Introduction
Telomeres are the ends of linear chromosomes in
eukaryotic cells and are characterized by the TTAGGG repeats
(1) that are bound by the shelterin
complex (2). Telomeric DNA shrinks
with each cellular division due to incomplete replication of TTAGGG
repeats, which eventually results in DNA damage and replicative
senescence (3). Telomerase is a
particular ribonucleoprotein complex which specifically adds TTAGGG
repeats to the ends of eukaryotic chromosomes, thus counteracts the
shortening of telomeric DNA during cell division. The activity of
telomerase is not detected in most human somatic cells (4). However, telomerase is reactivated in
vast majority of human cancer cells (5) and plays a critical role in the
progression of human cancer (6).
Recently telomerase has been found to exert extra-telomeric effects
via the modulation of NF-κB (7,8) and
Wnt/β-catenin signaling pathways (9,10).
These non-canonical functions are associated with the development
and progression of human cancer. In fact, telomerase has been
investigated as a potent target for anticancer therapy (11).
The major components of human telomerase are
tolemerase RNA (hTR) and telomerase reverse transcriptase (hTERT).
While hTR subunit serves as a template, hTERT subunit catalyzes the
elongation of telomeric DNA. Three main structural domains of hTERT
have been identified: NH2 terminus that binds DNA and
RNA, central catalytic RT region and a short COOH terminus
(12). Although the function of
COOH terminus is the least-characterized, it has been found to
mediate the nuclear translocation of telomerase (13) and is essential for the in
vivo activity of human telomerase (14). Recent studies showed that ectopic
expression of C27 (amino acid 882–1,132 of hTERT) reduces the
growth and tumorigenicity of tumor cells (15–19)
and sensitizes tumor cells to 5-fluorouracil-induced growth
inhibition and apoptosis (20). C27
does not affect telomerase activity. The unclearly illustrated
mechanisms include the induction of telomere dysfunction (15), triggering of apoptosis signal and
reduction of angiogenesis in tumor tissue (16), as well as involvement of immune
responses (18).
In the present study, a novel COOH-terminal
polypeptide (C197, amino acid 936–1,132) of hTERT was overexpressed
in HeLa cells using recombinant adenovirus Ad-EGFP-C197. Ectopic
expression of C197 was found to induce growth delay and promotes
apoptosis of HeLa cells in vitro, and in nude mice.
Furthermore, C197 suppressed the telomerase activity, as well as
downregulated the expression of hTERT and NF-κB p65 in HeLa cells,
demonstrating that the antitumor effect of C197 was associated with
the reduced expression of hTERT and NF-κB p65.
Materials and methods
Ethics statement
All animal protocols conformed to the EU guidelines
(2010/63/EU) and other EU recommendations, and were conducted with
the approval of the Ethics Committee of Animal Experiments,
Southern Medical University. All surgery was performed under sodium
pentobarbital anesthesia, and every effort was made to minimize
suffering.
Cell culture
HEK-293 (CRL-1573) and HeLa (CCL-2) cell lines were
obtained from American type culture collection (ATCC; Manassas, VA,
USA). Cells were cultivated in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) (both from
Gibco, USA) plus 100 U/ml penicillin and 100 μg/ml
streptomycin (Sigma, USA) at 37°C in a humidified atmosphere
containing 5% CO2.
Construction of Ad-EGFP-C197
The cDNA encoding C197 (hTERT amino acid 936–1,132)
was synthesized by Sangon Biotech (Shanghai, China), and was
identified by sequencing. A 6-histidine (6-His)-tag was added to
the N-terminus of C197 cDNA to facilitate the detection of C197
protein. The AdEasy Vector system was used for the construction of
recombinant adenovirus Ad-EGFP-C197. The construction, preparation
and titration of Ad-EGFP-C197 were conducted as previously
described (21), with the
substitution of 6-His-C197 cDNA for AT2R cDNA. The foreign genes
6-His-C197 and enhanced green fluorescent protein (EGFP) were
driven by a separate cytomegalovirus (CMV) promoter within the
recombinant Ad-EGFP-C197. The mock adenovirus Ad-CMV-EGFP was
prepared as previously described (21).
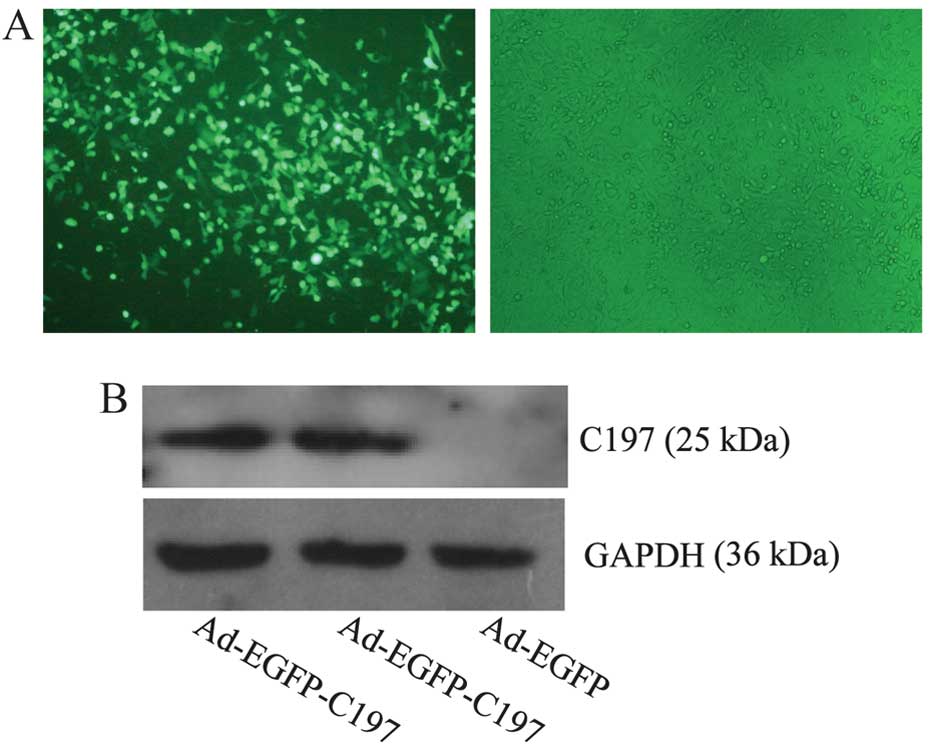
Detection of the expression of C197 in
HeLa cells
Cells (2×105/well) were seeded into
6-well tissue culture plates (Corning, Shanghai, China). The
following day, cells were infected with Ad-EGFP-C197 [multiplicity
of infection (MOI) of 100]. Forty-eight hours after infection,
cells were subject to the detection of C197 protein using
anti-6-His antibody by western blotting as described below.
