Introduction
Human malignant melanoma (HMM), the most deadly form
of skin cancer, is found in the outer layer of the skin and is
responsible for ~60% of lethal skin tumors (1). HMM incidence and mortality have
steadily increased over the last 50 years in the fair-skinned
population (2). The most common
risk factors for the development of HMM include exposure to
ultraviolet radiation (especially in childhood), fair skin,
dysplastic nevi syndrome, age and family history (3). Current treatment modalities for HMM,
including surgery, radiation and chemotherapy, are not efficient
enough to prevent the spread of metastases in up to 50% of patients
(4). For patients with stage IV
disease, the melanoma has spread beyond the local area into other
parts of the body or internal organs. Although surgery and
radiation therapy are the main treatment for malignant melanoma,
systemic therapy such as cytotoxic chemotherapy and immunotherapy
is the mainstay of treatment for most patients with stage IV
melanoma (5). Therefore, there is
an ongoing quest for novel effective chemotherapeutic agents for
HMM. One of the recently suggested agents for the treatment of
cancer is β-lapachone (β-lap).
β-lap
(3.4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione) is a
natural quinone derived from the bark of the Pink trumpet tree
(Tabebuia avellanedae), which has been used in traditional
medicine for centuries (6).
Previous studies have reported that β-lap has anti-bacterial
(7), anti-fungal (7), anti-inflammatory (8), anti-viral (9), anti-proliferative (10,11),
anti-psoriasis (12), and
anti-arthritic properties (13). In
addition, β-lap was also reported to sensitize cancer cells to
ionizing radiation (14), DNA
damaging agents (15) and increase
the generation of reactive oxygen species (16). In particular, the anti-proliferative
effect of β-lap on various cancer cell lines including prostate
cancer (10,11,17),
breast cancer (18,19), lung cancer (20) and gastric carcinoma (21) has been reported.
Specificity protein 1, a member of the specificity
protein/Kruppel-like factor family of transcription factors, is
responsible for a variety of cellular processes (22). Previous studies have reported that
Sp1 is related to tumorigenesis by modulating transcription
associated with cell growth and proliferation (23). Furthermore, Sp1 is highly expressed
in various cancer cell lines compared to normal cells. In this
regard, a decrease in cancer proliferation was found to occur
following inhibition of Sp1 by small interfering RNA in nude mice
(15,24–26).
Hence, many studies have revealed that regulation of the function
by Sp1 is a promising therapeutic target in cancer (27).
Although the anti-proliferative properties of β-lap
against several cancer cell lines have been demonstrated, its
effect on HMM and the mechanisms of β-lap-induced apoptosis are not
yet fully understood. In this study, we examined the effect of
β-lap on two HMM cell lines, G361 and SK-MEL-28. We determined that
β-lap inhibited cell viability and induced apoptosis in the G361
and SK-MEL-28 cells. The results from the present study provide
experimental evidence to support the hypothesis that β-lap
decreases Sp1 expression and inhibits HMM cell viability by
inducing cell cycle arrest and apoptosis. Our results reinforce the
potential pharmacological interest of β-lap, as confirmed by the
suppression of Sp1 in HMM cells.
Materials and methods
Cell culture
Human malignant melanoma (HMM) cell lines, G361 and
SK-MEL-28, were cultured in Hyclone Dulbecco's modified Eagle's
medium (DMEM; Welgene, Dea-gu, Korea) with 10% fetal bovine serum
(FBS) and 100 U/ml each of penicillin and streptomycin (Gibco,
Grand Island, NY, USA) at 37°C with 5% CO2 in a
humidified atmosphere.
