Introduction
Metastasis accounts for the majority of mortalities
of cancer patients. Thus, it is crucial to gain an understanding of
the relevant molecular and cellular mechanisms involved in the
metastatic process. The progression of cancer metastasis depends on
various factors, including major driver mutations in key regulatory
genes (ASPP2, smad7, PIK3CA, PTEN)
(1–5), microRNAs (miRNAs) (6), cancer stem cells (CSCs) (7) and the aberrant activation of multiple
signaling pathways as a consequence of the overexpression of
multiple tyrosine kinase receptors, such as the epidermal growth
factor (EGF) receptor (EGFR) and its ligands (8) and excessive production of transforming
growth factor-β (TGF-β) isoforms (9).
The growth factor control of cell fate is a pivotal
step in cancer progression. The high expression of EGFR in various
types of cancer has been associated with metastatic tumors and poor
clinical outcomes (10,11). The EGFR family members lead to
enhanced tyrosine kinase activity of the receptor complexes and
activation of multiple signaling pathways, such as
phosphatidylinositol 3′-kinase (PI3K), mitogen-activated protein
kinase (MAPK), p38 MAPK, Src and Crk (12–15).
TGF-β, a key regulator in epithelial-mesenchymal
transdifferentiation (EMT) and radiation resistance, also plays a
major role in the regulation of tumor initiation, progression and
metastasis (16). TGF-β can enhance
the invasion and migration of breast cancer cells and stimulate
breast cancer cell proliferation (17,18).
It activates serine-threonine kinases, which act through the smads
signaling pathways as well as non-smad signaling cascades including
MAPK/ERK, Rho-family of GTPases and PI3K/AKT on the tumor
microenvironment in breast cancers (19,20).
These results indicate that EGF-TGF-β 'cross talk' is important
with respect to cell-autonomous effects in breast cancer.
miRNAs are a family of small non-coding RNA
molecules that regulate gene expression by base pairing to the
3′-UTR of the target mRNA (21).
Recently, a series of miRNAs have been shown to play a critical
role in the progression and metastasis of human malignancies
(22–24). MicroRNA-21 (miR-21) is one of the
first miRNAs detected in the human genome, and is also known to be
upregulated in all types of human malignancies (25). Previous findings showed that miR-21
overexpression in breast cancer cells is a marker of the disease
aggressiveness (26,27). miR-21 regulates cell proliferation,
invasion and migration in the majority of cancer cells through its
downstream target proteins, such as tumor suppressor gene
tropomyosin 1 (TPM1), programmed cell death 4 (PDCD4),
MARCKS, maspin and phosphatase and tensin homolog deleted on
chromosome ten (PTEN) (4,28–30).
However, whether and how miR-21 and its target smad7 co-operate to
orchestrate breast cancer cell invasion and migration remains to be
determined.
On the basis of the above evidence, it appears that
miR-21, smad7, EGF and TGF-β are completely or partially involved
in the invasion and migration process of breast cancer cells. The
present study was undertaken determine the association of miR-21,
smad7, EGF and TGF-β with breast cancer cell invasion and migration
to provide insights into the causal mechanisms of breast cancer
invasion and metastasis.
Materials and methods
RT-qPCR assays and cell culture
Plasma from 16 normal controls, 20 patients with
fibroadenoma and 20 breast cancer patients (19 patients with
infiltrating ductal carcinoma and 1 patient with invasive lobular
carcinoma) was obtained from the Department of Breast Surgery, The
First Affiliated Hospital of Zhengzhou University (Henan, China),
from September, 2013 to May, 2014. The subjects included in the
three groups voluntarily joined the present study after providing
informed consent. The experimental procedure was carried out in
accordance with the Declaration of Helsinki and was approved by the
Medical Research Ethics Committee of the First Affiliated Hospital
of Zhengzhou University (approval no.: 2013-MR-0045). The miR-21
reverse transcription (RT) primer and RT-qPCR primers used were
described in a previous study (31). miRNAs were isolated from 50 ml of
plasma using a mirVana miRNA isolation kit (Applied Biosystems,
Foster City, CA, USA). RT-qPCR was performed to measure the
expression of mature miR-21 in cells, as previously described
(31). miR-21 levels were
normalized to U6 levels.
For mRNAs, total RNA from cells was isolated using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RT-qPCR reactions
were carried out as previously described (31). β-actin was used as an endogenous
control. MCF-7 cells were cultured as previously described
(31).
Constructions
The LightSwitch smad7 3′-UTR reporter (Switchgear
Genomics, Menlo Park, CA, USA) was utilized to assess whether there
is a direct interaction between miR-21 and smad7 3′-UTR.
Site-directed mutagenesis kits (Stratagene, La Jolla, CA, USA) were
used to introduce a three-base pair mutation in the seed-binding
site of smad7 3′-UTR. The pCMV-SPORT6-smad7 cDNA construct used in
the smad7 rescue experiment was purchased from Open Biosystems
(Huntsville, AL, USA).
Cell transfection
Human hsa-miR-21 (accession no.: MIMAT0000076)
mimics and negative control, and antagomir and negative control
were purchased from Ribobio (Guangzhou, China). The cells were
seeded in 6-well plates (50–60% confluency). miR-21 mimics or
negative control, and miR-21 antagomir or its negative control were
transfected into MCF-7 cells, as previously described (4,31).
Sismad7 or the scramble control siRNA sequence (control) was
designed, produced and annealed by Ribobio, and designed to target
smad7 (gene ID: 4092) was used to silence smad7 in MCF-7 cells. For
smad7 rescue experiments, MCF-7 cells were transfected with 40 nM
miR-21 mimics and 250 ng pCMV-SPORT6-smad7 cDNA (Invitrogen) and
Lipofectamine™ 2000, which was used in all the transfection
studies. After 3 days of transfection, the transfected cells were
measured.
Luciferase reporter assay
The cells in 24-well plates (60–70% confluency) were
co-transfected with miR-21 mimics (40 nM) and smad7 3′-UTR reporter
(200 ng). Luciferase activity was measured after 24 h, according to
the manufacturer's instructions, using a Dual-Glo luciferase assay
system (Promega, Madison, WI, USA).
Western blot analysis
Cells in the 6-well plates (60–70% confluency) were
lysed using RIPA lysis buffer (Sijiqing, Hangzhou, China).
According to our previous studies (4,31), 50
µg of each sample were separated by SDS-PAGE (10%) and
transferred to PVDF membranes. The membranes were incubated with
rabbit anti-human smad7 antibody (no: BA1399, 1:500) and rabbit
anti-human β-actin antibody (no: BA2305, 1:1,000) (both from
Boster, Wuhan, China), followed by an HRP-conjugated anti-rabbit
IgG secondary antibody (no.: BA1055, 1:2,500; Boster). The samples
were visualized by ECL chemiluminescence. β-actin was used as an
endogenous control.
Invasion and migration assay
Cell invasion and migration assays were performed as
previously described (31). For the
invasion assay, 2.5×104 cells were seeded on an
8-µm pore size Transwell filter insert (Corning Inc,
Corning, NY, USA) coated with ECM (1:7.5; Sigma, St. Louis, MO,
USA), while the cell migration assay was not coated with ECM. After
48 h of incubation at 37°C and 5% CO2, cells adherent to
the upper surface of the filter were removed and counted.
