Introduction
Cervical cancer is the second most common type of
cancer in females, with ~530,000 new cases each year and more than
274,000 mortalities worldwide (1).
Since cervical cancer is considered a radiosensitive tumor,
radiation therapy is the most widely used treatment modality in
patients with cervical cancer, particularly for patients at an
advanced stage or those who cannot be cured surgically (2). Therefore, cellular radiosensitivity
has been a long-term research focus, for it is critical for
therapeutic outcomes (3). However,
despite progress in radiation technology, local recurrence still
occurs in a large proportion of patients due to radioresistance
(4). Thus, it is urgent to uncover
new targets to enhance the cellular radiosensitivity in cervical
cancer.
The endoplasmic reticulum (ER) is an essential
subcellular compartment responsible for the synthesis and folding
of proteins (5). Different
physiological and pathological perturbations interfere with protein
folding processes in the ER lumen, leading to accumulation of
unfolded or misfolded proteins, a cellular condition termed ER
stress (5). ER stress triggers the
unfolded protein response (UPR), a transcriptional induction
pathway aimed at restoring normal ER functioning (6). If UPR is insufficient to recover ER
homeostasis, cells undergo apoptosis (5). The UPR is mediated by three ER stress
receptors: protein kinase RNA-like ER kinase (PERK),
inositol-requiring protein-1 (IRE1) and activating transcription
factor-6 (ATF6), representing three branches of the UPR (7). In the IRE1 branch of UPR, activation
of IRE1 leads to cleavage of a 26-nucleotide intron from the XBP1
mRNA. The spliced XBP1 mRNA encodes a stable, active transcription
factor that binds to many UPR target genes (8,9).
Recent studies have shown that ER stress induces apoptosis and
sensitizes tumor cells to ionizing radiation and chemotherapy
(10–12), suggesting that ER stress has the
potential as a novel target to improve cancer radiotherapy and
chemotherapy. In addition, radiotherapy reportedly induces ER
stress in cells (13).
Classical key tumor suppressor p53 is involved in
the response to a variety of cellular stresses (14). A number of stresses, including
damage to chromosomal DNA incurred by ionizing irradiation and
exposure to ultraviolet light may activate a p53-mediated
growth-suppressive response. Approximately half of all human
cancers harbor mutations in p53, which leads to loss of tumor
suppressor function and/or gain of new oncogenic activity (15). Loss of p53 function can contribute
not only to aggressive tumor behavior, but also to therapeutic
resistance (14).
Small-molecule RITA (reactivation of p53 and
induction of tumor cell apoptosis) has been shown to bind p53
directly, inducing a conformational change that prevents its
interaction with several inhibitory proteins including MDM2, Parc
and iASPP (16). RITA reportedly
induces p53-dependent apoptosis in tumor cells expressing wild-type
p53 (wtp53), as well as in tumor cells expressing mutant or null
p53 (15). A recent study
demonstrated that RITA increases radiosensitivity in head and neck
squamous cell carcinoma cells expressing mutant p53 (mtp53)
(17).
Commonly used human cervical cancer cell lines,
C-33A and HT-3, contain p53 codon mutations and are human
papillomavirus (HPV)-negative. In contrast, five HPV-positive
cervical cancer cell lines (HeLa S-3, Caski, SiHa, C-4I and ME-180)
contain wild-type p53 (18,19). It has been reported that RITA may
protect p53 from HPV-E6-mediated degradation in HPV-positive,
wtp53-expressing cervical cancer cells (20). However, the effect of RITA on mutant
p53 (mtp53)-expressing cervical cancer cells has not been
studied.
In the present study, we explored for the first time
the effects and the underlying mechanisms of RITA in regards to
radiosensitivity and ER stress in mtp53-expressing cervical cancer
cells.
Materials and methods
Cell culture and radiosensitivity
assay
Human cervix cancer cell lines C-33A (HTB-31) and
HT-3 (HTB-32) were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco's
modified Eagle medium (DMEM; Life Technologies, Carlsbad, CA, USA)
containing 10% heat-inactivated FBS (Life Technologies) and 100
U/ml penicillin-streptomycin (Sigma-Aldrich, Beijing, China) in an
incubator with a humidified atmosphere of 95% air and 5%
CO2 at 37°C. Cells were plated at 4×103
cells/well into 96-well plates and cultured for 3 h for the cells
to adhere to the plates. Then, the cells were cultured with or
without 1 µM of RITA for 24 h before irradiation, which was
performed at a dose rate of 200 cGy/min for the time required to
generate dose curves of 2, 4, 6 and 8 Gy with linear accelerator
Clinac 2100C (Varian Medical Systems, Palo Alto, CA, USA) operating
at 6 MV. Corresponding controls were sham-irradiated.
Colony-forming assays were performed immediately after irradiation
by plating the cells into 6-well culture dishes. After 17 days, the
colonies were fixed with 6.0% glutaraldehyde, stained with 0.5%
crystal violet and counted. Survival curves were fitted with
GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA).
Each experiment was repeated three times in duplicates.
Stable lentiviral transduction
The p53 (sc-29435-V) and the IRE1α (sc-40705-V)
shRNA lentiviral particles purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA) contain expression constructs encoding
target-specific shRNA designed to specifically knock down human p53
and IRE1α gene expression, respectively. The control shRNA
lentiviral particles (sc-108080; Santa Cruz Biotechnology) contain
a scrambled shRNA sequence that will not lead to specific
degradation of any cellular mRNA. Lentiviral transduction was
performed, and pools of stable transductants were generated via
selection with puromycin (6 µg/ml; Sigma-Aldrich) according
to the manufacturer's instructions (Santa Cruz Biotechnology).
Western blot analysis
C-33A and HT-3 cells was lysed with a hypotonic
buffer containing 2% Nonidet-P and a protease inhibitor cocktail
(Sigma-Aldrich) by sonication three times for 3 sec on ice. The
supernatant obtained after centrifugation at 2,000 × g for 15 min
at 4°C was used for protein concentration determination by the
Coomassie blue method. Equal amounts of proteins for each sample
were separated on 10% SDS-polyacrylamide gel and blotted onto a
polyvinylidene difluoride microporous membrane (Millipore,
Billerica, MA, USA). Membranes were incubated for 1 h with a
1:1,000 dilution of rabbit anti-human polyclonal p53 (FL-393;
sc-6243) antibody (Santa Cruz Biotechnology), rabbit anti-human
polyclonal IRE1α (H-190; sc-20790) antibody (Santa Cruz
Biotechnology) or mouse anti-human monoclonal
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (6C5; sc-32233)
antibody (Santa Cruz Biotechnology) and then washed and revealed
using bovine anti-rabbit (sc-2370) or anti-mouse (sc-2371)
secondary antibody (1:5,000, 1 h). Peroxidase was revealed with a
GE Healthcare ECL kit (Shanghai, China). Three independent
experiments were performed.
Real-time quantitative reverse
transcription PCR
RNA was prepared from the cells using TRIzol
reagent. The cDNAs were synthesized using SuperScript II reverse
transcriptase (Life Technologies). Real-time quantitative PCR was
performed on an Abi-Prism 7700 Sequence Detection System, using the
fluorescent dye SYBR-Green Master Mix (Applied Biosystems, Beijing,
China) as described by the manufacturer. The primers used were as
follows: for IRE1α, 5′-GCCACCCTGCAAGAGTATGT-3′ (forward) and
5′-ATGTTGAGGGAGTGGAGGTG-3′ (reverse); for spliced XBP1 mRNA
(XBP1spl), 5′-TGCTGAGTCCGCAGCAGGTG-3′ (forward) and
5′-GCTGGCAGGCTCTGGGGAAG-3′ (reverse); for GAPDH,
5′-GACTCATGACCACAGTCCATG C-3′ (forward) and
5′-AGAGGCAGGGATGATGTTCTG-3′ (reverse). Relative quantification of
the level of IRE1α mRNA or spliced XBP1 mRNA was determined using
the 2−ΔΔCt method and normalized against that of GAPDH
in the same sample (19). Each
experiment was repeated three times in duplicates.
