has
been showed that arecoline exposure stimulates fibroblast growth
(6). It was also reported that
arecoline depleted cellular glutathione (GTH) levels, and
subsequently induced various genotoxic and cytotoxic stimulation in
oral mucosal fibroblasts (7). A
recent study also demonstrated that arecoline induced
epithelial-mesenchymal transition (EMT)-related factors in primary
human buccal mucosal fibroblasts (6).
EMT is an indispensable mechanism during
morphogenesis, and is also a crucial event in oral squamous cell
carcinoma and OSF (8). Previous
studies demonstrated that upregulation of several molecules
involved in EMT, such as vimentin, were expressed in human buccal
mucosal fibroblasts following arecoline treatment (9), suggesting that the EMT process may be
directly involved in the pathogenesis of OSF. However, the
mechanisms underlying the EMT induced by arecoline remain
unknown.
MicroRNAs (miRNAs) are a class of short (~22 nt)
non-coding RNAs, which have been implicated in multiple cellular
processes, including survival, proliferation, apoptosis and EMT in
various types of cells (10).
Recently, a study found that arecoline induced expression changes
in miRNAs in normal mucosal cells (11). However, few studies have
investigated the functions and the mechanism of differentially
expressed miRNAs in OSF.
In the present study, we aimed to investigate the
role of miRNAs in arecoline-induced EMT in HaCaT cells.
Furthermore, we also explored the potential regulated targets of
the miRNAs. We found that miR-203 was significantly downregulated
in OSF tissues compared to that in normal buccal mucosa tissues,
and that miR-203 negatively regulated secreted frizzled-related
protein 4 (SFRP4), which is correlated with EMT-related gene
expression (12), and positively
regulated transmembrane-4 L six family member 1 (TM4SF1), a small
plasma membrane glycoprotein that regulates cell motility and
proliferation (13). Subsequently,
we observed that upregulation of miR-203 significantly decreased
the capacity of cell proliferation of HaCaT cells, and
significantly upregulated the expression of cytokeratin 19 (CK19)
and E-cadherin proteins, whereas it significantly downregulated the
expression of N-cadherin and vimentin compared to that of the
vehicle control cells. Thus, we provide evidence to illustrate that
miR-203 plays a role in the pathogenesis of OSF, which may be a
target for OSF management.
Materials and methods
Sample collection
A total of 6 OSF tissue samples and 6 normal buccal
mucosa tissues (Nor) were obtained from the Xiangya Hospital of
Central South University according to the legislation and the
Ethics Board of Xiangya Hospital. All subjects or their caregivers
provided written informed consent. All samples were collected and
identified by histopathological evaluation. All the samples were
stored at −80°C until being used.
Cell culture and treatment
HaCaT cells were purchased from ProCell Co. (Wuhan,
China). All the cells were cultured in MEM supplemented with 15%
fetal bovine serum in a 5% CO2 humidified atmosphere at
37°C. The cells were transfected with miR-203 mimics, miR-203
inhibitor, and a negative control (NC) using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) at a final concentration of 30 nM.
To detect the effect of arecoline on EMT, the HaCaT cells were
treated with arecoline (Selleck Chemicals, Houston, TX, USA) at the
indicated concentrations for 72 h.
Quantitative real-time polymerase chain
reaction (qPCR)
The RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA)
was used to extract total RNA according to the manufacturer’s
instructions. The miRNeasy Mini kit (Qiagen) was used for real-time
PCR to detect the expression of miR-203, miR30c and miR-206. The
specific primer sets for miRNA-203, miR30c, miR-206 and U6 were
purchased from GeneCopoeia. miR-203, miR30c and miR-206 expression
was normalized to that of U6. The FastLane Cell
SYBR®-Green kit (Qiagen) was used for real-time PCR to
detect the expression of TM4SF1 and SFRP4. The primers for TM4SF1,
SFRP4 and GAPDH were: TM4SF1 sense, GCTGGAACAGGATGACTGCT and
antisense, ACTCGGACCATGTGGAGGTA; SFRP4 sense, ATCTCGCCTGAAGCCATCG
and antisense, GGGGCTTAG GCGTTTACAGT; GAPDH sense,
CAATGACCCCTTCATTG ACC and antisense, GACAAGCTTCCCGTTCTCAG. TM4SF1
and SFRP4 expression was normalized to GAPDH. The 2−ΔΔCt
method was used to analyze the data.
Western blotting
RIPA lysis buffer (Cwbiotech, Wuhan, China) was used
to extract the total protein from the tissues and cells. The BCA
protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) was
used to measure the protein concentration. The total protein was
separated by 10% SDS-PAGE and then transferred to nitrocellulose
membranes. The membranes were blocked with 8% non-fat milk for 1 h
and incubated with the indicated primary antibody (anti-TM4SF1 and
anti-SFRP4 from Epitomics; rabbit, 1:1,000; anti-N-cadherin and
anti-vimentin from Abcam; rabbit, 1:500; anti-CK19, GAPDH and
anti-E-cadherin from Santa Cruz; mouse, 1:500) overnight at 4°C.
The membranes were washed and incubated with the appropriate
secondary antibody for 90 min at 37°C. The signals on the membranes
were detected by enhanced chemiluminescence (ECL) reagent. Data
were analyzed by densitometry using Image-Pro Plus software 6.0 and
normalized to internal control expression (GAPDH).
Dual luciferase reporter system
The wild-type 3′-UTR of TM4SF1 and SFRP4 was
inserted into the dual luciferase reporter vector. For the
luciferase assay, 105 cells were plated and cultured in
24-well plates to reach ~70% confluency. The cells were
co-transfected with miR-203 mimics and the TM4SF1 or SFRP4 dual
luciferase reporter vector, respectively. After a 48-h
transfection, the Luciferase Reporter Gene Assay kit (Global
Biotech, Shanghai, China) was used to determine the luciferase
activitiy on a luminometer (Roche). Renilla luciferase
activity was normalized to firefly luciferase activity.