Immunohistochemistry
Paraffin-embedded human breast cancer tissues were
obtained from the Department of Breast Surgery, The First
Affiliated Hospital of Zhengzhou University, and 5-µm
sections were stained for smad7 using the rabbit antihuman smad7
antibody (no.: BA1399, 1:100; Boster), as previously described
(32).
Xenografted mouse model
Four-week-old BALB/c nude mice were obtained from a
Shanghai Animal Laboratory (Shanghai, China). Animal welfare and
experimental procedures were carried out in accordance with the
Guide for the Care and Use of Laboratory Animals and were approved
by the Animal Ethics Committee of The First Affiliated Hospital of
Zhengzhou University (approval no.: 2013-MR-0045). Xenografted mice
were developed by injecting anti-miR-21 MCF-7 cells or its control
cells (the total cell number in each injection was
4×106) to the left side fat-pad of each nude mouse. The
tumor volume was determined weekly using digital caliper
measurements and was calculated using the formula: V (volume;
mm3) =1/2 × LD (longest diameter) 2 × SD (shortest
diameter). After five weeks, the mice were sacrificed and the
tumors were measured and analyzed.
Statistical analysis
Results are presented as mean ± SD. Statistical
differences between the groups were assessed using Student's
t-test, one- or two-way ANOVA, as indicated, by using statistical
analysis software SPSS 17.0. P≤0.05 was considered statistically
significant.
Results
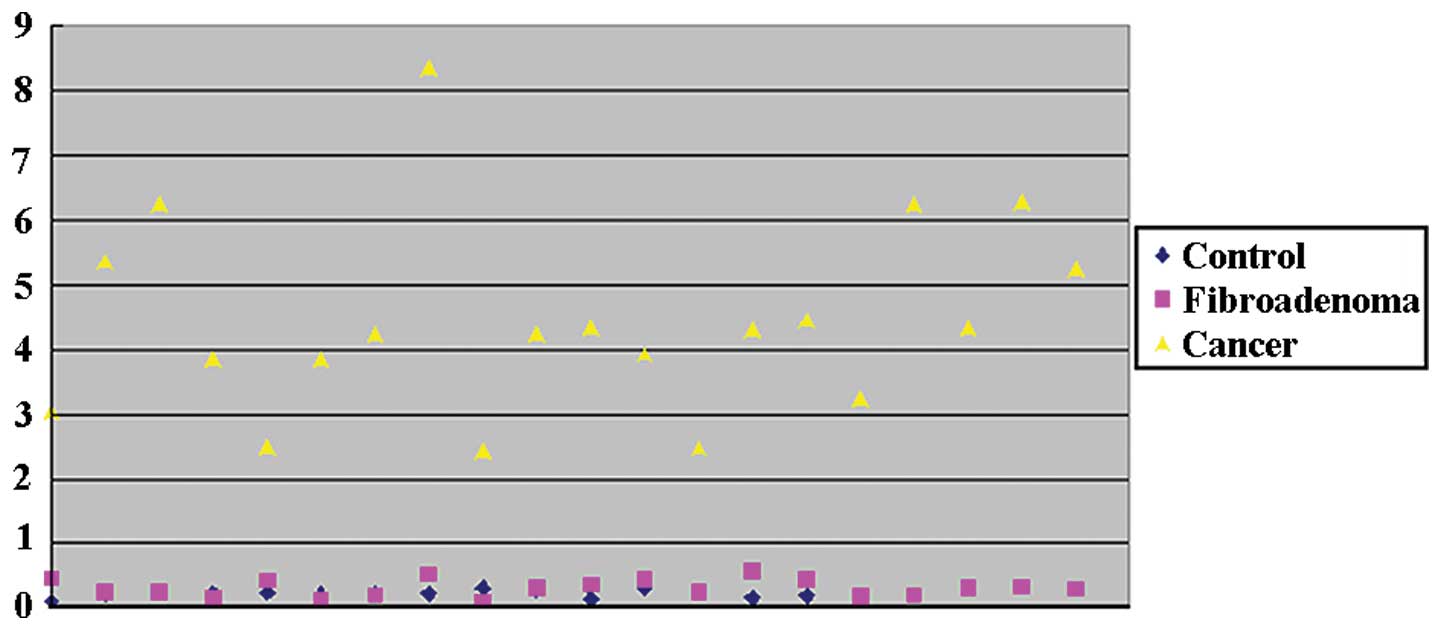
miR-21 is overexpressed in the plasma of
patients with breast cancer
To confirm that miR-21 was overexpressed in the
plasma of patients with breast cancer, we assayed the relative
levels of miR-21 in the plasma of 16 normal controls, 20 patients
with fibroadenoma and 20 patients with breast cancer, using
RT-qPCR. The relative expression of miR-21 was 4.44153±1.46336 in
breast cancer patients, significantly overexpressed as compared to
0.22116±0.05622 in the normal control group (>20-fold; P=0.0188;
Fig. 1) or 0.31409±0.13237 in the
fibroadenoma group (>14-fold; P=0.0192; Fig. 1).
