Introduction
Malignant pleural mesothelioma (MPM) is an
aggressive thoracic malignancy. Most patients are not eligible for
curative surgery due to the advanced stage of the disease (1), and the median survival of MPM with
standard chemotherapy (cisplatin combined with pemetrexed) is ~12
months (2). Despite its
aggressiveness, there is currently no established second-line
chemotherapy, which makes it difficult to manage this disease.
Therefore, new treatment strategies are required.
Clinical samples of MPM cells and cell lines were
frequently shown to express the HER family receptors EGFR and HER2
(3,4), which is why clinical studies using
EGFR-tyrosine kinase inhibitors (EGFR-TKIs) have been conducted in
patients with MPM. However, none of these studies showed any
clinical benefits of EGFR-TKIs, requiring other treatment
strategies for MPM (4,5). It was recently reported that a
sequential combination of lapatinib with trastuzumab enhanced
trastuzumab-mediated antibody-dependent cellular cytotoxicity
(ADCC) by upregulating HER2 expression in breast (6), esophageal (7) and gastric cancer (8) cell lines. In the present study, we
report that the dual EGFR/HER2-TKI lapatinib may upregulate the
expression of both EGFR and HER2, while neither the EGFR-TKI
gefitinib nor the pan-HER-TKI afatinib demonstrate the same effect.
Additionally, we have shown that lapatinib enhanced both
trastuzumab- and cetuximab-mediated ADCC in MPM cells isolated from
the patient. The sequential combination of lapatinib with
trastuzumab and/or cetuximab may prove to be a promising strategy
for MPM patients who have poor treatment options.
Materials and methods
Cell culture and reagents
The human MPM cell lines NCI-H28, NCI-H2052 and
MSTO211 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA), while the MESO1 (9) and MESO4 (9) cell lines were purchased from RIKEN BRC
through the National Bio-Resource Project of the MEXT (Tsukuba,
Japan). The genotypes of all cell lines were identified with the
GenePrint 10 STR system (Promega, Madison, WI, USA). All the cell
lines were maintained in culture in RPMI-1640 medium with 2 mM
L-glutamine supplemented with 10% FBS (both from Invitrogen,
Carlsbad, CA, USA) and 50 U/ml penicillin streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified
atmosphere with 5% CO2. For cell culturing, gefitinib
(Cayman Chemical, Ann Arbor, MI, USA), afatinib and lapatinib (both
from Selleckchem, Houston, TX, USA) were dissolved in
dimethylsulfoxide (DMSO) (Sigma-Aldrich). Rituximab, trastuzumab
(both from Roche, Basel, Switzerland) and cetuximab (Merck Serono,
Darmstadt, Germany) were dissolved in saline. Allophycocyanin
(APC)-labeled EGFR (G46-2.6), APC-labeled HER2 and phycoerythrin
(PE)-labeled HER3 as well as APC- and PE-labeled anti-mouse IgG1κ
(MOPC-21) or IgG2bκ (MOPC-173) (all from BioLegend, San Diego, CA,
USA) were used for flow cytometry. Fluorescein (FITC)-conjugated
trastuzumab and FITC-conjugated rituximab were kindly provided by
Professor Rolf Kiessling (Karolinska Institutet, Sweden). Cetuximab
was labeled with FITC using SureLINK FITC Labeling kit (KPL Inc.,
Gaithersburg, MD, USA) according to the manufacturer's
protocol.
Isolation of MPM cells from patients
The present study was approved by the Institutional
Review Board of the Kawasaki Medical School (no. 1433-3). After
written informed consent was given, tissue samples or malignant
effusions were collected from patients with MPM. Tumor tissue was
minced into pieces with ethylenediaminetetraacetic acid (EDTA;
Invitrogen) before the centrifugation. The MPM cells were isolated
through density centrifugation and were incubated. The MPM cells
were used for further experiments after the cells were grown and
reached high enough numbers.
WST cell proliferation assay
MPM cells were cultured in triplicate wells of
96-well flat-bottomed plates (Asahi Glass, Tokyo, Japan) with
0.01–10 µM afatinib, gefitinib or lapatinib in 100 µl
of culture medium for 48 h, then 10 µl of WST regent (Roche)
were added to wells for 4 h according to the manufacturer's
protocol. Colorimetric reaction was measured by a spectral scanning
Varioskan Flash multimode reader (Thermo Scientific, Waltham, MA,
USA). TKI-mediated inhibition of cell proliferation was calculated
with following formula: 100 × (absorbance of the wells treated with
TKI/absorbance of wells treated with DMSO).
Analysis of cell surface molecules by
flow cytometry
Tumor cells were washed in phosphate buffered
solution (PBS)(-) (Invitrogen), were detached using EDTA
(Sigma-Aldrich) and were analyzed for cell surface expression of
EGFR, HER2 and HER3 by direct immunofluorescence. Stained cells
were acquired on a FACSCanto II (BD Biosciences, Franklin Lakes,
NJ, USA) and analyzed using FlowJo software 6.4.7 (Tree Star,
Ashland, OR, USA). Both FITC-conjugated trastuzumab and cetuximab
were used to evaluate the expression of the trastuzumab-binding
site and the cetuximab-binding site, respectively, on the MPM
cells, while FITC-conjugated rituximab was used as an isotype
control.
