Introduction
As one of the most common malignant tumors, human
primary hepatocellular carcinoma (HCC) is the third leading cause
of cancer-related mortality worldwide (1,2), with
the incidence increasing, especially in East Asia and South Africa.
Approximately 50% of patients worldwide with HCC are located in
China, where HCC is the second leading cause of cancer-related
mortalities (3–5). Although marked progress has been made
in the treatment of HCC, many challenges still remain, such as the
difficulty of early diagnosis (6),
the high rate of metastasis when recurrence develops (7) and the lack of an effective therapeutic
target (8–10). Hepatic resection is a well accepted
therapy for HCC, however, many patients are diagnosed with advanced
HCC and are thus unable to undergo surgery (11,12).
Alternative treatments do not substantially improve patient
prognosis when HCC is deemed unresectable. Therefore, studies have
been conducted to identify novel genes and proteins associated with
the malignant proliferation of tumor cells in the pathogenesis of
HCC to develop more effective therapies.
The investigation of molecular mechanisms leading to
HCC development and progression is necessary to identify new
targets for early diagnosis and treatment (8). To gain new insight into the molecular
mechanisms underlying the pathogenesis of HCC, we searched for
HCC-specific molecules by screening genes that are differentialy
expressed between cancer and non-cancer counterparts of the liver
and identified a novel HCC-associated gene. We found that
ubiquitin-specific peptidase 14 (USP14), encoding an ~56 kDa
cytoplasmic protein (13,14), was upregulated in the liver of
patients with HCC. USP14 belongs to the family of deubiquitinating
enzymes (DUB) composed of His and Cys domains. USP14 removes the
(poly)ubiquitin moiety from protein substrates in a particular
form, as it is activated catalytically following specific
association with the 19S regulatory elements of the 26S proteasome
(15).
Dysfunction of the ubiquitin proteasome system (UPS)
is involved in numerous diseases, including cancer. The primary
means of selective intracellular protein degradation is through
proteasomal degradation after ubiquitin modification (16). Ubiquitination of proteins is a
dynamic balanced process. DUBs can reverse the ubiquitination
reaction because the deubiquitinating enzyme removes the ubiquitin
tag from a target protein that has been modified by ubiquitin
polymers (17,18). To maintain intracellular protein
stability and homeostasis, the UPS is precisely regulated. Some
studies have reported that DUBs can regulate cell cycle-associated
proteins by deubiquitination to maintain the stability of these
intracellular proteins, for example, cyclin-dependent kinase
inhibitors CDKN1A and CDKN1B (19)
and tumor-suppressor gene P53 (20). This influences the cell cycle as
well as cell proliferation. USP1 (21), USP9X (22), USP28 (23) and USP44 (24) have been reported to play important
roles in the development and progression of cancer. As vital
regulators of protein degradation, DUBs have also become potential
targets in drug design as inhibitors of malignant disease.
Recent studies suggest a high expression of USP14 in
some tumors, such as in colorectal cancer (25,26),
epithelial ovarian cancer (27),
lung adenocarcinoma (28) and
intrahepatic cholangiocarcinoma (29). Therefore, in the present study, we
examined the expression of USP14 in HCC. The biological role of
USP14 activation in the progression of HCC was investigated and the
association between the expression profile of USP14 and clinical
characteristics of HCC was also analyzed to explore the mechanisms
regulating HCC initiation and progression and to determine whether
USP14 may be a novel target in the diagnosis or treatment of
HCC.
Materials and methods
Patients and tissue samples
Formalin-fixed and paraffin-embedded liver tumor
specimens and matched tumor-adjacent normal tissues were obtained
for immunohistochemical analysis from 31 consecutive patients
diagnosed with HCC in the Eastern Hepatobiliary Surgery Hospital
(30), the Second Military Medical
University, Shanghai, China, from 2004 to 2007. To confirm the
expression level of USP14, stained sections were evaluated
according to the German immunoreactive scoring system using
immunoreactive scores (IRSs) as previously described (31,32).
Briefly, IRSs were assigned sub-scores for immunoreactivity
intensity (0–3) and distribution (0–4). These sub-scores were then
added to obtain the final IRS score. The sub-scores for
immunoreactive staining intensity were defined as: 0, no staining;
1, weakly stained; 2, moderately stained and 3, strongly stained.
The sub-scores for immunoreactivity distribution were defined as:
0, <5%, 1, 5–25%, 2, 25–50%, 3, 50–75% and 4, >75%.
Fresh-frozen liver tissues from 31 patients with HCC and normal
liver tissues from 6 patients who underwent primary hepatectomy
were used for RNA extraction and quantitative PCR (qPCR) assays.
For the 31 patients with HCC, 26 had been followed for 3 years and
their complete clinical data were electronically recorded. The
disease-free survival rate was defined as the interval between the
dates of surgery and recurrence, and where recurrence was not
diagnosed, patients were censored based on the dates of mortality
or the last follow-up. The present study was performed in
accordance with the ethical standards of the Human Experimentation
of the Second Military Medical University.
RNA isolation and cDNA synthesis
Total RNA was isolated from fresh-frozen HCC tumor
specimens, healthy control tissues and cell lines using TRIzol
(Invitrogen-Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. The extracted RNA samples (2
μg), which were kept at −80°C, were reverse-transcribed into
cDNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA)
in a final reaction volume of 20 μl. Reverse transcription
of total RNA was performed using random hexamers (Roche
Diagnostics, Penzberg, Germany).
qPCR analysis
For qPCR amplification, SYBR-Green (Takara, Otsu,
Japan) and 0.5 μl of cDNA per reaction were used. Primers of
human USP14 were: forward, 5′-GGCTTCAGCGCAGTATATTA-3′ and reverse,
5′-CAGATGAGGAGTCTGTCTCT-3′. The primers were synthesized by Sangon
(Shanghai, China). Reactions were performed under the following
conditions: 94°C for 6 min; 45 cycles of 94°C for 20 sec, 60°C for
31 sec, 72°C for 32 sec and 72°C for 10 min. Amplification and
detection of SYBR-Green were performed with a GeneAmp PCR System
5700 (Applied Biosystems). The human β-actin gene was used as an
internal control. Threshold changes relative to normal liver
controls were determined using cycle (Ct) values from triplicate
reactions and averaged. Fold was measured using the
2−ΔΔCT method.
