Introduction
Nasopharyngeal carcinoma (NPC) is a common head and
neck cancer derived from epithelium cells which were located in the
nasopharynx (1). NPC is notorious
for its metastatic potential at the early stages of the disease via
both lymph and blood vessels. Due to the recurrence and distant
metastasis, the prognosis of NPC patients is very poor (1). Furthermore, drug resistance may hamper
the efficacy of anti-cancer drugs (2). Therefore, there is a great and urgent
need to develop early diagnostic or predictive markers for NPC and
to elucidate the mechanisms that would allow the development of
efficient treatment options.
Recent studies of microRNAs (miRNAs) suggest a
potential role of these regulatory molecules as candidate oncogenes
or tumor suppressors in cancer progression (3–5).
miRNAs are small non-coding RNA molecules ~19–25 nucleotide, which
exist in many organisms and regulate gene expression through
degradation of the corresponding mRNAs and/or inhibiting their
translation by binding to their 3′-UTRs (1,6,7).
miRNAs have been reported to participate in a variety of pathways
in physiological and pathological processes such as cellular
differentiation, proliferation and apoptosis (1,8),
metastasis and resistance to therapy (6). Dysregulated expression of miRNAs has
been reported in most tumor types, including NPC (1). At present, several miRNAs have been
shown to target specific mRNAs to regulate the progression of NPC
(9). For example, miR-29c
suppressed metastasis by targeting TIAM1 and enhanced the
sensitivity to cisplatin based chemotherapy and radiotherapy in
nasopharyngeal carcinoma (10,11).
miR-451, by targeting MIF, inhibited cell growth and invasion and
was associated with survival of NPC (1). Furthermore, miR-218 (12), miR-26a/b (13,14),
miR-216b (15), miR-10b (16), miR-141 (17) and miR-200a (18) have been shown to have
tumor-suppressive functions in NPC. The dysregulated miRNAs are
involved in NPC development and progression by regulating cell
growth, proliferation, apoptosis, invasion and metastasis (1), indicating that miRNAs play important
roles in NPC tumorigenesis.
Recently, there has been increasing interest in
miR-338-3p as an anti-oncogene in different solid tumors, including
gastric cancer (19),
hepatocellular carcinoma (20) and
malignant melanoma (21). For
example, miR-338-3p suppressed liver cancer cell invasion by
targeting smoothened (7). Moreover,
miR-338 can decrease proliferative and migratory, invasive behavior
by attenuating the expression of NRP1 in gastric cancer (22). However, there is no study on the
functions and mechanisms of miR-338-3p in NPC development and
progression.
Hypoxia or low oxygen tension has emerged as a
specific and general feature of the microenvironment of many
malignant tumors (23), including
nasopharyngeal cancer (24). Tumor
hypoxia is known to be mainly responsible for tumor resistance to
radiotherapy and chemotherapy as well as to promote tumor phenotype
influencing invasiveness, metastasis and poor prognosis (24). Studies showed that 100% of primary
NPC and 58% of cervical nodal metastases of NPC were found with
hypoxic regions which increased distant metastases, as well as
resistance to chemotherapy in advanced NPC patients (25). There is emerging evidence shows that
the adaptive response to hypoxia is also mediated by
HIF-1-dependent pathways in cancer as well as in nasopharyngeal
carcinoma (24,26). Among the proteins associated with
tumor hypoxia, hypoxia-inducible factor 1-alpha (HIF-1α) is an
important hypoxia regulatory molecule promoting angiogenesis and
metastasis (25). HIF-1α is
commonly found overexpressed in NPC (27). HIF-1α has been reported as a
prognostic factor as well as a potential therapeutic target of NPC
(25). HIF-1α can be
transcriptionally and translationally regulated by signaling
molecules such as cytokines growth factors and microRNAs (28). However, the association of HIF-1α
with miR-338-3p has not been established in NPC.
In the present study, we found that decreased
expression of miR-338-3p had a causal role as a tumor suppressor in
NPC by target HIF-1α. miR-338-3p was downregulated in NPC tissues
and cells. We found that miR-338-3p regulated the expression levels
of HIF-1α of CNE2 cells under hypoxia, respectively. The luciferase
assay showed that HIF-1α was a direct target of miR-338-3p.
Overexpression of miR-338-3p suppressed the proliferation and
migration of CNE2 cells under hypoxia, whereas the inhibition of
miR-338-3p promoted these processes. Furthermore, our results
suggested that the chemo-sensitizing effect of miR-338-3p may be an
important feature for its potential therapeutic roles in NPC.
Materials and methods
Clinical specimens and cell culture
All NPC specimens and normal nasopharyngeal
epithelium samples were obtained from the Affiliated Hospital of
Nantong University. No patients had received any antitumor
treatments before biopsy. The patients were diagnosed via
histopathological evidence. The research protocols were approved by
the Academic Committee of Nantong University.
The human immortalized nasopharyngeal epithelial
cell line NP69 and NPC cell line CNE2 were received as a kind gift
from the Sun Yat-Sen University of China. The NPC cell lines CNE1
and 5–8F, 6–10B were a kind gift from the Xiang-Ya School of
Medicine, Central South University, China. The human immortalized
nasopharyngeal epithelial cell line NP69 was maintained in
keratinocyte/serum-free medium (Invitrogen) supplemented with
bovine pituitary extract (BD Biosciences), human NPC cell lines
(CNE-1, CNE-2, 5–8F and 6–10B) were cultured in RPMI-1640 (Gibco)
supplemented with 10% FBS (Gibco). 5–8F and 6–10B cells were
cultured in completed medium with 100 U/ml penicillin and 100
µg/ml streptomycin (Shanghai Genebase Gen-Tech Co., Ltd.,
Shanghai, China). All the cells were cultured at 37°C in a 5%
CO2 incubator.
Hypoxic condition was induced by exposing the cells
in a hypoxia modular incubator chamber (Billups-Rothenberg) that
provided hypoxic conditions with 1% O2, 5%
CO2 and 94% N2.
Cell transfection
CNE2 cells were seeded in 6-well plates and cultured
overnight at 37°C in a humidified atmosphere of 95% air and 5%
CO2. Subsequently, transfections were conducted for
miR-338-3p mimics, miR-338-3p inhibitor and non-specific control
(NC) using HiPerFect transfection reagent (Qiagen) according to the
manufacturer's instructions. Following culture for a further 48 h,
total RNA and cellular protein lysates were collected and used for
reverse transcription-quantitative PCR (RT-qPCR) and western blot
analysis, respectively.
Quantitative real-time PCR (qRT-PCR)
analysis
miR-338-3p expression in NPC cells and tissues
compared with normal was measured with SYBR qRT-PCR. Total RNA was
extracted with TRIzol (Invitrogen) according to the manufacturer's
instructions. miR-388-3p cDNA was synthesized from 2 µg of
total RNA. Subsequently, mRNA expression was analyzed by
quantitative PCR using primers designed and synthesized by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Briefly, 20
µl reactions containing 2 µl RT product, 9 µl
of SYBR-Green PCR Master Mix and 200 nM of primers were subjected
to 1 cycle of 95°C for 20 sec, and then 40 cycles of 95°C for 10
sec, 60°C for 20 sec and 70°C for 10 sec. miR-338-3p expression was
normalized to U6 RNA. The 2−ΔΔCT method was used to
quantify the expression changes of target genes. Three independent
experiments were performed.
