Introduction
The global prevalence of bladder cancer is estimated
at more than 1 million and is steadily increasing. At diagnosis,
20–25% of cases are non-muscle invasive bladder cancer (NMIBC), and
there is a high rate of tumor recurrence and progression even after
local surgical therapy. Thus, numerous patients require lifelong
follow-up examinations that include additional prophylactic
treatments in the event of recurrence. Intravesical Bacillus
Calmette-Guérin (BCG), a live attenuated Mycobacterium bovis
vaccine widely used to induce immunity against tuberculosis, is
currently the most common therapy employed for NMIBC, best known as
the most effective agent for the treatment of high-grade
superficial bladder cancer. As an adjunct to transurethral
resection, BCG is the treatment of choice for urothelial carcinoma
in situ (CIS) and for recurrent or multi-focal Ta and high
grade T1 bladder lesions (1). In
patients for whom radical cystectomy is not performed, BCG is
currently the first treatment option for high-risk NMIBC and CIS.
BCG therapy achieves 50–60% effectiveness against small residual
tumors, and a 70–75% complete response rate for CIS (2). Recent trials indicate that
immunotherapy with BCG is superior to chemotherapy in patients with
intermediate- to high-risk for recurrence (3).
Despite therapeutic application since 1976, the
mechanism of BCG action against bladder cancer remains unknown, yet
it is assumed that BCG-induced antitumor activity is dominated by
local non-specific immunological reactions reflecting the activity
of immunocompetent T cells (4). BCG
accumulates near and adheres to the bladder wall by binding to
fibronectin (5). After passage
through the GAG layer, BCG is internalized and processed by
professional APC and tumor cells, which then locally activate
numerous lymphocytes, macrophages, pleomorphic mononuclear and NK
cells (6,7). Th1-polarized cell-mediated immunity
appears to play a key role during BCG immunotherapy and
IFN-γ-producing, NK and CD8+ in addition to
CD4+ T cells are major cellular mediators of this
antitumor action (3).
However, a high percentage of patients fail initial
BCG therapy, and 40–50% of BCG responders develop recurrent tumors
within the first 5 years (8). In
addition, BCG, a viable living organism, causes infections.
Unfortunately, up to 90% of patients experience side-effects
ranging from bothersome cystitis in the majority of patients to
life-threatening complications such as sepsis in rare cases
(9). Where BCG is ineffective,
treatment schedules consisting of viable BCG and human lL-2 or
other Th1 cytokines are proven to be effective (10). Induction of Th1 immunity is required
for successful BCG immunotherapy of bladder cancer. The Th1
cytokine hIFN-α has been found to be safe and
tolerable when administered intravesically, alone or in combination
with BCG, in numerous controlled studies (11,12).
Additionally administration of IFN-α2b, both alone and in
combination with BCG, has been reported to achieve improved
clinical efficacy (13). The
side-effect profile of combination therapies is similar to BCG
monotherapy, and one phase III study has recommended combination
therapy with BCG and IFN-α in BCG non-responders or relapsers
(14). Although BCG and IFN-α
combination therapy may benefit patients with high-risk disease or
carcinoma in situ, the efficacy of cytokine perfusion is
limited by the high cost, short half-life and water solubility of
cytokines, which are readily lost to urine (15).
BCG has also been used as a live vehicle to deliver
multiple pathogen antigens due to the high immunogenicity and low
toxicity of this organism (16). We
thus sought to assess the efficacy of administration of a
previously described genetically engineered recombinant
hIFN-α2b-secreting BCG (rBCG) (17) in a murine model of bladder
cancer.
Materials and methods
BCG strains and culture
The Mycobacterium bovis (M. bovis) BCG
Danish 2 strain was purchased from the Shanghai Institute of
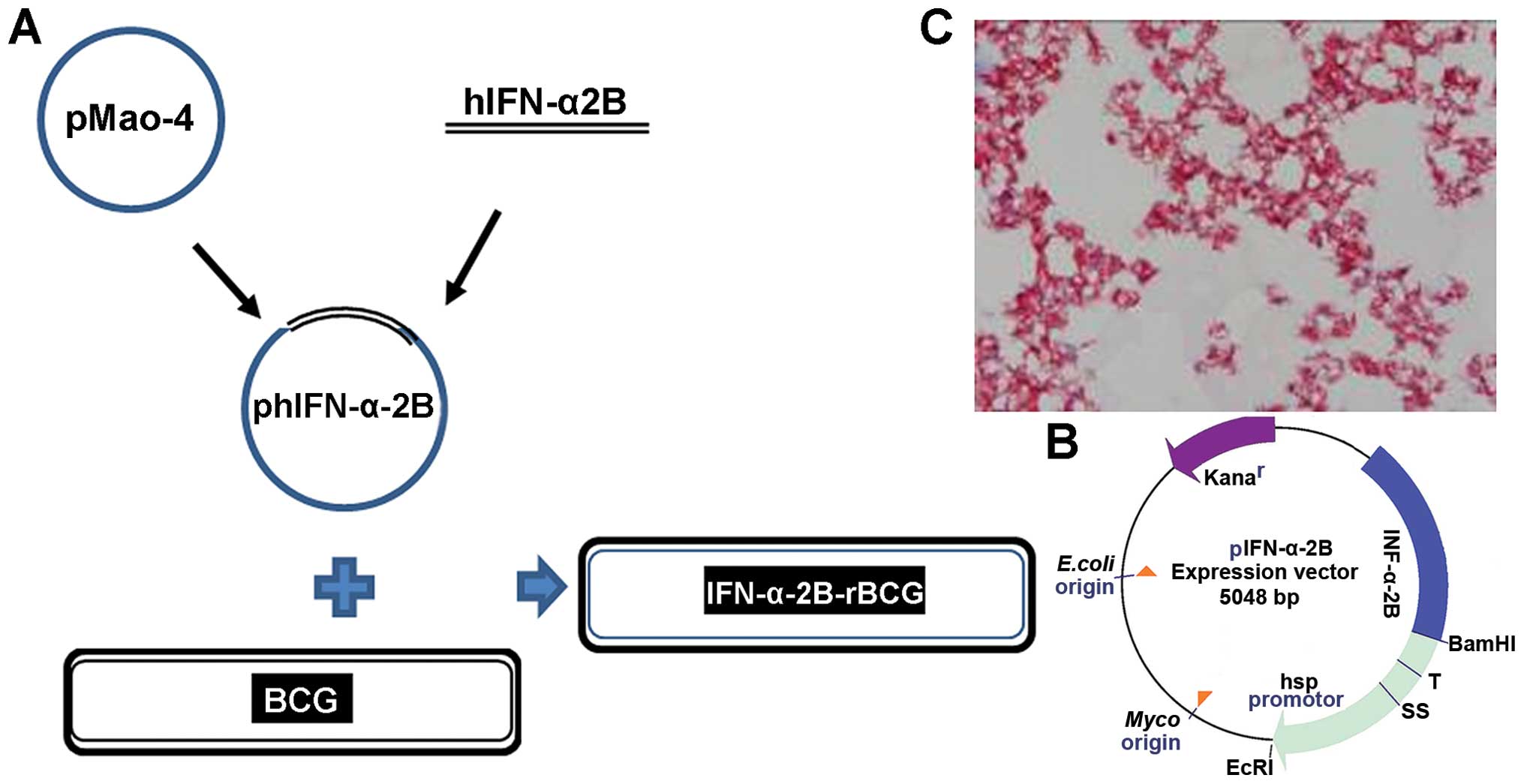
Biological Products. Recombinant hIFN-α2b-BCG was
constructed in-house. The hIFN-α2b fragment was
directionally cloned into the shuttle plasmid pMAO-4 to form
a recombinant plasmid phIFN-α-2B, which was extracted prior
to enrichment in DH5α-E. coli and electrically
transduced into BCG as previously described (17) (Fig.
1). BCG was recovered on 7H10 solid media, and cultured in
Middlebrock 7H9 broth media (both from Difco Laboratories, USA)
supplemented with 0.05% (by vol) glycerol, 10% (by vol)
albumin-dextrose-catalase (ADC) and 0.05% (by vol) Tween-80, at
37°C and 150 rpm until the log-stationary phase. rBCG was
supplemented with 15 μg/ml kanamycin (Sigma-Aldrich, St. Louis, MO,
USA), and then washed and dissolved in phosphate-buffered saline
(PBS). When A60 = 1, BCG/rBCG in the present
study was ~1.4×108 CFU/ml. Exogenous recombinant
IFN-α2b (Sigma-Aldrich) was applied by the same amount of
rBCG in the 7-day culture supernatant: ~1,000 IU in
~3×108 CFU/ml.
Bladder cancer cell line and culture
The mouse bladder tumor cell line MB49, originating
from the C57BL/6 mouse, was provided by Luo Yi of the Department of
Urology, University of Iowa, USA. Cells were grown in RPMI-1640
medium (Gibco, USA) supplemented with 10% fetal bovine serum, at
37°C in 5% CO2 in a humidified incubator. Cells in the
logarithmic growth phase were seeded at 1×106/ml, and at
90% confluency were co-cultured with BCG or rBCG at an MOI
of 1:1 at 37°C in 5% CO2 and saturated humidity, for 24,
48 and 72 h. PBS was used in place of BGC as a control.
Light and electron microscopy
MB49 cells were observed under an inverted
microscope and by transmission electron microscopy, as previously
described (18). Ultrathin sections
were stained with 0.5% uranyl acetate and 0.04% lead citrate and
observed under a transmission electron microscope JEM-2000EX (Jeol,
Ltd., Tokyo, Japan) at 80 kV.
CCK-8 tumor cell viability assay
Cellular viability was assessed using a colorimetric
cell counting kit, the Cell Counting Kit-8 (CCK-8; Dojindo, Japan),
according to the manufacturer’s instructions. MB49 cells
(1×105 cells/well) we r e seeded in 96-well plates. At
90% confluency, rBCG, BCG, BCG+hIFN-α2b or PBS
were added in triplicate to each well at an MOI of 10:1, and BCG
(rBCG, BCG+hIFN-α2b) alone in medium was used
as the blank control. CCK-8 solution (10 μl) was added to each
well, and after 4 h the absorbance was read on a microplate reader
at 450 nm. Cell growth inhibition rate (%) =
(1-Abstest/AbsPBS) x 100%.