Western blot analysis of USP14
The tissues or cells were lysed in RIPA buffer
containing a protease inhibitor (Roche Applied Science,
Indianapolis, In, USA) and homogenated. After incubation for 20 min
at 4°C and centrifugation at 10,800 × g for 25 min at 4°C, the
supernatant was collected. Protein concentrations were estimated
using the bicinchoninic acid (BCA) assay (Sango, China). For the
western blot analysis, proteins were loaded onto 10% SDS-PAGE gels
and transferred to polyvinylidene difluoride membranes. After
transfer, the membranes were incubated with a USP14 primary
antibody (Santa Cruz Biotechnology, Inc., CA, USA) for 1 h at room
temperature or overnight at 4°C on a shaker, followed by a
horseradish peroxidase-conjugated secondary antibody (Beyotime,
Shanghai, China). Protein bands were detected with ECL Plus
reagents (Amersham Biosciences, Switzerland).
Transfection
SMMC7721 cells were seeded in 6-well plates
(4×103 cells/well) in 0.5 ml of complete growth medium.
On the day of transfection, cell density was 50–80% confluent.
Suspensions of three USP14 shRNA lentiviruses (USP14-siRNA1,
USP14-siRNA2 and USP14-siRNA3) were added to different plates of
SMMC7721 cells and incubated for 3 h at 37°C. Fresh medium (2 ml)
was then added. After 24 h, 0.5 ml of complete growth medium was
replaced with growth medium.
Cell proliferation assay
Human SMMC7721 hepatocarcinoma cells
(4×103 cells/well) with various treatments were seeded
on a 96-well plate in DMEM (high glucose) supplemented with 10%
fetal bovine serum and 1% antibiotic/antimycotic solution
(Sigma-Aldrich, St. Louis, MO, USA). Cell proliferation was
detected over 5 days using the Cell Counting kit-8 (CCK-8) assay
according to the manufacturer's instructions.
Apoptosis analysis and clonogenic
assays
Apoptosis was monitored with an Annexin V-FITC
apoptosis detection kit (Invitrogen-Life Technologies) according to
the manufacturer's instructions. SMMC7721 cells with various
treatments were seeded in 6-well plates (4×103
cells/well) for clonogenic assays. After growing for 10 days, the
number of colonies was counted. A colony was defined as cell
clusters with ≥50 cells.
Cell cycle assay
SMMC7721 cells with various treatments were
incubated with propidium iodide (PI; Beyotime), according the
manufacturer's instructions. The samples were examined using a
fluorescence-activated cell sorting (FACS) assay and the results
were analyzed with CellQuest software (both from Becton-Dickinson,
Franklin Lakes, NJ, USA).
Statistical analysis
Survival curves were estimated using the
Kaplan-Meier method and the differences were compared with the
log-rank test. Statistical Package for the Social Sciences 15.0
software (SPSS Inc., Chicago, IL, USA) was used for the statistical
