Gastric cancer is the second most common cancer in
the Chinese population and the second leading cause of
cancer-related death worldwide. More than 90% of all gastric cancer
cases are gastric adenocarcinomas (GCs) (1), which are subdivided into intestinal-
and diffuse-type according to the clinical and pathological
characteristics (2). In recent
years, the overall incidence of gastric cancer has decreased
(3), yet the incidence of
diffuse-type gastric cancer particularly that of the gastric signet
ring cell carcinoma (GSRCC) has increased significantly, and tends
to affect younger population (4).
GSRCC is also called mucinous cell carcinoma, as cancerous cells
are filled with large amounts of mucus and the nucleus is squeezed
to one side and form a ring shape. Compared with the classic type
of gastric cancer, GSRCC is more invasive and usually develops drug
resistance; therefore, it is highly malignant and the prognosis is
poor. Due to its complexity, current studies have mainly focused on
the pathogenesis study of several disease-related genes (5–8).
microRNAs (miRNAs) are non-coding small
single-stranded RNAs with a length of ~20–25 nucleotides, found in
eukaryotes with functions of transcriptional and
post-transcriptional regulation of gene expression. miRNA
expression has been related to carcinogenesis and has been proposed
as a potential biomarker in numerous studies (9–11).
miRNAs promote mRNA degradation by inhibiting translation upon
binding to the 3′-untranslated region (3′-UTR) of their target mRNA
(12). Complementary binding of an
miRNA and mRNA is not exclusive; a single miRNA can target hundreds
of mRNAs, which was demonstrated in the present study and a single
mRNA can be affected by multiple miRNAs (13–17).
Consequently, miRNAs can act as tumor suppressors or possibly
oncogenes depending on the target mRNAs (18,19).
Studies have shown that miRNAs have carcinogenic effects on GC and
can be used as potential prognostic markers (20–23).
Some studies show that a number of miRNAs are related to the growth
and proliferation of GC (24–30),
yet reports on GSRCC are rare.
We speculated that the invasiveness and resistance
of GSRCC are likely due to the unique miRNA and mRNA regulation. In
order to conduct a comparative analysis with intestinal-type
gastric adenocarcinoma (IGA), we adopted microarray technology to
identify the unique miRNAs and mRNAs that are related to the
biological features of GSRCC. This should provide significant
understanding of the mechanisms involved in GSRCC metastasis and
chemotherapy resistance. Using the Agilent miRNA and mRNA
microarray technology, miRNA/mRNA expression and identification in
GSRCC and IGA were studied, respectively; bioinformatic analysis
was utilized for the miRNA-mRNA integration study. Differentially
expressed miRNAs in GSRCC were thoroughly studied to determine the
biological function of the targeted mRNAs in the occurrence of
cancer. miRNAs regulate genes that act as prognostic factors for
patients with GC (12). The present
study aimed to compare the miRNA profile of IGA and GSRCC tissues
and their targeted mRNAs.
Fifty-two human gastric tissue specimens were
collected from the Yantai Yuhuangding Hospital specimen bank from
26 subjects diagnosed with gastric cancer. The specimens included
13 GSRCC specimens (T1), 13 GSRCC paracancerous tissues (P1) from
the same group of subjects, 13 IGA samples (T2) and 13 IGA
paracancerous tissues (P2). All samples were collected and kept in
liquid nitrogen within 30 min after the tissue was assembled and
then transferred for storage at −80°C. All subjects were fully
informed and signed consent forms. The present study was approved
by the Human Research Ethics Committee of Yantai Yuhuangding
Hospital. Four samples were selected from each group for Agilent
miRNA and mRNA chip analysis.
According to the manufacturer’s instructions
(TRIzol; Invitrogen, Gaithersburg, MD, USA) total RNA was extracted
from 52 gastric cancer tissue samples individually. Samples were
then chosen for RNA column purification using
NucleoSpin® RNA Clean-up kit (Macherey-Nagel, Germany).
Samples were quantified with a spectrophotometer, and tested with
formaldehyde denaturing gel electrophoresis for quality inspection.
Four pairs of samples were randomly chosen from the GSRCC and IGA
groups, and prepared for cDNA synthesis using the EasyScript
First-Strand cDNA Synthesis SuperMix kit (Beijing Gold-Tide
Biotechnology Co., Ltd.).
The miRNA and mRNA synthetic products were
fluorescently-labeled and dissolved in hybridization solution.
After repeated washing and drying, the Agilent chips (Agilent
Technologies, Santa Clara, CA, USA) were scanned with Agilent chip
scanner (G2565CA), and hybridization images were obtained. The
images were analyzed and transferred into digital signals using the
Agilent Feature Extraction (v10.7) software. GeneSpring GX software
(Agilent Technologies) was then used for normalization using the
percentile shift method. The differences between the four groups
were analyzed using GeneSpring software. A p<0.05 and absolute
fold-change ≥2, and the flag marked as detected were used as
standards for differences in miRNA and mRNA screening.
All extracted samples from the GSRCC and IGA groups
were subjected to qRT-PCR. The Platinum® RTS
SYBR®-Green qPCR SuperMix-UDG kit (Invitrogen) was used
for miRNA and mRNA differential expression qRT-PCR verification
with a reaction system of 20 μl, and each miRNA and mRNA
sample was provided with 3-wells and repeated 3 times. Primer
designs are listed in Table I. U6
and GAPDH correction was used for targeted miRNA and mRNA
expression, respectively. Analysis was carried out using Rotor-Gene
Q Series software. ΔCt was calculated using the equation ΔCt =
CtmiRNA - CtmiR-U6; the relative miRNA
expression was calculated using the 2−ΔΔCt method.
The differentially expressed miRNAs were analyzed
and predicted with 10 online gene databases, including DIANAmT,
miRanda, miRDB, miRWalk, RNAhybrid, PICTAR4, PICTAR5, PITA, RNA22
and Targetscan (the differentially expressed miRNAs which were
successfully assigned to target genes by more than two database
were selected). Due to the fact that miRNAs negatively regulate
target genes, miRNAs and mRNAs with differential expression were
divided into groups of upregulation and downregulation, and applied
for GO/KO analysis (p<0.05 as the screening filter). The
selected gene was matched with those detected by microarray, and a
final target gene of GSRCC that was regulated by miRNA was
confirmed and further studied.