Western blot analysis
Cells were washed in PBS(-), lysed over 15 min with
CelLytic (Sigma-Aldrich) lysis buffer with a protease inhibitor
(protease inhibitor cocktail) and a phosphatase inhibitor
(phosphatase inhibitor cocktail 2) (both from Sigma-Aldrich). Cell
debris was removed at 12,000 × g for 15 min. The supernatants were
collected and the protein concentrations were analyzed using a BCA
protein assay (Takara Bio, Otsu, Japan) according to the
manufacturer's protocol. Equal amounts of protein were separated on
4–12% NuPAGE Bis-Tris acrylamide gels with MES running buffer and
transferred to polyvinylidene difluoride membranes (all from Life
Technologies, Carlsbad, CA, USA). Blots were blocked for 30 min in
PBS(-) with 0.05% of Tween-20 (Sigma-Aldrich) buffer (PBS-T) and 2%
non-fat dry milk, followed by incubation for 2 days at 4°C in PBS-T
with 2% non-fat dry milk and primary antibody against: EGFR,
phosphorylated EGFR (Tyr1068) (pEGFR), HER2, phosphorylated HER2
(Tyr1221/1222) (pHER2) or β-actin (Cell Signaling Technology,
Beverly, MA, USA). After two washes in PBS-T, membranes were
incubated with HRP-linked goat anti-rabbit or anti-mouse IgG
antibodies (Cell Signaling Technology) for 1 h at room temperature.
Blots were visualized by enhanced chemiluminescence with an ECL
prime system (GE Healthcare, Fairfield, CT, USA), and images were
captured using a LAS-4000 camera system (Fujifilm, Tokyo, Japan).
Membranes were stripped in stripping buffer (Takara Bio) for 30 min
at room temperature and reprobed up to two times.
ADCC assay
The present study was approved by the Institutional
Review Board of the Kawasaki Medical School (no. 1433-3).
Peripheral blood mononuclear cells (PBMCs) were collected from
healthy donors using density centrifugation, incubated overnight
with 100 IU/ml of human recombinant IL-2 (Teceleukin, Shionogi,
Osaka, Japan) and used as effector cells. MPM cell lines or MPM
cells from patients were cultured into a 6-well plate and treated
with DMSO or 1 µM of afatinib or lapatinib for 24 h, for
subsequent use as target cells. The ADCC was evaluated using an LDH
release assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay;
Promega) in 96-well U-bottom plate (BD Biosciences) with 100 ng/ml
of trastuzumab, cetuximab or rituximab as irrelevant control,
according to the manufacturer's protocol. After 4 h co-incubation,
LDH release in the supernatants was measured by Varioskan Flash
multimode reader. The percentage of specific lysis was calculated:
100 × (experimental release -spontaneous release)/(maximum release
- spontaneous release).
Statistical analysis
Differences in means were evaluated with two-way
ANOVA multiple comparisons, with a Bonferroni post test. All
analyses were performed using GraphPad Prism 5 software (GraphPad
Software Inc., La Jolla, CA, USA) at a significance level of 5%
(p<0.05).
Results
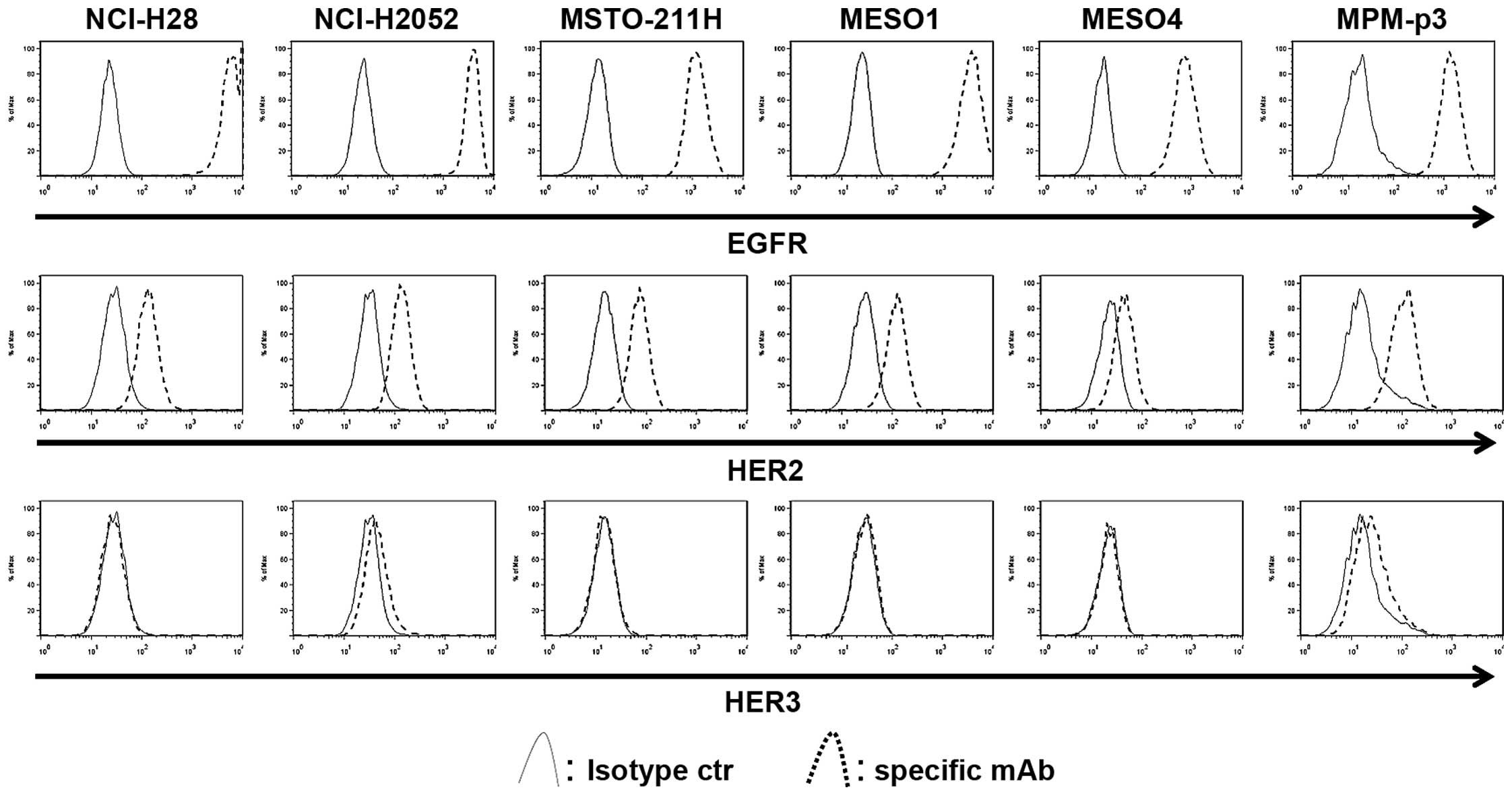
Both EGFR and HER2 are widely expressed,
while HER3 is not expressed on MPM cells
MPM tissue was collected from three patients,
however, we failed to acquire enough viable MPM cell samples from
all tissue samples. In contrast, we collected MPM cells from
malignant effusion which could be used for following experiments.