Western blot analysis
HIF-1α proteins were measured by western blot
analysis. Total protein was extracted from transfected cells using
the RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) according to the manufacturer's instructions. For western
blot analysis, equal amounts of protein samples (20 µg) were
separated by 10% SDS-PAGE and transferred onto PVDF membranes
(Millipore, Billerica, MA, USA). Membranes were blocked using with
5% skim milk in TBST. After 2 h at room temperature, the membranes
were incubated overnight with polyclonal primary antibodies. The
antibodies used were as follows: anti-HIF-1α (Abcam; 1:2,000
dilution), anti-p-ERK (Santa Cruz Biotechnology; 1:500 dilution),
anti-ERK (Santa Cruz Biotechnology; 1:500 dilution) and mouse
anti-β-actin (Santa Cruz Biotechnology; 1:1,000 dilution). Blots
were then incubated with goat anti-rabbit or anti-mouse secondary
antibody conjugated to horseradish peroxidase (Santa Cruz
Biotechnology; 1:1,000 dilution) and visualized by enhanced
chemiluminescence (ECL; Cell Signaling Technology). The values are
representative of at least three independent experiments.
Luciferase reporter assay
HEK293 cells were seeded in the inner wells of
24-well plates. Then, cells were co-transfected using Lipofectamine
2000 (Invitrogen) with 80 ng of the pGL3-vector plasmid harboring
the wild-type or mutant 3′-UTR of HIF-1α and 4 ng of the pRL-TK.
For each plate, the hsa-miR-338-3p mimics or mimics NC (Biomics
Biotechnologies Co., Ltd., Nantong, China) was co-transfected at a
final concentration of 50 pmol. Luciferase activities were measured
consecutively 48 h post-transfection using the Dual-Luciferase
reporter assay system (Promega, Southampton, UK) according to the
manufacturer's instructions. Luminescence signals served as a
measure for reporter activity normalized for transfection
efficiency.
Wound healing assay
Wound healing assay was conducted 48 h after cell
transfection. An artificial homogeneous wound was created on the
monolayer using a sterized 200 µl micropipette tip when the
cells reached ~90% confluency. Cell debris was removed by washing
with RPMI-1640 twice. Wound closure was recorded and observed by
light microscopy and images were captured at the indicated
time-points.
Immunocytochemical analysis
Cells cultured on glass cover-slips were transfected
with miR-338-3p mimics, miR-338-3p inhibitor and non-specific
control (NC) and then cultured under hypoxia condition for 24 h.
Glass coverslips were fixed with 4% paraformaldehyde and washed in
PBS. The cells were incubated with rabbit anti-HIF-1α antibody
(Abcam; 1:200 dilution) overnight at 4°C. PBS washed and incubated
with fluorescein isothiocyanate (FITC) labeled secondary antibodies
(EarthBox, Lancaster, PA, USA; 1:1,000) and at the same time the
nuclei were labeled with Hoechst (Invitrogen, Carlsbad, CA, USA).
The coverslips were then observed under an Olympus camera.
Cell viability assay (Cell Counting
kit-8)
Cells were seeded into 96-well plates
(1×104 cells/well) and treated as indicated. Cell
viability was assessed by Cell Counting kit-8 assay (Beyotime
Institute of Biotechnology, Shanghai, China). The absorbance of
each well was read on a microplate reader (F-2500 fluorescence
spectrophotometer; Hitachi) at 450 nm. Three independent
experiments were performed in quintuplicate.
Migration assays
For the Transwell migration assays, 5×104
CNE2 cells were plated in a serum-free medium in the top chamber
with a non-coated membrane (24-well insert; 8 µm pore size;
Millipore) and a medium supplemented with 10% serum was in the
lower chamber. The cells were incubated for 24 h under hypoxia
conditions. The non-migrated cells were removed from the upper
sides of the Transwell membrane filter inserts using cotton-tipped
swabs. The migrated/invaded cells on the lower sides were stained
with crystal violet and the cells were counted.
Flow cytometry
Cells were plated in 6-well plates at a specific
density. The cells were treated with miR-338-3p mimics, inhibitor
or miR-NC under hypoxic conditions for 24 h. After 24 h, the cells
were collected and analyzed using an Annexin V-FITC apoptosis
detection kit (BD Biosciences, Oxford, UK). The apoptotic cells
were detected by flow cytometry.
Statistical analysis
All the data are expressed as mean ± SEM. Data were
compared using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. Each experiment
consisted of at least three replicates per condition.
Results
miR-338-3p is downregulated in NPC
clinical specimens and cell lines
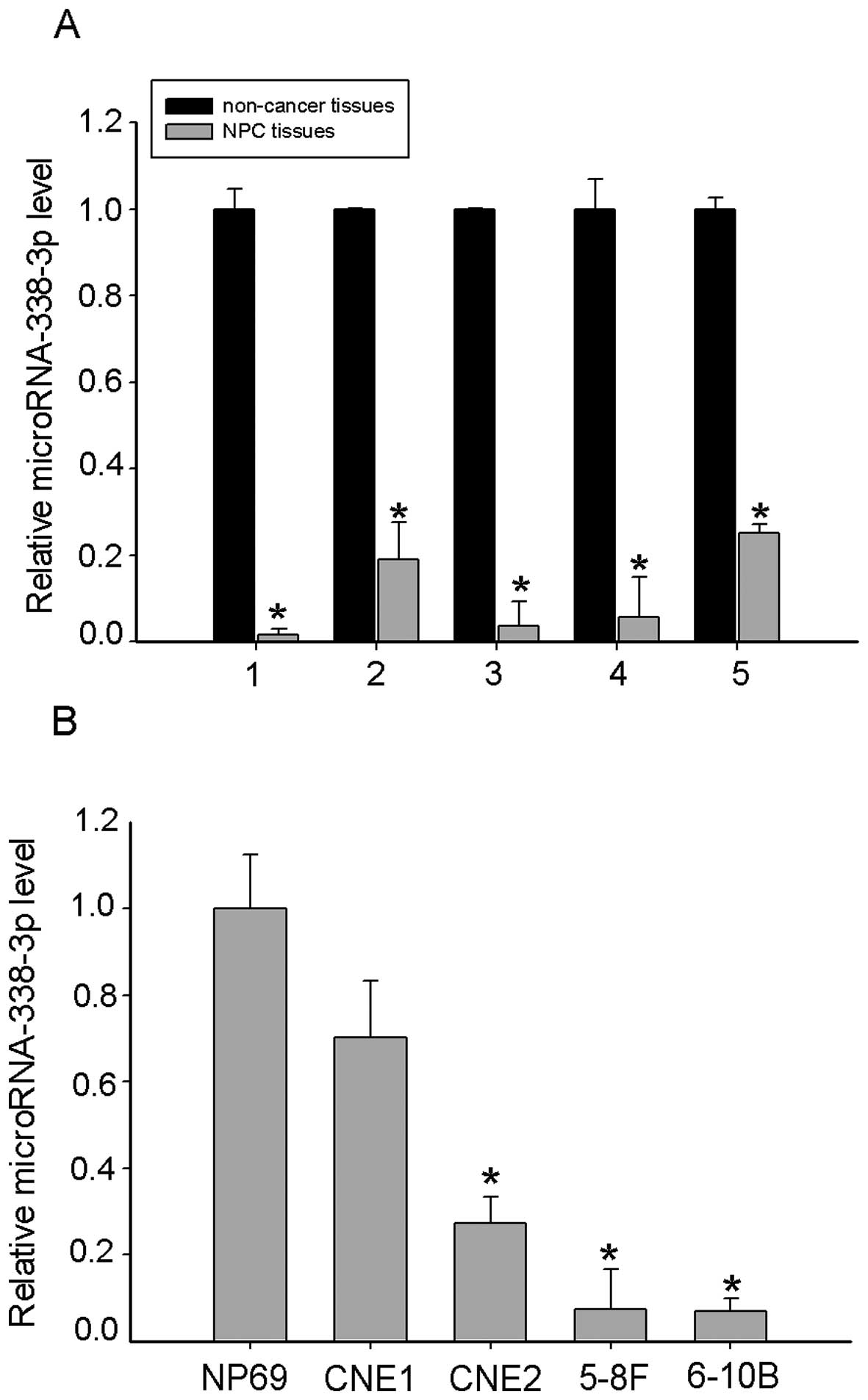
To determine whether miR-338-3p is involved in
regulation of human NPC tumorigenesis, we firstly tested miR-338-3p
expression in 5 freshly-frozen NPC and normal nasopharyngeal
epithelial tissue samples and found that the miR-338-3p expression
was significantly downregulated in NPC tissues (Fig. 1A). We also found miR-338-3p
under-expressed in NPC cell lines CNE1, CNE2, 6–10B and 5–8F,
compared to the normal nasopharyngeal epithelial cell line NP69
(Fig. 1B). Taken together, these
findings suggested that miR-338-3p was downregulated in NPC.
