Introduction
Lung cancer is the most common cause of cancer
mortality globally and the second most common type of cancer
(1). The high mortality associated
with lung cancer is primarily attributable to late diagnosis and
metastasis at the time of diagnosis. Identifying effective tumor
biomarkers of this malignancy in the early stages and preventing
metastasis may therefore be the best options for improving the
generally dismal survival rate of lung cancer patients.
Structural and numerical abnormalities of the
centrosome are significantly associated with chromosome
instability, aneuploidy and tumorigenesis (2,3,4–8).
The transforming acidic coiled-coil proteins (TACCs) family is
characterized by a highly conserved carboxyl-terminus coiled-coil
'TACC domain', which is important for the interaction with
spindle/centrosome dynamics (2,4,9). The
TACC family contains TACC1, TACC2 and TACC3 in mammals (2–4,10).
Although increasing evidence indicates that abnormalities in human
TACCs (TACC1-3) may correlate with the development of some human
malignancies, the functions of these three proteins in mammals are
largely unknown (11–13).
The human TACC1-3 genes are located on chromosome
8p11, 10q26 and 4p16.3, respectively (2,4,11–17).
TACC3 is mainly expressed in the developing embryo and in adult
testis, thymus, peripheral blood leukocytes, spleen and intestinal
tissues (4,17). Several studies had shown that TACC3
may be associated with some human tumor, but the expression status
of TACC3 in cancer remains controversial and unclear. For instance,
a microarray analysis suggested that TACC3 is upregulated during
the transition of ductal carcinoma in situ to invasive
ductal carcinoma, while an immunohistochemical analysis
demonstrated that TACC3 is downregulated in resected breast tumors
(18,19). A similar discrepancy was found in
the expression of TACC3 in ovarian tumor and thyroid cancer
(20–22). In addition, TACC3 has been
identified as a potential glioblastoma multiforme oncogene and may
be overexpressed in multiple myeloma cases and lung cancer
(23–25). Therefore, it is necessary to explore
the expression status of TACC3 in lung cancer and the relationship
between TACC3 expression and clinicopathological characteristic of
lung cancer.
In the present study, we focused on the expression
patterns of TACC3 in primary non-small cell lung cancer (NSCLC)
lesions and metastatic lymph nodes. Statistical analysis indicated
a relationship between high TACC3 expression levels and the
clinicopathological features of NSCLC. Multivariate Cox regression
analyses suggested that higher TACC3 expression in NSCLC predicts
poorer survival independent of other factors.
Materials and methods
Patients and tumor specimen
The present study is reported according to the
Reporting Recommendations for Tumor Marker Prognostic Studies
(REMARK) (26). A total of 207
consecutive cases of primary NSCLC with surgical resection were
collected from Sun Yat-sen University Cancer Center (SYSUCC;
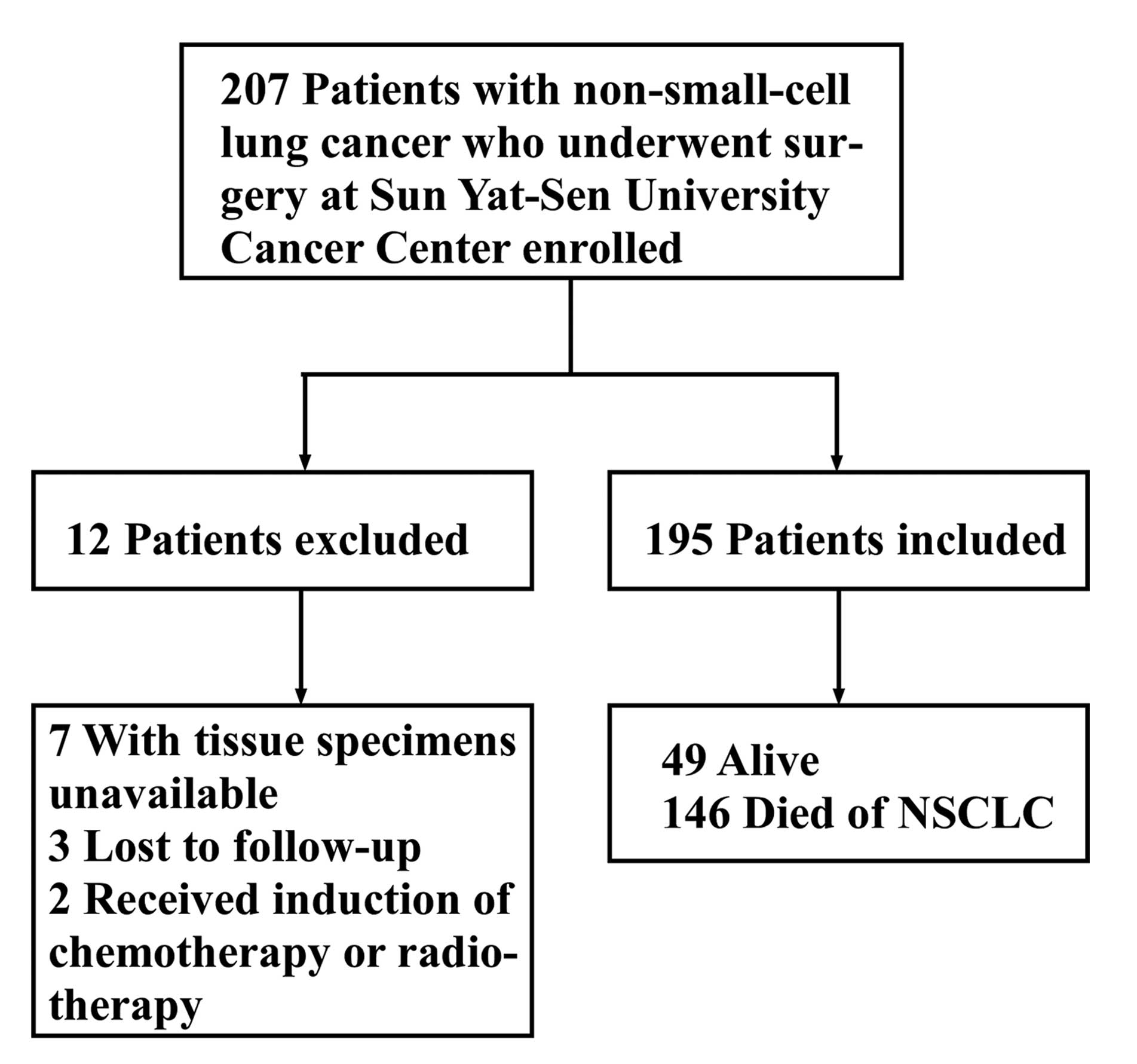
Guangzhou, China) between January 2003 and June 2004. Fig. 1 shows the inclusion and exclusion
criteria for the present study. Ultimately, a total of 195 patients
were enrolled.