Statistical analysis was performed using SPSS 17.0
software. All data were shown as mean ± SEM. The data were analyzed
for statistical significance using an unpaired Student’s t-test.
For all the experiments, any p-value <0.05 was considered to
indicate a statistically significant result.
Fifteen differentially expressed miRNAs were
recognized in the T1/P1 group (four pairs of sample) (p<0.05);
13 were upregulated and 2 were downregulated (Table II). In the T2/P2 group, 39 miRNAs
were upregulated whereas 6 were downregulated (p<0.05) (Table III). By comparing the two groups,
10 commonly expressed miRNAs were prominent, including 9 that were
upregulated and 1 that was downregulated.
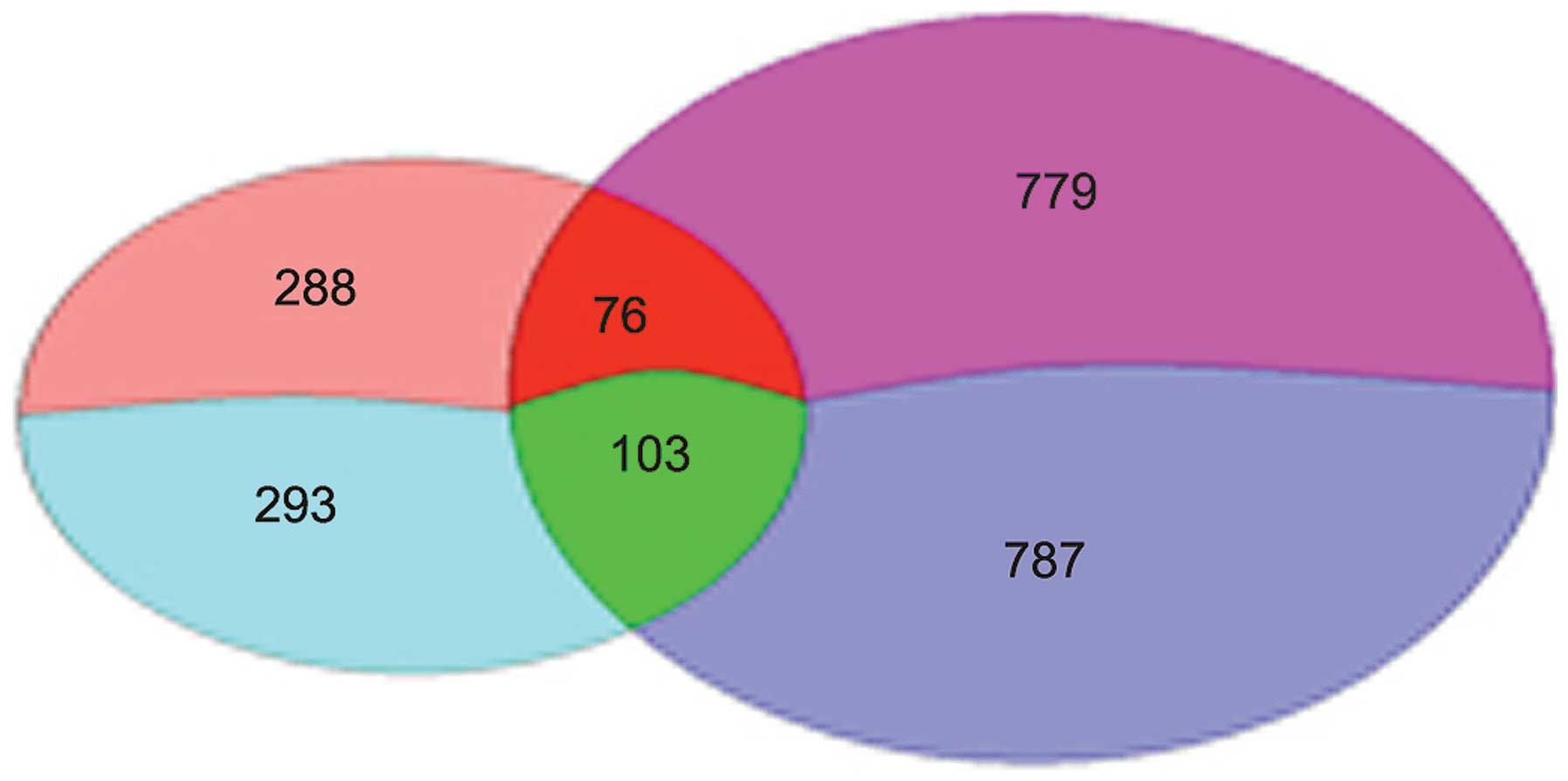
A total number of 760 differentially expressed genes
were identified between T1 and P1, including 364 upregulated genes
and 396 downregulated genes. Differentially expressed genes (1,745)
were identified between T2 and P2, including 855 upregulated and
890 downregulated genes. Overall, 581 genes were mutually expressed
between the two comparative groups, with 288 upregulated and 293
downregulated genes (Fig. 1).
Target gene prediction was carried out by comparison
of the binding site of the 10 T1/P1 specifically expressed miRNAs
(Table II) to a series number of
gene sequences in the databases. hsa-miR-665 was matched to 218
potential target genes, among them 134 were upregulated genes and
84 were downregulated; hsa-miR-95 was matched to a total number of
67 target genes with 47 being upregulated and 20 being
downregulated.