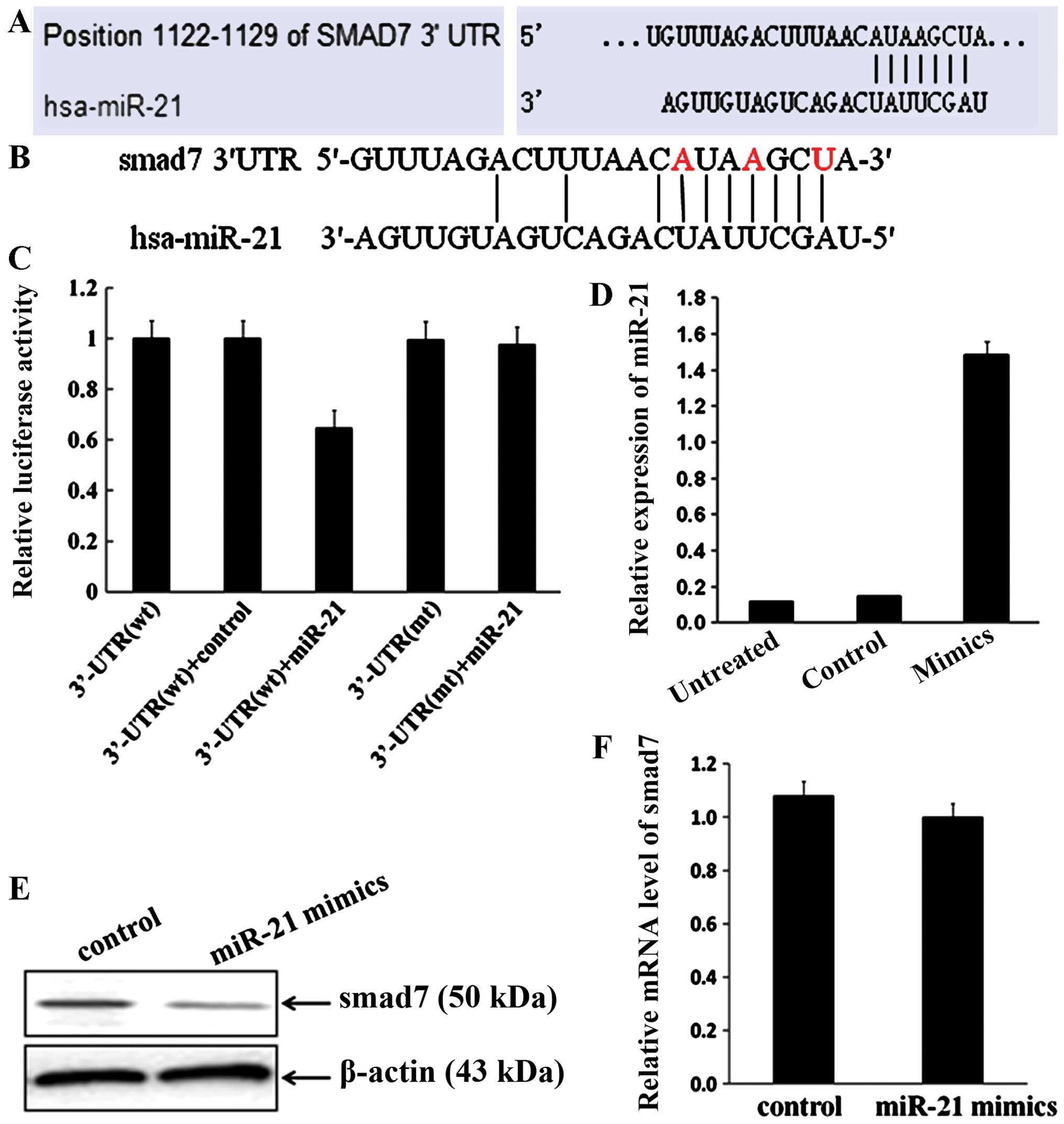
Smad7 is a direct downstream target of
miR-21
According to web-based predictive software
(http://www.targetscan.org/ and
http://www.mirbase.org/) and data from other
groups (33,34), smad7 is one of the downstream
targets of miR-21 (Fig. 2A). To
determine whether smad7 is a direct target of miR-21 during breast
cancer cell invasion and migration, luciferase assays were
performed using smad7 3′-UTR. The luciferase assays showed that
miR-21 only reduced luciferase activity in cells containing
wild-type smad7 3′-UTR, but not in cells containing mutant 3′-UTR
(the mutated nucleotide is marked as red; Fig. 2B and C).
The effects of miR-21 on smad7 expression were
further analyzed to determine whether miR-21 regulates smad7 during
cancer cell invasion and migration. Firstly, miR-21 mimics was
transfected into MCF-7 cells. The relative expression of miR-21 was
1.48398±0.31843 in MCF-7/miR-21 cells significantly upregulated as
compared to 0.14668±0.03017 in the negative control group
(>10-fold; P=0.0171; Fig. 2D),
which suggests that miR-21 mimics may enhance the activity of
miR-21 in MCF-7 cells, through various processes such as
gain-of-function. The results showed that miR-21 overexpression
significantly decreased the protein level of smad7 (Fig. 2E). Notably, the mRNA level of smad7
did not change in the miR-21-overexpressed cells (P=0.18697,
Fig. 2F).
miR-21 modulates the actions of EGF and
(or) TGF-β, and enhances breast cancer invasion and migration
To determine whether miR-21 enhanced breast cancer
invasion and migration, and whether it modulated the actions of EGF
or TGF-β, MCF-7/miR-21 and control cells were treated with EGF (1
nM) or TGF-β (0.5 nM) and measured using Transwell migration and
invasion assay. In MCF-7 cells, EGF enhanced invasion (P=0.0006,
Fig. 3A) and migration (P=0.01851,
Fig. 3B), TGF-β enhanced invasion
(P=0.00586, Fig. 3A) and migration
(P=0.0079, Fig. 3B), and their
combined action was greater than that of either growth factor alone
(invasion: P=0.00357, Fig. 3A;
migration: P=0.0038, Fig. 3B).
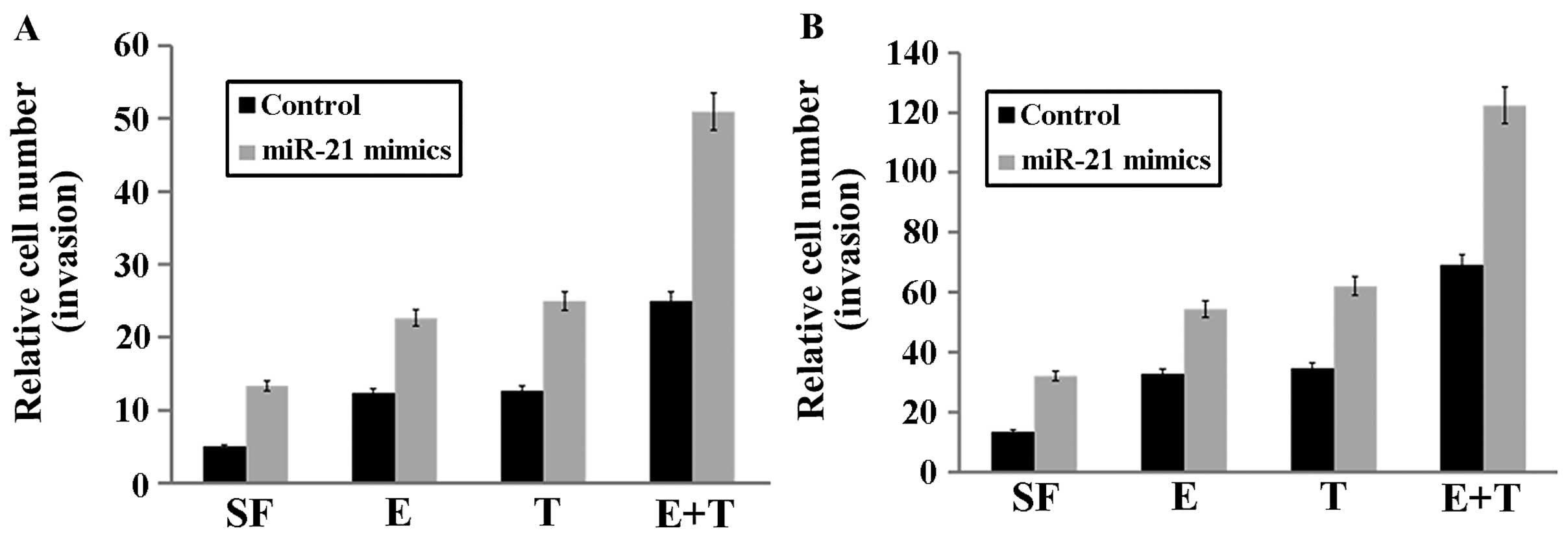 | Figure 3miR-21 modulates the actions of EGF
or TGF-β, as well as enhanced breast cancer cell invasion and
migration. (A) In MCF-7 cells, EGF (E, 1 nM) or TGF-β (T, 0.5 nM)
enhanced invasion, compared to the absence SF; and their combined
action (E+T) was greater than that of either growth factor alone.
