Introduction
Glioblastoma multiforme (GBM) is one of the most
malignant and difficult-to-treat tumors. Despite the availability
of multi-modality treatment involving surgical resection followed
by adjuvant radio- and chemotherapy, the outcome of GBM patients
remains poor with a median survival of ~15 months (1,2). GBM
patients succumb to brain tumor due to rapid, aggressive, and
infiltrative growth of the tumor, rendering the tumor unresectable
at the time of diagnosis in many cases (3).
MicroRNAs (miRNAs), a class of endogenous small
non-coding RNAs of ~23 nucleotides, play key roles in regulating
various biological processes (4,5), and
are involved in glioma migration and invasion (6–8).
miR-124 is abundantly expressed in normal brain tissue and plays a
crucial role in the differentiation and function of neurons, and
contributes to the pathogenesis of human brain disorders (9). Several studies indicated a correlation
between the downregulation of miR-124 and tumor grading in GBM
(6–8). These studies revealed a lower miR-124
expression in GBM with higher invasiveness (10).
The calpains are a family of calcium-dependent
neutral cysteine proteases and are active participants in processes
such as cell mobility and cell cycle progression. Calpain small
subunit 1 (Capn4) plays an essential role in maintaining calpain
stability and activity (11). Capn4
overexpression was observed in intrahepatic cholangiocarcinoma
tissues and involved in the metastasis of intrahepatic
cholangiocarcinoma (12). Higher
Capn4 expression was also identified in and associated with poor
overall survival (OS) of clear cell renal cell carcinoma patients
(13). In a previous study, we also
observed an increased expression of Capn4 in glioma tissues, which
was associated with a poorer OS and progression-free survival (PFS)
of GBM patients. Additionally, the downregulation of Capn4
suppressed the invasion and migration of glioma cells in
vitro (14). A tissue
microarray study by Bai et al revealed a correlation between
Capn4 expression and an invasive phenotype of hepatocellular
carcinoma (15).
Although miR-124 and Capn4 are invovled in the
invasiveness of GBM and other tumors, a connection between miR-124
and Capn4 has not been previously documented. In the present study,
we investigated the expression of miR-124 and Capn4 in 20 GBM
tissue specimens and 6 control tissue specimens and in queried data
on glioma from The Cancer Genome Atlas (TCGA). It was found that
miR-124 was downregulated whereas Capn4 was upregulatedin GBM
tissues. miR-124 expression correlated negatively with Capn4
protein levels. Our computer algorithm analysis revealed that Capn4
is a candidate target gene of miR-124. The results also indicated
that miR-124 suppressed the migration and invasion of glioma cells
in vitro by modulating Capn4.
Materials and methods
Tissue specimen acquisition
Twenty archived GBM specimens were obtained from the
tissue bank of Huashan Hospital, Fudan University, Shanghai, China.
These specimens were obtained from GBM patients who underwent
radical surgical resection between January 2011 and December 2012
at the Department of Neurosurgery, Huashan Hospital, and
snap-frozen. Six control brain specimens were obtained from trauma
patients or patients who received epilepsy surgery in the interim.
Acquisition of tissue specimens was approved by the hospital
Institutional Review Board and was performed in accordance with the
institutional and national regulations.
TCGA data retrieval
To investigate the expression of miR-124 and Capn4
in human GBM and normal brain tissues, we retrieved glioma data on
TCGA (https://tcga-data.nci.nih.gov/tcga/). The results were
based on data generated by the TCGA Research Network: http://cancergenome.nih.gov/. The resulting data set
was filtered for samples having expression data for miR-124 and
Capn4.
Computational algorithm analysis
The in silico prediction of predicted mRNA
target sites of miR124 on human mRNA transcripts was performed
using the algorithms TargetScan Human 5.1 (www.targetscan.org) and human microRNA (http://www.microrna.org/). The minimum free energy
predicted for hybridization was detected using BibiServ (http://bibiserv.techfak.uni-bielefeld.de/genefisher2/).
Cells and transfection
HEK293T and GBM cells, U251 and U87 (Cell Bank of
Chinese Academy of Sciences, Shanghai, China), were cultured in
DMEM with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA).
Capn4 was knocked down by shRNA in the U251 and U87
cells as previously described (14). The cells were transfected with
plasmid vectors expressing Capn4, pLenti-Capn4 or miR-124 mimics
(Genomeditech, Shanghai, China) using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA).
Dual luciferase reporter assays
The 3′-untranslated region (3′-UTR) of Capn4
containing the miR-124 target sequence (5′-GTGCCTT-3′) was
synthesized (Genomeditech) and inserted into the XbaI site
of the pGL3-control vector (Promega, Madison, WI, USA), with the
resultant plasmids pGL3-Capn4-wt. To determine sequence
specificity, the plasmid pGL3-Capn4-mt was constructed, in which
the conserved targeting sequence of miR-124 was mutated to
5′-ACACTCC-3′. miR-124 mimics or scrambled miRNA and reporter
plasmids were co-transfected into HEK-293 cells using Lipofectamine
2000. After 48 h, the cells were assayed with a dualluciferase
reporter assay system (Promega). Firefly luciferase activity for
each sample was normalized to Renilla luciferase activity.
Transfection was repeated three times in triplicate
independently.
