Introduction
Prostate cancer is one of the leading cause of
cancer-related death in males, second only to lung cancer (1). Multiple genetic and epigenetic factors
have been implicated in the oncogenesis and progression of prostate
cancer. Prostate cancer initially responds well to androgen
deprivation, but this treatment results in the emergence of
androgen-independent disease that is resistant to apoptosis
(2).
HS1-associated protein X-1 (HAX-1) originally
identified as a 35 kDa protein that interacts with HS1, is a
substrate of Src family tyrosine kinases (3). The HAX-1 gene is ubiquitously
expressed among tissues (4), and
its protein is localized mainly in mitochondria, and also in
endoplasmic reticulum and nuclear envelope in the cells.
HAX-1 was suggested to be involved in the regulation
of apoptosis (programmed cell death) (3). As an anti-apoptosis factor, the
anti-apoptotic, cell-protecting properties of HAX-1 as well as its
interactions with apoptosis-related proteins have been widely
reported (5,6). HAX-1 has been reported to protect the
cultured cells against the challenge of H2O2
(7). HAX-1 is highly expressed in
colorectal cancer and contributes to the malignant progression and
predict poor prognosis for patients with colorectal cancer
(8). So far, the expression levels
and the effect of HAX-1 in prostate cancer remain unclear.
Caspase-9, a protein also localized mainly in
mitochondria is a key regulatory player in the
mitochondria-mediated apoptosis pathway. Activated caspase-9
directly cleaves and activates caspase-3 and -7, resulting in the
biochemical destruction of the cells (9). The inhibitor of apoptosis proteins
(IAPs) have been thought to be the only class of proteins that
directly inhibits caspase-9 as previously described (10), however, new mechanistic evidence
showed that HAX-1 averts cell death by blocking the biological
activation of caspase-9.
In the present study, we investigated the expression
of HAX-1 in prostate cancer cell lines, PC3, VCaP and DU145. The
role and the underlying mechanism of HAX-1 and caspase-9
interaction in prostate cancer cell apoptosis were also
explored.
Materials and methods
Cell lines and cell culture
The primary human prostate epithelial cells and the
human prostate cancer cell lines PC3, VCaP and DU145 were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The primary human prostate epithelial cells were cultured in
Dulbecco's modified Eagle's medium (DMEM), and the human prostate
cancer cell lines PC3, VCaP and DU145 were cultured in RPMI-1640
supplemented with 10% fetal bovine serum (FBS), 2 mmol/l GlutaMAX
and 1% antibiotics (Invitrogen, Carlsbad, CA, USA) at 37°C in a
humidified 5% CO2 incubator. These cells were maintained
in the appropriate medium and passaged every three days.
siRNA transfection
HAX-1 and control siRNAs were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). For transfection,
5×104 cells were seeded in each well of 24-well
microplates and grown for 24 h to reach 60–65% confluency, and then
incubated with a mixture of siRNA and Lipofectamine 2000 reagent
(Invitrogen) in 100 µl of serum-free Opti-MEM according to
the manufacturer's instructions. The transfection efficiency was
examined by real-time PCR and western blotting.
Construction of expression vectors and
cell transfection
Total RNA from cells were isolated using TRIzol
reagent (Invitrogen), and then converted to cDNA using the
PrimeScript® RT reagent kit (Takara, Dalian, China) with
oligo(dT) primers. Then, the open reading frame of caspase-9 cDNA
was cloned and inserted into the pcDNA3.1 vector (Invitrogen) to
construct the recombinant pcDNA3.1-caspase-9 expression vector.
Control and pcDNA3.1 vector transfected cells were also prepared.
For cell transfection, cells were cultured to 60% confluency and
transfection was performed using the FuGENE HD transfection reagent
(Roche, Indianapolis, IN, USA) method as suggested by the
manufacturer.
Real-time PCR (RT-PCR)
Total RNA was isolated as previously described.
Primers for HAX-1 were designed as: 5′-AAC CAGAGAGGACAATGATCT-3′
(sense), and 5′-AAGTTGTCC AAAGAAACCTGT-3′ (antisense). β-actin was
used as the normalization control for HAX-1 and caspase-9 gene. The
25 µl reaction mixture contained 12.5 µl 2X OneStep
qRT-PCR buffer, 0.5 µM reverse and 0.5 µM forward
primer, 0.9 µl enzymix, 90 ng RNA template and 0.5 µM
probe. PCR conditions for the reverse transcription used to obtain
cDNA were as follows: 45°C for 10 min, pre-denaturation at 95°C for
10 min and then 45 cycles of 95°C for 15 sec and 60°C for 45 sec;
this was performed using the ABI 7500 Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA). Real-time RT-PCR was performed
in a Rotor-Gene RG-3000 Real-Time Thermal Cycler (Corbett Research,
Sydney, Australia).
Western blotting
The proteins were extracted from cells using RIPA
lysis buffer (Beyotime, Nantong, China). For western blotting,
equal amounts of proteins were separated on SDS-PAGE and blotted
onto a pre-wet nitrocellulose membrane (GE Healthcare, Germany),
followed by blocking of membranes in 10% defatted milk in
phosphate-buffered saline (PBS) at 4°C overnight, the membranes
were then probed with different primary antibodies. The primary
antibodies were as follows: mouse anti-HAX-1, mouse anti-Bcl-2,
mouse anti-Bax and mouse anti-β-actin antibodies (Santa Cruz
Biotechnology), rabbit anti-caspase-9 antibody (Abcam, Cambridge,
UK). After washing with TBST buffer, the membranes were incubated
for 1 h at 25°C with HRP-conjugated secondary antibodies. ECL
reagent was used for detection. The fluorescence was scanned using
a Typhoon scanner (Amersham Biosciences, Piscataway, NJ, USA). All
experiments were performed in triplicate.