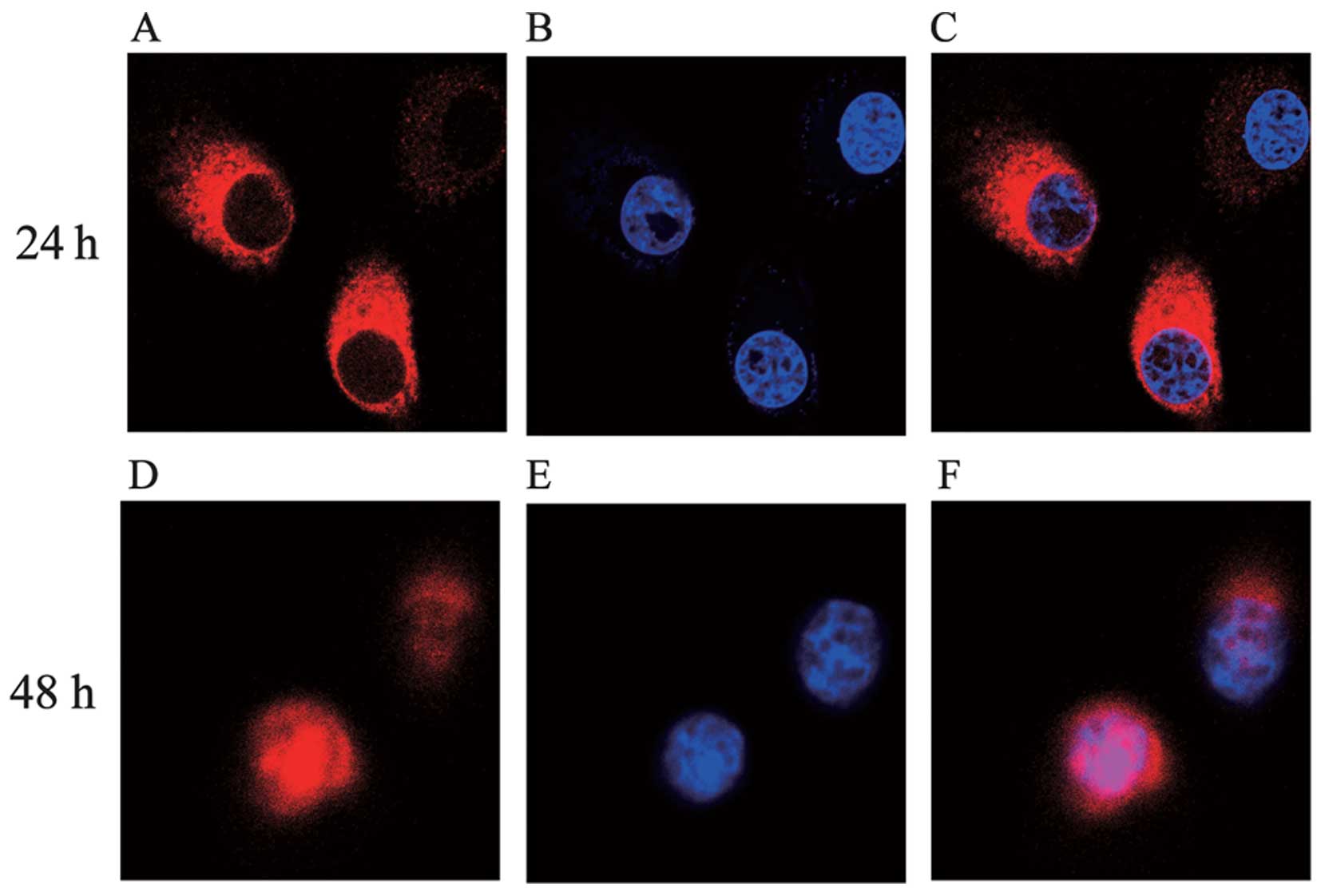
Determination of the sub-cellular
localization of C197
HeLa cells (1×105) were cultivated in a
10 mm glass bottom culture dish (Corning). The following day, cells
were infected with Ad-EGFP-C197 (MOI of 100). At the time point of
24 or 48 h after infection, cells were washed 3 times with
phosphate-buffered saline (PBS), then fixed in 4% paraformal-dehyde
for 15 min, followed by the incubation with 1% BSA (Sigma) for 30
min. Next, cells were incubated with primary antibody against
6-His-tag (mouse monoclonal IgG, 1:500 dilution; sc-57598; Santa
Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 1 h, washed
with PBS and incubated with TRITC-coupled secondary antibody (goat
polyclonal against mouse IgG, 1:100 dilution; BS11502; Bioworld
Technology) for 1 h at room temperature. Cell nuclei were
counterstained by phenylindole dihydrochloride (DAPI) (Sigma) for 5
min. After several washes with PBS, cells were examined under a
confocal microscope (Nikon, Japan).
In vitro proliferation and apoptosis
assay
For the cell proliferation assay, HeLa cells
(1×104 cells/well) were seeded in 96-well plates
(Corning). The following day, cells were infected with recombinant
adenovirus Ad-EGFP-C197 or mock adenovirus Ad-EGFP (MOI of 100).
Cells were cultivated for 7 days after infection. On each day cell
viability was examined by MTT (Sigma) method using a Multiskan
microplate reader (Benchmark Plus; Bio-Rad, USA).
For in vitro apoptosis assay, HeLa cells
(1×105 cells/well) were seeded in 6-well plates
(Corning). The following day, cells were infected with Ad-EGFP-C197
or Ad-EGFP (MOI of 100). Cells were cultivated for 3 days after
infection. Each day cell apoptosis was detected using Annexin V-PE
apoptosis detection kit I (BD Biosciences, USA) according to the
manufacturer's instructions, and analyzed by flow cytometry
(Bio-Rad) within 1 h.