Cell viability assay
Viability of the G361 and SK-MEL-28 cells treated
with β-lap was measured using the CellTiter96® Aqueous
One cell proliferation assay kit (Promega, Madison, WI, USA)
according to the manufacturer's instructions. The effect of β-lap
on the viability of the G361 and SK-MEL-28 cells was estimated by
3-(4,5-dimethylthiazol-2-yl)-5-(3-carb-oxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. G361 (3×103) and SK-MEL-28
(3×103) cells were seeded into 96-well plates and were
treated with various concentrations of β-lap for 24 and 48 h. MTS
reagent was added directly to the cells and incubated at 37°C.
Absorbance was measured using an absorbance microplate reader
(Biotek, Winooski, VT, USA) at 490 nm. All experiments were carried
out in triplicates, and the percentage of cell viability of the
β-lap-treated cells was normalized to that of the untreated control
cells.
DAPI staining
Chromatin condensation and nuclear fragmentation in
apoptotic cells were assessed by 4′,6-diamidino-2-phenylindole
(DAPI) staining. G361 and SK-MEL-28 cells were treated with various
concentrations of β-lap for 48 h and then harvested by
trypsinization. The detached cells were fixed in 100% methanol at
room temperature for 30 min, deposited on slides, and stained with
DAPI (Sigma-Aldrich, St. Louis, MO, USA) solution (2 µg/ml).
Cell morphology was observed under a FluoView confocal laser
microscope (Fluoview FV10i; Olympus Corporation, Tokyo, Japan).
Annexin V and Dead Cell assay
Annexin V and Dead Cell assay was performed using
Muse™ Cell Analyzer from Millipore (Billerica, MA, USA) following
the manufacturer's instructions. Briefly, after treatment with the
various concentrations of β-lap, the G361 and SK-MEL-28 cells were
incubated with Annexin V and Dead cell reagent (7-ADD) and the
events for dead, late apoptotic, early apoptotic, and live cells
were counted.
Mitochondrial membrane potential (MMP)
assay
MMP was determined with
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benz-imidazolyl-carbocyanine
iodine (JC-1) by using the Muse™ Cell Analyzer from Millipore
following the manufacturer's instructions. The samples were
analyzed by flow cytometry and counted (10,000 cells/sample). Loss
of MMP was quantified as the percentage of cells expressing JC-1
monomer fluorescence.
Western blot analysis
G361 and SK-MEL-28 cells were treated with β-lap,
and then the cells were washed twice with ice-cold PBS. Whole cell
lysate was prepared using M-PER® Mammalian Protein
Extraction reagent (Thermo Scientific, Rockford, IL, USA)
containing a protease inhibitor cocktail (Roche, Switzerland).
Protein concentrations were estimated using the BCA protein assay
kit (Thermo Scientific). The samples were separated by 8 or 12%
SDS-polyacrylamide gel electrophoresis, and were then transferred
to polyvinylidene difluoride (PVDF) membranes (Millipore). The
membranes were blocked with 5% (v/v) skim milk in TBS buffer
containing 0.1% Tween-20 and then incubated with the primary
antibody overnight at 4°C. Subsequently, the membranes were washed
5 times in TBS buffer including 0.1% Tween-20 for 10 min and
incubated with horseradish peroxidase-conjugated anti-mouse,
anti-rabbit or anti-goat IgG antibodies. The membranes were
visualized using a chemiluminescent ECL detection kit (Thermo
Scientific) and detected using ImageQuant Las 4000 Mini (GE
Healthcare Life Sciences) according to the manufacturer's
instructions.
Statistical analysis
Data are reported as the means ± SD of triplicate
independent experiment. Statistical significance was assessed using
the Student's t-test. A value of P<0.05 compared with the
untreated control was considered to be statistically
significant.