Reverse transcription-quantitative
PCR
Total RNA isolation, cDNA synthesis and reverse
transcription-quantitative PCR (RT-qPCR) was performed as
previously described (14), using
the primers: Capn4, 5′-ACCCACTCCGTAACCTC-3′ (sense) and
5′-GGGTAGCAACCGTGAA-3′ (antisense), GAPDH,
5′-TCCACCACCCTGTTGCTGTA-3′ (sense) and 5′-ACCACAGTCCATGCCATCAC-3′
(antisense), for miR-124 (DHM0032, Takara, Dalian, China) and the
universal miRNA control primer (D354, Takara). PCR was performed in
triplicate using the ABI PRISM 7900 Sequence Detection System
(Applied Biosystems, CA, USA). The relative amount of Capn4 mRNA,
standardized by GAPDH mRNA, was expressed as −∆CT=[CT
(Capn4)-CT (GAPDH)]. The ratio of the number of Capn4
mRNA copies to the number of GAPDH mRNA copies was then
calculated as 2−∆CTxK, where K is a constant.
Western blot assays
Western blotting was performed as previously
described (14) using the
antibodies: Capn4 (Abcam, MA, USA), E-cadherin,
N-cadherin, vimentin, MMP2, MMP9, FAK, and phospho-FAK (all
from Cell Signaling Technology, Beverly, MA, USA) and β-actin
(Abcam). The protein expression was normalized against β-actin and
densitometric analysis was performed using Image Pro plus 6.0.
Wound-healing and Transwell invasion
assays
Wound-healing and Transwell invasion assays were
performed as previously described (14). Briefly, the cells were seeded in
24-well plates and when the cells were 80–90% confluent, the
monolayer was scratched manually with a plastic pipette tip across
the diameter of each plate. After two washes with PBS, the cell
migration was observed by microscopy at 48 h. Transwell cell
invasion assays were performed using 24-well Transwells
(8-µm pore size; Millipore, Billerica, MA, USA) precoated
with Matrigel (BD Biosciences, San Jose, CA, USA). A total of
2×105 cells were suspended in 200 µl DMEM
containing 1% FBS and added to the upper chamber. The lower chamber
was filled with 600 µl DMEM containing 10% FBS acting as
chemoattractants. After 24 h of incubation, Matrigel and the cells
remaining in the upper chamber were removed by cotton swabs. The
cells on the lower surface of the membrane were fixed in 4%
paraformaldehyde and stained with Giemsa. Cells in 5 microscopic
fields were counted and imaged and expressed as the average number
of cells per field of view. The experiments were performed in
triplicate at least three times independently.
Statistical analysis
Data are presented as median and range or mean ± SD
where appropriate and analyzed using Student's t-test for
comparisons between groups. Spearman's correlation analysis was
performed between the Capn4 mRNA level, Capn4 protein level and
miR-124 level. P<0.05 was considered statistically
significant.
Results
miR-124 is significantly downregulated in
GBM tissues
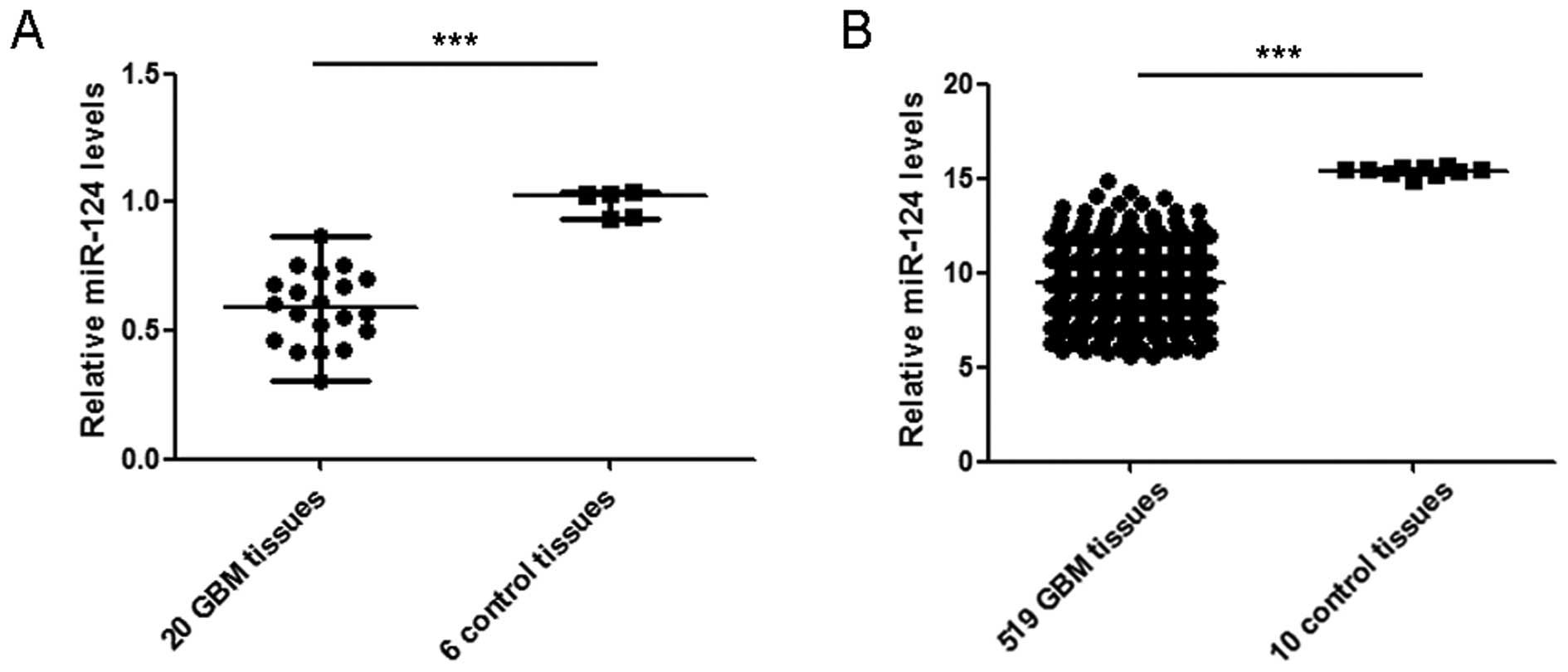
Analysis of 20 GBM tissue and 6 control tissue
specimens by RT-qPCR showed a median of 0.59 (range, 0.31–0.87) for
GBM and 1.03 (range, 0.94–1.04) for control brain tissues
(P<0.001) (Fig. 1A). The queried
data revealed a markedly lower level of miR-124 in GBM tissues
(n=519) than that of normal controls (n=10) (P<0.001) (Fig. 1B).