Overexpression of miR-21 increased invasion in MCF-7 cells, and
enhanced EGF-mediated invasion and TGF-β-mediated invasion.
Furthermore, the combination of the two growth factors exerted a
significantly greater stimulatory effect on invasion in cells with
high miR-21 levels. (B) The effects of miR-21, EGF and TGF-β on
breast cancer cell migration, which was similar to the invasion
assays. SF, serum-free. |
Overexpression of miR-21 increased cell invasion
(P=0.00126, Fig. 3A) and migration
(P=0.00274, Fig. 3B) in MCF-7
cells, enhanced EGF-mediated invasion (P=0.00771, Fig. 3A) and migration (P=0.01518, Fig. 3B) and TGF-β-mediated invasion
(P=0.00189, Fig. 3A) and migration
(P=0.00152, Fig. 3B). The
combination of the two growth factors exerted a significantly
greater stimulatory effect on invasion and migration in MCF-7 cells
with high miR-21 levels (invasion: P=0.00929, Fig. 3A; migration: P=0.00294, Fig. 3B).
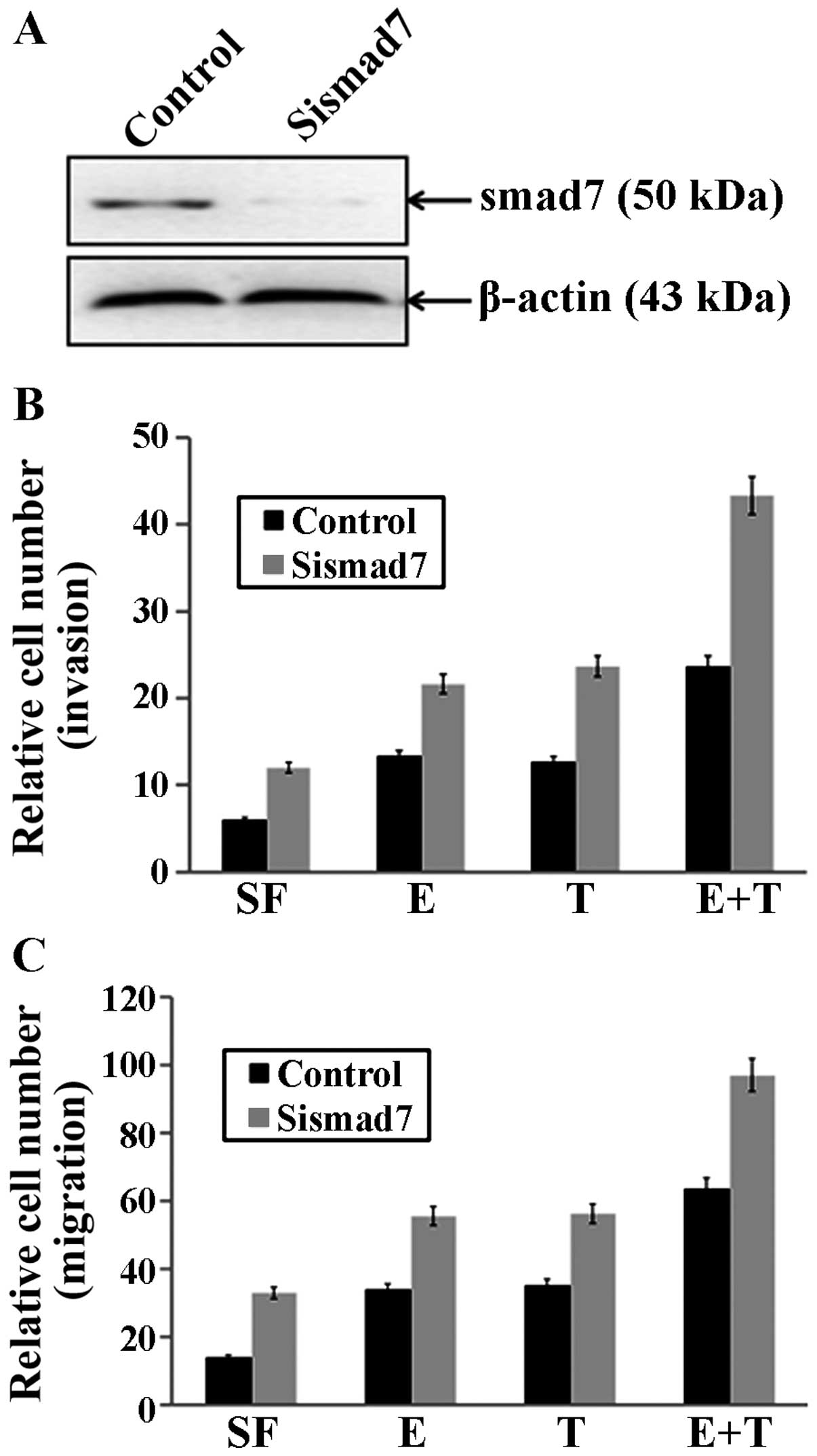
Smad7 is a negative regulator of breast
cancer invasion and migration, as well as the actions of EGF and
(or) TGF-β
To determine whether depletion of PTEN affected
breast cancer invasion and migration, as well as the actions of EGF
and (or) TGF-β, smad7 siRNA (sismad7), which targeted smad7 or the
control Ssismad7 was transfected into MCF-7 cells. After 24 h, the
cells were treated with EGF and (or) TGF-β, and measured uisng
Transwell migration and invasion assays, as described earlier. We
found that sismad7 significantly downregulated the expression of
smad7 (Fig. 4A), as compared to the
control groups. Notably, smad7 silencing by sismad7 enhanced breast
cancer cell invasion (P=0.03102, Fig.
4B) and migration (P=0.00281, Fig.
4C) in MCF-7 cells, and increased EGF-mediated invasion
(P=0.0301, Fig. 4B) and migration
(P=0.01058, Fig. 4C) and
TGF-β-mediated invasion (P=0.01746, Fig. 4B) and migration (P=0.00706, Fig. 4C). Moreover, the combination of the
two growth factors exerted a significantly greater stimulatory
effect on invasion and migration in MCF-7 cells with smad7
silencing (invasion: P=0.00577, Fig.
4B; migration: P=0.003306, Fig.
4C).