Apoptosis assay
Cells were seeded on coverslips in a 6-well plate,
cultured in 3 ml RPMI-1640. When cells adhered, BCG or rBCG
was added at an MOI of 1:1 and co-cultured for 24, 48 and 72 h.
After 4% paraformaldehyde fixation, 10 μl of acridine orange (AO)
(AppliChem, Gatersleben, Germany) dye mix (100 mg/l) was added to
each well, and after 5 min, the slides were rinsed twice in PBS
with 1% hydrochloric acid for 5 sec, then decolorized in
CaCl2 for 3–5 min. Staining with Hoechst 33258
(Sigma-Aldrich) was carried out according to the manufacturer’s
instructions, and the cells were observed under a fluorescence
microscope (Nikon 80i; Nikon, Japan) (excitation 340 nm for Hoechst
33258, 502 nm for AO).
Cells grown in 6-well plates (2×105
cells/well) were incubated in the presence or absence of inducers,
and then harvested by centrifugation at 4°C and 1,000 x g for 5 min
after trypsinization, and then rinsed twice with PBS. Cells were
suspended in 100 μl binding buffer, and then stained in triplicate
with the fluorescein isothiocyanate (FITC) Annexin V apoptosis
detection kit (BD Biosciences, Bedford, MA, USA) at room
temperature for 15 min. Thereafter, the flow cytometric analysis of
cells was performed with BD FACSVantage SE cytometer (BD
Biosciences) within 1 h.
FCM analysis of MHC-I expression
Cells were incubated with 5 μl FITC-anti-mouse MHC-I
(H-2Kb) Ab (BD Biosciences) for 20 min. After washing
with buffer [PBS containing 10 mmol/l ethylenediaminetetraacetic
acid (EDTA) and 0.1% sodium azide], the cells were analyzed using
the BD FACSVantage SE cytometer.
Murine orthotopic bladder cancer model
and BCG treatment
We used a well-defined murine syngeneic orthotopic
MB49 bladder cancer model to evaluate the role of rBCG in
vivo (19). In brief, 6- to
8-week-old SPF female C57BL/6 mice (Animal Laboratory Center,
Beijing Medical University, China) upon approval by the Animal Use
and Care Committee of Tianjin Medical University, were divided into
4 groups (n=15) and one group of controls (n=6). The 4 groups were
catheterized to receive an intravesical inoculate of 105
MB49 bladder tumor cells on day 0 following a 5-sec treatment of
the bladder wall with 30 μl of 0.2 M silver nitrite. On days 1, 8,
15 and 22 following tumor implantation, the mice were treated
intravesically with 100 μl of either 3×108 CFU/ml BCG,
3×108 CFU/ml rBCG, 3×108 CFU/ml
BCG+hIFN-α2b or PBS. Exogenous hIFN-α2b
was administered at the concentration achieved on day 7 of culture
for the same amount of rBCG.
Survival was recorded daily for 6 weeks. Bladders
were weighed to obtain the tumor growth inhibition rate (%) = (1 -
weighttreated group/weightcontrol group) x
100%. Twelve hours after the last perfusion, blood was taken from 6
mice in each group through the inner canthus venous plexus.
Hematoxylin and eosin (H&E) and
auramine O staining
The bladder, liver, spleen, kidney, lung and heart
of mice sacrificed on day 15 following the last perfusion and those
that died during the observation period were immediately removed.
Tissues were fixed in 4% paraformaldehyde, paraffin-embedded, and
then H&E stained for pathological observation under a light
microscope. For observation of mycobacteria in tissue, auramine O
was applied for staining as follows. The paraffin section was
dewaxed to water, dyed by auramine O solution for 5 min,
decolorized with 3% hydrochloric acid alcohol for 1–2 min, then
redyed 1–2 min in hematoxylin.
Immunohistochemistry
Mouse bladders were resected, shock frozen in liquid
nitrogen and stored at -80°C. Immuno-histochemical staining of 5-Am
cryostat sections with peroxidase-conjugated secondary antibodies
was carried out as previously described (20) with mouse CD3, CD20 and Gr1; and
mouse Fas (both from BD Biosciences, Heidelberg, Germany). The
number of infiltrating immune cells, expressed as average
number/high-power field (HP), was determined by examining 10
randomly selected non-overlapping microscopic fields at x400
magnification. To semi-quantify the cellular Fas expression, each
slide was scored on the basis of percentage and intensity of
stained tumor cells as previously described (21), as follows: no staining, 0; <20%,
1; 20–75%, 2; and >75%, 3. The intensity of staining was graded
on the following scale: negative, 0; low, 1; moderate, 2; and
strong, 3. The product of the scores for the intensity and positive
rate of staining was the total score. In the present study, a final
total score >2 for Fas expression was defined as
high-expression. All cell counting results were verified between
the manual and automated methods and are expressed as the viable
cell numbers for interpretation. Scoring was carried out in a
blinded manner by two independent individuals.
FCM analysis of T lymphocyte populations
in peripheral blood
Whole blood samples were treated with EDTA,
according to the manufacturer’s instructions. Whole blood (100 μl)
was incubated with directly conjugated fluorescent CD3/CD4/CD8
antibodies for 30 min in the dark at room temperature, and then red
cells were lysed using FACS Lyse (both from BD Biosciences).
Stained cells were washed and fixed in PBS with 1% formaldehyde,
and samples were acquired on FACSVantage. Fluorescence minus one
gating techniques were employed to establish staining thresholds
and aid gating of T cell subpopulations.
Detection of cytokine secretion by
ELISA
Heparinized whole blood supernatants (50 μl) were
harvested, and mTNF-α and mIL-12 levels were assayed
by ELISA (mouse TNF-α and IL-12 ELISA kit; Biosource, France)
according to the manufacturer’s instructions. The signal was
detected using the BioTek Epoch microarray reader, and the results
were analyzed using Gen5 software.
Statistical analyses
SPSS 18.0 software was used for data analysis.
Results displayed represent the mean ± SD. One-way ANOVA followed
by the Newman-Keuls post-hoc and Fisher exact test
probability were used to compare values and rates between groups,
respectively. ANOVA was employed for pairwise comparison of
repeated measurements. Survival of the mice was evaluated using
Kaplan-Meier plots and the logrank test. Statistical analyses are
given as two-sided p-values. p<0.05 was considered to indicate a
statistically significant result, and p<0.01 indicated extreme
significance.
Results
rBCG affects bladder cancer cell
morphology
Untreated MB49 cells were mostly round or of
irregular shape, began to adhere to the well wall after 1–2 h
culture, and extended cytoplasmic protrusions were observed after
12 h. MB49 cells demonstrated a high nuclear/cytoplasmic ratio,
uneven chromatin and the mitotic phase was occasionally observed
(Fig. 2A and D).
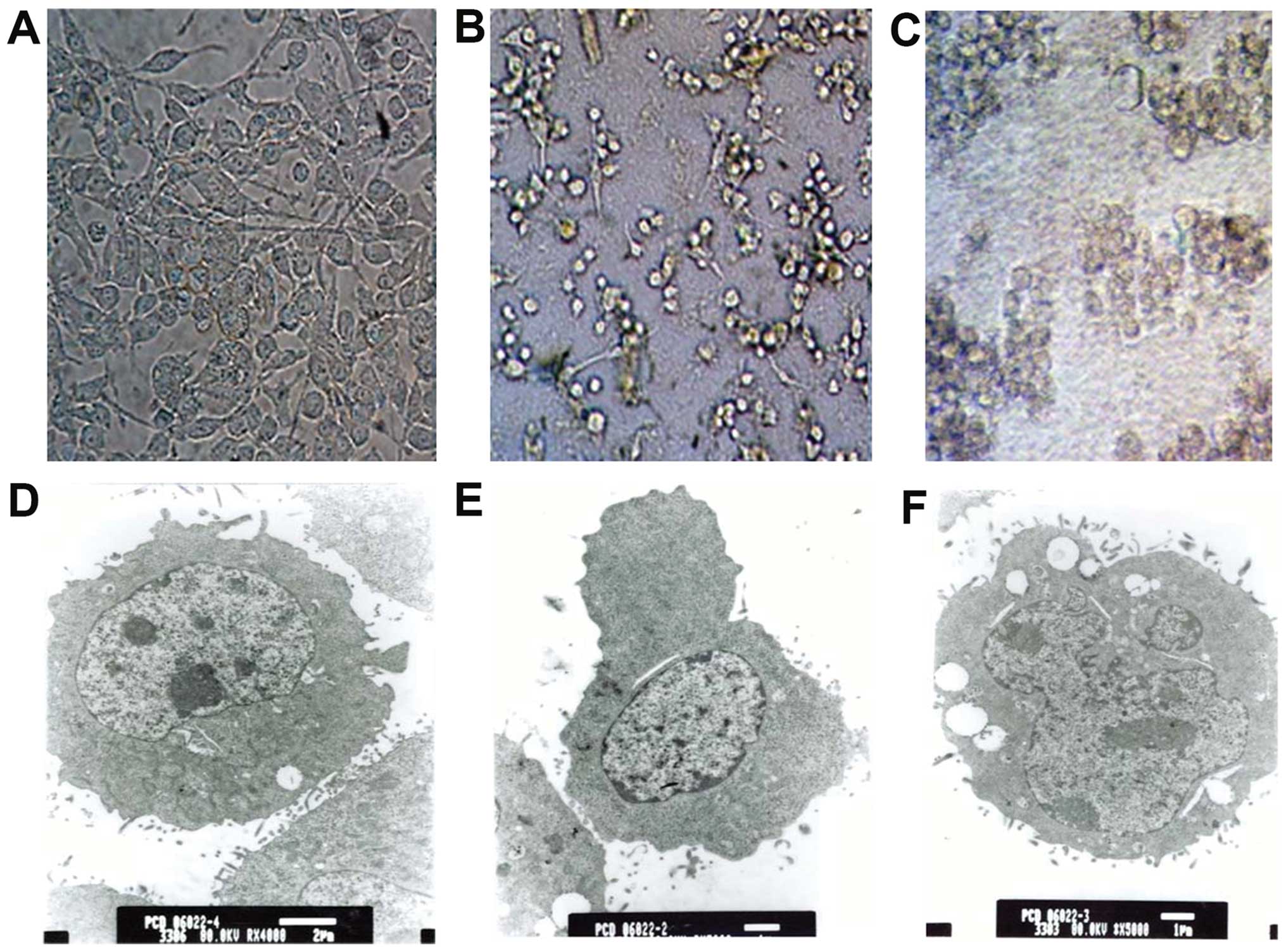 | Figure 2MB49 cell morphology: light
microscope (scale bar, 50 μm, A-C; magnification, x200) and TEM
(D-F). (A) Intact cells in the control group were generally bulky
and wall-attached. (B) Cells treated with rBCG for 48 h
shrunk, clumped and detached from the wall. (C) After 72 h,
cellular morphology was abnormal and cell death was observed. (D)
Intact cells contained ribosomes, mitochondria and endoplasmic
reticulum in plasma, and protuberances and microvilli were present
on the cell surface (scale bar, 2 μm; TEM magnification, x4,000).
