Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common type of cancer worldwide (1). HNSCC patients have benefited greatly
from recent advances in surgical techniques, radiotherapy and
chemotherapy. However, despite the advances in local control and
overall quality of life achieved with the use of combined
therapies, the survival rate for HNSCC has not improved
significantly over the last two decades (2). Invasion of normal adjacent tissue and
lymph node metastasis are the most adverse independent prognostic
factors for patients with HNSCC (3,4).
Although the current TNM staging system is used
routinely, its value for predicting clinical outcomes remains
modest, particularly among tumors of identical TNM stage (5,6). Thus,
there is a continuing need to identify biological markers that may
be able to augment the clinical staging system. It is also believed
that underlying molecular features of tumors play an essential role
in determining their aggressiveness.
Podoplanin is a mucin-type transmembrane
glycoprotein that is specifically expressed in lymphatic, but not
in blood endothelial cells (7). It
has been shown in a knockout animal model that podoplanin
deficiency causes lymphovascular malformations associated with
congenital lymphedema and dilation of lymphatic vessels, suggesting
that under physiological conditions, podoplanin may play an
important role in regulating peripheral lung cell proliferation and
lymphatic vascular development (8).
Recent studies have reported podoplanin expression in carcinomas of
the skin, lung, uterus, esophagus and squamous cell carcinomas of
the oral cavity (9–15). Moreover, high expression of
podoplanin was found to be significantly associated with poor
prognosis, suggesting that podoplanin may have biological functions
in tumor cells (9,12–15).
In the present study, we investigated the prognostic
influence of podoplanin expression in HNSCC and its association
with clinicopathological variables, particularly regional lymph
node metastasis, to determine its effectiveness as a marker of
high-risk HNSCC. To verify the involvement of podoplanin in the
metastasis of HNSCC, we examined cell wound healing migration and
invasion assays using both RNA interference and overexpression of
podoplanin.
Patients and methods
Patients
This retrospective study included 119 consecutive
treatment-naïve patients with biopsy confirmed primary HNSCC, who
were treated at the Department of Otolaryngology, Head and Neck
Surgery, Chungnam National University Hospital between 1998 and
2010. All patients underwent primary surgery including neck
dissection. Tumor staging was conducted according to the criteria
of the 7th edition of the AJCC (16). Clinicopathological parameters,
including histopathological and surgical studies, were obtained
from the medical charts.
Follow-up data were collected by a combination of
chart review and the local government office for the registration
of residents. At the time of the analysis, 51 (42.9%) patients had
succumbed to the disease; 45 (37.8%) of them due to the tumor.
Overall, 54 patients developed recurrent disease, including 21
(17.6%) local recurrences, 29 (24.3%) subsequent regional lymph
node metastases and 8 (6.7%) distant recurrences. The average
follow-up time was 40.6 (range, 2–144) months.
Tissue processing and immunohistochemical
analysis
The immunohistochemical (IHC) study of the HNSCC
patients was approved by the local institutional review board.
Formalin-fixed and paraffin-wax-embedded tissues from 119 HNSCC
lesions - 44 in the oral cavity, 17 in the oropharynx, 48 in the
larynx and 10 in the hypopharynx - were retrieved from the archives
of the Department of Pathology, Chungnam National University
Hospital, Korea. Sections (4 µm) of the
paraffin-wax-embedded tissue blocks were used for IHC studies
according to the procedures provided in the Vectastain ABC kit
(Vector, Burlingame. CA, USA). All slides were scored, as described
by Yuan et al (14), by at
least two pathologists who were blinded to the clinical information
of the patients. For scoring, representative areas of each tissue
section were selected and evaluated independently. Cytoplasm and/or
membrane immunoreactivity was considered to indicate podoplanin
expression. Quantitative scores of 0 to 5 were assigned when 0,
1–10, 11–30, 31–50, 51–80 or 81–100% of the tumor cells were
positive, respectively. The staining intensity was rated on a scale
of 0 to 3: 0 = negative, 1 = weak, 2 = moderate, and 3 = strong.
The raw data were then converted to a German Immunoreactive Score
(IRS) by multiplying the quantity and staining intensity scores.
Theoretically, the scores may range from 0 to 15. An IRS score
above the median (≥7) was considered high reactivity and 0–6, low.
Consensus opinions were used to assign final IRS scores in disputed
cases before data analysis.
Cell lines and culture conditions
The HNSCC cell lines FaDu and SNU-1041 were
purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA) and the Korean Cell line Bank (KClB; Seoul,
Korea) and maintained in DMEM and RPMI supplemented with 100 U/ml
penicillin, 100 µg/ml streptomycin, 25 ng/ml amphotericin B
and 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) at 37°C
in a humidified incubator with 5% CO2, respectively.
siRNA knockdown of podoplanin gene
expression
Podoplanin-specific siRNA and control siRNA were
purchased from Bioneer (Daejeon, Korea). The targeted sequences of
podoplanin were sense siRNA, 5′-GAU AAC ACG UGU GGU GAA CAA TT-3′
and antisense, 5′-UUG UUC ACC ACG UGU UAU GTT-3′. Podoplanin siRNA
transfection was performed in opti-MEM media with the transfection
reagent lipofectamine 2000 (Invitrogen life Technologies, Carlsbad,
CA, USA) following the manufacturer’s instructions.
Gene manipulation of podoplanin
Human podoplanin cDNA (NM_006474.4) was
PCR-amplified from a cDNA library which was purchased from Origene
(Rockville, MD, USA) and cloned into the NotI/NotI
site of pCMV6-Xl5 (Origene). The PDPN vector containing the
podoplanin coding region or the mock vector was transfected into
the FaDu or SNU-1041 cell line with lipofectamine 2000 according to
the manufacturer’s instructions, respectively.
RT-PCR
Oligonucleotides of the VEGF family genes such as
VEGF-A, -B, -C and -D were purchased from Bioneer. The primer
sequences for GAPDH were 5′-CCATCACCATCT TCCAGGAG-3′ (sense) and
5′-ACAGTCTTCTGGGTGGCA GT-3′ (antisense). The VEGF family-specific
primers used for PCR were described in a previous study (17). Relative gene expression levels were
normalized to GAPDH expression.
Western blot analysis
Sodium dodecyl sulfate-polyacryl-amide gel
electrophoresis (SDS-PAGE) was conducted using a Mini-Protean Tetra
Cell (Bio-Rad, Hercules, CA, USA) and a 12% gel according to the
manufacturer’s instructions. Proteins were transferred to a PVDF
membrane and probed with primary antibodies followed by an
HRP-conjugated secondary antibody. Immunolabeled proteins were
detected by incubation with enhanced chemiluminescence (ECl)
substrate followed by exposure of the membrane to autoradiography
film.