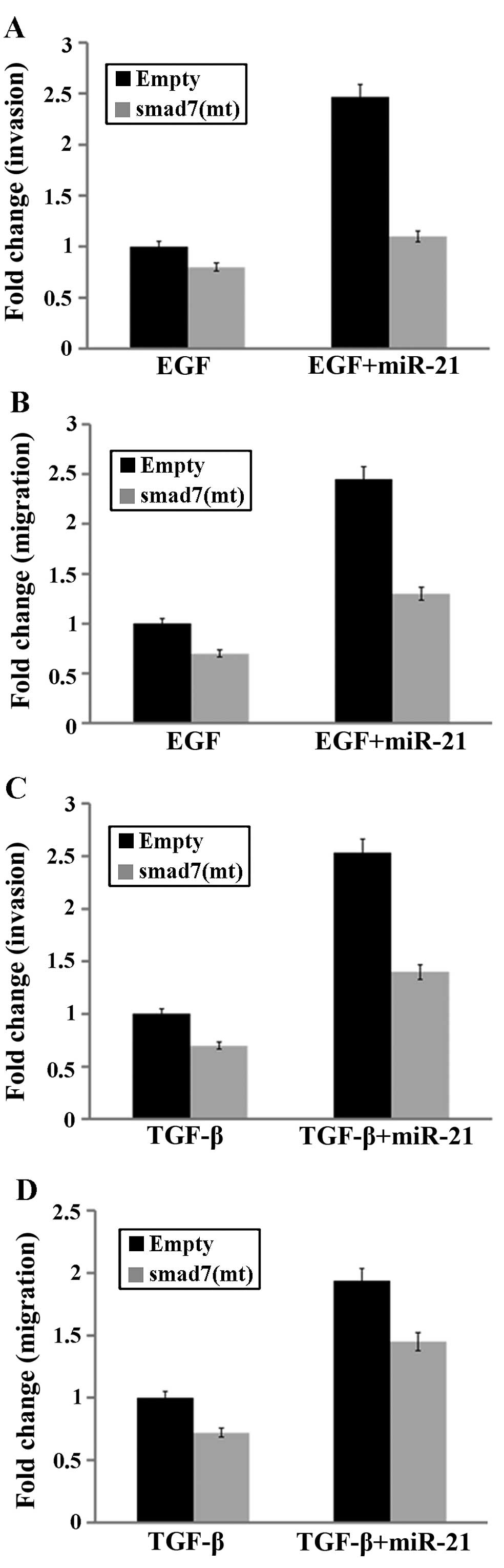
Smad7 downregulation is essential for
miR-21-induced stimulation of the actions of EGF and (or)
TGF-β
To confirm that smad7 downregulation was essential
for miR-21-induced stimulation of the actions of EGF and (or)
TGF-β, we used the pCMV-SPORT6-smad7 vector, which encodes a smad7
cDNA that is not regulated by miR-21 due to a mutated 3′-UTR
binding site. Experiments were carried out in the presence of EGF
or TGF-β, and in the absence or presence of transfected
miR-21mimics using cells expressing an empty vector or the
pCMV-SPORT6-smad7 vector. This experimental design allowed for the
specific evaluation of the consequence of expressing a smad7 that
was resistant to miR-21 upregulation. When pCMV-SPORT6-smad7 was
transfected into MCF-7 cells, the stimulatory effect of miR-21
overexpression on EGF- (or TGF-β-)mediated invasion and migration
was partly abrogated (Fig. 5).
Thus, smad7 is a functional target of miR-21 whose upregulation
promotes EGF (or TGF-β)-induced breast cancer cell invasion and
migration.
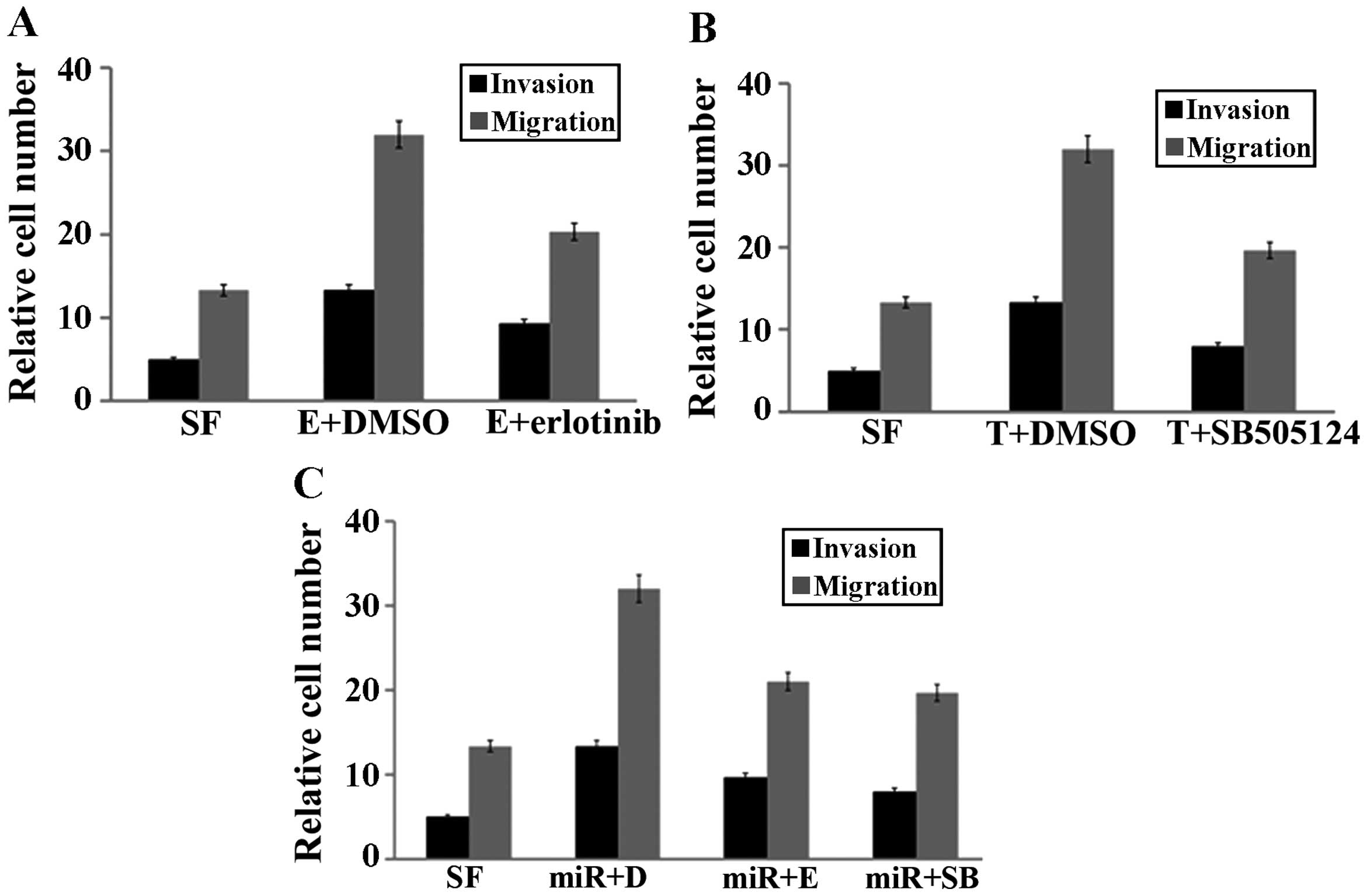
miR-21 regulates breast cancer cell
invasion and migration through EGF and TGF-β pathways
To investigate the roles of EGF and TGF-β pathways
in miR-21-regulating breast cancer cell invasion and migration, the
MCF-7 cells were transfected with miR-21 mimics at a concentration
of 40 nM for 72 h and then the cells were treated with erlotinib
(the inhibitor of EGFR) at 2 mM or SB505124 (the inhibitor of TGF-β
receptor) at 10 mM for 24 h. The EGFR inhibitor erlotinib (2 mM)
partly blocked EGF-induced invasion and migration in the presence
of miR-21 in MCF-7 cells (Fig. 6A).