Luciferase assay
Cells were transfected with a commercially available
human IRE1α promoter/luciferase reporter (S720185; SwitchGear
Genomics, Shanghai, China) using Lipofectamine 2000 transfection
reagent (Life Technologies) and then cultured for 24 h. Luciferase
assays were performed with the LightSwitch luciferase assay kit
(LS010; SwitchGear Genomics) according to the manufacturer's
instructions. Each experiment was repeated three times in
duplicates.
Transcriptional pulse-chase assay
A Click-iT Nascent RNA Capture kit (C-10365; Life
Technologies) was used to determine the stability of IRE1α mRNA
according to the manufacturer's instructions. Briefly, C-33A and
HT-3 cells were labeled with 0.2 mM ethynyl uridine (EU) and
incubated at 37°C for 4 h. The cells were then allowed to recover
in EU-free medium for 0, 1, 2 or 4 h, respectively. Total RNA was
extracted and 5 µg of total RNA was mixed with Click-iT
reaction cocktail. Immediately, the reaction buffer additive 1 was
added, followed by reaction buffer additive 2 exactly 3 min after
addition of the first additive and the reaction was carried out for
30 min at room temperature. Following incubation, the 'clicked' RNA
was re-purified by ammonium acetate precipitation and captured by
streptavidin magnetic beads. The captured RNA was in-bead converted
to cDNA using SuperScript III reverse transcriptase (Life
Technologies). Then, the level of the IRE1α mRNA or the spliced
XBP1 mRNA was determined with real-time quantitative reverse
transcription PCR as described above.
Cell apoptosis assay
Cells were plated at 4×103 cells/well
into 96-well plates and cultured for 3 h for the cells to adhere to
the plates. Then, the cells were cultured with or without 1
µM of RITA for 24 h before sham-irradiation or irradiation
at a dose of 6 Gy with linear accelerator Clinac 2100C operating at
6 MV. Cell apoptosis was measured 24 h after irradiation with a
microplate reader-based TiterTACS in situ apoptosis
detection kit (4822-96-K; R&D systems, Minneapolis, MN, USA)
according to the manufacturer's instructions (21). Each experiment was repeated three
times in duplicates.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 10.0 (SPSS Inc., Chicago, IL, USA). All data values are
expressed as means ± SD. Comparison of means between two groups was
performed with Student's t-tests. Comparisons of means among
multiple groups were performed with one-way ANOVA followed by post
hoc pairwise comparisons using Tukey's tests. A two-tailed
P<0.05 was considered statistically significant in the present
study.
Results
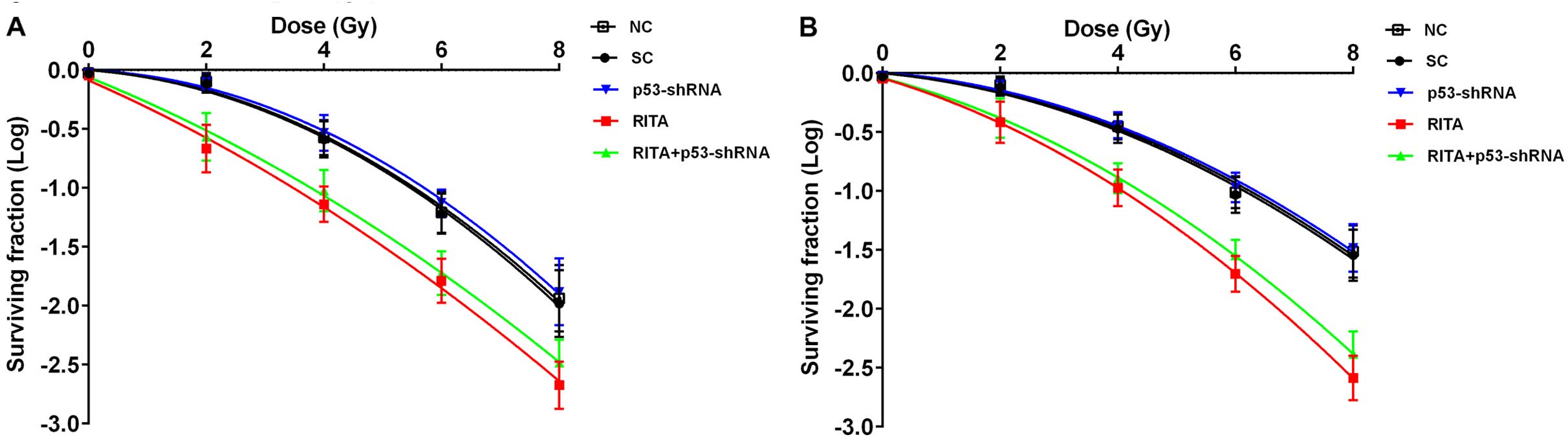
Effects of RITA on survival and apoptosis
of mtp53-expressing cervical cancer cells under irradiation