Results
β-lap inhibits the cell viability of HMM
cells
Previous studies have reported that β-lap has
anti-proliferative effects on several different cancer cells
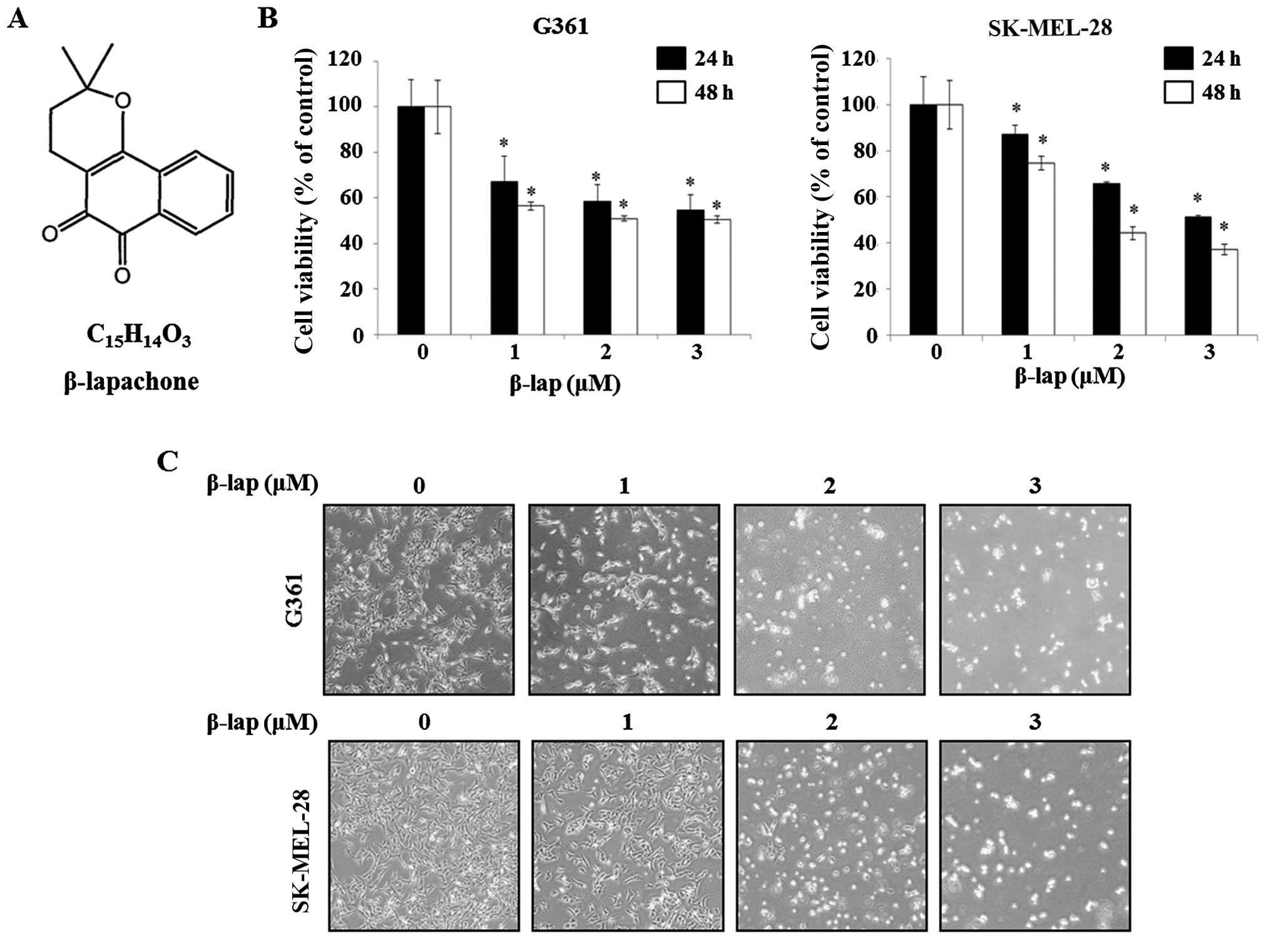
(10,11). The structure of β-lap is shown in
Fig. 1A. To investigate the effect
of β-lap on G361 and SK-MEL-28 cells, cell viability was measured
using MTS assay. The cell viability of G361 was 56.5±0.02,
50.9±0.01 and 50.5±0.01 following treatment with 1, 2 and 3
µM of β-lap, respectively, compared with the untreated
control cells at 48 h post-treatment. In the case of SK-MEL-28
cells, cell viability was 74.5±0.03, 44.1±0.03 and 37.0±0.02
following treatment with 1, 2 and 3 µM of β-lap for 48 h,
respectively, compared to that of the untreated control cells at 48
h post-treatment. The results indicated that β-lap decreased the
viability of G361 and SK-MEL-28 cells in a dose- and time-dependent
manner (Fig. 1B). Following, the
morphological alterations of the G361 and SK-MEL-28 cells were
observed using an optical microscope following treatment with β-lap
(1-3 µM) for 48 h. As shown Fig.