(E) After 48 h, the cell size was reduced, cytoplasm was condensed
and bulges rather than microvilli were visible on the cell surface
(scale bar, 1 μm; TEM magnification, x5,000). (F) After 72 h,
irregular nuclei and large vacuoles were observed in the condensed
cytoplasm (scale bar, 1 μm; TEM magnification, x5,000). TEM,
transmission electron microscopy. |
During co-culture with BCG, rBCG or
BCG+hIFN-α2b cellular refraction decreased,
cytoplasmic protrusions gradually disappeared, soma became stubby
and the cytoplasm became granular (Fig.
2B and E). After 48 h, the cells had clearly shrunk,
protuberances disappeared, cells rounded up and proliferation
slowed (Fig. 2B and E). Some cells
grew in suspension and formed clumps surrounded by bacteria. After
72 h, the cells were granular, sparsely distributed, and some
exhibited vacuolization, blebbing and lytic necrosis (Fig. 2C and F). The less damaged cells
showed mitochondrial swelling, large vacuolar degeneration,
dissolved nuclear chromatin, cytoplasm necrosis and surface
microvilli decrement (Fig. 2F).
rBCG inhibits tumor cell growth and
induces apoptosis
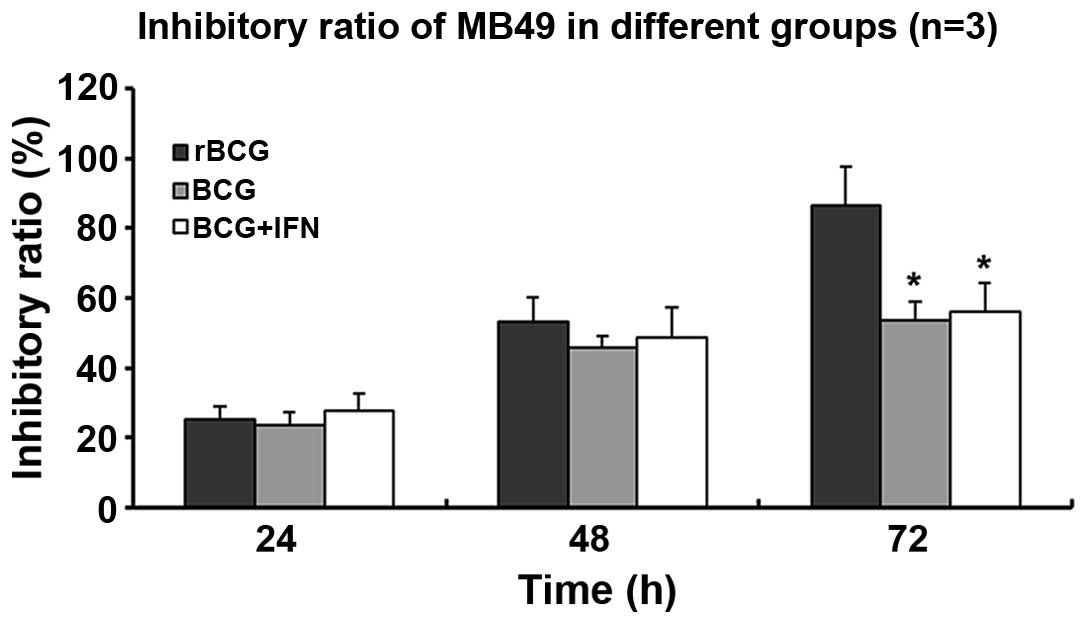
MB49 cells incubated with rBCG grew less
quickly than the controls incubated with PBS (Fig. 3). After 72 h, rBCG achieved a
significantly higher inhibitory ratio (86.37±3.67%) than BGC
(53.43±1.84%) or BCG+hIFN-α2b (56.03±2.79%) (all
p<0.05) (Fig. 3).
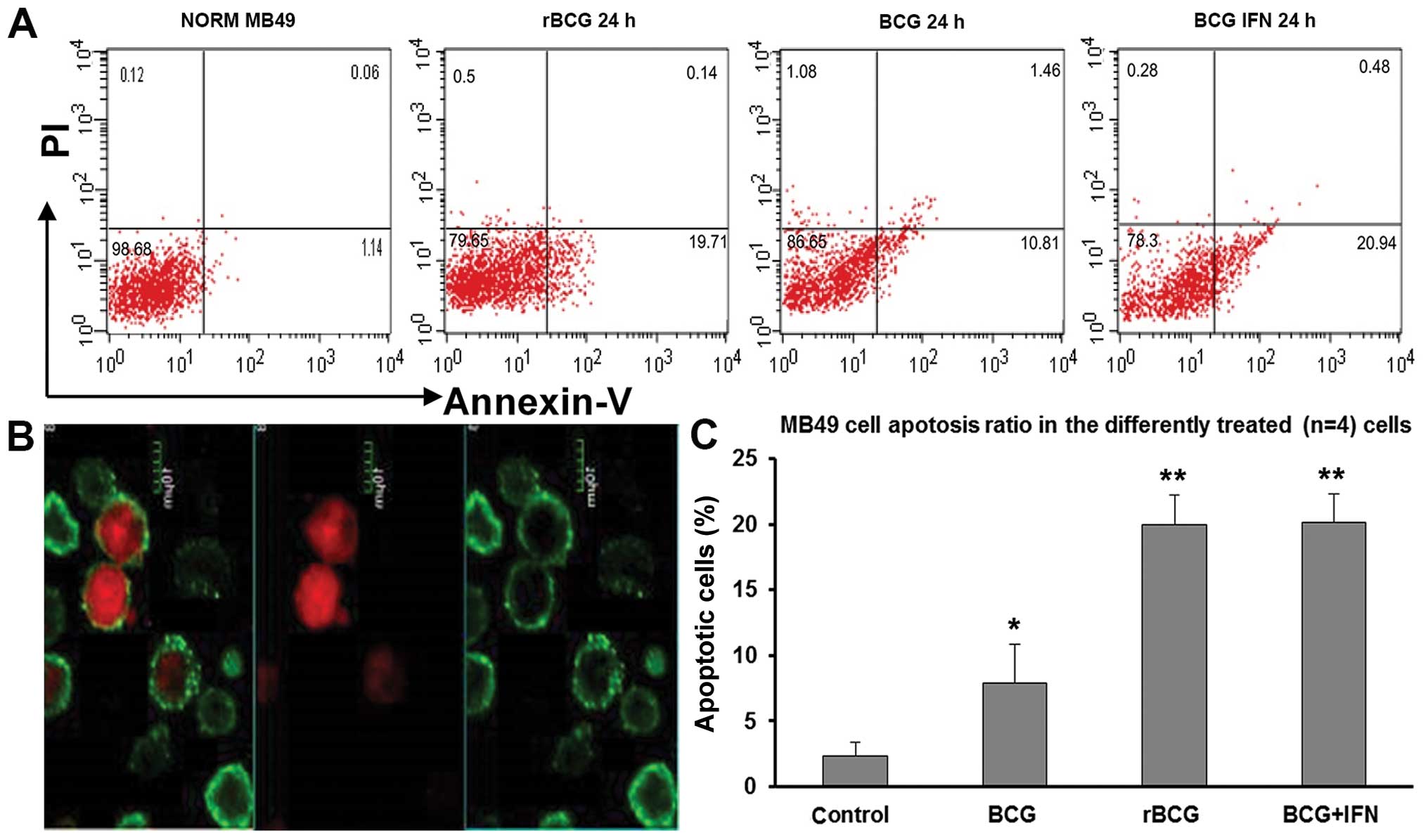
Intact MB49 cells stained with AO adhered and grew
vigorously. The nuclei emitted yellow or green fluorescence, and
the cytoplasm emitted orange red fluorescence, indicating a high
karyoplasmic ratio and cells were spindle shaped (Fig. 4A). MB49 cells incubated for 24 h
with rBCG, BCG or BCG+hIFN-α2b were not
obviously affected, yet after 48 h, the cells clumped, cytoplasmic
protuberances gradually disappeared, the karyoplasmic ratio
decreased, and vesicular bulging membranes and apoptotic bodies
were observed (Fig. 4B). After 72
h, the cells no longer adhered firmly, and the cell number and
karyoplasmic ratio were reduced (Fig.
4C and D). Hoechst 33258 staining revealed apoptotic bodies at
48 and 72 h (Fig. 4E–H).