miR-124 directly targets the 3′-UTR of
Capn4
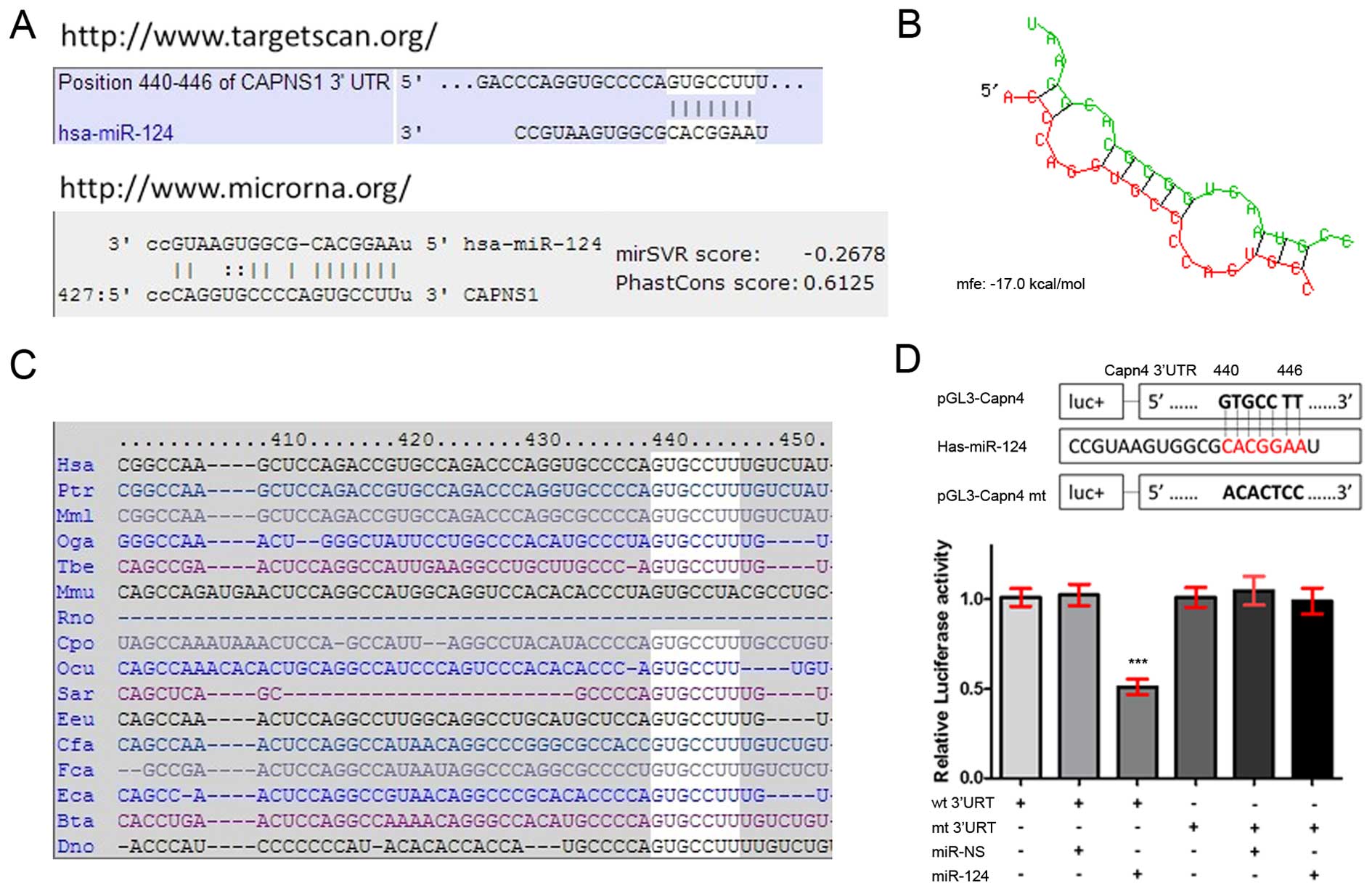
To determine the role of miR-124 in GBM. We searched
for candidate target genes of miR-124 using TargetScan and human
microRNA database. We found that the nucleotide 440–446 region
within the 3′-UTR of the Capn4 gene contained a candidate binding
site for miR-124 (Fig. 2A). The
BibiServ analysis revealed that the free energy (DG) was
approximately −17.0 kcal/mol for hybridization of the Capn4 3′-UTR
and miR-124 (Fig. 2B). The homology
analysis additionally revealed that the nucleotide 440–446 region
within the Capn4 3′-UTR was highly conserved across species
(Fig. 2C). The luciferase assays
showed that HEK293T cells co-transfected with pGL3-Capn4-wt and
miR-124 mimics expressed significantly decreased luciferase
activities compared to the cells co-transfected with pGL3-Capn4-mt
and miR-124 mimics or pGL3-Capn4-wt and scrambled miRNA controls
(Fig. 2D). These results
demonstrated that miR-124 directly targeted the 3′-UTR of
Capn4.