analyses and P<0.05 was considered significant. The Pearson's
χ2 test or Fisher's exact test was used to analyze the
relationship between USP14 expression and the HCC
clinicopathological characteristics. Data are presented as means ±
SE. other data were analyzed with one-way ANOVA. P<0.05 was
considered significant.
Results
USP14 expression in HCC specimens
To determine the expression level of USP14 in
hepatocellular carcinoma specimens, we detected USP14 expression
levels using RT-qPCR and immunohistochemistry (IHC) in livers
obtained from patients with HCC following liver resection. The RNA
was extracted from 31 cases of HCC specimens and 6 normal livers.
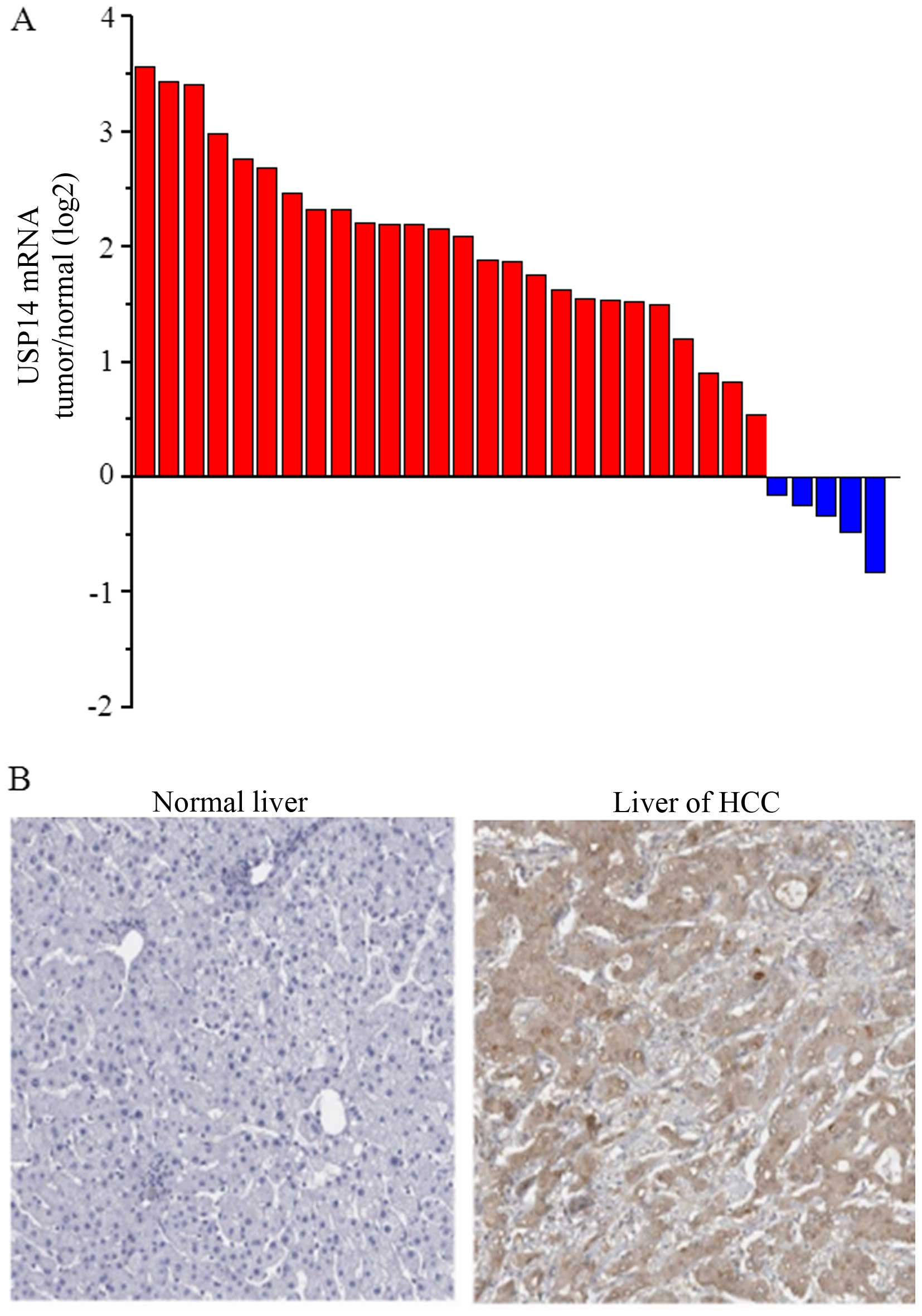
The USP14 mRNA expression was markedly increased in 26 of 31
(83.87%) HCC tissues compared with that in normal liver tissues
(Fig. 1A). We also examined the
protein expression of USP14 using IHC staining in 31 HCC tumor
tissues and matched tumor-adjacent normal tissues. A high USP14
protein expression was detected in the malignant cells, whereas
weak staining was observed throughout the matched normal controls
(Fig. 1B). USP14 expression was
observed in 83.87% of HCC specimens (26/31) and the
immunoreactivity was predominantly localized to the cytoplasm of
the malignant cells.
Association of USP14 expression with
clinicopathological characteristics
We evaluated the clinical significance of the USP14
expression in HCC in high and low USP14-expressing groups based on
the results of the expression levels of the USP14 protein. Thus,
patients were placed into a group with high intratumoral USP14
protein density or a group with low intratumoral USP14 protein
density. No significant correlation was detected between USP14
expression and the clinicopathological characteristics (Table I). No significant difference was
detected in tumor size, tumor thrombus or sub foci incidence
between the groups expressing high or low levels of USP14, although
these parameters showed an increasing trend (Table I). However, there was a trend for
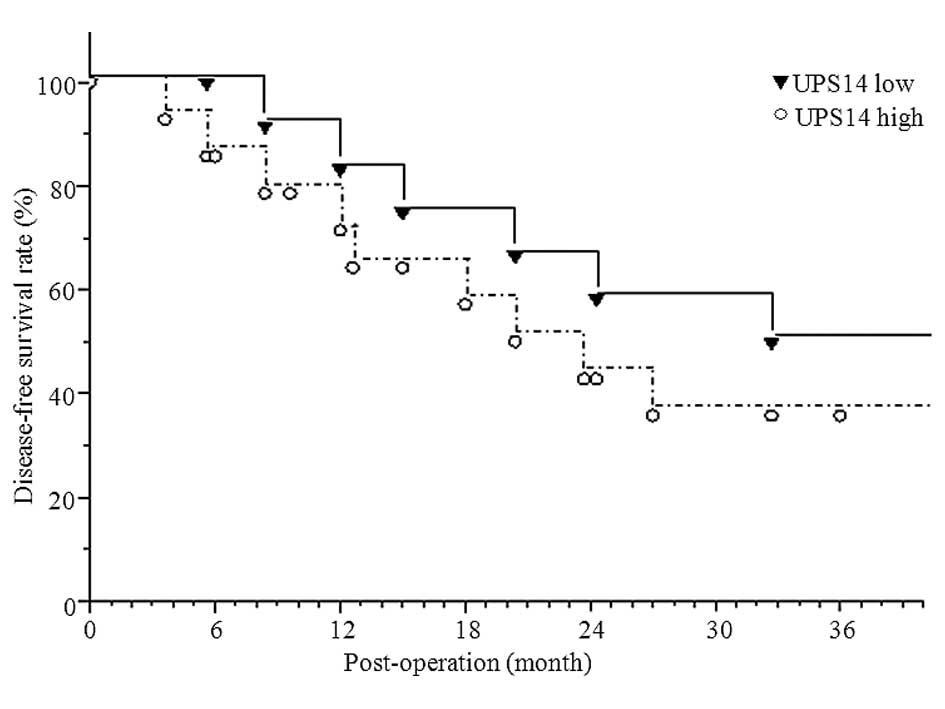
progression of HCC in the USP14 high-expressing group. Of the 31
patients with HCC 26 were followed for 3 years. During the 3-year
follow-up period, 15 out of 26 (57.69%) patients succumbed as a
result of disease progression. The Kaplan-Meier curve analysis
indicated that patients with a high USP14 expression (14 cases) had
a significantly shorter overall survival rate (35.71, P<0.05)
than those with a low USP14 expression (12 cases) (35.71 vs. 50%,
P<0.05) (Fig. 2).
 | Table IRelationship between USP14 protein
expression and HCC characteristics in training set (n=26). |
Table I
Relationship between USP14 protein
expression and HCC characteristics in training set (n=26).