Wound healing cell migration assays
Cells were plated on a 60-mm culture dish with 90%
confluence and an injury line with a width of 2 mm was made by
scraping across the cell monolayer with a yellow tip. After
floating cell debris was removed by washing with PBS, cell
migration was monitored under a phase-contrast microscope and
photographed.
Cell invasion assays
FaDu and SNU-1041 cells were cultured for 24 h after
the transfection of podoplanin siRNA or PDPN vector in growth
medium containing 10% FBS. The following procedures are described
in a previous study (18). The
cells were counted by taking photomicrographs at a magnification,
×100. Cells in five different fields of each well were counted with
two wells per treatment. The mean values were obtained from three
replicate experiments and were subjected to a t-test.
Statistical analysis
The SPSS software (ver. 14; SPSS, Chicago, Il, USA)
was used to perform statistical analyses. Univariate analysis of
podoplanin expression and clinicopathological parameters was
performed using the Fisher’s exact test. Significant variables in
the univariate analysis were included in a multinomial logistic
regression test (multivariate analysis). The Kaplan-Meier method
(assessed by log-rank test) and Cox regression model were used for
univariate and multivariate overall and disease-specific survival
analyses. A P-value <0.05 was considered to indicate statistical
significance.
Results
Upregulation of podoplanin expression in
HNSCC tissues and cell lines
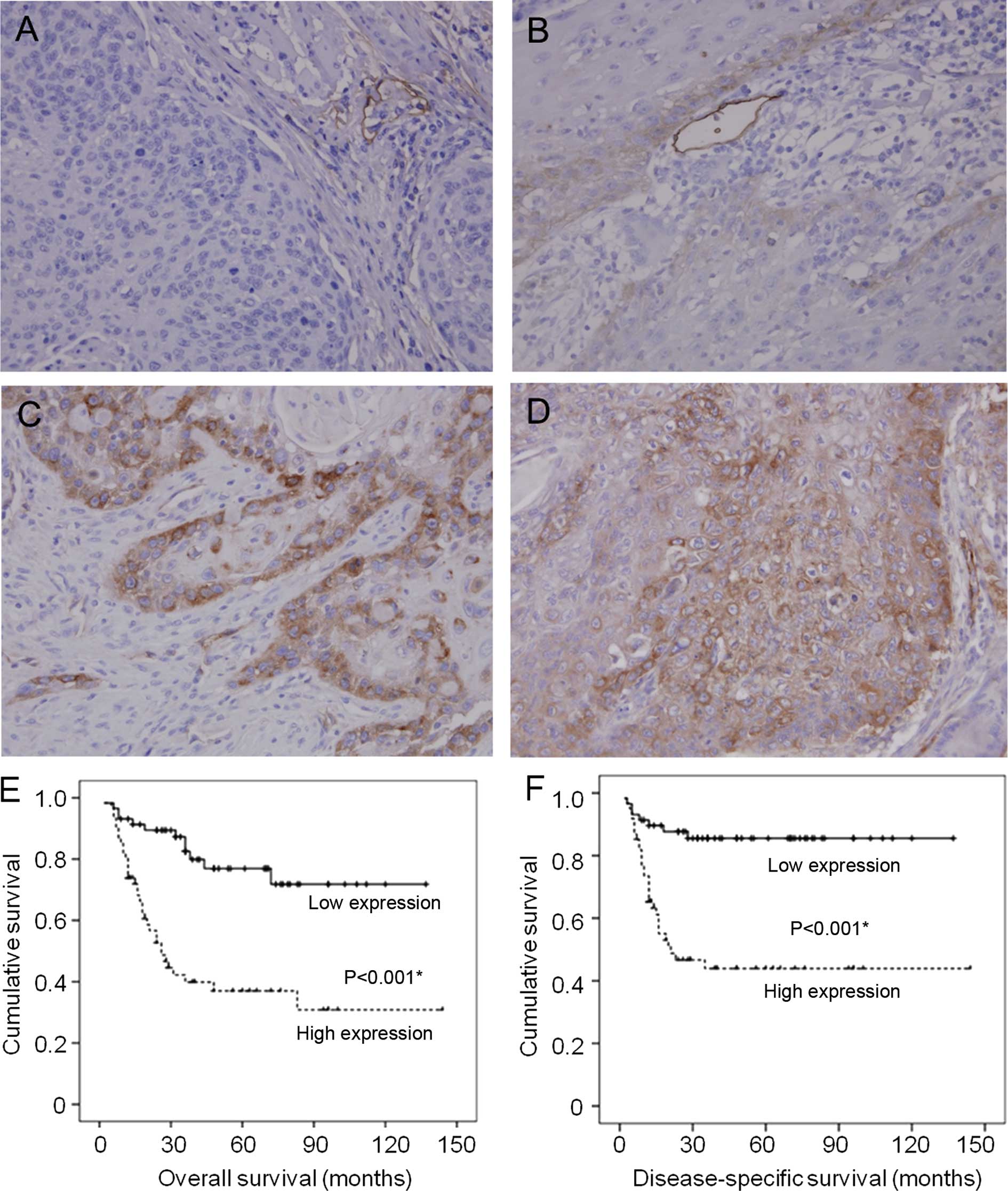
As expected, podoplanin was highly expressed in the
endothelial cells of lymphatic vessels, but was not detectable in
the endothelial cells of blood vessels. In histologically normal
squamous epithelium adjacent to the tumors, podoplanin expression
was either not detectable or extremely low in some basal cells. In
primary HNSCC, podoplanin expression was generally heterogeneous
and differentially increased (Fig.