To explore the effects of RITA on the
radiosensitivity of mtp53-expressing cervical cancer cells, we
treated human C-33A and HT-3 cervical cancer cells with 1 µM
of RITA for 24 h before irradiation at 2, 4, 6 and 8 Gy. As shown
in Fig. 1, RITA markedly decreased
cell survival under irradiation compared with the controls,
indicating that RITA may increase the radiosensitivity of C-33A and
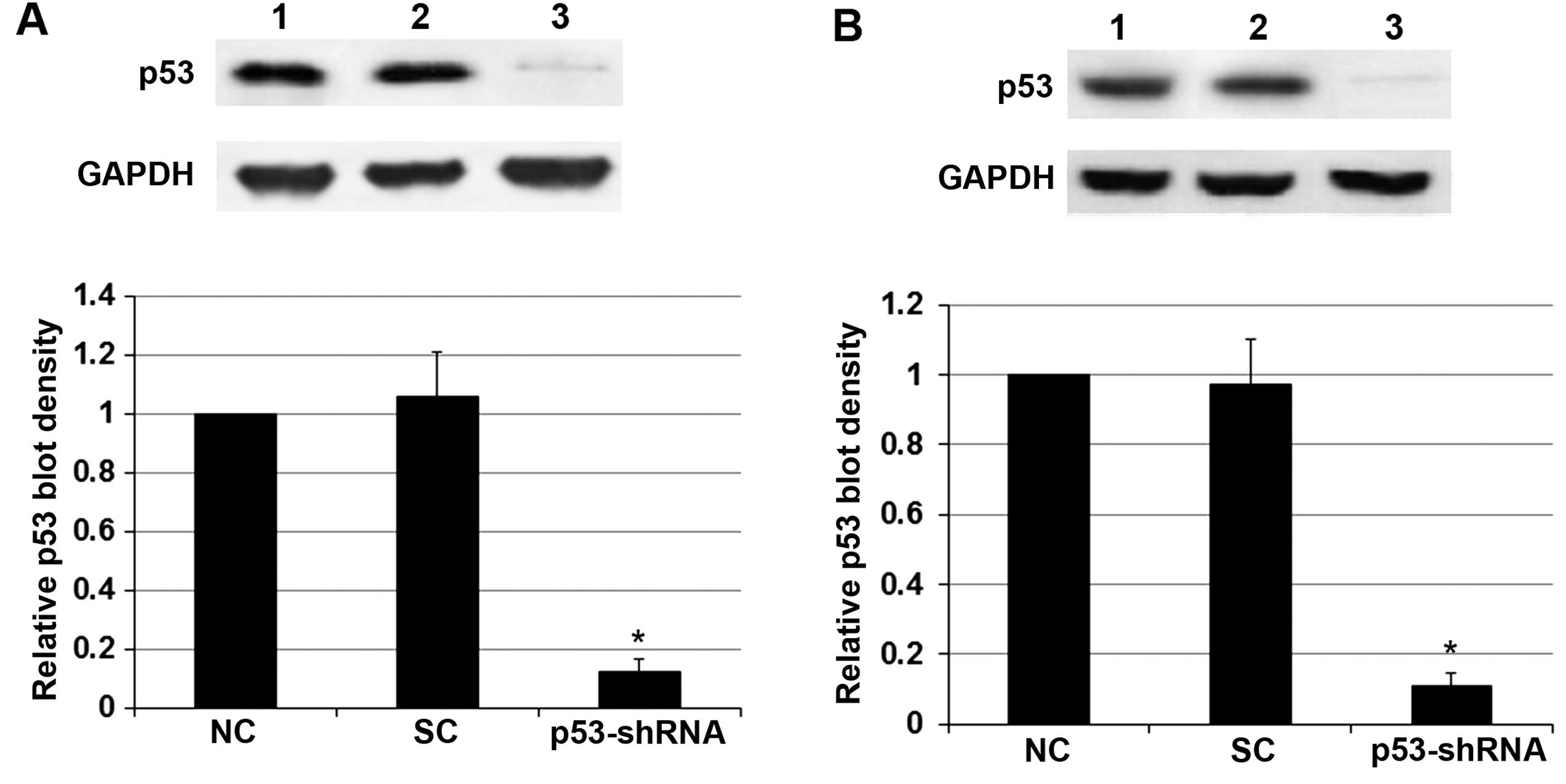
HT-3 cells. To determine the potential role of p53 in the
radiosensitivity-enhancing effect of RITA, we knocked down p53 in
both cell lines, even though the cells only express mtp53. As shown
in Fig. 2, stable transduction of
lentiviral p53-shRNA knocked down the endogenous p53 expression by
over 85% in both the C-33A and HT-3 cells. Obviously, knockdown of
p53 did not show any significant effect on RITA-induced
radiosensitivity in the cells (Fig.
1).
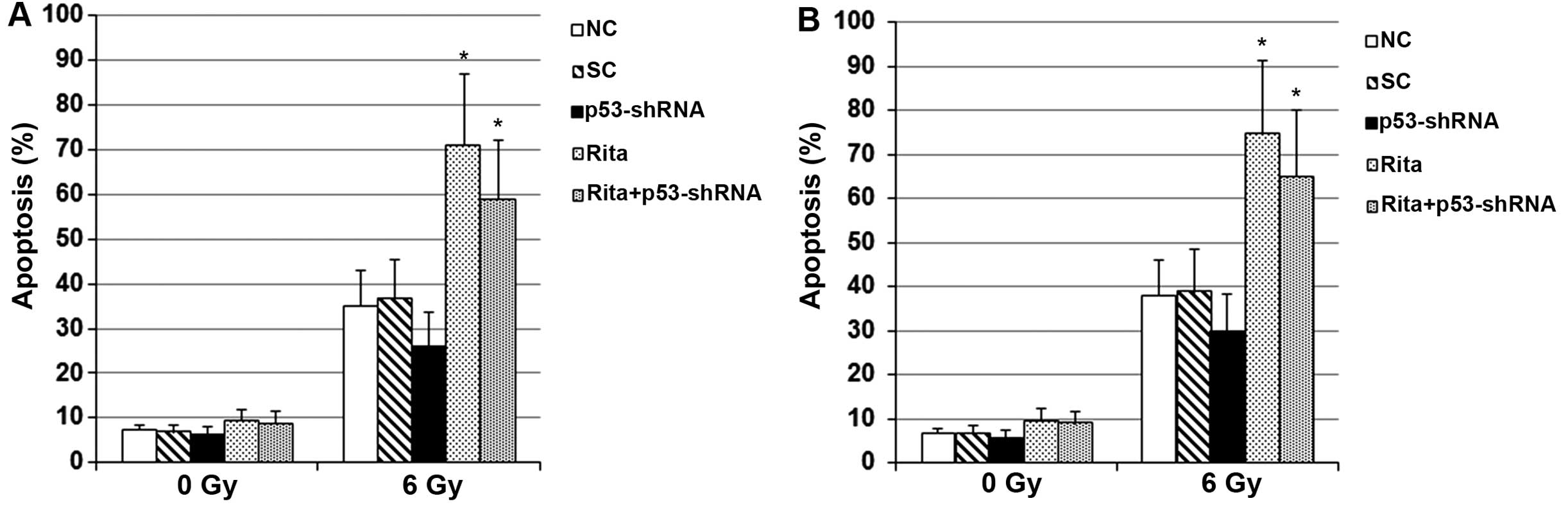
To explore the effects of RITA on
irradiation-induced apoptosis in mtp53-expressing cervical cancer
cells, we next measured apoptosis in the C-33A and HT-3 cells with
or without RITA treatment under sham 0 or 6 Gy of irradiation. As
shown in Fig. 3, compared with the
controls, RITA treatment at 1 µM for 24 h showed no
significant effect on apoptosis in the C-33A and HT-3 cells under
sham irradiation. The RITA IC50 value for C-33A and HT-3
cells under sham irradiation was calculated to be 15.6 and 18.3
µM, respectively. However, in the cells under irradiation,
RITA treatment at 1 µM for 24 h increased the apoptosis rate
by ~2-fold in both cell lines compared with the controls (Fig. 3). Knockdown of p53 did not
significantly alter the apoptotic effect of RITA on the cells under
irradiation (Fig. 3).
Collectively, the findings suggested that RITA
induced radiosensitivity and irradiation-induced apoptosis in
mtp53-expressing cervical cancer cells by a p53-independent
mechanism.
Effects of RITA/IRE1α signaling on
survival and apoptosis of mtp53-expressing cervical cancer cells
under irradiation
As shown in Fig. 4,
compared with the controls, RITA showed no significant effect on
the protein level of IRE1α in the C-33A and HT-3 cells under sham
irradiation. However, in cells under irradiation, RITA increased
the protein level of IRE1α by 2.5-fold in the C-33A cells and by
~3.6-fold in the HT-3 cells, respectively (Fig. 4). Notably, compared with the
controls under sham irradiation, the irradiation treatment
increased the protein level of IRE1α by ~3-fold in the C-33A and by
~2-fold in the HT-3 cells, respectively (Fig. 4). Knockdown of p53 did not
significantly alter the effect of RITA on the expression of IRE1α
in the presence or absence of irradiation (Fig. 4).