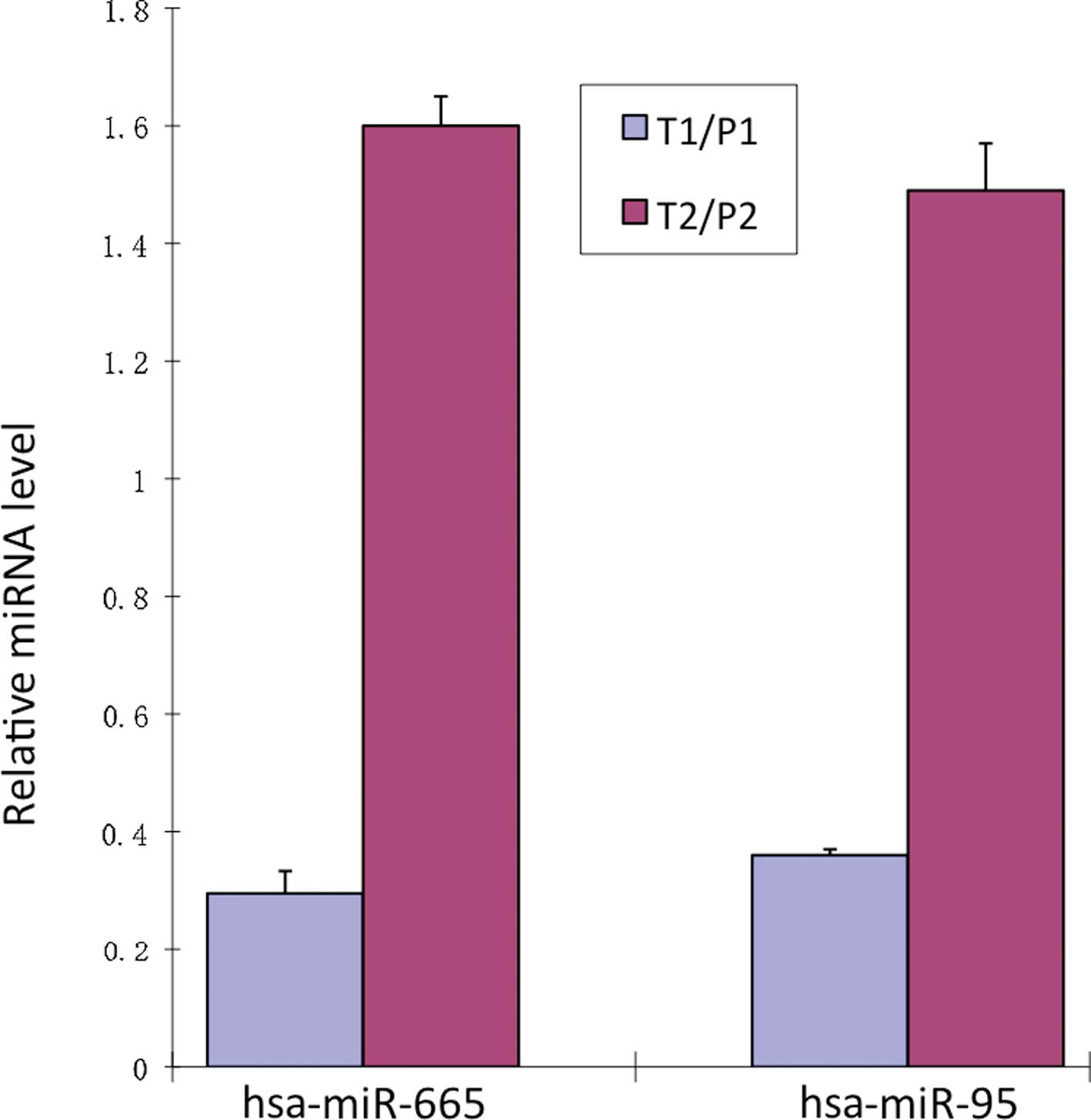
qRT-PCR showed that the expression of hsa-miR-665
and hsa-miR-95 was relatively reduced by 0.30±0.04- and
0.36±0.05-fold in the T1/P1 groups. In the T2/P2 groups hsa-miR-665
and hsa-miR-95 were relatively increased by 1.60±0.01- and
1.49±0.08-fold (Fig. 2).
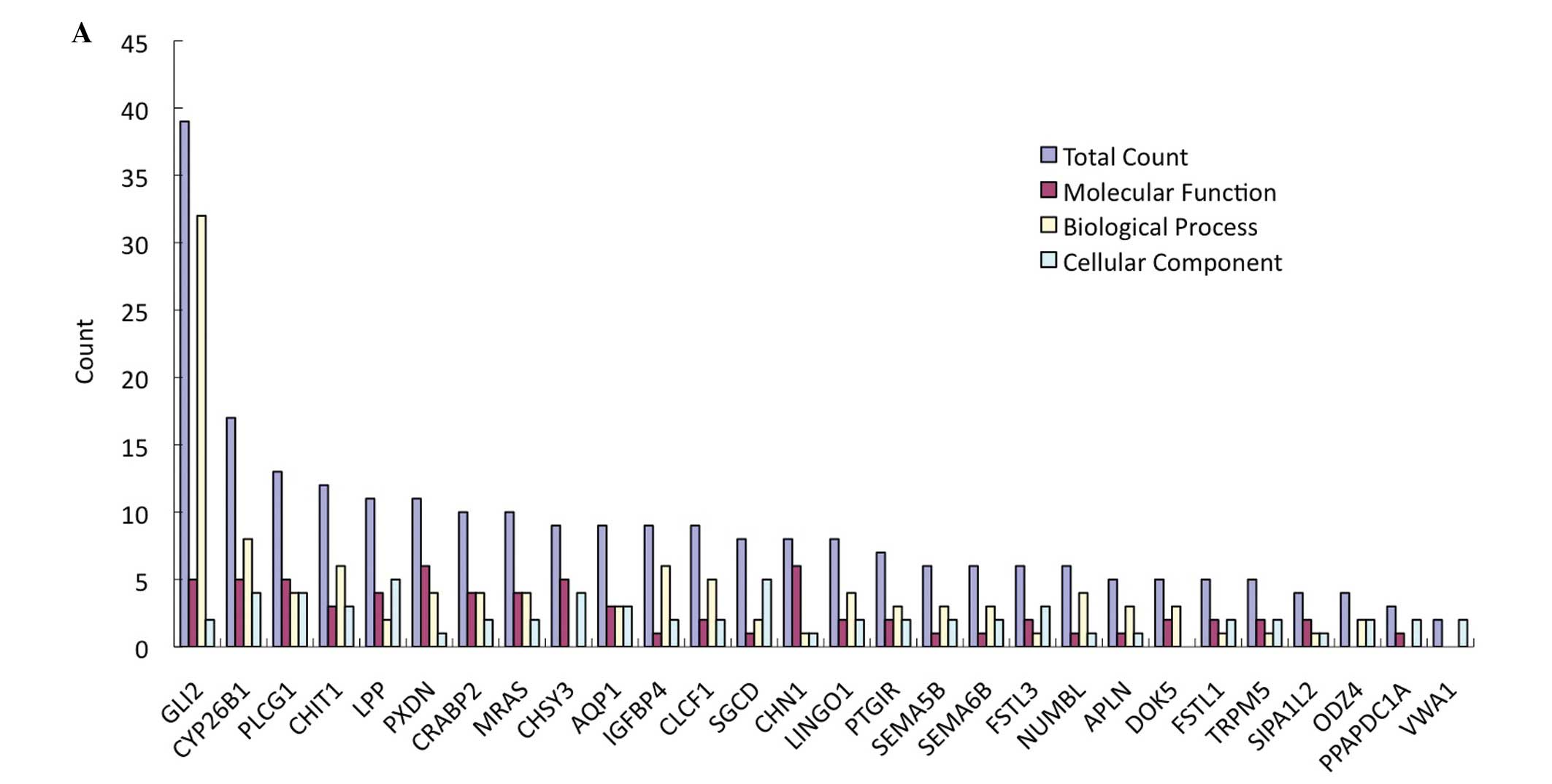
Twenty-eight upregulated target genes were selected
for hsa-miR-665 from T1/P1; 7 were also upregulated in hsa-miR-95
(Table IV). Comprehensive
networking of the functional features related to carcinogenesis
were produced and the genes with the highest interest in the
present study, GLI2 and PLCG1, were selected and GO and KO plots
were constructed (Fig. 4). GO
analysis showed that the functions of the GLI2 gene related to
tumors mainly include: transcription factor activation, ion