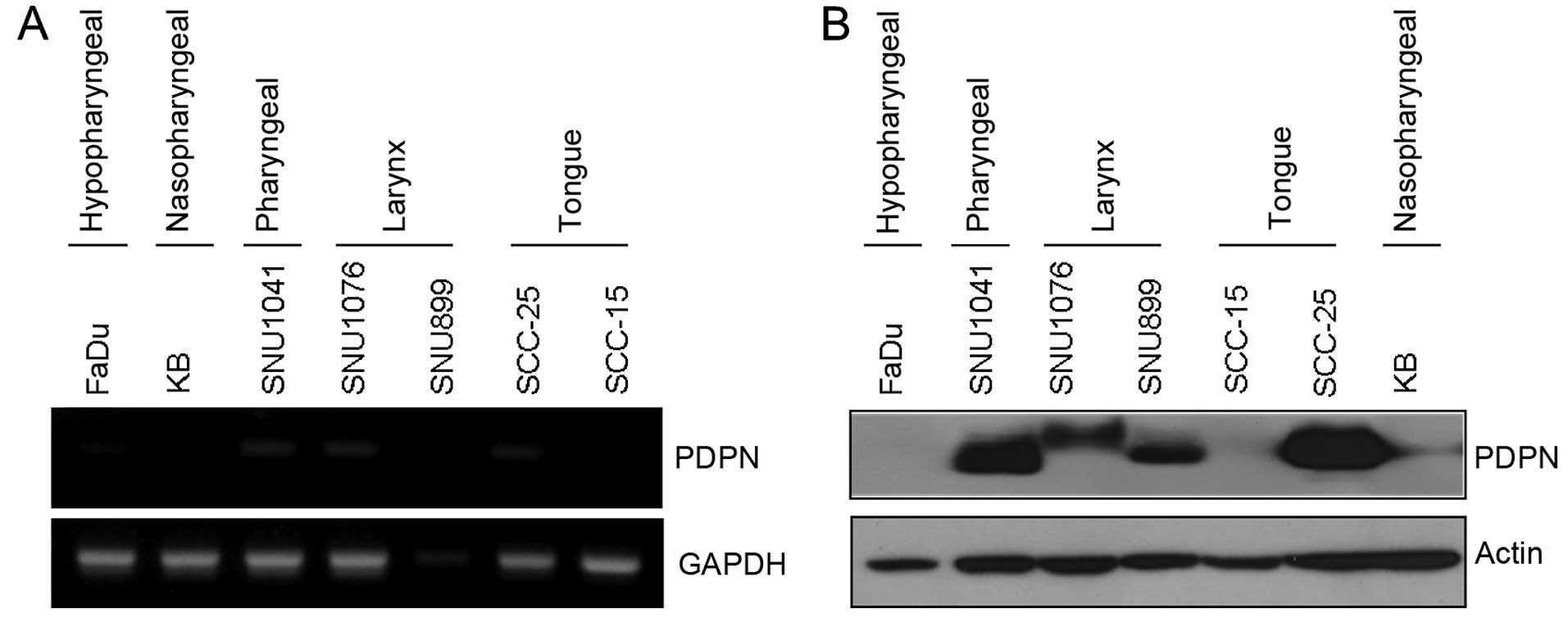
1). Podoplanin transcript and translational levels were
expressed differentially in the HNSCC cell lines (Fig. 2).
Relationships of podoplanin expression
with clinicopathological features in patients with HNSCC
Immunohistochemical staining images are shown in
Fig. 1. Of the 119 cases, 26
(21.8%) showed no podoplanin expression, 32 (26.9%) had weak, 37
(31.1%) moderate and 24 (20.2%) strong expression. For statistical
purposes, we divided the cases into two groups; those with scores
(IRS) ≤6 (the median value) were considered to have low podoplanin
expression, whereas those with scores >6 were considered to have
high expression. Thus, podoplanin expression was low in 58 (48.7%)
cases and high in 61 (51.3%).
Relationships between the degree of podoplanin
expression and the clinicopathological features of the 119 HNSCC
patients are shown in Table I.
There was a statistically significant correlation between high
podoplanin expression and the presence of lymph node metastasis,
advanced AJCC stage, and poor histological grade. In the
multivariate analysis, high podoplanin expression was significantly
associated with ~3- and 5-fold increases in the presence of
positive lymph node metastasis and poor histological grade,
respectively (P<0.05; Table
II).
 | Table IAssociations between podoplanin
expression and the clinicopathological features of the patients
with HNSCC. |
Table I
Associations between podoplanin
expression and the clinicopathological features of the patients
with HNSCC.
| Variables | No. of
patients | Podoplanin
expression
| P-value |
|---|
| Low | High |
|---|
| Age (years) |
| <65 | 63 | 28 | 35 | 0.361 |
| ≥65 | 56 | 30 | 26 | |
| Gender |
| Male | 105 | 49 | 56 | 0.262 |
| Female | 14 | 9 | 5 | |
| T stage |
| I+II | 66 | 36 | 30 | 0.197 |
| III+IV | 53 | 22 | 31 | |
| lymph node
metastasis |
| No | 60 | 38 | 22 | 0.002a |
| yes | 59 | 20 | 39 | |
| AJCC stage |
| I+II | 41 | 27 | 14 | 0.008a |
| III+IV | 78 | 31 | 47 | |
| Histological
grade |
| Well | 33 | 23 | 10 | 0.007a |
| Moderate | 56 | 26 | 30 | |
| Poor | 30 | 19 | 21 | |
| Primary site | | | | 0.465 |
| Oral cavity | 44 | 24 | 20 | |
| Oropharynx | 17 | 10 | 7 | |
| Hypopharynx | 10 | 4 | 6 | |
| Larynx | 48 | 20 | 28 | |
 | Table IIMultinomial logistic regression for
the associations of podoplanin expression with lymph node
metastasis, AJCC stage and histological grade. |
Table II
Multinomial logistic regression for
the associations of podoplanin expression with lymph node
metastasis, AJCC stage and histological grade.
| Factor | β | P-value | Exp(β) | 95% CI |
|---|
| Positive lymph node
metastasis | 1.153 | 0.031a | 3.168 | (1.108–9.059) |
| Advanced AJCC
stage | 0.005 | 0.993 | 1.005 | (0.326–3.098) |
| Poor histological
grade | 1.604 | 0.006a | 4.972 | (1.581–15.630) |
Correlation of podoplanin expression and
the overall survival and disease-specific survival rates of the
HNSCC patients
High podoplanin expression had a marked impact on
the overall (P<0.001) and disease-specific survival rate
(P<0.001; Fig. 1E and F). The
5-year overall and disease-specific survival rates were 77 and 86%,
respectively, in patients with low podoplanin expression, while 37
and 44%, respectively, in those with high podoplanin
expression.