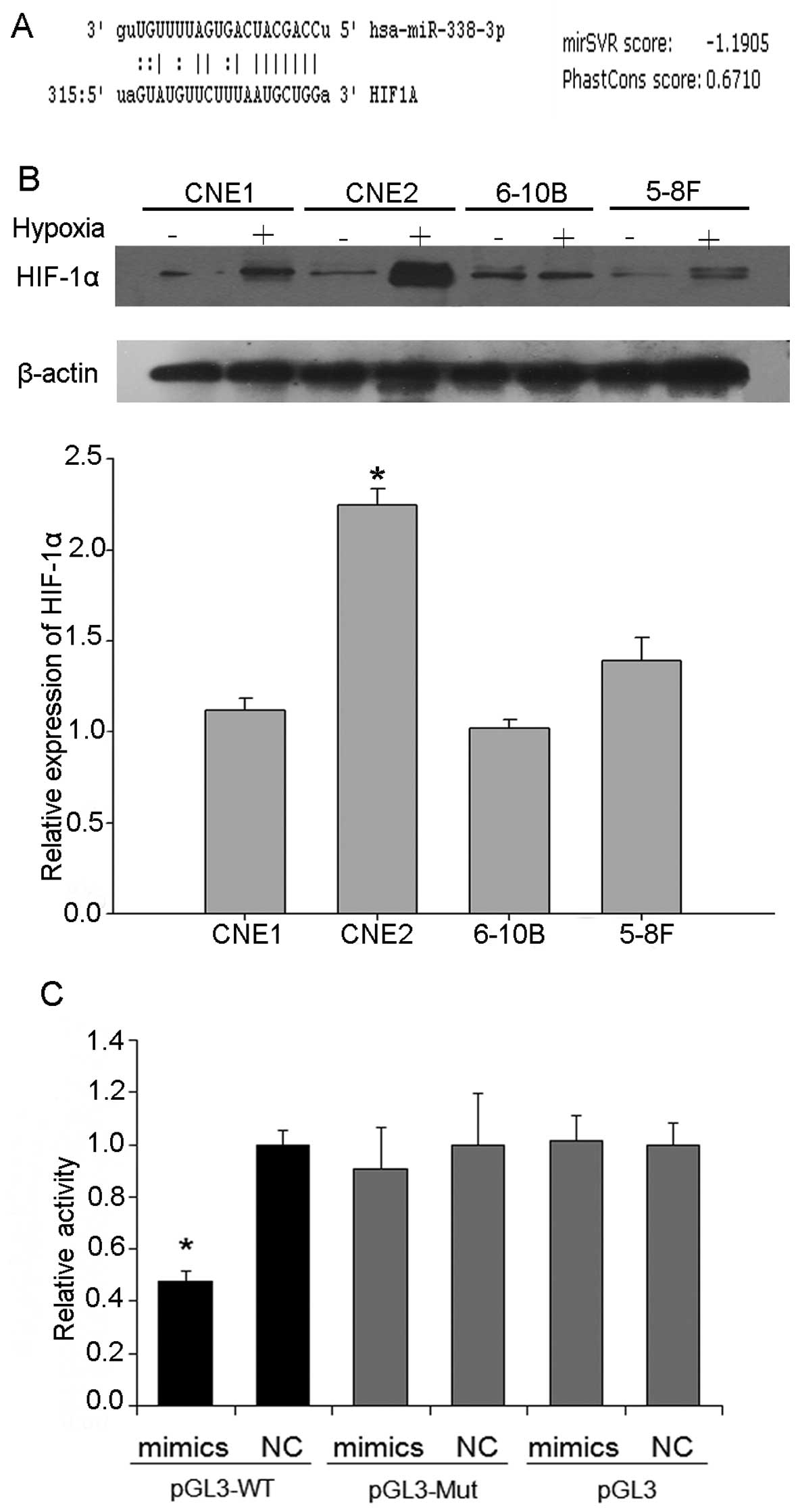
miR-338-3p directly targets oncogenic
HIF-1α
miRNAs have been shown to target specific genes to
regulate the progression of NPC. To identify miR-338-3p target
genes, we used the target prediction program, Bioinformatic
analysis using the TargetScan (http://www.targetscan.org) and miRanda algorithm
(http://www.microrna.org) indicated that the
3′-UTR of HIF-1α contained a predicted binding site for miR-338-3p
(Fig. 2A). HIF-1α increased in the
NPC cells under hypoxic conditions. Compared with the other NPC
cell lines CNE1, 6–10B and 5–8F, as shown by western blot analysis,
HIF-1α increased most in CNE2 cells (Fig. 2B). Then CNE2 cells were chosen for
the following experiments. HIF-1α has been reported to be an
important molecule that drives cancer cell proliferation, migration
and invasion including NPC. Using prediction tools, we predicted
that the target of miR-338-3p is HIF-1α. To demonstrate that
miR-338-3p directly targets HIF-1α by interacting with its 3′-UTR,
we co-transfected the pGL luciferase reporter plasmid harboring the
wild-type or mutant 3′-UTR of HIF-1α, along with miR-338-3p or NC
miRNA (Fig. 2C). Overexpressed
miR-338-3p resulted in significant reduction of HIF-1α 3′-UTR
firefly luciferase reporter activity containing wild-type but not
mutant binding sites compared to that of NC-miRNA. In summary,
these results indicate that HIF-1α was a direct target gene of
miR-338-3p in NPC cells.