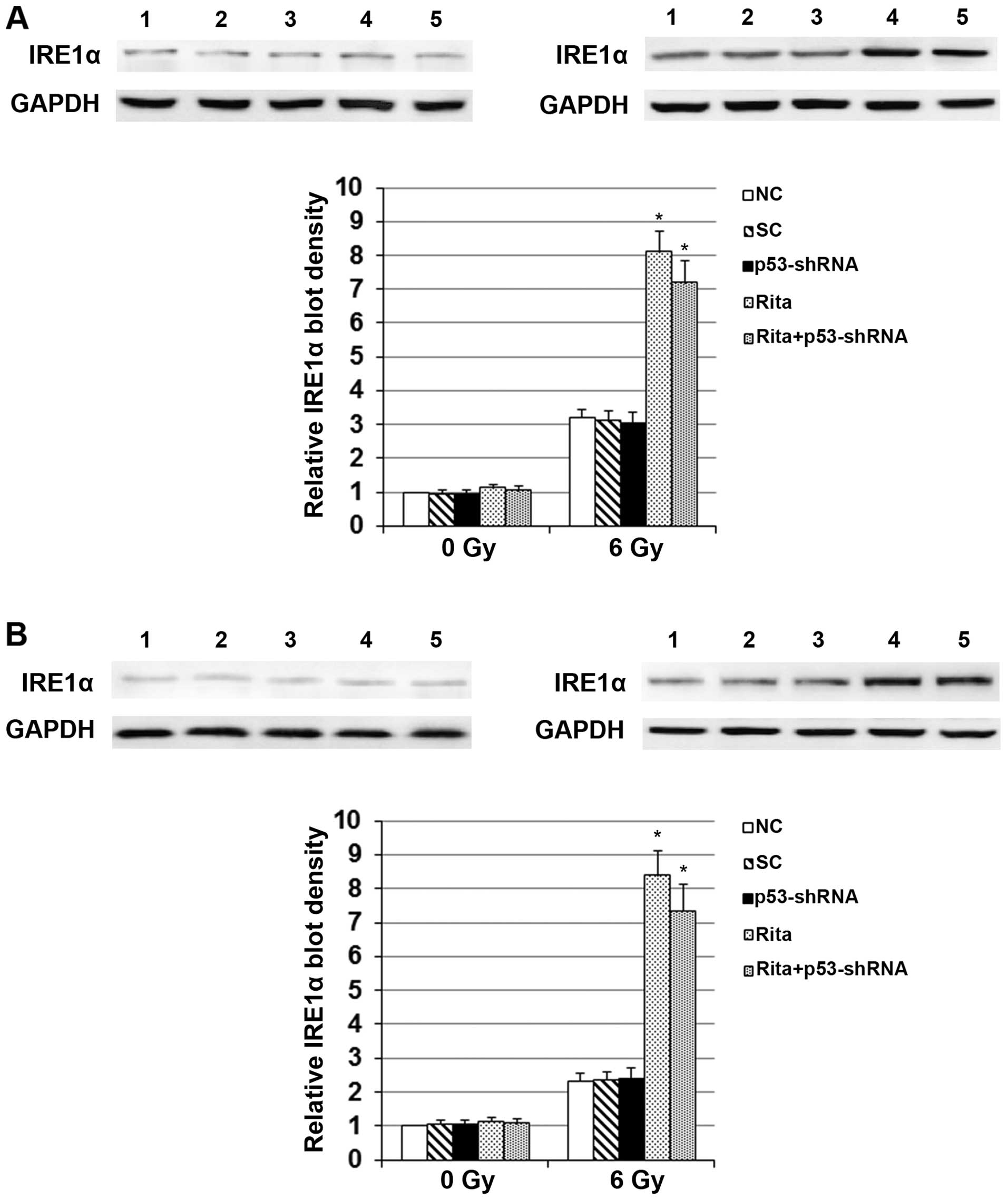 | Figure 4Effects of RITA on the protein levels
of IRE1α in cervical cancer cells in the presence or absence of
irradiation. (A) C-33A and (B) HT-3 cells with or without stable
transduction of lentiviral p53-shRNA were cultured with or without
1 µM of RITA for 24 h before irradiation at 0 (sham
irradiation) (left panel) or 6 Gy (right panel) with a linear
accelerator operating at 200 cGy/min and 6 MV. The protein levels
of IRE1α were determined by western blot analyses 24 h after
irradiation in normal control cells (NC, lane 1), cells stably
transduced with scramble control shRNA (SC, lane 2), cells stably
transduced with lentiviral p53-shRNA (lane 3), cells treated with 1
µM of RITA for 24 h before irradiation (lane 4) and cells
stably transduced with lentiviral p53-shRNA and treated with 1
µM of RITA for 24 h before irradiation (RITA+p53-shRNA, lane
5). GAPDH blotting was used as a loading control. Density of the
western blot analysis was measured by densitometry, and the density
of the IRE1α blot was normalized against that of the GAPDH blot in
the same sample to obtain a relative blot density, which was
expressed as a fold-change to that of NC at 0 Gy (designated as 1).
*P<0.05 vs. controls (NC and SC). NC, normal control
cells; SC, cells stably transduced with scramble control shRNA;
p53-shRNA, cells stably transduced with lentiviral p53-shRNA; RITA,
cells treated with 1 µM of RITA for 24 h before irradiation;
RITA+p53-shRNA, cells stably transduced with lentiviral p53-shRNA
and treated with 1 µM of RITA for 24 h before irradiation;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
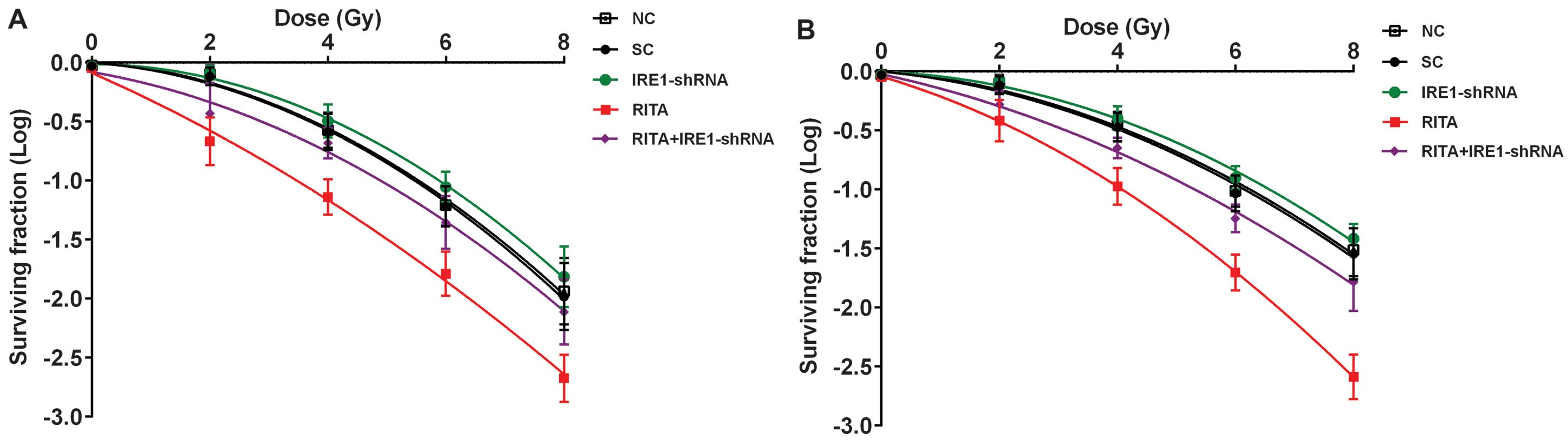
To determine the potential role of IRE1α in the
radio-sensitivity-enhancing effect of RITA on mtp53-expressing
cervical cancer cells, we knocked down IRE1α in the C-33A and HT-3
cells. As shown in Fig. 5, even
under irradiation, stable transduction of lentiviral IRE1α-shRNA
knocked down the endogenous IRE1α expression by ~80% in both the
C-33A and HT-3 cells; the addition of RITA treatment (1 µM
for 24 h) only partially restored the expression of IRE1α by 28% in
the C-33A cells and by 37% in the HT-3 cells, respectively
(Fig. 5). As shown in Fig. 6, RITA markedly decreased cell
survival under irradiation compared with the controls, which was
largely reversed by the knockdown of IRE1α. Notably, knockdown of
IRE1α itself did not significantly improve cell survival compared
with the controls (Fig. 6).