Cox proportional hazards regression analysis was
performed to determine whether the effect of podoplanin expression
on the disease-specific survival rate was dependent on other known
risk factors. Poor histological grade and high podoplanin
expression were independent significant factors for worse
disease-specific survival rates (P<0.05; Table III). The disease-specific death
risk in HNSCC patients with high podoplanin expression was ~3-fold
higher than in those with a lower podoplanin expression (Table III).
 | Table IIIMultivariate Cox regression analysis
of disease-specific death events in the HNSCC patients (n=119). |
Table III
Multivariate Cox regression analysis
of disease-specific death events in the HNSCC patients (n=119).
| Parameters | Risk ratio
(RR) | 95% CI | P-value |
|---|
| Age (years) | 1.195 | 0.598–2.388 | 0.614 |
| Gender | 0.421 | 0.122–1.449 | 0.170 |
| Advanced T
stage | 0.607 | 0.284–1.293 | 0.196 |
| Lymph node
metastasis | 2.293 | 0.952–5.520 | 0.064 |
| Advanced AJCC
stage | 4.438 | 0.993–19.831 | 0.051 |
| Poor histological
grade | 17.027 | 2.220–130.574 | 0.006a |
| High podoplanin
expression | 2.981 | 1.320–6.732 | 0.009a |
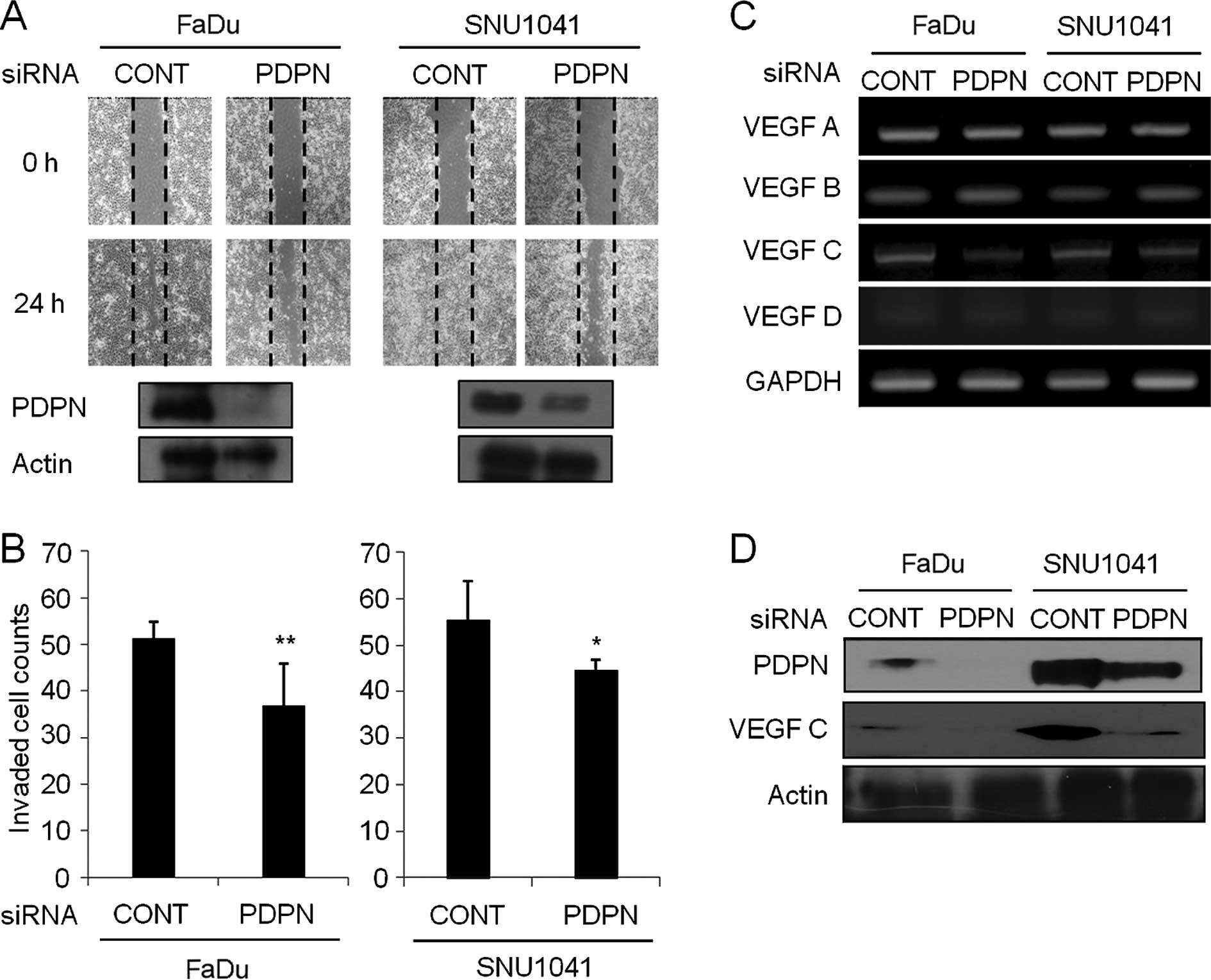
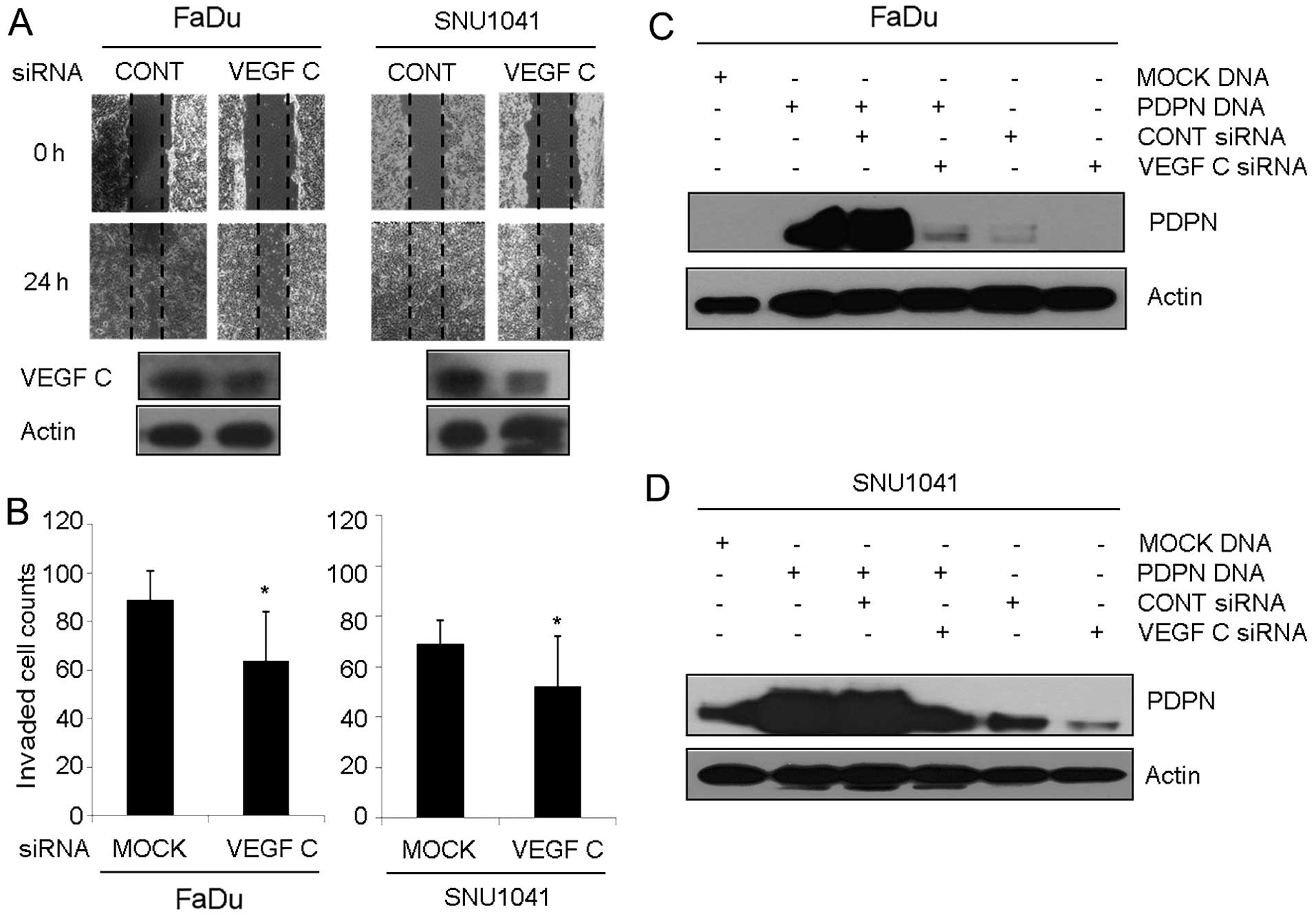
Silencing of podoplanin suppresses wound
healing cell migration and invasion in HNSCC
After cells were transfected with the podoplanin
siRNA for 24 h, total protein was extracted, and western blot