Capn4 is upregulated in GBM tissues at
the post-transcriptional level
Given that the dysregulated expression of Capn4 has
been shown to be a predictor of adverse prognosis of glioma
patients (14) and our finding that
Capn4 was a direct target of miR-124, we examined whether Capn4 was
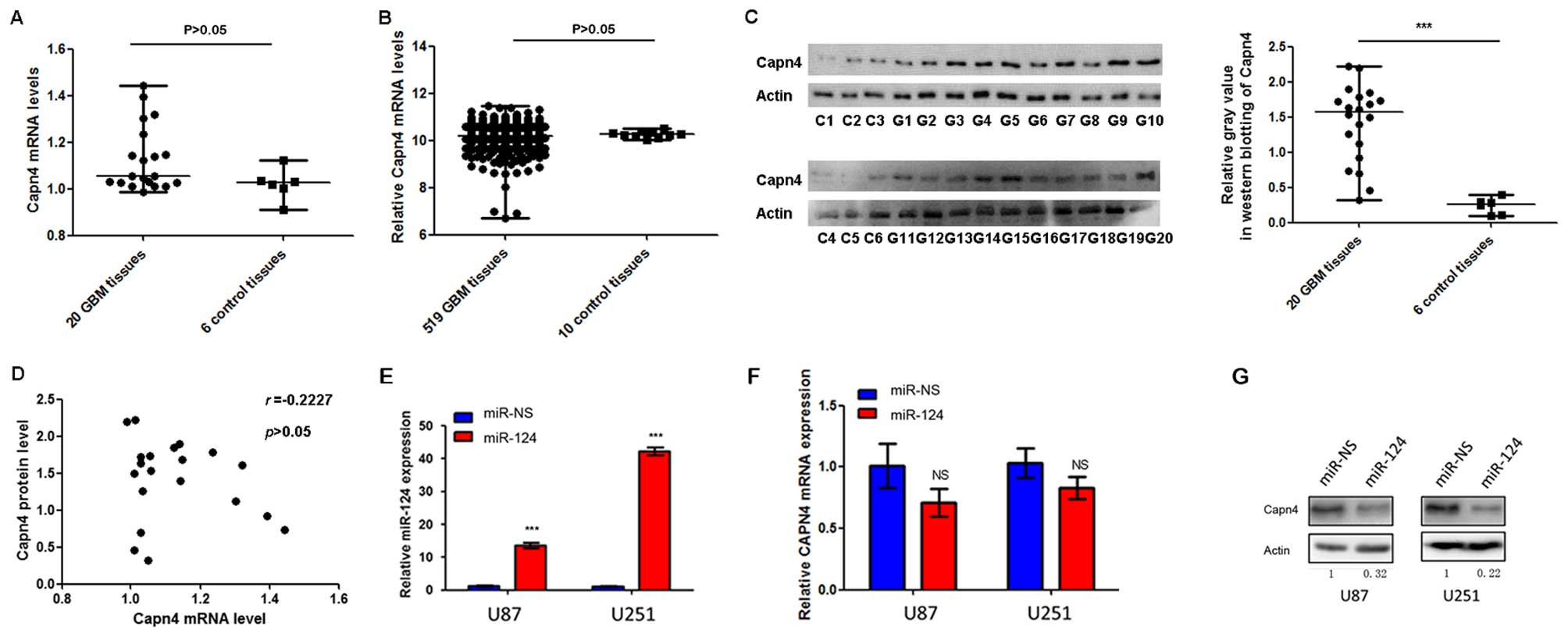
dysregulated in GBM. The RT-qPCR analysis demonstrated a median
Capn4 mRNA transcript level of 1.06 (range, 0.99–1.44) for 20 GBM
tissue specimens and 1.03 (range, 0.91–1.12) for 6 control brain
tissue specimens (P>0.05) (Fig.
3A). Query of data from the TCGA revealed a median Capn4 mRNA
transcript level of 10.21 (range, 6.73–11.48) for 519 GBM tissue
specimens and 10.25 (range, 10.04–10.52) for 10 normal brain tissue
specimens (P>0.05) (Fig. 3B). By
contrast, the immunoblotting assays showed a median Capn4 level of
1.60 (range, 0.32–2.22) for GBM tissue specimens and 0.26 (range,
0.01–0.40) for normal specimens (P<0.001) (Fig. 3C). Spearman's correlation analysis
found no significant correlation between the mRNA transcript levels
and protein levels of Capn4 in GBM tissues (r=0.2227, P>0.05)
(Fig. 3D).