Telomerase activity assay
HeLa cells (1×105 cells/well) were seeded
into 6-well plates (Corning). The following day, cells were
infected with Ad-EGFP-C197 or Ad-EGFP (MOI of 100). Forty-eight
hours after infection, cell lysates were prepared and telomerase
activity was determined using a TRAPeze® telomerase
detection kit (S7700; Millipore, Billerica, MA, USA) as described
in the manufacturer's instructions. Briefly, protein was extracted
from cells and suspended in 1X CHAPS in a concentration of 700
ng/μl. PCR was performed in 50 μl reaction system
(including 2 μl protein solution). Amplification reactions
were performed for 30 cycles of 94°C for 30 sec, 59°C for 30 sec
and 72°C for 10 min. Products were separated by electrophoresis on
12.5% polyacrylamide gels, stained with EB for 30 min and scanned
with a gel documentation system (Bio-Rad).
Detection of the expression of hTERT and
NF-κB p65
HeLa cells (1×105 cells/well) were seeded
into 6-well plates (Corning). The following day, cells were
infected with Ad-EGFP-C197 or Ad-EGFP (MOI of 100). Forty-eight
hours after infection, cell lysates were prepared to detect the
expression of hTERT. For the detection of NF-κB p65, TNF-α
(PeproTech, USA) was added to the medium (final concentration 10
ng/ml) and cells were cultivated for another 12 h. Cells were then
collected to prepare cell lysates. The expression of hTERT and
NF-κB p65 were detected by western blotting as described below.
Western blot analysis
Western blotting was performed according to standard
protocols. Briefly, total protein was extracted from the cells by
repeated freezing and thawing, or from tumor tissues by
homogenization, and was quantified using the Bradford method.
Samples were separated by SDS-PAGE and transferred onto a
polyvinylidene fluoride (PVDF) membrane (Millipore) using Mini
Trans-Blot (Bio-Rad). The membrane was blocked with 5% non-fat dry
milk (Sigma) and incubated with primary antibodies, followed by the
incubation with horseradish peroxidase (HRP)-conjugated secondary
antibodies. The film was developed using the enhanced
chemiluminescence (ECL) kit (Vazyme, Nanjing, China). Primary
antibodies included mouse monoclonal antibodies (IgG) against
6-his-tag (1:500 dilution, sc-57598)/human GAPDH (1:500 dilution,
sc-365062)/human β-actin (1:500 dilution, sc-130301)/hTERT (1:100
dilution, sc-393013) (all from Santa Cruz Biotechnology), and
rabbit monoclonal IgG against human NF-κB p65 (1:1,000 dilution,
#8242; Cell Signaling Technology). The secondary antibodies used
were HRP-conjugated goat polyclonal antibody against mouse IgG
(1:5,000 dilution, BS12478) and HRP-conjugated goat polyclonal
antibody against rabbit IgG (1:500 dilution, BS13278) (both from
Bioworld Technology).
Animal studies
Male athymic nude mice (BALB/c-nu/nu) aged 4–6 weeks
(Center of Laboratory Animals, Southern Medical University,
Guangzhou, China) were housed in sterile cages with a 12:12 h
light-dark cycle. Animals were fed autoclaved chow and water ad
libitum. The tumors were established by subcutaneous injection
of 1×107 HeLa cells into the right flank of each mouse.
When the tumor nodules reached 5–6 mm in diameter, mice were
randomly divided into two groups of six mice. Each mouse received
intratumor injection of Ad-EGFP-C197 or Ad-EGFP (1×109
pfu) twice a week for 4 weeks. Animals were observed for one week
after the last injection. Tumor volumes were determined once a week
by measuring in two dimensions and calculated as tumor volume =
length x (width)2/2 (22). At the end of the experiment, mice
were sacrificed with sodium pentobarbital and tumor nodules were
separated and weighed. Tumor tissues were subjected to TUNEL assay
and immunohistochemistry analysis, and a fraction was homogenized
for protein extraction and immunodetection of β-actin and
6-His-C197 by western blotting, as previously described.
TUNEL assay
Tumor tissues were fixed with 4% paraformal-dehyde
(Sigma) and were embedded in paraffin (Sigma). After dehydration,
tissue sections (6-μm thick) were prepared and mounted on
polylysine (Sigma) coated slides. Sections were then subject to
deparaffinization and rehydration, followed by the microwave
antigen retrieval with citric acid. TUNEL assay was conducted using
the DeadEnd Colorimetric TUNEL System (Promega, Beijing, China) as
described in the manufacturer's instructions. Stained sections were
analyzed with a light microscope (Nikon). At least 3-high
magnification fields were examined in each section (500 cells
altogether), and the apoptotic index (AI) was calculated as the
percentage of TUNEL-positive cells.
Immunohistochemistry analysis
Tissue sections were prepared as described above.
After incubation with 3% H2O2 for 10 min and
blocking with 5% BSA for 30 min, sections were incubated with
primary antibody against NF-κB p65 (rabbit monoclonal IgG, 1:800
dilution, #8242; Cell Signaling Technology) for 12 h at 4°C,
followed by the incubation with HRP-conjugated goat polyclonal
antibody against rabbit IgG (1:500 dilution, BS13278, Bioworld
Technology) for 30 min at 37°C. Next, 3,3′-diaminobenzidine (DAB;
Sigma) was added to visualize the brown color. Finally, sections
were counter-stained with hematoxylin to show cell nuclei and
examined with a light microscope (Nikon). The total integrate
optical density (IOD) and total area of each section was determined
with an Image-Pro Plus 6.0 software. The mean optical density (MOD)
was employed to show the relative amount of p65 protein in each
section using the following formula: mean optical density = total
IOD/total area.
Statistical analysis
Data are represented as mean ± standard deviation
(SD). All statistical analyses were carried out using SPSS 13.0
(IBM, USA). Differences between groups were assessed using
independent samples t-test and were considered to indicate a
statistically significant result when the P-value was <0.05.