Cell viability
The cell viability measurements were carried out
using the MTT assay. Approximately 5×104 cells were
seeded into 96-well plates, washed twice with PBS and 10 µl
of MTT was added to each well. Then, the cells were incubated at
37°C for 2 h and 100 µl dimethylsulfoxide (DMSO) was added
to dissolve the formazan crystals. Absorbance was measured at 560
nm with a SpectraMax Paradigm Multi-Mode Reader (Molecular Devices,
Austria).
Cell apoptosis
Flow cytometry was used to analyze cell apoptosis
and Annexin V-propidium iodide (AV-PI) staining was performed.
Briefly, after treatment cells were harvested and washed three
times with PBS. Following centrifugation for 10 min, cells were
resuspended in 500 µl of binding buffer including 5
µl FITC-conjugated Annexin V, the mixture was incubated in
the dark for 10 min, and then 5 µl of PI was added.
Ultimately, all specimens were assessed by flow cytometry with a
FACSCalibur using CellQuest software (BD Biosciences, San Jose, CA,
USA), and all the results are shown as a percentage of total cells,
to quantitatively evaluate the rate of apoptosis.
Caspase-9 activity
The caspase-9 activity was assayed using the
Caspase-9 Assay kit (Abcam), according to the manufacture's
instruction. The fresh protein lysates from cells were prepared
using cell lysis buffer. Then, 85 µl of reaction buffer and
5 µl of LEHD-pNA (Leu-Glu-His-Asp-p-nitroanilide) were added
to each sample and incubated at 37°C for 2 h. The absorbance was
measured in an ELISA reader (Labsystems, Helsinki, Finland) at 405
nm.
Statistical analysis
The SPSS version 19.0 software (SPSS, Inc., Chicago,
IL, USA) was used to analyze the related data with χ2 or
t-tests. The results were considered to indicate a statistically
significant result at P<0.05.
Results
HAX-1 is highly expressed in human
prostate cancer cell lines
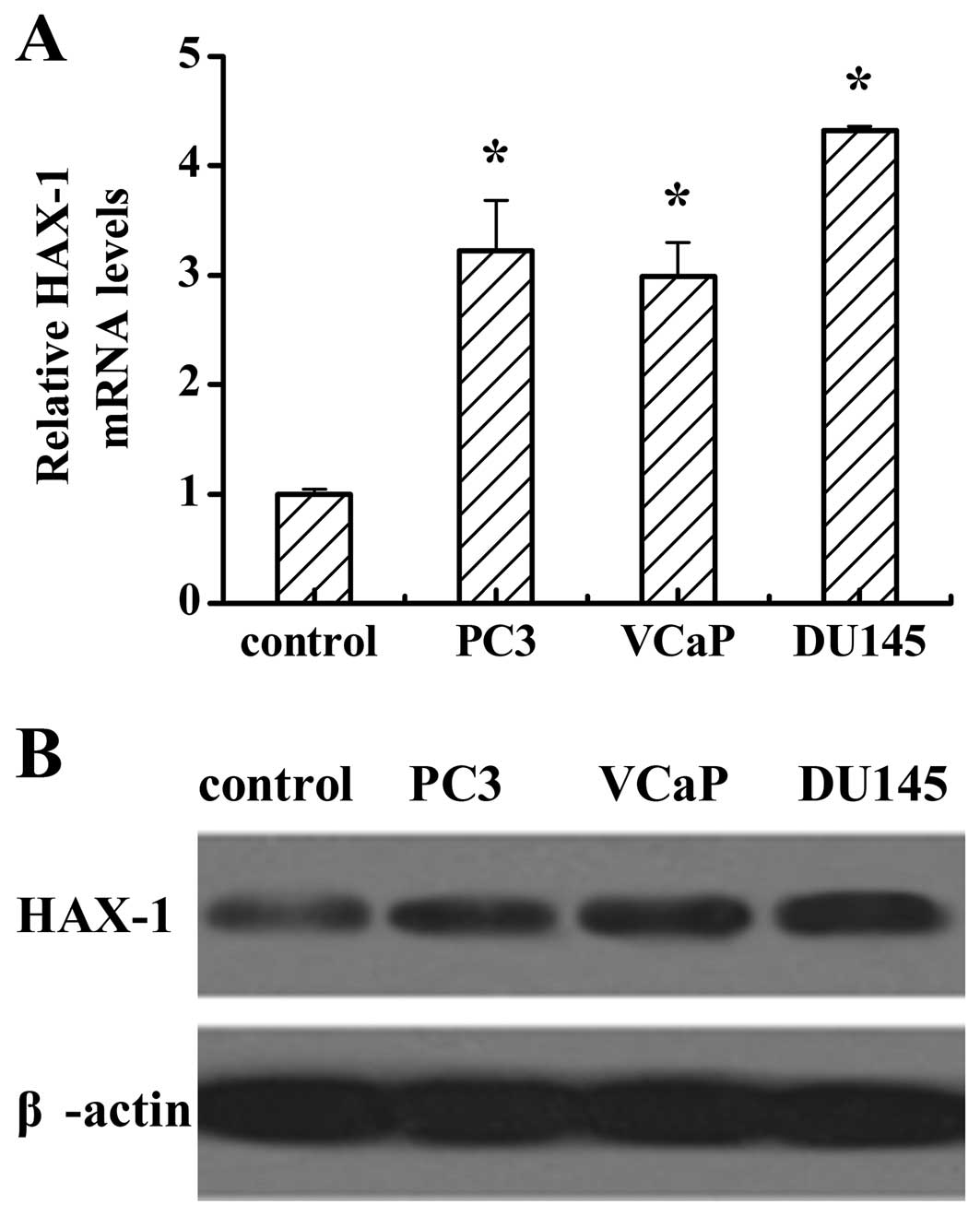
To investigate the expression of HAX-1 in human
prostate cancer cells, we used RT-PCR to detect HAX-1 mRNA levels
and western blotting to analyze the protein levels in normal human
primary prostate epithelial cells, and the prostate cancer cell
lines PC3, VCaP and DU145. RT-PCR analysis showed that the mRNA
levels of HAX-1 in the prostate cancer cell lines were higher than
that in the normal cells (Fig. 1A).
