Introduction
Lung cancer is one of the leading causes of
cancer-related death worldwide, and has the highest cancer
mortality rate among all cancers in males worldwide and the highest
mortality rate among females in developing countries (1,2).
Non-small cell lung cancer is the most common type of lung cancer.
Therefore, effective treatments for preventing non-small cell lung
cancer and promoting the survival rate of patients have been
extensively investigated. As multiple genetic alterations are
involved in a chronic process that leads to cancers, it could be
suggested that the regulation of non-small cell lung
cancer-associated genes may contribute to non-small cell lung
cancer therapy. RNA interference (RNAi)-mediated cancer therapy
whose function is to downregulate relevant transcripts has been
identified as a novel and effective process in regards to
therapeutic strategy (3). To date,
numerous genes influencing non-small cell lung cancer cells have
been confirmed, such as nuclear factor of activated T cells (NFAT)
(4), nuclear factor,
erythroid-derived 2, like 2 (NRF2) (5) and metastasis-associated protein 1
(MTA1) (6). These developments
herald a promising future for gene-targeted therapy for non-small
cell lung cancer.
NIN/RPN12 binding protein 1 (NOB1p) encoded by
NOB1 was first identified in Saccharomyces cerevisiae
by the two-hybrid screening method as a binding protein that
interacts with Nin1p/Rpn12p which is a subunit of 19S regulatory
particle of the yeast 26S proteasome (7). NOB1p joins the 20S proteasome with the
19S regulatory particle and promotes the maturation of the 20S
proteasome, and then NOB1p is internalized into the 26S proteasome
and degraded to complete 26S proteasome biogenesis in eukaryotes
(8). Additionally, as a ribosome
assembly factor, NOB1p is essential for processing of the 20S
pre-rRNA to the mature 18S rRNA (9,10).
NOB1p also serves as a part of a pre-40S ribosomal particle that is
transported from the nucleus to the cytoplasm and consequently
cleaves site D at the 3′ end of mature 18S rRNA (9,10).
This evolutionarily conserved protein contains a PIN domain which
is required for pre-rRNA cleavage, RNAi process and
nonsense-mediated mRNA decay (9,11). The
human NOB1 is located on human chromosome 16q22.1 and
includes nine exons. The length of the cDNA sequence is 1,749 bp
and contains an open reading frame 1,239-bp long. The NOB1
mRNA is mainly expressed in the liver, lung and spleen, and is
localized in the nucleus (12).
Furthermore, the expression of NOB1p in papillary thyroid carcinoma
cells (13) and breast cancer cells
(14) is significantly higher than
that in normal tissue cells.
In the past few years, many efforts have been made
to indicate the role of NOB1 in tumor development. Thus, in
the present study, to investigate the biological function of
NOB1 in non-small cell lung cancer, we employed
lentivirus-mediated short hairpin RNA (shRNA) to silence
NOB1 expression in two established non-small cell lung
cancer cell lines. Then the effects of NOB1 knockdown on the
proliferation, colony formation and cell cycle progression of
non-small cell lung cancer cells were studied.
Materials and methods
Reagents and plasmids
Dulbecco's modified Eagle's medium (DMEM) and
RPMi-1640 medium were obtained from Hyclone (Beijing, China).
Opti-MEM medium and fetal calf serum (FCS) were obtained from Gibco
(Cambrex, USA). Lipofectamine 2000 and ΤRIzol were purchased from
Invitrogen (Carlsbad, CA, USA). Isopropanol and crystal violet were
obtained from Sinopharm Chemical Reagent Co., Ltd., and Beyotime
Institute of Biotechnology, respectively. All other reagents were
purchased from Sigma (St. Louis, MO, USA). pFH-L, pCMVΔR8.92 and
pVSVG-I plasmids as well as helper plasmids (pHelper 1.0 and
pHelper 2.0) were purchased from Hollybio (Shanghai, China).
Immunohistochemistry (IHC)
Twenty-nine non-small cell lung cancer specimens
were collected for immunohistochemistry (15 males, 14 females; 16
specimens from patients younger than 60 years of age, 13 from
patients older than 60 years of age). Ten normal lung specimens
were used as control. All of the above tissue samples were provided
by the Department of Thoracic Surgery at the First Hospital of
Shanghai, and patients provided informed consent forms conforming
to the guidelines of the Declaration of Helsinki. The tissues were
fixed with formalin and embedded in paraffin. Before
immunostaining, the paraffin was removed from the samples. Samples
were blocked and incubated with the primary antibody against NOB1
(cat no. GTX120935, dilution 1:100; GeneTex) overnight at 4°C and a
biotinylated secondary antibody for 30 min at room temperature.
After reacting with streptavidin-peroxidase conjugate for 10 min at
room temperature, DAB staining was performed. All samples were
counterstained with hematoxylin.
Cell culture
Human embryonic kidney cell line 293T (HEK293T) and
human non-small cell lung cancer cell lines A549 and H1299 were
obtained from the Cell Bank of the Shanghai Institute of Cell
Biology, Chinese Academy of Sciences (Shanghai, China). HEK293T and
A549 cells were cultured in DMEM supplemented with 10% FBS at 37°C
in a 5% CO2 humidified incubator. H1299 cells were
cultured in RPMi-1640 supplemented with 10% FBS at 37°C in a 5%
CO2 humidified incubator.