In addition, we transfected U87 and U251 cells with
miR-124 mimics or scrambled miRNA controls (Fig. 3E). The RT-qPCR analysis revealed
that miR-124 failed to markedly suppress the endogenous expression
of Capn4 mRNA transcripts compared to the cells transfected with
scrambled miRNA controls (P>0.05) (Fig. 3F). Consistent with our findings in
GBM tissues, miR-124 mimics significantly reduced the expression of
Capn4 protein in the U87 and U251 cells (P<0.001 vs. scrambled
miRNA controls) (Fig. 3G). The
above findings together indicated that Capn4 was upregulated in GBM
cells or tissues at the post-transcriptional level and not at the
transcriptional level.
miR-124 expression correlates negatively
with Capn4 protein levels in GBM
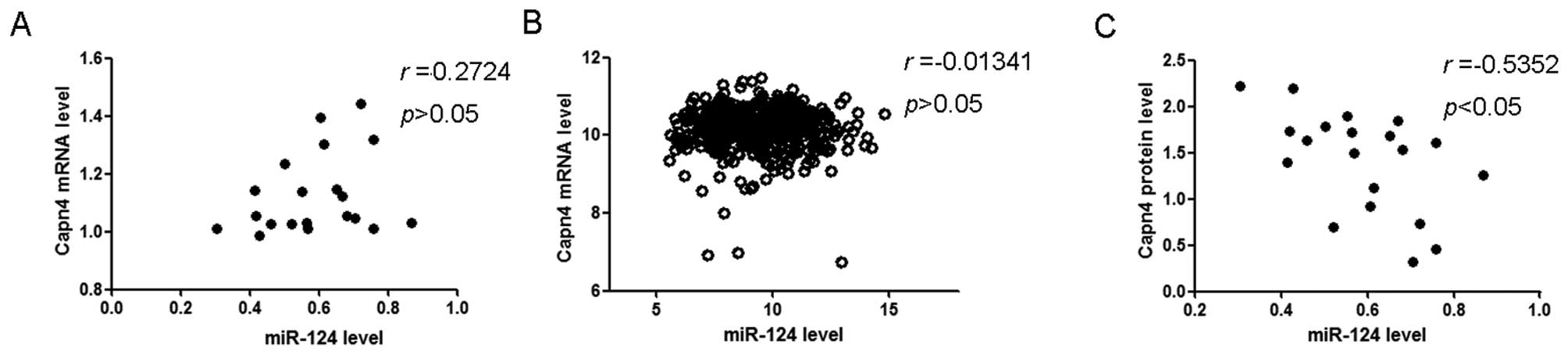
We examined whether miR-124 expression correlated
with Capn4 expression in GBM tissues. The Spearman's correlation
analysis revealed that Capn4 mRNA transcript levels did not
correlate with miR-124 levels in GBM tissues from our cohort
(r=0.2724, P>0.05) (Fig. 4A).
Analysis of the queried data from the TCGA also failed to reveal
significant correlation between Capn4 mRNA transcript levels and
miR-124 levels in GBM tissues (r=0.0134, P>0.05) (Fig. 4B). By contrast, a significant
negative correlation was demonstrated between Capn4 protein levels
and mR-124 levels in the 20 GBM tissue specimens from the present
study (r=0.5352, P<0.05) (Fig.
4C).
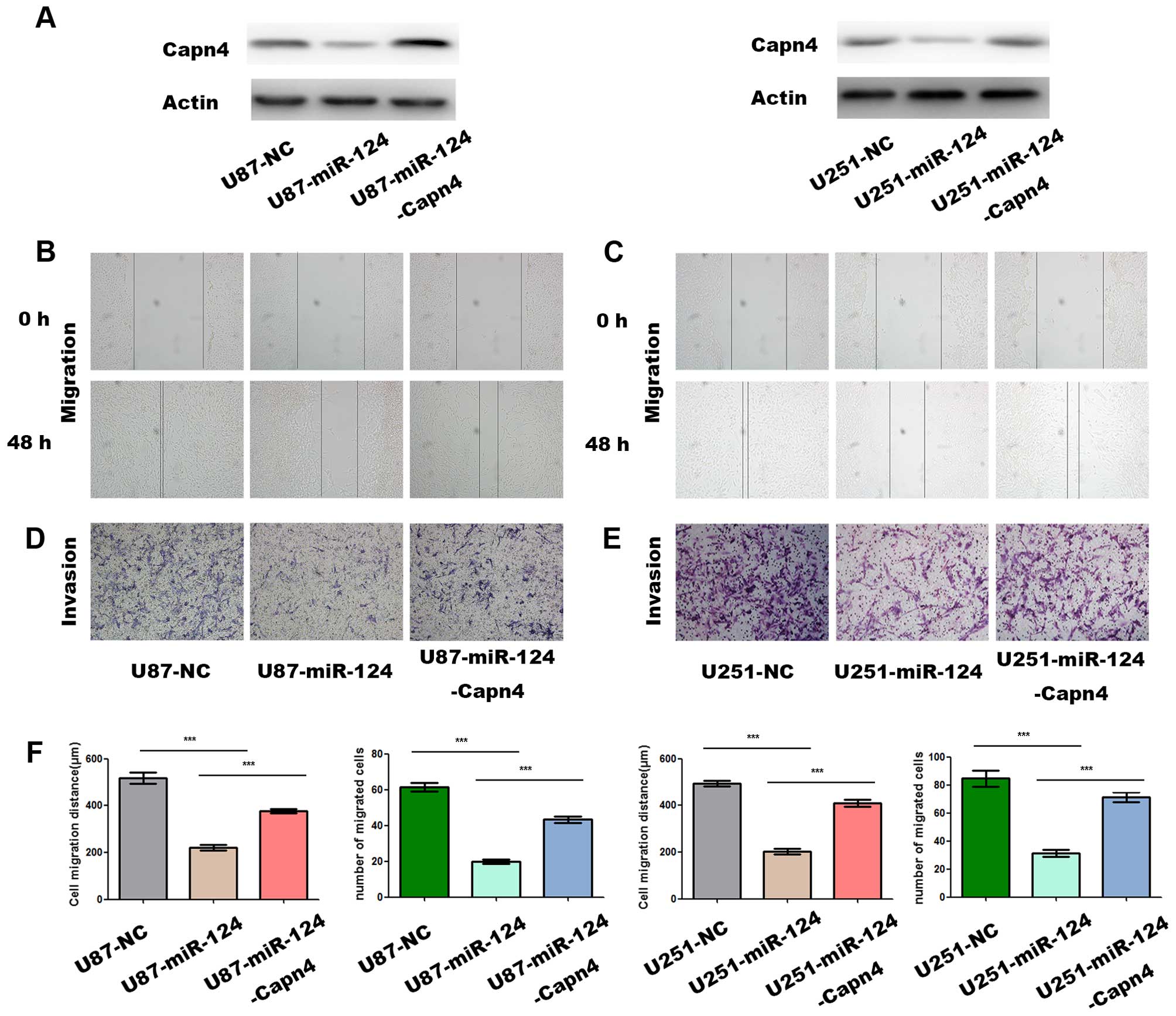
miR-124 suppresses the migration and
invasion of glioma cells by modulating Capn4
miR-124 has been shown to inhibit the migration and
invasion of U87 and U251 cells (7).
We determined whether Capn4 played any role in the miR-124-mediated
suppression of the migration and invasion of glioma cells.
Transfection of U87 and U251 cells with miR-124 mimics caused an
apparent reduction in the level of Capn4 protein in the two cells,
which, however, was abrogated by co-transfection with pLenti-Capn4
(Fig. 5A). The wound-healing assays
showed that Capn4 downregulation by miR-124 mimics suppressed the
migration of U87 (Fig. 5B) and U251
cells (P<0.001 vs. controls) (Fig.
5C). The decrease in the migration of U87 and U251 cells was
markedly attenuated by co-transfection with pLenti-Capn4
(P<0.001 vs. miR-124 mimics) (Fig.
5F). In addition, Transwell assays demonstrated that miR-124
