Introduction
Breast cancer is one of the most common malignant
cancers worldwide, and is the leading cause of cancer-related death
in women (1). Despite great
advances in the treatment of breast cancer in recent years, the
development of drug resistance and relapse is a major hurdle in the
treatment of breast cancer (2).
Recent studies have shown that cancer stem cells (CSCs), a rare
subpopulation of cells with tumorigenic potential, are resistant to
chemotherapy, thereby allowing tumor regrowth (3–5).
Therefore, targeting chemotherapy-resistant breast CSCs will be
essential to prevent breast cancer resistance and relapse.
The Hedgehog (Hh), Notch, and Wnt signaling pathways
are crucial to cell proliferation, apoptosis, and differentiation
during embryonic development, and play an important role in CSC
maintenance and carcinogenesis (6–8).
Recently, the Hh signaling pathway has attracted extensive
attention in the CSC research. Aberrant activation of the Hh
pathway has been found in many tumors, such as gastric carcinoma,
pancreatic cancer, esophageal carcinoma, and small-cell lung cancer
(9–12). In addition, it has been reported
that Hh signaling activation is required for human glioma growth
and survival as well as CSC self-renewal and tumorigenicity
(13). Tanaka et al reported
that the Hh signaling pathway played an essential role in
maintaining the highly tumorigenic populations of breast cancer
cells, including the side population and the
CD44+CD24−/low subpopulation (14). Therefore, targeting Hh signaling
pathway represents a novel and promising therapeutic strategy for
the treatment of breast cancer. Currently, Hh pathway inhibitors
are undergoing preclinical and clinical studies as anticancer
agents (15).
Salinomycin (SAL), a carboxylic polyether ionophore,
has recently been identified as a highly effective inhibitor of
breast CSCs by high-throughput screening (16). Subsequently, SAL has been shown to
selectively kill CSCs in many other cancers including colorectal
cancer, gastric cancer, pancreatic cancer, head and neck squamous
cell carcinoma, and endometrial cancer (17–21).
Nevertheless, the mechanisms underlying selective toxicity of SAL
for CSCs remain poorly understood. It has been reported that SAL
inhibits cancer cell growth and migration by promoting oxidative
stress, and inducing apoptosis and autophagy (22–26).
In addition, Lu et al reported that SAL inhibited the Wnt
signaling pathway and selectively induced cell apoptosis in chronic
lymphocytic leukemia cells (27).
SAL has been reported to selectively inhibit osteosarcoma stem
cells and downregulate Wnt signaling (28). However, it remains unclear whether
the Hh signaling pathway is involved in SAL-induced toxicity for
breast CSCs.
In the present study, we cultured breast cancer
MCF-7 cells in suspension in serum-free medium to obtain
BCSC-enriched MCF-7 mammospheres (MCF-7 MS), and examined the
effect of SAL on proliferation, apoptosis, migration and invasion
of MCF-7 MS cells. More importantly, we investigated the
role/involvement of the Hh signaling pathway in SAL-induced
selective cytotoxicity against MCF-7 MS cells. Our study showed
that the Hh signaling pathway was highly activated in BCSC-enriched
MCF-7 MS. The inhibition of the Hh signaling pathway mediated by
SAL was critical for SAL-induced selective cytotoxicity to breast
CSCs.
Materials and methods
Cell culture and mammosphere
generation
The human breast cancer MCF-7 cell line was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen, USA) containing 10%
fetal bovine serum (Hyclone, USA), 100 U/ml penicillin, and 100
mg/ml streptomycin in a humidified atmosphere with 5%
CO2 at 37°C. Mammospheres were cultured as previously
reported by Ponti et al (29). Briefly, MCF-7 cells
(5×104/ml) were cultured in suspension in serum-free
DMEM-F12 (Gibco, USA), supplemented with 2% B27 (Invitrogen) and 20
ng/ml EGF and 10 ng/ml bFGF (both from Peprotech, USA). Cells were
grown in these conditions as non-adherent spherical clusters of
cells, the MCF-7 mammospheres (MCF-7 MS). MCF-7 MS cells were
enzymatically dissociated every 5–6 days with 0.25% trypsin and
subcultured in DMEM-F12 with growth factors as described above.
Flow cytometric analysis
Flow cytometry was performed to determine the
expression of CD44 and CD24 in MCF-7 and MCF-7 MS cells, apoptosis,
and cell cycle change in the SAL-treated MCF-7 MS cells. For
analysis of CD44 and CD24 expression, cells were suspended at a
density of 1×106 cells/ml in 100 µl PBS and
incubated with fluorescence isothiocyanate (FITC)-conjugated
antibodies against CD44 (1:20) and phycoerythrin (PE)-conjugated
antibodies against CD24 (1:10) (both from BD Pharmingen, USA) for
30 min at 4°C in the dark. The cells were washed in PBS and
centrifuged at 800 × g for 5 min. Single-cell suspensions were
analyzed by flow cytometry using FACSCalibur
(Becton-Dickinson).
MCF-7 MS cells were treated with 30 and 60 nM SAL
for 48 h. DMSO was used as a negative control. Cells were the
harvested by centrifugation, and washed twice with cold PBS. For
apoptosis analysis, cells were resuspended in 250 µl Annexin
V binding buffer at a density of 1×106 cells/ml. The
suspension (100 µl) was incubated in the dark at room
temperature for 15 min with a solution of Annexin V-FITC (2.5
µg/ml) and PI (5 µg/ml). Cells were analyzed for
apoptosis by flow cytometer. For cell cycle analysis, cells were
fixed with 70% ethanol and stored at 4°C overnight. Cells were then
rehydrated with PBS for 10 min, and stained with propidium iodide
(PI, 50 µg/ml) for 15 min at 37°C in PBS containing 2
µg/ml RNase A and 0.2% NP-40. Cell cycle analysis was
performed by flow cytometry.
Soft agar colony formation assay
MCF-7 and MCF-7 MS cells (103 cells/ml)
were suspended in 0.6% agar with culture medium (1:1), and layered
on preformed 1.2% agar with culture medium (1:1) base layer.
Culture medium was added on the top agar layer every 3–4 days.
After incubation for 3 weeks at 37°C, the colonies/well was counted
from 8 different random fields under an inverted microscope (Nikon
TE2000-U; Nikon Japan).
Cell Counting Kit-8 (CCK-8/WST-8)
assay
Cell viability was measured by a Cell Counting Kit-8
(CCK-8; Dojindo, Japan). MCF-7 or MCF-7 MS cells (8,000 cells/well)
were seeded into 96-well ultra-low adherent plates (Corning,
Lowell, MA, USA), and allowed to grow in the growth medium for 24
h. To determine the IC50 value of SAL, cells were
treated with various concentrations of SAL (10, 30, 100, 300,
1,000, 3,000 and 10,000 nM; Sigma, USA) for 48 h. To investigate
the effect of Shh on SAL-induced inhibition on MCF-7 MS
proliferation, MCF-7 MS cells were treated with SAL (60 nM), Shh (3
µg/ml; R&D Systems, Minneapolis, MN, USA), SAL (60 nM) +
Shh (3 µg/ml), or vehicle control (DMSO) for 48 h. Cells in
each well were then incubated with WST-8
(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium)
for 4 h. Plates were read at 450 nm wavelength in an Anthos 2010
microplate reader (Anthos Labtec Instruments GmbH, Austria).
Transwell migration and invasion
assays
Transwell migration and invasion assays were
conducted as described by Fan et al (30). Briefly, the upper chambers were
plated with 4×104 MCF-7 cells in 0.5 ml serum-free DMEM
medium or 4×104 MCF-7 MS cells in 0.5 ml serum-free
DMEM/F12 medium. The lower chambers were filled with 0.5 ml cell
culture medium containing 10% FBS. To test the effect of SAL on
migration and invasion of MCF-7 MS cells, MCF-7 MS cells were
pretreated with 30 and 60 nM SAL, or vehicle control (DMSO) for 48
h. Cells were allowed to migrate toward the lower chamber for 24 h
at 37°C. The number of cells migrating through the membrane was
counted under a light microscope (×200 magnification, five random
fields per well), and were analyzed using ImageJ software.