We evaluated conventional clinical characteristics
as shown in Table I. All 195 cases
were reevaluated with respect to histological subtype,
differentiation and tumor stage. The follow-up interval ranged from
0.9 to 139.4 months (median 27.3 months, mean 49.5 months). The
5-year cumulative survival rate for all patients was 30%.
 | Table ICorrelation between TACC3 expression
and clinicopathological characteristics. |
Table I
Correlation between TACC3 expression
and clinicopathological characteristics.
|
Characteristics | TACC3 expression:
no. of patients (%)
| P-valueb |
|---|
| Lowa (101) | Higha (94) |
|---|
| Gender |
| Male | 76 (49.7) | 77 (50.3) | 0.258 |
| Female | 25 (59.5) | 17 (40.5) | |
| Age (years) |
| ≤60 | 54 (50.5) | 53 (49.5) | 0.682 |
| >60 | 47 (53.4) | 41 (46.6) | |
| Smoking
statusc |
| No | 40 (63.5) | 23 (36.5) | 0.027 |
| Yes | 61 (46.6) | 70 (53.4) | |
|
Differentiation |
| Well-moderate | 53 (63.9) | 30 (36.1) | 0.004 |
| Poor | 48 (42.9) | 64 (57.1) | |
| T staged |
| T1 | 23 (69.7) | 10 (30.3) | 0.024 |
| T2–4 | 78 (48.1) | 84 (51.9) | |
| N staged |
| N0 | 54 (56.8) | 41 (43.2) | 0.168 |
| N1–3 | 45 (46.9) | 51 (53.1) | |
| M staged |
| M0 | 82 (49.7) | 83 (50.3) | 0.169 |
| M1 | 19 (63.3) | 11 (36.7) | |
| Clinical
staged |
| I | 36 (66.7) | 18 (33.3) | 0.001 |
| II | 18 (32.1) | 38 (67.9) | |
| III | 28 (50.0) | 28 (50.0) | |
| IV | 19 (65.5) | 10 (34.5) | |
| Histological
type |
|
Adenocarcinoma | 67 (59.3) | 46 (40.7) | 0.004 |
| SCC | 34 (41.5) | 48 (58.5) | |
| SCCAe |
| (−) | 49 (53.8) | 42 (46.2) | 0.320 |
| (+) | 6 (40.0) | 9 (60.0) | |
| CEAf |
| (−) | 40 (55.6) | 32 (44.4) | 0.741 |
| (+) | 30 (52.6) | 27 (47.4) | |
| CYFRA
21-1g |
| (−) | 11 (73.3) | 4 (26.7) | 0.025 |
| (+) | 7 (35.0) | 13 (65.0) | |
A total of 195 paraffin-embedded NSCLC tissue
samples and 11 matched adjacent paraffin-embedded non-cancerous
human lung tissues were obtained from the archives of the
Department of Sample Resources, SYSUCC. These samples had been
histologically and clinically diagnosed. Fifty metastatic lymph
nodes were also obtained from the above-mentioned patients. Eleven
paired freshly frozen lung carcinoma and non-cancerous tissues
adjacent to cancer lesions were obtained from the same patients.
The present study was conducted with the consent of the patients
and the approval of the Institute Research Ethics Committee of
SYSUCC.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (27). The relevant,
sections were incubated with an anti-TACC3 monoclonal antibody
(1:800; Abcam, Cambridge, UK) at 4°C overnight. Immunohistochemical
kit (SP-9001 rabbit SP kit, lot: 50581654) was obtained from
Zhongshan Golden Bridge Biotechnology Co. Ltd. (Beijing, China). As
a negative control, the primary antibody was replaced by IgG.
The degree of immunostaining was examined and
evaluated independently by two pathologists without knowledge of
the clinical characteristics of the cases. The proportion of
positively stained tumor cells varied from 0 to 100%, and the
intensity of staining varied from weak to strong. The proportion of
stained tumor cells was graded according to the following criteria:
0 (no positive tumor cells), 1 (≤10% positive tumor cells), 2
(11–25% positive tumor cells), 3 (26–40% positive tumor cells) and
4 (≥41% positive tumor cells). The intensity of staining was
quantified using the following score system: 0 (no staining), 1
(weak staining, light yellow), 2 (moderate staining, yellowish
brown) and 3 (intense staining, brown). The TACC3 expression index
was calculated by multiplying the two scores to obtain a final
score of 0, 1, 2, 3, 4, 6, 9 or 12. The cut-off value for high and
low levels of expression was based on a measurement of
heterogeneity using a log-rank test statistical analysis with
respect to the overall survival (OS) rate. The carcinomas were
finally classified as low expression (score <5) or high
expression (score ≥6).
Western blotting
Western blot analysis was performed as previously
described (27). The membrane was
incubated with primary antibodies (1:1,000) at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated goat
anti-mouse or anti-rabbit IgG secondary antibody (1:3,000) (both
from Abcam) at room temperature for 1 h. A mouse β-actin monoclonal
antibody (1:4,000; Cell Signaling Technology, Danvers, MA, USA) was
used as an internal loading control. The membrane was detected
using an enhanced chemiluminescence (ECL; Amersham Pharmacia
Biotech, UK) detection system (Amersham Biosciences Europe,
Freiburg, Germany) according to the manufacturer's
instructions.
RNA extraction and quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from 11 paired freshly
frozen lung cancer and noncancerous tissues using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. The RNA was treated with RNA-free
DNase, and 2.0 µg of total RNA was used to generate cDNA
using the RETROscript kit (Promega, Madison, WI, USA). The
full-length open reading frame of TACC3 was PCR amplified from cDNA
samples. RT-PCR was performed using iQ SYBR-Green Supermix on a
MYiQ qPCR machine (both from Bio-Rad, Hercules, CA, USA). The
following primers were used to amplify TACC3: forward,
5′-CCTCTTCAAGCGTTTTGAGAAAC-3′ and reverse,
5′-GCCCTCCTGGGTGATCCTT-3′. The following primers were used to
amplify β-actin: forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′. The primer sets were designed and
produced by PrimerDesign Ltd. (Southampton, UK). Cycles were as
follows: 95°C for 3 min (1 cycle), 94°C for 30 sec followed by 60°C
for 10 sec and 72°C for 15 sec (40 cycles), and 95°C for 30 sec (1
cycle). Melt curve analysis was performed for each reaction to
ensure amplification specificity.
Statistical analysis
All statistical analyses were performed using the
SPSS version 16.0 statistical software package (SPSS, Inc.,
Chicago, IL, USA).
The data are presented as the mean ± SEM unless
otherwise indicated. Differences among variables were identified by
χ2 analysis or two-tailed Student's t-tests.
Correlations between TACC3 expression and clinicopathological
factors were assessed by χ2 analysis and Fisher's exact
test. Survival curves were plotted using Kaplan-Meier survival
analysis and compared using a log-rank test. Further analyses of
survival curves were calculated based on stratifying age, TNM
classifications, histological type and clinical stages. Univariate
and multivariate Cox proportional hazards models were used to
evaluate the relative risks (RRs) of death associated with TACC3
expression and other variables. In our analyses, we defined the RR
of 1.000 as baseline for factors including gender (male), age (≤60
years), smoking status (non-smoker), differentiation
(well-moderate), SCC (0–15.0 ng/ml), T1, N0, M0, clinical stage
(I–II), and low level of TACC3 expression. In all cases, P<0.05
was considered to indicate a statistically significant result.
Results
Immunohistochemistry reveals high TACC3
expression in NSCLC tissue samples
As shown in Fig. 2A,
normal lung alveolar epithelium did not express TACC3 protein
(Fig. 2A-a, -c and -d). TACC3
immunoreactivity was generally confined to the cytoplasm of tumor
cells in NSCLC lesions and metastatic lymph nodes (Fig. 2A-b, -c, -d, -e and -f). In all NSCLC
tissue samples, TACC3 protein was observed in 180 of 195 (92.3%)
human NSCLC samples, and strong cytoplasmic staining of TACC3
protein was detected in 64 (35.5%) tumors. Among the corresponding
metastatic lymph nodes, 47 of 50 (94%) cases displayed positive
staining (Fig. 2A-e and -f), and
48.9% (23 of 47) of cases exhibited strong staining.