CCK-8 cell proliferation assay
Cells (1,000) were seeded in each well of 96-well
plates for 12 h. Then, following the indicated treatment, the cells
were further incubated for 0, 12, 24, 48, 72 and 96 h,
respectively. CCK-8 reagent (10 μl) (Dojindo, Tokyo, Japan)
was added to the well at 1 h before the end of the incubation. The
optical density (OD) value at 490 nm of each well was detected by
an enzyme immunoassay analyzer.
Statistical analysis
Statistical analysis was performed by GraphPad Prism
5 and SPSS 16.0 softwares. The Student’s t-test or one-way ANOVA
was used depending on the experimental conditions. Data are
expressed as mean ± SD. Compared to the controls, a P-value of
<0.05 was considered to indicate a statistically significant
result.
Results
miRNAs are differentially expressed in
OSF tissues
We performed qPCR assay to detect the changes in
miRNAs in 6 OSF tissues. Compared to the average expression in the
normal oral mucosa tissues, the expression of miR-206 was
significantly upregulated in 2 cases and slightly upregulated in 4
cases of OSF; the expression of miR-30c was significantly
upregulated in 5 cases and slightly upregulated in 1 case of OSF;
the expression of miR-203 was significantly upregulated in all of
the 6 cases (Fig. 1A–C). We also
detected the expression of potential target genes of these miRNAs
by qPCR and western blotting in OSF tissues. We found that SFRP4
was increased in 5 of the 6 cases of OSF tissues at the mRNA level
and upregulated in all the OSF tissues at the protein level
compared with that in the normal tissues (Fig. 1D, F and G).
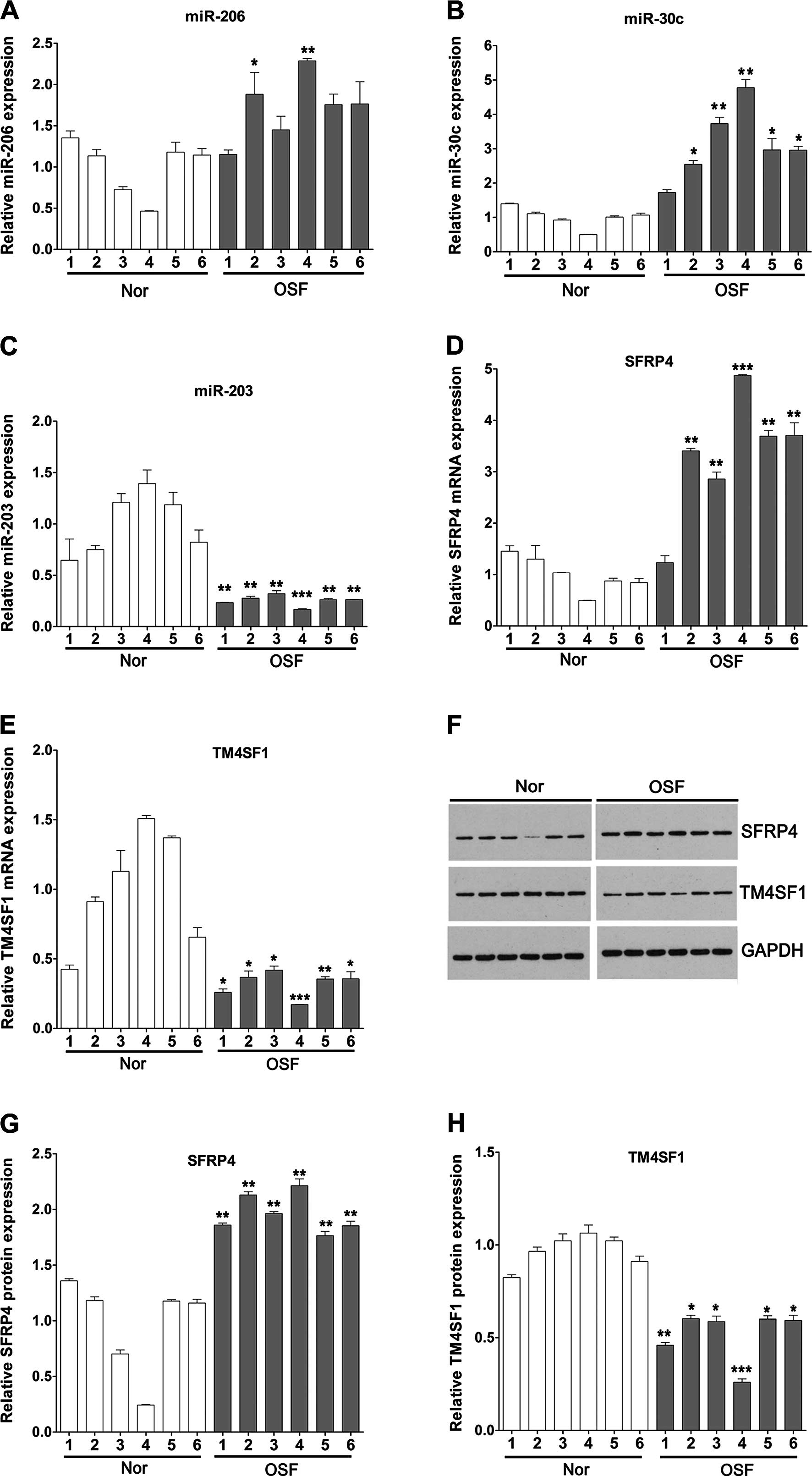 | Figure 1Expression of miR-206, miR-30c,
miR-203, SFRP4 and TM4SF1 in OSF tissues and normal oral mucosa
tissues. qPCR was utilized to detect the expression of (A) miR-206,
(B) miR-30c, (C) miR-203, (D) SFRP4 and (E) TM4SF1 in OSF tissues
and normal oral mucosa tissues. (F) Western blotting was used to
analyze the expression of SFRP4 and TM4SF1 protein in OSF and
normal oral mucosa tissues and quantification was carried out for
(G) SFRP4 and (H) TM4SF1. Data are expressed as the means ± SD.