Consistent with the results of mRNA levels, the protein levels in
different prostate cancer cells were higher than that in the normal
cells (Fig. 1B). It is noteworthy,
that both the mRNA and protein levels of HAX-1 in DU145 were higher
than those in PC3 and VCaP.
Expression levels of HAX-1 in HAX-1
knockdown DU145 cells
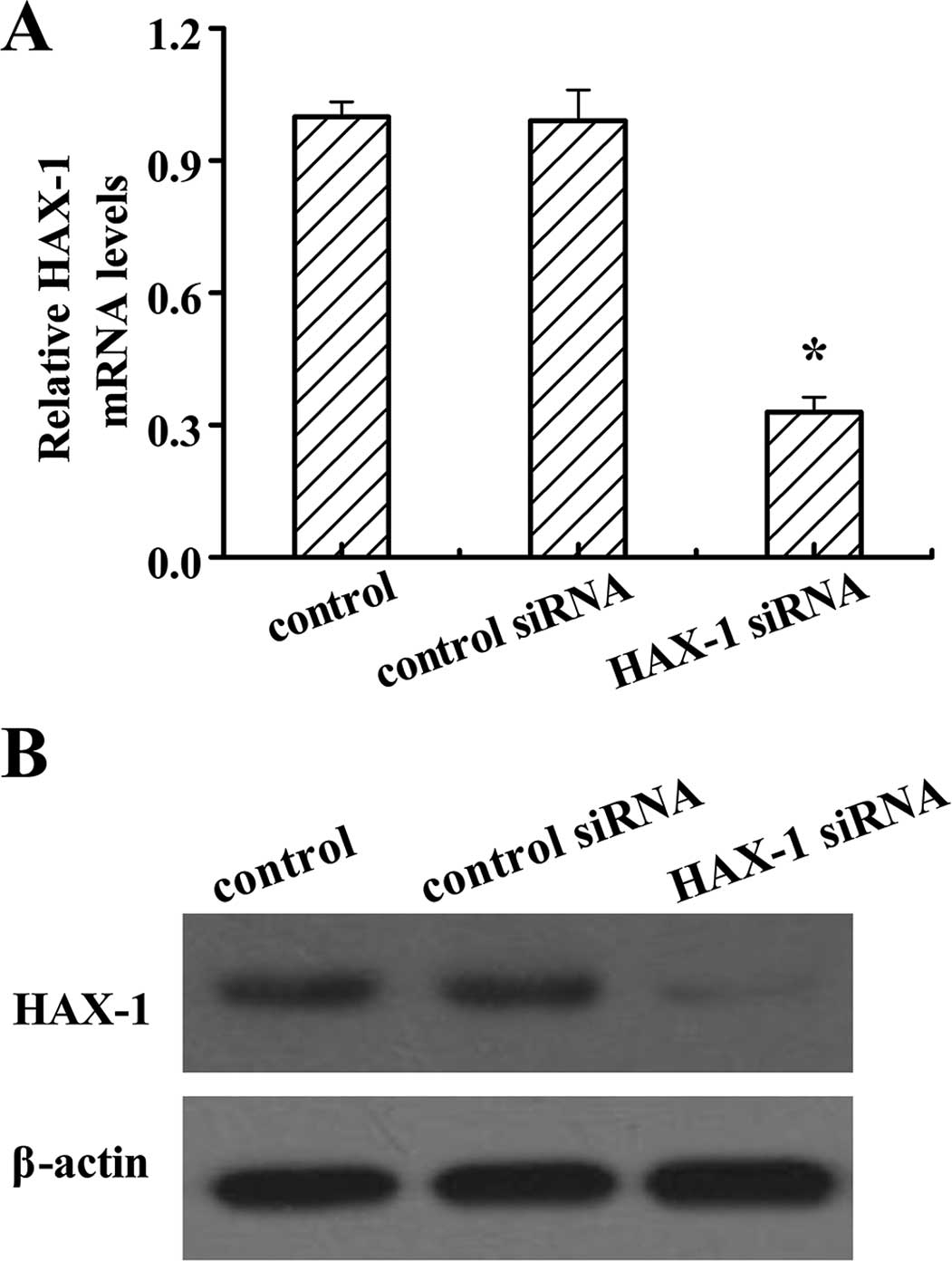
On the basis that the expression of HAX-1 is higher
in the DU145 cells than the other two prostate cancer cell lines,
PC3 and VCaP, we generated HAX-1 knockdown DU145 cells by HAX-1
siRNA to investigate the function of HAX-1 in prostate cancer
cells. After HAX-1 siRNA treatment, the mRNA and protein levels of
HAX-1 were significantly decreased (Fig. 2). These results confirmed that the
HAX-1 knockdown-DU145 cells were successfully established.