Total and nuclear proteins extraction and
western blot analysis
Cells were harvested and total proteins were
extracted was carried out as previously described (31), and nuclear proteins were extracted
according to the manufacturer's protocol from nuclear protein
extraction kit (Pierce Biotechnology, Rockford, IL, USA). Proteins
were resolved by SDS-PAGE, and transferred onto polyvinylidene
fluoride membranes by electroblotting. Membranes were blocked with
5% milk in Tris-buffered saline with 0.1% Tween-20, and then
incubated with primary antibodies against OCT4 (1:1,000; Cell
Signaling Technology), Gli1 (1:500), Gli2 (1:800), PTCH (1:1,000)
and SMO (1:1,000) (all from Abcam), C-myc (1:1,000) and Bcl-2
(1:1,000) (both from Cell Signaling Technology), cyclin D1
(1:1,000; Beyotime Biotechnology), Snail (1:300; Abcam), GAPDH
(1:6,000; Santa Cruz Biotechnology) and histone H3 (1:1,000;
Beyotime Biotechnology) overnight at 4°C. Membranes were then
incubated with horseradish peroxidase-conjugated goat anti-rabbit
or goat anti-mouse secondary antibodies (dilution 1:5,000; Abcam)
at room temperature for 1 h. Bands were visualized using an
enhanced chemiluminescence detection system (Amersham, Freiburg,
Germany). The results were quantitatively analyzed using Scion
Image software (Scion Corporation, Frederick, MA, USA).
Mammosphere formation assay
Single-cell suspensions of MCF-7 MS cells were
thoroughly suspended and plated in 6-well ultra-low adherent plates
(Corning) at 1×105 cells/well in 4 ml of sphere
formation medium. After 24 h, cells were treated with SAL, Shh, or
DMSO as a control for 48 h. Cells were then collected, digested
into single cells and plated in 6-well ultra-low adherent plates
with 2,000 cells/well in mammosphere formation medium (2 ml). Fresh
medium (1 ml) was added into the plates every 3–4 days. After
culture for 8 days, the number of the mammospheres/2,000 cells was
counted for the primary mammosphere formation assay under an
inverted microscope (Nikon TE2000-U; Nikon). The above mammospheres
in each group were collected, digested into single cells and plated
in 6-well ultra-low adherent plates with 2,000 cells/well in
mammosphere formation medium (2 ml) for the secondary mammosphere
formation assay.
Immunofluorescence
MCF-7 MS cells were treated with 30 and 60 nM SAL,
or DMSO as a control for 48 h. After the treatment, cells were
collected and rinsed in PBS before incubation in 4%
paraformaldehyde for 30 min and embedded in paraffin wax. Sections
(4 µm) were cut and subjected to immunofluorescence
staining. Cells were permeabilized with 0.5% Triton X-100 (Sigma)
for 10 min, rinse in PBS, and blocked with normal goat serum for 1
h at room temperature. The sections were incubated overnight at 4°C
with primary antibodies against PTCH (1:20), SMO (1:100), Gli1
(1:100) and Gli2 (1:100) (all from Abcam). After primary antibody
was removed by washing in PBS, immunoreactivity was detected by
incubation with FITC-conjugated secondary antibodies (1:300;
Invitrogen) for 1 h at room temperature. Nuclei were counterstained
using DAPI for 15 min. Fluorescence was detected using a Nikon
Eclipse 80i microscope (Japan).
In vivo xenograft experiments
For the study of the tumorigenic ability of MCF-7
and MCF-7 MS cells, equal numbers of MCF-7 cells or MCF-7 MS cells
(2×103, 2×104, 2×105 and
2×106 cells) were suspended in PBS and Matrigel (1:1; BD
Biosciences), and subcutaneously inoculated into the flank of
female BALB/c athymic nude mice (n=6 mice per group). The presence
or absence of a visible or palpable tumor was evaluated 6 weeks
after the initial injection of the cells. Mice (n=5 mice per group)
inoculated with 2×106 MCF-7 or MCF-7 MS cells were
sacrificed 6 weeks after the initial injection of the cells, and
tumors were weighed and harvested for subsequent western blot
analysis. All mice were bred in pathogen-free conditions at the
Animal Center of China Medical University. All animal studies were
carried out in accordance with the National Institute of Health
Guide for the Care and Use of Laboratory Animals.
Statistical analysis
Statistical analyses were performed using the SPSS
statistics 16.0 software package. Data are presented as mean ±
standard deviation (SD). Student's t-test was used to compare
differences between two groups. One-way analysis of variance
(ANOVA) was used to compare differences among more than two groups.
Statistical significance was considered at P<0.05.
Results
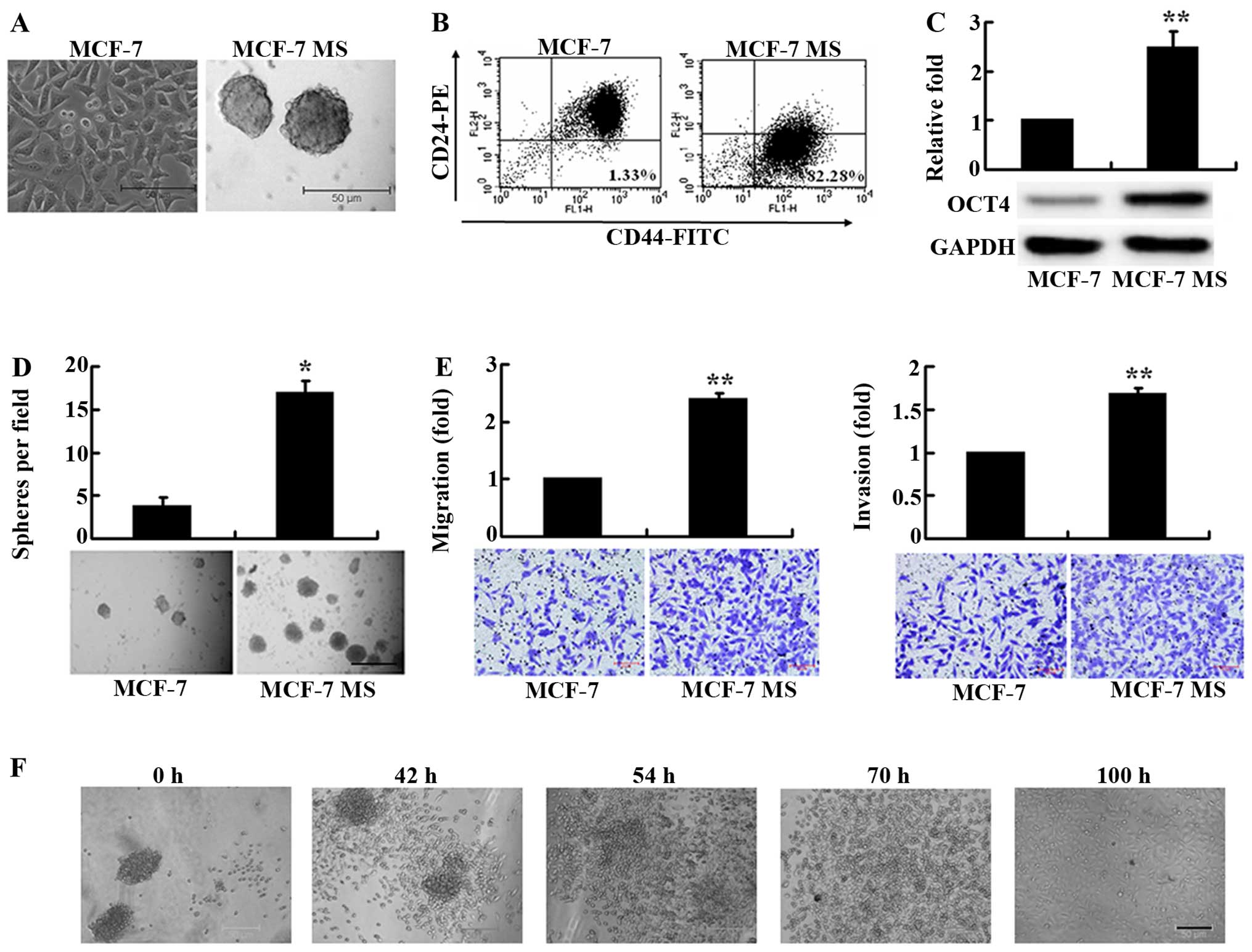
MCF-7 MS cells possess breast CSC-like
properties
MCF-7 cells cultured in suspension in serum-free
DMEM/F12 medium supplemented with growth factors formed tight
sphere-like mammospheres after 7–8 passages (Fig. 1A). It has been shown that breast
CSCs have a CD44+CD24− phenotype (32), so that we examined the presence of
CD44 and CD24 in MCF-7 MS cells using flow cytometry. We found that
the proportion of CD44+CD24− cells in MCF-7
MS cells was as high as 81.4±11.7%, >30-fold greater than that
(2.53±1.28%) in the parental MCF-7 cells (Fig. 1B), indicating that MCF-7 MS cells
expressed breast CSC-specific markers.