*P<0.05, **P<0.01,
***P<0.001. SFRP4, secreted frizzled-related protein
4; TM4SF1, transmembrane-4 L six family member 1; OSF, oral
submucous fibrosis. |
In all of the 6 cases of OSF tissues and normal oral
mucosa tissues, we found that TM4SF1 was significantly decreased at
the mRNA and protein levels in the OSF tissues compared with levels
in the normal tissues (Fig. 1E, F and
H).
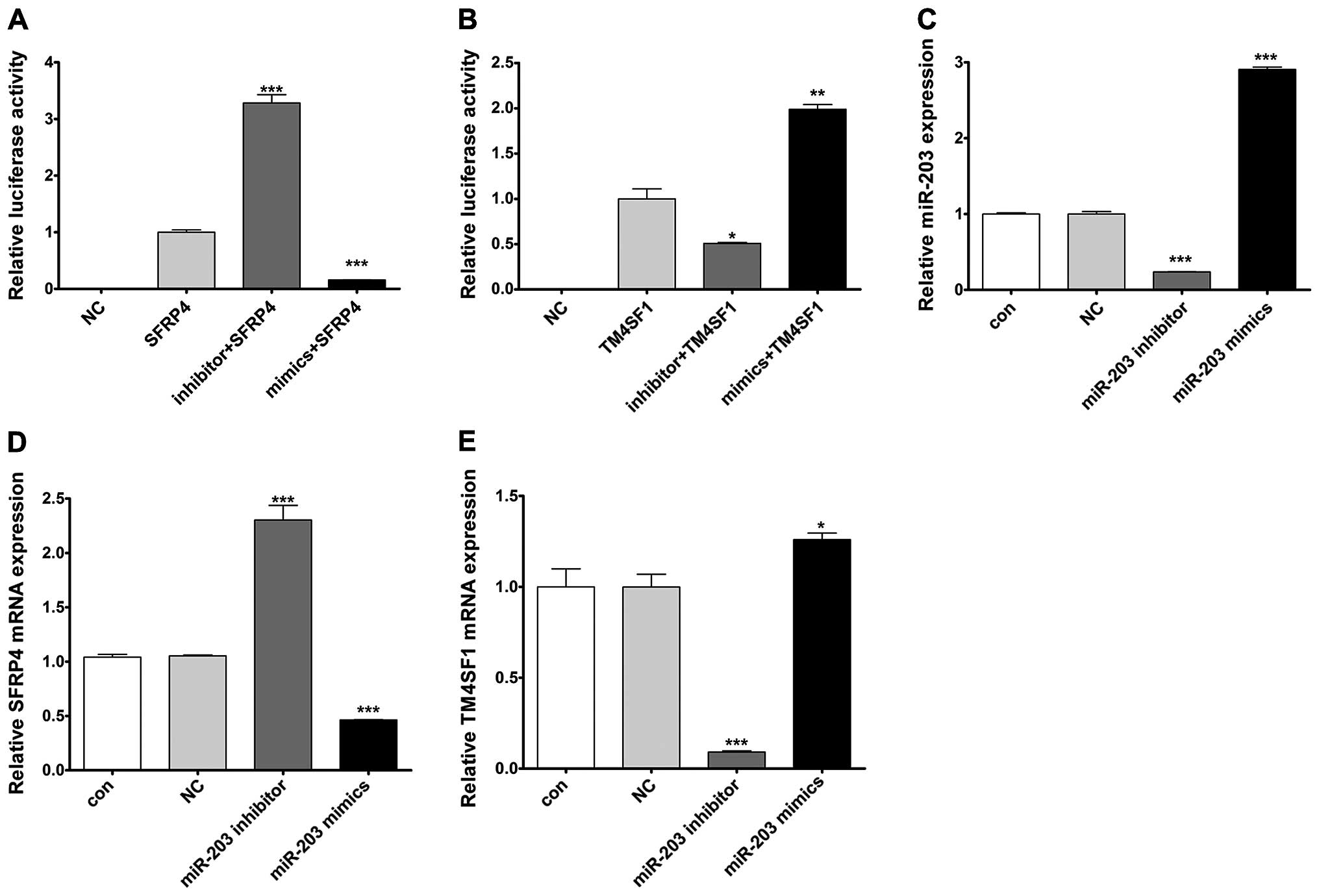
miR-203 negatively regulates SFRP4 and
positively regulates TM4SF1
To investigate whether miR-203 regulates the
expression of SFRP4 and TM4SF1, we cloned the 3′UTR of SFRP4 and
TM4SF1 downstream to the luciferase reporter gene. The constructed
vectors were co-transfected with miR-203 mimics or inhibitor into
the HaCaT cells. The luciferase activity of cells transfected with
the miR-203 mimics and SFRP4 was significantly decreased compared
with the cells that were transfected with SFRP4 alone, whereas the
luciferase activity of cells transfected with miR-203 inhibitor and
SFRP4 was significantly increased compared with the cells that were
transfected with SFRP4 alone (Fig.
2A). In contrast to SFRP4, however, the luciferase activity of
cells transfected with TM4SF1 was induced by co-transfection with
the miR-203 mimics, while it was reduced by co-transfected with the
miR-203 inhibitor (Fig. 2B). In
order to further illustrate the regulatory relationship between
miR-203 and SFRP4 and TM4SF1, we transfected the miR-203 mimics or
inhibitor into HaCaT cells to upregulate or downregulate miR-203
expression. The efficiency of transfection was satisfied for
further analysis (Fig. 2C). qPCR
showed that enhanced or repressed miR-203 significantly decreased
or increased SFRP4 mRNA levels, respectively, compared to the cells
transfected with NC in the HaCaT cells (Fig. 2D). In addition, upregulation or
downregulation of miR-203 significantly induced or reduced TM4SF1
mRNA levels, respectively, compared to the cells transfected with
NC in the HaCaT cells (Fig. 2E).
These results revealed that miR-203 negatively regulated SFRP4 and
positively regulated TM4SF1 at the transcriptional levels.