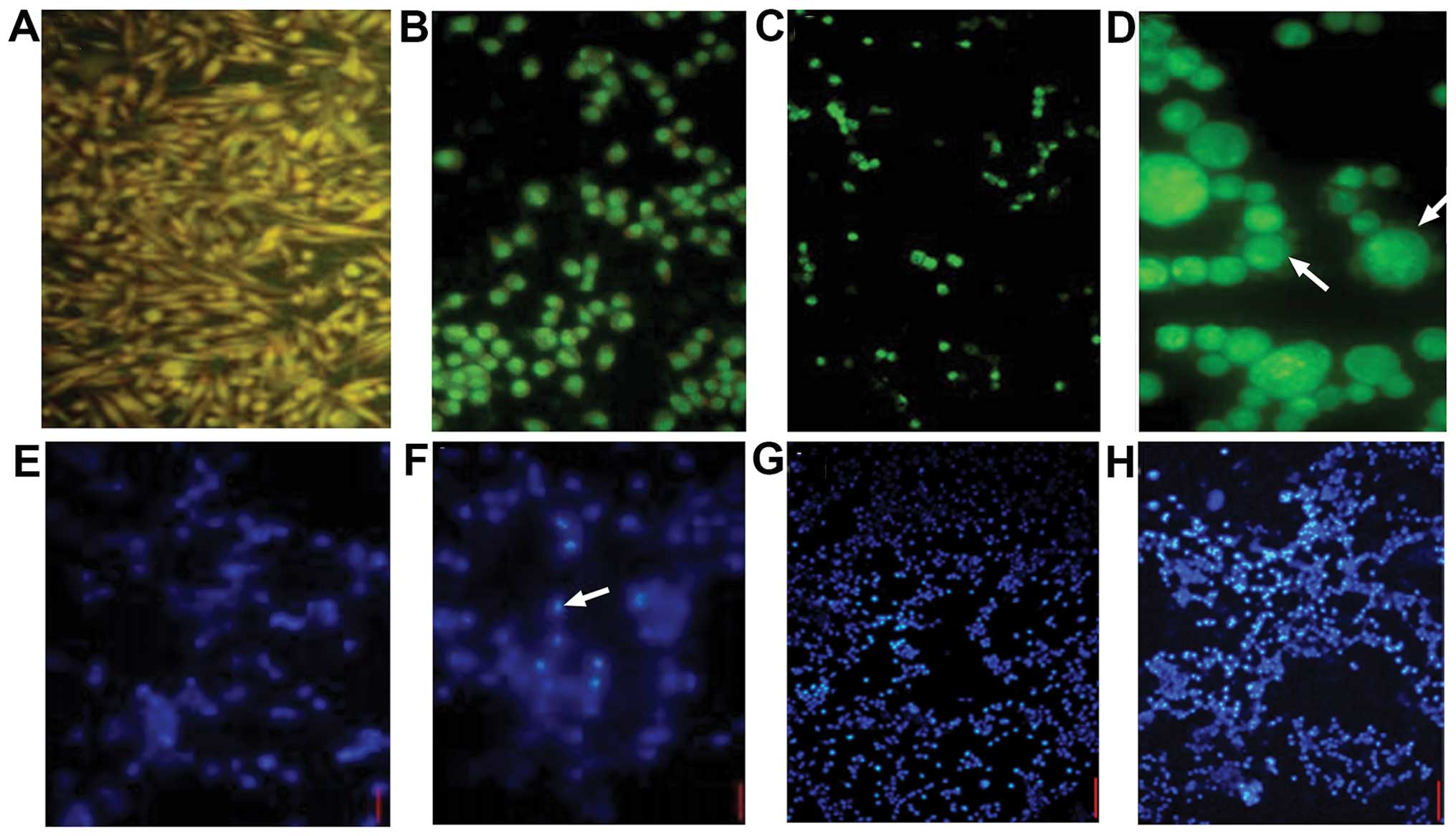 | Figure 4Fluorescence imaging of bladder tumor
cells stained with (A-D) AO and (E-H) Hoechst 33258. (A) Intact
MB49 cells were spindle-shaped and nuclei fluoresced yellow/green
and the cytoplasm emitted orange/red fluorescence, indicating a
high karyoplasmic ratio (FSM, x100). (B) After 48 h in the presence
of rBCG, the cells became rounded and clumped, cytoplasmic
protuberances disappeared, the karyoplasmic ratio increased and
vesicular bulging membrane and apoptotic bodies were observed (FSM,
x200), (C) After 72 h, significantly fewer cells were observed
(FSM, x100). (D) Apoptotic cells forming typical petal-like
structures (FSM, x400). (E) Intact cells showed faint blue
luminescence under UV (FSM, x200). (F) Luminous apoptotic bodies
were observed (FSM, x200). (G and H) Cells incubated with
rBCG for 48 and 72 h (FSM, x40). |
The rate of apoptosis was 2.31±1.02% in the control
cells, but after 24 h the presence of BGC, rBCG and
BCG+hIFN-α2b significantly increased the rate of
apoptosis to 7.9±0.97% (p<0.05), 19.92±0.77% (p<0.01) and
20.11±0.74% (p<0.01), respectively (Fig. 5).
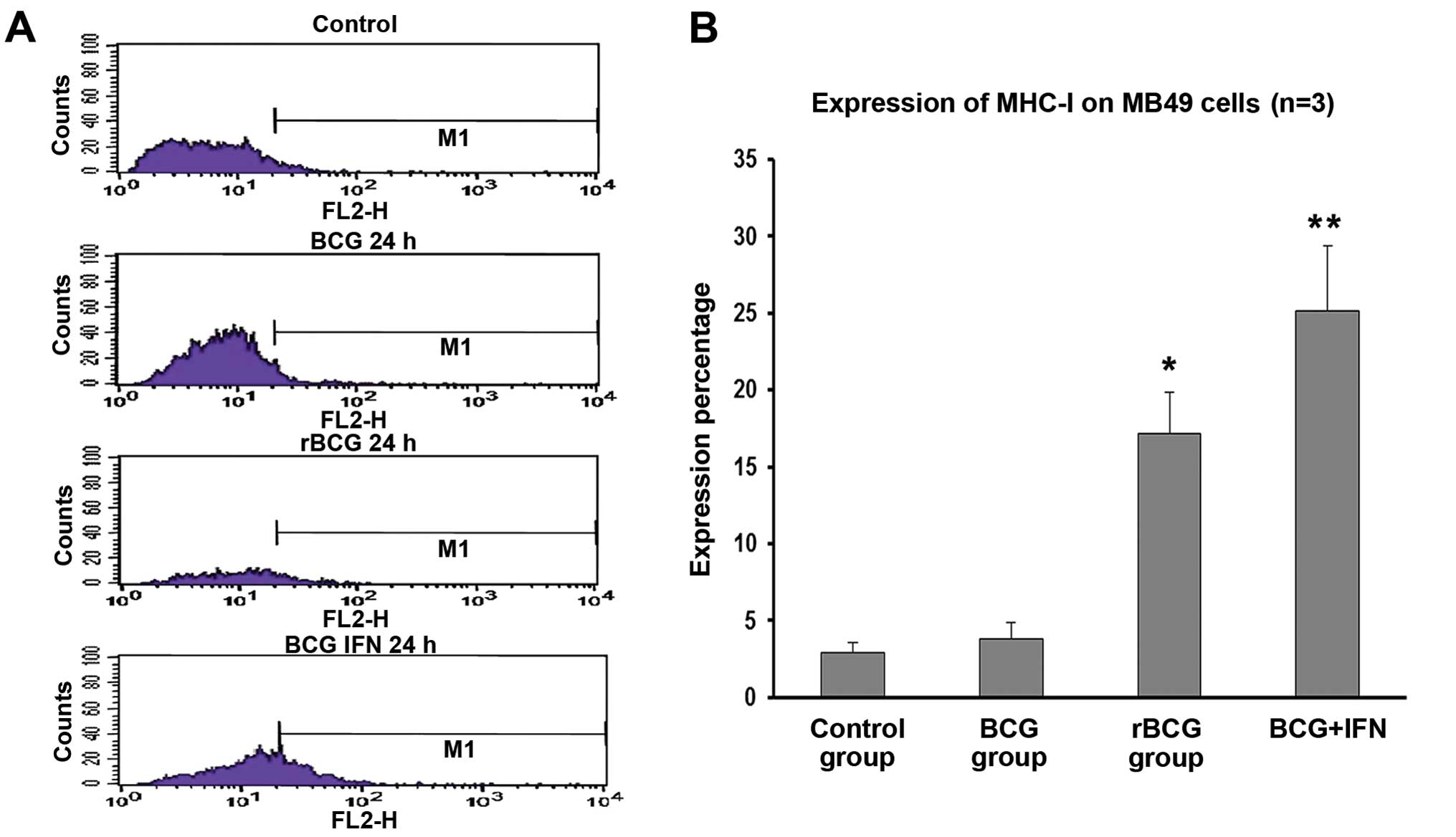
rBCG promotes MHC-I expression on MB49
cells
MB49 cells were incubated with MHC-I-directed
antibody and staining was detected by flow cytometry. A 24-h
incubation with BCG did not significantly influence the fraction of
MHC-I+ cells (2.89±0.24%). However, rBCG and
BCG+hIFN-α2b increased the fraction of
MHC-I+ cells to 17.18±0.88% (p<0.05) and 25.13±1.42%
(p<0.01), respectively (Fig.
6).
rBCG inhibits tumor growth and promotes
survival in a mouse model of orthotopic bladder cancer
After 2 weeks of modeling, C57BL/6 mice exhibited
different degrees of hema-turia. After 6 weeks, several mice
developed a hypogastric mass of 0.5–2 cm upon palpation. Mice
administered rBCG (n=5) survived for significantly longer
than mice administered BCG (n=7) (p<0.001), yet did not survive
significantly longer than mice administered
BCG+hIFN-α2b (n=8) (Fig.
7A).