Results
Expression and sub-cellular localization
of C197 in HeLa cells
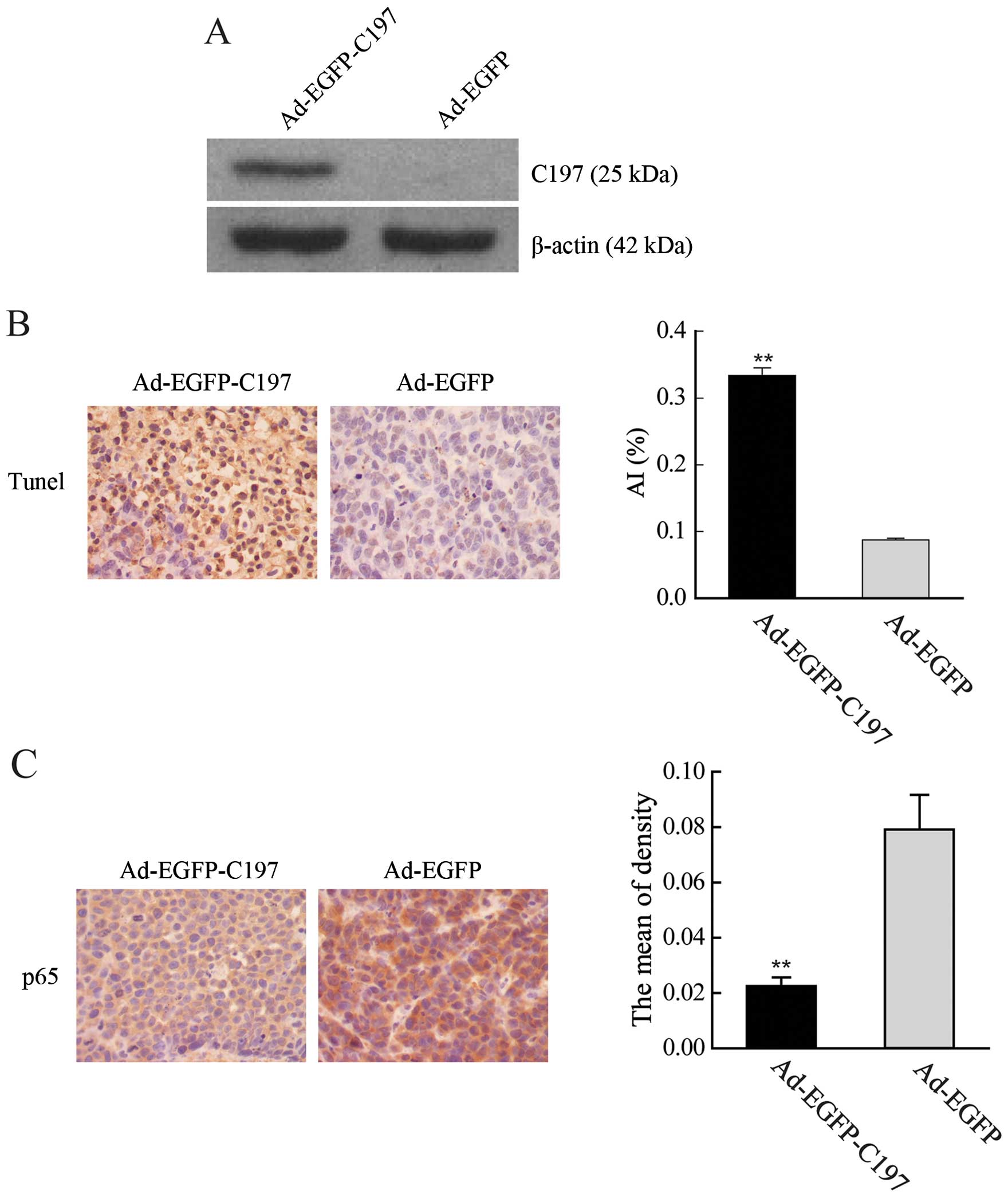
As shown in Figs. 1
and 2, high level expression of
C197 was achieved in HeLa cells by the infection of Ad-EGPF-C197.
Within the first 24 h after infection, C197 accumulated
predominantly in the cytoplasm, and appeared in the cell nucleus 48
h after infection (Fig. 2)
demonstrating that C197 was capable of entering the nucleus.
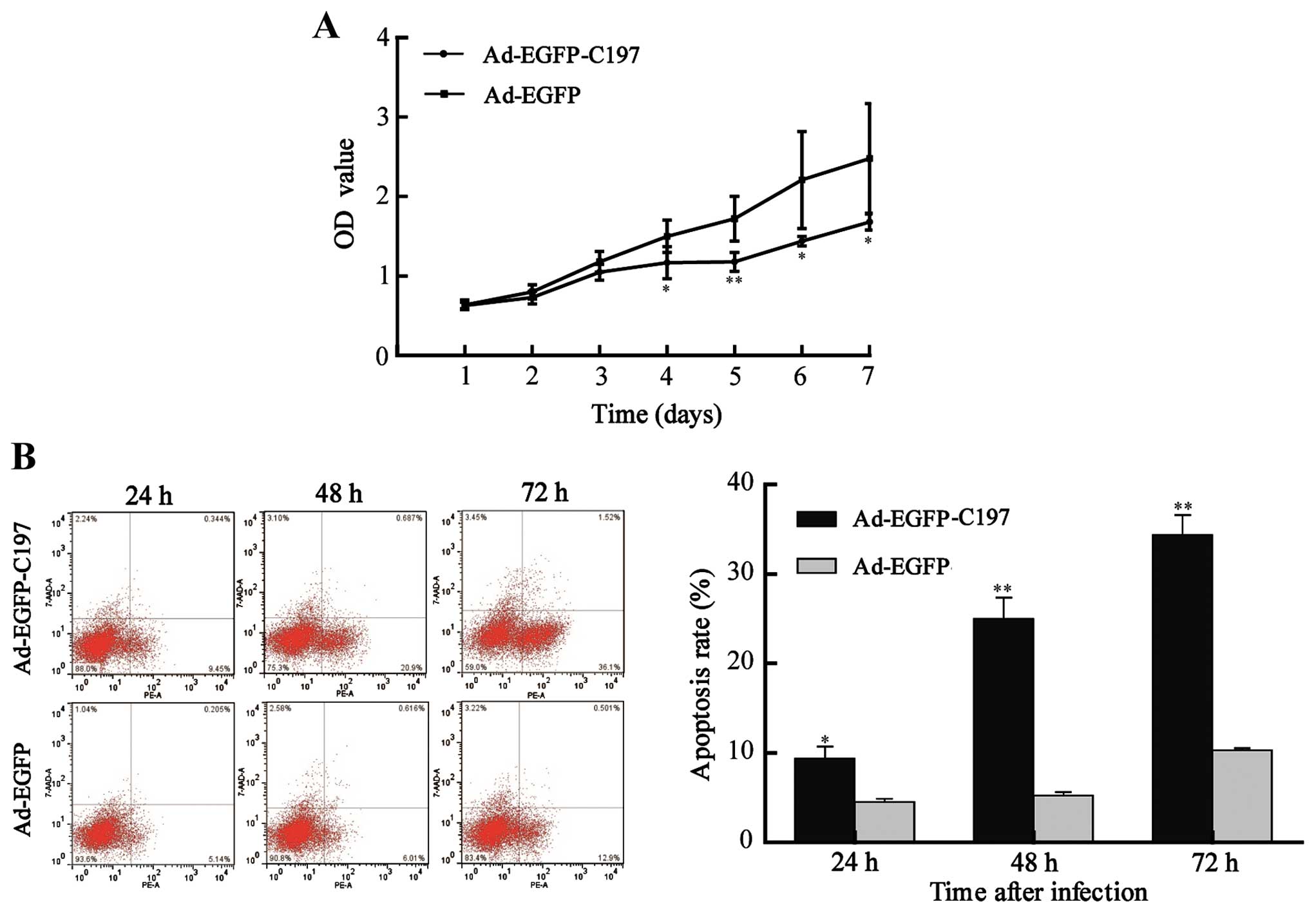
Ad-EGFP-C197 induces growth delay and
apoptosis of HeLa cells in vitro
We next evaluated the effect of C197 on the
proliferation of HeLa cells. The results in MTT assay showed that
Ad-EGFP-C197 significantly inhibited the proliferation of cells
[optical density (OD) value, 1.17±0.20, 1.18±0.12, 1.44±0.06 and
1.68±0.10 at the 4, 5, 6 and 7 days after infection, respectively]
compared to Ad-EGFP (OD value, 1.50±0.20, 1.72±0.28, 2.21±0.61 and
2.48±0.69 at the 4, 5, 6 and 7 days after infection, respectively,
P<0.05 or P<0.01, Fig.