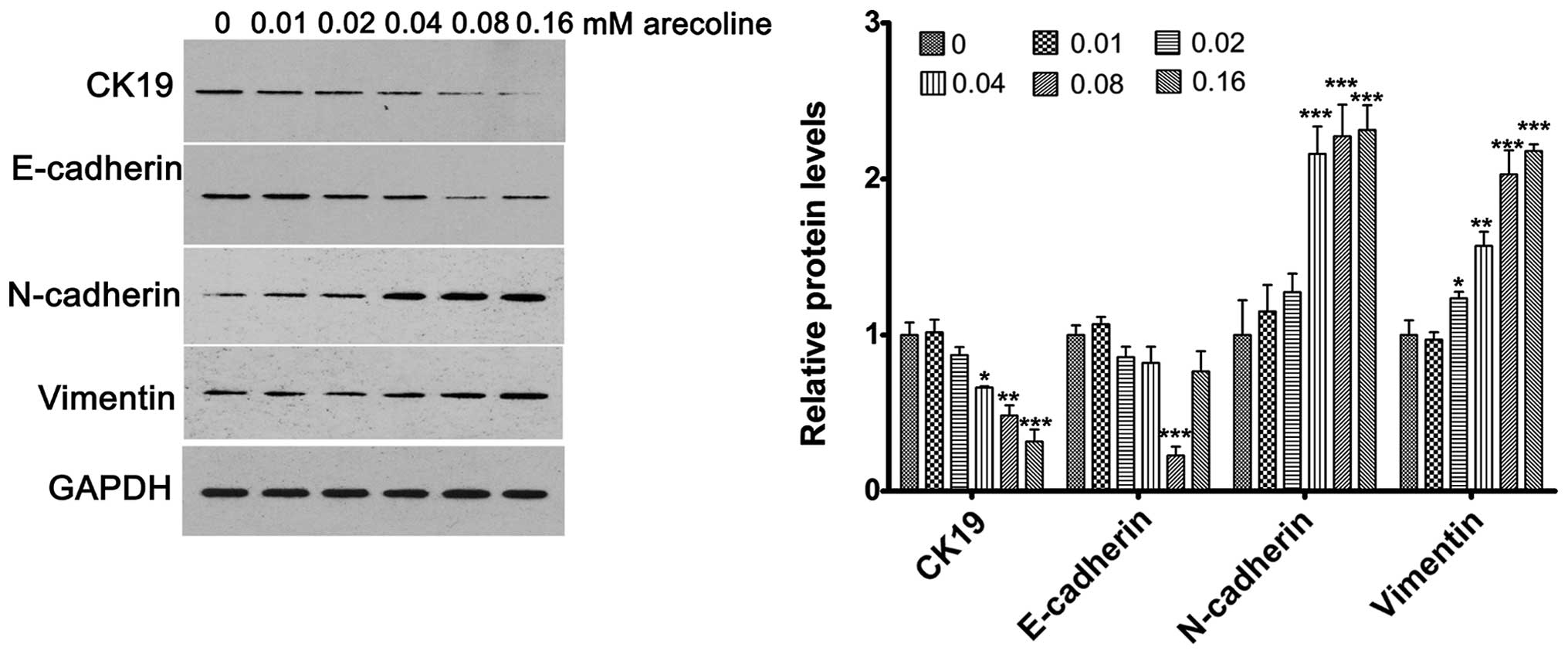
Arecoline affects the expression of
EMT-related genes in a dose-dependent manner
To determine the effects of arecoline on EMT in
HaCaT cells, the HaCaT cells were treated with a series of
increased doses of arecoline for 72 h. Western blotting was used to
evaluate the changes in expression of the EMT-related genes,
including CK19, E-cadherin, N-cadherin and vimentin. The results
showed that CK19 expression was significantly decreased with
increased concentrations of arecoline; E-cadherin expression was
markedly reduced at 0.08 mM; while the expression of N-cadherin and
vimentin was significantly upregulated with increasing
concentrations of arecoline (Fig.
3). Downregulated expression of CK19 and E-cadherin, and
upregulated expression of N-cadherin and vimentin are important
promotive factors of EMT. Thus, the concentration of arecoline at
0.08 mM was chosen for further analysis.
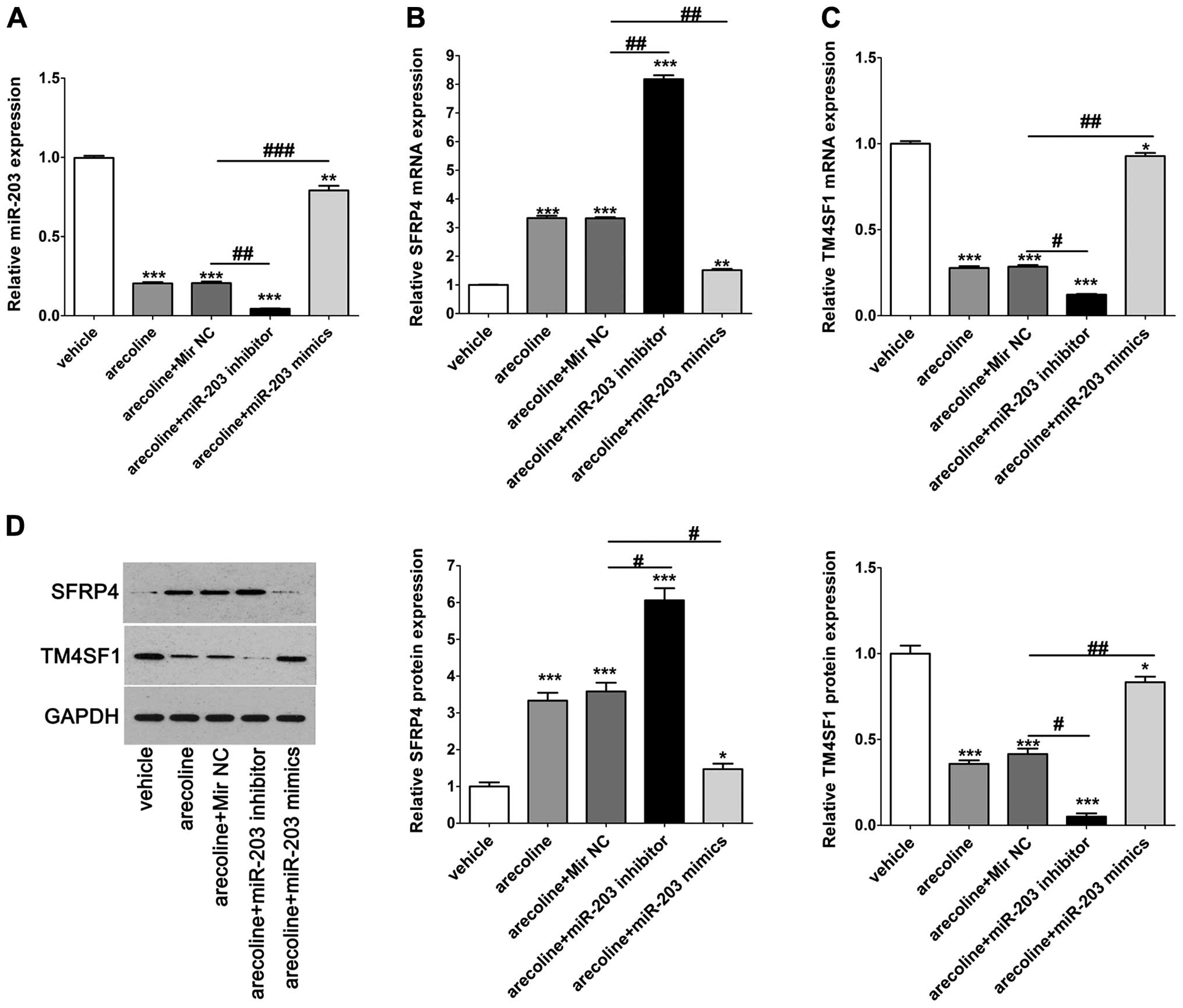
Effects of arecoline on miR-203, SFRP4
and TM4SF1 in the HaCaT cells
To further analyze the effects of arecoline on
miR-203, SFRP4 and TM4SF1 in the HaCaT cells, cells were treated
with arecoline alone or co-treated with miR-203 mimics/inhibitor.
We found that arecoline significantly decreased the expression of
miR-203 compared with the vehicle control, and had a synergistic
effect with the miR-203 inhibitor while co-treatment with miR-203
mimics rescued the expression of miR-203 that was decreased by
arecoline (Fig. 4A). qPCR and