It is well known that both EGFR and HER2 are widely expressed in
MPM tissue (3,4). First, the basal expressions of EGFR,
HER2 and HER3 were determined in five MPM cell lines and the MPM
cells from a patient (MPM-p3) using flow cytometry. The EGFR was
commonly highly expressed in all MPM cells, while HER2 was also
expressed in all MPM cells. In contrast, the expression of HER3 was
rare and its expression level was quite low (Fig. 1).
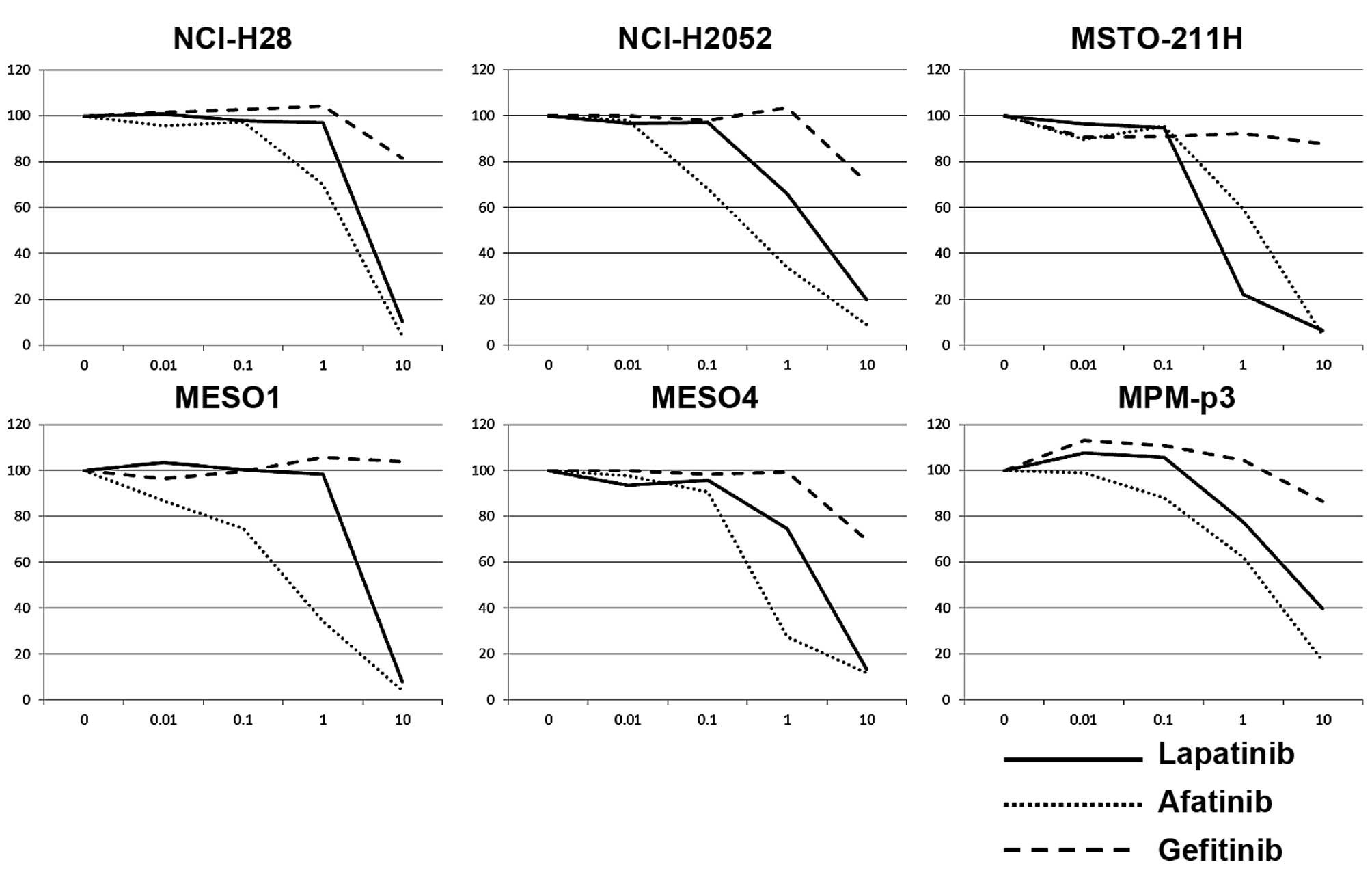
Lapatinib and afatinib strongly inhibit
the cell proliferation of MPM cell lines, but gefitinib does
not
The first-generation EGFR-TKI, gefitinib, is a
powerful tool for EGFR driver mutation-positive non-small cell lung
cancer (NSCLC) but invalid for NSCLC with an EGFR gatekeeper
mutation such as T790M (10), while
a second generation pan-HER inhibitor afatinib overcame T790M in
NSCLC cells (11). Lapatinib is a
dual EGFR/HER2-TKI with clinical benefit for HER2 over-expressing
breast cancer (12) but resistance
to T790M (13). Five MPM cell
lines, as well as MPM-p3 cells, were treated with various
concentrations of TKIs. Both afatinib and lapatinib clearly
inhibited cell proliferation of MPM cells, while gefitinib had a
slight effect on cell proliferation of MPM cells (Fig. 2).