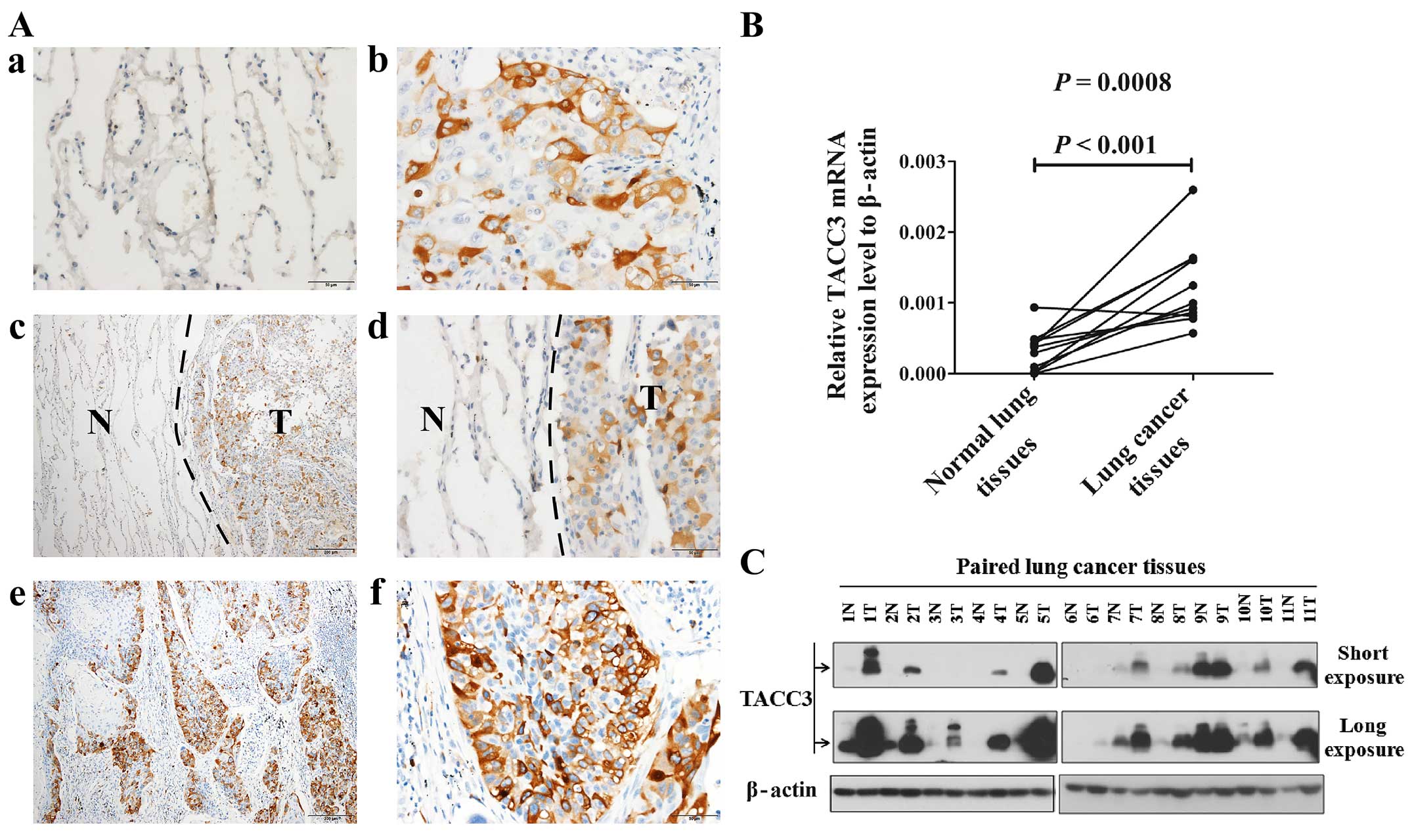 | Figure 2The expression of TACC3 is elevated
in fresh frozen primary NSCLC tissues compared with non-cancerous
tissues adjacent to the cancer lesions. (A) Expression of TACC3 in
different tissues as determined by immunohistochemical staining. a,
Lack of TACC3 staining in the alveolar epithelium of a normal lung
(magnification, ×400). b, Cytoplasmic TACC3 staining in primary
lung cancer lesions (magnification, ×400). c and d, Higher
expression of the TACC3 protein in primary lung cancer lesions
compared with adjacent non-cancerous tissues (c, magnification,
×100; d, magnification, ×400). e and f, Strong cytoplasmic TACC3
staining in the matched metastatic lymph nodes of adenocarcinomas
(e, magnification, ×100; f, magnification, ×400). (B) Expression
analysis of TACC3 mRNA in paired and fresh frozen primary NSCLC (T)
and non-cancerous tissues (N) from the same patient by real-time
RT-PCR. β-actin was used as an internal control. The reactions were
performed in triplicate in three independent experiments. (C)
Expression analysis of TACC3 protein in paired and fresh frozen
primary NSCLC tissues (T) and non-cancerous tissues (N) from the
same patient by western blotting. β-actin was used as an internal
reference. |
TACC3 expression is upregulated in NSCLC
tissue samples as determined by real-time RT-PCR and western
blotting
To investigate the mRNA and protein expression
levels of TACC3 in NSCLC lesions, real-time RT-PCR and western blot
analyses were performed in paired freshly frozen NSCLC tissues and
adjacent non-cancerous tissues from the same patients. As
determined by real-time RT-PCR analysis, all 11 human NSCLC samples
exhibited higher TACC3 mRNA expression compared to paired
non-cancerous tissues (Fig. 2B).
Western blot analysis demonstrated that TACC3 protein was markedly
overexpressed in the NSCLC lesions but weakly detected in adjacent
paired non-cancerous tissues (Fig.
2C).
Correlation between the
clinicopathological features and TACC3 expression levels
The immunoreactivity results of TACC3 expression
levels were statistically analyzed to determine their relationships
with the clinical characteristics of the NSCLC patients. As
presented in Table I, the
expression of TACC3 was strongly correlated with smoking status,
histological classification, differentiation, CYFRA21-1 levels, T
stage and the clinical stage of NSCLC patients. However, there was
no significant correlation between the expression level of TACC3
protein and gender, age, squamous cell carcinoma antigen (SCCA),
carcinoembryonic antigen (CEA), tumor supplies group of factors
(TSGF), N classification or distant metastasis of NSCLC
patients.
TACC3 expression is correlated with poor
overall and recurrence-free survival
After demonstrating the correlation of TACC3
expression with clinicopathological characteristics, we proceeded
to examine the relationship between TACC3 expression level and the
patients survival. The patients OS time was defined as the time
from the day of surgery to (i) the point of death due to recurrence
or metastasis or (ii) to the end point of the most recent follow-up
if the patient was alive. Recurrence-free survival (RFS) was
calculated from the date of curative surgery to the day of disease
recurrence, disease progression, death or the most recent
follow-up. Patients with metastases were excluded from the RFS
analysis.