3A).
We further assayed the apoptosis rate of cells.
Apoptotic rate was calculated using the following formula:
apoptotic rate = (cells undergoing apoptosis and cells in end-stage
apoptosis)/total cells. Ad-EGFPC197 induced a higher apoptosis rate
(9.36±2.32, 25.03±4.00 and 38.07±2.59% at 24, 48 and 72 h after
infection, respectively) compared to Ad-EGFP (4.53±0.59, 5.24±0.68
and 10.33±0.40% at 24, 48 and 72 h after infection, respectively,
P<0.05 or P<0.01, Fig. 3B),
implying that apoptosis contributed to the growth inhibition
induced by C197 in vitro.
Ad-EGFP-C197 suppresses telomerase
activity and the expression of hTERT and NF-κB p65 in HeLa cells in
vitro
Telomerase activity was detected by TRAP assay using
TRAPeze kit, which generated a ladder of products with six-base
increments starting at 50 nucleotides. K1 primer and TSK1 template
in each reaction mixture allowed for the amplification of 36 bp
nucleotides as standard of internal control (S-IC). The results
(Fig. 4A) showed that telomerase
activity in Ad-EGFP infected cells was unaffected when compared to
untreated cells. While in cells infected with Ad-EGFP-C197,
telomerase activity was significantly decreased at 48 and 72 h
after infection, showing that the expression of C197 inhibited the
telomerase activity in HeLa cells.
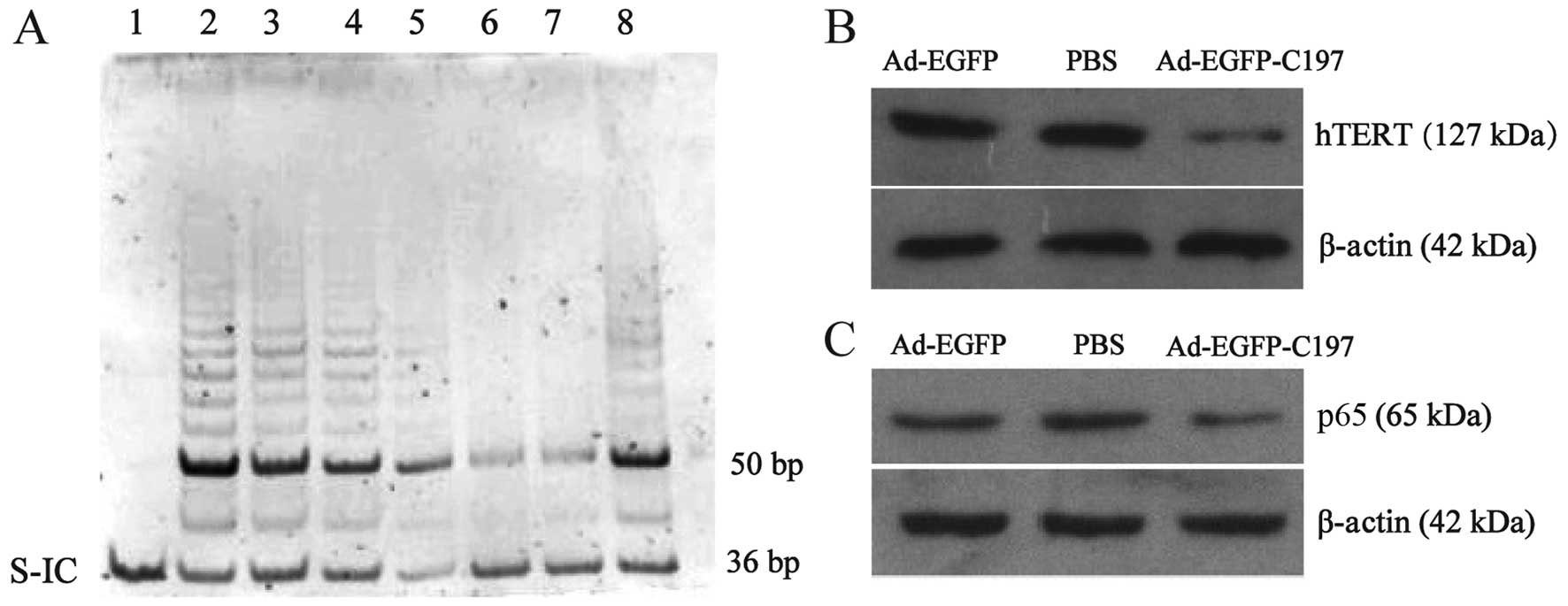 | Figure 4Ad-EGFP-C197 suppresses telomerase
activity and expression of hTERT and NF-κB p65 in HeLa cells in
vitro. (A) Ad-EGFP-C197 suppresses telomerase activity. The
36-bp internal positive control band (S-IC) is seen in every lane.