Construction of recombinant
lentivirus
shRNA for the human NOB1 gene (NM_020143) and
the non-silencing control shRNA were: 5′-CTAGCCCGGTTCTCCGAACGTGTCAC
GTATCTCGAGATACGTGACACGTTCGGAGAATTTTTT TAAT-3′ and
5′-CTAGCCCGGCCAAGGAAGTGCAATT GCATACTCGAGTATGCAATTGCACTTCCTTGGTTTTT
TGTTAAT-3′, respectively. Subsequently, they were inserted into the
lentiviral vector pFH-L. HEK293T cells were cultured in 10-cm
dishes at the concentration of 6×105 cells/ml for 24 h.
Two hours before transfection, the medium was replaced by
serum-free DMEM. The modified pFH-L plasmids, lentiviral packing
vector pCMVΔR8.92, pVSVG-I plasmids and helper plasmids (pHelper
1.0 and pHelper 2.0) were transected into 70–80% confluent HEK293T
cells via Lipofectamine 2000 to generate the recombinant
lentivirus. After incubation for 48 h, the lentivirus was
harvested, collected and concentrated by Centricon-Plus-20 filter
devices (Millipore, USA).
Lentivirus-mediated infection in
non-small cell lung cancer cells
Lentivirus-mediated NOB1 and non-silencing
control shRNA were infected into A549 and H1299 cells and seeded
into 6-well plates at a density of 5×104 cells/well by
replacing the medium with Opti-MEM medium containing Polybrene (5
µg/ml). After 48 h, the medium was replaced with fresh
medium and incubated for another 48 h. Then the cells were examined
under a fluorescence microscope (CKX41; Olympus, Japan) by
observing the green fluorescence emitted by the green fluorescent
protein (GFP) in the lentivirus particles.
RNA extraction and real-time PCR
A549 and H1299 cells were cultured in 6-well plates
and infected with recombinant lentivirus for 6 days. The cells were
lysed with TRIzol reagent and total RNA was isolated from the
lysate. The cDNA was synthesized from total RNA (2 µg) using
Promega M-MLV cDNA Synthesis kit according to the manufacturer's
instructions. NOB1 mRNA expression was determined by
real-time PCR (CFX96; Bio-Rad, USA) using SYBR-Green PCR core
reagents with Bio-Rad Connect Real-Time PCR platform. For
NOB1 detection, forward, 5′-GAAAGAACAACGCCCTGGAG-3′ and
reverse, 5′-CAGCCTTGAGATGACCTAAGC-3′were designed, respectively.
Parallel reactions were performed using primers (forward,
5′-GTGGACATCCGCAAAGAC-3′ and reverse, 5′-AAAGGGTGTAACGCAACTA-3′)
for actin as an internal control. The relative NOB1 mRNA
expression level as compared with actin was evaluated using the
2−ΔΔCt analysis method.
Western blot analysis
A549 and H1299 cells were cultured in 6-well plates
and infected with the recombinant lentivirus for 6 days. The cells
were then washed twice with ice-cold PBS and lysed in 2X SDS sample
buffer (10 mM EDTA, 4% SDS, 10% glycine in 100 mM Tris-HCl buffer,
pH 6.8) for 1 h at 4°C. Total cell lysates were then centrifuged
(12,000 rpm, 15 min, 4°C), and the supernatants were employed for
further processing. The protein concentration was determined by
using the BCA protein assay kit. Equal amounts of proteins (30
µg) were loaded and separated on 10% SDS-PAGE gels and
transferred onto PVDF membranes (Millipore). Proteins were probed
overnight at 4°C with primary antibodies: anti-NOB1 (1:5,000, cat
no. GTX120935; GeneTex), anti-CDK4 (1:500, #2906; Cell Signaling
Technology), anticyclin D1 (CCND1) (1:1,000, cat no. 60186-1-1g;
Proteintech Group, inc.), anti-CDKN2B (1:1,000, #4822; Cell
Signaling Technology), anti-CDKN2A (1:3,000, cat no. 10883-1-AP;
Proteintech Group, inc.) and anti-GAPDH (1:80,000, cat no.
10494-1-AP; Proteintech Group, inc.), followed by incubation with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000,
cat no. SC-2004; Santa Cruz) at room temperature for 1 h. ECL
reaction was performed using enhanced chemiluminescence (Amersham).
Experiments were repeated at least three times.
Cell proliferation assay
Briefly, non-small cell lung cancer cells infected
with NOB1 shRNA lentivirus (Lv-shNOB1) or non-silencing
shRNA lentivirus (Lv-shCon), and non-infected cells (Con) were
seeded in a 96-well plate at an initial density of 2×103
cells/well. At specified incubation time-points, 20 µl
methylthiazol tetrazolium (MTT) solution (5 mg/ml) was added to
each well. Following incubation at 37°C for 4 h, 100 µl
acidified isopropanol was added to the well to terminate the
reaction, and then the samples were measured by a microplate reader
(BioTek Epoch; BioTek, USA) at 595 nm.
Colony formation assay
A549 cells from different groups were seeded into
6-well plates at a density of 200 cells/well. The cells were
cultured for 9 days to form colonies with the medium changed every
2 days. Then the cells were washed twice with PBS and fixed with
700 µl 4% paraformaldehyde for 10 min. After being washed
twice with PBS to remove the paraformaldehyde, the cells were
stained with 700 µl Giemsa solution for 5 min, and rinsed
with PBS three times. The number of colonies containing >50
cells was counted under a microscope (CKX41; Olympus, Japan).
Cell cycle analysis
After infection for 4 days, A549 cells were seeded
in 6-cm dishes at a density of 5×104 cells/dish. After
the density reached ~80%, the A549 cells were collected,
re-suspended in cold PBS and fixed with pre-cold 75% ethanol for 30
min at 4°C. Samples were washed with PBS and incubated with PBS
containing RNAase and propidium iodide (PI) at 4°C overnight in the
dark. Cell cycle progression was monitored using a flow cytometer
(Beckman Coulter, Miami, FL, USA). Experiments were repeated at
least three times.