mimics significantly reduced the number of migrated U87 (Fig. 5D) and U251 cells (P<0.001 vs.
controls) (Fig. 5E). The reduction
in the number of migrated glioma cells was lessened by
co-transfection with pLenti-Capn4 (P<0.001 vs. miR-124 mimics)
(Fig. 5F). The results suggested
that miR-124 modulated the migration and invasion ability of
gliomas cells via Capn4.
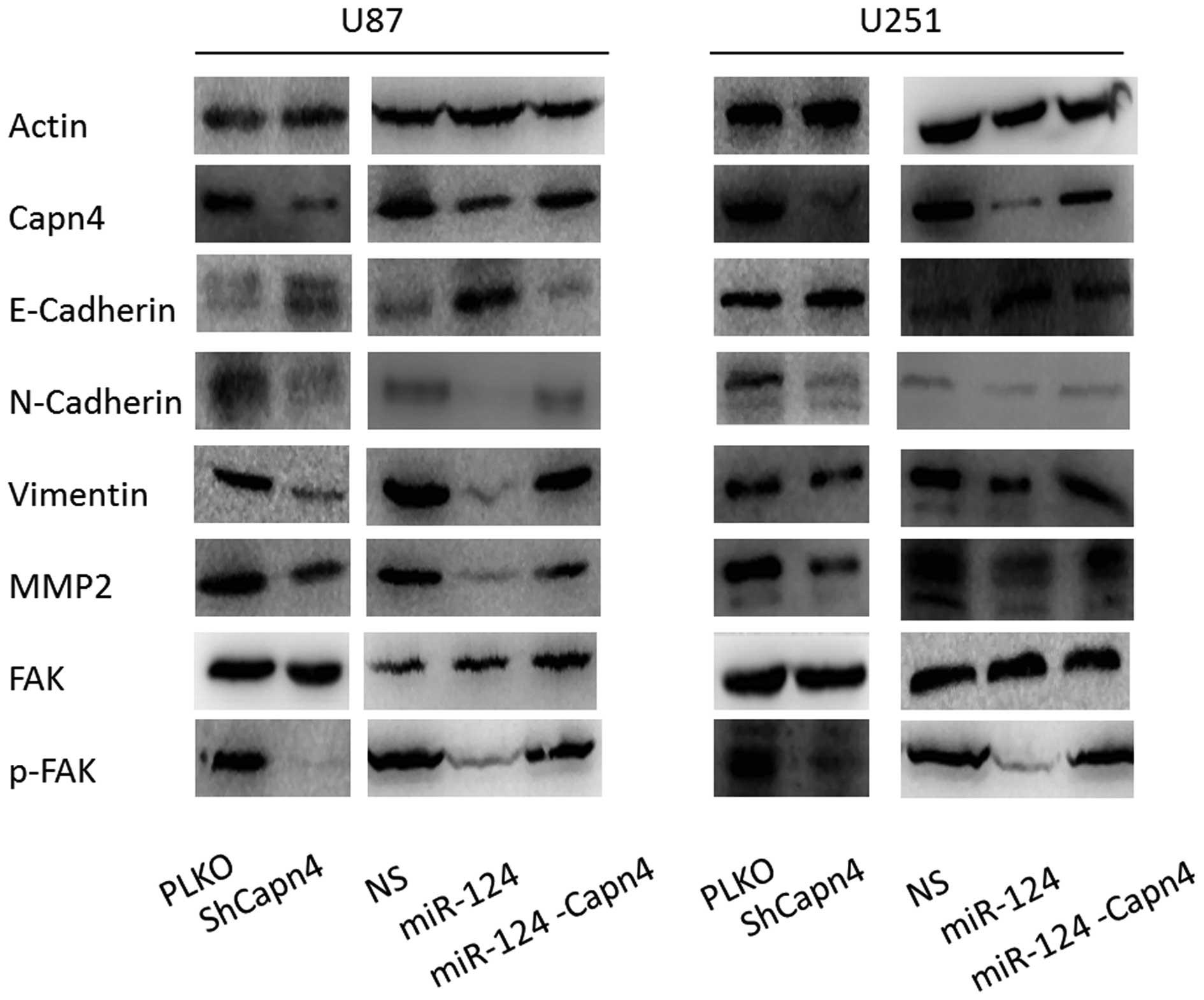
Capn4 is involved in the EMT of glioma
cells
miR-124 has been demonstrated to reverse the EMT
(16). We investigated whether
Capn4 was involved in this process. Capn4 knockdown by shRNA was
associated with an increase in E-cadherin and a decrease in
N-cadherin and vimentin. Capn4 downregulation also caused an
apparent reduction in the level of phospho-FAK and MMP2 in U87
cells and U251 cells. Similar findings were observed in U87 cells
and U251 cells transfected with miR-124 mimics. These changes were
attenuated by co-transfection with pLenti-Capn4 (Fig. 6). The results indicated that Capn4
was involved in the EMT of glioma cells and was modulated by
miR-124 in the process.
Discussion
The highly infiltrative and invasive nature of
malignant gliomasis a significant contributor to the poor outcome
of the disease. miR-124 and Capn4 are involved in the migration and
invasion of glioma cells and their aberrant expression in glioma
tissues has been found to be associated with the outcome of the
disease (7,10). To the best of our knowledge, the
present study provides the first piece of evidence that miR-124
directly targets Capn4 in modulating the migratory and invasive
activities of glioma cells in vitro. Additionally, miR-124
and Capn4 are aberrantly expressed in GBM tissues and miR-124
expression negatively correlates with Capn4 protein levels.
Understanding the molecular mechanisms of gliomagenesis and tumor
progression is necessary to explore more effective diagnostic and
therapeutic strategies. In a previous study, it was shown that
alterations in Capn4 expression correlated with glioma grade and
patient survival (13). In the
present study, we have shown that the Capn4 protein levels, but not
the mRNA levels, in GBM were higher than the control tissues, which
is consistent with our immunohistochemical findings in our previous
report (13).
Currently, studies on the role of miR-124 in glioma
involve only a small number of patients. Zhao et al studied