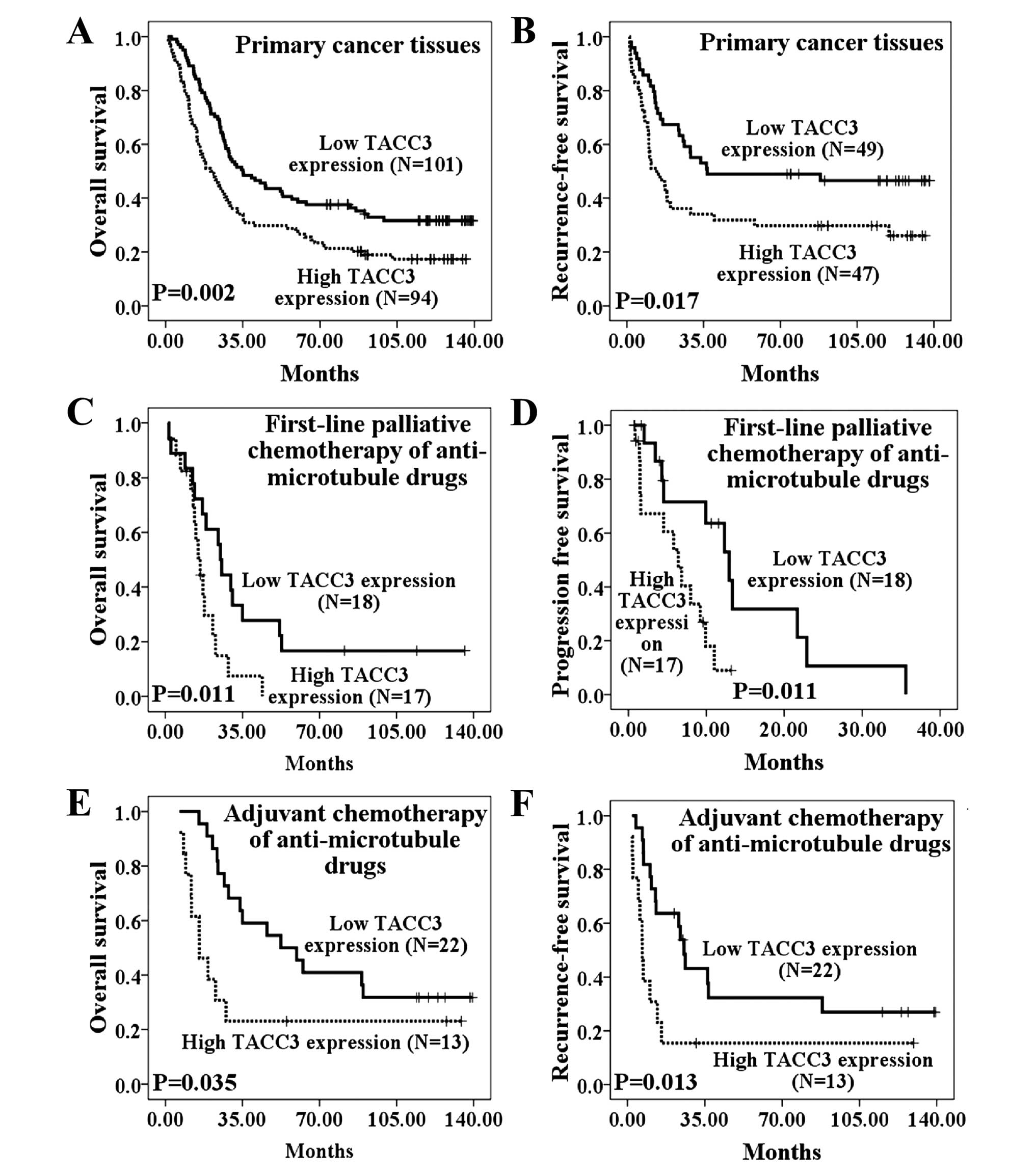
The relationship between TACC3 expression levels and
OS time (P=0.002) is presented in Fig.
3A. In primary NSCLC lesions, the low TACC3 expression group
had better OS (median, 35.10 months), whereas the high TACC3
expression group had poor OS (median, 20.33 months). The cumulative
5-year survival rate was 38.6% in the low TACC3 expression group
and 25.5% in the high TACC3 expression group. Furthermore, patients
with a low TACC3 expression had better OS regardless of the
patients age, histological type, tumor differentiation, N stage and
clinical stage. Moreover, the OS showed a statistically significant
difference between the high and low TACC3 expression groups in the
non-smoking group, early T stage (T1+T2) and M0 stage subgroups
(Fig. 4A–P).
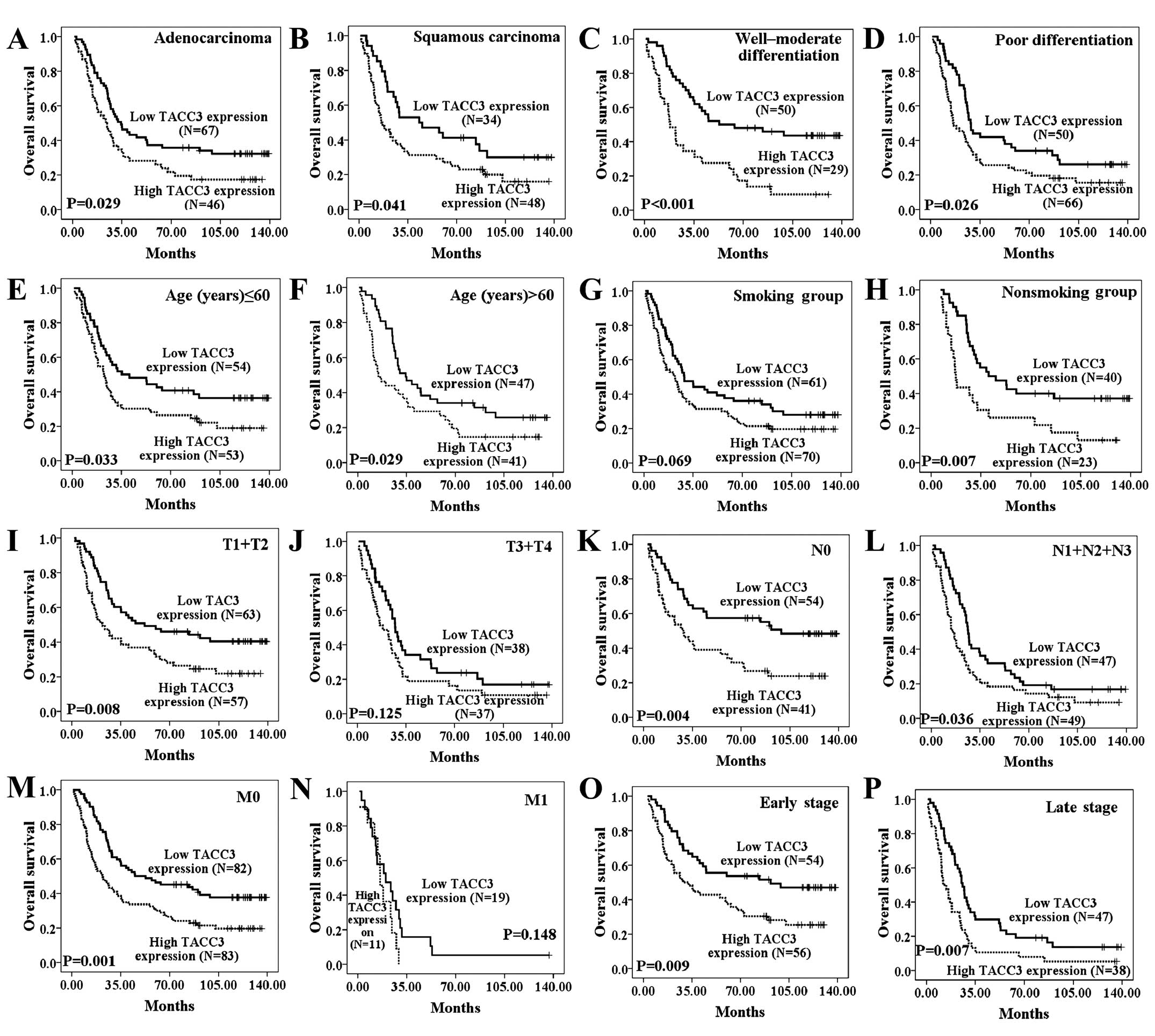 | Figure 4The influence of TACC3 expression on
overall survival of NSCLC patients stratified by
clinicopathological characteristics. Kaplan-Meier curves were
stratified by TACC3 expression level according to histological
type, tumor differentiation, age, smoking status, as well as T, N,
M and clinical stage (A–P). Patients with high TACC3 expression had
poor OS regardless of histological type (A and B), tumor
differentiation (C and D), age (E and F), N stage (K and L) and
clinical stage (O and P). Patients with high TACC3 expression have
poorer OS in the non-smoking group (H), early T stage (T1+T2) (K),
and M0 stage (M) subgroups. TACC3 low expression, score <5; high
expression, score ≥6. Smoking status is unknown for 1 patient. N
stage is unknown for 4 patients. |
As shown in Fig. 3B,
RFS also differed significantly between patients with high and low
TACC3 expression levels (P=0.017). RFS also significantly differed
between the high and low TACC3 expression groups in the younger age
group (≤60 years), among cases with well-moderated differentiation,
and in the early T stage (T1+T2), N0 and M0 stages, and early
clinical stage (I+II) subgroups (Fig.