Effect of HAX-1 knockdown on cell
proliferation
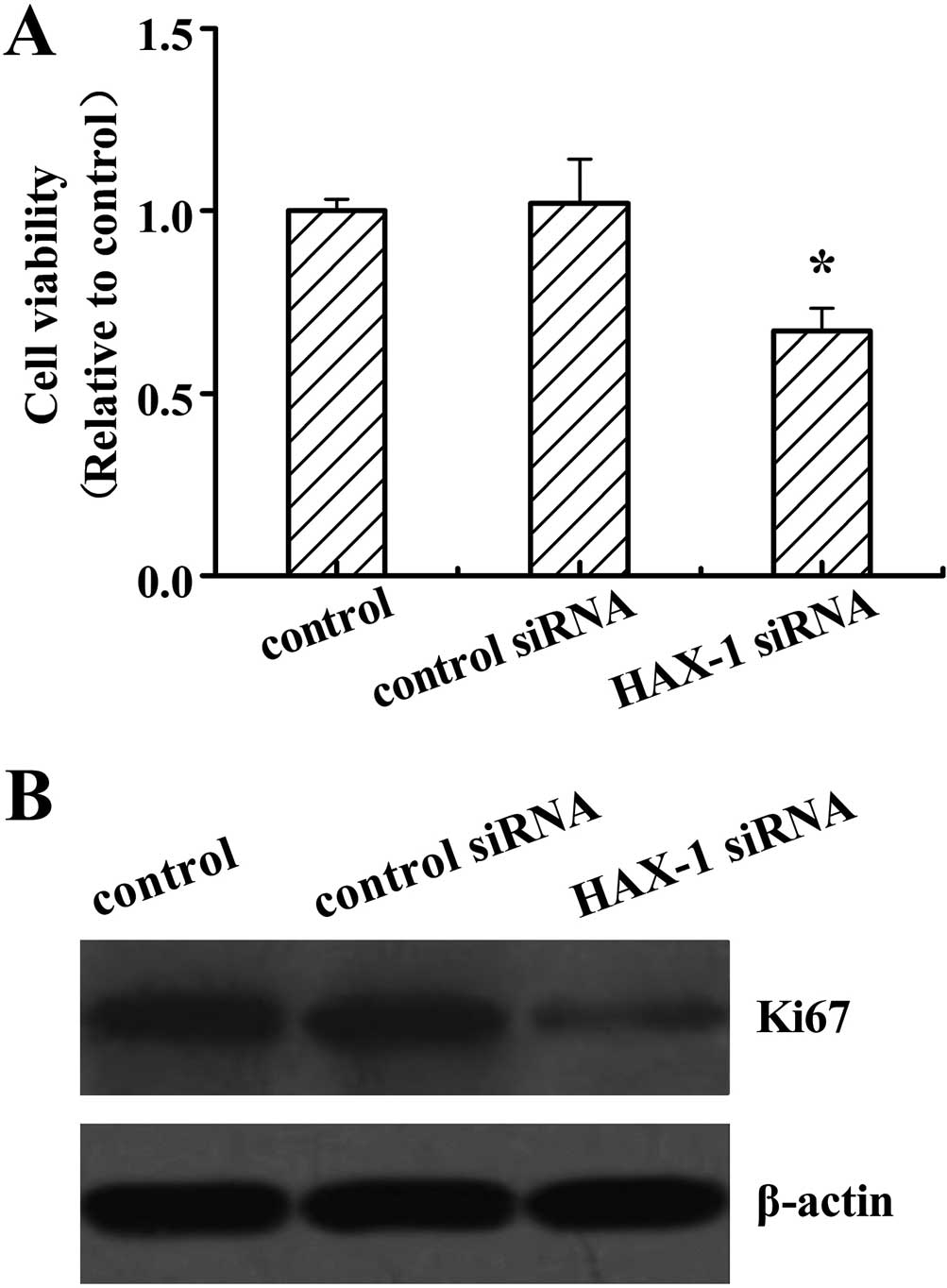
Cell proliferation was assessed in DU145 cells after
HAX-1 siRNA treatment by the MTT analysis. The results revealed
that HAX-1 knockdown significantly decreased the cell viability
compared to the other two groups (P<0.05; Fig. 3A). No differences were detected
between the control and control siRNA-treated cells. Ki67 has been
used as a marker for cell proliferation in cancer. We further
analyzed the Ki67 protein expression levels, and revealed that
HAX-1 siRNA suppressed the Ki67 protein expression (Fig. 3B).