Statistical analysis
The data are presented as mean ± SD from at least
three independent experiments. Statistical analysis was performed
using the Student's t-test and SPSS 17.0 software, and P<0.05
was considered to indicate a statistically significant result.
Results
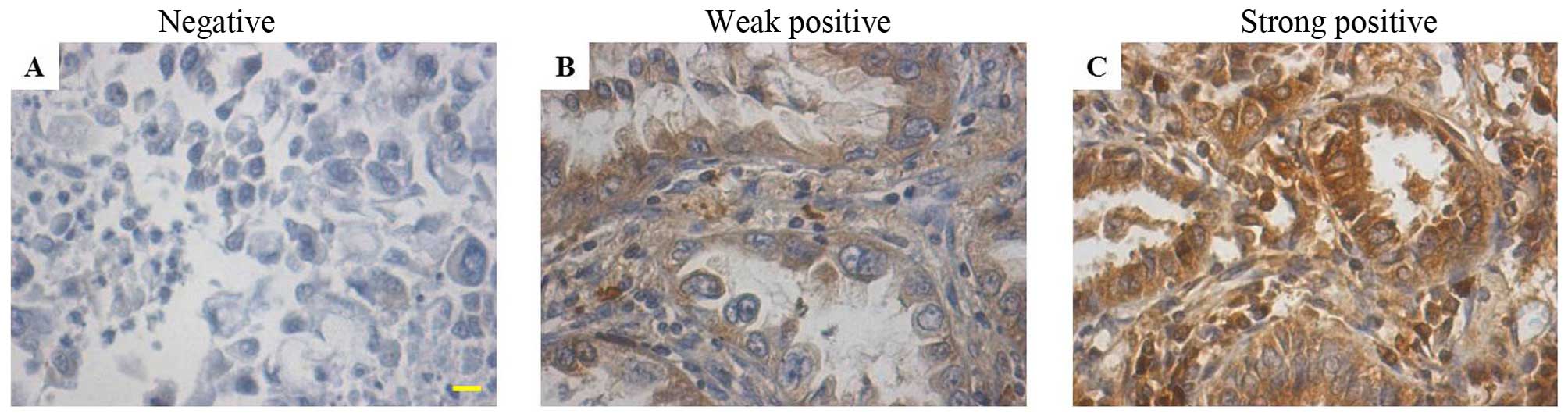
NOB1 is highly expressed in non-small
cell lung cancer
Previous studies have indicated that the expression
of NOB1 is involved in several types of carcinomas. To investigate
the function of NOB1 in non-small cell lung cancer, we evaluated
the expression in 29 non-small cell lung cancer specimens using
immunohistochemical staining. Of the 29 non-small cell lung cancer
samples, 4 (13.7%) were hadro-positive, 21 (72.4%) were positive
and 2 (6.9%) were weak positive, which was significantly higher
than the levels in the normal lung tissue samples [none were
hadro-positive, 2 out of 10 (20.0%) were positive and 8 out of 10
(80.0%) were negative] (Fig. 1 and
Table I). These results suggest
that NOB1 is highly expressed in non-small cell lung cancer. The
high expression level of NOB1 in non-small cell lung cancer
suggests that it may be involved in the pathogenesis of non-small
cell lung cancers.
 | Table IExpression of NOB1 in the lung
carcinoma specimens. |
Table I
Expression of NOB1 in the lung
carcinoma specimens.
| Sample | N | NOB1 immunostaining
| Chi-square | P-valua |
|---|
Negative
(−)
n (%) | Weak positive (− to
+)
n (%) |
Positive(+)
n (%) |
Hadro-positive(++)
n (%) |
|---|