We next assessed the expression of the stem cell
marker OCT4 in MCF-7 MS cells. Western blot analysis showed that
the expression of OCT4 was significantly higher in MCF-7 MS cells
compared with MCF-7 cells (Fig.
1C). Furthermore, we measured the colony-forming ability of
MCF-7 MS cells, using soft agar colony formation assay. The MCF-7
MS cells formed significantly more colonies than MCF-7 cells
(Fig. 1D). These data suggested
that MCF-7 MS cells exhibited breast CSC-like self-renewal
capacity.
We further investigated the migration and invasion
capacity of MCF-7 MS cells, using Transwell migration and invasion
assays. The number of MCF-7 MS cells that migrated and invaded into
the lower Transwell chamber was significantly greater than that of
MCF-7 cells (Fig. 1E), suggesting
that MCF-7 MS cells exhibited increased migration and invasion.
We also examined the re-differentiation potential of
the MCF-7 MS cells by culturing MCF-7 MS in DMEM culture medium
with 10% FBS. After culture for 42 h, some spherical MCF-7 MS cells
began to grow adherently, and exhibited differentiation properties.
After culture for 100 h, MCF-7 MS cells completely lost the
spheroid characteristics, grew adherently, and exhibited morphology
similar to MCF-7 cells (Fig. 1F).
The finding that MCF-7 MS cells could re-differentiate into MCF-7
cells under serum-rich conditions suggested that MCF-7 MS cells
have the CSC-like differentiation potential.
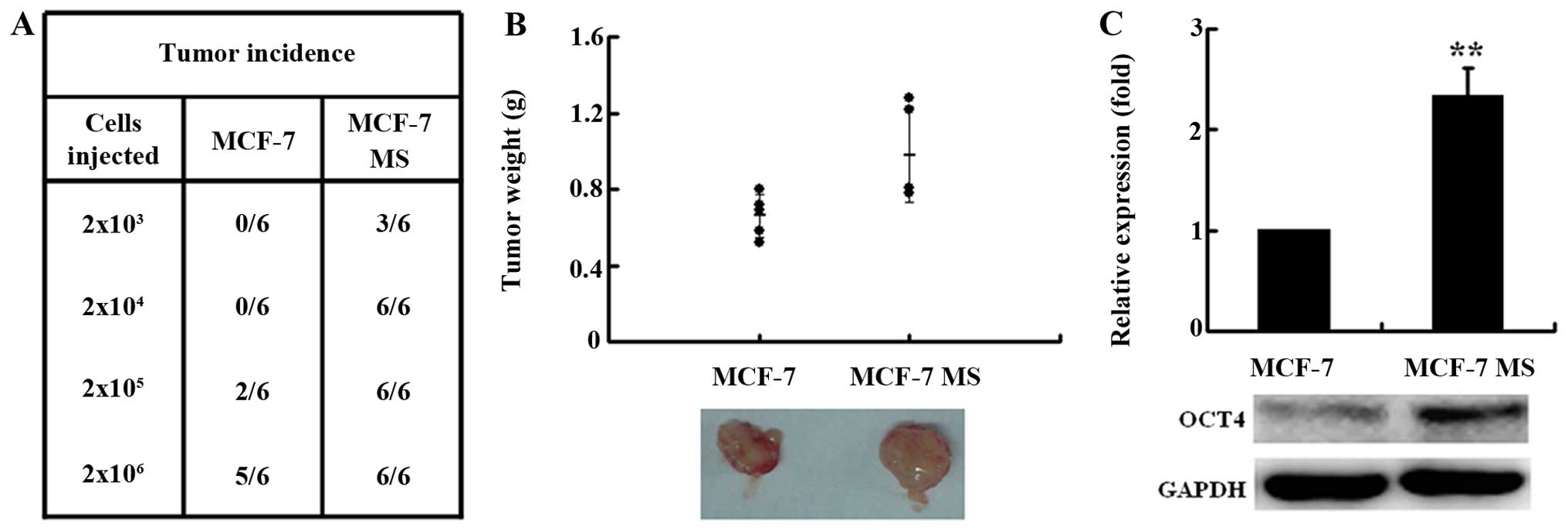
To further investigate the in vivo
tumorigenic ability of MCF-7 MS, we subcutaneously inoculated MCF-7
MS cells or MCF-7 cells into the flank of nude mice. MCF-7 MS cells
formed tumors in mice administered 2×103 cells, whereas
2×105 parental MCF-7 cells were required to generate
tumors (Fig. 2A). With a given
number of xenografted cells, MCF-7 MS cells generated tumors at a
higher frequency in mouse xenografts than MCF-7 cells (Fig. 2A). Six weeks after inoculation of
2×106 cells, the average weight of MCF-7 MS cell-induced
tumors (0.98±0.25 g) was significantly higher than that of MCF-7
cell-induced tumors (0.66±0.11 g) (Fig.
2B). The expression of OCT4 was also significantly higher in
tumors transplanted with MCF-7 MS cells than in those transplanted
with MCF-7 cells. These results showed that MCF-7 MS cells had
stronger tumorigenicity.
Taken together, our data showed that MCF-7 MS cells
obtained from serum-free suspension culture possessed breast
CSC-like properties such as self-renewal, differentiation
potential, strong migration and invasion capacities, and high
tumorigenicity.