Effect of HAX-1 knockdown on cell
apoptosis
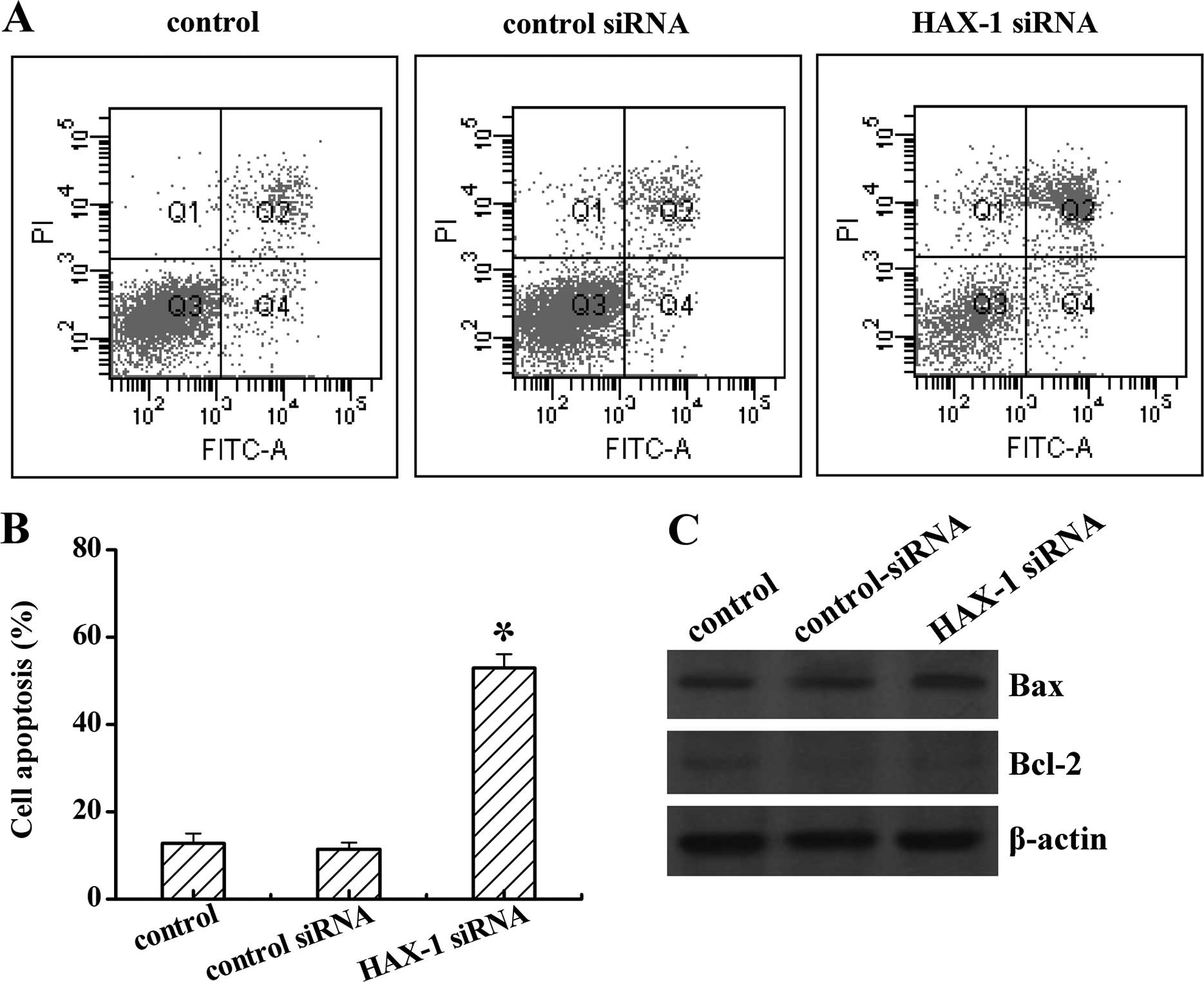
We next investigated the role of HAX-1 on DU145
apoptosis, after HAX-1 siRNA treatment. As shown in Fig. 4, TRAF4 knockdown notably increased
cell apoptosis from 13 to 56% when compared to the control groups.
No difference was found between the control and control siRNA
groups (P<0.05; Fig. 4A and B).
Moreover, HAX-1 siRNA also induced the pro-apoptosis protein Bax,
whereas inhibited the anti-apoptosis protein Bcl-2 expression
(Fig. 4C). These data reflected
that HAX-1 is an anti-apoptosis factor in prostate cancer.
HAX-1 knockdown enhances viability and
inhibits apoptosis in prostate cancer cells by caspase-9
inhibition
Caspase-9 is a critical regulator of
mitochondria-mediated apoptosis, and HAX-1 is one of the molecules
that interacts with caspase-9 (11). To determine whether HAX-1 interacts
with caspase-9 in the progress of prostate cancer viability and