1C, the numbers of irregular and rounded cells were increased
by β-lap (0, 1, 2 and 3 µM). Thus, these results determined
that β-lap inhibited the cell viability of the HMM cells.
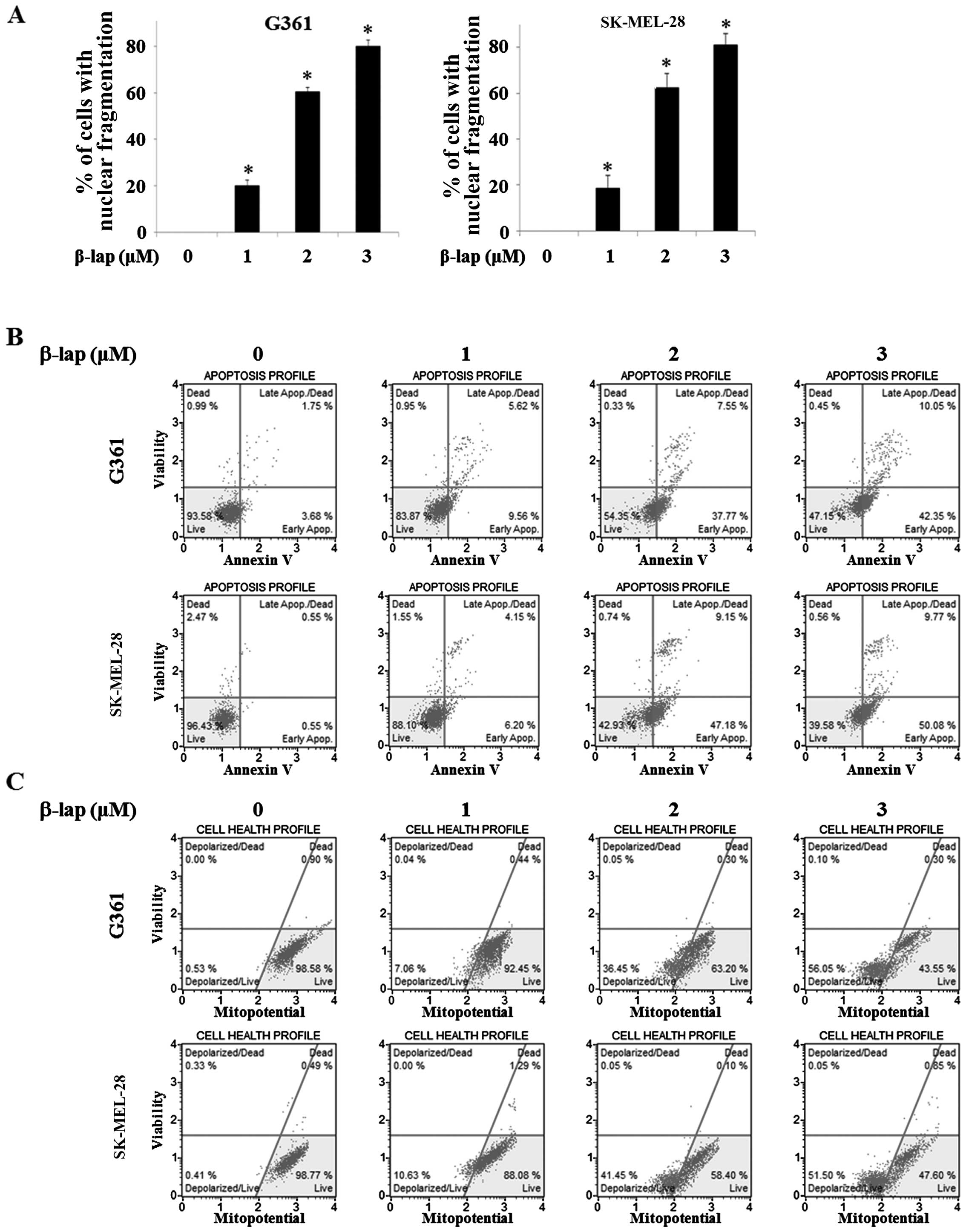
β-lap induces apoptosis in HMM cells
In order to investigate apoptotic morphological
alterations in the G361 and SK-MEL-28 cells, they were treated with
β-lap at various concentrations (0, 1, 2 and 3 µM). The
cells were stained with DAPI and then observed under a FluoView
confocal laser microscope. As shown in Fig. 2A, the percentage of cells with
nuclear condensation and perinuclear apoptotic bodies was increased
in a β-lap dose-dependent manner in the HMM cells.
We confirmed that apoptosis was mainly induced upon
exposure to β-lap by the Annexin V and Dead Cell assay and MMP
assay in the G361 and SK-MEL-28 cells. The upper right quadrant and