| Lung cancer | 29 | 2
(6.9) | 2 (6.9) | 21 (72.4) | 4 (13.8) | 21.03 | 0.0001 |
| Normal tissues | 10 | 8 (80.0) | 0 (0.0) | 2
(20.0) | 0
(0.0) | | |
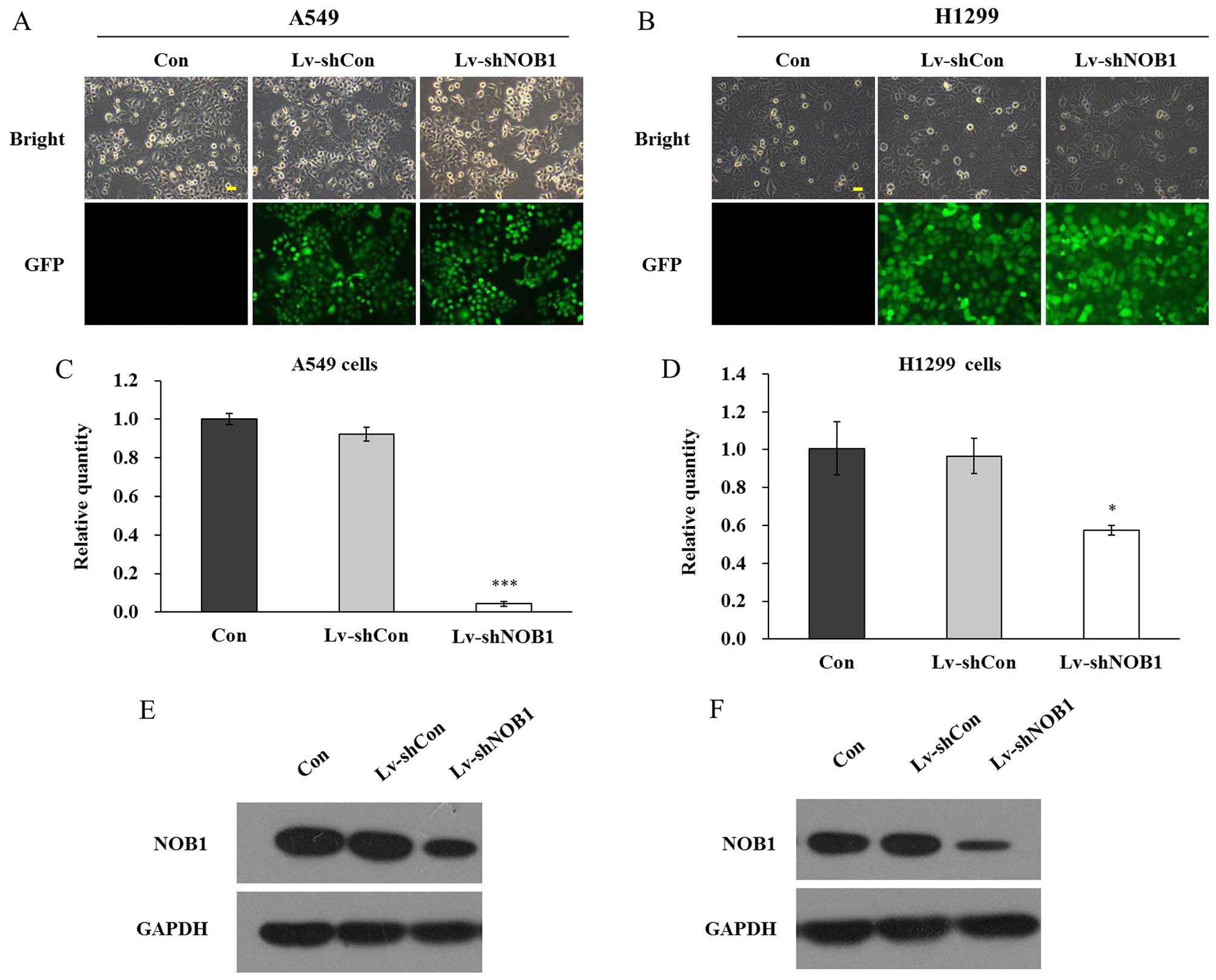
Lentivirus-mediated RNAi inhibits NOB1
expression
Herein, NOB1 shRNA targets were cloned into
the recombinant lentivirus plasmid, which was utilized to infect
two established non-small cell lung cancer cell lines A549 and
H1299. The non-silencing sequences were also inserted into the
vector as a control. GFP florescence imaging was used to indicate
the lentivirus infection as GFP was transfected into the cancer
cells together with NOB1 shRNA. As shown in Fig. 2A and B, >80% cells were infected
by the lentivirus as assessed by GFP fluorescence, indicating the
successful transfection in both A549 and H1299 cells. NOB1
knockdown efficiency was determined by real-time PCR and western
blotting. Lentivirus-mediated RNAi markedly decreased endogenous
NOB1 mRNA expression, by 95.4% in the A549 cells and 40.7%
in the H1299 cells (Fig. 2C and D).
The protein level of NOB1 was also significantly reduced in both
cell lines after lentivirus infection (Fig. 2E and F). Hence, lentivirus infection
was confirmed to be effective to inhibit the expression of
NOB1 in non-small cell lung cancer cells.
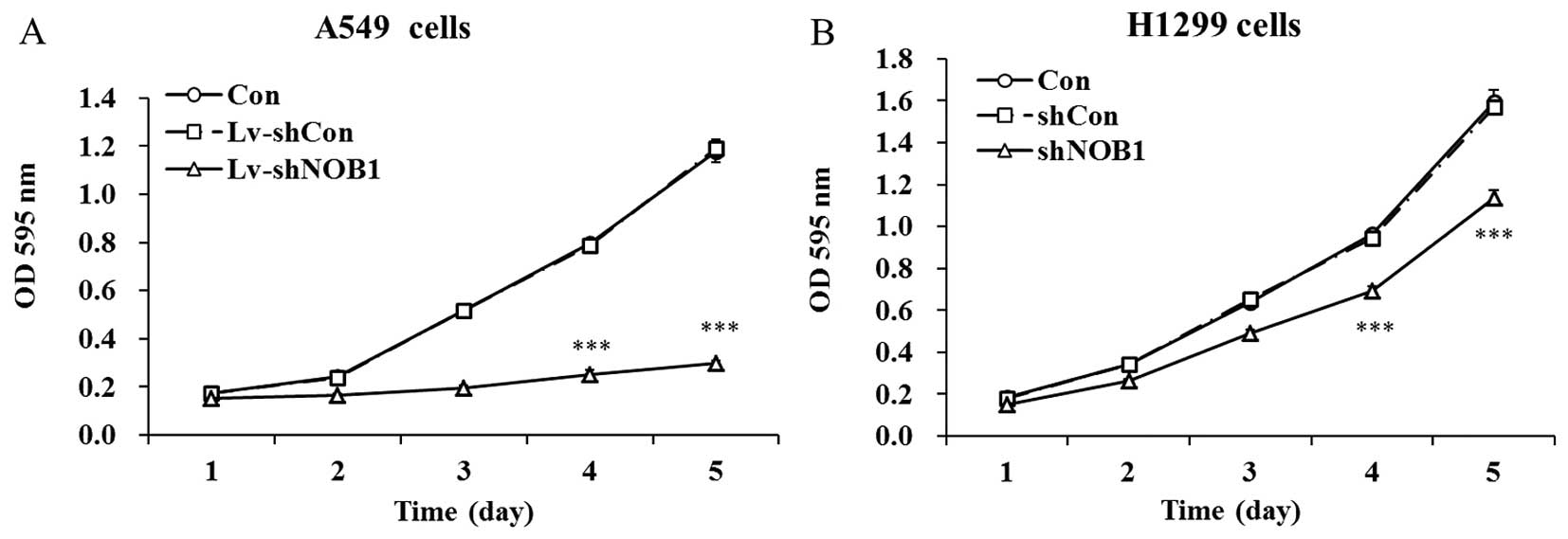
NOB1 knockdown inhibits non-small cell
lung cancer cell proliferation
The effect of the silencing of NOB1 on
non-small cell lung cancer cell proliferation was examined by MTT