binding, regulation of RNA polymerase II promoter, axon guidance,
cell proliferation and DNA replication. KO analysis showed that
PLCG1 was mainly involved in the signaling pathway of tumors
including the phosphoinositide, ErbB and calcium signaling
pathways, VEGF pathway, and Fcε RI signaling pathways. As for
hsa-mir-95, there were only two genes, TNFRSF19 and RAB11FIP4,
predicted with the criteria, and only RAB11FIP4 was predicted as a
target gene by over four databases, and both were comparatively
irrelevant. Therefore, they were not further validated.
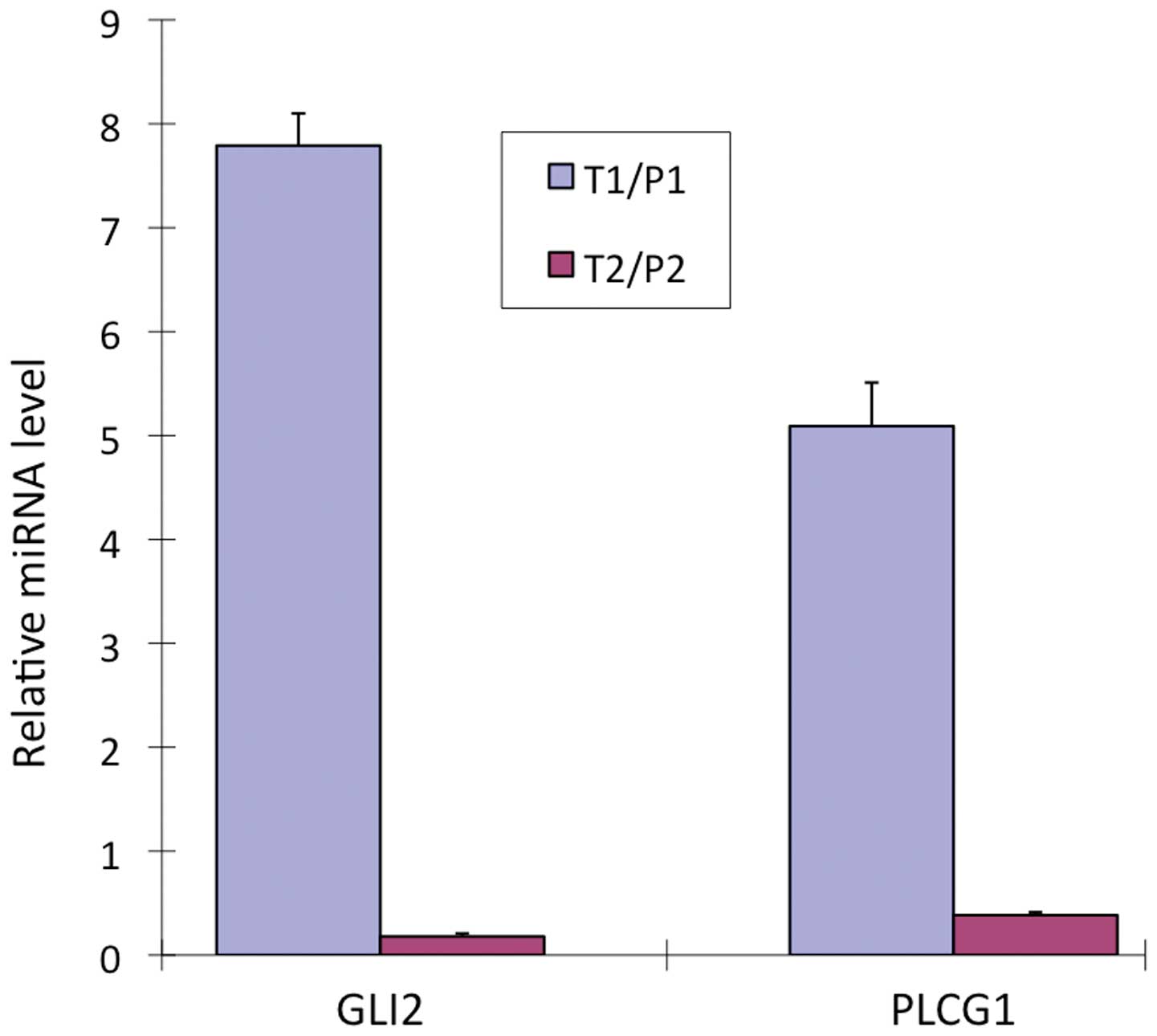
GLI2 and PLCG1 were upregulated in the T1/P1 group
by 7.79±0.31- and 5.09±0.03-fold, relatively. In the T2/P2 groups,
both were downregulated by 0.18±0.42- and 0.38±0.03-fold,
respectively (Fig. 3).