Salinomycin inhibits proliferation,
induces apoptosis, and reduces migration and invasion of MCF-7 MS
cells
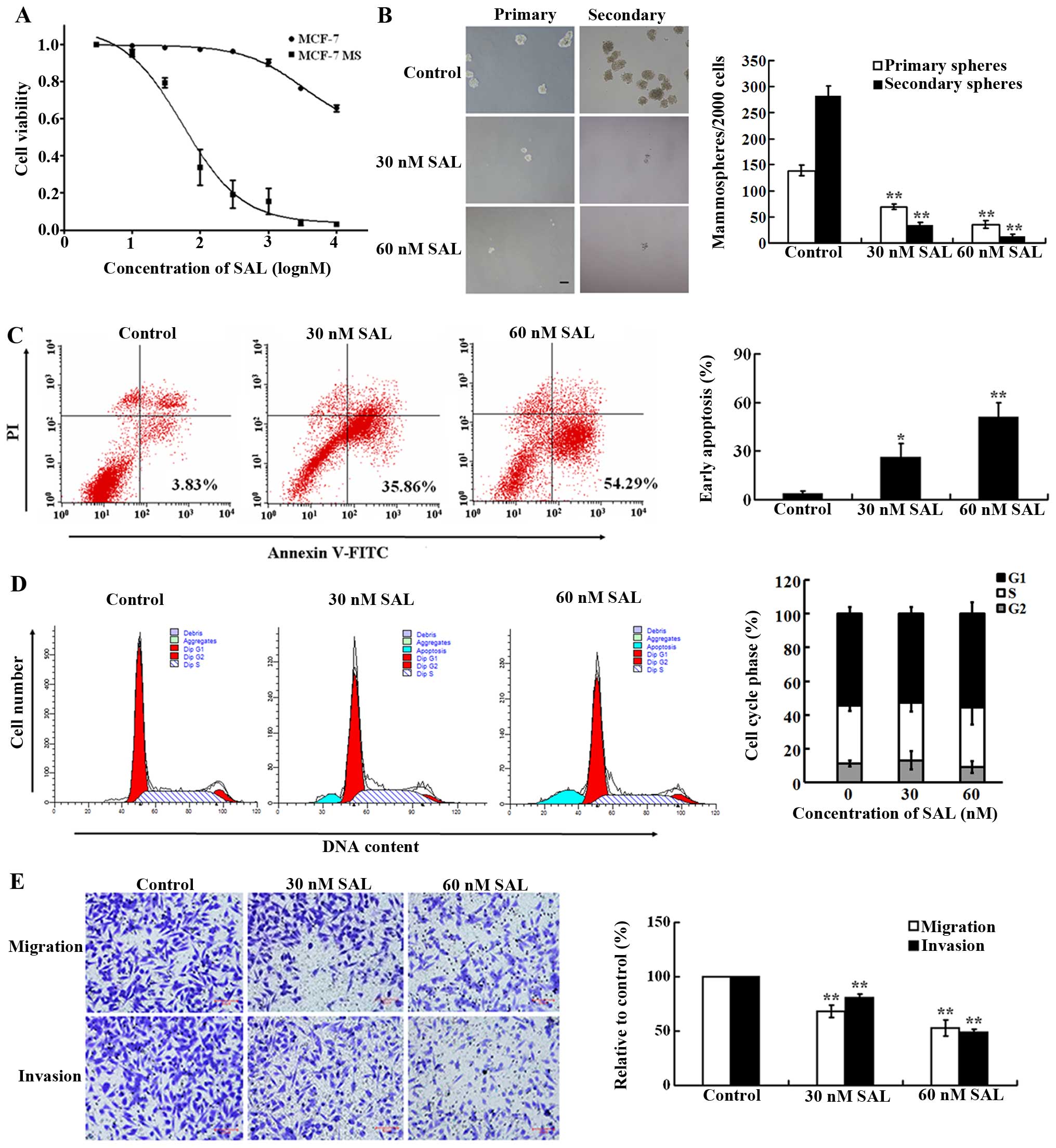
It is known that SAL can selectively kill BCSCs
(16). To investigate whether SAL
selectively killed CSC-like MCF-7 MS cells obtained from serum-free
suspension culture, we tested the sensitivity of MCF-7 and MCF-7 MS
cells to SAL. Cells were treated with various concentrations of SAL
(10–10,000 nM) for 48 h, and cell viability was examined using
CCK-8 assay. The survival rates of both cells decreased in a
dose-dependent manner. The IC50 value for SAL in MCF-7
MS cells was 99 nM, which was ~82-fold lower than that in MCF-7 MS
cells (8,113 nM) (Fig. 3A),
suggesting that SAL selectively killed MCF-7 MS cells. Furthermore,
we examined the effect of SAL on mammosphere formation of MCF-7 MS
cells. SAL (30 and 60 nM) significantly inhibited the primary and
secondary mammosphere formation (Fig.
3B), further suggesting that SAL inhibited proliferation of
MCF-7 MS cells.
We next examined the effect of SAL on the apoptosis
of MCF-7 MS cells, using flow cytometry. SAL (30 and 60 nM)
treatment for 48 h significantly increased the percentage of early
apoptotic MCF-7 MS cells compared with the vehicle control
(Fig. 3C). SAL treatment increased
apoptosis of MCF-7 MS cells in a dose-dependent manner. However,
compared with vehicle controls, SAL (30 and 60 nM) treatment for 48
h did not result in a significant change in the proportions of
MCF-7 MS cells in G1, S, and G2 phases of the cell cycle (Fig. 3D).
We then investigated the effects of SAL on migration
and invasion of MCF-7 MS cells using Transwell migration and
invasion assays. Compared with vehicle controls, SAL (30 and 60 nM)
treatment for 48 h resulted in a significantly lower number of
MCF-7 MS cells that migrated into the lower chambers (Fig. 3E). SAL-induced inhibition of cell
migration and invasion was dose-dependent.
The Hh signaling pathway is highly
activated in MCF-7 MS cells and its activation can be effectively
inhibited by salinomycin
The Hh signaling pathway regulates cell
proliferation, apoptosis, and differentiation during normal
development, and plays an important role in CSC maintenance and
carcinogenesis (6,8). Thus, we presumed that the Hh signaling
pathway may be involved in SAL-induced cytotoxicity toward MCF-7 MS
cells. We examined the protein expression of the main components of
the Hh signaling pathway in MCF-7 and MCF-7 MS cells, including the
Patched (PTCH) receptor, Smoothened (SMO), Gli1, and Gli2. Western
blot analysis showed that the expression levels of PTCH, SMO, Gli1,
and Gli2 were significantly higher in MCF-7 MS cells than in MCF-7
cells (Fig. 4A), suggesting that
the Hh signaling pathway was highly activated in MCF-7 MS cells. As
expected, SAL (30 and 60 nM) effectively inhibited the expression
of PTCH, SMO, Gli1, and Gli2 in MCF-7 MS cells dose-dependenly
(Fig. 4B). Consistently with
western blot results, immunofluorescence results showed that the
expression of PTCH, SMO, Gli1, and Gli2 was substantially decreased
in MCF-7 MS cells after the treatment of SAL (Fig. 4C). In addition, we examined the
nuclear expression of Gli1, which more reliably reflects Hh
signaling activation. The nuclear expression of Gli1 was
significantly inhibited by SAL treatment (Fig. 4D).