Similarly, the TGF-β receptor inhibitor SB505124 (2 µM)
partly blocked TGF-β-induced invasion and migration in the presence
of miR-21 in MCF-7 cells (Fig. 6B).
In addition, the effects of miR-21 on EGF (or TGF-β) actions on
invasion and migration were markedly attenuated by erlotinib and
(or) SB505124 (Fig. 6C), indicating
that EGF and TGF-β act through their respective receptors to cross
talk and enhance breast cancer cell invasion and migration.
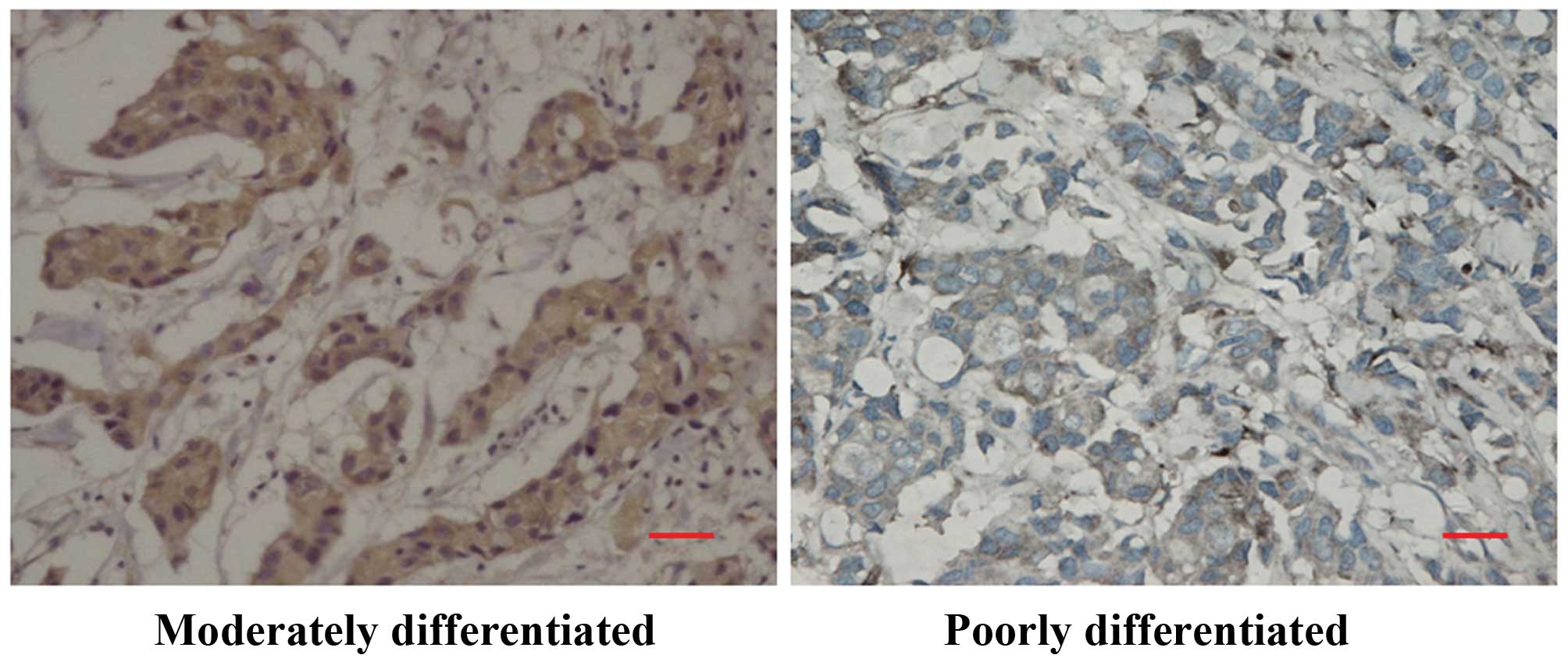
Smad7 downregulation in poorly
differentiated breast cancer
We determined whether smad7 was expressed in human
breast cancer samples using the same highly specific antibody that
was used in the immunohistochemical experiments. Smad7 was readily
visible in the cytoplasm of many cancer cells but not in the
adjoining stroma. A high smad7 expression was principally
identified in well- to moderately differentiated breast cancer
(Fig. 7), whereas a low smad7
expression was observed in poorly differentiated breast cancer
(Fig. 7).
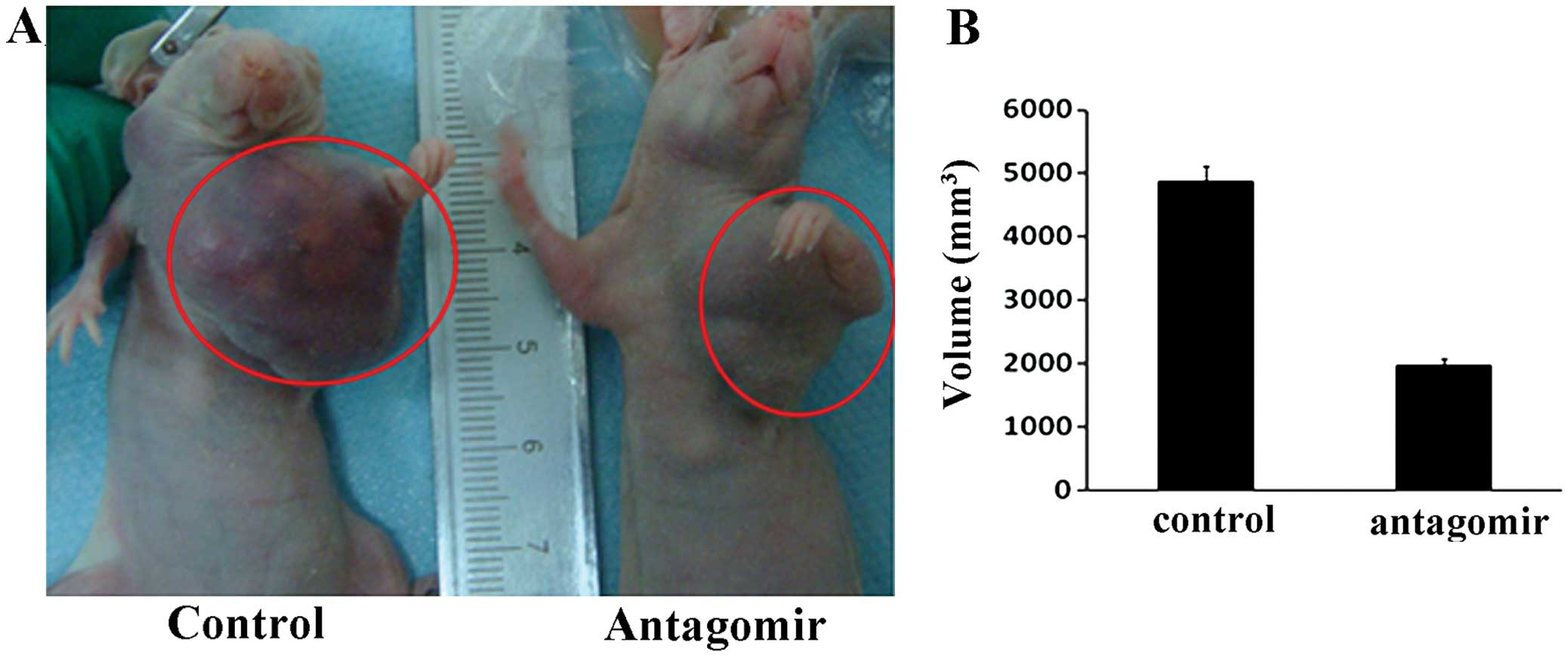
Antagonism of miR-21 inhibits breast
cancer biological aggressiveness in a nude mouse model
To validate the contribution of miR-21 to breast
cancer biological aggressiveness, we carried out an in vivo
study in an orthotopic model using nude mice (Fig. 8A). Three-fifths of the mice with
miR-21 antagomir MCF-7 cells and four-fifths of the mice with MCF-7
control cells formed breast tumors and the presence of miR-21
antagomir resulted in retarded growth and smaller tumors
(P=0.00033, Fig. 8A and B).