western blotting were performed to detect the expression of SFRP4
and TM4SF1 in the HaCaT cells after the indicated treatments. We
found that arecoline significantly increased the expression of
SFRP4 compared with that in the vehicle control, and had a
synergistic effect with the miR-203 inhibitor; while co-treatment
with miR-203 mimics reversed the upregulated expression of SFRP4
that was induced by arecoline (Fig.
4B). Inversely, arecoline significantly reduced the expression
of TM4SF1 compared with the vehicle control, and had a synergistic
effect with the miR-203 inhibitor; while co-treatment with miR-203
mimics reversed the downregulated expression of TM4SF1 that was
decreased by arecoline (Fig. 4C).
The results of the western blotting further validated the
phenomenon mentioned above at the translational level (Fig. 4D). These results indicated that
arecoline affected the expression of miR-203, and subsequently
regulated the expression of SFRP4 and TM4SF1.
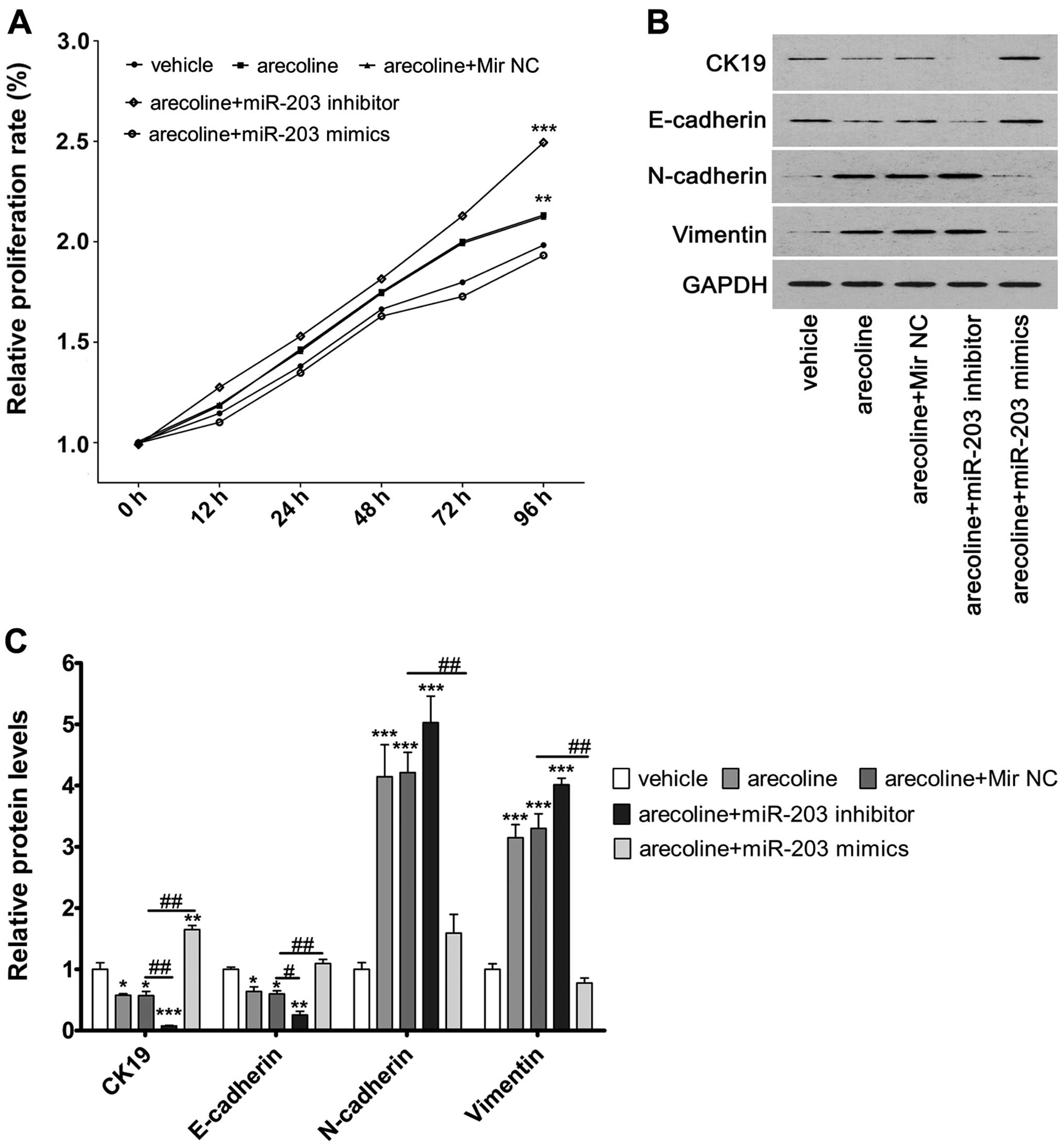
miR-203 regulates cell proliferation, and
regulates the expression of CK19, E-cadherin, N-cadherin and
vimentin in the HaCaT cells
We observed that downregulation of miR-203 mediated
by arecoline or co-treatment with the miR-203 inhibitor
significantly increased cell proliferation ability in the HaCaT
cells compared to the vehicle control cells. Furthermore, we
observed that co-treatment of miR-203 mimics and arecoline
significantly decreased the upregulation of cell proliferation
induced by arecoline (Fig. 5A).