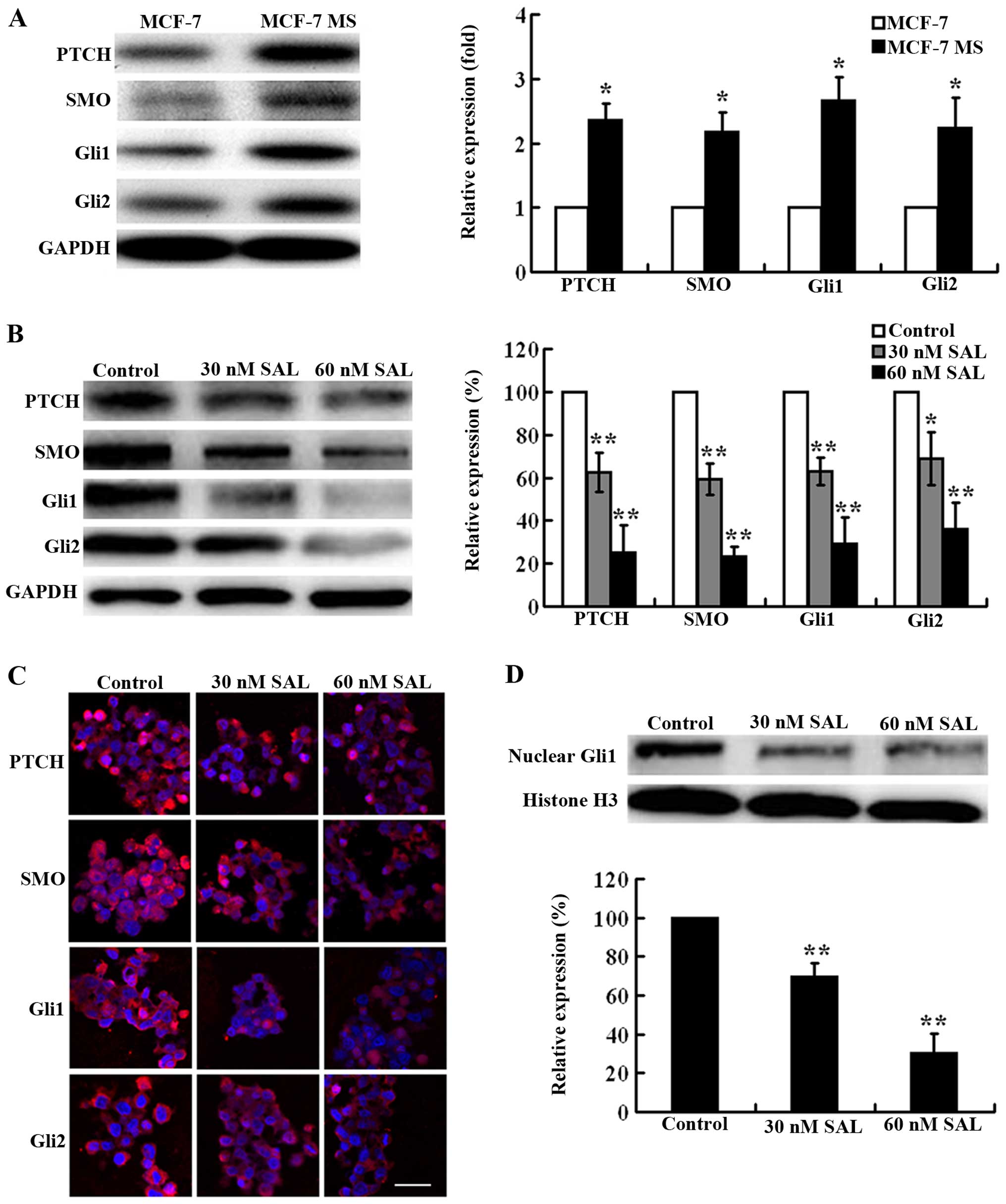 | Figure 4Salinomycin (SAL) inhibits Hh
signaling activation in MCF-7 MS cells. (A) Western blot analysis
showing the expression of PTCH, SMO, Gli1, and Gli2 in MCF-7 and
MCF-7 MS cells. GAPDH is a loading control. The expression level of
PTCH, SMO, Gli1, and Gli2 was normalized to that of GAPDH. The
expression levels of these proteins in MCF-7 cells were set as 1.
(B) Western blot analysis showing the expression of PTCH, SMO,
Gli1, and Gli2 in MCF-7 MS cells treated with 30 and 60 nM SAL or
DMSO as a control for 48 h. GAPDH was a loading control. The
expression level of PTCH, SMO, Gli1, and Gli2 was normalized to
that of GAPDH l. The expression levels of these proteins in control
cells were set as 100%. (C) Representative immunofluorescence
images showing the expression of PTCH, SMO, Gli1 and Gli2 in MCF-7
MS cells treated with 30 and 60 nM SAL or DMSO as a control for 48
h (scale bar, 10 µm). (D) Western blot analysis showing the
nuclear expression of Gli1 in MCF-7 MS cells treated with 30 and 60
nM SAL or DMSO as a control for 48 h. Histone H3 is a loading
control. The expression level of Gli1 was normalized to that of
histone H3. The expression levels of Gli1 in control cells were set
as 100%. Data are presented as mean ± SD from there independent
experiments. *P<0.05, **P<0.01 vs.
control. |
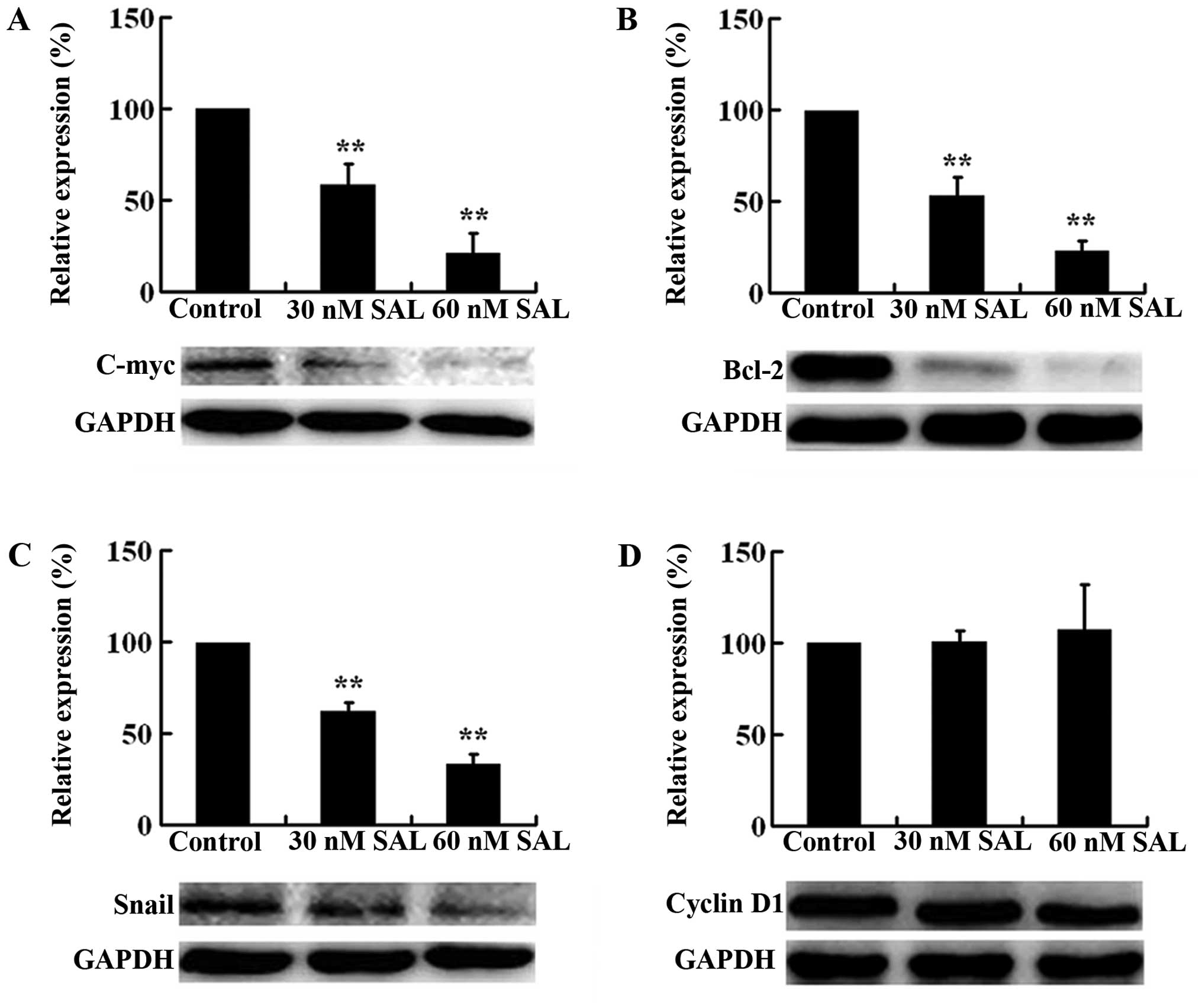
Oncogene C-myc, anti-apoptotic gene Bcl-2, cell
cycle regulator cyclin D1, and transcription factor Snail are
important downstream target genes of the Hh/Gli signaling pathway
(33–36). To further demonstrate SAL-induced
inhibition on Hh signaling activation, we investigated the effect
of SAL on the protein expression of C-myc, Bcl-2, cyclin D1, and
Snail. Western blot analysis showed that SAL (30 and 60 nM)
significantly reduced the expression of C-myc, Bcl-2, and Snail,
but not cyclin D1 (Fig. 5). The
inhibitory effect of SAL on the expression of C-myc, Bcl-2, and
Snail was dose-dependent. These findings suggested that salinomycin
could effectively inhibit the activation of Hh signaling pathway in
MCF-7 MS cells.