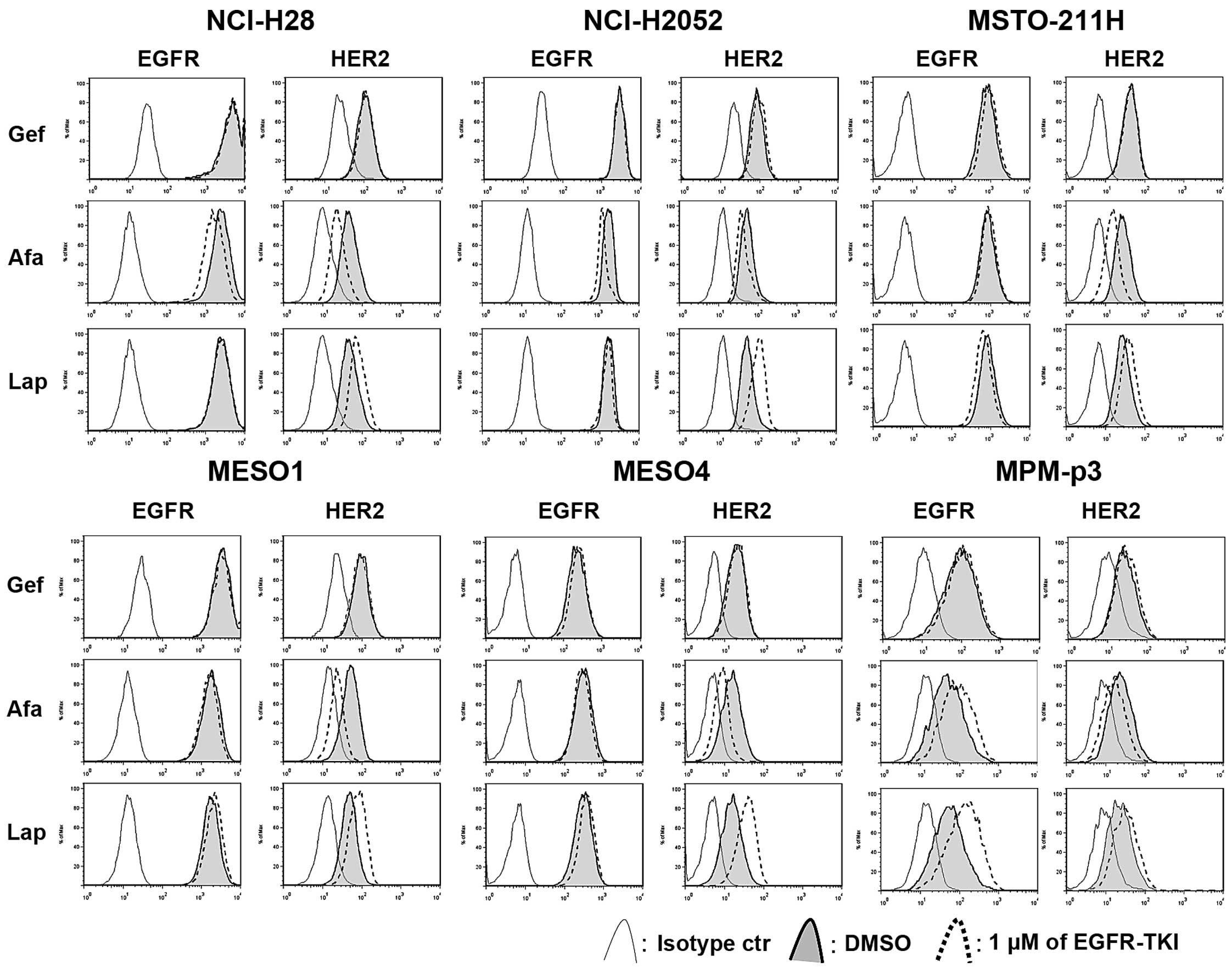
Lapatinib upregulates HER2 expression
while afatinib downregulates HER2 expression in MPM cells
We assessed whether each TKI affected on the
expression of EGFR and HER2 in MPM cells (Fig. 3). Tumor cells were treated with a
TKI for 24 h, then the expression of EGFR and HER2 was assessed by
flow cytometry. Notably, afatinib downregulated the expression of
EGFR in NCI-H28 and NCI-H2052 cells and also downregulated the
expression of HER2 in all cell lines, while lapatinib clearly
upregulated HER2 expression in all the cell lines. In MPM-p3 cells,
afatinib upregulated EGFR and downregulated HER2, while lapatinib
upregulated expression of both EGFR and HER2. Gefitinib had no
effect on the expression of EGFR and HER2 in the cell lines, or in
the MPM-p3 cells.