assay. As shown in Fig. 3, there
was no significant difference in cell viability between the
Lv-shCon infected and uninfected cells, suggesting no cytotoxic
effect of the lentiviral system on both cell lines. Whereas, the
proliferation rates of NOB1-silenced A549 cells and H1299
cells were significantly reduced as compared with the control
groups from day 3. On day 5, the cell viability was decreased by
74.9% in the A549 cells and 27.5% in the H1299 cells after
lentivirus infection, respectively. The inhibitory rate in the A549
cells was higher than the rate in the H1299 cells, consistent with
the suppression of NOB1 expression by Lv-shNOB1. These
results revealed the important functional role of NOB1 in
the proliferation of non-small cell lung cancer cells, and its
inhibitory effect was dependent on the specific cell line.
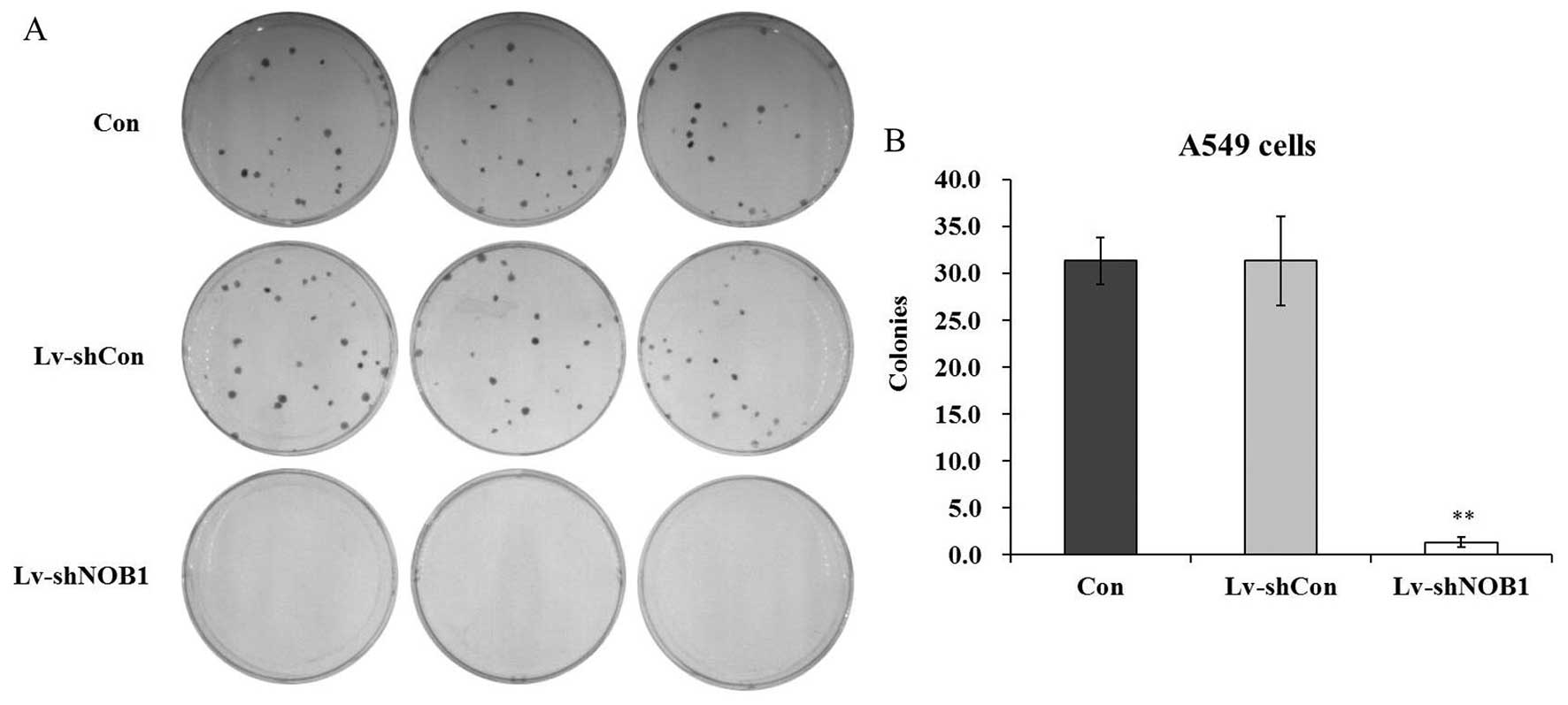
NOB1 depletion suppresses non-small cell
lung cancer cell colony formation
The colony formation assay by Giemsa staining was
performed to evaluate the effect of NOB1 depletion on the
colony forming capability of A549 cells. Three groups of A549 cells
(Con, Lv-shCon and Lv-shNOB1) were cultured for 9 days. The number
of colonies in the Lv-shNOB1-infected A549 cells was markedly
reduced by 96.8% as compared with the control groups as observed
under a light microscope (Fig. 4A and
B). The results indicated that knockdown of NOB1 could
also inhibit the colony formation of non-small cell lung cancer
cells, representing its oncogenicity in vitro.