Lane 1, negative control without any product except S-IC. Lane 2,
telomerase-positive control cell showing a ladder of PCR products
with 6 base increments starting at 50 nucleotides. Lane 3, HeLa
cells infected with Ad-EGFP. Lanes 4 and 5, HeLa cells 24 and 48 h
after infection with Ad-EGFP-C197, respectively. Lanes 6 and 7,
HeLa cells 72 h after infection with Ad-EGFP-C197. Lane 8, HeLa
cells without any treatment. (B and C) Ad-EGFP-C197 suppresses the
expression of hTERT and NF-κB p65. HeLa cells were treated with
Ad-EGFP, PBS or Ad-EGFP-C197. The expression of hTERT and NF-κB p65
was determined by western blot analysis. β-actin served as an
internal control. hTERT, human telomerase reverse transcriptase;
S-IC, standard of internal control; PBS, phosphate-buffered
saline. |
Based on the fact that hTERT is critical to the
telomerase activity, we asked whether C197 affected the expression
of hTERT. The results showed that infection of AD-EGFP-C197 reduced
the expression of hTERT protein (Fig.
4B), which accounts for the suppressed telomerase activity.
In contrast, depressed telomerase activity impaired
the telomere elongation, yet this could not explain the rapid
antiproliferative effect of C197 (as shown in Fig. 2A), since there is a long lag period
between the initiation of telomerase inhibition and the observing
of growth arrest effect (23).
Since there is a positive feedback between hTERT and NF-κB
signaling pathway in the development of tumors (24), and suppression of NF-κB activation
results rapidly in apoptosis in HeLa cells (25–27),
we further asked whether the expression of NF-κB p65 would be
affected by C197 in HeLa cells. As shown in Fig. 4C, Ad-EGFP did not affect the
expression of NF-κB p65, while Ad-EGFP-C197 significantly reduced
the expression of NF-κB p65 suggesting that C197 has an impact on
NF-κB signaling pathway.
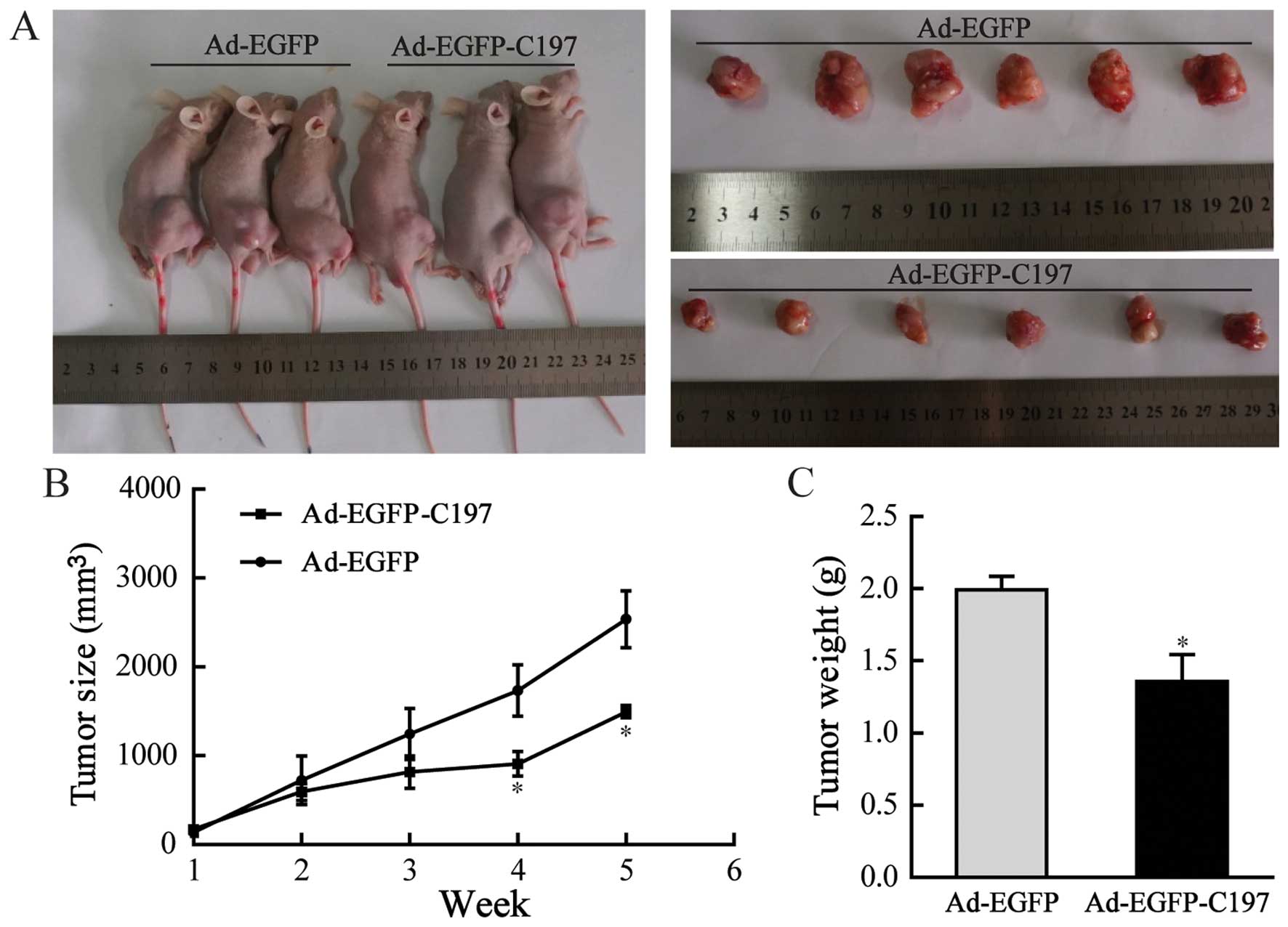
Ad-EGFP-C197 inhibits tumorigenicity of
HeLa cells in vivo
HeLa cells were subcutaneously injected into athymic
nude mice to establish xenograft tumor models, followed by
injecting the resulting tumors with Ad-EGFP or Ad-EGFP-C197.