|
Characteristics | No. of patients
(%) | USP 14
| P-value |
|---|
| Low (n=12) | High (n=14) |
|---|
| Age (years) | | 50.87±10.1 | 49.7±11.9 | 0.576 |
| Gender | | | | 0.657 |
| Male | 21 (80.77) | 10 | 11 | |
| Female | 5 (19.23) | 2 | 3 | |
| HBV | | | | 0.417 |
| Negative | 19 (73.08) | 9 (75) | 10 (71.43) | |
| Positive | 7 (26.92) | 3 (25) | 4 (28.57) | |
| AFP (ng/ml) | | 65.9 | 55.7 | 0.949 |
| Tumor size
(cm) | | 4.8±1.9 | 5.2±1.7 | 0.206 |
| Sub foci | 4 (15.38) | 1 (8.33) | 3 (21.43) | 0.078 |
| Tumor thrombus | 3 (11.54) | 1 (8.33) | 2 (14.29) | 0.258 |
Silencing USP14 impairs HCC cell growth
in vitro and in vivo
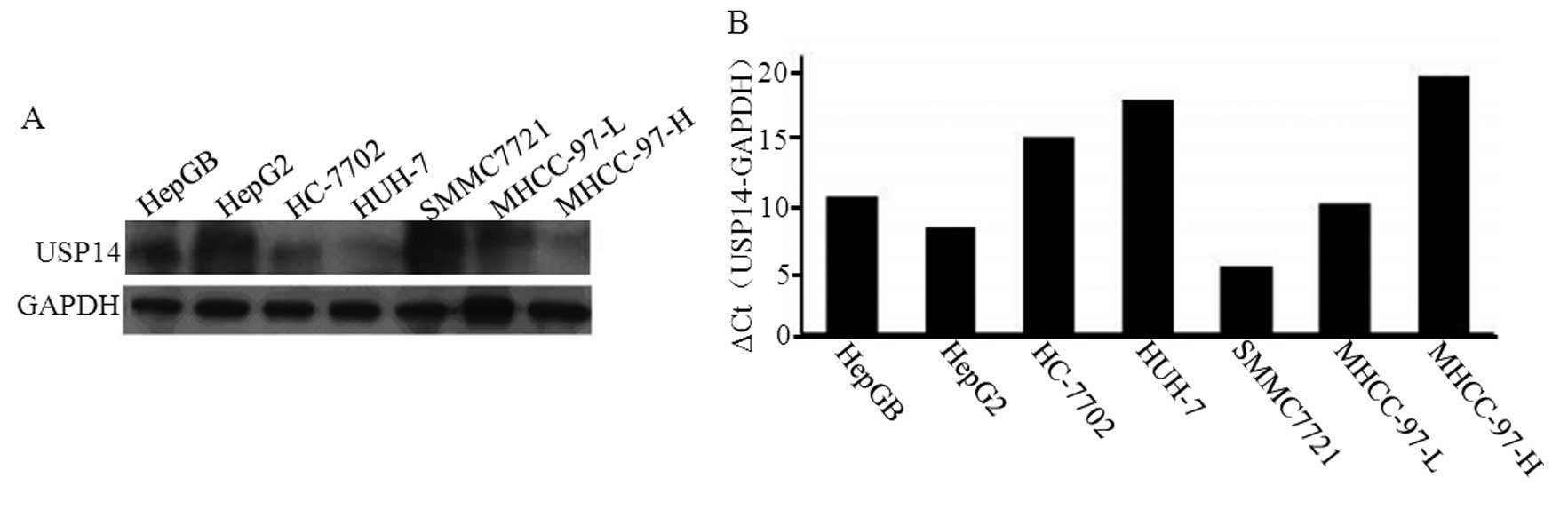
The expression of the USP14 protein was detected in
seven HCC cell lines. The level of USP14 protein expression was
markedly upregulated in the HepGB, HepG2, SMMC7721 and MHCC-97-L
cell lines (Fig. 3A). We also
detected the expression of USP14 mRNA in these seven HCC cell
lines. The relative expression levels of the USP14 mRNAs were
similar to those of the protein (Fig.
3B), with USP14 expression being highest in SMMC7721 cells
(Fig. 3). Therefore, SMMC7721 cells
were selected for the transfection of three USP14 shRNA
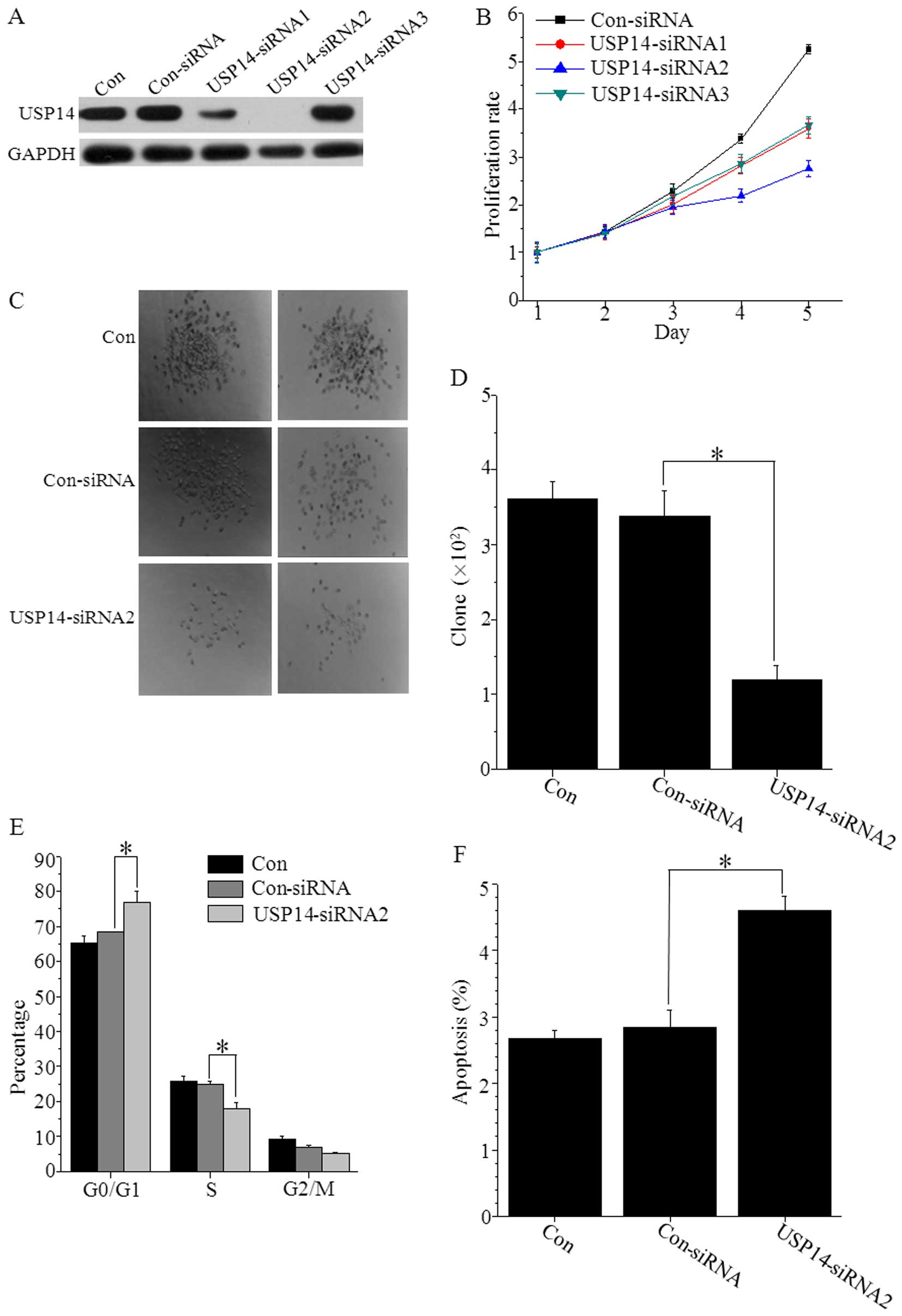
lentiviruses (USP14-siRNA1, USP14-siRNA2 and USP14-siRNA3). The
USP14-siRNA2 significantly suppressed USP14 expression levels
(Fig. 4A). The analyses of the cell
growth curves under USP14 silencing conditions indicated that the
proliferation of USP14-siRNA2 cells was significantly decreased
compared with that in the control cells on days 4 and 5 (P<0.01
for both) (Fig. 4B).