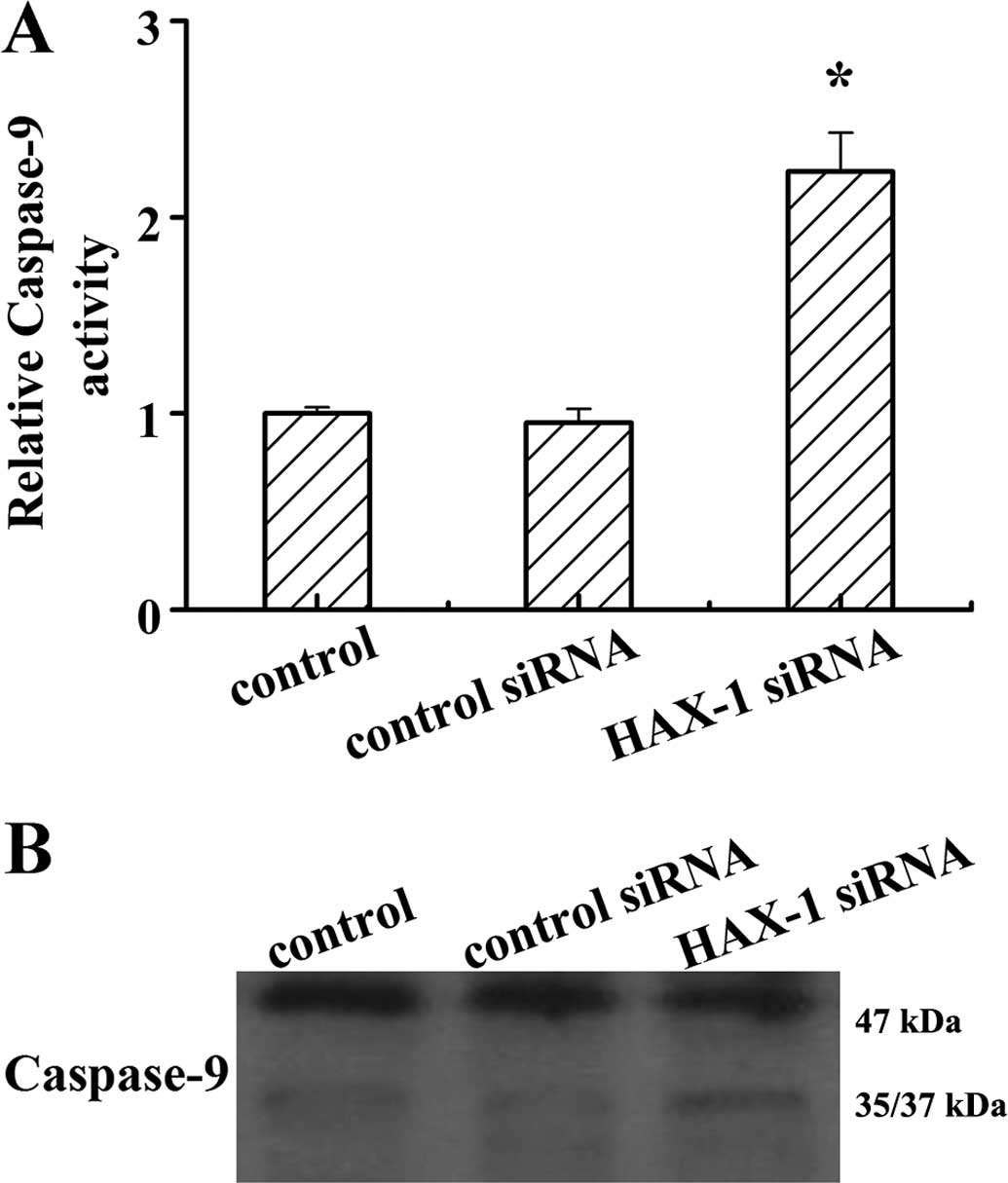
apoptosis, the activity of caspase-9 was determined in DU145 cells
after HAX-1 knockdown. The results showed that HAX-1 knockdown
markedly increased caspase-9 activity (P<0.05; Fig. 5A). The results of western blotting
showed that HAX-1 knockdown promoted caspase-9 processing in DU145
cells. These results indicated that HAX-1 is a negative regulator
of caspase-9 activation.
HAX-1 enhances prostate cancer cell
viability and apoptosis through inhibit caspase-9 activation
To further explore the function of caspase-9
signaling in HAX-1-induced prostate cancer viability and apoptosis,
caspase-9, HAX-1 and HAX-1 siRNA were co-transfected to DU145
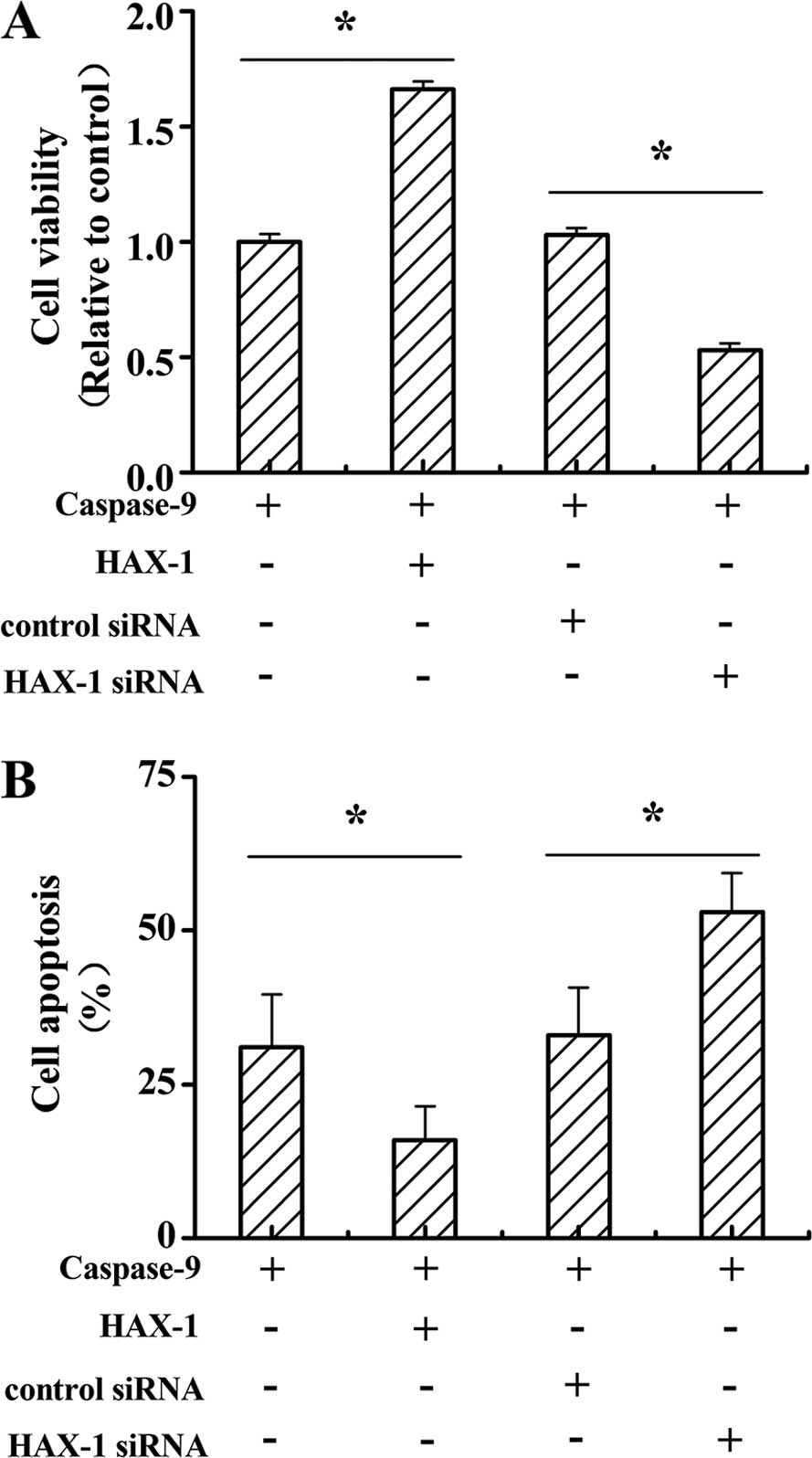
cells. As shown in Fig. 6A, cell
viability was significantly increased in caspase-9 and HAX-1
co-transfected cells compared to caspase-9 only transfected cells.