Shh-mediated Hh signaling activation
largely reverses SAL-induced cytotoxicity toward MCF-7 MS
cells
To determine whether SAL-induced inhibition of the
Hh signaling pathway is required for its selective cytotoxicity
against MCF-7 MS cells, we conducted a series of rescue assays. Shh
is a ligand that can activate the Hh signaling pathway (37), and therefore we used it for the
rescue assays. As shown in Fig. 6A,
Shh (3 µg/ml) significantly increased the expression of
PTCH, SMO, Gli1, and Gli2, indicating that Shh could activate the
Hh signaling pathway in MCF-7 MS cells. As expected, Shh treatment
could largely reverse SAL-induced inhibition on the expression of
PTCH, SMO, Gli1, Gli2 (Fig. 6A) and
downstream target genes, C-myc, Bcl-2, and Snail (Fig. 6B) in MCF-7 MS cells, suggesting that
Shh prevented SAL-induced inhibition on Hh signaling
activation.
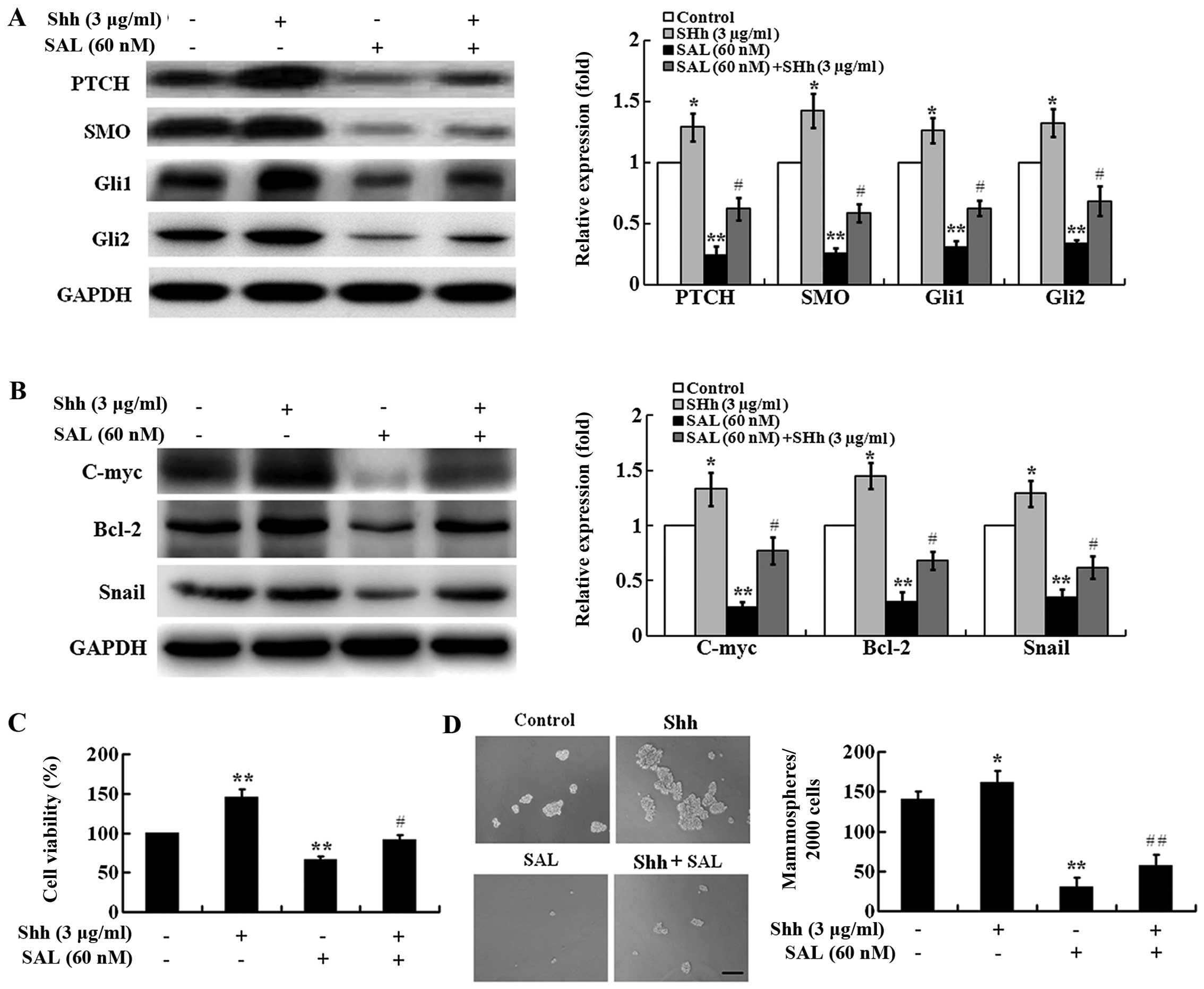 | Figure 6Hh signaling activation decreases
SAL-induced cytotoxicity in MCF-7 MS cells. (A) Western blot
analysis showing the expression of PTCH, SMO, Gli1, and Gli2 in
MCF-7 MS cells treated with SAL (60 nM), Shh (3 µg/ml), SAL
(60 nM) + Shh (3 µg/ml), or DMSO as a control for 48 h.
GAPDH is a loading control. The expression level of PTCH, SMO,
Gli1, and Gli2 was normalized to that of GAPDH. The expression
levels of these proteins in control cells were set as 1. (B)
Western blot analysis showing the expression of C-myc, Bcl-2, and
Snail in MCF-7 MS cells treated with SAL (60 nM), Shh (3
µg/ml), SAL (60 nM) + Shh (3 µg/ml), or DMSO as a
control for 48 h. GAPDH is a loading control. The expression level
of C-myc, Bcl-2, and Snail was normalized to that of GAPDH. The
expression levels of these proteins in control cells were set as 1.
(C) Cell viability of MCF-7 MS cells after treatment with SAL (60
nM), Shh (3 µg/ml), SAL (60 nM) + Shh (3 µg/ml), or
DMSO as a control for 48 h was measured by CCK-8 assay. (D)
Mammosphere formation assay of MCF-7 MS cells after treatment with
SAL (60 nM), Shh (3 µg/ml), SAL (60 nM) + Shh (3
µg/ml), or DMSO as a control for 48 h (scale bar, 100
µm). Data are presented as mean ± SD from there independent
experiments. *P<0.05, **P<0.01 vs.
control. #P<0.05, ##P<0.01 vs. SAL
alone. |
We then investigated the effect of Shh on
SAL-induced cytotoxicity in MCF-7 MS cells. Shh (3 µg/ml)
significantly promoted cell viability of MCF-7 MS cells compared
with the vehicle control (Fig. 6C).
Moreover, Shh treatment could largely reverse SAL-induced decrease
in cell viability of MCF-7 MS cells (Fig. 6C). In addition, we found that Shh (3
µg/ml) significantly promoted mammosphere formation and Shh
treatment could largely reverse SAL-induced inhibition on
mammosphere formation (Fig. 6D).
These results suggest that the Hh signaling pathway is critical for
SAL-induced selective cytotoxicity against BCSC-enriched MCF-7 MS
cells.
Discussion
Recent studies proposed that CSCs are responsible
for tumor chemoresistance, recurrence, and metastasis (3,5,38). A
subpopulation of breast cancer with the expression of the surface
marker CD44+CD24−/low has been shown to
display stem cell-like properties with tumorigenic potential
(32). However, CSCs are rare,
making them very difficult to isolate and study. Ponti et al
have reported that CD44+CD24−/low cells with
stem cell-like properties are enriched in mammospheres obtained
from culturing of breast cancer samples and breast cancer MCF-7
cells in suspension in serum-free medium (29). In the present study, we applied a
similar procedure for culturing MCF-7 cells and obtained
CD44+CD24−/low cell-enriched mammospheres. In
addition, MCF-7 MS cells are featured with high expression of the
stem cell marker OCT4, increased colony-forming ability, strong
migration and invasion capabilities, re-differentiation potential,
and strong tumorigenicity in vivo. These properties are
typical characteristics of breast CSCs (29,39,40).