Similarly, in the cell apoptosis assays, whereas knockdown of IRE1α
reversed the apoptotic effect of RITA in the C-33A and HT-3 cells
under irradiation, it did not significantly decrease cell apoptosis
compared with the controls. Collectively, the findings suggested
that RITA enhanced radiosensitivity and irradiation-induced
apoptosis in the mtp53-expressing cervical cancer cells largely
through inducing the expression of IRE1α.
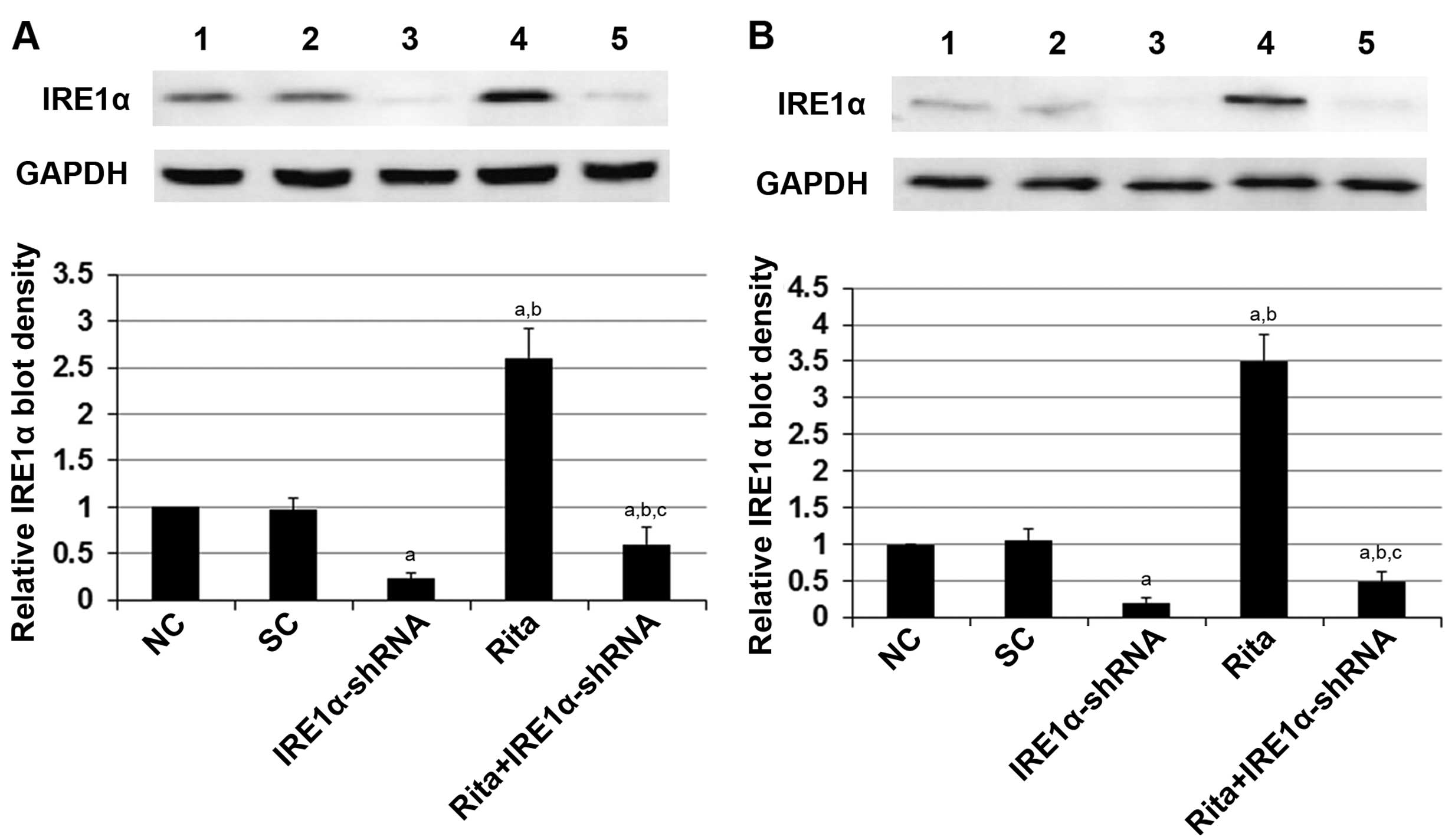 | Figure 5Knockdown of IRE1α in cervical cancer
cells. (A) C-33A and (B) HT-3 cells stably transduced with
lentiviral IRE1α-shRNA were cultured with or without 1 µM of
RITA for 24 h before irradiation at 6 Gy with a linear accelerator
operating at 200 cGy/min and 6 MV. Twenty-four hours after
irradiation, the protein levels of IRE1α were determined by western
blot analyses 24 h after irradiation in normal control cells (NC,
lane 1), cells stably transduced with scramble control shRNA (SC,
lane 2), cells stably transduced with lentiviral IRE1α-shRNA (lane
3), cells treated with 1 µM of RITA for 24 h before
irradiation (lane 4) and cells stably transduced with lentiviral
IRE1α-shRNA and treated with 1 µM of RITA for 24 h before
irradiation (RITA+IRE1α-shRNA, lane 5). GAPDH blotting was used as
a loading control. Density of the western blot analysis was
measured by densitometry and the density of the IRE1α blot was
normalized against that of the GAPDH blot in the same sample to
obtain a relative blot density, which was expressed as a
fold-change to that of NC (designated as 1). aP<0.05
vs. controls (NC and SC); bP<0.05 vs. IRE1α-shRNA;
cP<0.05 vs. RITA. NC, normal control cells; SC, cells
stably transduced with scramble control shRNA; RITA, cells treated
with 1 µM of RITA for 24 h before irradiation;
RITA+p53-shRNA, cells stably transduced with lentiviral p53-shRNA
and treated with 1 µM of RITA for 24 h before irradiation;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
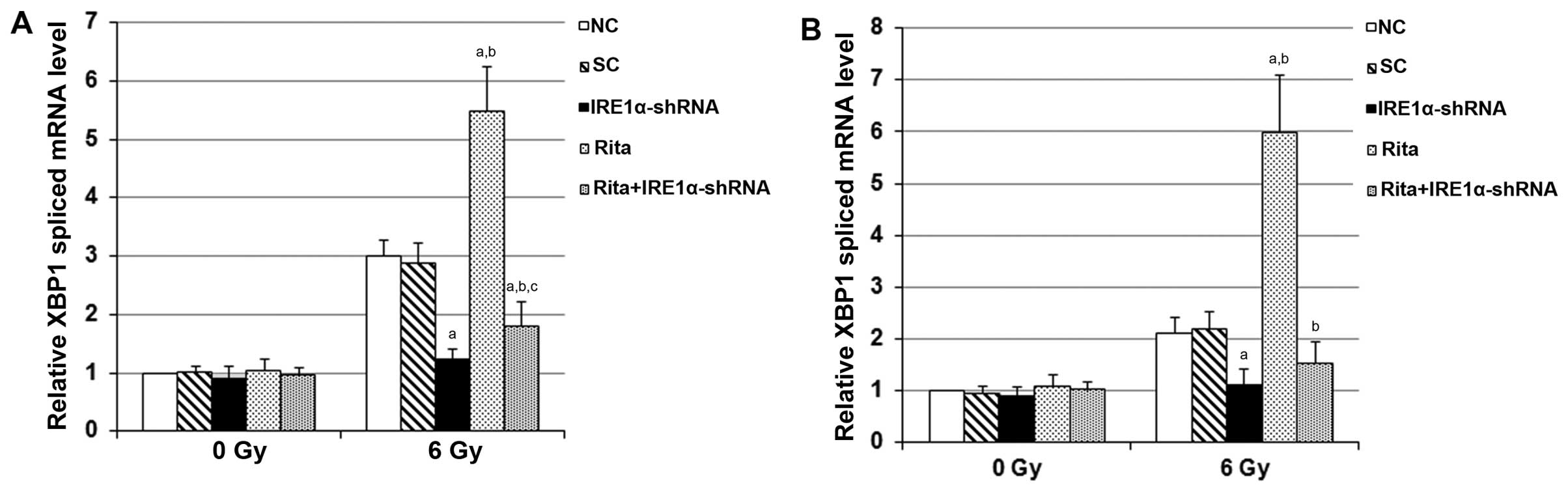
In the IRE1 branch of ER stress-triggered UPR,
activation of IRE1 leads to cleavage of a 26-nucleotide intron from
the XBP1 mRNA. The spliced XBP1 mRNA is considered to be an
important marker for ER stress, particularly for IRE1-mediated UPR
(8,9). We next examined whether RITA enhanced
ER stress in the C-33A and HT-3 cells under irradiation, using XBP1
as a marker. As shown in Fig. 8,
compared with the controls, RITA showed no significant effect on
the spliced mRNA level of XBP1 in the C-33A and HT-3 cells under
sham irradiation. However, in cells under irradiation, RITA
increased the spliced mRNA level of XBP1 by ~1.8-fold in the C-33A
cells and by ~3-fold in the HT-3 cells, respectively, which was
completely abolished by the knockdown of IRE1α (Fig. 8). The findings suggested that RITA
enhances irradiation-induced ER stress through the IRE1α/XBP1
branch and promotes apoptosis in mtp53-expressing cervical cancer
cells.