GSRCC is a unique pathological type of gastric
carcinoma. The IGA related overexpression of EGF and mutation of
the anticancer gene P53 have also been detected in GSRCC,
suggesting a common pathogenesis (31,32).
However, as one of the most highly malignant tumors, GSRCC also
presents powerful features of invasion, metastasis and
chemoresistance. At the early stage, the tumor often fails to
present typical clinical manifestations, but progresses rapidly
with metastasis. Therefore, the majority of patients are diagnosed
with advanced stage and lose the chance for surgical treatment.
Patients with GSRCC are relatively insensitive to chemotherapy and
radiotherapy; hence, poor prognosis becomes a huge hurdle in the
clinical treatment for this type of tumor. Previous studies have
shown that increased expression of PKM2 and E-cadherin, and
decreased expression of TMEM207 in GSRCC are closely related to its
characteristics of invasion and metastasis (33–35).
Another study also showed that inherent chemoresistance of GSRCC
and a delay in definitive surgery may favor tumor progression
(36). Although these studies
indicate a direction for GSRCC research, the occurrence of GSRCC
involves a variety of abnormally expressed tumor-related genes, in
a multi-stage process. Therefore, it is imperative to screen for
novel biomarkers that are differentially expressed in GSRCC, and
investigate drugs that target chemoresistance in GSRCC. With a very
limited number of studies in this area, the present study aimed to
identify specifically expressed biomarkers between GSRCC and IGA,
and identify unique biological features of this malignancy.
Some recent studies have demonstrated that miRNA
expression is linked to carcinogenesis by regulating its target
genes. These miRNAs also play a part as tumor-suppressor genes,
such as miRNA-370, miRNA-520d-3p and miR-181a-5p (37–39);
or as oncogenes, such as miRNA-21, miR-18a and miR-544a (40–42).
We randomly selected 4 pairs of GSRCC samples and 4 pairs of IGA
samples, and conducted a comparative analysis of the differentially
expressed miRNAs using microarray analysis. As a result, two
miRNAs, hsa-miR-665 and hsa-miR-95, were selected for further
verification.
hsa-miR-665 is located at 14q32.2, with a length of
20 amino acids. Its expression has been reported to inhibit the
expression of B7-H3 in breast cancer (17), yet its mechanism remains unknown. In
the present study, qRT-PCR results showed that hsa-miR-665 was
downregulated in GSRCC, whereas microarray analysis showed
contradictory results. Repeated qRT-PCR verification of hsa-miR-665
also showed a decreased expression of hsa-miR-665, which is
consistent with our previous results and that reported in breast
cancer. Thus, it was speculated that hsa-miR-665 may play a role as
a tumor-suppressor gene. In order to predict its mechanism of
action, a number of potential target genes were evaluated. Some
studies have reported upregulation of hsa-miR-665 expression in a
series of cancers, for example in esophageal squamous cell
carcinoma (43) and bladder
urothelial carcinoma (BUC) (44).
hsa-miR-95 is located at 4p16.1, with a length of 22 amino acids.
hsa-miR-95 plays a role as a proto-oncogene in the occurrence and
development of a number of tumors, such as colon, cervical and
pancreatic cancer, in which upregulation of hsa-miR-95 was observed
(45–47). Recent studies found that hsa-miR-95
is a target for the treatment of liver cancer with Brucein D, and
it is also closely related to radiotherapy and chemotherapy
tolerance (48–50). In addition, downregulation of
hsa-miR-95 expression was detected in human glioblastoma (51). Therefore, hsa-miR-95 may have
different functions in different types of tumors. qRT-PCR
verification showed downregulation of hsa-miR-665 and hsa-miR-95 in
the GSRCC samples, and upregulation in IGA samples, which was
different from the results obtained by the microarray analysis.
Repeated qRT-PCR showed a similar pattern of expression, which may
be explained by different functional features of these two miRNAs
in different types of gastric cancer. In order to establish
possible functions of hsa-miR-665 and hsa-miR-95 in GSRCC, target
genes were predicted by filtering potential genes from a number of
databases and GO/KO analysis. Preliminary results revealed two
genes, GLI2 and PLCG1, with the greatest connections in terms of
tumorigenesis.
GLI2 is located in 2q14, a member of the zinc finger
transcription factor GLI family. Studies found that there was
increased GLI2 activity in gastric cancer, lung squamous cell,
pancreatic and basal cell carcinoma, and melanoma and other tumors,
possibly by the activation of Hedgehog signaling pathway to promote
tumor invasion and induce chemoresistance (52–56).
PLCG1, also called PLCγ1, is located in 20q12-q13.1. PLC-γ1 is a
subtype of phospholipase (PLC). Many cytokines reply on PLC to
regulate cell metabolism. Studies have found that PLC-γ1 was
upregulated in breast and oral cancer and other tumors (57,58),
and mutations in PLC-γ1 are associated with the incidence of many
types of cancers. It also promotes tumor growth and migration
(58–62). The activation of PLC-γ1 promotes
tumor cell metastasis by triggering a series of signaling pathways
including the PKC signaling pathway, calcium signaling pathway,
Ras/ERK, VEGF signaling and MAPK pathways; it also promotes
upregulation of the MDR1 gene and induces resistance of tumor cells
to chemotherapeutic drugs (63–67).
qRT-PCR verification of GLI2 and PLCG1 showed
upregulation in GSRCC but downregulation in IGA samples. The
relative expression of GLI2 was inversely regulated by hsa-miR-665
and hsa-miR-95; PLCG1 expression was inversely regulated by
hsa-miR-665. Together with the results of the GO/KO analysis, it
was speculated that hsa-miR-665 and hsa-miR-95 promote metastasis
and chemoresistance in GSRCC by upregulating its target genes, in
this case GLI2 and PLCG1. And the distinct expression of these
miRNAs could be the key to the different molecular pathogenesis of
GSRCC and IGA. Moreover, it was observed that the results of the
qRT-PCR verification was not consistent with that of the microarray
analysis. This could be due to relatively a high false-positive
rate of the array analysis, or the 4 pairs of samples chosen from
each GC type did not represent all samples in this study.