analysis was performed. There were various degrees of podoplanin
protein suppression, depending on the cell types and there was no
change in the cellular morphology of cells that were treated with
podoplanin siRNA (data not shown). As shown in Fig. 3A, control siRNA-expressing FaDu or
SNU-1041 cells repaired the wounded area rapidly at 24 h.
Meanwhile, treatment with podoplanin siRNA in FaDu and SNU-1041
cells inhibited wound repair significantly at 24 h, respectively.
In the Transwell invasion assay, the percentages of the invasive
cells in the podoplanin siRNA-expressing FaDu and SNU-1041 were
decreased to 72 and 80.7% of that in the control siRNA-expressing
FaDu and SNU-1041 cells, respectively (Fig. 3B). In addition, inhibition of
podoplanin led to suppression of the VEGF-C transcript level
(Fig. 3C) and protein expression
(Fig. 3D) in the FaDu and SNU-1041
cells, respectively. Other VEGF family such as VEGF-A, -B and -D
were not altered in the podoplanin siRNA-expressing cells when
compared to levels in the control cells (Fig. 3C).
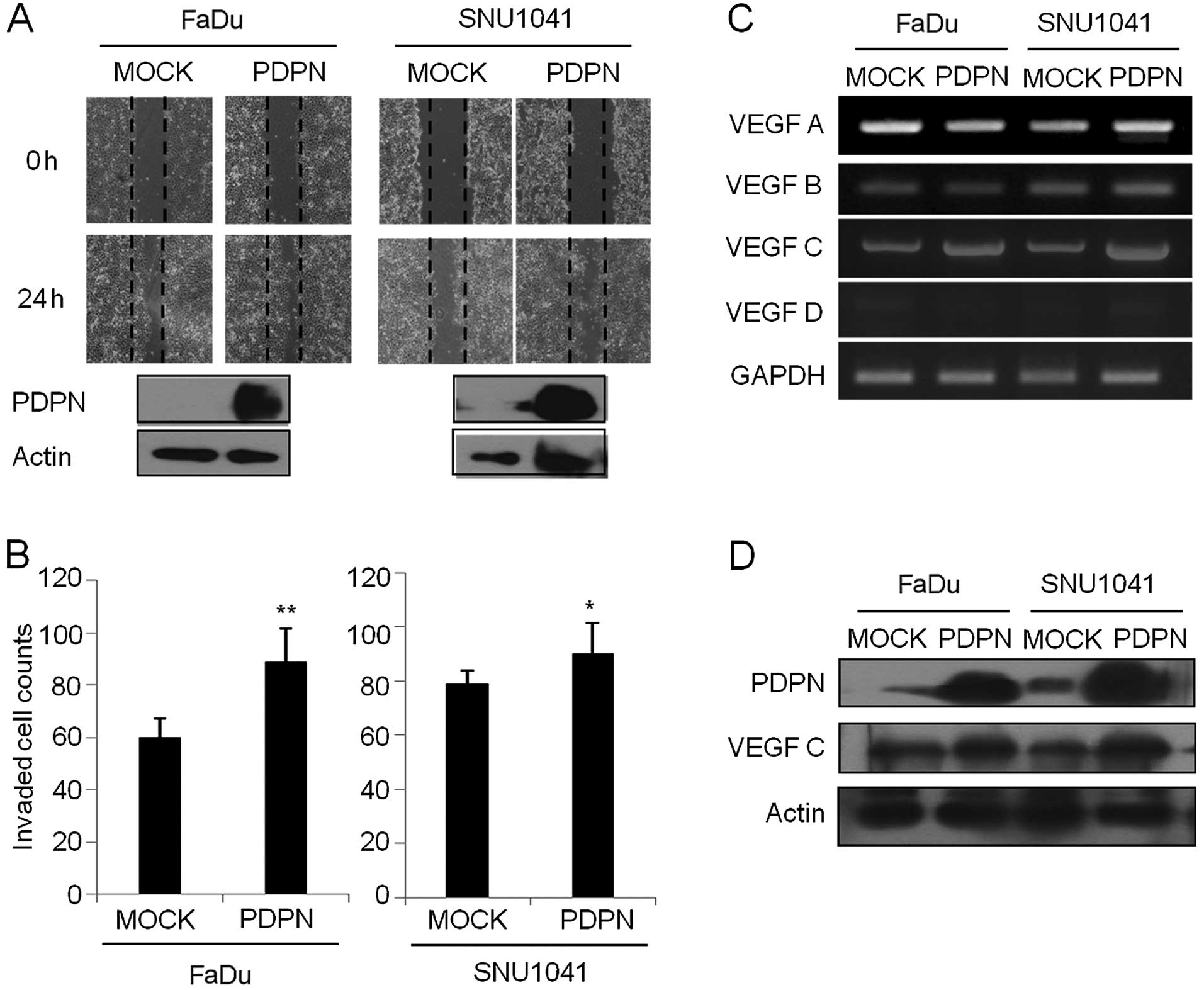
Overexpression of podoplanin induces
wound healing cell migration and invasion in the HNSCC cells
As shown in Fig. 4A,
mock vector-expressing FaDu or SNU-1041 cells slightly repaired the
wounded area at 24 h. However, PDPN vector-overexpressing FaDu and
SNU-1041 cells induced wound repair rapidly at 24 h, respectively.