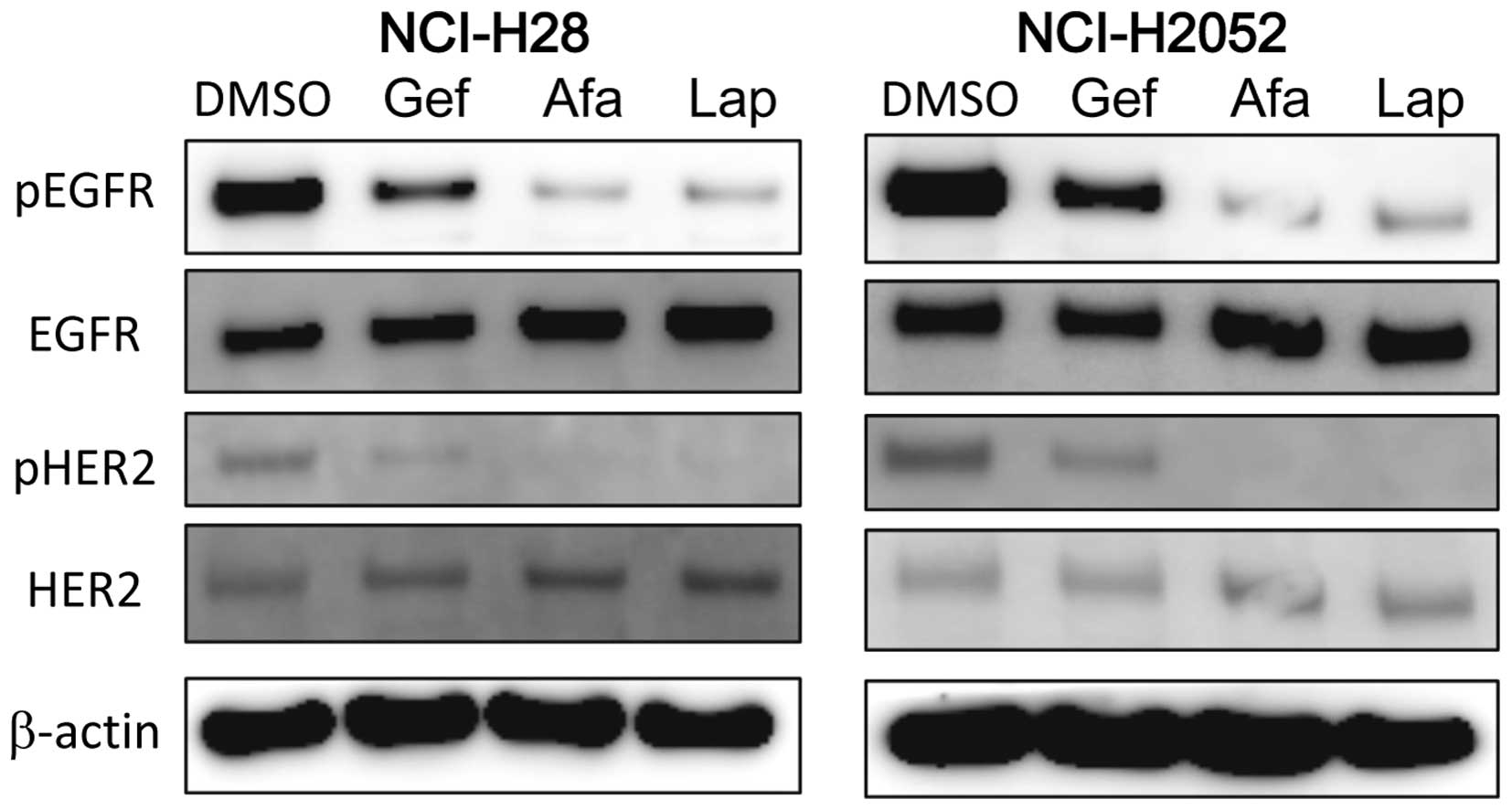
TKIs have different effect on inhibition
of EGFR/HER2
To investigate why the effects on the expression of
HER2 were different between TKIs, MPM cells were treated with 1
µl of each TKI for 1 h, then both EGFR and HER2 signaling
were assessed by western blot analysis in NCI-H28 and NCI-H2052
cells. Gefitinib weakly inhibited the phosphorylation of both EGFR
and HER2. Notably, both afatinib and lapatinib strongly and
similarly inhibited the phosphorylation of EGFR and HER2 in both
cell lines (Fig. 4).
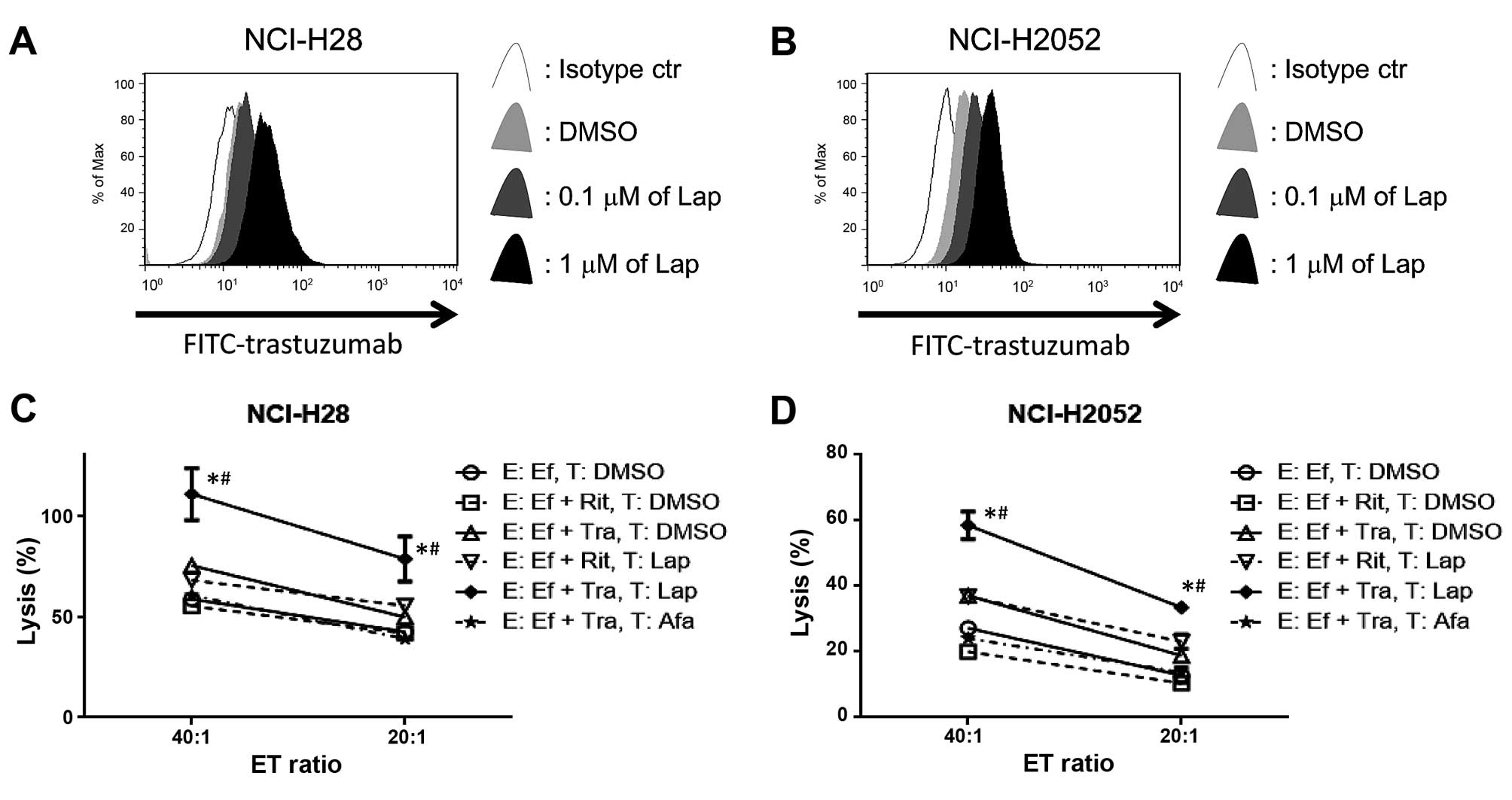
Lapatinib enhances trastuzumab binding
with MPM cells, resulting in enhanced trastuzumab-mediated ADCC in
MPM cell lines
To evaluate whether lapatinib enhances trastuzumab
binding with tumor cells, we used FITC-conjugated trastuzumab in
two MPM cell lines (NCI-H28 and NCI-H2052) in which lapatinib
clearly upregulated HER2 expression for flow cytometry. In line