In addition, miRNA verification showed differential
expression of hsa-miR-665 and hsa-miR-95 in both the GSRCC and
intestinal IGA, yet the difference was more apparent in GSRCC.
Retrospective analysis of the miRNA results of the target genes
showed that genes differentially expressed in GSRCC were also
present in the intestinal type, which is distinctive from the
results from the array analysis. This is possibly due to different
sensitivity between the detection methods.
In summary, invasion and metastasis are the main
characteristics of GSRCC, which also explains its poor prognosis
and high mortality rate. Chemoresistance is a major hurdle in
clinical treatment of most cancers. For the first time, the present
study showed that hsa-miR-665 and hsa-miR-95 are downregualted in
the GSRCC type of gastric cancer, but upregulated in IGA, and the
target genes present with the opposite pattern of expression.
Therefore, the relatively differential expression of miRNAs
negatively controlling their target genes could be closely related
to the high invasive metastasis and chemoresistance of GSRCC. The
mechanism of its molecular pathogenesis requires further study.
Using microarray technology, the present study identified for the
first time that hsa-miR-665 and hsa-miR-95 exhibit a significant
differential expression pattern in GSRCC compare to intestinal IGA.
Target gene screening by bioinformatic analysis of corresponding
miRNAs showed that differentially expressed miRNAs and mRNAs are
likely to have a significant correlation with invasion and
metastasis and multidrug resistance of gastric signet ring cell,
but its mechanism of action remains to be further evaluated.
Overall, these two markers identified in the present study should
provide information concerning the unique biological
characteristics of GSRCC, which could provide the theoretical basis
for therapeutic development for this type of cancer.
This study was supported by the National Natural
Science Foundation of China (no. 81071758), Shandong Province Young
and Middle-Aged Scientists Research Awards Fund (BS2009SW052,
2008BS02012), and the Yantai Science and Technology Program
(2012085).
|
1
|
Piazuelo MB and Correa P: Gastric cáncer:
Overview. Colomb Med. 44:192–201. 2013.
|
|
2
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim BS, Oh ST, Yook JH and Kim BS: Signet
ring cell type and other histologic types: Differing clinical
course and prognosis in T1 gastric cancer. Surgery. 155:1030–1035.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon KJ, Shim KN, Song EM, Choi JY, Kim
SE, Jung HK and Jung SA: Clinicopathological characteristics and
prognosis of signet ring cell carcinoma of the stomach. Gastric
Cancer. 17:43–53. 2014. View Article : Google Scholar
|
|
6
|
Tabouret T, Dhooge M, Rouquette A,
Brezault C, Beuvon F, Chaussade S and Coriat R: Gastric signet ring
cell adenocarcinoma: A distinct entity. Presse Med. 43:353–357.
2014.In French. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huh CW, Jung H, Kim JH, Lee YC, Kim H, Kim
H, Yoon SO, Youn YH, Park H, Lee SI, et al: Signet ring cell mixed
histology may show more aggressive behavior than other histologies
in early gastric cancer. J Surg Oncol. 107:124–129. 2013.
View Article : Google Scholar
|
|
8
|
Hass HG, Smith U, Jäger C, Schäffer M,
Wellhäuber U, Hehr T, Markmann HU, Nehls O and Denzlinger C: Signet
ring cell carcinoma of the stomach is significantly associated with
poor prognosis and diffuse gastric cancer (Lauren’s): Single-center
experience of 160 cases. Onkologie. 34:682–686. 2011. View Article : Google Scholar
|
|
9
|
Llauradó M, Majem B, Altadill T, Lanau L,
Castellví J, Sánchez-Iglesias JL, Cabrera S, De la Torre J,
Díaz-Feijoo B, Pérez-Benavente A, et al: MicroRNAs as prognostic
markers in ovarian cancer. Mol Cell Endocrinol. 390:73–84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mulrane L, Klinger R, McGee SF, Gallagher
WM and O’Connor DP: microRNAs: A new class of breast cancer
biomarkers. Expert Rev Mol Diagn. 14:347–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue Y, Abou Tayoun AN, Abo KM, Pipas JM,
Gordon SR, Gardner TB, Barth RJ Jr, Suriawinata AA and Tsongalis
GJ: MicroRNAs as diagnostic markers for pancreatic ductal
adenocarcinoma and its precursor, pancreatic intraepithelial
neoplasm. Cancer Genet. 206:217–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Meng F, Ma J, Yu Y, Hua X, Qin J and
Li Y: Insulin receptor substrate-1 and Golgi phosphoprotein 3 are
downstream targets of miR-126 in esophageal squamous cell
carcinoma. Oncol Rep. 32:1225–1233. 2014.PubMed/NCBI
|
|
15
|
Konno Y, Dong P, Xiong Y, Suzuki F, Lu J,
Cai M, Watari H, Mitamura T, Hosaka M, Hanley SJ, et al:
MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation,
invasion and stem cell-like phenotype of aggressive endometrial
cancer cells. Oncotarget. 5:6049–6062. 2014.PubMed/NCBI
|
|
16
|
Zhong D, Huang G, Zhang Y, Zeng Y, Xu Z,
Zhao Y, He X and He F: MicroRNA-1 and microRNA-206 suppress
LXRα-induced lipogenesis in hepatocytes. Cell Signal. 25:1429–1437.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nygren MK, Tekle C, Ingebrigtsen VA,
Mäkelä R, Krohn M, Aure MR, Nunes-Xavier CE, Perälä M, Tramm T,
Alsner J, et al: Identifying microRNAs regulating B7-H3 in breast
cancer: The clinical impact of microRNA-29c. Br J Cancer.
110:2072–2080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garzon R, Heaphy CE, Havelange V, Fabbri
M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA,
et al: MicroRNA 29b functions in acute myeloid leukemia. Blood.
114:5331–5341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishiguro H, Kimura M and Takeyama H: Role
of microRNAs in gastric cancer. World J Gastroenterol.