In a Transwell invasion assay, the percentages of invasive cells in
the podoplanin-expressing FaDu and SNU-1041 were increased to 148.3
and 113.9% of that in the mock vector-expressing FaDu and SNU-1041
cells, respectively (Fig. 4B).
Furthermore, overexpression of podoplanin led to an increase in the
VEGF-C transcript level (Fig. 4C)
and protein expression (Fig. 4D) in
the FaDu and SNU-1041 cells, respectively. Other VEGF family such
as VEGF-A, -B and -D were not altered in the
podoplanin-overexpressing cells compared to that in the mock cells
(Fig. 4C). These results suggest
that podoplanin regulates cell wound healing activity and
invasiveness through interaction of VEGF-C in HNSCC cells.
Podoplanin regulates the metastatic
process through interaction of VEGF-C in the HNSCC cells
To determine whether podoplanin modulates HNSCC cell
metastasis, we examined cell wound healing migration and a
Transwell invasion assay in VEGF-C-suppressed cells transfected
with VEGF-C siRNA. We confirmed that VEGF-C protein expression was
suppressed in the VEGF-C siRNA-expressing FaDu and SNU-1041 cells
compared with levels in the control siRNA-expressing FaDu and
SNU-1041 cells, respectively (Fig.
5A). Control siRNA-expressing FaDu and SNU-1041 cells repaired
the wound area rapidly at 24 h, while the VEGF-C siRNA-expressing
FaDu and SNU-1041 cells suppressed the wound repair significantly
at 24 h, respectively (Fig. 5A). In
addition, the percentage of invasive cells in the VEGF-C
siRNA-expressing FaDu and SNU-1041 were decreased to 71.9 and 75.4%
of that in the control siRNA-expressing FaDu and SNU-1041 cells,
respectively (Fig. 5B).
Additionally, inhibition of VEGF-C led to suppression of podoplanin
expression in the FaDu (Fig. 5C)
and SNU-1041 (Fig. 5D) cells,
respectively.
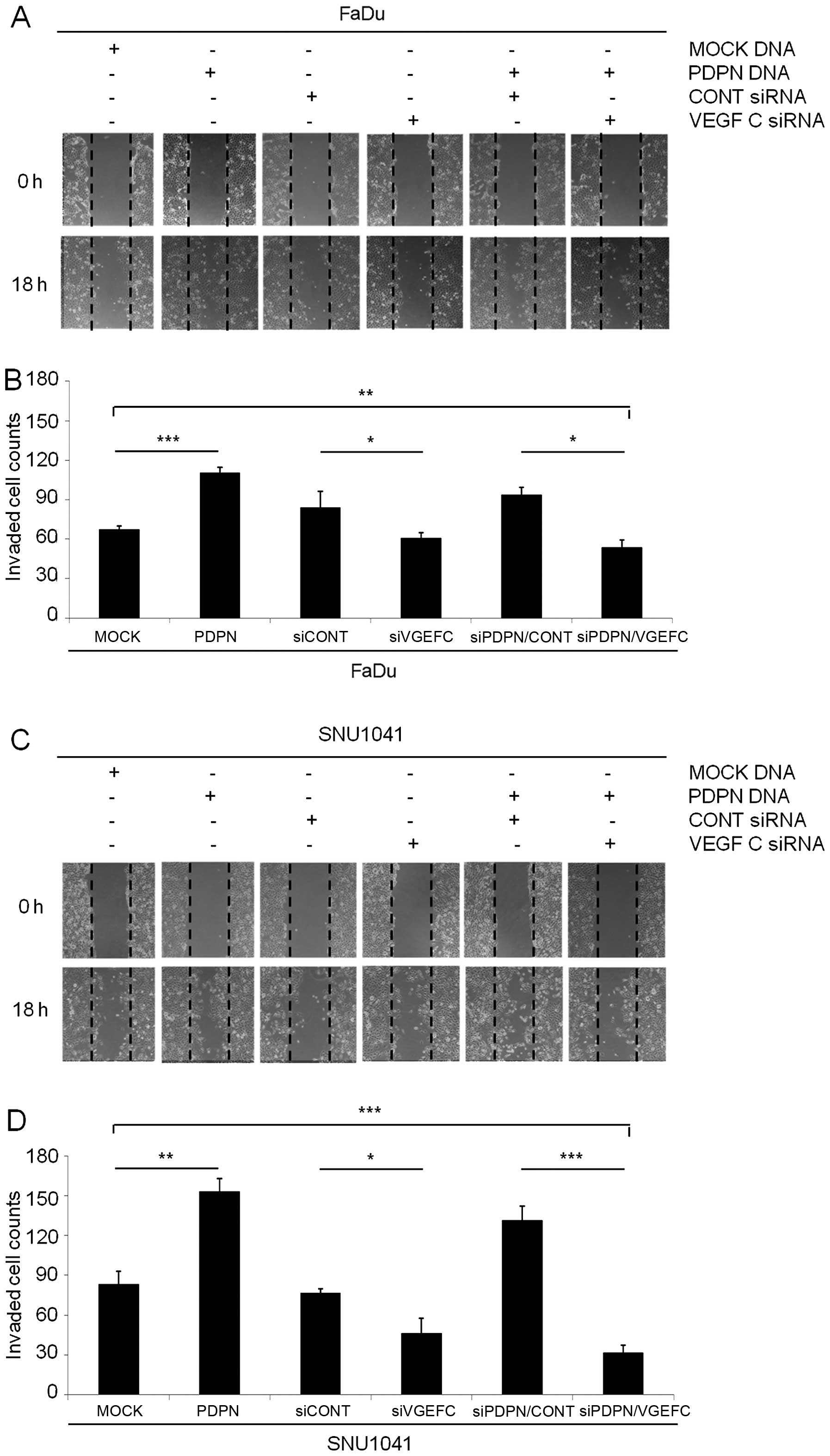
Next, to determine a correlation between podoplanin
and VEGF-C, we examined the cell wound healing migration and
invasion assays in both podoplanin overexpression vector and VEGF-C
siRNA-cotransfected FaDu and SNU-1041 cells, respectively. The
podoplanin-overexpressing FaDu and SNU-1041 cells rapidly repaired
the wound area compared with this rate in the mock cells,
respectively (Fig. 6A and C). In
contrast, both podoplanin vector and VEGF-C siRNA-coexpressing FaDu
or SNU-1041 cells inhibited wound repair compared with the
podoplanin vector-expressing FaDu and SNU-1041 cells (Fig. 6A and C). In addition, the
percentages of invasive cells in the podoplanin vector-expressing
FaDu and SNU-1041 were increased to 163.9 and 184% of that in the
mock vector-expressing FaDu and SNU-1041 cells (Fig. 6B and D). In the cell wound healing
assay, the percentages of invasive cells in both the podoplanin
vector and VEGF-C siRNA-coexpressing FaDu and SNU-1041 cells were
significantly decreased to 48.8 and 20.7% of these percentage in
the podoplanin vector-expressing FaDu and SNU-1041 cells,
respectively (Fig. 6B and D). These
results indicated that podoplanin regulates metastasis via
interaction of VEGF-C in HNSCC cells.