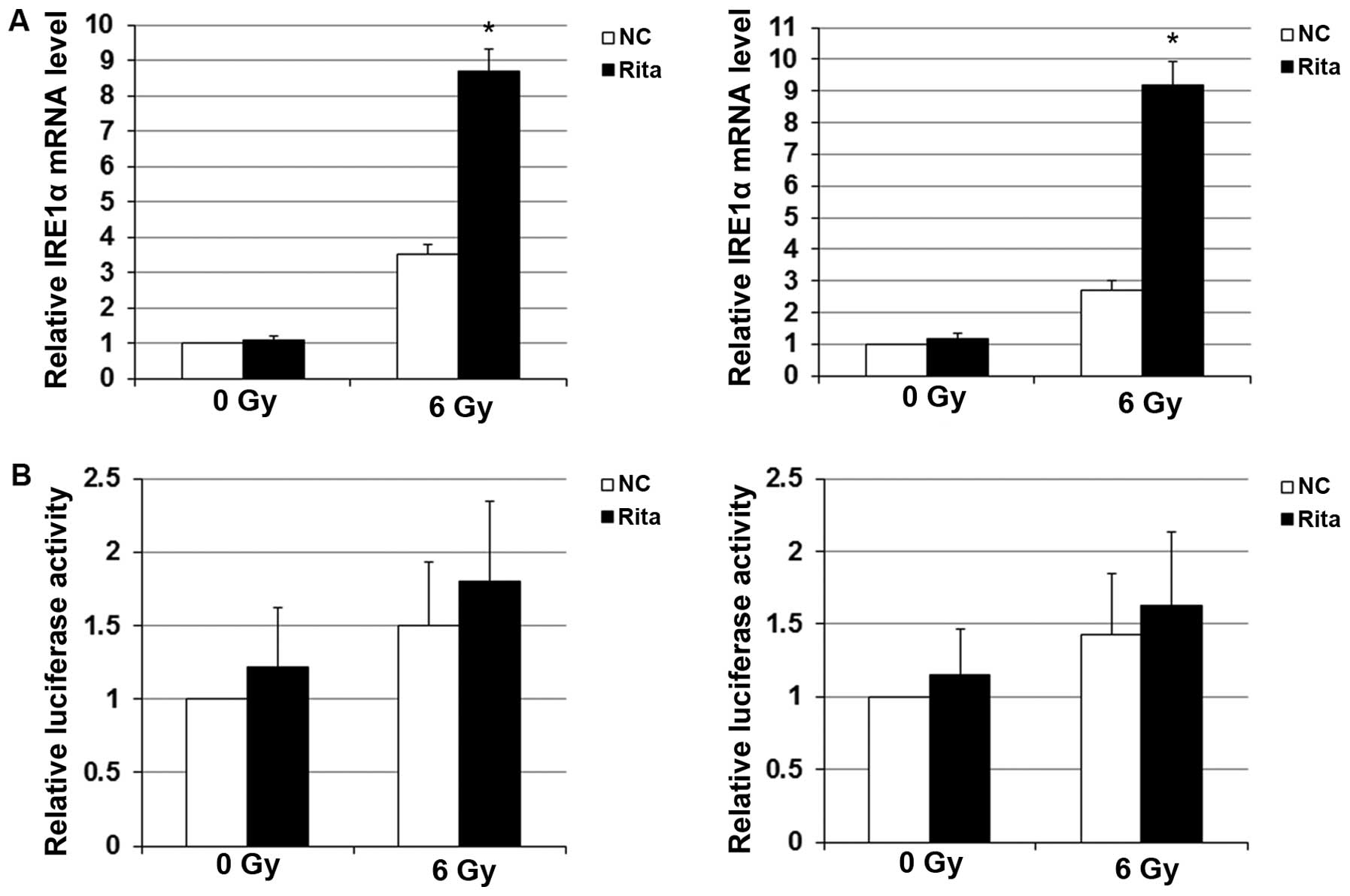
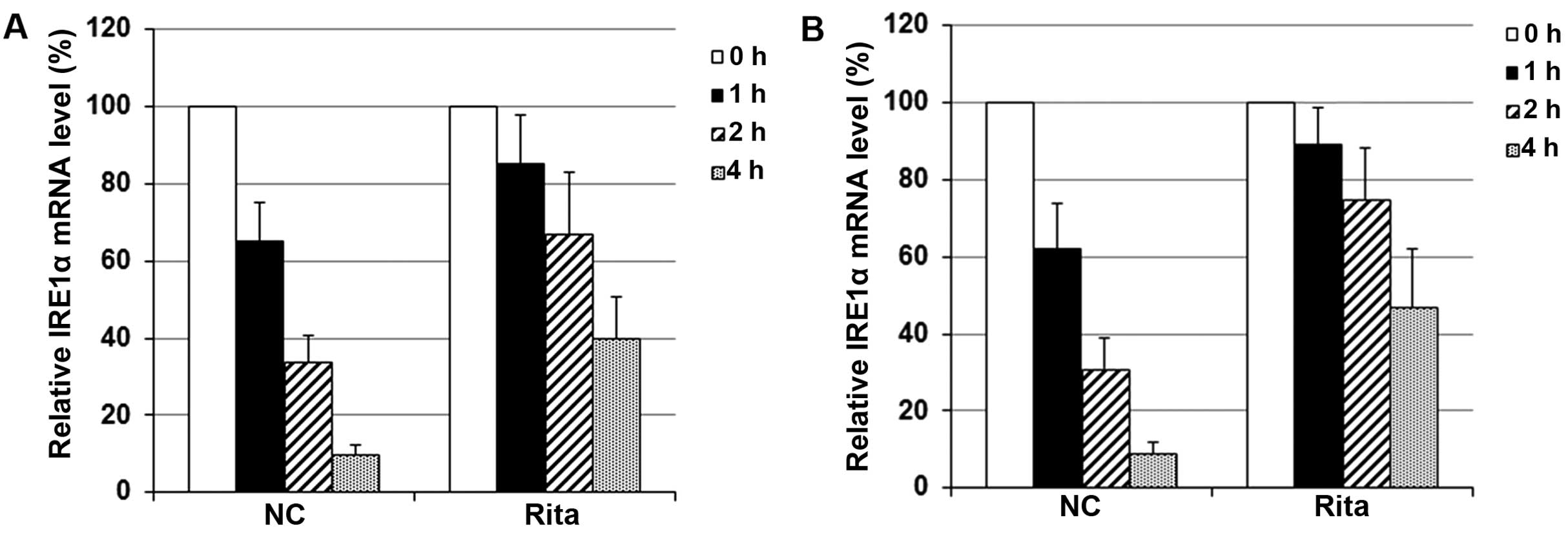
Effects of RITA on the stability of IRE1α
mRNA in mtp53-expressing cervical cancer cells under
irradiation
As shown in Fig. 9A,
compared with the controls, RITA markedly elevated the mRNA level
of IRE1α in the C-33A and HT-3 cells under irradiation, indicating
that RITA induced the expression of IRE1α at the mRNA level in
mtp53-expressing cervical cancer cells under irradiation. To test
whether RITA exerted this effect through transactivation of the
IRE1α gene promoter, we transfected the C-33A and HT-3 cells with a
human IRE1α gene promoter/luciferase reporter. As shown in Fig. 9B, luciferase reporter assays
revealed that RITA had no significant effect on the IRE1α promoter
activity in the presence or absence of irradiation treatment,
suggesting that RITA did not upregulate the IRE1α mRNA level in the
irradiated C-33A and HT-3 cells at the gene promoter/transcription
level. We next examined the effect of RITA on the stability of
IRE1α mRNA with transcriptional pulse-chase assays, using a
Click-iT Nascent RNA Capture kit (Life Technologies). Briefly,
immediately after irradiation treatment, the C-33A and HT-3 cells
were labeled with EU and incubated at 37°C for 4 h. Cells were then
allowed to recover in EU-free medium for 0, 1, 2 or 4 h,
respectively. Then, the labeled RNA was captured and subjected to
real-time reverse transcription PCR assays to determine the IRE1α
mRNA. As shown in Fig. 10, at 1 h,
the IRE1α mRNA level in the control cells dropped to 62–65% of that
at 0 h; at 4 h, the IRE1α mRNA level in the control cells dropped
to ~10% of that at 0 h. In cells treated with RITA, however, the
IRE1α mRNA level at 1 h remained above 85% of that at 0 h; at 4 h,
the IRE1α mRNA level remained above 40% of that at 0 h. The
findings suggested that in mtp53-expressing cervical cancer cells
under irradiation, RITA induced the expression of IRE1α mainly by
increasing its mRNA stability.
Discussion
In the present study, we demonstrated that RITA
enhanced irradiation-induced apoptosis in mtp53-expressing cervical
cancer cells mainly by upregulating the expression of IRE1α and
IRE1α/XBP1 signaling.
RITA has been shown to reactivate wtp53 function as
well as to rescue the function of p53 mutants in different types of
human tumor cells and induce p53-dependent apoptosis (22). It reportedly may protect p53 from
HPV-E6-mediated degradation in HPV-positive wtp53-expressing
cervical cancer cells (20).
However, few studies have explored the effect of RITA on
mtp53-expressing cervical cancer cells. Thus, we employed C-33A and
HT-3 cells, two human cervical cancer cell lines expressing mtp53,
as cell models in the present study (18,19).
At the concentration of 1 µM, RITA markedly enhanced
apoptosis in the mtp53-expressing cervical cancer cells under
irradiation, but not in those under sham irradiation. The
radiosensitivity-enhancing effect of RITA at 1 µM was
obviously p53-independent, since it was not altered by knockdown of
p53. Much higher concentrations were needed for RITA to induce
significant apoptosis in the mtp53-expressing cervical cancer cells
under sham irradiation in the present study (15.6 for C-33A and