20:5694–5699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brenner B, Hoshen MB, Purim O, David MB,
Ashkenazi K, Marshak G, Kundel Y, Brenner R, Morgenstern S, Halpern
M, et al: MicroRNAs as a potential prognostic factor in gastric
cancer. World J Gastroenterol. 17:3976–3985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW,
Choi SH, Lee JS and Cho JY: Overexpression of miR-196b and HOXA10
characterize a poor-prognosis gastric cancer subtype. World J
Gastroenterol. 19:7078–7088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
microRNA-503 inhibits gastric cancer cell growth and
epithelial-to-mesenchymal transition. Oncol Lett. 7:1233–1238.
2014.PubMed/NCBI
|
|
25
|
Shen ZY, Zhang ZZ, Liu H, Zhao EH and Cao
H: miR-375 inhibits the proliferation of gastric cancer cells by
repressing ERBB2 expression. Exp Ther Med. 7:1757–1761.
2014.PubMed/NCBI
|
|
26
|
Ren J, Huang HJ, Gong Y, Yue S, Tang LM
and Cheng SY: MicroRNA-206 suppresses gastric cancer cell growth
and metastasis. Cell Biosci. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng Y, Guo JJ, Liu YM and Wu XL:
MicroRNA-34A inhibits the growth, invasion and metastasis of
gastric cancer by targeting PDGFR and MET expression. Biosci Rep.
34:342014. View Article : Google Scholar
|
|
28
|
Tsai MM, Wang CS, Tsai CY, Chen CY, Chi
HC, Tseng YH, Chung PJ, Lin YH, Chung IH, Chen CY, et al:
MicroRNA-196a/-196b promote cell metastasis via negative regulation
of radixin in human gastric cancer. Cancer Lett. 351:222–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu
W, Shu Y and Liu P: MiR-503 regulates cisplatin resistance of human
gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J.
127:2357–2362. 2014.PubMed/NCBI
|
|
30
|
Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding
Y, Shu Y and Liu P: miR-1271 regulates cisplatin resistance of
human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR, and
BCL2. Anticancer Agents Med Chem. 14:884–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aizawa K, Muto I, Suzuki S, Tanaka N,
Yabusaki H, Tanaka S, Katayanagi N, Suzuki T, Tanaka O and Muto T:
Augmentation of 5-fluorouracil cytotoxicity by epidermal growth
factor in a newly established human signet-ring cell carcinoma of
the stomach in culture. Surg Today. 24:420–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimada S, Mimata A, Sekine M, Mogushi K,
Akiyama Y, Fukamachi H, Jonkers J, Tanaka H, Eishi Y and Yuasa Y:
Synergistic tumour suppressor activity of E-cadherin and p53 in a
conditional mouse model for metastatic diffuse-type gastric cancer.
Gut. 61:344–353. 2012. View Article : Google Scholar
|
|
33
|
Takeuchi T, Adachi Y and Nagayama T: A
WWOX-binding molecule, transmembrane protein 207, is related to the
invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis.
33:548–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Humar B, Blair V, Charlton A, More H,
Martin I and Guilford P: E-cadherin deficiency initiates gastric
signet-ring cell carcinoma in mice and man. Cancer Res.
69:2050–2056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW,
Choi SH and Cho JY: Overexpression of the M2 isoform of pyruvate
kinase is an adverse prognostic factor for signet ring cell gastric
cancer. World J Gastroenterol. 18:4037–4043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piessen G, Messager M, Le Malicot K, Robb
WB, Di Fiore F, Guilbert M, Moreau M, Christophe V, Adenis A and
Mariette C: Phase II/III multicentre randomised controlled trial
evaluating a strategy of primary surgery and adjuvant chemotherapy
versus peri-operative chemotherapy for resectable gastric signet
ring cell adenocarcinomas - PRODIGE 19 - FFCD1103 - ADCI002. BMC
Cancer. 13:2812013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen XP, Chen YG, Lan JY and Shen ZJ:
MicroRNA-370 suppresses proliferation and promotes endometrioid
ovarian cancer chemosensitivity to cDDP by negatively regulating
ENG. Cancer Lett. 353:201–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li R, Yuan W, Mei W, Yang K and Chen Z:
MicroRNA 520d-3p inhibits gastric cancer cell proliferation,
migration, and invasion by downregulating EphA2 expression. Mol
Cell Biochem. 396:295–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Korhan P, Erdal E and Atabey N:
MiR-181a-5p is downregulated in hepatocellular carcinoma and
suppresses motility, invasion and branching-morphogenesis by
directly targeting c-Met. Biochem Biophys Res Commun.
450:1304–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang CH, Yue J, Pfeffer SR, Fan M, Paulus
E, Hosni-Ahmed A, Sims M, Qayyum S, Davidoff AM, Handorf CR, et al:
MicroRNA-21 promotes glioblastoma tumorigenesis by downregulating
IGFBP3. J Biol Chem. 289:25079–25087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen X, Wang J, Cheng L and Lu MP: miR-18a
downregulates DICER1 and promotes proliferation and metastasis of
nasopha-ryngeal carcinoma. Int J Clin Exp Med. 7:847–855. 2014.
|
|
42
|
Mo X, Zhang F, Liang H, Liu M, Li H and
Xia H: miR-544a promotes the invasion of lung cancer cells by
targeting cadherina 1 in vitro. Onco Targets Ther. 7:895–900. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zang W, Wang Y, Du Y, Xuan X, Wang T, Li
M, Ma Y, Li P, Chen X, Dong Z, et al: Differential expression
profiling of microRNAs and their potential involvement in
esophageal squamous cell carcinoma. Tumour Biol. 35:3295–3304.
2014. View Article : Google Scholar
|
|
44
|
Cheng W, Gao J, Zhang Z, Ge J, Xu F and
Wei Z: Study on microRNAs in urothelial carcinoma (II grade) of the
bladder. J Med Postgraduates China. 23:48–52. 2010.
|
|
45
|
Huang Z, Huang S, Wang Q, Liang L, Ni S,
Wang L, Sheng W, He X and Du X: MicroRNA-95 promotes cell
proliferation and targets sorting Nexin 1 in human colorectal
carcinoma. Cancer Res. 71:2582–2589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yao T, Rao Q, Liu L, Zheng C, Xie Q, Liang
J and Lin Z: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in cervical cancer. Virol J. 10:1752013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li WG, Yuan YZ, Qiao MM and Zhang YP: High
dose glargine alters the expression profiles of microRNAs in
pancreatic cancer cells. World J Gastroenterol. 18:2630–2639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xiao Z, Ching Chow S, Han Li C, Chun Tang
S, Tsui SK, Lin Z and Chen Y: Role of microRNA-95 in the anticancer
activity of Brucein D in hepatocellular carcinoma. Eur J Pharmacol.