We assessed the effects of USP14 depletion on colony
formation in the SMMC7721 cell line (Fig. 4C and D). The depletion of USP14
markedly reduced colony formation in SMMC7721 cells. We also
identified the role of USP14 in the SMMC7721 cell cycle using a
FACS assay. The results demonstrated that the cell number in the S
phase was significantly decreased (P<0.05), while the cell
number in G0/G1 phase was significantly increased (P<0.05) after
transfection with the USP14-shRNA lentivirus (Fig. 4E). We also examined the cells for
apoptosis using Annexin V staining. We found that apoptosis in
SMMC7721 cells was markedly increased after USP14-RNAi-2 knockdown
compared with that in the control cells (Fig. 4F), demonstrating that the cell
survival rate was significantly reduced when USP14 was
downregulated.
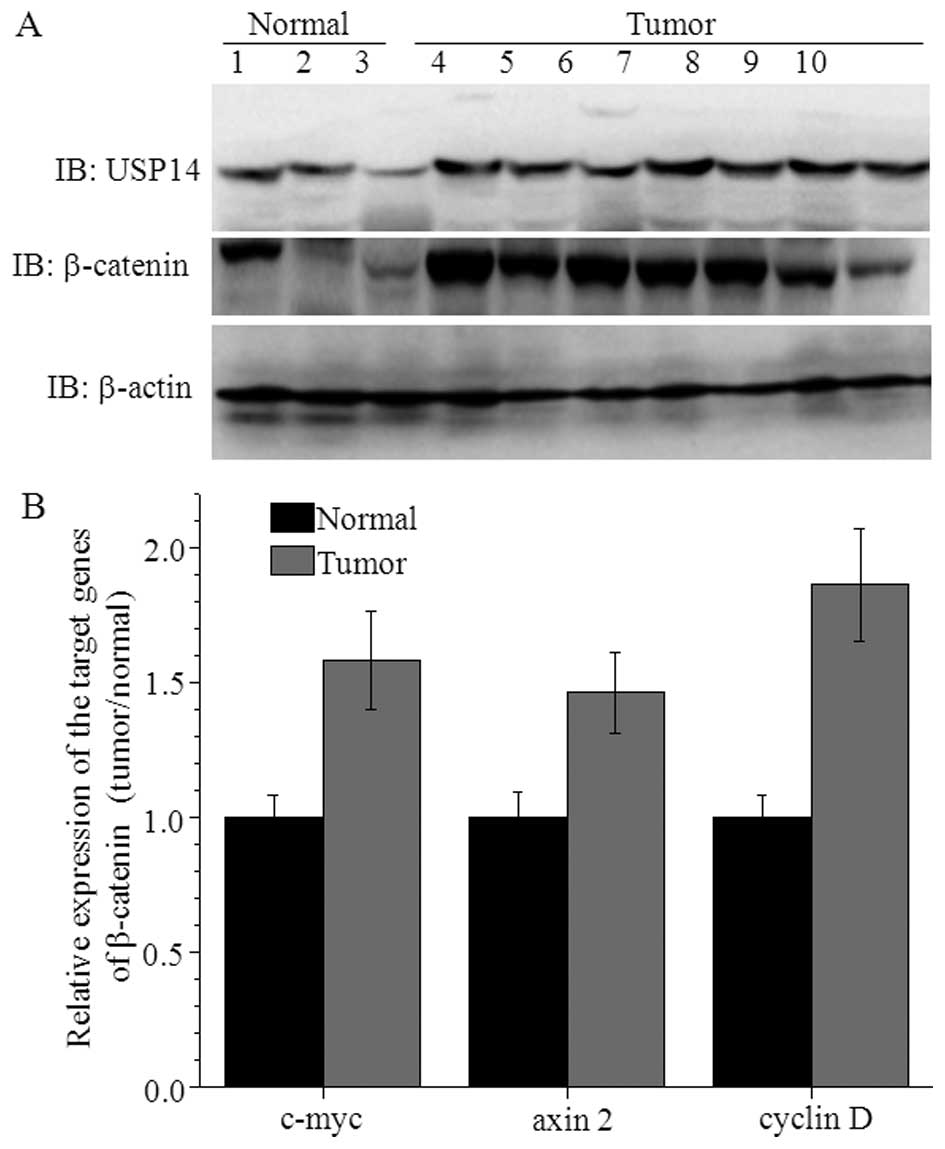
USP14 induces HCC by increasing β-catenin
levels
USP14 activates the Wnt/β-catenin signaling pathway
through deubiquitination (33). In
A549 cancer cells, β-catenin protein levels were decreased
following USP14 knockdown (28).