the lower right quadrant, respectively, indicated late and early
apoptotic cells and the percentage of these apoptotic cells was
increased by β-lap (1, 2 and 3 µM) when compared to the
cells without treatment (Fig. 2B).
The lower left quadrant indicated depolarized cells and the
percentage of these cells was increased by β-lap (0, 1, 2 and 3
µM) (Fig. 2C). These results
indicated that β-lap treatment effectively inhibited cell
proliferation, leading to apoptosis in the HMM cells.
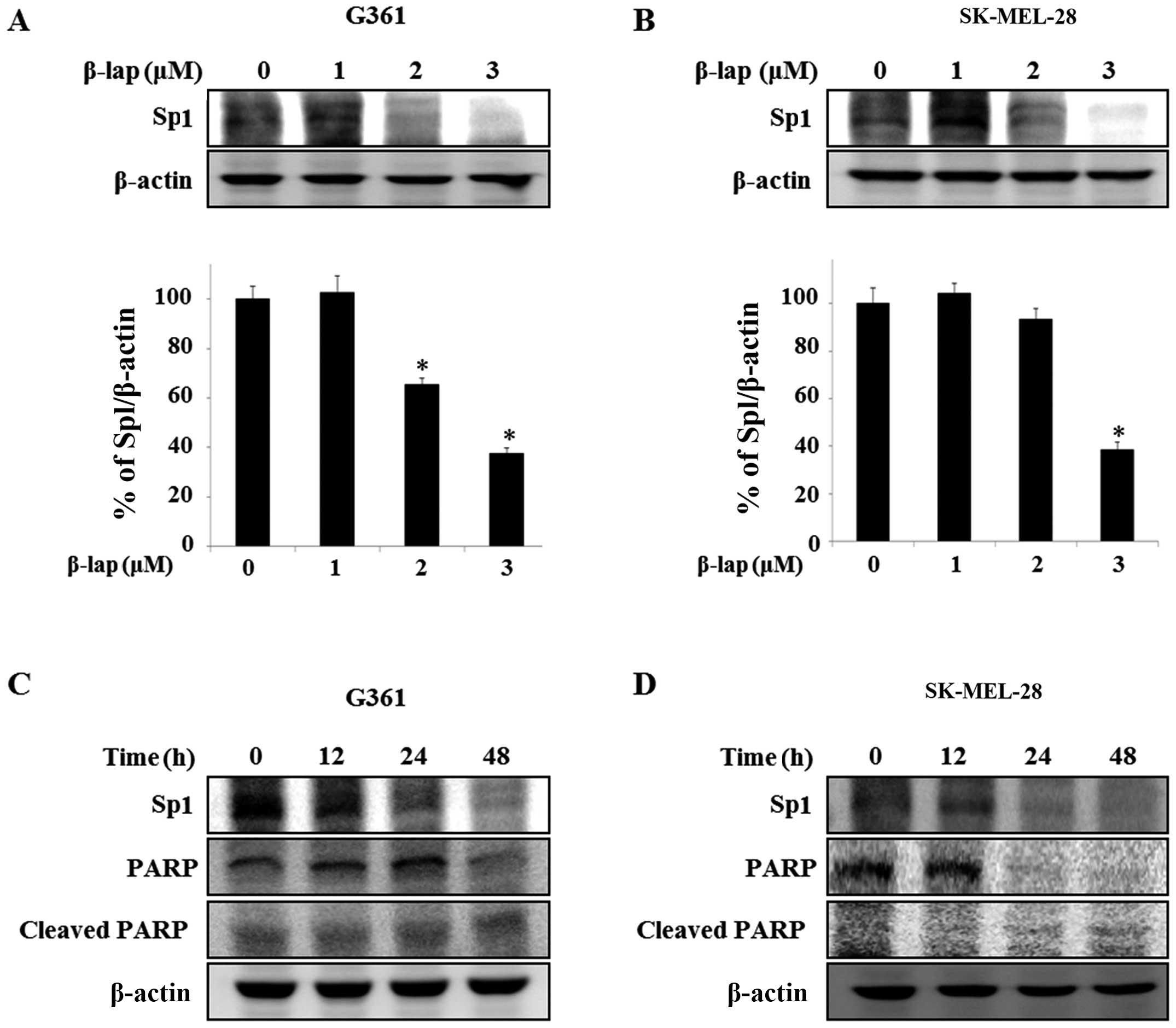
Expression of Sp1 is suppressed by β-lap
in HMM cells
Sp1 is overexpressed in various human cancers,
including human glioblastoma (28),
lung cancer (24) and pancreatic
cancer (29), and is regulated by
chemotherapeutic agents (30) and
natural compounds, including honokiol and esculetin (27,31).
Thus, we investigated whether the expression level of Sp1 was
regulated by β-lap in the HMM G361 and SK-MEL-28 cells. We
determined whether the expression level of Sp1 was altered by β-lap
in the G361 and SK-MEL-28 cells. We detected Sp1 expression levels
by western blot analysis. The expression levels were significantly
reduced in the treated cells, with a maximum decrease of 62.4±0.01%
in the G361 cells compared to the untreated cells and a maximum
decrease of 61.6±0.01% was noted in the SK-MEL-28 cells compared to
the untreated group (Fig. 3A and
B). To further determine the apoptotic effect by the
downregulation of Sp1 in the cells treated with β-lap, we treated
the G361 and SK-MEL-28 cells with 3 µM β-lap for different
times (0, 12, 24 and 48 h) and investigated the expression levels
of PARP and cleaved PARP (Fig. 3C and
D). Thus, these results demonstrated that β-lap induces
apoptosis through downregulation of Sp1.