with our FACS data in Fig. 3,
lapatinib upregulated the trastuzumab binding site in both NCI-H28
and NCI-H2052 cells (Fig. 5A and
B). Next, trastuzumab-mediated ADCC was evaluated. Lapatinib
enhanced trastuzumab-mediated ADCC compared with target cells
treated with DMSO in both cell lines, while afatinib did not
enhance trastuzumab mediated-ADCC (Fig.
5C and D).
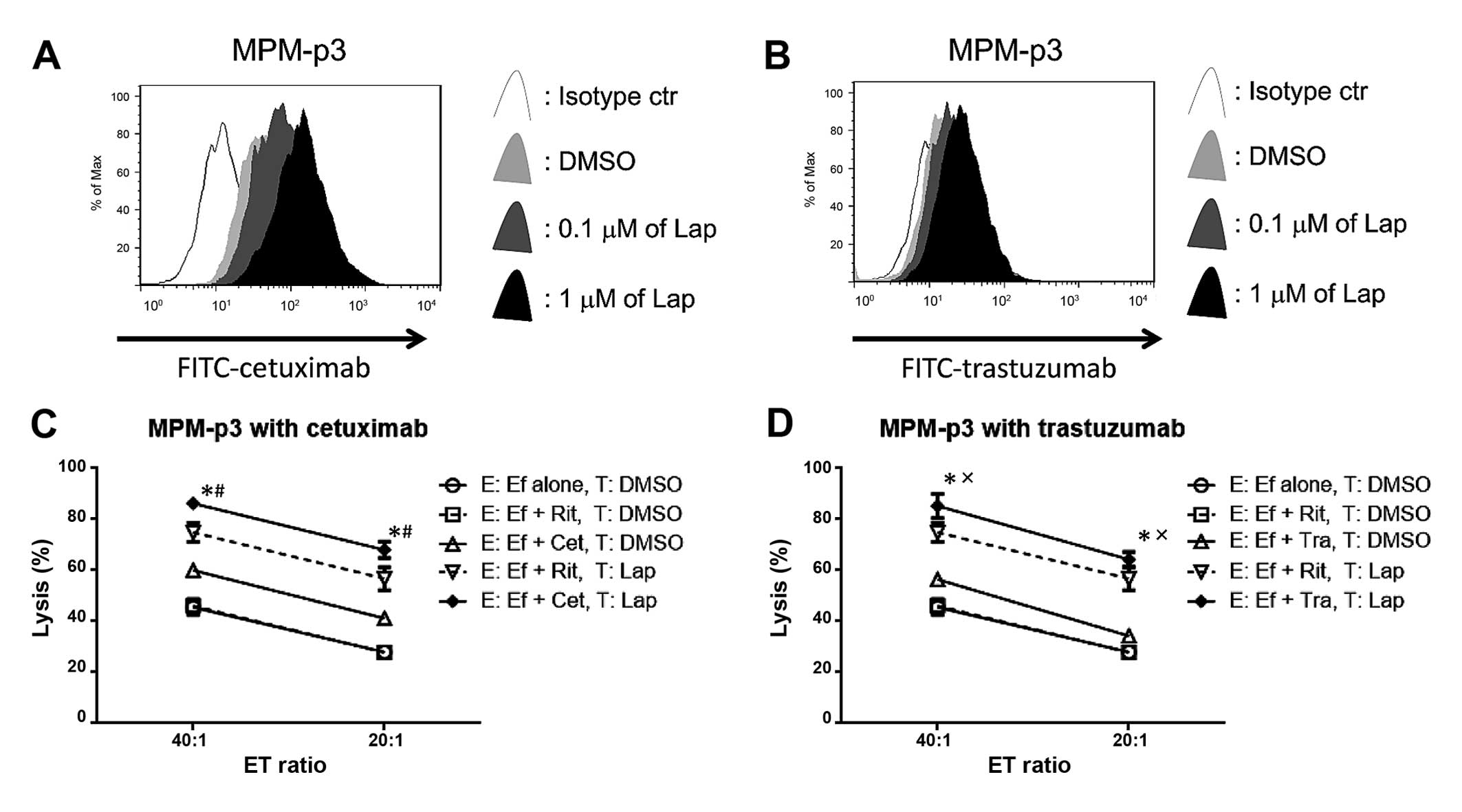
Lapatinib enhances trastuzumab or
cetuximab to bind with MPM cells from a patient, resulted in
enhanced cetuximab- or trastuzumab-mediated ADCC
Finally, MPM-p3 cells, which were collected from a
malignant effusion of a patient with MPM, were assessed. Both EGFR
and HER2 were upregulated by lapatinib in MPM-p3 cells (Fig. 3). In line with these results,
lapatinib upregulated both the cetuximab and trastuzumab binding
sites (Fig. 6A and B), resulting in
enhanced cetuximab- or trastuzumab-mediated ADCC in MPM-p3 cells
(Fig. 6C and D).