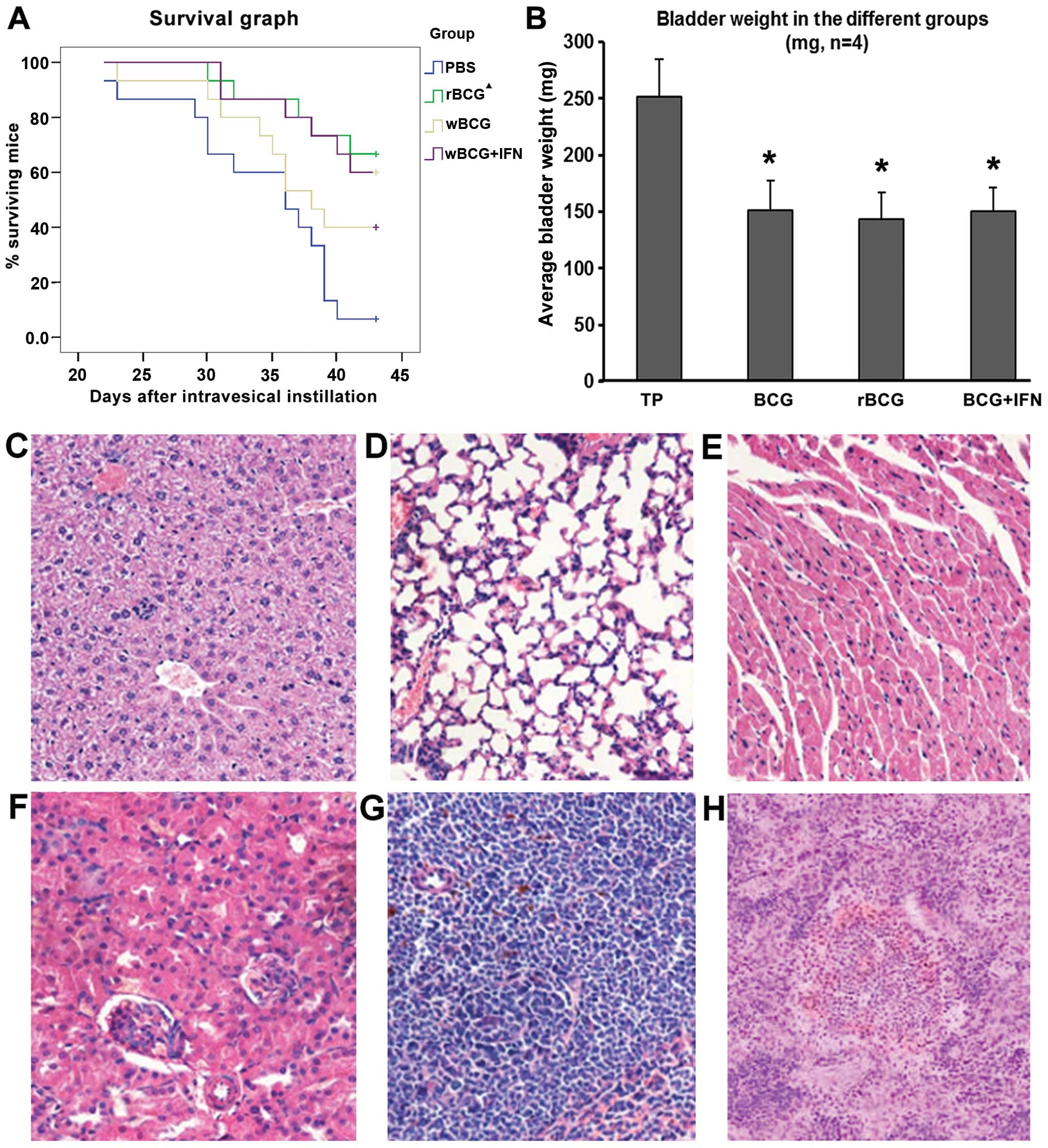 | Figure 7Tumor growth and survival in the
C57BL/6 mice and microscopic morphology of the organ tissues. (A)
Survival of mice following tumor establishment. Black triangles
indicate that the survival of mice administered rBCG was
higher than mice administered BCG (p<0.05), and did not differ
significantly from the mice administered BCG+IFN. (B) Average
bladder weight (mean ± SD, n=4). *p<0.001 vs. the TP
group. TP group, tumor-bearing mice administered PBS. (C) Liver,
(D) lung, (E) heart, (F) kidney and (G) spleen of the tumor-bearing
mice administered rBCG all showed no obvious abnormal, no
tumor metastasis and miliary pattern. (H) Individual mouse spleen
showed hyperplasia (H, magnification, x100; C-G, magnification,
x200). |
The average bladder weight was significantly lower
in mice administered rBCG (143.6±1.6 mg) than that in mice
administered PBS (n=14) (251.5±2.2 mg, p<0.001, Fig. 7B). Average bladder weight was
reduced by 39.9% in mice administered BCG, 42.9% in mice
administered rBCG and 40.2% in mice administered
BCG+hIFN-α2b.
The bladder, liver, spleen, kidney, lung and heart
of the mice sacrificed following the last perfusion and those that
died during the observation period indicated no tumor metastasis
and miliary nodules. No significant difference in pathological
features was observed among the mice treated with BCG, rBCG
or BCG+hIFN-α2b (Fig.
7C-H).
rBCG induces immunity in the bladder
The pathological morphology of the bladder tissue
indicated diffuse infiltration of tumor cells with high-grade
malignancy (Fig. 8A and B).
Administration of BCG, rBCG and BCG+hIFN-α2b
induced bladder inflammation. Migrating inflammatory cells were
observed in the submucosa. The local inflammatory reaction in
response to BCG was characterized by an initial increase in blood
flow, enhanced vascular permeability characterized by edema, and an
influx of effector cells. Vasodilation was evident and leukocytes
populated the submucosal layer (Fig.
8C). After administration of BCG into the mouse bladder,
auramine O staining-positive bacteria were detected within and
underlying urothelial cells, indicating that BCG was taken up by
the epithelium (Fig. 8D).
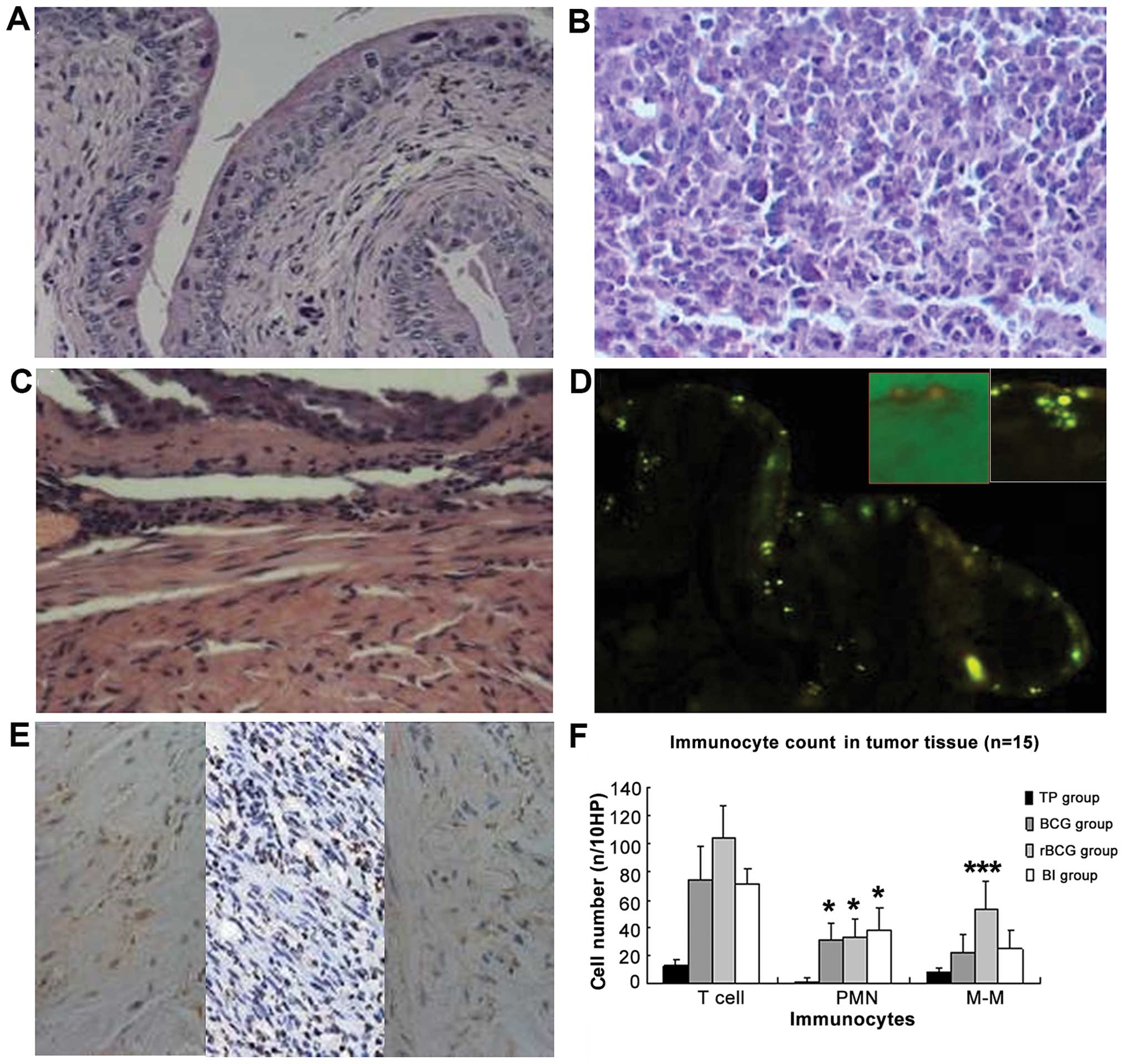 | Figure 8H&E staining and immunocyte
content of the bladder and cancer tissues in the C57BL/6 mice (A-C,
magnification, x200; F, magnification, x100). (A) Untreated bladder
exhibited normal morphology. (B) Tumor tissue formed by seeded MB49
cells did not affect normal bladder structure; tumor cells were
diffusely infiltrated. (C) After BCG administration, submucosal
edema appeared, vasodilation was evident and inflammatory cells
infiltrated the submucosal layer. (D) After 24 h of BCG
administration, auramine O-positive bacteria were detected within
and underlying the urothelial cells. The red frame is the merge of
the bright and fluorescent field 2 h after BCG administration,
indicating BCG adhesion to the intimal. The white frame displays
BCG taken up by the epithelium. (E) IHC image of CD20, CD3 and Gr1
staining in the rBCG-treated bladder (magnification, x400).