This indicated that miR-203 regulated the proliferation of HaCaT
cells.
To explore the potential molecular mechanisms
underlying miR-203-induced cell proliferation and EMT, we assessed
the expression of CK19, E-cadherin, N-cadherin and vimentin in
HaCaT cells. Downregulation of miR-203 induced by arecoline or
miR-203 inhibitor significantly downregulated the expression of
CK19 and E-cadherin proteins compared to these levels in the
vehicle control cells, whereas it significantly upregulated the
expression of N-cadherin and vimentin. Reversal of the
downregulation of miR-203 in the arecoline-treated cells by miR-203
mimic transfection significantly upregulated the expression of CK19
and E-cadherin proteins compared to these levels in the vehicle
control cells, whereas it significantly downregulated the
expression of N-cadherin and vimentin (Fig. 5B and C).
Discussion
miRNAs have been implicated in inflammation,
fibrosis, EMT and various types of cancers, such as oral cancer.
Some miRNA families have gained attention for their clear function
in tissue fibrosis. miR-30c has been reported to be a tumor
suppressor in endometrial cancer (14) and to act as an independent predictor
for the clinical benefit of tamoxifen therapy in patients with
advanced breast cancer (15). A
previous study showed that miR-206 overexpression inhibited
estrogen receptor-α-dependent proliferation, impaired invasiveness
and induced cell cycle arrest of estrogen receptor-α-positive
endometrial endometrioid adenocarcinoma cell lines (16). miR-203 has previously been shown to
play an important role in epithelial cell biology (17). miR-203 was differentially regulated
in gingival epithelial cells in the presence of P.
gingivalis. Silencing of miR-203 diminished the activation of
signal transducer and activator of transcription 3 (Stat3),
suggesting that alterations in miRNAs modulate important host
signaling responses in pathological processes of severe periodontal
disease (18). During master
transcription factor-induced EMT in MCF7 breast cancer cells,
miR-203 was repressed in a time-dependent manner. Dynamic
simulations revealed stable epithelial and mesenchymal states, and
underscored the crucial role of miR203 in state transitions
underlying epithelial plasticity (19). Thus, according to these findings, we
hypothesized that miR-203 plays a critical role in oral submucous
fibrosis by regulating EMT-related genes via downstream target
molecules.
In the present study, we found that the expression
of miR-203 was significantly upregulated in all 6 OSF tissues,
whereas upregulated expression of miR-206 and miR-30c was observed
in 2 and 5 cases, respectively. Collectively, we therefore focused
on the role of miR-203 in EMT of HaCaT cells for further analysis.
We also found that SFRP4 was increased, while TM4SF1 was
significantly decreased in OSF tissues at the mRNA and protein
levels compared with that in the normal tissues. By dual luciferase
report assay, we demonstrated that miR-203 negatively regulated
SFRP4 and positively regulated TM4SF1 at the transcriptional
levels. Secreted frizzled related protein (SFRP) proteins are
characterized by a frizzled-like cysteine-rich domain and form a
family of soluble proteins (SFRP1-5) (20). Upregulation of SFRP4 has been
observed both in the skin of systemic sclerosis patients (21) and kidney fibrosis (22). Matsushima et al demonstrated
that intramuscular administration of recombinant SFRP4 reduced
fibrosis scar size and ameliorated cardiac function after ischemic
injury (23). By oligonucleotide
microarray that containing 15 cases of OSF tissues and 14 normal
buccal mucosa tissues, our previously study found that expression
of EMT-related gene SFRP4 was significantly upregulated in the OSF
tissues compared with that in the normal mucosa tissues, which was
validated by RT-PCR (24). This
suggested that SFRP4 abnormalities in EMT may play an important
role in the pathogenesis and malignant transformation of OSF.
Transmembrane-4 L six family 1 (TM4SF1), the founding member of the
L6 family, was originally identified as a protein abundantly
expressed in a variety of epithelial cancer cells, and was found to
share certain tetraspanin functions including roles in cell growth,
motility and metastasis (25,26).
Previous findings indicated that TM4SF1 serves as a surface protein
marker which identified mesenchymal stem cells from diverse cell
sources, in particular, fibroblast-rich connective tissues
(27). Thus, these data suggest
that TM4SF1 is involved in the cell proliferation of epithelial
cells during the processes of EMT. miRNAs regulate gene expression
by inhibition of gene translation or facilitation of mRNA
degradation. Our recent results showed that miR-203 directly
targets the 3′UTR of SFRP4, and subsequently downregulated its
expression. However, we also found that miR-203 upregulated TM4SF1
expression. We infer that miR-203 regulates the expression of
TM4SF1 in an indirect manner, and further research should validate
this.