Periodic measurement showed that the sizes of tumors in
Ad-EGFP-C197-treated mice (909.18±309.65 mm3 at 4 weeks,
and 1496.18±153.35 mm3 at 5 weeks) were significantly
smaller than in Ad-EGFP-treated mice (1736.02±643.67 mm3
at 4 weeks, and 2538.26±715.6 mm3 at 5 weeks, P<0.05,
Fig. 5B). At 5 weeks the weight of
tumors in animals injected with Ad-EGFP-C197 (1.36±0.46 g) were
significantly lower than in animals injected with Ad-EGFP
(1.99±0.23 g, P<0.05, Fig.
5C).
Ad-EGFP-C197 promoted tumor cell
apoptosis and decreased expression of NF-κB p65 in tumor
tissues
After 4 weeks injection of Ad-EGFP-C197 or Ad-GFP,
tumor tissues were subjected to TUNEL assays and
immunohistochemistry analysis. In TUNEL assay, TUNEL-positive cells
were more abundant in tumors injected with Ad-EGFP-C197 (AI,
0.334±0.023) than tumors injected with Ad-EGFP (AI, 0.088±0.005,
P<0.01, Fig. 6B). In
immunohistochemistry examination, the staining of NF-κB p65 protein
was significantly lower in Ad-EGFP-C197-treated tumor tissues (MOD,
0.022±0.006) than in tissues treated with Ad-EGFP (MOD,
0.079±0.025, P<0.01, Fig.
6C).
Discussion
In the present study, we report that the ectopic
expression of the novel C197 COOH-terminal polypeptide (amino acid
936–1,132) of hTERT, induced growth delay and apoptosis of HeLa
cells both in vitro and in nude mice. Furthermore, C197
suppressed the telomerase activity which we believe was induced by
the declined expression of hTERT. In contrast, previous studies
showed that C27 did not affect the telomerase activity. One
possible explanation of the different effects of C197 and C27 on
telomerase activity is that there may be different conformations
between C197 and C27, since C27 contains additional 54 amino acids
(D and E conserved short motifs of reverse transcriptase domain) at
the NH2 terminus (15).
Since telomeric DNA progressively erodes at a rate
of 30–120 bp with each cell cycle (28–30),
there is a long lag period between the initiation of telomerase
inhibition and the observing of growth arrest effect (23,31).
While in the present study, C197 induced growth arrest and
apoptosis of HeLa cells within a short time in vitro
(significant effects were observed 4 days after the infection of
Ad-EGFP-C197). Therefore, although telomerase activity and hTERT
protein were decreased, the rapid antiproliferative effect of C197
may be independent of telomere-repairing effect of hTERT.
In the present study, ectopic expression of C197
reduced the expression of NF-κB p65, implying that C197 has an
impact on NF-κB signaling pathway. NF-κB signaling pathway is known
as a master regulator of cellular and developmental events and
suppression of NF-κB activation results rapidly in apoptosis of
HeLa cells (25–27). Although further study is needed to
elucidate the mechanisms, the present data suggest that the
anticancer effect of C197 is associated with the downregulation of
p65 which results in the impaired function of NF-κB signaling
pathway.
In recent years, it was found that hTERT and NF-κB
pathway was often deregulated and overexpressed in many cancer
cells, and there is a forward feedback between hTERT and NF-κB
pathway (7,24). NF-κB p65 modulates hTERT expression
and nuclear translocation from cytoplasm in tumor cells (32,33),
and hTERT has been shown to contribute to cancer development and
progression as a transcriptional modulator of the NF-κB signaling
pathway in a manner independent of telomerase activity (7,8). In
the present study, we speculated that the C197 interrupts the
forward feedback between hTERT and NF-κB signaling pathway which
contributes to the antitumor effect of C197. Reduced expression of
NF-κB p65 leads to the decreased expression and activity of hTERT.
In return, decreased hTERT protein in nucleus attenuates the effect
of NF-κB pathway. Furthermore, it has been reported that
COOH-terminus of hTERT contains binding sites for 14-3-3, a protein
that prevents telomerase exporting from the nucleus (13). C197 also contains these binding
sites. The present data showed that C197 was able to find its way
to enter the nucleus. Nucleus accumulation of C197 binds most of
14-3-3, therefore inhibits the nuclear localization of hTERT, which
further suppresses the interaction between hTERT and NF-κB
pathway.
In general, our studies showed that
adenovirus-mediated overexpression of C197 inhibited the growth and
promoted apoptosis of HeLa cells in vitro and in
vivo. Although further study is needed to elucidate the more
detailed mechanisms, the present study demonstrated that the
antitumor effect of C197 is associated with the downregulation of
hTERT and NF-κB p65 protein. Our results, along with the previous
study, suggest the potential of COOH-terminal of hTERT in the
treatment of tumors.
Acknowledgments
The present study was supported by a grant from the
Science and Technology Program of Guangzhou City, China (grant no.
2014J4100195).
References
|
1
|
Moyzis RK, Buckingham JM, Cram LS, Dani M,
Deaven LL, Jones MD, Meyne J, Ratliff RL and Wu JR: A highly
conserved repetitive DNA sequence, (TTAGGG)n, present at
the telomeres of human chromosomes. Proc Natl Acad Sci USA.
85:6622–6626. 1988. View Article : Google Scholar
|
|
2
|
de Lange T: Shelterin: The protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shay JW: Telomerase therapeutics:
Telomeres recognized as a DNA damage signal: Commentary re: K.
Kraemer et al, antisense-mediated hTERT inhibition specifically
reduces the growth of human bladder cancer cells. Clin Cancer Res.
9:3794–3800. 2003.
Clin Cancer Res. 9:3521–3525. 2003.
|
|
4
|
Wright WE, Piatyszek MA, Rainey WE, Byrd W
and Shay JW: Telomerase activity in human germline and embryonic
tissues and cells. Dev Genet. 18:173–179. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Low KC and Tergaonkar V: Telomerase:
Central regulator of all of the hallmarks of cancer. Trends Biochem
Sci. 38:426–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghosh A, Saginc G, Leow SC, Khattar E,
Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, et al: Telomerase
directly regulates NF-κB-dependent transcription. Nat Cell Biol.