18.3 µM for HT-3 cells, respectively). Collectively, the
findings suggest that RITA is an effective enhancer of
radiosensitivity for mtp53-expressing cervical cancer cells and
therefore if used in conjunction with radiotherapy, may be
potentially beneficial for patients with mtp53-expressing cervical
cancer. This is important since radiation therapy is the most
broadly used treatment for patients with cervical cancer,
particularly patients at an advanced stage or those who cannot be
cured surgically (2).
ER stress induced by protein misfolding is an
important mechanism in cellular stress (23). ER stress triggers the UPR to restore
normal ER functioning (6). The UPR
is mediated by three principal classes of stress sensors including
PERK, ATF6 and IRE1 (8), which
operate in parallel and use unique mechanisms of signal
transduction to orchestrate adaptation to ER stress (5,8).
However, if UPR is insufficient to reverse the ER stress or recover
ER homeostasis, the cell fate switches to apoptosis (5). Recent studies have shown that ER
stress induces apoptosis and sensitizes tumor cells to ionizing
radiation (10–12), suggesting that ER stress has the
potential as a novel target to improve cancer radiotherapy, which
reportedly induces cellular ER stress (13). In the present study, irradiation led
to irreversible ER stress in the C-33A and HT-3 cells, as indicated
by the significantly increased IRE1α/XBP1 ER stress signaling and
apoptosis in the irradiated cells compared with that in the
sham-irradiated cells. RITA markedly enhanced IRE1α/XBP1 signaling
and apoptosis in the irradiated C-33A and HT-3 cells, suggesting
that RITA enhanced apoptosis in irradiated mtp53-expressing
cervical cancer cells by enhancing irradiation-induced ER stress,
mainly through the IRE1α/XBP1 signaling pathway. Knockdown of p53
did not alter this effect of RITA, confirming that it was through a
p53-independent mechanism. Some previous studies have shown that ER
stress sensitizes or stimulates wtp53-dependent apoptosis (24,25),
while others have shown that ER stress prevents or inhibits
wtp53-dependent apoptosis (26–29).
The discrepancies can be attributed to different cell models or
treatments/stimuli used in the studies. Nevertheless, since RITA is
an established activator of p53, it may be intriguing to determine
whether and how RITA may affect radiosensitivity and apoptosis in
irradiated wtp53-expressing cervical cancer cells. We will focus on
these issues in future studies.
We found that RITA increased the expression of IRE1α
in the irradiated C-33A and HT-3 cells at both the protein and the
mRNA levels. Transcriptional pulse-chase assays immediately after
irradiation revealed that treatment with 1 µM of RITA for 24
h before irradiation significantly increased the stability of IRE1α
mRNA. This effect was manifested at the IRE1α protein level,
spliced XBP1 mRNA level and the cell apoptosis level 24 h after
irradiation, confirming RITA as an effective and efficient enhancer
of irradiation-induced ER stress/apoptosis in mtp53-expressing
cervical cancer cells. The mechanism of how RITA increases IRE1α
mRNA stability in irradiated mtp53-expressing cervical cancer cells
is still unclear and will be explored in our future studies.