CK19 is expressed exclusively by epithelial cells
and derived cancers (28). Previous
research indicated that CK19 expression may be implicated in the
retention of proliferative potential or undifferentiated character
in oral non-keratinized mucosa (29). We previously reported that the
expression of CK19 at the mRNA or protein levels was shown to be
significantly downregulated in the OSF basal cell layer, which is
the proliferative invasive layer, than levels in normal buccal
non-keratinized mucosa, indicating that the self-renewal capacity
of the basal cell layer of OSF through stem cells was obviously
inhibited (30). Similarly,
stimulation by the areca nut also depressed the constant
regeneration of OSF mucosa and promoted the atrophy of the oral
epithelium. E-cadherin, a calcium-dependent cell-surface
glycoprotein, is critical for maintaining epithelial cell-cell
adhesion, cellular polarity differentiation, growth, cell migration
and seems to be the most common target for various EMT signaling
pathways (31). Downregulation of
E-cadherin has been considered as a hallmark of EMT (32). During EMT, the epithelium expresses
mesenchymal markers and becomes motile, and causes expression of
N-cadherin which is present in mesenchymal cells (33). Enhanced expression of N-cadherin in
epithelial cells has been shown to decrease the endogenous levels
of E-cadherin (34). Vimentin, one
of the four types of intermediate filaments, is considered to play
an important role in structural maintenance and adhesion in many
cells originating from the mesenchymal (35). A previous immunohistochemical assay
revealed that vimentin expression is significantly increased in OSF
specimens than that in normal buccal mucosa. The upregulation of
vimentin following arecoline exposure was consistent with that
noted for fibroblasts cultured from OSF patients (9). By evaluating the changes in EMT
hallmarks, CK19 and E-cadherin, and mesenchymal markers, N-cadherin
and vimentin, using western blotting, we validated that arecoline
induced EMT in the HaCaT cells. We found that arecoline
significantly decreased the expression of miR-203 and SFRP4, and
increased the expression of TM4SF1. Furthermore, arecoline
treatment markedly enhanced the cell growth of HaCaT cells.
Importantly, we found that restoration of downregulated miR-203
induced by arecoline increased the expression of SFRP4, and
decreased the expression of TM4SF1, and attenuated the cell
proliferation of HaCaT cells. Moreover, the restoration of miR-203
expression in the arecoline-treated cells significantly upregulated
the expression of CK19 and E-cadherin proteins, whereas it
significantly downregulated the expression of N-cadherin and
vimentin. Our results suggest that miR-203 inhibits EMT by
regulating the expression of SFRP4 and TM4SF1 in HaCaT cells.
In conclusion, the present study provides evidence
that miR-203 plays a critical role in arecoline-induced OSF by
regulating the process of EMT, at least partially, via targeting
SFRP4 and TM4SF1. Thus, miR-203 may be a target for the prevention
and therapy of OSF.
Acknowledgments
This study was supported by the National Natural
Sciences Foundation of China (no. 81260166, 81041052 and
30572044).
References
|
1
|
Lunde ML, Roman E, Warnakulasuriya S,
Mehrotra R, Laranne J, Vasstrand EN and Ibrahim SO: Profiling of
chromosomal changes in potentially malignant and malignant oral
mucosal lesions from South and South-East Asia using
array-comparative genomic hybridization. Cancer Genomics
Proteomics. 11:127–140. 2014.PubMed/NCBI
|
|
2
|
Hosthor SS, Mahesh P, Priya SA, Sharada P,
Jyotsna M and Chitra S: Quantitative analysis of serum levels of
trace elements in patients with oral submucous fibrosis and oral
squamous cell carcinoma: A randomized cross-sectional study. J Oral
Maxillofac Pathol. 18:46–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohammed F, Manohar V, Jose M, Fairozekhan
Thapasum A, Mohamed S, Halima Shamaz B and D’Souza N: Estimation of
copper in saliva and areca nut products and its correlation with
histological grades of oral submucous fibrosis. J Oral Pathol Med.
44:208–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou ZS, Li M, Gao F, Peng JY, Xiao HB,
Dai LX, Lin SR, Zhang R and Jin LY: Arecoline suppresses HaCaT cell
proliferation through cell cycle regulatory molecules. Oncol Rep.
29:2438–2444. 2013.PubMed/NCBI
|
|
5
|
Wang TN, Huang MS, Lin MC, Duh TH, Lee CH,
Wang CC, Chen PH, Chiang SL, Sheu CC, Chen VC, et al: Betel chewing
and arecoline affects eotaxin-1, asthma and lung function. PLoS
One. 9:e918892014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang YC, Tsai CH, Lai YL, Yu CC, Chi WY,
Li JJ and Chang WW: Arecoline-induced myofibroblast
transdifferentiation from human buccal mucosal fibroblasts is
mediated by ZEB1. J Cell Mol Med. 18:698–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsai CH, Yang SF, Chen YJ, Chu SC, Hsieh
YS and Chang YC: Regulation of interleukin-6 expression by
arecoline in human buccal mucosal fibroblasts is related to
intracellular glutathione levels. Oral Dis. 10:360–364. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanjia H and Xinchun J: The role of
epithelial-mesenchymal transition in oral squamous cell carcinoma
and oral submucous fibrosis. Clin Chim Acta. 383:51–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang YC, Tsai CH, Tai KW, Yang SH, Chou
MY and Lii CK: Elevated vimentin expression in buccal mucosal
fibroblasts by arecoline in vitro as a possible pathogenesis for
oral submucous fibrosis. Oral Oncol. 38:425–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das RK, Anura A, Pal M, Bag S, Majumdar S,
Barui A, Chakraborty C, Ray AK, Sengupta S, Paul RR, et al:
Epitheliomesenchymal transitional attributes in oral sub-mucous
fibrosis. Exp Mol Pathol. 95:259–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu B, Chen J and Jian X: Changes of miRNA
after oral submucous fibrosis co-cultured with Salvia and low-dose
prednisolone. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 39:471–476.