Discussion
Podoplanin is a 38-kDa type I transmembrane
glycoprotein consisting of 162 amino acids that is expressed almost
exclusively in lymphatic endothelial cells (19). In other normal human tissues,
podoplanin is expressed by various cells, including kidney
podocytes, lung alveolar type I cells, basal epithelial
keratinocytes of the skin, cervix and esophagus and myoepithelial
cells of the breast gland and salivary glands (20). Due to its specific expression in
lymphatic endothelium, podoplanin has been widely used as a
specific marker of lymphatic endothelial cells and
lymphangiogenesis under physiological and pathological conditions.
Thus, over the past few decades, podoplanin has been used to assess
tumoral lymphatic vessel density in many types of cancer and
correlates with lymph node metastasis and poor prognosis (14,21–23).
Podoplanin expression is upregulated in a number of
human cancer types, including squamous cell carcinomas of the oral
cavity, lung, cervix, esophagus and the skin, in dysgerminomas of
the ovary and granulosa cell tumors, in mesothelioma and in many
tumors of the central nervous system (9–15,24–27).
Recent experimental results have also demonstrated that podoplanin
mediates a pathway leading to collective cell migration and
invasion in vivo and in vitro (27). Some investigators have demonstrated
a possible relationship between podoplanin expression and tumor
invasion or metastasis (11,12,14,21).
In oral leukoplakia, high podoplanin expression has been associated
with an increased risk of progression to invasive cancer,
suggesting that podoplanin may be a powerful biomarker of the risk
of oral cancer development in patients with oral leukoplakia
(15). This evidence supports the
importance of podoplanin in oral tumorigenesis and malignant
transformation. Recent studies have also shown that high levels of
podoplanin expression in primary oral SCC are associated with
advanced T stage, lymphatic spread to the cervical region and a
poor clinical outcome (14,28). Tong et al demonstrated a
significant association between the level of podoplanin expression
and depth of tumor invasion, lymph node status, lymphatic vessel
density, progressive TNM stage and disease-free survival time in
patients with esophageal SCC (29).
Thus, they suggested that podoplanin may be useful as an
independent prognostic factor for esophageal SCC. In the present
study, we also found that podoplanin was strongly associated with
lymph node metastasis and poor histological grade in HNSCC
patients. More importantly, high levels of podoplanin expression
were associated with decreased overall and disease-specific
survival rate in patients with HNSCC.
However, in contrast to these findings, Dumoff et
al (11) reported a strong
correlation between low expression of podoplanin and both lymphatic
invasion and nodal metastasis in uterine cervical cancer. Rodrigo
et al (30) reported that
laryngeal cancer patients with high podoplanin expression showed
prolonged disease-specific survival. These findings suggest that
the biological function of podoplanin may vary according to cancer
type. Considering that only 48 laryngeal carcinoma cases were
investigated in the present study, high podoplanin expression was
also significantly correlated with poor survival outcome, in
contrast to the results of Rodrigo et al (30). This may have been since most of the
laryngeal carcinoma patients had advanced-stage disease and
underwent total laryngectomies, and the case number of laryngeal
carcinoma patients was small in our study population. The
prognostic effects of podoplanin expression should be investigated
in larger populations of laryngeal carcinoma patients.
Our results suggest that podoplanin plays a role in
lymphatic spread and tumor progression in HNSCC, while the exact
mechanism is unclear. It has been demonstrated that podoplanin
contributes to tumor invasion by binding ERM proteins, such as
ezrin, radixin and moesin to activate RhoA, resulting in
epithelial-mesenchymal transition (EMT) (31). However, Wicki et al (27) suggested that podoplanin induced
collective tumor cell invasion in the absence of a cadherin switch
or EMT. Podoplanin is also involved in the aggregation of platelets
and may therefore enhance the arrest, extravasation and subsequent
metastasis of podoplanin-expressing tumor cells circulating in the
blood (32). In the present study,
we found that silencing of podoplanin led to suppression of VEGF-C
expression resulting in inhibition of cell wound healing repair and
invasion (Fig. 3) and that
overexpression of podoplanin led to elevation of VEGF-C expression
resulting in induction of metastatic processes such as wound
healing migration and invasion in HNSCC cells (Fig. 4). As shown in Fig. 5 and 6, treatment of VEGF-C siRNA and
overexpression of podoplanin suppressed wound repair activity and
invasiveness in both FaDu and SNU-1041 cells. Based on these
results, we suggest that podoplanin regulates metastatic processes
such as migration and invasion via interaction of VEGF-C in HNSCC
cells. Research is ongoing to elucidate the detailed molecular
mechanisms underlying the relationship between podoplanin and
VEGF-C in the metastatic process of HNSCC.
In conclusion, our findings suggest that high
podoplanin expression is associated with aggressive tumor behavior,
poor prognosis of head and neck cancer and regulation of metastasis
through VEGF-C modulation in HNSCC. Podoplanin may be a potential
regulator of VEGF-C in HNSCC metastasis and may be used as a
prognostic biomarker for HNSCC patients.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MSIP) (nos. 2013R1A2A2A01015281 and 2012R1A1A2005393)
and partially supported by a grant from the National R&D
Program for Cancer Control, Ministry of Health, Welfare and Family
Affairs, Republic of Korea (0720560).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
3
|
Capote A, Escorial V, Muñoz-Guerra MF,
Rodríguez-Campo FJ, Gamallo C and Naval L: Elective neck dissection
in early-stage oral squamous cell carcinoma - does it influence
recurrence and survival? Head Neck. 29:3–11. 2007. View Article : Google Scholar
|
|
4
|
Kowalski LP, Bagietto R, Lara JR, Santos
RL, Silva JF Jr and Magrin J: Prognostic significance of the
distribution of neck node metastasis from oral carcinoma. Head
Neck. 22:207–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lothaire P, de Azambuja E, Dequanter D,
Lalami Y, Sotiriou C, Andry G, Castro G Jr and Awada A: Molecular
markers of head and neck squamous cell carcinoma: Promising signs
in need of prospective evaluation. Head Neck. 28:256–269. 2006.