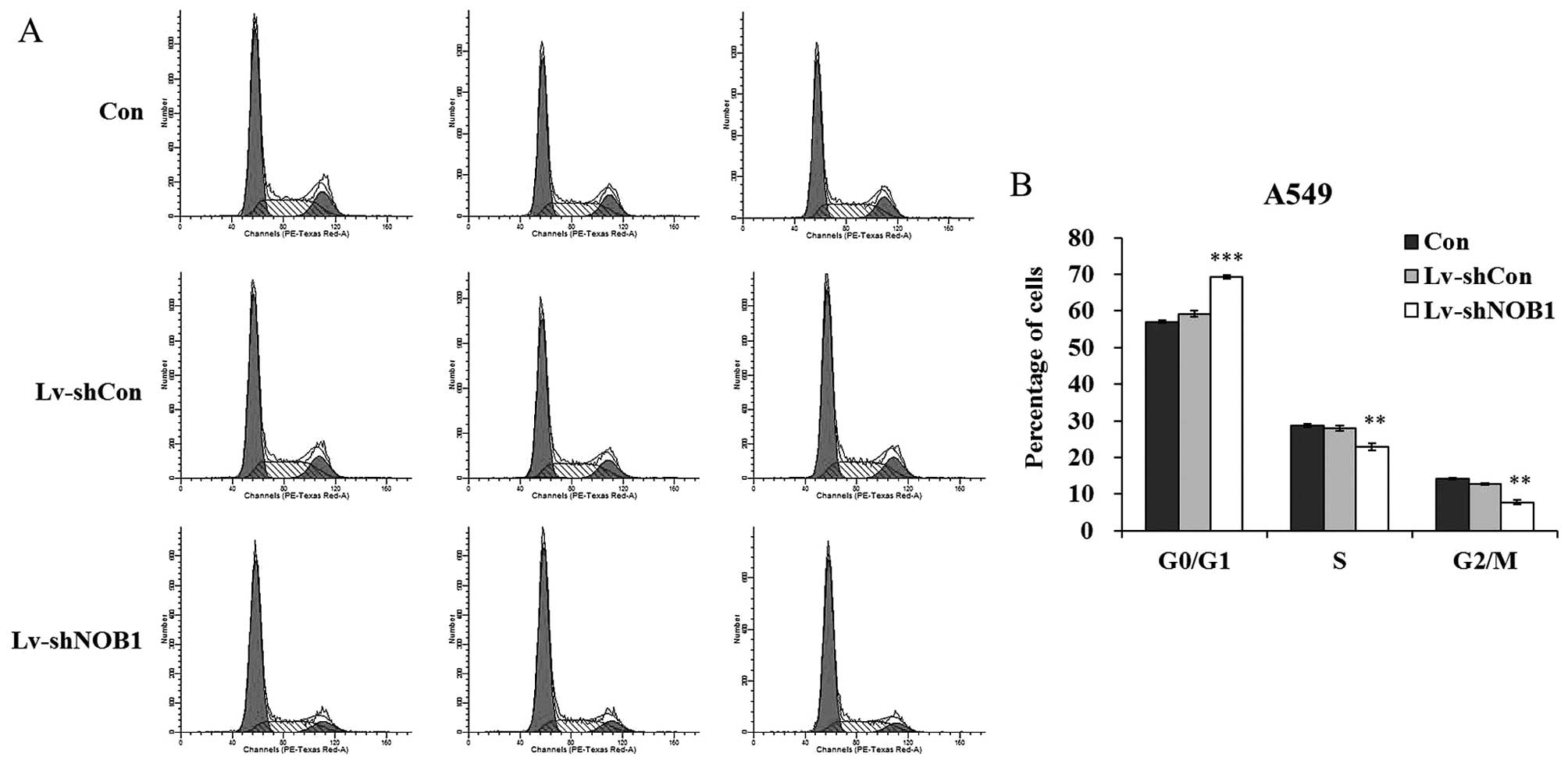
NOB1 suppression leads to G0/G1 cell
cycle arrest
To ascertain the underlying mechanisms involved in
the cell growth inhibition induced following NOB1 silencing,
we analyzed the cell cycle distribution of the A549 cells after
lentivirus infection (Fig. 5A). As
shown in Fig. 5B, a higher
percentage of cells was accumulated in the G0/G1 phase of the cell
cycle (69.32±0.45%) after Lv-shNOB1 infection, compared with the
the percentage in the Con group (57.05±0.37%) and Lv-shCon group
(59.21±0.86%). The percentages of cells in the S phase and G2/M
phase were markedly decreased after lentivirus infection. These
results suggest that knockdown of NOB1 suppressed the growth
of non-small cell lung cancer cells possibly via induction of cell
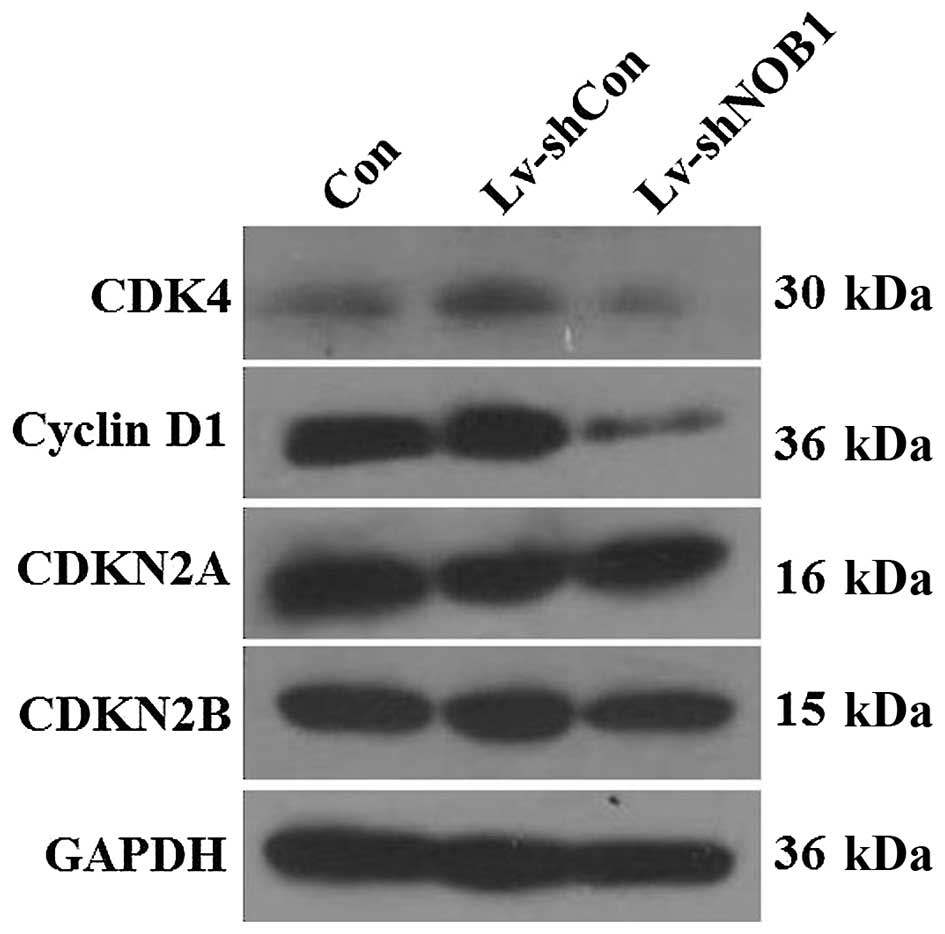
cycle arrest. Furthermore, alterations in the expression of cell
cycle markers were detected in the A549 cells, including cyclin D1,
CDK4, CDKN2A, and CDKN2B. Western blotting showed that depletion of
NOB1 resulted in a significant decrease in cyclin D1 and CDK4
expression, while no significant change was observed in CDKN2A and
CDKN2B expression (Fig. 6). These
results suggest that knockdown of NOB1 in non-small cell lung
cancer cells blocks cell cycle progression via downregulation of
cyclin D1 and CDK4.
Discussion
NOB1 was first identified as an essential
gene encoding NOB1p in Saccharomyces cerevisiae. NOB1p, as a
nuclear protein in mammalian cells, has been proven to play a
crucial part in proteasome biogenesis (8). In the present study, we found that
NOB1 was highly expressed in the non-small cell lung cancer samples
and ~13.8% of the non-small cell lung cancer samples exhibited
aberrantly strong NOB1 expression. Recently, it was reported that
NOB1 is responsible for the high proliferation rate of
cancer cells and repression of NOB1 could suppress breast
cancer and ovarian cancer cell survival (14,15).
In the present study, NOB1 was predicted to be an oncogenic