(F) Comparison of immunocyte content in the tumor tissues. Data are
shown as means ± SD (n/10 HP). *p<0.05;
***p<0.001 vs. the TP group. TP group, tumor-bearing
mice administered PBS; BI group, BCG+IFN group. H&E,
hematoxylin and eosin. PMN, polymorphonuclear leukocytes; M-M,
monocytes/macrophages. |
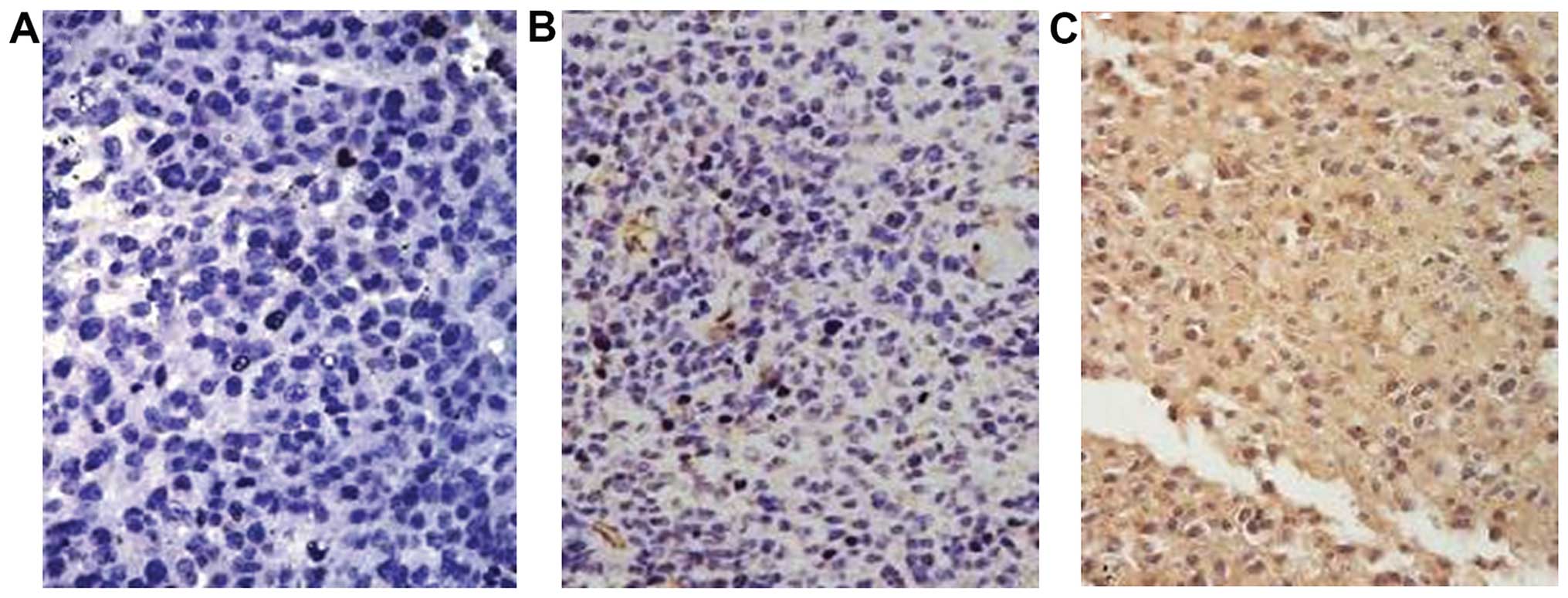
Immunohistochemical staining indicated infiltration
of CD3+ lymphocytes, CD20+ monocytes and
Gr1+ polymorpho-nuclear leukocytes (PMNs) (Fig. 8E and F). PMN, monocyte and T
lymphocyte infiltration increased significantly in the treated
groups, compared with the infiltration in the control group (all
p≤0.05). Monocyte and T cell counts were significantly higher in
mice administered rBCG than these counts in the mice administered
BCG or BCG+hIFN-α2b (both p=0.000). However, PMN
counts did not differ significantly between the treated
animals.
rBCG increases the expression of Fas
Fas expression was initially low in the bladder
tumor tissues. Administration of BCG, rBCG and
BCG+hIFN-α2b significantly increased Fas expression
(p=0.000), but the intensity of Fas staining did not differ
significantly between the three treated groups (Fig. 9 and Table I).
 | Table IFas expression in the tumors (cases)
according to the IHC results. |
Table I
Fas expression in the tumors (cases)
according to the IHC results.
| Groups | Low expression | High
expression |
|---|
| TP | 14 | 1 |
| BCGa | 4 | 11 |
| rBCGa | 1 | 13 |
| BIa | 4 | 10 |
rBCG increases the ratio of
CD4+/CD8+ in peripheral blood
In comparison to normal mice, the tumor-bearing mice
had depressed levels of peripheral blood CD4+ cells and
lower CD4+/CD8+ ratios. However,
administration of BCG, rBCG or BCG+hIFN-α2b
elevated CD4+ cell counts and
CD4+/CD8+ ratios to near-normal levels
(Fig. 10A-C) (all p<0.01).
However, no significant difference in these values was detected
between the three treatment groups.
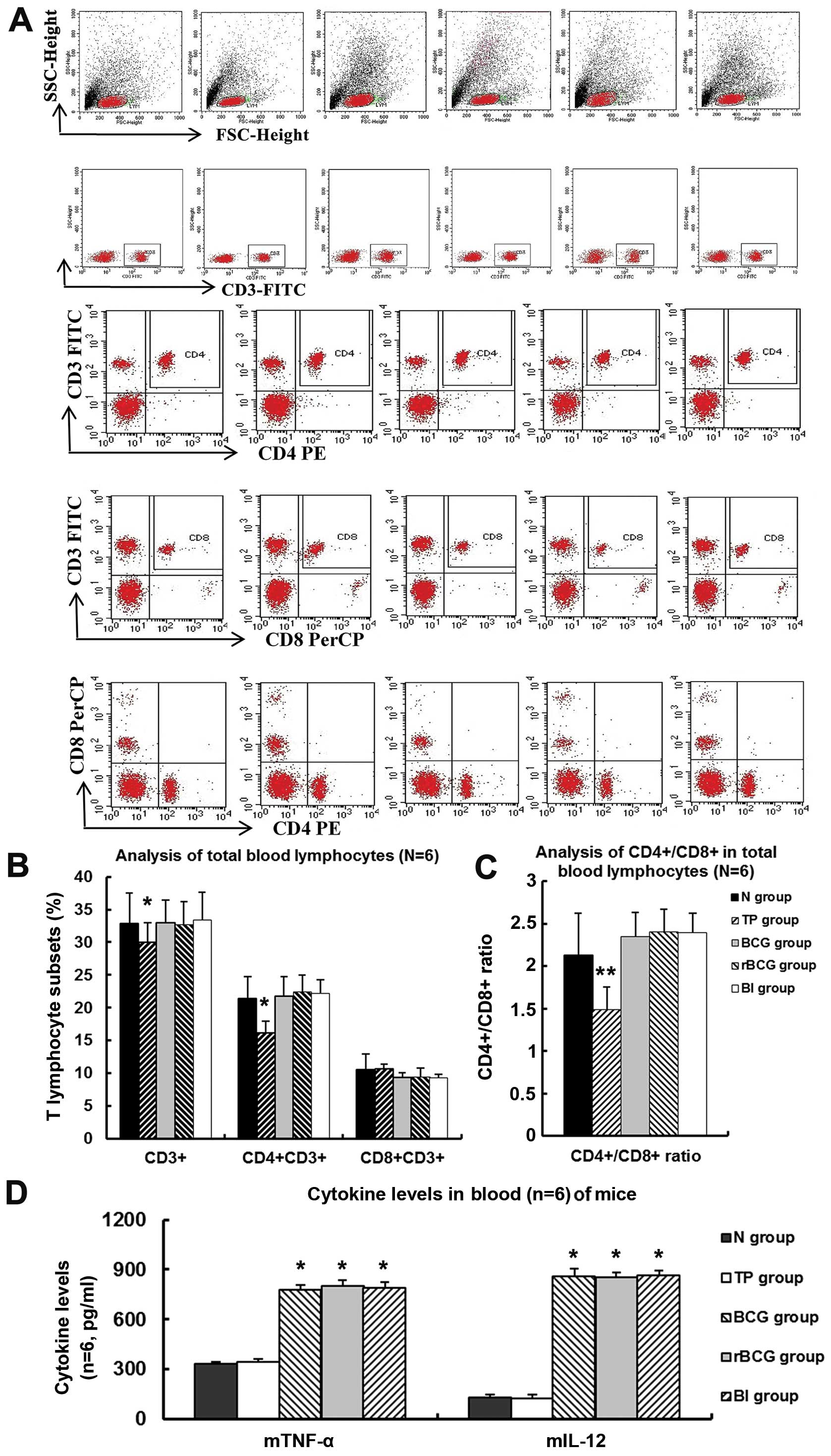 | Figure 10T lymphocyte subsets and cytokines in
peripheral blood of C57BL/6 mice administered BCG, rBCG or
BCG+IFN as analyzed by FCM. (A) Representative images of
CD3+, CD3+CD8+ and
CD3+CD8+ lymphocytes in the peripheral blood
of mice administered BCG, rBCG or BCG+IFN, and (B) the
percentage of CD3+, CD3+CD8+ and
CD3+CD8+ lymphocytes in the peripheral blood
of mice administered BCG, rBCG or BCG+IFN (mean ± SD) (n=6).
*p<0.05 vs. normal. (C) CD4+/CD8+ ratio,
mean ± SD (n=6). **p<0.01 vs. normal. (D) The level of
mTNF-α and mIL-12 in the peripheral blood of the
C57BL/6 mice administered BCG, rBCG or BCG+IFN, mean ± SD
(n=6, pg/ml). *p<0.001 vs. normal. N, normal group; TP group,
tumor-bearing mice administered PBS; BI group, BCG+IFN group |
rBCG increases the level of TNF-a and
IL-12
rBCG administration increased circulating
levels of mTNF-α and mIL-12 to 02.33±11.00 and
854.46±4.56 pg/ml, respectively (Fig.
10D). However the circulating levels of these cytokines did not
differ significantly among mice administered rBCG, BCG or
BCG+hIFN-α2b.
Discussion
BCG prevents bladder cancer-related metastasis and
decreases bladder cancer-associated mortality. Although systemic
reactions have been reported, the likely mechanism of BCG action
involves local inflammation (1). We
sought to further characterize the mechanism of action of BCG in a
bladder cancer cell line and an orthotopic murine bladder cancer
model.
Due to the involvement of the host immune system in
BCG efficacy, immunodeficient mice are not suitable for
investigation of its mechanism of action. We instead established an
orthotopic bladder tumor model in which the mouse bladder tumor
cell line MB49 was implanted into the C57BL/6 mouse bladder.
C57BL/6 mice were chosen, as they are widely used, permitting
direct comparison with previously established clinical baselines
(22). Following chemical injury
and cell transplantation (19),
tumor cell proliferation was observed, yet no tumor metastasis and
miliary nodules were found in all groups.
A wide range of rBCG vaccine candidates
expressing bacterial, viral, parasitic antigens have previously
been developed, and rBCG strains secreting mouse and human
cytokines, primarily Th1 cytokines (e.g., IL-2, IL-18, IFN-γ and
IFN-α), have previously been investigated (10). We studied the influence of
hIFN-α2b-rBCG on tumor growth in vitro
and both tumor growth and the systemic and local immune response
in vivo. We used the BCG Shanghai substrain, derived
from the Danish 2 strain that has been used for tuberculosis
prevention and immune-modulation universally in China. We
engineered a strain of BCG Danish 2 to secrete high levels
of recombinant hIFN-α2b.