14:1270–1281. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding D, Xi P, Zhou J, Wang M and Cong YS:
Human telomerase reverse transcriptase regulates MMP expression
independently of telomerase activity via NF-κB-dependent
transcription. FASEB J. 27:4375–4383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JI, Venteicher AS, Hong JY, Choi J,
Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Listerman I, Gazzaniga FS and Blackburn
EH: An investigation of the effects of the core protein telomerase
reverse transcriptase on Wnt signaling in breast cancer cells. Mol
Cell Biol. 34:280–289. 2014. View Article : Google Scholar :
|
|
11
|
Li Y and Tergaonkar V: Noncanonical
functions of telomerase: Implications in telomerase-targeted cancer
therapies. Cancer Res. 74:1639–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Autexier C and Lue NF: The structure and
function of telomerase reverse transcriptase. Annu Rev Biochem.
75:493–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seimiya H, Sawada H, Muramatsu Y, Shimizu
M, Ohko K, Yamane K and Tsuruo T: Involvement of 1-3-3 proteins in
nuclear localization of telomerase. EMBO J. 19:2652–2661. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banik SS, Guo C, Smith AC, Margolis SS,
Richardson DA, Tirado CA and Counter CM: C-terminal regions of the
human telomerase catalytic subunit essential for in vivo enzyme
activity. Mol Cell Biol. 22:6234–6246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang JJ, Lin MC, Bai YX, Jing DD, Wong
BC, Han SW, Lin J, Xu B, Huang CF and Kung HF: Ectopic expression
of a COOH-terminal fragment of the human telomerase reverse
transcriptase leads to telomere dysfunction and reduction of growth
and tumorigenicity in HeLa cells. Cancer Res. 62:3226–3232.
2002.PubMed/NCBI
|
|
16
|
Ng SS, Gao Y, Chau DH, Li GH, Lai LH,
Huang PT, Huang CF, Huang JJ, Chen YC, Kung HF, et al: A novel
glioblastoma cancer gene therapy using AAV-mediated long-term
expression of human TERT C-terminal polypeptide. Cancer Gene Ther.
14:561–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao Y, Ng SS, Chau DH, Yao H, Yang C, Man
K, Huang PT, Huang C, Huang JJ, Kung HF, et al: Development of
recombinant adeno-associated virus and adenovirus cocktail system
for efficient hTERTC27 polypeptide-mediated cancer gene therapy.
Cancer Gene Ther. 15:723–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He L, Gong HX, Li XP, Wang YD, Li Y, Huang
JJ, Xie D, Kung HF and Peng Y: Inhibition of hepatocellular
carcinoma growth by adenovirus-mediated expression of human
telomerase reverse transcriptase COOH-27 terminal polypeptide in
mice. Oncol Lett. 6:748–752. 2013.PubMed/NCBI
|
|
19
|
Huo L, Yao H, Wang X, Wong GW, Kung HF and
Lin MC: Inhibition of melanoma growth by subcutaneous
administration of hTERTC27 viral cocktail in C57BL/6 mice. PLoS
One. 5:e127052010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin G, Chen Q, Yu S, Lin S, Yao H, Ding Z,
Chen S, Lin MC and Wang X: Overexpression of human telomerase
reverse transcriptase C-terminal polypeptide sensitizes HeLa cells
to 5-fluorouracil-induced growth inhibition and apoptosis. Mol Med
Rep. 9:279–284. 2014.
|
|
21
|
Li HW, Gao YX, Raizada MK and Sumners C:
Intronic enhancement of angiotensin II type 2 receptor transgene
expression in vitro and in vivo. Biochem Biophys Res Commun.
336:29–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoon JW, Lee JS, Kim BM, Ahn J and Yang
KM: Catechin-7-O-xyloside induces apoptosis via endoplasmic
reticulum stress and mitochondrial dysfunction in human non-small
cell lung carcinoma H1299 cells. Oncol Rep. 31:314–320. 2014.
|
|
23
|
Damm K, Hemmann U, Garin-Chesa P, Hauel N,
Kauffmann I, Priepke H, Niestroj C, Daiber C, Enenkel B, Guilliard
B, et al: A highly selective telomerase inhibitor limiting human
cancer cell proliferation. EMBO J. 20:6958–6968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu XQ, Huang C, He X, Tian YY, Zhou DX, He
Y, Liu XH and Li J: Feedback regulation of telomerase reverse
transcriptase: new insight into the evolving field of telomerase in
cancer. Cell Signal. 25:2462–2468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu W, Zhang G, Zhang R, Flores LG II,
Huang Q, Gelovani JG and Li C: Tumor site-specific silencing of
NF-kappaB p65 by targeted hollow gold nanosphere-mediated
photothermal transfection. Cancer Res. 70:3177–3188. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Xing D, Chen Q and Chen WR:
Enhancement of chemotherapeutic agent-induced apoptosis by
inhibition of NF-kappaB using ursolic acid. Int J Cancer.
127:462–473. 2010.
|
|
27
|
Juneja M, Vanam U, Paranthaman S,
Bharathan A, Keerthi VS, Reena JK, Rajaram R, Rajasekharan KN and
Karunagaran D: 4-Amino-2-arylamino-5-indoloyl/cinnamoythiazoles,
analogs of topsentin-class of marine alkaloids, induce apoptosis in
HeLa cells. Eur J Med Chem. 63:474–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hastie ND, Dempster M, Dunlop MG, Thompson
AM, Green DK and Allshire RC: Telomere reduction in human
colorectal carcinoma and with ageing. Nature. 346:866–868. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Counter CM, Avilion AA, LeFeuvre CE,
Stewart NG, Greider CW, Harley CB and Bacchetti S: Telomere
shortening associated with chromosome instability is arrested in
immortal cells which express telomerase activity. EMBO J.
11:1921–1929. 1992.PubMed/NCBI
|
|
31
|
Hahn WC, Stewart SA, Brooks MW, York SG,
Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M and
Weinberg RA: Inhibition of telomerase limits the growth of human
cancer cells. Nat Med. 5:1164–1170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo QP, Liu SK, Li ZJ, Li B, Zhou YL, Guo
R and Huang LH: NF-kappaB p65 modulates the telomerase reverse
transcriptase in the HepG2 hepatoma cell line. Eur J
Pharmacol. 672:113–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akiyama M, Hideshima T, Hayashi T, Tai YT,
Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC and
Anderson KC: Nuclear factor-kappaB p65 mediates tumor necrosis
factor alpha-induced nuclear translocation of telomerase reverse
transcriptase protein. Cancer Res. 63:18–21. 2003.PubMed/NCBI
|