Our findings suggest that RITA may be useful as an
enhancer of radiosensitivity for mtp53-expressing cancer cells.
Since approximately half of all human cancers harbor mutations in
p53 (15) and we only tested
mtp53-expressing cervical cancer cell models in the present study,
it would be beneficial to ascertain whether RITA can enhance
irradiation-induced ER stress and apoptosis in other
irradiation-sensitive cancers harboring mtp53. Moreover, as certain
chemotherapeutic agents reportedly induce tumor cell apoptosis
mainly through stimulation of ER stress, it may also be beneficial
to discover whether RITA can enhance chemotherapeutic agent-induced
ER stress/apoptosis in cancers harboring mtp53. Furthermore, PERK,
ATF6 and IRE1 represent three branches of the UPR (5,8). We
found in the present study that RITA is mainly affected through the
IRE1 branch of UPR. Whether the PERK and the ATF6 branches of UPR
could be critically involved in the potential effects of RITA on
irradiation- or chemotherapeutic agent-induced ER stress/apoptosis
in other types of mtp53-expressing cancers still needs to be
elucidated in future studies.
In conclusion, the present study provides initial
evidence that RITA upregulates the expression level of IRE1α by
increasing the stability of IRE1α mRNA in irradiated
mtp53-expressing cervical cancer cells; the effect leads to
enhanced IRE1α/XBP1 ER stress signaling and increased apoptosis in
the cells. The present study offers novel insight into the
pharmacological potential of RITA in the radiotherapy for cervical
cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372428), the
Natural Science Foundation of Hunan Province (grant no. 11JJ5080),
and the Science and Technology Plan Fund of Hunan Province (grant
nos. 2010RS4031 and 2011FJ3028), China.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
3
|
Hu Q and Hill RP: Radiosensitivity,
apoptosis and repair of DNA double-strand breaks in
radiation-sensitive Chinese hamster ovary cell mutants treated at
different dose rates. Radiat Res. 146:636–645. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiang L, Xie G, Liu C, Zhou J, Chen J, Yu
S, Li J, Pang X, Shi H and Liang H: Knock-down of glutaminase 2
expression decreases glutathione, NADH, and sensitizes cervical
cancer to ionizing radiation. Biochim Biophys Acta. 1833:2996–3005.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hetz C, Martinon F, Rodriguez D and
Glimcher LH: The unfolded protein response: Integrating stress
signals through the stress sensor IRE1α. Physiol Rev. 91:1219–1243.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar
|
|
10
|
Yamamori T, Meike S, Nagane M, Yasui H and
Inanami O: ER stress suppresses DNA double-strand break repair and
sensitizes tumor cells to ionizing radiation by stimulating
proteasomal degradation of Rad51. FEBS Lett. 587:3348–3353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson GG, White MC and Grimaldi M:
Stressed to death: Targeting endoplasmic reticulum stress response
induced apoptosis in gliomas. Curr Pharm Des. 17:284–292. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang HJ, Lin CC, Chou HC, Chen YW, Lin
ST, Lin YC, Lin DY, Lyu KW and Chan HL: Proteomic analysis of
rhein-induced cyt: ER stress mediates cell death in breast cancer
cells. Mol Biosyst. 10:3086–3100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saglar E, Unlu S, Babalioglu I, Gokce SC
and Mergen H: Assessment of ER stress and autophagy induced by
ionizing radiation in both radiotherapy patients and ex vivo
irradiated samples. J Biochem Mol Toxicol. 28:413–417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Deiry WS: The role of p53 in
chemosensitivity and radiosensitivity. Oncogene. 22:7486–7495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weilbacher A, Gutekunst M, Oren M,
Aulitzky WE and van der Kuip H: RITA can induce cell death in
p53-defective cells independently of p53 function via activation of
JNK/SAPK and p38. Cell Death Dis. 5:e13182014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Issaeva N, Bozko P, Enge M, Protopopova M,
Verhoef LG, Masucci M, Pramanik A and Selivanova G: Small molecule
RITA binds to p53, blocks p53-HDM-2 interaction and activates p53
function in tumors. Nat Med. 10:1321–1328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chuang HC, Yang LP, Fitzgerald AL, Osman
A, Woo SH, Myers JN and Skinner HD: The p53-reactivating small
molecule RITA induces senescence in head and neck cancer cells.
PLoS One. 9:e1048212014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yaginuma Y and Westphal H: Analysis of the
p53 gene in human uterine carcinoma cell lines. Cancer Res.
51:6506–6509. 1991.PubMed/NCBI
|
|
19
|
Srivastava S, Tong YA, Devadas K, Zou ZQ,
Chen Y, Pirollo KF and Chang EH: The status of the p53 gene in
human papilloma virus positive or negative cervical carcinoma cell
lines. Carcinogenesis. 13:1273–1275. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao CY, Szekely L, Bao W and Selivanova
G: Rescue of p53 function by small-molecule RITA in cervical
carcinoma by blocking E6-mediated degradation. Cancer Res.
70:3372–3381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Byun K, Bayarsaikhan E, Kim D, Kim CY,
Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, et al:
Induction of neuronal death by microglial AGE-albumin: Implications
for Alzheimer's disease. PLoS One. 7:e379172012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao CY, Grinkevich VV, Nikulenkov F, Bao
W and Selivanova G: Rescue of the apoptotic-inducing function of
mutant p53 by small molecule RITA. Cell Cycle. 9:1847–1855. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Schadewijk A, van't Wout EF, Stolk J
and Hiemstra PS: A quantitative method for detection of spliced
X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic
reticulum (ER) stress. Cell Stress Chaperones. 17:275–279. 2012.
View Article : Google Scholar :
|
|
24
|
Lin WC, Chuang YC, Chang YS, Lai MD, Teng
YN, Su IJ, Wang CC, Lee KH and Hung JH: Endoplasmic reticulum
stress stimulates p53 expression through NF-κB activation. PLoS
One. 7:e391202012. View Article : Google Scholar
|
|
25
|
Mlynarczyk C and Fåhraeus R: Endoplasmic
reticulum stress sensitizes cells to DNA damage-induced apoptosis
through p53-dependent suppression of p21(CDKN1A). Nat Commun.
5:50672014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu L, Huang S, Baltzis D, Rivas-Estilla
AM, Pluquet O, Hatzoglou M, Koumenis C, Taya Y, Yoshimura A and
Koromilas AE: Endoplasmic reticulum stress induces p53 cytoplasmic
localization and prevents p53-dependent apoptosis by a pathway
involving glycogen synthase kinase-3beta. Genes Dev. 18:261–277.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pluquet O, Qu LK, Baltzis D and Koromilas
AE: Endoplasmic reticulum stress accelerates p53 degradation by the
cooperative actions of Hdm2 and glycogen synthase kinase 3beta. Mol
Cell Biol. 25:9392–9405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan JM, Zhou L, Wang GB, Xia GW, Xue K,
Cui XG, Shi HZ, Liu JH and Hu J: Fatsioside A inhibits the growth
of glioma cells via the induction of endoplasmic reticulum
stress-mediated apoptosis. Mol Med Rep. 11:3493–3498.
2015.PubMed/NCBI
|
|
29
|
Chiu HW, Tseng YC, Hsu YH, Lin YF, Foo NP,
Guo HR and Wang YJ: Arsenic trioxide induces programmed cell death
through stimulation of ER stress and inhibition of the
ubiquitinproteasome system in human sarcoma cells. Cancer Lett.
356:762–772. 2015. View Article : Google Scholar
|