5A–O).
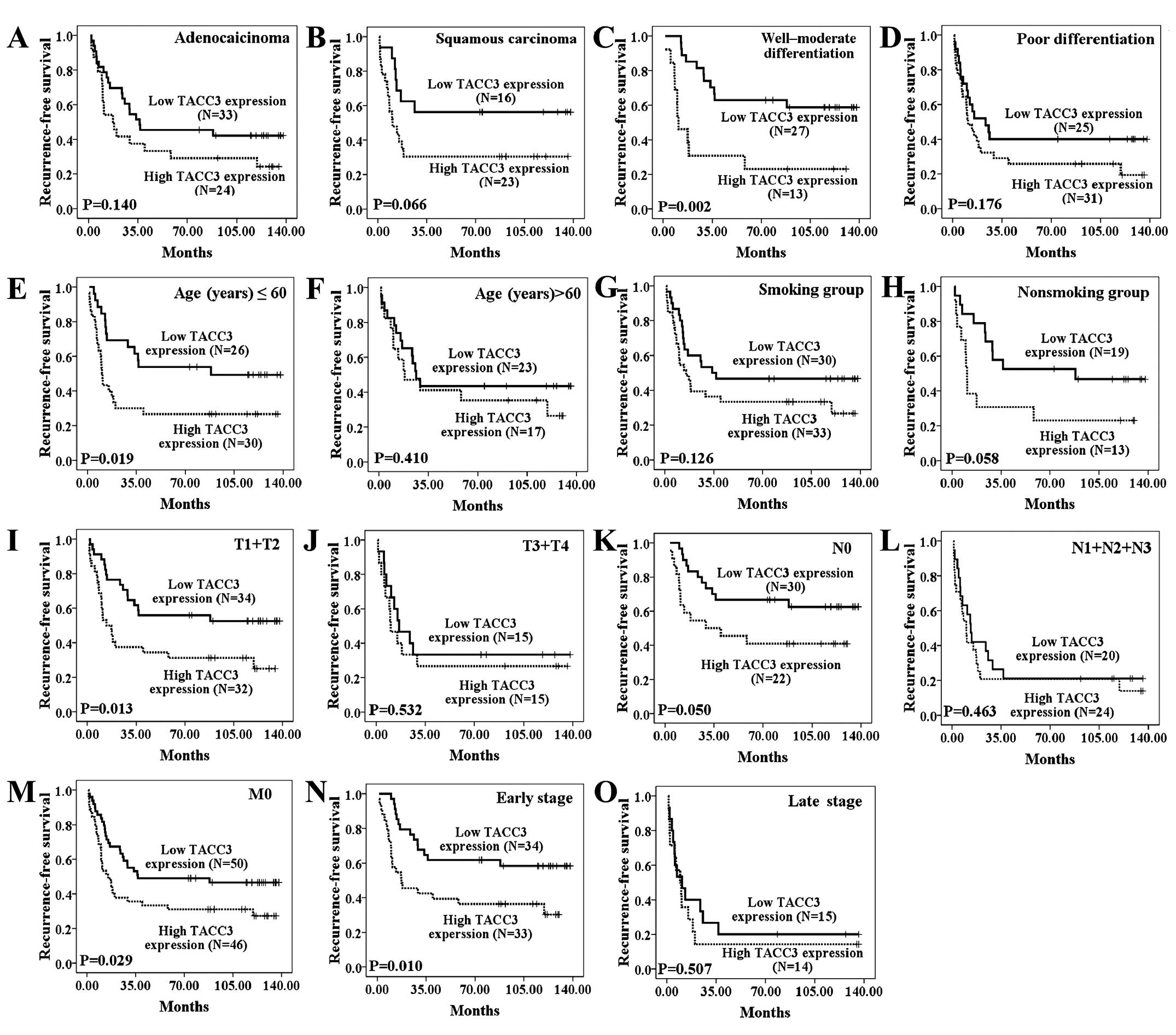 | Figure 5The influence of TACC3 expression on
recurrence-free survival of NSCLC patients stratified by
clinicopathological characteristics. (A and B) The duration of RFS
did not differ significantly between the two groups among the
histological type subgroups (P=0.140 and P=0.066). In addition, the
duration of RFS differed significantly between the high and low
TACC3 expression groups among the well-moderate differentiation
(C), younger age (≤60 years) (E), early T stage (T1+T2) (I), N0
stage (K), M0 stage (M), and early clinical stage (I+II) (N)
subgroups. The duration of RFS did not differ significantly between
the high and low TACC3 expression groups among the poor
differentiation (D), older age (>60 years) (F), smoking (G) or
non-smoking groups (H), or the late T stage (T3+T4) (J), advanced N
stage (N1+N2+N3) (L) and late clinical stage (III+IV) (O)
subgroups. TACC3 low expression, score <5; high expression,
score ≥6. |
Univariate and multivariate analyses
indicate an independent role of TACC3 in the prognosis of
NSCLC
As shown in Tables
II and III, univariate Cox
regression analyses revealed that a higher level of TACC3 was
associated with a significantly increased risk of cancer-related
death (P=0.002, RR=1.663; P=0.019, RR=1.849) in NSCLC patients. The
RRs indicated that the level of SCCA, differentiation and clinical
staging were also effective predictors. Multivariate Cox regression
analyses suggested that higher TACC3 expression in NSCLC predicted
worse survival independent of other factors (P=0.005, RR=1.999;
P=0.022, RR=1.825). As expected, clinical staging was also
recognized as independent prognostic factors.
 | Table IIUnivariate and multivariate Cox
regression analysis of potential prognostic parameters for NSCLC
patients regarding overall survival. |
Table II
Univariate and multivariate Cox
regression analysis of potential prognostic parameters for NSCLC
patients regarding overall survival.