728:141–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang X, Taeb S, Jahangiri S, Emmenegger
U, Tran E, Bruce J, Mesci A, Korpela E, Vesprini D, Wong CS, et al:
miRNA-95 mediates radioresistance in tumors by targeting the
sphingolipid phosphatase SGPP1. Cancer Res. 73:6972–6986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen X, Chen S, Hang W, Huang H and Ma H:
MiR-95 induces proliferation and chemo- or radioresistance through
directly targeting sorting nexin1 (SNX1) in non-small cell lung
cancer. Biomed Pharmacother. 68:589–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Skalsky RL and Cullen BR: Reduced
expression of brain-enriched microRNAs in glioblastomas permits
targeted regulation of a cell death gene. PLoS One. 6:e242482011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wan J, Zhou J, Zhao H, Wang M, Wei Z, Gao
H, Wang Y and Cui H: Sonic hedgehog pathway contributes to gastric
cancer cell growth and proliferation. Biores Open Access. 3:53–59.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang L, Walter V, Hayes DN and Onaitis M:
Hedgehog-GLI signaling inhibition suppresses tumor growth in
squamous lung cancer. Clin Cancer Res. 20:1566–1575. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
An Y, Cai B, Chen J, Lv N, Yao J, Xue X,
Tu M, Tang D, Wei J, Jiang K, et al: MAP3K10 promotes the
proliferation and decreases the sensitivity of pancreatic cancer
cells to gemcitabine by upregulating Gli-1 and Gli-2. Cancer Lett.
329:228–235. 2013. View Article : Google Scholar
|
|
55
|
Luongo C, Ambrosio R, Salzano S, Dlugosz
AA, Missero C and Dentice M: The sonic hedgehog-induced type 3
deiodinase facilitates tumorigenesis of basal cell carcinoma by
reducing Gli2 inactivation. Endocrinology. 155:2077–2088. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Perrot CY, Gilbert C, Marsaud V, Postigo
A, Javelaud D and Mauviel A: GLI2 cooperates with ZEB1 for
transcriptional repression of CDH1 expression in human melanoma
cells. Pigment Cell Melanoma Res. 26:861–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lattanzio R, Marchisio M, La Sorda R,
Tinari N, Falasca M, Alberti S, Miscia S, Ercolani C, Di Benedetto
A, Perracchio L, et al CINBO (Consorzio Interuniversitario
Nazionale per Bio-Oncologia): Overexpression of activated
phospholipase Cγ1 is a risk factor for distant metastases in T1–T2,
N0 breast cancer patients undergoing adjuvant chemotherapy. Int J
Cancer. 132:1022–1031. 2013. View Article : Google Scholar
|
|
58
|
Ma LW, Zhou ZT, He QB and Jiang WW:
Phospholipase C-γ1 expression correlated with cancer progression of
potentially malignant oral lesions. J Oral Pathol Med. 42:47–52.
2013. View Article : Google Scholar
|
|
59
|
Behjati S, Tarpey PS, Sheldon H,
Martincorena I, Van Loo P, Gundem G, Wedge DC, Ramakrishna M, Cooke
SL, Pillay N, et al: Recurrent PTPRB and PLCG1 mutations in
angiosarcoma. Nat Genet. 46:376–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vaqué JP, Gómez-López G, Monsálvez V,
Varela I, Martínez N, Pérez C, Domínguez O, Graña O,
Rodríguez-Peralto JL, Rodríguez-Pinilla SM, et al: PLCG1 mutations
in cutaneous T-cell lymphomas. Blood. 123:2034–2043. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Meyer RD, Husain D and Rahimi N: c-Cbl
inhibits angiogenesis and tumor growth by suppressing activation of
PLCγ1. Oncogene. 30:2198–2206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Phillips-Mason PJ, Kaur H, Burden-Gulley
SM, Craig SE and Brady-Kalnay SM: Identification of phospholipase C
gamma1 as a protein tyrosine phosphatase mu substrate that
regulates cell migration. J Cell Biochem. 112:39–48. 2011.
View Article : Google Scholar :
|
|
63
|
Yang J, Song X, Chen Y, Lu XA, Fu Y and
Luo Y: PLCγ1-PKCγ signaling-mediated Hsp90α plasma membrane
translocation facilitates tumor metastasis. Traffic. 15:861–878.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choi AY, Choi JH, Hwang KY, Jeong YJ, Choe
W, Yoon KS, Ha J, Kim SS, Youn JH, Yeo EJ, et al: Licochalcone A
induces apoptosis through endoplasmic reticulum stress via a
phospholipase Cγ1-, Ca2+-, and reactive oxygen
species-dependent pathway in HepG2 human hepatocellular carcinoma
cells. Apoptosis. 19:682–697. 2014. View Article : Google Scholar
|
|
65
|
Kortum RL, Rouquette-Jazdanian AK, Miyaji
M, Merrill RK, Markegard E, Pinski JM, Wesselink A, Nath NN,
Alexander CP, Li W, et al: A phospholipase C-γ1-independent,
RasGRP1-ERK-dependent pathway drives lymphoproliferative disease in
linker for activation of T cells-Y136F mutant mice. J Immunol.
190:147–158. 2013. View Article : Google Scholar
|
|
66
|
Zhang Q, Yu C, Peng S, Xu H, Wright E,
Zhang X, Huo X, Cheng E, Pham TH, Asanuma K, et al: Autocrine VEGF
signaling promotes proliferation of neoplastic Barrett’s epithelial
cells through a PLC-dependent pathway. Gastroenterology.
146:461–472.e6. 2014. View Article : Google Scholar
|
|
67
|
Yang JM, Vassil AD and Hait WN: Activation
of phospholipase C induces the expression of the multidrug
resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol.
60:674–680. 2001.PubMed/NCBI
|