Thus, we examined the expression profile of β-catenin in seven
patients with HCC. Compared with normal liver tissues, the
expression level of β-catenin was increased in HCC patients with
high USP14 expression levels. The target genes of β-catenin (such
as c-myc, axin 2 and cyclin D) were also increased in these
patients (Fig. 5).
Discussion
HCC is a common malignant disease with high rates of
metastasis and relapse. The mortality rate ranks third in the world
and second in China (4), where
>100,000 people succumb annually from HCC. The early diagnosis
of HCC is difficult, with most cases undetected until the advanced
stages (6). No satisfactory
treatment for advanced HCC is currently available (34). Therefore, a new target therapy in
HCC is required.
In the present study, we reported that the
expression of USP14 was significantly higher in HCC than in normal
liver tissues. We also analyzed the prognostic value of USP14 in
HCC. In the present study, we examined the correlation between the
molecular features of USP14 and clinical outcomes in HCC. We found
no significant difference in tumor size between the groups
expressing high or low levels of USP14. However, the group with a
high USP14 expression showed a greater disease progression. The
Kaplan-Meier curve analysis indicated that patients with a high
USP14 expression (14 cases) had a significantly shorter overall
survival rate (35.71, P<0.05) than those with a low USP14
expression (12 cases) (35.71 vs. 50%, P<0.05).
USP14 is highly expressed in some tumors. Ishiwata
et al were the first to study the upregulation of USP14
expression in leukemia cells (35).
The high expression of USP14 was also found in a variety of colon
cancer cells and in colorectal tissues of patients with colorectal
cancer (25,26). The clinical prognosis in patients
with colorectal cancer is poor when USP14 expression is high. The
expression of USP14 in colorectal cancer is strong and positively
correlated with liver or lymph node metastasis or both. The
mechanism of this metastasis may be associated with the regulation
of tumor infiltration mediated by metastasis-associated protein
MMP9 (26). Chuensumran et
al (29) reported that
pathological grading was directly associated with the expression
level of USP14 in patients with intrahepatic cholangiocarcinoma.
Another study using retroviral expression library screening
indicated that USP14 may be important in the occurrence of ovarian
cancer (27).
The present study found that USP14 was highly
expressed in the SMMC7721 cell line. The growth of SMMC7721 cells
was markedly decreased in vitro after knocking down the
expression of USP14 with USP14-siRNA. After USP14 silencing, the
suppression of SMMC7721 cell proliferation was >50%, the cell
cycle was clearly altered and the apoptotic ratio was greatly
increased. In addition, the ability of the SMMC7721 cells to form
colonies was significantly inhibited. D'Arcy et al reported
that b-AP15, a specific inhibitor of USP14 and HCHL5, effectively
inhibits a variety of allogeneic transplantation tumor growths
(36). The inhibitor b-AP15
activates caspase-3 in the cytosol, leading to the downstream
accumulation in the cytosol of P53, CDKN1A and CDKN1B. Lee et
al found that this specific small molecule inhibitor of USP14
increased the activity of proteasomes by promoting the
ubiquitination of its substrate protein cyclin B to effectively
inhibit allogeneic transplantation tumor growth (37). Wu et al reported that the
overexpression of USP14 was highly associated with poor prognosis
in patients with non-small cell lung cancer (NSCLC) (28). The high expression of USP14 in
patients with NSCLC promoted tumor cell proliferation through the
accumulation of β-catenin. USP14 regulates cell proliferation and
apoptosis in epithelial ovarian cancer (27). Auranofin, a clinically used
antirheumatic agent, is a proteasomal deubiquitinase inhibitor that
inhibits tumor growth (38,39).
Previous findings have shown that a deubiquitinating
enzyme influenced the stability of cell cycle-associated proteins
to regulate the cell cycle and this was closely associated with the
occurrence and development of tumors (40). Many cell cycle-related proteins
(such as cyclin A, D, B, E and cyclin-dependent kinases) play vital
roles in regulating the transformation process. Their dysregulation
can lead to the occurrence and development of many tumors (41–46).
The intracellular half-life of these cell cycle-related proteins is
short because they are normally selectively degraded by proteasomes
following ubiquitination. Thus, with the overexpression of DUBs,
ubiquitination-dependent degradation may be inhibited, leading to
the accumulation of cell cycle-related proteins in cells. USP9X can
stabilize myeloid cell leukemin-1 to promote the growth of tumor
cells (22). The USP28 protein
inhibited the ubiquitination activity of Fbxw7 ligase to stabilize
cyclin-E1 and c-Myc protein in patients with colon or breast
cancer, in which the USP14 protein was overexpressed (23). USP44 protein was also shown to
inhibit the activity of the anaphase-promoting protein APC/C via
removal of the effects of the ubiquitin modification effect of
Cdc20 to prevent premature failure of the spindle checkpoint
(24).
The ubiquitin proteasome system is a major
intracellular protein degradation pathway involved in the
regulation of the cell cycle, immune response, signal transduction
and DNA repair in eukaryotic cells. The DUBs function to reverse
the process of the UPS, ensuring a dynamic balance in the
degradation of proteins. The UPS is strictly regulated to maintain
protein homeostasis in cells. Thus, the dysregulation of the UPS
pathway may induce a variety of diseases, including cancer. The UPS
has proven to be an important target for cancer treatment. Many
studies have screened compounds or drugs based on their ability to
inhibit proteasome activity. For example, bortezomib, a drug
targeting the ubiquitin-proteasome pathway, was approved for the
treatment of multiple myeloma (MM) and mantle cell lymphoma (MCL)
by the Food and Drug Administration (FDA) over 12 years ago
(47). Subsequently, carfilzomib,
another drug targeting the ubiquitin-proteasome pathway, was
approved by the FDA for the treatment of patients with relapsed and
refractory MM who received prior bortezomib and lenalidomide or
thalidomide (48). Thousands of
patients suffering from myeloma or lymphoma have benefited from
bortezomib- or carfilzomib-based therapy and the overall survival
rate of MM has been significantly increased in the last decade
(48–50). In addition, copper pyrithione, a
potent inhibitor of proteasome-specific UCHL5 and USP14, inhibits
tumor growth in vivo (51).