| Univariate analysis
| Multivariate
analysis
|
|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Gender |
| Male | 1.000 | 0.108 | | |
| Female | 0.716
(0.477–1.077) | | | |
| Age, years |
| ≤60 | 1.000 | 0.441 | | |
| >60 | 1.137
(0.820–1.575) | | | |
| Smoking status |
| No | 1.000 | 0.186 | | |
| Yes | 1.268
(0.891–1.804) | | | |
| Histology |
|
Adenocarcinoma | 1.000 | 0.475 | | |
| Squamous
carcinoma | 1.132
(0.806–1.589) | | | |
|
Differentiation |
| Well-moderate | 1.000 | 0.077 | | |
| Poor | 1.376
(0.966–1.961) | | | |
| SCCAa |
| (−) | 1.000 | 0.006 | 1.000 | 0.009 |
| (+) | 2.336
(1.280–4.264) | | 2.304
(1.233–4.304) | |
| CEAb |
| (−) | 1.000 | 0.082 | | |
| (+) | 1.433
(0.955–2.150) | | | |
| CYFRA 21-1c |
| (−) | 1.000 | 0.753 | | |
| (+) | 1.142
(0.499–2.611) | | | |
| Clinical
staged |
| I–II | 1.000 |
<0.001 | 1.000 |
<0.001 |
| III–IV | 2.269
(1.629–3.161) | | 3.255
(1.990–5.323) | |
| TACC3
expressione |
| Low | 1.000 | 0.002 | 1.000 | 0.005 |
| High | 1.663
(1.199–2.306) | | 1.999
(1.231–3.248) | |
 | Table IIIUnivariate and multivariate Cox
regression analysis of potential prognostic parameters for NSCLC
patients regarding recurrence-free survival. |
Table III
Univariate and multivariate Cox
regression analysis of potential prognostic parameters for NSCLC
patients regarding recurrence-free survival.
| Univariate analysis
| Multivariate
analysis
|
|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Gender |
| Male | 1.000 | 0.361 | | |
| Female | 0.751
(0.406–1.389) | | | |
| Age, years |
| ≤60 | 1.000 | 0.802 | | |
| >60 | 0.973
(0.560–1.565) | | | |
| Smoking status |
| No | 1.000 | 0.849 | | |
| Yes | 1.054
(0.651–1.807) | | | |
| Histology |
|
Adenocarcinoma | 1.000 | 0.575 | | |
| Squamous
carcinoma | 1.127
(0.669–1.896) | | | |
|
Differentiation |
| Well-moderate | 1.000 | 0.016 | 1.000 | 0.297 |
| Poor | 1.911
(1.128–3.235) | | 1.336
(0.760–2.456) | |
| SCCAa |
| (−) | 1.000 | 0.122 | | |
| (+) | 2.136
(0.737–6.270) | | | |
| CEAb |
| (−) | 1.000 | 0.302 | | |
| (+) | 1.329
(0.743–2.611) | | | |
| CYFRA 21-1c |
| (−) | 1.000 | 0.706 | | |
| (+) | 1.709
(0.167–3.336) | | | |
| Clinical
staged |
| I–II | 1.000 |
<0.001 | 1.000 |
<0.001 |
| III–IV | 2.640
(1.566–4.451) | | 2.614
(1.560–4.408) | |
| TACC3
expressione |
| Low | 1.000 | 0.019 | 1.000 | 0.022 |
| High | 1.849
(1.107–3.086) | | 1.825
(1.093–3.047) | |
Taken together, the univariate and multivariate
analyses suggested that TACC3 may be a useful biomarker for the
prognosis of NSCLC patients.
TACC3 expression levels are correlated
with sensitivity to anti-microtubule drugs
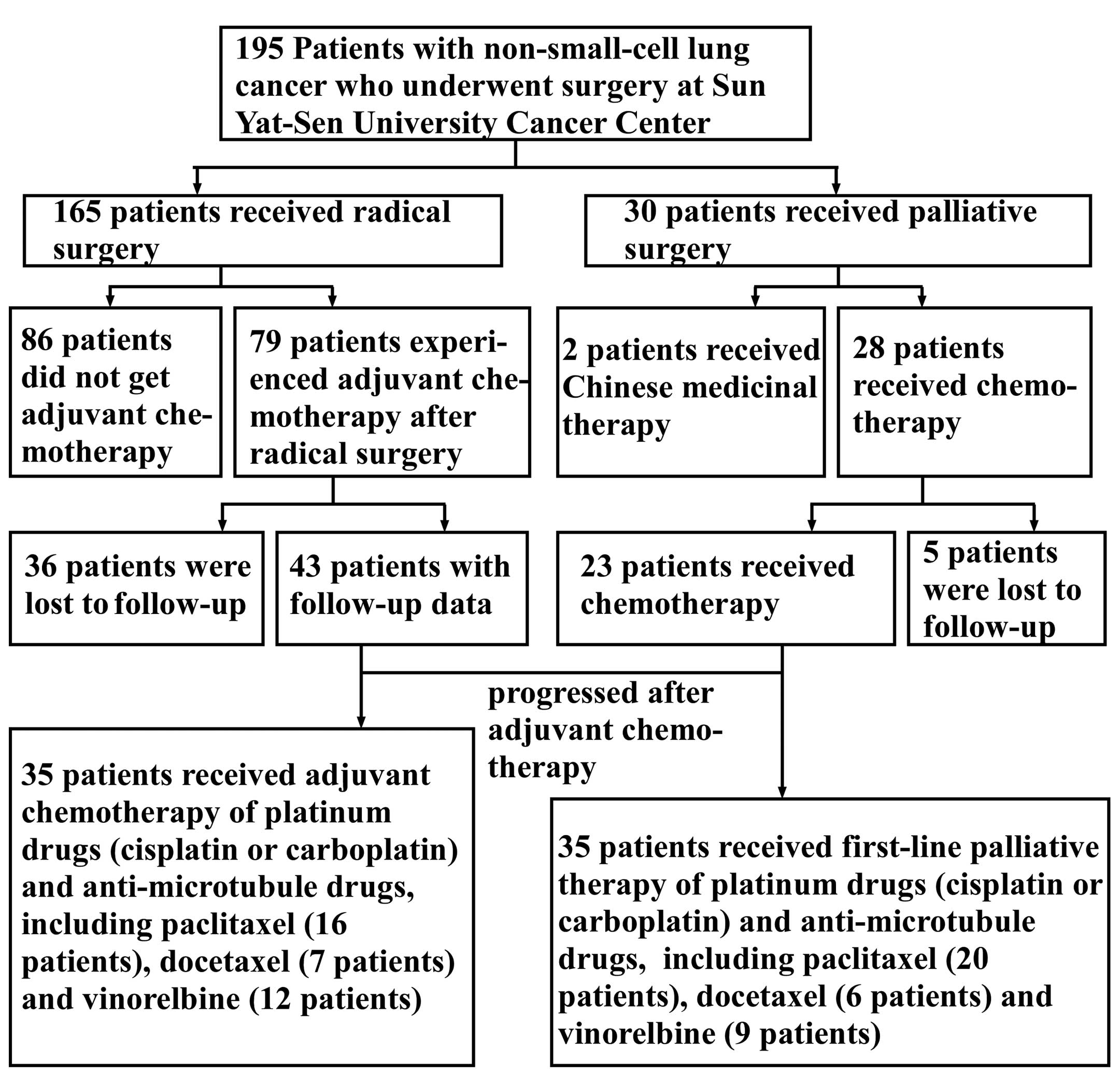
As shown in Fig. 6,
35 patients received adjuvant chemotherapy with platinum drugs
(cisplatin or carboplatin) and anti-microtubule drugs, including
paclitaxel (16 patients), docetaxel (7 patients) and vinorelbine
(12 patients). Among the patients who progressed after adjuvant
chemotherapy and those who received palliative therapy, 35 received
first-line palliative therapy with platinum and anti-microtubule
drugs, including paclitaxel (20 patients), docetaxel (6 patients)
and vinorelbine (9 patients).