Direct effects on tumor cells
We found that both BCG and rBCG inhibited growth of
a mouse bladder cancer cell line, inducing morphological changes
and apoptosis, while rBCG was significantly more potent. IFN
induces tumor cell apoptosis by promoting protooncogenes and TNF-α
receptor expression, or by inhibiting intracellular proteins
(23,24). Following administration to mice, we
observed BCG adherence to the bladder intima prior to bladder
pathologic changes, a process that has previously been reported to
involve non-specific, physicochemical and specific
receptor-ligand-mediated events (7,25).
Phospholipids, lipids, wax D, bacterial proteins and
lipopolysaccharides in BCG are all strongly immunogenic (26–28),
and BCG cell wall protein and Arabian-polysaccharide
pathogen-associated molecular patterns induce expression of
lysosomal membrane protein and apoptosis in host cells (27–29). A
BCG cell wall glycolipid, trehalose dimycolate, has also been
reported to damage host cells by attacking the mitochondrial
membrane, affecting cellular respiration and energy metabolism,
destroying microsomal enzymes and inducing programmed cell death
(30–33). Furthermore, internalized BCG
increased production of intracellular cytotoxic nitric oxide, which
at a high concentration, causes DNA damage, and cytostatic and
cytotoxic effects (7). BCG has also
been reported to induce upregulation of certain surface molecules
and cytokines in epithelial cells (34,35),
and activation of the signal transduction pathways involving
activator protein 1 and NFκB (36,37).
We also observed cytoplasmic Fas in mouse bladder
tumor cells, and administration of rBCG and BCG upregulated
Fas expression on the surface of the tumor cells. Fas typically is
highly expressed in rapidly proliferating cells and injured
tissues, and the triggering of Fas by its ligand induces apop-tosis
in target cells (38). Expression
of Fas can be upregulated by IL-2 and IFNs (39), but we observed no significant
difference between Fas expression in the rBCG- and
BCG-treated cells, potentially due to the species specificity of
human IFN.
Immunomodulation
BGC has been hypothesized to ameliorate the
aberrant imbalance of T helper and cytotoxic T cell subsets
observed in the bladder during proliferation of cancer cells
(3). Systemic immunity is induced
by administration of BGC in patients with bladder cancer and a
cytokine profile typical of active tuberculosis can be observed
(40). In our mouse model, the
circulating levels of CD3+ and CD4+ cells,
and the CD4+/CD8+ ratio was lower in the
tumor-bearing mice than in the normal animals. This immune response
was likely caused by activation and proliferation of
antigen-specific T lymphocytes, followed by immune tolerance or
apoptosis, and depletion of T lymphocytes by activation-induced
cell death. Administration of both BCG and rBCG induced
lymphocyte proliferation, and regulated lymphocyte subsets,
adjusting cellular immune function. CD4+ and
CD3+ cell counts recovered to the levels observed in
healthy control animals, yet the level of CD8+ cells was
not significantly influenced. The CD4+/CD8+
ratio was also increased, but no significant difference was
observed between the effect of BCG and rBCG.
Local immune responses
A small number of local lymphocytes are usually
observed in the tumor tissue of patients with bladder tumors,
mainly distributed in the submucosa (41). Administration of BCG in our mouse
model of bladder cancer caused extensive local inflammation in the
bladder wall. Expression of CD3, CD20 and Gr1 in the tumors was
negative or weak, yet administration of BCG or rBCG induced influx
of PMNs and other inflammatory cells. BCG appears to activate local
non-specific Th1 type cells and cytokines (42). We observed that BCG and rBCG
induced acute inflammation of the bladder, characterized by a
strong vascular component and edema, followed by a gradual influx
of mono-cytes/macrophages, T lymphocytes and NK cells, which form
chronic granuloma-like cellular infiltrates in the suburothelial st
roma (3).
In vitro, BCG also induced MHC-I expression
on mouse MB49 cells, as previously reported by Shankaran et
al (43), and we found that
rBCG did so more potently than BCG. Tumor cells often
downregulate MHC-I to avoid immune surveillance (44), yet IFN is well known to induce MHC-I
(45). Increased expression of
MHC-I on the tumor cell surface likely enhances the immunogenicity
of these cells.
Cytokine release
Patients with bladder tumors often exhibit a marked
polarization towards expression of Th2 type cyto-kines while
expression of Th1 cytokines are suppressed (46). Administration of BCG adjusted the
imbalance of Th1 cyto-kines (IL-2, IL-12, IFN-γ and TNF-α) leading
to detection of these cytokines in the urine of BCG-treated
patients (7). The induction of
proinfammatory cytokines, specifically IFN-γ, TNF-α and IL-2, are
crucial for the cytotoxic effect of live BCG-activated cells
(47). BCG-activated lymphocytes
and macrophages are the most likely sources of these cytokines, but
at present, other cellular sources such as urothelial cells cannot
be ruled out (7,46). We found that administration of BCG
and rBCG promoted Th1 type immunity in the tumor-bearing
mice. In vitro, IFN-α2b enhances the BCG induction of
Th1 immune responses in human PBMCs (48), yet this effect was not observed in
the peripheral blood of our experimental animals. T cell-mediated
cell lysis and release of regulatory cytokines have been shown to
represent late acquired immune events of the antitumor effector
phase (49), to induce tumor
apoptosis and prevent tumor growth. For example, IL-12 promotes the
activation of effector cells and induces IFN-γ, IL-2 and TNF-α
(50). TNF-α kills tumor cells
directly and also induces apoptosis by the Fas/FasL-mediated path
or others, which all could be strengthened by IFN.
In conclusion, our findings suggest that the
therapeutic effects of BCG can be attributed in part to the rapid
accumulation of antigen-presenting, and activated immune cells
responsible for the production of a multiphasic immune response as
demonstrated by the presence of the Th1 or Th2 phenotype. IFN
directly inhibits proliferation and angio-genesis (51), and further immunomodulation of tumor
MHC expression (52), could be
advantageous in bladder cancer treatment. According to our results
rBCG also exhibited antitumor activity in the bladder of
mice transplanted with a bladder cancer cell line. Mice
administered rBCG survived longer than those administered
BCG plus endogenous IFN. Although the capacity of rBCG to
inhibit tumor growth was not sup er ior t o t hat of BCG, t he in
fluence of t he secreted cytokine may have been limited by species
specificity. As a novel immune-modulatory agent for the treatment
of human bladder cancer, rBCG has excellent prospects for
development, but more in-depth exploration of its function and
mechanisms is needed.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81402095). We thank
Professor M.A. O’Donnell and Professor Yi Luo for kindly providing
plasmid pMAO-4 to construct the recombinant
hIFN-α2b-secreting BCG.
References
|
1
|
Gandhi NM, Morales A and Lamm DL: Bacillus
Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int.
112:288–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hall MC, Chang SS, Dalbagni G, Pruthi RS,
Seigne JD, Skinner EC, Wolf JS Jr and Schellhammer PF: Guideline
for the management of nonmuscle invasive bladder cancer (stages Ta,
T1, and Tis): 2007 update. J Urol. 178:2314–2330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alexandroff AB, Nicholson S, Patel PM and
Jackson AM: Recent advances in bacillus Calmette-Guerin
immunotherapy in bladder cancer. Immunotherapy. 2:551–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haley JL, Young DG, Alexandroff A, James K
and Jackson AM: Enhancing the immunotherapeutic potential of
mycobacteria by transfection with tumour necrosis factor-alpha.
Immunology. 96:114–121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ratliff TL, Kavoussi LR and Catalona WJ:
Role of fibronectin in intravesical BCG therapy for superficial
bladder cancer. J Urol. 139:410–414. 1988.PubMed/NCBI
|
|
6
|
Kresowik TP and Griffith TS: Bacillus
Calmette-Guerin immunotherapy for urothelial carcinoma of the
bladder. Immunotherapy. 1:281–288. 2009. View Article : Google Scholar :
|
|
7
|
Bevers RF, Kurth KH and Schamhart DH: Role
of urothelial cells in BCG immunotherapy for superficial bladder
cancer. Br J Cancer. 91:607–612. 2004.PubMed/NCBI
|
|
8
|
Williams SK, Hoenig DM, Ghavamian R and
Soloway M: Intravesical therapy for bladder cancer. Expert Opin
Pharmacother. 11:947–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delimpoura V, Samitas K, Vamvakaris I,
Zervas E and Gaga M: Concurrent granulomatous hepatitis,
pneumonitis and sepsis as a complication of intravesical BCG
immunotherapy. BMJ Case Rep. 10:20132013.
|
|
10
|
Kawai K, Miyazaki J, Joraku A, Nishiyama H
and Akaza H: Bacillus Calmette-Guerin (BCG) immunotherapy for
bladder cancer: Current understanding and perspectives on
engineered BCG vaccine. Cancer Sci. 104:22–27. 2013. View Article : Google Scholar
|
|
11
|
Joudi FN, Smith BJ and O’Donnell MA;
National BCG-Interferon Phase 2 Investigator Group: Final results
from a national multicenter phase II trial of combination bacillus
Calmette-Guérin plus interferon alpha-2B for reducing recurrence of
superficial bladder cancer. Urol Oncol. 24:344–348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nepple KG, Lightfoot AJ, Rosevear HM,
O’Donnell MA and Lamm DL; Bladder Cancer Genitourinary Oncology
Study Group: Bacillus Calmette-Guérin with or without interferon
α-2b and megadose versus recommended daily allowance vitamins
during induction and maintenance intravesical treatment of
nonmuscle invasive bladder cancer. J Urol. 184:1915–1919. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lam JS, Benson MC, O’Donnell MA, Sawczuk
A, Gavazzi A, Wechsler MH and Sawczuk IS: Bacillus Calmete-Guérin
plus interferon-alpha2B intravesical therapy maintains an extended
treatment plan for superficial bladder cancer with minimal
toxicity. Urol Oncol. 21:354–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bazarbashi S, Soudy H, Abdelsalam M,
Al-Jubran A, Akhtar S, Memon M, Aslam M, Kattan S and Shoukri M:
Co-administration of intravesical bacillus Calmette-Guérin and
interferon α-2B as first line in treating superficial transitional
cell carcinoma of the urinary bladder. BJU Int. 108:1115–1118.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Askeland EJ, Newton MR, O’Donnell MA and
Luo Y: Bladder cancer immunotherapy: BCG and beyond. Adv Urol.