View Article : Google Scholar
|
|
6
|
Lopes MA, Nikitakis NG, Reynolds MA, Ord
RA and Sauk J Jr: Biomarkers predictive of lymph node metastases in
oral squamous cell carcinoma. J Oral Maxillofac Surg. 60:142–147;
discussion 147–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schacht V, Ramirez MI, Hong YK, Hirakawa
S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, et
al: T1alpha/podoplanin deficiency disrupts normal lymphatic
vasculature formation and causes lymphedema. EMBO J. 22:3546–3556.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durchdewald M, Guinea-Viniegra J, Haag D,
Riehl A, Lichter P, Hahn M, Wagner EF, Angel P and Hess J:
Podoplanin is a novel fos target gene in skin carcinogenesis.
Cancer Res. 68:6877–6883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimada Y, Ishii G, Nagai K, Atsumi N,
Fujii S, Yamada A, Yamane Y, Hishida T, Nishimura M, Yoshida J, et
al: Expression of podoplanin, CD44, and p63 in squamous cell
carcinoma of the lung. Cancer Sci. 100:2054–2059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dumoff KL, Chu CS, Harris EE, Holtz D, Xu
X, Zhang PJ and Acs G: Low podoplanin expression in pretreatment
biopsy material predicts poor prognosis in advanced-stage squamous
cell carcinoma of the uterine cervix treated by primary radiation.
Mod Pathol. 19:708–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuang WY, Yeh CJ, Wu YC, Chao YK, Liu YH,
Tseng CK, Chang HK, Liu HP and Hsueh C: Tumor cell expression of
podoplanin correlates with nodal metastasis in esophageal squamous
cell carcinoma. Histol Histopathol. 24:1021–1027. 2009.PubMed/NCBI
|
|
13
|
Rahadiani N, Ikeda J, Makino T, Tian T,
Qiu Y, Mamat S, Wang Y, Doki Y, Aozasa K and Morii E: Tumorigenic
role of podoplanin in esophageal squamous-cell carcinoma. Ann Surg
Oncol. 17:1311–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawaguchi H, El-Naggar AK,
Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim E, Hong
WK, Lippman SM, et al: Podoplanin: A novel marker for oral cancer
risk in patients with oral premalignancy. J Clin Oncol. 26:354–360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byrd DR, Compton CC, Fritz AG, Greene FL
and Trotti A: AJCC Cancer Staging Manual. 7th edition. Springer;
New York, NY: 2009
|
|
17
|
Koo BS, Kim JM, Seo ST, Yoon YH, Kwon KR,
Kim SH, Kwon HW, Bae WJ and Lim YC: Upregulation of HGF and c-MET
is associated with subclinical central lymph node metastasis in
papillary thyroid microcarcinoma. Ann Surg Oncol. 21:2310–2317.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang YH, Ji NY, Han SR, Lee CI, Kim JW,
Yeom YI, Kim YH, Chun HK, Kim JW, Chung JW, et al: ESM-1 regulates
cell growth and metastatic process through activation of NF-κB in
colorectal cancer. Cell Signal. 24:1940–1949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schoppmann SF, Birner P, Studer P and
Breiteneder-Geleff S: Lymphatic microvessel density and
lymphovascular invasion assessed by anti-podoplanin immunostaining
in human breast cancer. Anticancer Res. 21:2351–2355.
2001.PubMed/NCBI
|
|
20
|
Raica M, Cimpean AM and Ribatti D: The
role of podoplanin in tumor progression and metastasis. Anticancer
Res. 28:2997–3006. 2008.PubMed/NCBI
|
|
21
|
Tomita N, Matsumoto T, Hayashi T, Arakawa
A, Sonoue H, Kajiyama Y and Tsurumaru M: Lymphatic invasion
according to D2-40 immunostaining is a strong predictor of nodal
metastasis in superficial squamous cell carcinoma of the esophagus:
Algorithm for risk of nodal metastasis based on lymphatic invasion.
Pathol Int. 58:282–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schacht V, Dadras SS, Johnson LA, Jackson
DG, Hong YK and Detmar M: Up-regulation of the lymphatic marker
podoplanin, a mucin-type transmembrane glycoprotein, in human
squamous cell carcinomas and germ cell tumors. Am J Pathol.
166:913–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erovic BM, Neuchrist C, Kandutsch S,
Woegerbauer M and Pammer J: CD9 expression on lymphatic vessels in
head and neck mucosa. Mod Pathol. 16:1028–1034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo
T and Osawa M: Enhanced expression of Aggrus (T1alpha/podoplanin),
a platelet-aggregation-inducing factor in lung squamous cell
carcinoma. Tumour Biol. 26:195–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura N and Kimura I: Podoplanin as a
marker for mesothelioma. Pathol Int. 55:83–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibahara J, Kashima T, Kikuchi Y, Kunita
A and Fukayama M: Podoplanin is expressed in subsets of tumors of
the central nervous system. Virchows Arch. 448:493–499. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: Podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kreppel M, Scheer M, Drebber U, Ritter L
and Zöller JE: Impact of podoplanin expression in oral squamous
cell carcinoma: Clinical and histopathologic correlations. Virchows
Arch. 456:473–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong L, Yuan S, Feng F and Zhang H: Role
of podoplanin expression in esophageal squamous cell carcinoma: A
retrospective study. Dis Esophagus. 25:72–80. 2012. View Article : Google Scholar
|
|
30
|
Rodrigo JP, García-Carracedo D, González
MV, Mancebo G, Fresno MF and García-Pedrero J: Podoplanin
expression in the development and progression of laryngeal squamous
cell carcinomas. Mol Cancer. 9:482010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martín-Villar E, Megías D, Castel S,
Yurrita MM, Vilaró S and Quintanilla M: Podoplanin binds ERM
proteins to activate RhoA and promote epithelial-mesenchymal
transition. J Cell Sci. 119:4541–4553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cueni LN, Hegyi I, Shin JW, Albinger-Hegyi
A, Gruber S, Kunstfeld R, Moch H and Detmar M: Tumor
lymphangiogenesis and metastasis to lymph nodes induced by cancer
cell expression of podoplanin. Am J Pathol. 177:1004–1016. 2010.
View Article : Google Scholar : PubMed/NCBI
|