Moreover, the cell viability in caspase-9 and HAX-1 siRNA
co-transfected cells was sharply decreased when compared to
caspase-9 and control siRNA co-transfected cells (P<0.05). The
cell apoptosis in caspase-9 and HAX-1 co-transfected cells was
markedly decreased when compared to the caspase-9 only transfected
cells. Furthermore, cell apoptosis in caspase-9 and HAX-1 siRNA
co-transfected cells was notably increased when compared to the
caspase-9 and control siRNA co-transfected cells (P<0.05).
Discussion
Induction of differentiation and apoptosis in cancer
cells is a critical approach in cancer therapy (12). Substantial research has focused on
the induction of apoptosis in prostate cancer. For example, Hsieh
and Wu showed that resveratrol treatment is able to induce
apoptosis in prostate cancer (13),
and Hsu et al showed that the cyclooxygenase-2 inhibitor
celecoxib induces apoptosis in human prostate cancer cells by
blocking Akt activation (14). In
fact, the regulation of apoptosis relies on multiple cell signaling
mechanisms, cancer cells can employ a number of different
strategies to suppress a protective apoptotic response,
contributing to cancer development by promoting cell survival and
resistance to antineoplastic drugs (15–17).
In this regard, new anticancer therapeutics are needed to focus on
the induction of cancer cell apoptosis through activation of the
apoptotic pathway (18).
HAX-1 is a family of apoptotic regulators in disease
including cancer (19–21). It has been reported that HAX-1 is
highly expressed in different human melanoma cell lines (22). In this research, we first
investigated that HAX-1 is highly expressed in prostate cancer
cells, and further analysis found that HAX-1 knockdown sharply
decreased prostate cancer cell proliferation and the expression of
cell proliferating marker, Ki67. Moreover, HAX-1 knockdown
significantly promoted apoptosis, enhanced the pro-apoptosis
protein Bax, and inhibited the anti-apoptosis protein Bcl-2
expression in prostate cancer. These data indicate that HAX-1 is an
anti-apoptosis molecule in prostate cancer. Cell apoptosis is a
complex process regulated by several molecules that function as
either promoters, including Bax, Bak and caspases, or inhibitors of
the cell death process such as Bcl-2, Bcl-xL and the IAPs (23–27).
In addition, HAX-1 has been shown to interact with a number of
cellular and viral proteins, thus confirming its involvement in
multiple signaling pathways and cellular processes (28,29).
In recent years, many cellular factors involved in
apoptosis have been identified and their roles in the apoptotic
pathway has been elucidated (30).
Mitochondria is a key participant in cell death, and apoptotic cell
death is characterized by a host of morphological and biochemical
features, mitochondrial outer membrane permeabilization (MOMP) and
the release of pro-apoptotic proteins (31). Activation of the BCL-2 family
members Bax and Bak results in MOMP, and release of the
pro-apoptotic proteins, such as cytochrome c from the
inter-membrane space into the cytosol. Cytochrome c binds
Apaf-1 forming the apoptosome and activating caspase-9. Once
active, caspase-9 directly cleaves and activates caspase-3 and
caspase-7 (32–34). It has been found that HAX-1 directly
binds to caspase-9, leading to post-mitochondrial inactivation of
caspase-9, thus blocking the caspase-9-mediated cell apoptosis
pathway in cardiac myocytes (9). It
is noteworthy that we found, that HAX-1 knockdown enhanced
caspase-9 activity, further experiments demonstrated that prostate
cancer cell co-transfected with HAX-1 and caspase-9 promoted cell
viability and reduced cell apoptosis. In contract, co-transfection
of caspase-9 and HAX-1 siRNA suppressed cell viability and enhanced
apoptosis. This phenomenon, provides evidence that the HAX-1,
inhibits cell apoptosis through caspase-9 inactivation.
In summary, in the present study we propose a new
mechanism to account for apoptosis in prostate cancer by HAX-1
inhibition. A better understanding of this process could lead to
the development of novel therapeutic strategies aimed at reducing
tumor growth and the development of prostate cancer.
References
|
1
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai HT, Penson DF, Makambi KH, Lynch JH,
Van Den Eeden SK and Potosky AL: Efficacy of intermittent androgen
deprivation therapy vs conventional continuous androgen deprivation
therapy for advanced prostate cancer: A meta-analysis. Urology.
82:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki Y, Demoliere C, Kitamura D,
Takeshita H, Deuschle U and Watanabe T: HAX-1, a novel
intracellular protein, localized on mitochondria, directly
associates with HS1, a substrate of Src family tyrosine kinases. J
Immunol. 158:2736–2744. 1997.PubMed/NCBI
|
|
4
|
Mirmohammadsadegh A, Tartler U, Michel G,
Baer A, Walz M, Wolf R, Ruzicka T and Hengge UR: HAX-1, identified
by differential display reverse transcription polymerase chain
reaction, is overexpressed in lesional psoriasis. J Invest
Dermatol. 120:1045–1051. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharp TV, Wang H-W, Koumi A, Hollyman D,
Endo Y, Ye H, Du MQ and Boshoff C: K15 protein of Kaposi's
sarcoma-associated herpesvirus is latently expressed and binds to
HAX-1, a protein with antiapoptotic function. J Virol. 76:802–816.