factor in non-small cell lung cancer and silencing of NOB1
may inhibit non-small cell lung cancer cell proliferation.
Non-small cell lung cancer has emerged as one of the
leading causes of cancer-related death in the world. The influence
of NOB1 downregulation on the growth of two non-small cell
lung cancer cell lines A549 and H1299 which express significantly
high expression of NOB1 was investigated. As a novel
strategy in cancer treatment, gene-level approaches have generated
increased attention. RNAi technology has been proven to be an
efficient, specific and stable method to silence target genes
(3). Taking advantage of the
prevalence and availability of RNAi technology in cancer therapy as
well as the relatively high and stable transfection rate of viral
vectors (16), a lentivirus shRNA
system was used to knock down NOB1 expression in non-small
cell lung cancer cells. The real-time PCR and western blotting
results demonstrated that the expression of NOB1 was
sufficiently suppressed in the non-small cell lung cancer cells,
which guaranteed the subsequent assays. The notably reduced
proliferation of both non-small cell lung cancer cell lines was
observed by MTT assay as the expression of NOB1 was
decreased. It was also confirmed that the colony formation capacity
of the A549 cells was inhibited following knockdown of NOB1.
NOB1 silencing led to A549 cell cycle arrest in the G0/G1
phase, which contributed to the cell growth inhibition. Cyclin D1
and CDK4 are key molecules for G1-S and G2-M transition during the
cell cycle, respectively (17,18).
CDKN2A and CDKN2B are potent cyclin-dependent kinase inhibitors,
and their induction may also cause cell cycle arrest (19). Western blotting showed that
depletion of NOB1 resulted in a significant increase in CDKN2A
expression, and a slight decrease in cyclin D1 and CDK4 expression,
which contributed to cell cycle arrest. Therefore, NOB1
plays an important role in the growth and cell cycle progression of
non-small cell lung cancer cells.