OS of patients who received chemotherapy was defined
as the time from the day of the first chemotherapy treatment to (i)
the point of death due to recurrence or metastasis or (ii) to the
end point of the most recent follow-up if the patient was alive.
Progression-free survival (PFS) of patients who received palliative
chemotherapy was defined as the interval between the first
chemotherapy treatment and disease progression or last contact. RFS
of patients who received adjuvant chemotherapy was calculated from
the date of the first chemotherapy treatment to the day of disease
recurrence, disease progression, death or the most recent
follow-up.
To explore whether expression of TACC3 was
correlated with sensitivity to anti-microtubule drugs, we used
Kaplan-Meier analysis and log-rank tests to examine the
relationship between the TACC3 expression level and survival in
patients who received anti-microtubule chemotherapy. As shown in
Fig. 3C and D, the low TACC3
expression group exhibited better OS (P=0.011) and better PFS
(P=0.011) among patients who received anti-microtubule chemotherapy
as a first-line palliative therapy. Among patients who received
anti-microtubule chemotherapies as adjuvant chemotherapy, low TACC3
expression was also correlated with longer OS time (Fig. 3E, P=0.035) and longer RFS time
(Fig. 3F, P=0.013) compared with
patients expressing high TACC3 levels.
Discussion
Members of the TACC family play important roles in
cell growth, differentiation and gene regulation by affecting
centrosome/spindle dynamics (10,17,28–29).
In addition, the deregulation of human TACCs (TACC1-3) may be
involved in the development and progression of some human
malignancies (3,10,13,15,25,30,31).
A previous immunohistochemical analysis of lung
cancer showed TACC3 expression is higher in primary lung cancer
tissues than in normal lung tissues and high level of TACC3 is
associated with a poor prognosis (25). However, that study did not use
western blot analysis and real-time RT-PCR to verify the conclusion
and did not analysis the expression of TACC3 in metastatic lymph
node tissues. In the present study, we performed western blot
analysis and real-time RT-PCR as well as immunohistochemical
analysis to determine whether TACC3 expression is upregulated in
primary NSCLC lesions compared with normal lung tissues. The
present study indicated that high TACC3 expression in primary NSCLC
lesions is correlated with poorer OS (25). We present that high TACC3 expression
in primary NSCLC lesions is also associated with poorer RFS, which
has not been reported. These data provide strong evidence for a
relationship between high TACC3 expression levels and poor
prognosis in NSCLC patients and indicate that the TACC3 expression
level is an independent prognostic factor for NSCLC patients. In
addition, we compared the expression of TACC3 in metastatic lymph
nodes tissues with normal lung tissues and demonstrated that
expression of TACC3 is also upregulated in metastatic lymph nodes
tissues. These results indicate that alterations in TACC3 protein
levels may be involved in both tumor development and
progression.
We observed a strong correlation between more
advanced clinical staging and higher T classification and higher
TACC3 expression. These results may indicate a correlation between
TACC3 overexpression and clinical progression in NSCLC. Recent
studies in cervical cancer have suggested that TACC3 overexpression
promotes epithelial-mesenchymal transition (EMT) by activating the
phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular
signal-regulated protein kinases (ERKs) signal transduction
pathways (32,33). As EMT plays a major role during
cancer invasion and metastases, it is worthwhile to further
investigate the role of TACC3 in the progression of NSCLC.
TACC3 expression has been observed in various cell
lines and tissues, particularly those undergoing rapid growth and
differentiation. Sadek et al found that TACC3 is upregulated
during the early differentiation of many varied cell types
(17). Another study showed TACC3
plays an important role in murine embryonic development and may be
involved in differentiation (34,35).
These observations showed the potential relationship between TACC3
and differentiation stage in normal tissues. However, the
correlation between TACC3 and the differentiation status in tumor
tissues has not been studied to date. The present study
demonstrated that high levels of TACC3 expression were
significantly correlated with poor differentiation in lung cancer,
which had not been reported. Further experiments are needed to
explore the molecular mechanisms of relationship between TACC3
expression and differentiation in tumor tissues.
Furthermore, the present study revealed that
patients who smoked, squamous cell carcinoma patients and those
with elevated CYFRA21-1 levels tend to have high expression levels
of TACC3. More analysis is required to investigate the mechanisms
underlying changes in TACC3 expression in NSCLC patients.
The phosphorylation and localization of TACC3 are
novel pharmacodynamic indicators of Aurora A activity (36). Yim et al identified TACC3 as
a possible direct target of anti-microtubule drugs such as
paclitaxel in cervical carcinoma cells (37). Subsequent studies have demonstrated
that paclitaxel induces strong polyploidy and apoptosis in
TACC3-depleted murine fibroblast cells and that the downregulation
of TACC3 enhances paclitaxel chemosensitivity in breast carcinoma
cells (38,39). However, if the expression of TACC3
in tumor tissue by immunohistochemical analysis correlates with the
sensitivity to anti-microtubule drugs has not been reported yet.
Our results indicate that low TACC3 expression may enhance
chemosensitivity of anti-microtubule drugs in patients of lung
cancer. However, larger samples ofpatients and further experiments
in vitro are needed to verify the correlation between TACC3
expression status and chemosensitivity to anti-microtubule
drugs.
In conclusion, we demonstrated that TACC3 expression
is associated with the clinicopathological classification of NSCLC
and that TACC3 is an independent prognostic factor for patient
survival. In addition, TACC3 overexpression may be associated with
chemosensitivity to anti-microtubule drugs. Our findings suggest
that the TACC3 expression level may be a potential prognostic
factor and a new therapeutic target for NSCLC.
Acknowledgments
The present study was supported by the National
Scientific Foundation of China (no. 81372887), the National Basic
Research Program of China (grant no. 2013CB910500), and the
Guangdong Science and Technology Project (no. 2013B021800167).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gergely F: Centrosomal TACCtics.
BioEssays. 24:915–925. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peset I and Vernos I: The TACC proteins:
TACC-ling microtubule dynamics and centrosome function. Trends Cell
Biol. 18:379–388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ha GH, Kim JL and Breuer EK: Transforming
acidic coiled-coil proteins (TACCs) in human cancer. Cancer Lett.
336:24–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albee AJ and Wiese C: Xenopus TACC3/maskin
is not required for microtubule stability but is required for
anchoring microtubules at the centrosome. Mol Biol Cell.
19:3347–3356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Vaart B, Akhmanova A and Straube
A: Regulation of microtubule dynamic instability. Biochem Soc
Trans. 37:1007–1013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duensing S, Lee BH, Dal Cin P and Münger
K: Excessive centro-some abnormalities without ongoing numerical
chromosome instability in a Burkitt's lymphoma. Mol Cancer.
2:30–36. 2003. View Article : Google Scholar
|
|
8
|
Trachana V, van Wely KH, Guerrero AA,
Fütterer A and Martínez-A C: Dido disruption leads to centrosome
amplification and mitotic checkpoint defects compromising
chromosome stability. Proc Natl Acad Sci USA. 104:2691–2696. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gergely F, Karlsson C, Still I, Cowell J,
Kilmartin J and Raff JW: The TACC domain identifies a family of
centrosomal proteins that can interact with microtubules. Proc Natl
Acad Sci USA. 97:14352–14357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raff JW: Centrosomes and cancer: Lessons
from a TACC. Trends Cell Biol. 12:222–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Still IH, Vince P and Cowell JK: The third
member of the transforming acidic coiled coil-containing gene
family, TACC3, maps in 4p16, close to translocation breakpoints in
multiple myeloma, and is upregulated in various cancer cell lines.