2002. View Article : Google Scholar
|
|
6
|
Trebinska A, Rembiszewska A, Ciosek K,
Ptaszynski K, Rowinski S, Kupryjanczyk J, Siedlecki JA and
Grzybowska EA: HAX-1 overexpression, splicing and cellular
localization in tumors. BMC Cancer. 10:762010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jing YY, Li XL, Shi Q, Wang ZY, Guo Y, Pan
MM, Tian C, Zhu SY, Chen C, Gong HS, et al: A novel PrP partner
HS-1 associated protein X-1 (HAX-1) protected the cultured cells
against the challenge of H2O2. J Mol
Neurosci. 45:216–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei XJ, Li SY, Yu B, Chen G, Du JF and Cai
HY: Expression of HAX-1 in human colorectal cancer and its clinical
significance. Tumour Biol. 35:1411–1415. 2014. View Article : Google Scholar
|
|
9
|
Shaw J and Kirshenbaum LA: HAX-1 represses
post-mitochondrial caspase-9 activation and cell death during
hypoxia-reoxygenation. Circ Res. 99:336–338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahdavi M, Davoodi J, Zali MR and
Foroumadi A: Concomitant activation of caspase-9 and
down-regulation of IAP proteins as a mechanism of apoptotic death
in HepG2, T47D and HCT-116 cells upon exposure to a derivative from
4-aryl-4H-chromenes family. Biomed Pharmacother. 65:175–182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han Y, Chen YS, Liu Z, Bodyak N, Rigor D,
Bisping E, Pu WT and Kang PM: Overexpression of HAX-1 protects
cardiac myocytes from apoptosis through caspase-9 inhibition. Circ
Res. 99:415–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elstner E, Müller C, Koshizuka K,
Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D and
Koeffer HP: Ligands for peroxisome proliferator-activated
receptorgamma and retinoic acid receptor inhibit growth and induce
apoptosis of human breast cancer cells in vitro and in BNX mice.
Proc Natl Acad Sci USA. 95:8806–8811. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsieh TC and Wu JM: Differential effects
on growth, cell cycle arrest, and induction of apoptosis by
resveratrol in human prostate cancer cell lines. Exp Cell Res.
249:109–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu AL, Ching TT, Wang DS, Song X,
Rangnekar VM and Chen CS: The cyclooxygenase-2 inhibitor celecoxib
induces apoptosis by blocking Akt activation in human prostate
cancer cells independently of Bcl-2. J Biol Chem. 275:11397–11403.
2000. View Article : Google Scholar
|
|
15
|
Fulda S: Evasion of apoptosis as a
cellular stress response in cancer. Int J Cell Biol.
2010:3708352010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plati J, Bucur O and Khosravi-Far R:
Apoptotic cell signaling in cancer progression and therapy. Integr
Biol Camb. 3:279–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar
|
|
18
|
Vidya Priyadarsini R, Senthil Murugan R,
Maitreyi S, Ramalingam K, Karunagaran D and Nagini S: The flavonoid
quercetin induces cell cycle arrest and mitochondria-mediated
apoptosis in human cervical cancer (HeLa) cells through p53
induction and NF-κB inhibition. Eur J Pharmacol. 649:84–91. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li WB, Feng J, Geng SM, Zhang PY, Yan XN,
Hu G, Zhang CQ and Shi BJ: Induction of apoptosis by Hax-1 siRNA in
melanoma cells. Cell Biol Int. 33:548–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yap SV, Koontz JM and
Kontrogianni-Konstantopoulos A: HAX-1: A family of apoptotic
regulators in health and disease. J Cell Physiol. 226:2752–2761.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Tang Y, Zang W, Xuan X, Wang N, Ma
Y, Wang Y, Dong Z and Zhao G: Analysis of HAX-1 gene expression in
esophageal squamous cell carcinoma. Diagn Pathol. 8:472013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Philchenkov A: Caspases: Potential targets
for regulating cell death. J Cell Mol Med. 8:432–444. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin C, Knudson CM, Korsmeyer SJ and Van
Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in
vivo. Nature. 385:637–640. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. PubMed/NCBI
|
|
26
|
Zong WX, Li C, Hatzivassiliou G, Lindsten
T, Yu QC, Yuan J and Thompson CB: Bax and Bak can localize to the
endoplasmic reticulum to initiate apoptosis. J Cell Biol.
162:59–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fadeel B and Grzybowska E: HAX-1: A
multifunctional protein with emerging roles in human disease.
Biochim Biophys Acta. 1790:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cilenti L, Soundarapandian MM, Kyriazis
GA, Stratico V, Singh S, Gupta S, Bonventre JV, Alnemri ES and
Zervos AS: Regulation of HAX-1 anti-apoptotic protein by Omi/HtrA2
protease during cell death. J Biol Chem. 279:50295–50301. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta K, Thakur VS, Bhaskaran N, Nawab A,
Babcook MA, Jackson MW and Gupta S: Green tea polyphenols induce
p53-dependent and p53-independent apoptosis in prostate cancer
cells through two distinct mechanisms. PLoS One. 7:e525722012.
View Article : Google Scholar
|
|
31
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srinivasula SM, Ahmad M, Fernandes-Alnemri
T and Alnemri ES: Autoactivation of procaspase-9 by Apaf-1-mediated
oligomerization. Mol Cell. 1:949–957. 1998. View Article : Google Scholar : PubMed/NCBI
|