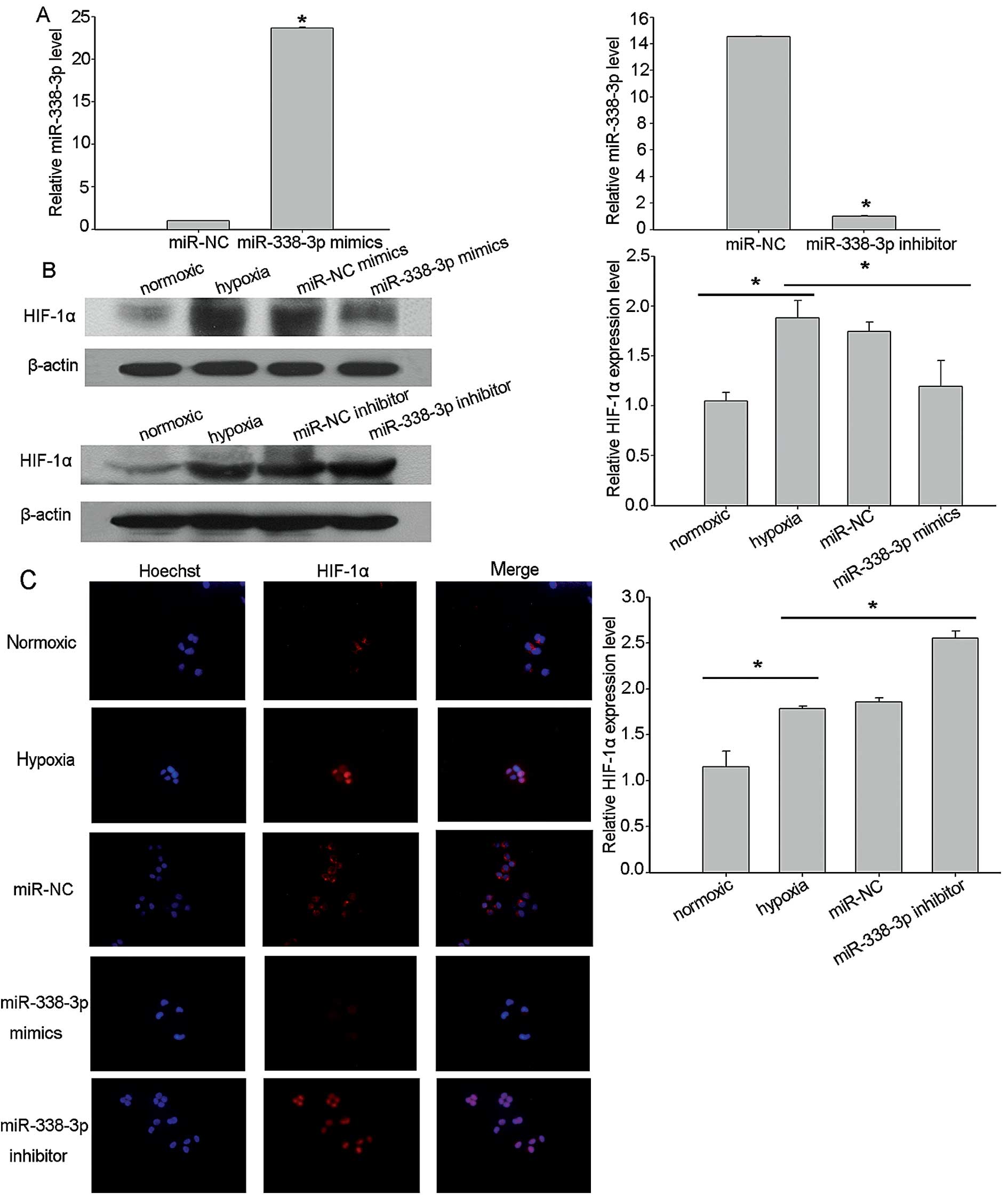
To examine the functional significance of miR-338-3p
in NPC, we infected the CNE2 cells with miR-338-3p mimics and
inhibitor, using miR-67 as control. Using RT-qPCR, we confirmed
that the miR-338-3p level had increased >20-fold after
transfection with miR-338-3p mimics compared with the expression of
cont-miR, while the level was downregulated after the miR-338-3p
inhibitor was delivered into CNE2 cells (Fig. 3A). To further investigate the
antitumor effect of miR-338-3p under hypoxic conditions and to
monitor the effect of miR-338-3p overexpression on HIF-1α
expression in target cells expressing low levels of miR-338-3p, NPC
cells transfected with miR-338-3p mimics, inhibitors and scrambled
control were exposed to hypoxic conditions for 24 h. To confirm
that miR-338-3p regulates HIF-1α expression, we assessed HIF-1α
protein levels in CNE2 cells expressing ectopic miR-338-3p, using
western blot analysis. The results showed that HIF-1α levels, under
hypoxia, were consistently downregulated by overexpressed
miR-338-3p in CNE2 cell lines (Fig.
3B). These results were substantiated by immunofluorescence
microscopy (Fig. 3C).
Immunofluorescence staining revealed that HIF-1α expression was
inhibited by transfection with miR-338-3p mimics and was promoted
by transfection with miR-338-3p inhibitor.
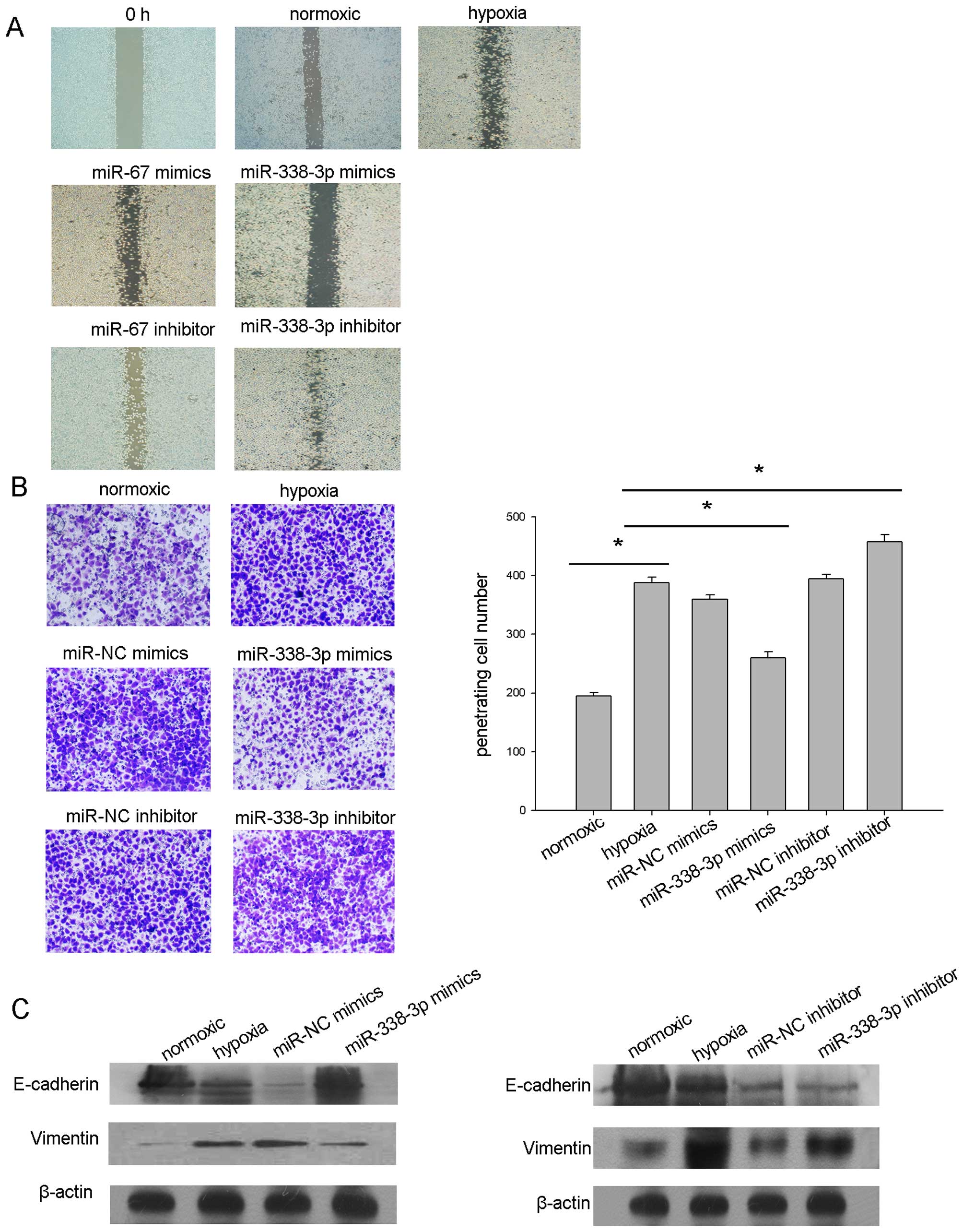
miR-338-3p regulates the metastasis
potential of human NPC cell lines in vitro
To examine the functional significance of
overexpressed miR-338-3p in NPC, we first investigated the
metastasis potential of CNE2 cells with exceptional expression of
miR-338-3p. Wound healing tests demonstrated that hypoxic condition
increased the metastasis capacity of CNE2 cells. However, the
overexpression of miR-338-3p significantly reduced the metastasis
of the CNE2 cells under hypoxia (Fig.