Salinomycin (SAL) has been identified as a selective
inhibitor of breast CSCs (16), and
its selective inhibition on CSCs has also been observed in other
cancers including colorectal cancer, gastric cancer, pancreatic
cancer, head and neck squamous cell carcinoma (17–21).
Here we showed that SAL exerted selective cytotoxicity to MCF-7 MS
cells with an IC50 value of 99 nM, which was ~82-fold
lower compared with parental MCF-7 cells, suggesting that SAL
selectively killed MCF-7 MS cells. In addition, Dong et al
found that SAL selectively targeted CD133+ cell
subpopulations and reduced cell migration in colorectal cancer
cells (17). In the present study,
we found that SAL reduced migration and invasion of MCF-7 MS cells.
These studies suggest that SAL may prevent cancer metastasis.
Additionally, SAL selectively induces cell apoptosis in chronic
lymphocytic leukemia cells (27).
Similarly, we found that SAL induced apoptosis in MCF-7 MS
cells.
The mechanisms underlying SAL-induced cytotoxicity
to CSCs remain unclear. Lu et al reported that SAL inhibited
the Wnt signaling pathway in chronic lymphocytic leukemia cells
(27). In addition, SAL has been
found to inhibit CSCs in osteosarcoma and endometrial cancer and
downregulate Wnt signaling (21,28).
It is well known that similar to the Wnt signaling pathway, the Hh
signaling pathway plays an important role in maintaining
self-renewal of stem cells (37,41).
The Hh signaling pathway is activated by binding of ligands to the
PTCH receptor and subsequently alleviating inhibition of SMO, thus
regulating the expression of Gli transcription factors (33–36).
It has been reported that the expression of PTCH, SMO, Gli1 and
Gli2 are upregulated in breast CSCs (37). In the present study, we found that
the expression of PTCH, SMO, Gli1, and Gli2 was significantly
higher in MCF-7 MS cells, T47D MS cells and MCF-7 MS xenograft
tumors, suggesting that the Hh signaling pathway is activated in
breast CSCs. In addition, we found that SAL inhibited Hh signaling
activation, and Hh signaling activation reduced SAL-induced
cytotoxicity in MCF-7 MS cells, suggesting that the Hh signaling
pathway is involved in SAL-induced cytotoxicity to breast CSCs.
Tanaka et al reported that inhibition of the Hh signaling
pathway decreased proliferation of
CD44+CD24−/low breast cancer cells (14), suggesting that the Hh signaling
pathway plays an important role in maintaining proliferation of
breast CSCs. In agreement with their findings, we found that SAL
inhibited Hh signaling activation, and decreased
CD44+CD24−/low cell-enriched mammosphere
formation, suggesting that SAL reduces proliferation of breast CSCs
via inhibition of the Hh signaling pathway. Recently, Lu et
al reported that salinomycin exerted anticancer effects on
MCF-7 cells via modulation of Hedgehog signaling (42). However, their study focused on the
anticancer effects of salinomycin on MCF-7 cells, not breast cancer
stem cells. While in the present study we demonstrated that
salinomycin selectively induced cytotoxicity to BCSC-enriched MCF-7
mammosphere cells through targeting the Hedgehog signaling
pathway.
Hh/Gli signaling activation results in an increase
in the expression of many downstream target genes including C-myc,
Bcl-2, cyclin D1, and Snail, which regulate cell proliferation,
apoptosis, cell cycle, migration, and epithelial-mesenchymal
transition (EMT) (33–36). It has been reported that C-myc is
required for proliferation and self-renewal of normal stem cell and
CSCs (43,44). Our findings that SAL significantly
inhibited cell proliferation and reduced the expression of C-myc in
MCF-7 MS cells suggest that SAL may inhibit breast CSC
proliferation via the downregulation of C-myc. In addition, we also
found that SAL induced cell apoptosis and downregulated the
expression of anti-apoptotic Bcl-2 proteins in MCF-7 MS cells,
suggesting that SAL may induce breast CSC apoptosis via the
downregulation of Bcl-2. Consistently with our findings, Fu et
al found that inhibition of Bcl-2 expression promoted
pancreatic CSC apoptosis (45).
Furthermore, we found that SAL inhibited cell migration and
invasion in MCF-7 MS cells and reduced the expression of Snail, a
transcription factor that regulates EMT (46,47).
It has been reported that blockade of Hh signaling downregulates
the expression of Snail, and inhibits pancreatic cancer invasion
and metastases (48). Therefore,
SAL may inhibit breast CSC migration and invasion by inhibiting the
expression of Snail. Taken together, these results suggests that
SAL may produce cytotoxicity to MCF-7 MS cells via repressing the
Hh/Gli signaling pathway by inhibiting C-myc expression to reduce
cell proliferation, inhibiting Bcl-2 expression to promote cell
apoptosis, and inhibiting Snail expression to reduce cell migration
and invasion.
Cell cycle regulator cyclin D1 is one of the
downstream target genes of the Hh signaling pathway (35). However, in the present study,
although SAL inhibited Hh signaling activation in MCF-7 MS cells,
SAL did not alter the expression of cell cycle regulator cyclin D1,
and did not cause cell cycle arrest measured by flow cytometry.
Similarly, SAL-induced apoptosis is not accompanied by cell cycle
arrest in human Molt-4 leukemia cells (49). In contrast, it has been reported
that SAL downregulates the expression of cyclin D1 in ovarian
cancer and endometrial cancer cells, and induces apoptosis via cell
cycle arrest at G1 in ovarian cancer cells (50). The effect of SAL on cell cycle
regulation seems to be cell-context dependent.
In summary, we found that SAL exerted cytotoxicity
to MCF-7 MS cells by inhibiting proliferation, inducing apoptosis,
and reducing migration and invasion, but not affecting the cell
cycle. SAL-induced cytotoxicity was associated with inhibition of
Hh signaling activation and the expression of downstream target
genes including C-myc, Bcl-2 and Snail, but not cyclin D1.
Therefore, our studies not only revealed a novel molecular
mechanism underlying SAL-induced selective cytotoxicity to BCSCs,
but also suggest that the Hh signaling pathway likely plays an
important role in the maintenance of CSC properties of breast
cancer cells, and this pathway is a possible drug target for the
treatment of breast cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81373427
and 31300693), Program for Liaoning Innovative Research Team in
University (grant no. LT2014016), Program for Liaoning Excellent
Talents in University (grant no. LJQ2014084), the Natural Science
Foundation of Liaoning Province (grant no. 2014021085) and the
S&T Projects in Shenyang, China (grant no. F14-232-6-05).
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marquette C and Nabell L:
Chemotherapy-resistant metastatic breast cancer. Curr Treat Options
Oncol. 13:263–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou J, Zhang H, Gu P, Bai J, Margolick JB
and Zhang Y: NF-kappaB pathway inhibitors preferentially inhibit
breast cancer stem-like cells. Breast Cancer Res Treat.
111:419–427. 2008. View Article : Google Scholar
|
|
4
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sampieri K and Fodde R: Cancer stem cells
and metastasis. Semin Cancer Biol. 22:187–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varjosalo M and Taipale J: Hedgehog:
Functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dontu G, Jackson KW, McNicholas E,
Kawamura MJ, Abdallah WM and Wicha MS: Role of Notch signaling in
cell-fate determination of human mammary stem/progenitor cells.