Discussion
In the present study, we found that miR-21 is
overexpressed in the plasma of patients with breast cancer.
Furthermore, it was demonstrated that smad7 is a direct downstream
target of miR-21. Functionally, miR-21 enhanced EGF-mediated
invasion and migration, as well as TGF-β-mediated invasion and
migration in breast cancer cells and these effects were due to
miR-21-induced downregulation of smad7.
miR-21 is expressed at high levels in cancer cells
in invasive breast cancers (35)
and findings of previous studies (including our previous study)
have shown that miR-21 plays an important role in breast cancer
metastasis (36–38). In the present study, miR-21 was
overexpressed in the plasma of patients with breast cancer,
demonstrating that miR-21 plays an important role in cancer cell
invasion and migration, as metastasis, indicating that plasma
miR-21 levels may serve as a diagnostic marker in breast
cancer.
Web-based predictive software and data from other
groups (33,34) suggest that, smad7 is one of the
downstream targets of miR-21. In the present study, the
co-transfection of MCF-7 cells with the smad7 3′-UTR construct and
miR-21 precursor caused an approximately 40% decrease in luciferase
activity as compared to the negative control and this suppression
of smad7 expression was completely reversed by the three-nucleotide
substitution in the core binding site. Additionally, he results
showed that miR-21 overexpression significantly decreased the level
of smad7. Notably, the mRNA level of smad7 was not altered in the
miR-21 overexpressed cells. These results demonstrated that smad7
is a downstream target of miR-21 and miR-21 is capable of
decreasing the smad7 level by inhibiting mRNA translation, rather
than by mRNA decay.
We determined whether TGF-β increased EGF-mediated
cell invasion and migration, and whether this effect was markedly
enhanced by high levels of miR-21. Of note, the combination of EGF,
TGF-β and miR-21 induced a marked increase in cancer cell invasion
and migration. Thus, miR-21 facilitated deleterious cross-talk
between EGF and TGF-β in a manner that promotes breast cancer cell
invasion and migration. Given that miR-21, EGFR and TGF-β are often
overexpressed in breast cancers (12,18,20,26,35,39),
these observations suggest that suppression of miR-21 may prevent
invasion and possibly metastasis in breast cancers, while
interrupting deleterious EGF-TGF-β interactions that have the
potential to contribute to breast cancer cell proliferation in
vivo.
We also determined whether smad7 is a functional
target of miR-21 whose downregulation promotes EGF-induced (or
TGF-β-induced) breast cancer cell invasion and migration. Specific
inhibitors were subsequently used to confirm that EGF and TGF-β
acted through their respective receptors with respect to their
individual and combined stimulatory effects on invasion in the
presence of miR-21. Thus, miR-21 exerts multiple deleterious
actions in breast cancers, which include the upregulation of EGF
and TGF-β signaling, thereby contributing to increased breast
cancer cell migration and invasion.
Lower smad7 levels were associated with a more
poorly differentiated histology and antagonism of miR-21 MCF-7
cells exhibited decreased breast cancer cell proliferation and
fat-pad tumor growth. These observations indicate that miR-21 may
promote breast cancer cell proliferation, as breast cancer
metastasis, by suppressing smad7, which was consistent with the
ability of smad7 to inhibit metastasis of mouse mammary carcinoma
JygMC(A) cells (40).
In conclusion, the results have shown that by
targeting smad7, miR-21 regulates breast cancer cell migration and
invasion by promoting EGF and TGF-β actions, which suggested that
targeting miR-21 may serve to suppress cancer metastasis and to
interrupt deleterious EGF-TGF-β cross-talk, suggesting a molecular
pathway that may relieve the malignant biological behaviors of
breast cancers.
Acknowledgments
The present study was supported in part by the Youth
Innovation Foundation of The First Affiliated Hospital of Zhengzhou
University, China. We would also like to thank the members of the
Department of Breast Surgery, The First Affiliated Hospital of
Zhengzhou University, for their critical technical support and
financial assistance.
References
|
1
|
Wang Y, Bu F, Royer C, Serres S, Larkin
JR, Soto MS, Sibson NR, Salter V, Fritzsche F, Turnquist C, et al:
ASPP2 controls epithelial plasticity and inhibits metastasis
through β-catenin-dependent regulation of ZEB1. Nat Cell Biol.
16:1092–1104. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lamora A, Talbot J, Bougras G, Amiaud J,
Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann
MF, et al: Overexpression of smad7 blocks primary tumor growth and
lung metastasis development in osteosarcoma. Clin Cancer Res.
20:5097–5112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markou A, Farkona S, Schiza C, Efstathiou
T, Kounelis S, Malamos N, Georgoulias V and Lianidou E: PIK3CA
mutational status in circulating tumor cells can change during
disease recurrence or progression in patients with breast cancer.
Clin Cancer Res. 20:5823–5834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y,
Zhao L, Qu H, Fan Y and Wu C: Antagonism of miR-21 reverses
epithelial-mesenchymal transition and cancer stem cell phenotype
through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One.
7:e395202012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mulrane L, McGee SF, Gallagher WM and
O'Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lowery AJ, Miller N, McNeill RE and Kerin
MJ: MicroRNAs as prognostic indicators and therapeutic targets:
Potential effect on breast cancer management. Clin Cancer Res.