4A), the results were confirmed by Transwell analysis (Fig. 4B).
To further explore the molecular mechanism of
miR-338-3p on hypoxic signaling in NPC cells, the factors
responsible for hypoxia were investigated after the treatment of
hypoxic CNE2. Our previous study by western blot analysis disclosed
that miR-338-3p downregulated the expression level of HIF-1α,
showed that hypoxia selects for survival the more aggressive tumor
cells and induces epithelial to mesenchymal transition (EMT)
(29). We suspected that miRNAs
would affect EMT under hypoxia since hypoxia is a key factor in the
process of EMT. As shown here, exposured CNE2 cells to hypoxia
resulted in the transition to a mesenchymal morphology, significant
loss of E-cadherin and increased expression of vimentin. To assess
the effect of miR-338-3p on hypoxia-mediated EMT, miR-338-3p
mimics, inhibitor and scrambled control was transfected into CNE2
cells, and the cells were moved to the hypoxic incubator (1%
O2, 5% CO2, 37°C) for another 24-h
incubation. As shown in Fig. 4C,
the downstream E-cadherin was upregulated and vimentin was
downregulated in miR-338-3p mimic-pretreated CNE2,
respectively.
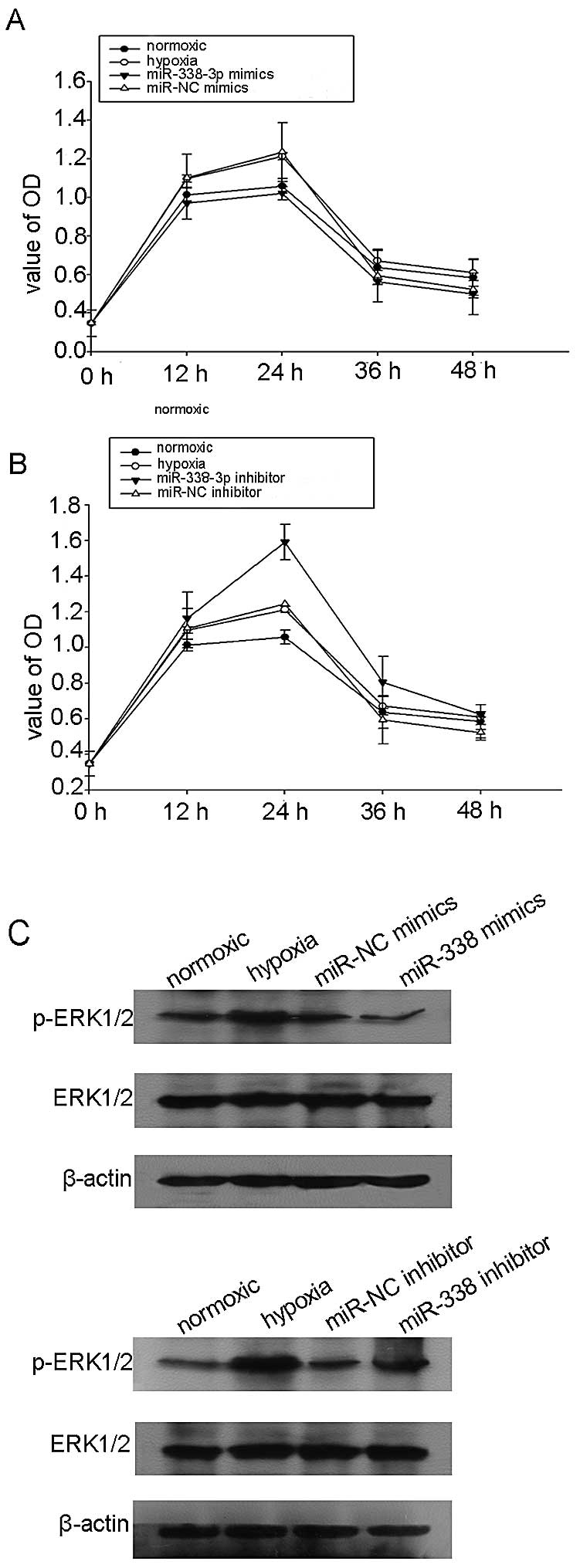
miR-338-3p suppresses NPC cell viability
under hypoxic conditions
In order to investigate the effect of miR-338-3p on
NPC cell proliferation, CCK8 assays were employed to analyze cell
proliferation. As shown in Fig. 5A,
overexpression of miR-338-3p in CNE2 cells significantly inhibited
cell proliferation at first 24 h under hypoxic conditions
(P<0.05), compared with the NC transfection. To further
demonstrate the effect of miR-338-3p on cell proliferation,
miR-338-3p inhibitor was transfected into CNE2 cells. Compared with
the mock group, the proliferation of the miR-338-3p inhibitor
transfected cells significantly increased (Fig. 5B). Previous evidence showed that
miR-338-3p regulated the phosphorylation of ERK1/2 to mediate tumor
cell metastasis and proliferation in gastric cancer (22). Thus, we assumed that miR-338-3p
could decrease the expression of the phosphorylation of Erk1/2 by
targeting HIF-1α under hypoxic conditions. Our data showed that the
overexpression of miR-338-3p inhibited the phosphorylation of
Erk1/2, but the relative expression level of total Erk1/2 was not
significantly altered (Fig. 5C).