In the present study we detected the expression
profile of USP14 during the progression of HCC, and analyzed the
relationship between USP14 and the malignant transformation of
hepatocytes. Furthermore, we found that USP14 promoted HCC
development by increasing HCC cell proliferation, altering the cell
cycle and reducing apoptosis. These results indicate that USP14 may
be a novel therapeutic target for preventing or combating HCC, as
well as a novel target for use in the clinical diagnosis or
prognostic analysis of HCC to assist in designing personalized
therapies for patients. The present study also provides evidence
for the mechanism of HCC progression.
Abbreviations:
|
BCA
|
bicinchoninic acid
|
|
CCK-8
|
cell counting kit-8
|
|
DFS
|
disease-free survival
|
|
DUB
|
deubiquitinating enzymes
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FDA
|
food and drug administration
|
|
HCC
|
hepatocellular carcinoma
|
|
IHC
|
immunohistochemistry
|
|
IRSs
|
immunoreactive scores
|
|
MCL
|
mantle cell lymphoma
|
|
MM
|
multiple myeloma
|
|
NSCLC
|
non-small cell lung cancer
|
|
UPS
|
ubiquitin proteasome system
|
|
USP14
|
ubiquitin-specific peptidase 14
|
Acknowledgments
The present study was supported by grants awarded by
the national natural Science Foundation of China (no. 81201555),
the Major State Basic Research Development Program of China (973
Program; no. 2014CB542102), the Science Fund for Creative Research
Groups of the National Natural Science Foundation of China (no.
81201940) and the State Key Infectious Disease Project of China
(nos. 2012ZX10002010 and 2012ZX10002016).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao J, Xie L, Yang WS, Zhang W, Gao S,
Wang J and Xiang YB: Risk factors of hepatocellular carcinoma -
current status and perspectives. Asian Pac J Cancer Prev.
13:743–752. 2012. View Article : Google Scholar
|
|
5
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakamoto M: Early HCC: Diagnosis and
molecular markers. J Gastroenterol. 44(Suppl 19): 108–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Portolani N, Coniglio A, Ghidoni S,
Giovanelli M, Benetti A, Tiberio GA and Giulini SM: Early and late
recurrence after liver resection for hepatocellular carcinoma:
Prognostic and therapeutic implications. Ann Surg. 243:229–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giordano S and Columbano A: Met as a
therapeutic target in HCC: Facts and hopes. J Hepatol. 60:442–452.
2014. View Article : Google Scholar
|
|
9
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Newell P, Villanueva A and Llovet JM:
Molecular targeted therapies in hepatocellular carcinoma: From
pre-clinical models to clinical trials. J Hepatol. 49:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azoulay D: Resection for hepatocellular
carcinoma with hepatic vein tumour thrombus: Pushing the limits
beyond the guidelines frontiers. J Hepatol. 61:462–463. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cucchetti A, Djulbegovic B, Tsalatsanis A,
Vitale A, Hozo I, Piscaglia F, Cescon M, Ercolani G, Tuci F, Cillo
U, et al: When to perform hepatic resection for intermediate-stage
hepatocellular carcinoma. Hepatology. 61:905–914. 2015. View Article : Google Scholar
|
|
13
|
Borodovsky A, Kessler BM, Casagrande R,
Overkleeft HS, Wilkinson KD and Ploegh HL: A novel active
site-directed probe specific for deubiquitylating enzymes reveals
proteasome association of USP14. EMBO J. 20:5187–5196. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu M, Li P, Song L, Jeffrey PD, Chenova
TA, Wilkinson KD, Cohen RE and Shi Y: Structure and mechanisms of
the proteasome-associated deubiquitinating enzyme USP14. EMBO J.
24:3747–3756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peth A, Besche HC and Goldberg AL:
Ubiquitinated proteins activate the proteasome by binding to
Usp14/Ubp6, which causes 20S gate opening. Mol Cell. 36:794–804.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: Destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura Y, Yashiroda H, Kudo T, Koitabashi
S, Murata S, Kakizuka A and Tanaka K: An inhibitor of a
deubiquitinating enzyme regulates ubiquitin homeostasis. Cell.
137:549–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Love KR, Catic A, Schlieker C and Ploegh
HL: Mechanisms, biology and inhibitors of deubiquitinating enzymes.
Nat Chem Biol. 3:697–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheaff RJ, Singer JD, Swanger J,
Smitherman M, Roberts JM and Clurman BE: Proteasomal turnover of
p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell.
5:403–410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maki CG, Huibregtse JM and Howley PM: In
vivo ubiquitination and proteasome-mediated degradation of p53(1).
Cancer Res. 56:2649–2654. 1996.PubMed/NCBI
|
|
21
|
Huang TT, Nijman SM, Mirchandani KD,
Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R and
D'Andrea AD: Regulation of monoubiquitinated PCNA by DUB
autocleavage. Nat Cell Biol. 8:339–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwickart M, Huang X, Lill JR, Liu J,
Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F,
Eastham-Anderson J, et al: Deubiquitinase USP9X stabilizes MCL1 and
promotes tumour cell survival. Nature. 463:103–107. 2010.
View Article : Google Scholar
|
|
23
|
Popov N, Wanzel M, Madiredjo M, Zhang D,
Beijersbergen R, Bernards R, Moll R, Elledge SJ and Eilers M: The
ubiquitin-specific protease USP28 is required for MYC stability.
Nat Cell Biol. 9:765–774. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y,
Keough S, Wong M, Rowitch D and Bonni A: A centrosomal Cdc20-APC
pathway controls dendrite morphogenesis in postmitotic neurons.
Cell. 136:322–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishiwata S, Ozawa Y, Katayama J, Kaneko S,
Shindo H, Tomioka Y, Ishiwata T, Asano G, Ikegawa S and Mizugaki M:
Elevated expression level of 60-kDa subunit of tRNA-guanine
transglycosylase in colon cancer. Cancer Lett. 212:113–119. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shinji S, Naito Z, Ishiwata S, Ishiwata T,
Tanaka N, Furukawa K, Suzuki H, Seya T, Matsuda A, Katsuta M, et
al: Ubiquitin-specific protease 14 expression in colorectal cancer
is associated with liver and lymph node metastases. Oncol Rep.