Discussion
In the present study, we reported that lapatinib
enhanced trastuzumab-mediated ADCC in MPM cell lines as well as
trastuzumab- and cetuximab-mediated ADCC in patient-derived MPM
cells. Although EGFR is frequently expressed in MPM patient cells,
EGFR targeting therapy using first-generation EGFR-TKIs gefitinib
or erlotinib have failed to show any clinical benefits in
previously conducted studies (4,5). HER2
is also expressed in MPM cells, however, there are no reported
clinical studies using trastuzumab or lapatinib. Thus, targeting
both EGFR and HER2 in patients with MPM is still an undeveloped yet
promising strategy. Other EGFR- or HER2-targeting regents are
antibody drugs targeting EGFR or HER2. The main mechanism behind
the therapeutic potential of the anti-HER2 antibody drug
trastuzumab in HER2 overexpressing breast cancer cells is ADCC
(14), which is also a crucial
mechanism for the antitumor effects of anti-EGFR antibody drug
cetuximab in both NSCLC (15) and
MPM cells (16). For antibody
drugs, not the driver mutation, but the overexpression of the
targeting molecule is important for clinical benefit, suggesting
that the upregulation of the expression of EGFR or HER2 by TKI is a
reasonable strategy.
We have shown that MPM cells frequently expressed
both EGFR and HER2 molecules, but did not express HER3, suggesting
that both EGFR and HER2 could be promising targets for the
treatment of MPM. We have also shown that dual EGFR/HER2-TKI
lapatinib enhanced the expression of EGFR or HER2, however, the
EGFR-TKI gefitinib and pan-HER TKI afatinib did not enhance either
receptor in MPM cells.
Although these results are in line with the recent
studies showing that lapatinib enhanced trastuzumab-mediated ADCC
in breast cancer or gastrointestinal cancers (6–8), we
believe that the present study is promising as there is no
established second-line therapy for patients with MPM, and new
treatment strategies are urgently required.
Another important finding is that dual EGFR/HER2-TKI
lapatinib enhances both EGFR and HER2, while neither the EGFR-TKI
gefitinib nor pan-HER TKI afatinib have shown this effect. Our
immunoblotting results show the phosphorylation of both EGFR and
HER2 were weakly inhibited by gefitinib, while strongly inhibited
by afatinib or lapatinib, suggesting that gefinitib had weaker
effects on EGFR/HER2 signaling than afatinib or lapatinib in MPM
cells. Notably, afatinib shows a similar effect to that of
lapatinib on both pEGFR and pHER2, but differed from that of
lapatinib on the expression of HER2. Rimawi et al showed
that afatinib monotherapy decreased the HER2 dimer in breast cancer
tissue collected from patients (17). It was also reported that lapatinib
blocks the internalization of HER2, resulting in enhanced
stabilization of inactive HER2 homo- and heterodimers in the plasma
membrane of breast cancer cells (6). Based on these reported findings, the
possible mechanism is that lapatinib keeps homo- or heterodimers on
the cell surface via the inhibition of HER2 internalization,
resulting in the enhancement of HER2 expression. However, if
afatinib does not keep the dimerization, then HER2 may be
internalized and degradated.
It is well known that the first-generation EGFR-TKIs
gefitinib and erlotinib exhibit a significant response in NSCLC
cells having the EGFR driver mutation. However, this mutation is
quite rare in MPM cells; therefore, first-generation EGFR-TKIs have
no clinical benefits for patients with MPM. In contrast, the
second-generation EGFR-TKI afatinib can inhibit EGFR signaling in
NSCLC cells without EGFR driver mutation or with both EGFR driver
mutation and a resistant mutation such as the T790M mutation.
According to our data, both afatinib and lapatinib clearly
inhibited the cell proliferation of MPM cells, suggesting that
these TKIs may show the same clinical benefits as monotherapy.
Additionally, we have shown in the present study that lapatinib
enhanced trastuzumab- or cetuximab-mediated ADCC while afatinib did
not. This suggests that lapatinib combined with trastuzumab and/or
cetuximab is a very promising strategy, as we can expect the dual
EGFR/HER2 blocking effect of lapatinib, as well as trastuzumab- or
cetuximab-mediated ADCC with this combination. We should pursue
optimal effect with least toxicity for MPM patients, and lapatinib
has been shown to be an ideal partner drug for cetuximab or
trastuzumab to enhance ADCC for MPM treatment.