Taken together, these results suggested anti-cell growth properties
of miR-338-3p in CNE2 cells.
miR-338-3p sensitizes NPC cells to
cisplatin
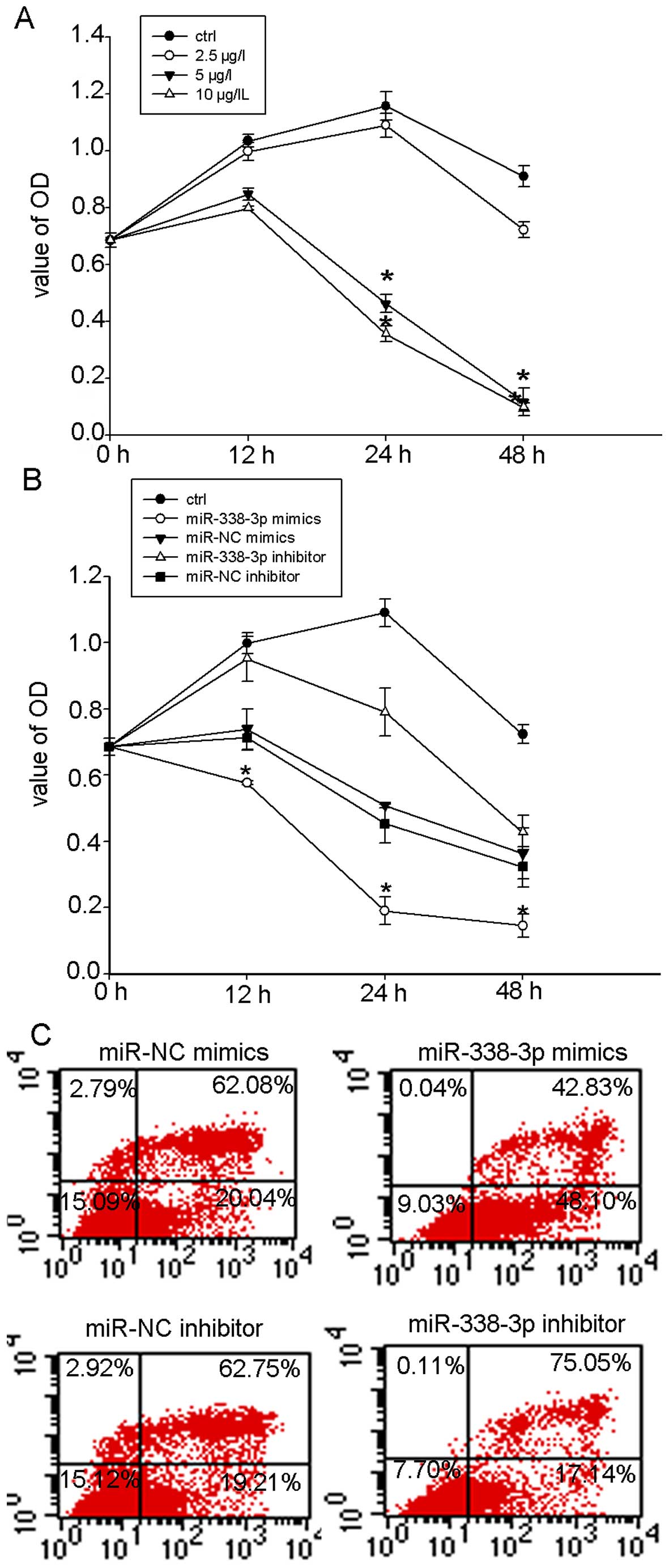
Because recent studies have reported that
destabilization of HIF-1α can overcome hypoxia-mediated cisplatin
resistance in non-small cell lung carcinoma (30), we then tested whether miR-338-3p
could sensitize NPC cells to chemotherapy treatment of cisplatin.
CCK8 assay showed that 5 and 10 µg/l cisplatin inhibited the
proliferation of CNE-2 cells in a dose- and time-dependent manner
(Fig. 6A). However, the inhibition
effect of 2.5 µg/l cisplatin on hypoxic CNE2 cells was not
obvious. To determine the chemo-sensitizing effects of miR-338-3p
on hypoxic NPC cells, we chose the treatment dose at 2.5
µg/l for the following experiments. We treated miR-338-3p
transfected cells and NC cells with cisplatin and measured the cell
viability. Cell viability (CCK8 assay) showed that non-transfected
CNE2 cells were highly resistant to cisplatin (2.5 µg/l)
under hypoxia and that transfection with miR-338-3p mimics
significantly reduced cisplatin resistance (Fig. 6B). To determine whether the
chemosensitizing effects of miR-338-3p on NPC cells result from the
induction of apoptosis, we treated miR-338-3p transfected CNE-2
cells in hypoxic conditions for 24 h. As shown in Fig. 6C, the rate of apoptotic cell death
was significant higher in cells treated with miR-338-3p mimics
(Fig. 6C). The above data supported
the indicated potential applications for miR-338-3p in anticancer
therapy.
Discussion
The present study focused on the antitumor effect of
miR-338-3p in NPC. We demonstrated that the overexpressed
miR-338-3p was able to inhibit the proliferation and migration of
hypoxic CNE2 cells as well as overcome hypoxia-mediated cisplatin
resistance.
NPC is a highly invasive malignancy, and up to 70%
of patients with NPC present with a locally advanced stage or with
cervical lymph node metastasis at the time of diagnosis (2,25).
Those patients have a poor outcome, and drug resistance may hamper
the efficacy of anticancer drugs (2). Therefore, clarification of the
molecular pathogenesis and mechanism of NPC is crucial for
developing effective therapy strategies to improve the outcome of
patients with this disease.
Growing evidence indicates that miRNAs hold great
promise for novel therapeutic approaches for treating human cancers
(28) and that hypoxia regulated
miRNAs exhibit induction in response to HIF activation and
participate in the development of angiogenesis and tumorigenesis
(31). Previous studies have
demonstrated that miR-15b, miR-16, miR-20a, and miR-20b are sharply
downregulated in CNE cells during hypoxia (31).
Many studies have reported on the tumor suppressive
effects of miR-338-3p in the malignant processes of various
cancers, such as gastric cancer (19) hepatocellular carcinoma (20) and malignant melanoma (21). However, there is little knowledge on
miR-338-3p and its targets in NPC. Our data showed that miR-338-3p
expression is markedly downregulated in NPC patient samples and NPC
cell lines as compared to immortalized nasopharyngeal epithelial
cells.
Predicted targets of miR-338-3p are elements
involved in many biological processes, such as cell proliferation,
differentiation, metastasis and cell death in various types of
cancer (28,32). Similar to previous studies, our data
identified HIF-1α as a key target of miR-338-3p. HIF-1α is an
important hypoxia transcription molecule promoting angiogenesis and
metastasis.
Hypoxia is one of the fundamental biological
properties that are associated with the development and
aggressiveness of a variety of solid tumors (33,34),
including NPC (24). Previous study
shows that all primary NPC tumors contain hypoxic regions which
result in decreased local control and increased distant metastases,
as well as resistance to chemotherapy in NPC patients (25). It was recently reported that hypoxia
inducible factor (HIF) results in global transcriptional changes in
gene expression, playing an important role in promoting tumor
progression, angiogenesis and metastasis (34,35).
In the present study, we showed evidence that the HIF-1α levels
were consistently downregulated by overexpressed miR-338-3p in CNE2
cell lines under hypoxia conditons. Previous studies have shown
that the oxygen-sensitive HIF-1α subunit accumulates and
trans-locates into the nucleus under hypoxia conditions, where it
binds to the constitutively expressed HIF-1β, forming the active
HIF-1 heterodimer (36). In the
present study, immunofluorescence staining results revealed that
HIF-1α was accumulated into the nuclei in hypoxia cells. However,
the overall staining and nuclear accumulation of HIF-1α was reduced
after miR-338-3p transfection. Furthermore, overexpressed
miR-338-3p inhibited cell viability as well as metastasis ability
by directly targeting HIF-1α.
HIF-1α activates some important oxygen modulated
genes critically involved in tumor angiogenesis and metastasis
(37). EMT is clearly associated
with pathological processes requiring epithelial cell migration and
invasion (2,38). Furthermore, HIF-1α also initiates
endothelial to mesenchymal transition (39). Our data showed that the
over-expressed miR-338-3p inhibited the hypoxia-induced EMT.