2014.In Chinese. PubMed/NCBI
|
|
12
|
Ford CE, Jary E, Ma SS, Nixdorf S,
Heinzelmann-Schwarz VA and Ward RL: The Wnt gatekeeper SFRP4
modulates EMT, cell migration and downstream Wnt signalling in
serous ovarian cancer cells. PLoS One. 8:e543622013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CI, Merley A, Sciuto TE, Li D, Dvorak
AM, Melero-Martin JM, Dvorak HF and Jaminet SC: TM4SF1: A new
vascular therapeutic target in cancer. Angiogenesis. 17:897–907.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong X, Xu X, Yan Y, Guo F, Li J, Hu Y,
Zhou H and Xun Q: Estrogen regulates the tumour suppressor
MiRNA-30c and its target gene, MTA-1, in endometrial cancer. PLoS
One. 9:e908102014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodríguez-González FG, Sieuwerts AM, Smid
M, Look MP, Meijer-van Gelder ME, de Weerd V, Sleijfer S, Martens
JW and Foekens JA: MicroRNA-30c expression level is an independent
predictor of clinical benefit of endocrine therapy in advanced
estrogen receptor positive breast cancer. Breast Cancer Res Treat.
127:43–51. 2011. View Article : Google Scholar
|
|
16
|
Chen X, Yan Q, Li S, Zhou L, Yang H, Yang
Y, Liu X and Wan X: Expression of the tumor suppressor miR-206 is
associated with cellular proliferative inhibition and impairs
invasion in ERα-positive endometrioid adenocarcinoma. Cancer Lett.
314:41–53. 2012. View Article : Google Scholar
|
|
17
|
McKenna DJ, McDade SS, Patel D and McCance
DJ: MicroRNA 203 expression in keratinocytes is dependent on
regulation of p53 levels by E6. J Virol. 84:10644–10652. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moffatt CE and Lamont RJ: Porphyromonas
gingivalis induction of microRNA-203 expression controls suppressor
of cytokine signaling 3 in gingival epithelial cells. Infect Immun.
79:2632–2637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moes M, Le Béchec A, Crespo I, Laurini C,
Halavatyi A, Vetter G, Del Sol A and Friederich E: A novel network
integrating a miRNA-203/SNAI1 feedback loop which regulates
epithelial to mesenchymal transition. PLoS One. 7:e354402012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tendeng C and Houart C: Cloning and
embryonic expression of five distinct sfrp genes in the zebrafish
Danio rerio. Gene Expr Patterns. 6:761–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bayle J, Fitch J, Jacobsen K, Kumar R,
Lafyatis R and Lemaire R: Increased expression of Wnt2 and SFRP4 in
Tsk mouse skin: Role of Wnt signaling in altered dermal fibrillin
deposition and systemic sclerosis. J Invest Dermatol. 128:871–881.
2008. View Article : Google Scholar
|
|
22
|
Surendran K, Schiavi S and Hruska KA:
Wnt-dependent beta-catenin signaling is activated after unilateral
ureteral obstruction, and recombinant secreted frizzled-related
protein 4 alters the progression of renal fibrosis. J Am Soc
Nephrol. 16:2373–2384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsushima K, Suyama T, Takenaka C,
Nishishita N, Ikeda K, Ikada Y, Sawa Y, Jakt LM, Mori H and
Kawamata S: Secreted frizzled related protein 4 reduces fibrosis
scar size and ameliorates cardiac function after ischemic injury.
Tissue Eng Part A. 16:3329–3341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu Y, Jian X, Peng J, Jiang X, Li N and
Zhou S: Gene expression profiling of oral submucous fibrosis using
oligonucleotide microarray. Oncol Rep. 20:287–294. 2008.PubMed/NCBI
|
|
25
|
Xu L, Li Q, Xu D, Wang Q, An Y, Du Q,
Zhang J, Zhu Y and Miao Y: hsa-miR-141 downregulates TM4SF1 to
inhibit pancreatic cancer cell invasion and migration. Int J Oncol.
44:459–466. 2014.
|
|
26
|
Tu SH, Huang HI, Lin SI, Liu HY, Sher YP,
Chiang SK, Chong P, Roffler S, Tseng GC, Chen HW, et al: A novel
HLA-A2-restricted CTL epitope of tumor-associated antigen L6 can
inhibit tumor growth in vivo. J Immunother. 35:235–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bae S, Shim SH, Park CW, Son HK, Lee HJ,
Son JY, Jeon C and Kim H: Combined omics analysis identifies
transmembrane 4 L6 family member 1 as a surface protein marker
specific to human mesenchymal stem cells. Stem Cells Dev.
20:197–203. 2011. View Article : Google Scholar
|
|
28
|
Fuchs E, Tyner AL, Giudice GJ, Marchuk D,
RayChaudhury A and Rosenberg M: The human keratin genes and their
differential expression. Curr Top Dev Biol. 22:5–34. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Michel M, Török N, Godbout MJ, Lussier M,
Gaudreau P, Royal A and Germain L: Keratin 19 as a biochemical
marker of skin stem cells in vivo and in vitro: Keratin 19
expressing cells are differentially localized in function of
anatomic sites, and their number varies with donor age and culture
stage. J Cell Sci. 109:1017–1028. 1996.PubMed/NCBI
|
|
30
|
Li N, Jian X, Hu Y, Xu C, Yao Z and Zhong
X: Discovery of novel biomarkers in oral submucous fibrosis by
microarray analysis. Cancer Epidemiol Biomarkers Prev.
17:2249–2259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu CH, Tang SC, Wang PH, Lee H and Ko JL:
Nickel-induced epithelial-mesenchymal transition by reactive oxygen
species generation and E-cadherin promoter hypermethylation. J Biol
Chem. 287:25292–25302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Islam S, Carey TE, Wolf GT, Wheelock MJ
and Johnson KR: Expression of N-cadherin by human squamous
carcinoma cells induces a scattered fibroblastic phenotype with
disrupted cell-cell adhesion. J Cell Biol. 135:1643–1654. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guarino M, Tosoni A and Nebuloni M: Direct
contribution of epithelium to organ fibrosis:
Epithelial-mesenchymal transition. Hum Pathol. 40:1365–1376. 2009.
View Article : Google Scholar : PubMed/NCBI
|