9 GBM patients (6) while An et
al studied 16 glioma patients (10) including eight high-grade glioma
patients. We studied 20 archived GBM tissue specimens and showed
that miR-124 was significantly downregulated while Capn4 was
markedly upregulated in GBM tissues. This finding was further
supported by our analysis of 519 GBM patients in TGCA. We and other
authors have shown that miR-124 suppresses the migration and
invasion of glioma cells. An et al found that this
inhibitory effect of miR-124 on glioma cell migration and invasion
was exerted via ROC1 (10), a
protein involved in the regulation of cell motility. By contrast,
Zhao et al, showed that miR-124 inhibited the invasiveness
of glioma cells by targeting PPP1R3L (6), an inhibitory member of the
apoptosis-stimulating protein of the p53 family that also regulates
growth, cell cycle progression, metastasis and apoptosis of various
types of cancer. The role of Capn4 in regulating the invasiveness
of glioma cells has not been well elucidated, as identified in our
previous study, where the results demonstrated that downregulation
of Capn4 suppressed the invasion and migration of glioma cells
in vitro. To the best of our knowledge, no other study has
focused on the role of Capn4 in glioma. In this study, we
demonstrated that miR-124 targeted Capn4 in regulating the
migration and invasion of glioma cells.
Dai et al, demonstrated that Capn4 promoted
the metastasis of hepatocellular carcinoma cells via activation of
the FAK-Src signaling pathway and MMP2 (17). We also found that Capn4
downregulation by shRNA was associated with reduced levels of
phospho-FAK and MMP2. Similarly, miR-124 mimics suppressed the
levels of phospho-FAK and MMP2 in U87 cells, which was attenuated
by co-transfection with plasmid vectors expressing Capn4. An
increasing number of studies have shed light on the mechanisms
whereby glioma cells infiltrate normal brain. Glioma invasion is a
multistep process that involves detachment from the tumor mass,
remodeling of the extracellular matrix, and migration of glioma
cells (18). Several factors,
including CD44 (19), cadherins
(20), and matrix
metalloproteinases (21), have been
shown to mediate these processes. In this study, the results showed
that the exo genous overexpression of miR-124 suppressed the
mesenchymal phenotype of U87 cells by inhibiting Capn4.