HIF-1α has attracted considerable interest as a
potential target in cancer therapy (37). Notably, growing evidence suggests
that higher HIF-1α levels in tumors are related to radioresistance
and poor clinical outcome in different tumor entities (40). Some evidence shows that
HIF-1α-deficient cells are more sensitive to radiotherapy and
chemotherapy compared with normal cells (41), however, the mechanism needs further
investigation. Therefore, the specific inhibition of HIF may
enhance cancer radiosensitivity in clinical settings. Our results
showed that miR-338-3p down-regulated the level of HIF-1α in CNE2
under hypoxic conditions and could overcome hypoxia-mediated
cisplatin resistance.
Previous studies have shown that other cell
regulatory elements such as cyclin D, and smoothened, are also
targets of miR-338-3p that are aberrantly expressed due to the
down-regulation of miR-338-3p in HCC (28). Undoubtedly, regulation of these
other targets may contribute to the inhibitory effects of
miR-338-3p on NPC. However, considering our observation that HIF-1α
overexpression rescued the cell from the anti-NPC activity of
miR-338-3p, it is likely that regulation of HIF-1α by miR-338-3p is
a key antitumor aspect in NPC. Our further studies will focus on
other targets of miR-338-3p and their specific roles under both
normoxic and hypoxic conditions.
In conventional clinical therapy of NPC,
radiotherapy is the primary therapeutic approach, while using
radiotherapy combined with chemotherapy is recommended for the
treatment of advanced carcinoma (42). Treatment failure rates remain high
and the 5-year survival rate is extremely low (42). One reason for the treatment failure
may be attributed to the drug resistance and distant metastasis
(42). Evidence shows that
destabilization of HIF-1α can overcome hypoxia-mediated cisplatin
resistance in non-small cell lung carcinoma (30). We want to determine whether
miR-338-3p potentiates sensitivity of NPC cells to cisplatin, which
is clinically used as adjuvant therapy for NPC in order to induce
tumor cell death (42). Our results
showed that under hypoxic conditions, CNE2 cells are highly
resistant to cisplatin in vitro. Moreover, cells
pre-transfected with miR-338-3p can overcome hypoxia-mediated
cisplatin resistance. These results enriched the function of
miR-338-3p in addition to its role as a tumor suppressor.
In conclusion, we found that miR-338-3p was
downregulated in NPC clinical samples and cell lines. HIF-1α was
verified as a direct target of miR-338-3p, and involved in NPC cell
growth and invasion. Furthermore, our data suggest that miR-338-3p
and/or its target gene HIF-1α could represent important therapeutic
targets in NPC. We explored its effects on cell growth, invasive
and chemotherapy resistance. The miR-338-3p/HIF-1α pathway may help
to understand the mechanisms of NPC progression and would provide a
novel therapeutic strategy for NPC.
Acknowledgments
The present study was supported by grants from the
Chinese National Natural Science Foundation (nos. 81172841,
81202368 and 81471603), the China Postdoctoral Science Foundation
(2013M541708); the project of '333 Natural Science Foundation' of
Jiangsu Grant (BRA2013286); the 'Top Six Types of Talents'
Financial Assistance of Jiangsu Province Grant (no. 6); the project
of Jiangsu Provincial Health Department (Z201005); the innovative
project o Nantong University postgraduate students (13025043); the
Jiangsu province's Outstanding Medical Academic Leader Program
(LJ201136); and the Natural Science Foundation of Jiangsu Province
(SBK2015022581).
References
|
1
|
Liu N, Jiang N, Guo R, Jiang W, He QM, Xu
YF, Li YQ, Tang LL, Mao YP, Sun Y, et al: MiR-451 inhibits cell
growth and invasion by targeting MIF and is associated with
survival in nasopharyngeal carcinoma. Mol Cancer. 12:1232013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aga M, Bentz GL, Raffa S, Torrisi MR,
Kondo S, Wakisaka N, Yoshizaki T, Pagano JS and Shackelford J:
Exosomal HIF1α supports invasive potential of nasopharyngeal
carcinoma-associated LMP1-positive exosomes. Oncogene.
33:4613–4622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krutilina R, Sun W, Sethuraman A, Brown M,
Seagroves TN, Pfeffer LM, Ignatova T and Fan M: MicroRNA-18a
inhibits hypoxia-inducible factor 1α activity and lung metastasis
in basal breast cancers. Breast Cancer Res. 16:R782014. View Article : Google Scholar
|
|
4
|
Long Z, Wang B, Tao D, Huang Y and Tao Z:
Hypofractionated radiotherapy induces miR-34a expression and
enhances apoptosis in human nasopharyngeal carcinoma cells. Int J
Mol Med. 34:1388–1394. 2014.PubMed/NCBI
|
|
5
|
Zhou W, Fong MY, Min Y, Somlo G, Liu L,
Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al:
Cancer-secreted miR-105 destroys vascular endothelial barriers to
promote metastasis. Cancer Cell. 25:501–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suh YE, Raulf N, Gäken J, Lawler K, Urbano
TG, Bullenkamp J, Gobeil S, Huot J, Odell E and Tavassoli M:
MicroRNA-196a promotes an oncogenic effect in head and neck cancer
cells by suppressing annexin A1 and enhancing radioresistance. Int
J Cancer. 137:1021–1034. 2014. View Article : Google Scholar
|
|
7
|
Huang XH, Chen JS, Wang Q, Chen XL, Wen L,
Chen LZ, Bi J, Zhang LJ, Su Q and Zeng WT: miR-338–3p suppresses
invasion of liver cancer cell by targeting smoothened. J Pathol.
225:463–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu ZJ, Lu LG, Tao KZ, Chen DF, Xia Q, Weng
JJ, Zhu F, Wang XP and Zheng P: MicroRNA-185 suppresses growth and
invasion of colon cancer cells through inhibition of the
hypoxia-inducible factor-2α pathway in vitro and in vivo. Mol Med
Rep. 10:2401–2408. 2014.PubMed/NCBI
|
|
9
|
Luo Z, Dai Y, Zhang L, Jiang C, Li Z, Yang
J, McCarthy JB, She X, Zhang W, Ma J, et al: miR-18a promotes
malignant progression by impairing microRNA biogenesis in
nasopharyngeal carcinoma. Carcinogenesis. 34:415–425. 2013.
View Article : Google Scholar
|
|
10
|
Liu N, Tang LL, Sun Y, Cui RX, Wang HY,
Huang BJ, He QM, Jiang W and Ma J: MiR-29c suppresses invasion and
metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer
Lett. 329:181–188. 2013. View Article : Google Scholar
|
|
11
|
Zhang JX, Qian D, Wang FW, Liao DZ, Wei
JH, Tong ZT, Fu J, Huang XX, Liao YJ, Deng HX, et al: MicroRNA-29c
enhances the sensitivities of human nasopharyngeal carcinoma to
cisplatin-based chemotherapy and radiotherapy. Cancer Lett.
329:91–98. 2013. View Article : Google Scholar
|
|
12
|
Alajez NM, Lenarduzzi M, Ito E, Hui AB,
Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al: MiR-218
suppresses nasopha-ryngeal cancer progression through
downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res.