Genomics. 58:165–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Still IH, Hamilton M, Vince P, Wolfman A
and Cowell JK: Cloning of TACC1, an embryonically expressed,
potentially transforming coiled coil containing gene, from the 8p11
breast cancer amplicon. Oncogene. 18:4032–4038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng S, Douglas-Jones A, Yang X, Mansel
RE and Jiang WG: Transforming acidic coiled-coil-containing protein
2 (TACC2) in human breast cancer, expression pattern and
clinical/prognostic relevance. Cancer Genomics Proteomics. 7:67–73.
2010.PubMed/NCBI
|
|
14
|
Conte N, Delaval B, Ginestier C, Ferrand
A, Isnardon D, Larroque C, Prigent C, Séraphin B, Jacquemier J and
Birnbaum D: TACC1-chTOG-Aurora A protein complex in breast cancer.
Oncogene. 22:8102–8116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Line A, Slucka Z, Stengrevics A, Li G and
Rees RC: Altered splicing pattern of TACC1 mRNA in gastric cancer.
Cancer Genet Cytogenet. 139:78–83. 2002. View Article : Google Scholar
|
|
16
|
Dhanasekaran SM, Barrette TR, Ghosh D,
Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA and Chinnaiyan
AM: Delineation of prognostic biomarkers in prostate cancer.
Nature. 412:822–826. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadek CM, Pelto-Huikko M, Tujague M,
Steffensen KR, Wennerholm M and Gustafsson JA: TACC3 expression is
tightly regulated during early differentiation. Gene Expr Patterns.
3:203–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conte N, Charafe-Jauffret E, Delaval B,
Adélaïde J, Ginestier C, Geneix J, Isnardon D, Jacquemier J and
Birnbaum D: Carcinogenesis and translational controls: TACC1 is
down-regulated in human cancers and associates with mRNA
regulators. Oncogene. 21:5619–5630. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma XJ, Salunga R, Tuggle JT, Gaudet J,
Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, et
al: Gene expression profiles of human breast cancer progression.
Proc Natl Acad Sci USA. 100:5974–5979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lauffart B, Vaughan MM, Eddy R, Chervinsky
D, DiCioccio RA, Black JD and Still IH: Aberrations of TACC1 and
TACC3 are associated with ovarian cancer. BMC Womens Health.
5:8–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peters DG, Kudla DM, Deloia JA, Chu TJ,
Fairfull L, Edwards RP and Ferrell RE: Comparative gene expression
analysis of ovarian carcinoma and normal ovarian epithelium by
serial analysis of gene expression. Cancer Epidemiol Biomarkers
Prev. 14:1717–1723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arlot-Bonnemains Y, Baldini E, Martin B,
Delcros JG, Toller M, Curcio F, Ambesi-Impiombato FS, D'Armiento M
and Ulisse S: Effects of the Aurora kinase inhibitor VX-680 on
anaplastic thyroid cancer-derived cell lines. Endocr Relat Cancer.
15:559–568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duncan CG, Killela PJ, Payne CA, Lampson
B, Chen WC, Liu J, Solomon D, Waldman T, Towers AJ, Gregory SG, et
al: Integrated genomic analyses identify ERRFI1 and TACC3 as
glioblastoma-targeted genes. Oncotarget. 1:265–277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stewart JP, Thompson A, Santra M, Barlogie
B, Lappin TR and Shaughnessy J Jr: Correlation of TACC3, FGFR3,
MMSET and p21 expression with the t(4;14)(p16.3;q32) in multiple
myeloma. Br J Haematol. 126:72–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung CK, Jung JH, Park GS, Lee A, Kang CS
and Lee KY: Expression of transforming acidic coiled-coil
containing protein 3 is a novel independent prognostic marker in
non-small cell lung cancer. Pathol Int. 56:503–509. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM; Statistics Subcommittee of the NCI-EORTC
Working Group on Cancer Diagnostics: Reporting recommendations for
tumor marker prognostic studies (REMARK). J Natl Cancer Inst.
97:1180–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuang BH, Zhang MQ, Xu LH, Hu LJ, Wang HB,
Zhao WF, Du Y and Zhang X: Proline-rich tyrosine kinase 2 and its
phosphorylated form pY881 are novel prognostic markers for
non-small-cell lung cancer progression and patients' overall
survival. Br J Cancer. 109:1252–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gangisetty O, Lauffart B, Sondarva GV,
Chelsea DM and Still IH: The transforming acidic coiled coil
proteins interact with nuclear histone acetyltransferases.
Oncogene. 23:2559–2563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piekorz RP, Hoffmeyer A, Duntsch CD, McKay
C, Nakajima H, Sexl V, Snyder L, Rehg J and Ihle JN: The
centrosomal protein TACC3 is essential for hematopoietic stem cell
function and genetically interfaces with p53-regulated apoptosis.
EMBO J. 21:653–664. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richter JD and Theurkauf WE: Development.
The message is in the translation. Science. 293:60–62. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hood FE and Royle SJ: Pulling it together:
The mitotic function of TACC3. BioArchitecture. 1:105–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ha GH, Park JS and Breuer EK: TACC3
promotes epithelial-mesenchymal transition (EMT) through the
activation of PI3K/Akt and ERK signaling pathways. Cancer Lett.
332:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ha GH, Kim JL and Breuer EK: TACC3 is
essential for EGF-mediated EMT in cervical cancer. PLoS One.
8:e703532013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sadek CM, Jalaguier S, Feeney EP, Aitola
M, Damdimopoulos AE, Pelto-Huikko M and Gustafsson JA: Isolation
and characterization of AINT: A novel ARNT interacting protein
expressed during murine embryonic development. Mech Dev. 97:13–26.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McKeveney PJ, Hodges VM, Mullan RN,
Maxwell P, Simpson D, Thompson A, Winter PC, Lappin TR and Maxwell
AP: Characterization and localization of expression of an
erythropoietin-induced gene, ERIC-1/TACC3, identified in erythroid
precursor cells. Br J Haematol. 112:1016–1024. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
LeRoy PJ, Hunter JJ, Hoar KM, Burke KE,
Shinde V, Ruan J, Bowman D, Galvin K and Ecsedy JA: Localization of
human TACC3 to mitotic spindles is mediated by phosphorylation on
Ser558 by Aurora A: A novel pharmacodynamic method for
measuring Aurora A activity. Cancer Res. 67:5362–5370. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yim EK, Tong SY, Ho EM, Bae JH, Um SJ and
Park JS: Anticancer effects on TACC3 by treatment of paclitaxel in
HPV-18 positive cervical carcinoma cells. Oncol Rep. 21:549–557.
2009.PubMed/NCBI
|
|
38
|
Schneider L, Essmann F, Kletke A, Rio P,
Hanenberg H, Schulze-Osthoff K, Nürnberg B and Piekorz RP: TACC3
depletion sensitizes to paclitaxel-induced cell death and overrides
p21WAF-mediated cell cycle arrest. Oncogene. 27:116–125.
2008. View Article : Google Scholar
|
|
39
|
Schmidt S, Schneider L, Essmann F, Cirstea
IC, Kuck F, Kletke A, Jänicke RU, Wiek C, Hanenberg H, Ahmadian MR,
et al: The centrosomal protein TACC3 controls paclitaxel
sensitivity by modulating a premature senescence program. Oncogene.
29:6184–6192. 2010. View Article : Google Scholar : PubMed/NCBI
|