Breast Cancer Res. 6:R605–R615. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taipale J and Beachy PA: The Hedgehog and
Wnt signalling pathways in cancer. Nature. 411:349–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma X, Sheng T, Zhang Y, Zhang X, He J,
Huang S, Chen K, Sultz J, Adegboyega PA, Zhang H, et al: Hedgehog
signaling is activated in subsets of esophageal cancers. Int J
Cancer. 118:139–148. 2006. View Article : Google Scholar
|
|
11
|
Fukaya M, Isohata N, Ohta H, Aoyagi K,
Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto
H, et al: Hedgehog signal activation in gastric pit cell and in
diffuse-type gastric cancer. Gastroenterology. 131:14–29. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kayed H, Kleeff J, Keleg S, Guo J,
Ketterer K, Berberat PO, Giese N, Esposito I, Giese T, Büchler MW,
et al: Indian hedgehog signaling pathway: Expression and regulation
in pancreatic cancer. Int J Cancer. 110:668–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka H, Nakamura M, Kameda C, Kubo M,
Sato N, Kuroki S, Tanaka M and Katano M: The Hedgehog signaling
pathway plays an essential role in maintaining the
CD44+CD24−/low subpopulation and the side
population of breast cancer cells. Anticancer Res. 29:2147–2157.
2009.PubMed/NCBI
|
|
15
|
Sheikh A, Alvi AA, Aslam HM and Haseeb A:
Hedgehog pathway inhibitors - current status and future prospects.
Infect Agent Cancer. 7:292012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong TT, Zhou HM, Wang LL, Feng B, Lv B
and Zheng MH: Salinomycin selectively targets 'CD133+'
cell subpopulations and decreases malignant traits in colorectal
cancer lines. Ann Surg Oncol. 18:1797–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhi QM, Chen XH, Ji J, Zhang JN, Li JF,
Cai Q, Liu BY, Gu QL, Zhu ZG and Yu YY: Salinomycin can effectively
kill ALDH(high) stem-like cells on gastric cancer. Biomed
Pharmacother. 65:509–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen
G, Zhang TP, Kang T and Zhao YP: Combination of salinomycin and
gemci-tabine eliminates pancreatic cancer cells. Cancer Lett.
313:137–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo SZ, Blair KJ, Rahimy E, Kiang A,
Abhold E, Fan JB, Wang-Rodriguez J, Altuna X and Ongkeko WM:
Salinomycin induces cell death and differentiation in head and neck
squamous cell carcinoma stem cells despite activation of
epithelial-mesenchymal transition and Akt. BMC Cancer. 12:5562012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kusunoki S, Kato K, Tabu K, Inagaki T,
Okabe H, Kaneda H, Suga S, Terao Y, Taga T and Takeda S: The
inhibitory effect of salinomycin on the proliferation, migration
and invasion of human endometrial cancer stem-like cells. Gynecol
Oncol. 129:598–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim KY, Yu SN, Lee SY, Chun SS, Choi YL,
Park YM, Song CS, Chatterjee B and Ahn SC: Salinomycin-induced
apoptosis of human prostate cancer cells due to accumulated
reactive oxygen species and mitochondrial membrane depolarization.
Biochem Biophys Res Commun. 413:80–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lieke T, Ramackers W, Bergmann S,
Klempnauer J, Winkler M and Klose J: Impact of salinomycin on human
cholangiocarcinoma: Induction of apoptosis and impairment of tumor
cell proliferation in vitro. BMC Cancer. 12:4662012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL,
Wu SM, Cheng P, Zhang Y, Shen M, et al: Salinomycin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells in vitro and in vivo. PLoS One. 7:e506382012.
View Article : Google Scholar
|
|
26
|
Verdoodt B, Vogt M, Schmitz I, Liffers ST,
Tannapfel A and Mirmohammadsadegh A: Salinomycin induces autophagy
in colon and breast cancer cells with concomitant generation of
reactive oxygen species. PLoS One. 7:e441322012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ,
Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, et al: Salinomycin
inhibits osteo-sarcoma by targeting its tumor stem cells. Cancer
Lett. 311:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan X, Chen X, Deng W, Zhong G, Cai Q and
Lin T: Up-regulated microRNA-143 in cancer stem cells
differentiation promotes prostate cancer cells metastasis by
modulating FNDC3B expression. BMC Cancer. 13:612013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He M, Sun HG, Hao JY, Li YL, Yu JK, Yan
YY, Zhao L, Li N, Wang Y, Bai XF, et al: RNA interference-mediated
FANCF silencing sensitizes OVCAR3 ovarian cancer cells to
adriamycin through increased adriamycin-induced apoptosis dependent
on JNK activation. Oncol Rep. 29:1721–1729. 2013.PubMed/NCBI
|
|
32
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hatton BA, Knoepfler PS, Kenney AM,
Rowitch DH, de Alborán IM, Olson JM and Eisenman RN: N-myc is an
essential downstream effector of Shh signaling during both normal
and neoplastic cerebellar growth. Cancer Res. 66:8655–8661. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bigelow RL, Chari NS, Unden AB, Spurgers
KB, Lee S, Roop DR, Toftgard R and McDonnell TJ: Transcriptional
regulation of bcl-2 mediated by the sonic hedgehog signaling
pathway through gli-1. J Biol Chem. 279:1197–1205. 2004. View Article : Google Scholar
|
|
35
|
Duman-Scheel M, Weng L, Xin S and Du W:
Hedgehog regulates cell growth and proliferation by inducing cyclin
D and cyclin E. Nature. 417:299–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Deng W, Nail CD, Bailey SK, Kraus
MH, Ruppert JM and Lobo-Ruppert SM: Snail induction is an early
response to Gli1 that determines the efficiency of epithelial
transformation. Oncogene. 25:609–621. 2006.
|
|
37
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu H, Patel MR, Prescher JA, Patsialou A,
Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, et al:
Cancer stem cells from human breast tumors are involved in
spontaneous metastases in orthotopic mouse models. Proc Natl Acad
Sci USA. 107:18115–18120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cariati M, Naderi A, Brown JP, Smalley MJ,
Pinder SE, Caldas C and Purushotham AD: Alpha-6 integrin is
necessary for the tumourigenicity of a stem cell-like subpopulation
within the MCF7 breast cancer cell line. Int J Cancer. 122:298–304.
2008. View Article : Google Scholar
|
|
40
|
Zhao XQ, Dai CL, Ohnuma S, Liang YJ, Deng
W, Chen JJ, Zeng MS, Ambudkar SV, Chen ZS and Fu LW: Tandutinib
(MLN518/CT53518) targeted to stem-like cells by inhibiting the
function of ATP-binding cassette subfamily G member 2. Eur J Pharm
Sci. 49:441–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takashima S, Mkrtchyan M,
Younossi-Hartenstein A, Merriam JR and Hartenstein V: The behaviour
of Drosophila adult hindgut stem cells is controlled by Wnt and Hh
signalling. Nature. 454:651–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu Y, Ma W, Mao J, Yu X, Hou Z, Fan S,
Song B, Wang H, Li J, Kang L, et al: Salinomycin exerts anticancer
effects on human breast carcinoma MCF-7 cancer stem cells via
modulation of Hedgehog signaling. Chem Biol Interact. 228:100–107.
2015. View Article : Google Scholar
|
|
43
|
Wang J, Wang H, Li Z, Wu Q, Lathia JD,
McLendon RE, Hjelmeland AB and Rich JN: c-Myc is required for
maintenance of glioma cancer stem cells. PLoS One. 3:e37692008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murphy MJ, Wilson A and Trumpp A: More
than just proliferation: Myc function in stem cells. Trends Cell
Biol. 15:128–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu J, Rodova M, Roy SK, Sharma J, Singh
KP, Srivastava RK and Shankar S: GANT-61 inhibits pancreatic cancer
stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice
xenograft. Cancer Lett. 330:22–32. 2013. View Article : Google Scholar
|
|
46
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesen-chymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feldmann G, Dhara S, Fendrich V, Bedja D,
Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C,
Jimeno A, et al: Blockade of hedgehog signaling inhibits pancreatic
cancer invasion and metastases: A new paradigm for combination
therapy in solid cancers. Cancer Res. 67:2187–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Sali-nomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee
CH and Kim YN: Salinomycin induces cell death via inactivation of
Stat3 and downregulation of Skp2. Cell Death Dis. 4:e6932013.
View Article : Google Scholar : PubMed/NCBI
|