2012:1819872012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chapman R, Chege G, Shephard E, Stutz H
and Williamson AL: Recombinant Mycobacterium bovis BCG as an HIV
vaccine vector. Curr HIV Res. 8:282–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun E, Nian X, Liu C, Fan X and Han R:
Construction of recombinant human IFNa-2b BCG and its antitumor
effects on bladder cancer cells in vitro. Genet Mol Res.
14:3436–3449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mugabe C, Raven PA, Fazli L, Baker JH,
Jackson JK, Liggins RT, So AI, Gleave ME, Minchinton AI, Brooks DE,
et al: Tissue uptake of docetaxel loaded hydrophobically
derivatized hyper-branched polyglycerols and their effects on the
morphology of the bladder urothelium. Biomaterials. 33:692–703.
2012. View Article : Google Scholar
|
|
19
|
Luo Y, Chen X and O’donnell MA: Use of
prostate specific antigen to measure bladder tumor growth in a
mouse orthotopic model. J Urol. 172:2414–2420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bayne LJ and Vonderheide RH:
Immunohistochemical assessment of immune cells in mouse tumor
tissue. Cold Spring Harb Protoc. 2013:843–848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohuchida K, Mizumoto K, Ishikawa N, Fujii
K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M and Tanaka M: The
role of S100A6 in pancreatic cancer development and its clinical
implication as a diagnostic marker and therapeutic target. Clin
Cancer Res. 11:7785–7793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saban MR, Simpson C, Davis C, Wallis G,
Knowlton N, Frank MB, Centola M, Gallucci RM and Saban R:
Discriminators of mouse bladder response to intravesical Bacillus
Calmette-Guerin (BCG). BMC Immunol. 8:62007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanase N, Hayashida M, Kanetaka-Naka Y,
Hoshika A and Mizuguchi J: PKC-δ mediates interferon-α-induced
apoptosis through c-Jun NH2-terminal kinase activation.
BMC Cell Biol. 13:72012. View Article : Google Scholar
|
|
24
|
Yanase N, Kanetaka Y and Mizuguchi J:
Interferon-α-induced apoptosis via tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-dependent and -independent
manner. Oncol Rep. 18:1031–1038. 2007.PubMed/NCBI
|
|
25
|
Chen F, Zhang G, Iwamoto Y and See WA:
Bacillus Calmette-Guerin initiates intracellular signaling in a
transitional carcinoma cell line by cross-linking alpha 5 beta 1
integrin. J Urol. 170:605–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Villeneuve C, Gilleron M,
Maridonneau-Parini I, Daffé M, Astarie-Dequeker C and Etienne G:
Mycobacteria use their surface-exposed glycolipids to infect human
macrophages through a receptor-dependent process. J Lipid Res.
46:475–483. 2005. View Article : Google Scholar
|
|
27
|
Moriwaki Y, Begum NA, Kobayashi M,
Matsumoto M, Toyoshima K and Seya T: Mycobacterium bovis Bacillus
Calmette-Guerin and its cell wall complex induce a novel lysosomal
membrane protein, SIMPLE, that bridges the missing link between
lipopolysaccharide and p53-inducible gene, LITAF(PIG7), and
estrogen-inducible gene, EET-1. J Biol Chem. 276:23065–23076. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato T, Bilim V, Yuuki K, Naito S,
Yamanobe T, Nagaoka A, Yano I, Akaza H and Tomita Y: Bacillus
Calmette-Guerin and BCG cell wall skeleton suppressed viability of
bladder cancer cells in vitro. Anticancer Res. 30:4089–4096.
2010.PubMed/NCBI
|
|
29
|
Ishibashi T, Yamada H, Harada S, Harada Y,
Takamoto M and Sugiyama K: Comparison of the mode of
immunopotentiating action of BCG and wax D. II. Effect on the
methylcholanthrene carcinogenesis. Jpn J Exp Med. 47:435–440.
1977.PubMed/NCBI
|
|
30
|
Kato M: Action of a toxic glycolipid of
Corynebacterium diph-theriae on mitochondrial structure and
function. J Bacteriol. 101:709–716. 1970.PubMed/NCBI
|
|
31
|
Fujita Y, Okamoto Y, Uenishi Y, Sunagawa
M, Uchiyama T and Yano I: Molecular and supra-molecular structure
related differences in toxicity and granulomatogenic activity of
mycobacterial cord factor in mice. Microb Pathog. 43:10–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryll R, Watanabe K, Fujiwara N, Takimoto
H, Hasunuma R, Kumazawa Y, Okada M and Yano I: Mycobacterial cord
factor, but not sulfolipid, causes depletion of NKT cells and
upregulation of CD1d1 on murine macrophages. Microbes Infect.
3:611–619. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamasaki N, Isowa K, Kamada K, Terano Y,
Matsumoto T, Arakawa T, Kobayashi K and Yano I: In vivo
administration of mycobacterial cord factor (Trehalose
6,6′-dimycolate) can induce lung and liver granulomas and thymic
atrophy in rabbits. Infect Immun. 68:3704–3709. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saban MR, Hellmich HL, Simpson C, Davis
CA, Lang ML, Ihnat MA, O’Donnell MA, Wu XR and Saban R: Repeated
BCG treatment of mouse bladder selectively stimulates small GTPases
and HLA antigens and inhibits single-spanning uroplakins. BMC
Cancer. 7:2042007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyazaki J, Kawai K, Kojima T, Oikawa T,
Joraku A, Shimazui T, Nakaya A, Yano I, Nakamura T, Harashima H, et
al: The lipo-some-incorporating cell wall skeleton of Mycobacterium
bovis bacillus Calmette-Guéin can directly enhance the
susceptibility of cancer cells to lymphokine-activated killer cells
through up-regulation of natural-killer group 2, member D ligands.
BJU Int. 108:1520–1526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen FH, Crist SA, Zhang GJ, Iwamoto Y and
See WA: Interleukin-6 production by human bladder tumor cell lines
is up-regulated by bacillus Calmette-Guérin through nuclear
factor-kappaB and Ap-1 via an immediate early pathway. J Urol.
168:786–797. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang G, Chen F, Cao Y and See WA:
Bacillus Calmette-Guérin induces p21 expression in human
transitional carcinoma cell lines via an immediate early, p53
independent pathway. Urol Oncol. 25:221–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He C, Jiang H, Geng S, Sheng H, Shen X,
Zhang X, Zhu S, Chen X, Yang C and Gao H: Expression and prognostic
value of c-Myc and Fas (CD95/APO1) in patients with pancreatic
cancer. Int J Clin Exp Pathol. 7:742–750. 2014.PubMed/NCBI
|
|
39
|
Belardelli F and Ferrantini M: Cytokines
as a link between innate and adaptive antitumor immunity. Trends
Immunol. 23:201–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elsäßer J, Janssen MW, Becker F, Suttmann
H, Schmitt K, Sester U, Stöckle M and Sester M: Antigen-specific
CD4 T cells are induced after intravesical BCG-instillation therapy
in patients with bladder cancer and show similar cytokine profiles
as in active tuberculosis. PLoS One. 8:e698922013. View Article : Google Scholar
|
|
41
|
Ingersoll MA and Albert ML: From infection
to immunotherapy: Host immune responses to bacteria at the bladder
mucosa. Mucosal Immunol. 6:1041–1053. 2013.PubMed/NCBI
|
|
42
|
Monjazeb AM, Hsiao HH, Sckisel GD and
Murphy WJ: The role of antigen-specific and non-specific
immunotherapy in the treatment of cancer. J Immunotoxicol.
9:248–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shankaran V, Ikeda H, Bruce AT, White JM,
Swanson PE, Old LJ and Schreiber RD: IFNgamma and lymphocytes
prevent primary tumour development and shape tumour immunogenicity.
Nature. 410:1107–1111. 2001. View Article : Google Scholar
|
|
44
|
D’Orazio SE, Halme DG, Ploegh HL and
Starnbach MN: Class Ia MHC-deficient BALB/c mice generate
CD8+ T cell-mediated protective immunity against
Listeria monocytogenes infection. J Immunol. 171:291–298. 2003.
View Article : Google Scholar
|
|
45
|
Kamat AM and Lamm DL: Immunotherapy for
bladder cancer. Curr Urol Rep. 2:62–69. 2001. View Article : Google Scholar
|
|
46
|
Satyam A, Singh P, Badjatia N, Seth A and
Sharma A: A disproportion of TH1/TH2 cytokines with predominance of
TH2, in urothelial carcinoma of bladder. Urol Oncol. 29:58–65.
2011. View Article : Google Scholar
|
|
47
|
Abadie V, Badell E, Douillard P,
Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B
and Winter N: Neutrophils rapidly migrate via lymphatics after
Mycobacterium bovis BCG intradermal vaccination and shuttle live
bacilli to the draining lymph nodes. Blood. 106:1843–1850. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo Y, Chen X, Downs TM, DeWolf WC and
O’Donnell MA: IFN-alpha 2B enhances Th1 cytokine responses in
bladder cancer patients receiving Mycobacterium bovis bacillus
Calmette-Guérin immunotherapy. J Immunol. 162:2399–2405.
1999.PubMed/NCBI
|
|
49
|
Böhle A and Brandau S: Immune mechanisms
in bacillus Calmette-Guerin immunotherapy for superficial bladder
cancer. J Urol. 170:964–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Luo Y, Chen X and O’Donnell MA: Role of
Th1 and Th2 cytokines in BCG-induced IFN-gamma production: Cytokine
promotion and simulation of BCG effect. Cytokine. 21:17–26. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Panaretakis T, Pokrovskaja K, Shoshan MC
and Grandér D: Interferon-alpha-induced apoptosis in U266 cells is
associated with activation of the proapoptotic Bcl-2 family members
Bak and Bax. Oncogene. 22:4543–4556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Manna SK, Mukhopadhyay A and Aggarwal BB:
IFN-alpha suppresses activation of nuclear transcription factors
NF-kappa B and activator protein 1 and potentiates TNF-induced
apoptosis. J Immunol. 165:4927–4934. 2000. View Article : Google Scholar : PubMed/NCBI
|