71:2381–2391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF, et al: MiR-26a inhibits cell
growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji Y, He Y, Liu L and Chong X: MiRNA-26b
regulates the expression of cyclooxygenase-2 in
desferrioxamine-treated CNE cells. FEBS Lett. 584:961–967. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng M, Tang H, Zhou Y, Zhou M, Xiong W,
Zheng Y, Ye Q, Zeng X, Liao Q, Guo X, et al: miR-216b suppresses
tumor growth and invasion by targeting KRAS in nasopharyngeal
carcinoma. J Cell Sci. 124:2997–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li G, Wu Z, Peng Y, Liu X, Lu J, Wang L,
Pan Q, He ML and Li XP: MicroRNA-10b induced by Epstein-Barr
virus-encoded latent membrane protein-1 promotes the metastasis of
human nasopharyngeal carcinoma cells. Cancer Lett. 299:29–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Deng T, Li X, Liu H, Zhou H, Ma
J, Wu M, Zhou M, Shen S, Li X, et al: microRNA-141 is involved in a
nasopharyngeal carcinoma-related genes network. Carcinogenesis.
31:559–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia H, Ng SS, Jiang S, Cheung WK, Sze J,
Bian XW, Kung HF and Lin MC: miR-200a-mediated downregulation of
ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma
cell growth, migration and invasion. Biochem Biophys Res Commun.
391:535–541. 2010. View Article : Google Scholar
|
|
19
|
Guo B, Liu L, Yao J, Ma R, Chang D, Li Z,
Song T and Huang C: miR-338–3p suppresses gastric cancer
progression through a PTEN-AKT axis by targeting P-REX2a. Mol
Cancer Res. 12:313–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang XH, Wang Q, Chen JS, Fu XH, Chen XL,
Chen LZ, Li W, Bi J, Zhang LJ, Fu Q, et al: Bead-based microarray
analysis of microRNA expression in hepatocellular carcinoma:
miR-338 is downregulated. Hepatol Res. 39:786–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caramuta S, Egyházi S, Rodolfo M, Witten
D, Hansson J, Larsson C and Lui WO: MicroRNA expression profiles
associated with mutational status and survival in malignant
melanoma. J Invest Dermatol. 130:2062–2070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
MicroRNA-338 inhibits growth, invasion and metastasis of gastric
cancer by targeting NRP1 expression. PLoS One. 9:e944222014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kucharzewska P, Christianson HC, Welch JE,
Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain
E, Bengzon J and Belting M: Exosomes reflect the hypoxic status of
glioma cells and mediate hypoxia-dependent activation of vascular
cells during tumor development. Proc Natl Acad Sci USA.
110:7312–7317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Li X, Wu S, Xu G, Zhou Y, Gong L,
Li Z and Yang D: Expression of HIF-1α and CAIX in nasopharyngeal
carcinoma and their correlation with patients' prognosis. Med
Oncol. 31:3042014. View Article : Google Scholar
|
|
25
|
Pan WL, Wong JH, Fang EF, Chan YS, Ng TB
and Cheung RC: Preferential cytotoxicity of the type I ribosome
inactivating protein alpha-momorcharin on human nasopharyngeal
carcinoma cells under normoxia and hypoxia. Biochem Pharmacol.
89:329–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xueguan L, Xiaoshen W, Yongsheng Z, Chaosu
H, Chunying S and Yan F: Hypoxia inducible factor-1 alpha and
vascular endothelial growth factor expression are associated with a
poor prognosis in patients with nasopharyngeal carcinoma receiving
radiotherapy with carbogen and nicotinamide. Clin Oncol (R Coll
Radiol). 20:606–612. 2008. View Article : Google Scholar
|
|
27
|
Hui EP, Chan AT, Pezzella F, Turley H, To
KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, et al: Coexpression of
hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX,
and vascular endothelial growth factor in nasopharyngeal carcinoma
and relationship to survival. Clin Cancer Res. 8:2595–2604.
2002.PubMed/NCBI
|
|
28
|
Xu H, Zhao L, Fang Q, Sun J, Zhang S, Zhan
C, Liu S and Zhang Y: MiR-338–3p inhibits hepatocarcinoma cells and
sensitizes these cells to sorafenib by targeting hypoxia-induced
factor 1α. PLoS One. 9:e1155652014. View Article : Google Scholar
|
|
29
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for Gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fischer C, Leithner K, Wohlkoenig C,
Quehenberger F, Bertsch A, Olschewski A, Olschewski H and Hrzenjak
A: Panobinostat reduces hypoxia-induced cisplatin resistance of
non-small cell lung carcinoma cells via HIF-1α destabilization. Mol
Cancer. 14:42015. View Article : Google Scholar
|
|
31
|
Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L,
Wang H, Huang C and Sun S: Hypoxia-induced down-regulation of
microRNA-34a promotes EMT by targeting the Notch signaling pathway
in tubular epithelial cells. PLoS One. 7:e307712012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun K, Deng HJ, Lei ST, Dong JQ and Li GX:
miRNA-338–3p suppresses cell growth of human colorectal carcinoma
by targeting smoothened. World J Gastroenterol. 19:2197–2207. 2013.
View Article : Google Scholar :
|
|
33
|
Bao B, Azmi AS, Ali S, Ahmad A, Li Y,
Banerjee S, Kong D and Sarkar FH: The biological kinship of hypoxia
with CSC and EMT and their relationship with deregulated expression
of miRNAs and tumor aggressiveness. Biochim Biophys Acta.
1826:272–296. 2012.PubMed/NCBI
|
|
34
|
King HW, Michael MZ and Gleadle JM:
Hypoxic enhancement of exosome release by breast cancer cells. BMC
Cancer. 12:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Li K, Gu Y, Feng B, Ren G, Zhang
L, Wang Y, Nie Y and Fan D: Transcriptional up-regulation of RhoE
by hypoxia-inducible factor (HIF)-1 promotes epithelial to
mesenchymal transition of gastric cancer cells during hypoxia.
Biochem Biophys Res Commun. 415:348–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo Z, Bai M, Xiao X, Zhang W, Liu X, Yang
X, Li S, Huan Y, Wu Z, Zhang X, et al: Silencing of HIF-1α enhances
the radiation sensitivity of human glioma growth in vitro and in
vivo. Neuropharmacology. 89:168–174. 2015. View Article : Google Scholar
|
|
38
|
Li X, Zhang Z, Beiter T and Schluesener
HJ: Nanovesicular vaccines: Exosomes. Arch Immunol Ther Exp
(Warsz). 53:329–335. 2005.
|
|
39
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor -1α in hepatocellular carcinoma. BMC
Cancer. 13:1082013. View Article : Google Scholar
|
|
40
|
Meijer TW, Kaanders JH, Span PN and
Bussink J: Targeting hypoxia, HIF-1, and tumor glucose metabolism
to improve radiotherapy efficacy. Clin Cancer Res. 18:5585–5594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasabe E, Zhou X, Li D, Oku N, Yamamoto T
and Osaki T: The involvement of hypoxia-inducible factor-1alpha in
the susceptibility to gamma-rays and chemotherapeutic drugs of oral
squamous cell carcinoma cells. Int J Cancer. 120:268–277. 2007.
View Article : Google Scholar
|
|
42
|
Zhang P, Liu H, Xia F, Zhang QW, Zhang YY,
Zhao Q, Chao ZH, Jiang ZW and Jiang CC: Epithelial-mesenchymal
transition is necessary for acquired resistance to cisplatin and
increases the metastatic potential of nasopharyngeal carcinoma
cells. Int J Mol Med. 33:151–159. 2014.
|