The proteolysis of intracellular proteolysis is
mainly through the ubiquitin-proteasome pathway, and the proteome
is confirmed to control various proteins involved in cycle
progression and apoptosis such as the cyclins, caspases, nuclear
factor κB (NF-κB) and apoptosis proteins (20,21).
NOB1p facilitates the maturation of the 20S proteasome, and then
regulates the biogenesis of the 26S proteasome which contributes to
protein degradation by the ubiquitin-proteasome system (UPS) in
universal biological processes including cell cycle progression in
eukaryotes (22,23). Thus, the inhibitory effect on the
proliferation of non-small cell lung cancer cells induced by
NOB1 repression may be attributed to the influences on
degradation of cell cycle proteins and various complex aspects in
cell cycle progression. Subsequent research is needed to elucidate
the mechanism involved in the regulation of the cell cycle of
non-small cell lung cancer cells by NOB1 and its underlying
relationship with proteasome-mediated degradation.
In conclusion, the present study demonstrated that
lentivirus-mediated NOB1 knockdown inhibited the growth of
non-small cell lung cancer cells along with cell cycle arrest in
the G0/G1 phase. These results suggest that NOB1 may be
considered as a potential target for non-small cell lung cancer
therapy.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mello CC and Conte D Jr: Revealing the
world of RNA interference. Nature. 431:338–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu JF, Zhao SH and Wu SS: Depleting NFAT1
expression inhibits the ability of invasion and migration of human
lung cancer cells. Cancer Cell Int. 13:412013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martens-de Kemp SR, Nagel R, Stigter-van
Walsum M, van der Meulen IH, van Beusechem VW, Braakhuis BJ and
Brakenhoff RH: Functional genetic screens identify genes essential
for tumor cell survival in head and neck and lung cancer. Clin
Cancer Res. 19:1994–2003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Tian H, Yue W, Li L, Gao C, Si L, Li
W, Hu W, Qi L and Lu M: Down-regulation of MTA1 protein leads to
the inhibition of migration, invasion, and angiogenesis of
non-small-cell lung cancer cell line. Acta Biochim Biophys Sin
(Shanghai). 45:115–122. 2013. View Article : Google Scholar
|
|
7
|
Tone Y, Tanahashi N, Tanaka K, Fujimuro M,
Yokosawa H and Toh-E A: Nob1p, a new essential protein, associates
with the 26S proteasome of growing Saccharomyces cerevisiae cells.
Gene. 243:37–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tone Y and Toh-E A: Nob1p is required for
biogenesis of the 26S proteasome and degraded upon its maturation
in Saccharomyces cerevisiae. Genes Dev. 16:3142–3157. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fatica A, Tollervey D and Dlakić M: PIN
domain of Nob1p is required for D-site cleavage in 20S pre-rRNA.
RNA. 10:1698–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamanna AC and Karbstein K: Nob1 binds the
single-stranded cleavage site D at the 3′-end of 18S rRNA with its
PIN domain. Proc Natl Acad Sci USA. 106:14259–14264. 2009.
View Article : Google Scholar
|
|
11
|
Clissold PM and Ponting CP: PIN domains in
nonsense-mediated mRNA decay and RNAi. Curr Biol. 10:R888–R890.
2000. View Article : Google Scholar
|
|
12
|
Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan
Y, Tang W, Yu L and Zhao S: Cloning, expression and
characterization of the human NOB1 gene. Mol Biol Rep. 32:185–189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin S, Meng W, Zhang W, Liu J, Wang P, Xue
S and Chen G: Expression of the NOB1 gene and its clinical
significance in papillary thyroid carcinoma. J Int Med Res.
41:568–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang WY, Chen DH, Ning L and Wang LW:
siRNA mediated silencing of NIN1/RPN12 binding protein 1 homolog
inhibits proliferation and growth of breast cancer cells. Asian Pac
J Cancer Prev. 13:1823–1827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Y, Peng S, Yu H, Teng H and Cui M:
RNAi-mediated down-regulation of NOB1 suppresses the growth and
colony-formation ability of human ovarian cancer cells. Med Oncol.
29:311–317. 2012. View Article : Google Scholar
|
|
16
|
Tomar RS, Matta H and Chaudhary PM: Use of
adeno-associated viral vector for delivery of small interfering
RNA. Oncogene. 22:5712–5715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan J, Yan R, Krämer A, Eckerdt F, Roller
M, Kaufmann M and Strebhardt K: Cyclin B1 depletion inhibits
proliferation and induces apoptosis in human tumor cells. Oncogene.
23:5843–5852. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Chen X, Li T, Li Y, Wang R, He D,
Luo W, Li X and Wu X: Screening a phage display library for a novel
FGF8b-binding peptide with anti-tumor effect on prostate cancer.
Exp Cell Res. 319:1156–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
20
|
Machuy N, Thiede B, Rajalingam K, Dimmler
C, Thieck O, Meyer TF and Rudel T: A global approach combining
proteome analysis and phenotypic screening with RNA interference
yields novel apoptosis regulators. Mol Cell Proteomics. 4:44–55.
2005. View Article : Google Scholar
|
|
21
|
Adams J: The proteasome: A suitable
antineoplastic target. Nat Rev Cancer. 4:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lehman NL: The ubiquitin proteasome system
in neuropathology. Acta Neuropathol. 118:329–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clurman BE, Sheaff RJ, Thress K, Groudine
M and Roberts JM: Turnover of cyclin E by the ubiquitin-proteasome
pathway is regulated by cdk2 binding and cyclin phosphorylation.
Genes Dev. 10:1979–1990. 1996. View Article : Google Scholar : PubMed/NCBI
|