15:539–543. 2006.PubMed/NCBI
|
|
27
|
Wada T, Yamashita Y, Saga Y, Takahashi K,
Koinuma K, Choi YL, Kaneda R, Fujiwara S, Soda M, Watanabe H, et
al: Screening for genetic abnormalities involved in ovarian carcino
genesis using retroviral expression libraries. Int J Oncol.
35:973–976. 2009.PubMed/NCBI
|
|
28
|
Wu N, Liu C, Bai C, Han YP, Cho WC and Li
Q: Overexpression of deubiquitinating enzyme USP14 in lung
adenocarcinoma promotes proliferation through the accumulation of
β-catenin. Int J Mol Sci. 14:10749–10760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chuensumran U, Saelee P, Punyarit P,
Wongkham S, Pairojkul C, Chauin S and Petmitr S: Ubiquitin-specific
protease 14 expression associated with intrahepatic
cholangiocarcinoma cell differentiation. Asian Pac J Cancer Prev.
12:775–779. 2011.PubMed/NCBI
|
|
30
|
Zhang C, Ling Y, Zhang C, Xu Y, Gao L, Li
R, Zhu J, Fan L and Wei L: The silencing of RECK gene is associated
with promoter hypermethylation and poor survival in hepatocellular
carcinoma. Int J Biol Sci. 8:451–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong LW, Hou YJ, Tan YX, Tang L, Pan YF,
Wang M and Wang HY: Prognostic significance of Beclin 1 in
intrahepatic cholangiocellular carcinoma. Autophagy. 7:1222–1229.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Tan YX, Ren YB, Dong LW, Xie ZF,
Tang L, Cao D, Zhang WP, Hu HP and Wang HY: Zinc finger protein
ZBTB20 expression is increased in hepatocellular carcinoma and
associated with poor prognosis. BMC Cancer. 11:2712011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung H, Kim BG, Han WH, Lee JH, Cho JY,
Park WS, Maurice MM, Han JK, Lee MJ, Finley D, et al:
Deubiquitination of Dishevelled by Usp14 is required for Wnt
signaling. Oncogenesis. 2:e642013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Germano D and Daniele B: Systemic therapy
of hepatocellular carcinoma: Current status and future
perspectives. World J Gastroenterol. 20:3087–3099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishiwata S, Katayama J, Shindo H, Ozawa Y,
Itoh K and Mizugaki M: Increased expression of queuosine
synthesizing enzyme, tRNA-guanine transglycosylase, and queuosine
levels in tRNA of leukemic cells. J Biochem. 129:13–17. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Arcy P, Brnjic S, Olofsson MH, Fryknäs
M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson
R, et al: Inhibition of proteasome deubiquitinating activity as a
new cancer therapy. Nat Med. 17:1636–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee BH, Lee MJ, Park S, Oh DC, Elsasser S,
Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, et al: Enhancement
of proteasome activity by a small-molecule inhibitor of USP14.
Nature. 467:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu N, Li X, Huang H, Zhao C, Liao S, Yang
C, Liu S, Song W, Lu X, Lan X, et al: Clinically used antirheumatic
agent auranofin is a proteasomal deubiquitinase inhibitor and
inhibits tumor growth. Oncotarget. 5:5453–5471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X, Shi X, Zhao C, Li X, Lan X, Liu S,
Huang H, Liu N, Liao S, Zang D, et al: Anti-rheumatic agent
auranofin induced apoptosis in chronic myeloid leukemia cells
resistant to imatinib through both Bcr/Abl-dependent and
-independent mechanisms. Oncotarget. 5:9118–9132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: cell-cycle control and cancer. Nat Rev cancer. 6:369–381.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Husdal A, Bukholm G and Bukholm IR: The
prognostic value and overexpression of cyclin A is correlated with
gene amplification of both cyclin A and cyclin E in breast cancer
patient. Cell Oncol. 28:107–116. 2006.PubMed/NCBI
|
|
42
|
Nauman A, Turowska O, Poplawski P, Master
A, Tanski Z and Puzianowska-Kuznicka M: Elevated cyclin E level in
human clear cell renal cell carcinoma: Possible causes and
consequences. Acta Biochim Pol. 54:595–602. 2007.PubMed/NCBI
|
|
43
|
Chang KC, Chang Y, Jones D and Su IJ:
Aberrant expression of cyclin a correlates with morphogenesis of
reed-sternberg cells in Hodgkin lymphoma. Am J Clin Pathol.
132:50–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cooley A, Zelivianski S and Jeruss JS:
Impact of cyclin E overexpression on Smad3 activity in breast
cancer cell lines. Cell Cycle. 9:4900–4907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang LH, Huang W, Lai MD and Su IJ:
Aberrant cyclin A expression and centrosome overduplication induced
by hepatitis B virus pre-S2 mutants and its implication in
hepatocarcinogenesis. Carcinogenesis. 33:466–472. 2012. View Article : Google Scholar
|
|
46
|
Freije A, Ceballos L, Coisy M, Barnes L,
Rosa M, De Diego E, Blanchard JM and Gandarillas A: Cyclin E drives
human keratinocyte growth into differentiation. Oncogene.
31:5180–5192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Richardson PG, Barlogie B, Berenson J,
Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina
M, Alexanian R, et al: A phase 2 study of bortezomib in relapsed,
refractory myeloma. N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thompson JL: Carfilzomib: A
second-generation proteasome inhibitor for the treatment of
relapsed and refractory multiple myeloma. Ann Pharmacother.
47:56–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Richardson PG, Mitsiades C, Hideshima T
and Anderson KC: Bortezomib: Proteasome inhibition as an effective
anticancer therapy. Annu Rev Med. 57:33–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cavo M: Proteasome inhibitor bortezomib
for the treatment of multiple myeloma. Leukemia. 20:1341–1352.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu N, Liu C, Li X, Liao S, Song W, Yang
C, Zhao C, Huang H, Guan L, Zhang P, et al: A novel proteasome
inhibitor suppresses tumor growth via targeting both 19S proteasome
deubiquitinases and 20S proteolytic peptidases. Sci Rep.
4:52402014.PubMed/NCBI
|