In conclusion, this study has demonstrated the
functions of miR-124 in inhibiting the invasive propensity of
glioma cells. miR-124 controls the invasive phenotype of glioma
cells via one of its downstream targets, Capn4. In view of its
frequent downregulation in GBM, it is likely that brain-enriched
miR-124 may not only be important for neural development but may
also serve as a putative tumor suppressor miRNA that prevents
gliomagenesis by inhibiting the invasiveness of glioma cells. The
results suggest a new avenue for elucidating the molecular
mechanism of glioma cell invasion and developing therapeutic
strategies against glioma by restoring the level of miR-124 and
suppressing Capn4.
Acknowledgments
This study was supported by 'China National Funds
for Distinguished Young Scientists' (81025013 to Ying Mao), 'The
major research and development project of innovative drugs,
Ministry of Science and Technology' (2012ZX09303004-001) and
Shanghai Municipal Commission of Health and Family Planning
(20124354).
Abbreviations:
|
GBM
|
glioblastoma multiforme
|
|
miRNA
|
microRNA
|
|
3′-UTR
|
3′ untranslated region
|
|
TGCA
|
The Cancer Genome Atlas
|
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riddick G and Fine HA: Integration and
analysis of genome-scale data from gliomas. Nat Rev Neurol.
7:439–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Wang B, Shi Y, Xu C, Xiao HL, Ma
LN, Xu SL, Yang L, Wang QL, Dang WQ, et al: Oncogenic miR-20a and
miR-106a enhance the invasiveness of human glioma stem cells by
directly targeting TIMP-2. Oncogene. 34:1407–1419. 2015. View Article : Google Scholar
|
|
5
|
Mao J, Zhang M, Zhong M, Zhang Y and Lv K:
MicroRNA-204, a direct negative regulator of ezrin gene expression,
inhibits glioma cell migration and invasion. Mol Cell Biochem.
396:117–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao WH, Wu SQ and Zhang YD:
Downregulation of miR-124 promotes the growth and invasiveness of
glioblastoma cells involving upregulation of PPP1R13L. Int J Mol
Med. 32:101–107. 2013.PubMed/NCBI
|
|
7
|
Xia H, Cheung WK, Ng SS, Jiang X, Jiang S,
Sze J, Leung GK, Lu G, Chan DT, Bian XW, et al: Loss of
brain-enriched miR-124 microRNA enhances stem-like traits and
invasiveness of glioma cells. J Biol Chem. 287:9962–9971. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Chen P, Li XY, Zhang LY, Xiong W,
Zhou M, Xiao L, Zeng F, Li XL, Wu MH, et al: Grade-specific
expression profiles of miRNAs/mRNAs and docking study in human
grade I–III astrocytomas. OMICS. 15:673–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonntag KC, Woo TU and Krichevsky AM:
Converging miRNA functions in diverse brain disorders: A case for
miR-124 and miR-126. Exp Neurol. 235:427–435. 2012. View Article : Google Scholar :
|
|
10
|
An L1, Liu Y, Wu A and Guan Y:
microRNA-124 inhibits migration and invasion by down-regulating
ROCK1 in glioma. PLoS One. 8:e694782013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Storr SJ, Carragher NO, Frame MC, Parr T
and Martin SG: The calpain system and cancer. Nat Rev Cancer.
11:364–374. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Bai DS, Huang XY, Shi GM, Ke AW,
Yang LX, Yang XR, Zhou J and Fan J: Prognostic significance of
Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One.
8:e546192013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang Q, Qian X, Cao Y, Fan M, Xu X and
He X: Capn4 mRNA level is correlated with tumour progression and
clinical outcome in clear cell renal cell carcinoma. J Int Med Res.
42:282–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai JJ, Qi ZX, Hua W, Zhu JJ, Zhang X, Yao
Y and Mao Y: Increased expression of Capn4 is associated with the
malignancy of human glioma. CNS Neurosci Ther. 20:521–527. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai DS, Dai Z, Zhou J, Liu YK, Qiu SJ, Tan
CJ, Shi YH, Huang C, Wang Z, He YF, et al: Capn4 overexpression
underlies tumor invasion and metastasis after liver transplantation
for hepatocellular carcinoma. Hepatology. 49:460–470. 2009.
View Article : Google Scholar
|
|
16
|
Qin W, Pan Y, Zheng X, Li D, Bu J, Xu C,
Tang J, Cui R, Lin P and Yu X: MicroRNA-124 regulates TGF-α-induced
epithelial-mesenchymal transition in human prostate cancer cells.
Int J Oncol. 45:1225–1231. 2014.PubMed/NCBI
|
|
17
|
Dai Z, Zhou SL, Zhou ZJ, Bai DS, Xu XY, Fu
XT, Chen Q, Zhao YM, Zhu K, Yu L, et al: Capn4 contributes to
tumour growth and metastasis of hepatocellular carcinoma by
activation of the FAK-Src signalling pathways. J Pathol.
234:316–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pietras A, Katz AM, Ekström EJ, Wee B,
Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and
Holland EC: Osteopontin-CD44 signaling in the glioma perivascular
niche enhances cancer stem cell phenotypes and promotes aggressive
tumor growth. Cell Stem Cell. 14:357–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barami K, Lewis-Tuffin L and Anastasiadis
PZ: The role of cadherins and catenins in gliomagenesis. Neurosurg
Focus. 21:E132006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Könnecke H and Bechmann I: The role of
microglia and matrix metalloproteinases involvement in
neuroinflammation and gliomas. Clin Dev Immunol. 2013:9141042013.
View Article : Google Scholar : PubMed/NCBI
|