14:360–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: Multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hardy KM, Booth BW, Hendrix MJ, Salomon DS
and Strizzi L: ErbB/EGF signaling and EMT in mammary development
and breast cancer. J Mammary Gland Biol Neoplasia. 15:191–199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Javelaud D, Alexaki VI, Dennler S,
Mohammad KS, Guise TA and Mauviel A: TGF-β/SMAD/GLI2 signaling axis
in cancer progression and metastasis. Cancer Res. 71:5606–5610.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brand TM, Iida M, Luthar N, Starr MM,
Huppert EJ and Wheeler DL: Nuclear EGFR as a molecular target in
cancer. Radiother Oncol. 108:370–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boudot A, Kerdivel G, Lecomte S, Flouriot
G, Desille M, Godey F, Leveque J, Tas P, Le Dréan Y and Pakdel F:
COUP-TFI modifies CXCL12 and CXCR4 expression by activating EGF
signaling and stimulates breast cancer cell migration. BMC Cancer.
14:4072014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foley J, Nickerson NK, Nam S, Allen KT,
Gilmore JL, Nephew KP and Riese DJ II: EGFR signaling in breast
cancer: Bad to the bone. Semin Cell Dev Biol. 21:951–960. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolch W and Pitt A: Functional proteomics
to dissect tyrosine kinase signalling pathways in cancer. Nat Rev
Cancer. 10:618–629. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner JP, Wolf-Yadlin A, Sevecka M,
Grenier JK, Root DE, Lauffenburger DA and MacBeath G: Receptor
tyrosine kinases fall into distinct classes based on their inferred
signaling networks. Sci Signal. 6:ra582013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-β actions.
Oncogene. 33:4664–4674. 2014. View Article : Google Scholar :
|
|
16
|
Javelaud D, Alexaki VI and Dennler S,
Javelaud D, Alexaki VI and Dennler S: TGF-β/SMAD/GLI2 signaling
axis in cancer progression and metastasis. Cancer Res.
71:5606–5610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moses H and Barcellos-Hoff MH: TGF-beta
biology in mammary development and breast cancer. Cold Spring Harb
Perspect Biol. 3:a0032772011. View Article : Google Scholar
|
|
18
|
Drabsch Y and ten Dijke P: TGF-β signaling
in breast cancer cell invasion and bone metastasis. J Mammary Gland
Biol Neoplasia. 16:97–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Connolly EC, Freimuth J and Akhurst RJ:
Complexities of TGF-β targeted cancer therapy. Int J Biol Sci.
8:964–978. 2012. View Article : Google Scholar
|
|
20
|
Kang JS, Liu C and Derynck R: New
regulatory mechanisms of TGF-beta receptor function. Trends Cell
Biol. 19:385–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilczynska A and Bushell M: The complexity
of miRNA-mediated repression. Cell Death Differ. 22:22–33. 2015.
View Article : Google Scholar
|
|
22
|
Yang F, Zhang W, Shen Y and Guan X:
Identification of dysregulated microRNAs in triple-negative breast
cancer (Review). Int J Oncol. 46:927–932. 2015.PubMed/NCBI
|
|
23
|
Farazi TA, Horlings HM, Ten Hoeve JJ,
Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F,
van Kouwenhove M, et al: MicroRNA sequence and expression analysis
in breast tumors by deep sequencing. Cancer Res. 71:4443–4453.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang K, Zhang Y, Liu C, Xiong Y and Zhang
J: MicroRNAs in the diagnosis and prognosis of breast cancer and
their therapeutic potential (Review). Int J Oncol. 45:950–958.
2014.PubMed/NCBI
|
|
25
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang TH, Wu F, Loeb GB, Hsu R,
Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo YY, Goga A, et
al: Up-regulation of miR-21 by HER2/neu signaling promotes cell
invasion. J Biol Chem. 284:18515–18524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi L, Bart J, Tan LP, Platteel I, Sluis T,
Huitema S, Harms G, Fu L, Hollema H and Berg A: Expression of
miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia
of the breast in relation to ductal carcinoma in situ and invasive
carcinoma. BMC Cancer. 9:1632009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li T, Li D, Sha J, Sun P and Huang Y:
MicroRNA-21 directly targets MARCKS and promotes apoptosis
resistance and invasion in prostate cancer cells. Biochem Biophys
Res Commun. 383:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han M, Liu M, Wang Y, Mo Z, Bi X, Liu Z,
Fan Y, Chen X and Wu C: Re-expression of miR-21 contributes to
migration and invasion by inducing epithelial-mesenchymal
transition consistent with cancer stem cell characteristics in
MCF-7 cells. Mol Cell Biochem. 363:427–436. 2012. View Article : Google Scholar
|
|
32
|
Chun HK, Jung KU, Choi YL, Hong HK, Kim
SH, Yun SH, Kim HC, Lee WY and Cho YB: Low expression of
transforming growth factor beta-1 in cancer tissue predicts a poor
prognosis for patients with stage III rectal cancers. Oncology.
86:159–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Zhang D, Wang Y, Sun P, Hou X,
Larner J, Xiong W and Mi J: miR-21/Smad 7 signaling determines
TGF-β1-induced CAF formation. Sci Rep. 3:20382013.
|
|
34
|
Wang JY, Gao YB, Zhang N, Zou DW, Wang P,
Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY, et al: miR-21
overexpression enhances TGF-β1-induced epithelial-to-mesenchymal
transition by target smad7 and aggravates renal damage in diabetic
nephropathy. Mol Cell Endocrinol. 392:163–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Petrović N, Mandušić V, Dimitrijević B,
Roganović J, Lukić S, Todorović L and Stanojević B: Higher miR-21
expression in invasive breast carcinomas is associated with
positive estrogen and progesterone receptor status in patients from
Serbia. Med Oncol. 31:9772014. View Article : Google Scholar
|
|
36
|
Han M, Wang Y, Liu M, Bi X, Bao J, Zeng N,
Zhu Z, Mo Z, Wu C and Chen X: MiR-21 regulates
epithelial-mesenchymal transition phenotype and hypoxia-inducible
factor-1α expression in third-sphere forming breast cancer stem
cell-like cells. Cancer Sci. 103:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marino AL, Evangelista AF, Vieira RA,
Macedo T, Kerr LM, Abrahão-Machado LF, Longatto-Filho A, Silveira
HC and Marques MM: MicroRNA expression as risk biomarker of breast
cancer metastasis: A pilot retrospective case-cohort study. BMC
Cancer. 14:7392014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petrović N, Mandušić V, Stanojević B,
Lukić S, Todorović L, Roganović J and Dimitrijević B: The
difference in miR-21 expression levels between invasive and
non-invasive breast cancers emphasizes its role in breast cancer
invasion. Med Oncol. 31:8672014. View Article : Google Scholar
|
|
39
|
Chen J and Wang X: MicroRNA-21 in breast
cancer: Diagnostic and prognostic potential. Clin Transl Oncol.
16:225–233. 2014. View Article : Google Scholar
|
|
40
|
Azuma H, Ehata S, Miyazaki H, Watabe T,
Maruyama O, Imamura T, Sakamoto T, Kiyama S, Kiyama Y, Ubai T, et
al: Effect of Smad7 expression on metastasis of mouse mammary
carcinoma JygMC(A) cells. J Natl Cancer Inst. 97:1734–1746. 2005.
View Article : Google Scholar : PubMed/NCBI
|