Acknowledgments
We thank Ms. Maitani and the staff of the Tissue
Culture and Immunology, Biochemistry and Tissue Biology and
Electron Microscopy Research Center (Kawasaki Medical School) for
technical assistance. We also thank Editage for English language
editing. This study was supported by JSPS Kakenhi Grant no.
25462189, Kawasaki Medical School Project Grant (25–34), the Ryobi
Teien Research Grant and The Okayama Medical Foundation (to R.O.),
and the Strategic Research Foundation Grant-aided Project for
Private Universities from Ministry of Education, Culture, Sport,
Science and Technology (to M.N.).
Abbreviations:
|
MPM
|
malignant pleural mesothelioma
|
|
TKI
|
tyrosine kinase inhibitor
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
DMSO
|
dimethylsulfoxide
|
|
APC
|
allophycocyanin
|
|
PE
|
phycoerythrin
|
|
FITC
|
fluorescein isothiocyanate
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
PBS
|
phosphate-buffered saline
|
|
p-
|
phosphorylated
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
NSCLC
|
non-small cell lung cancer
|
|
E:T ratio
|
effector/target ratio
|
References
|
1
|
Rusch VW and Venkatraman ES: Important
prognostic factors in patients with malignant pleural mesothelioma,
managed surgically. Ann Thorac Surg. 68:1799–1804. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Destro A, Ceresoli GL, Falleni M, Zucali
PA, Morenghi E, Bianchi P, Pellegrini C, Cordani N, Vaira V,
Alloisio M, et al: EGFR overexpression in malignant pleural
mesothelioma. An immunohistochemical and molecular study with
clinico-pathological correlations. Lung Cancer. 51:207–215. 2006.
View Article : Google Scholar
|
|
4
|
Garland LL, Rankin C, Gandara DR, Rivkin
SE, Scott KM, Nagle RB, Klein-Szanto AJ, Testa JR, Altomare DA and
Borden EC: Phase II study of erlotinib in patients with malignant
pleural mesothelioma: A Southwest Oncology Group Study. J Clin
Oncol. 25:2406–2413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Govindan R, Kratzke RA, Herndon JE II,
Niehans GA, Vollmer R, Watson D, Green MR and Kindler HL: Cancer
and Leukemia Group B (CALGB 30101): Gefitinib in patients with
malignant mesothelioma: A phase II study by the Cancer and Leukemia
Group B. Clin Cancer Res. 11:2300–2304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scaltriti M, Verma C, Guzman M, Jimenez J,
Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S,
Arribas J, et al: Lapatinib, a HER2 tyrosine kinase inhibitor,
induces stabilization and accumulation of HER2 and potentiates
trastuzumab-dependent cell cytotoxicity. Oncogene. 28:803–814.
2009. View Article : Google Scholar
|
|
7
|
Mimura K, Kono K, Maruyama T, Watanabe M,
Izawa S, Shiba S, Mizukami Y, Kawaguchi Y, Inoue M, Kono T, et al:
Lapatinib inhibits receptor phosphorylation and cell growth and
enhances antibody-dependent cellular cytotoxicity of EGFR-and
HER2-overexpressing esophageal cancer cell lines. Int J Cancer.
129:2408–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiraishi K, Mimura K, Izawa S, Inoue A,
Shiba S, Maruyama T, Watanabe M, Kawaguchi Y, Inoue M, Fujii H, et
al: Lapatinib acts on gastric cancer through both antiproliferative
function and augmentation of trastuzumab-mediated
antibody-dependent cellular cytotoxicity. Gastric Cancer.
16:571–580. 2013. View Article : Google Scholar
|
|
9
|
Usami N, Fukui T, Kondo M, Taniguchi T,
Yokoyama T, Mori S, Yokoi K, Horio Y, Shimokata K, Sekido Y, et al:
Establishment and characterization of four malignant pleural
mesothelioma cell lines from Japanese patients. Cancer Sci.
97:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Ambrogio L, Shimamura T, Kubo S,
Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A,
Himmelsbach F, et al: BIBW2992, an irreversible EGFR/HER2 inhibitor
highly effective in preclinical lung cancer models. Oncogene.
27:4702–4711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gilmer TM, Cable L, Alligood K, Rusnak D,
Spehar G, Gallagher KT, Woldu E, Carter HL, Truesdale AT, Shewchuk
L, et al: Impact of common epidermal growth factor receptor and
HER2 variants on receptor activity and inhibition by lapatinib.
Cancer Res. 68:571–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clynes RA, Towers TL, Presta LG and
Ravetch JV: Inhibitory Fc receptors modulate in vivo cytotoxicity
against tumor targets. Nat Med. 6:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurai J, Chikumi H, Hashimoto K, Yamaguchi
K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M, et
al: Antibody-dependent cellular cytotoxicity mediated by cetuximab
against lung cancer cell lines. Clin Cancer Res. 13:1552–1561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurai J, Chikumi H, Hashimoto K, Takata M,
Sako T, Yamaguchi K, Kinoshita N, Watanabe M, Touge H, Makino H, et
al: Therapeutic antitumor efficacy of anti-epidermal growth factor
receptor antibody, cetuximab, against malignant pleural
mesothelioma. Int J Oncol. 41:1610–1618. 2012.PubMed/NCBI
|
|
17
|
Rimawi MF, Aleixo SB, Rozas AA, Nunes de
Matos Neto J, Caleffi M, Figueira AC, Souza SC, Reiriz AB,
Gutierrez C, Arantes H, et al: A neoadjuvant, randomized,
open-label phase II trial of afatinib versus trastuzumab versus
lapatinib in patients with locally advanced HER2-positive breast
cancer. Clin Breast Cancer. 15:101–109. 2015. View Article : Google Scholar
|