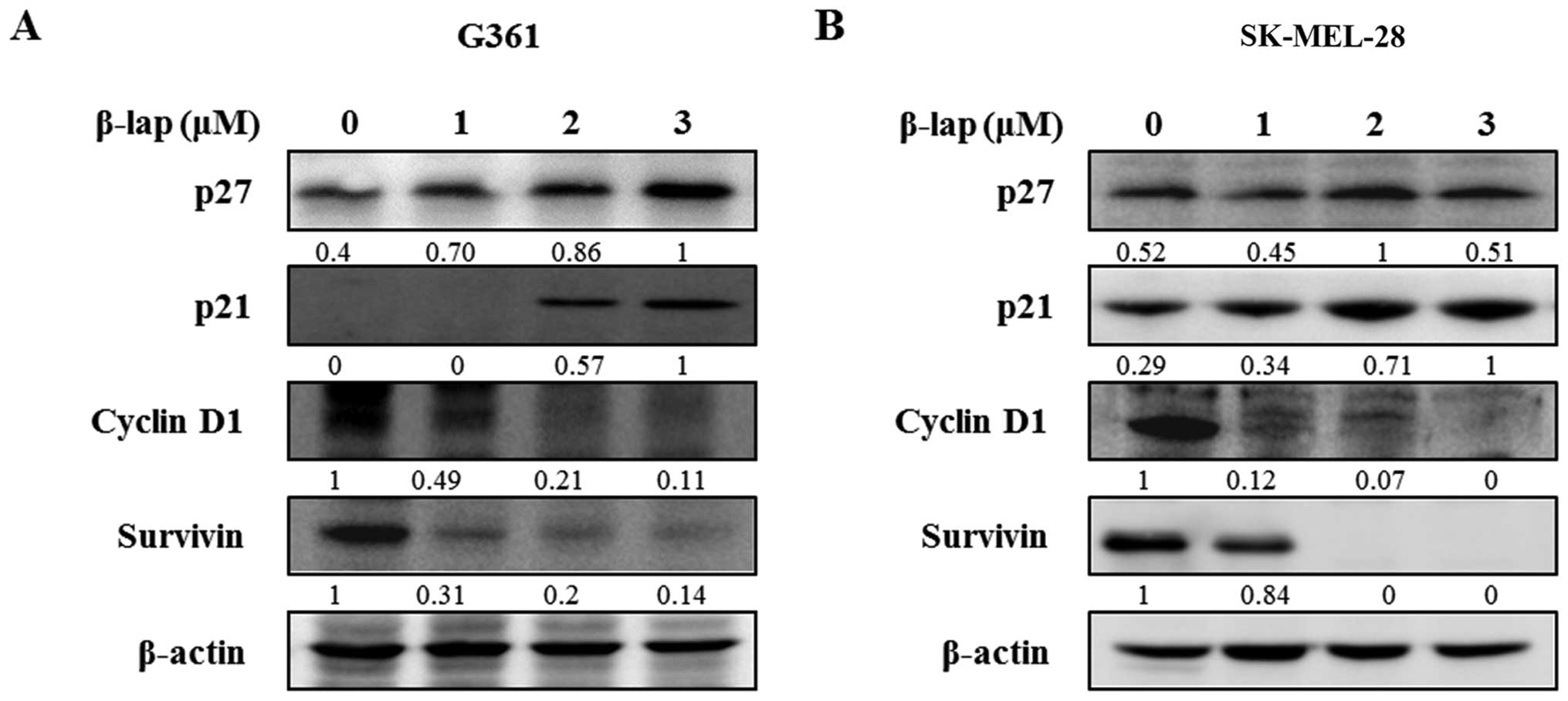
β-lap regulates cell cycle arrest and
apoptosis in the HMM cells
To investigate the regulatory effect of β-lap, we
examined the changes in expression levels of Sp1 downstream targets
and apoptosis-related proteins. The cell cycle arrest proteins,
including p27 and p21, were significantly increased following
treatment with different concentrations of β-lap, whereas cell
proliferation and survival-related proteins, cyclin D1 and
survivin, were decreased by β-lap in a concentration-dependent
manner (Fig. 4). In addition, we
assessed the expression levels of several pro- and anti-apoptotic
proteins in the β-lap-treated G361 and SK-MEL-28 cells. As shown in
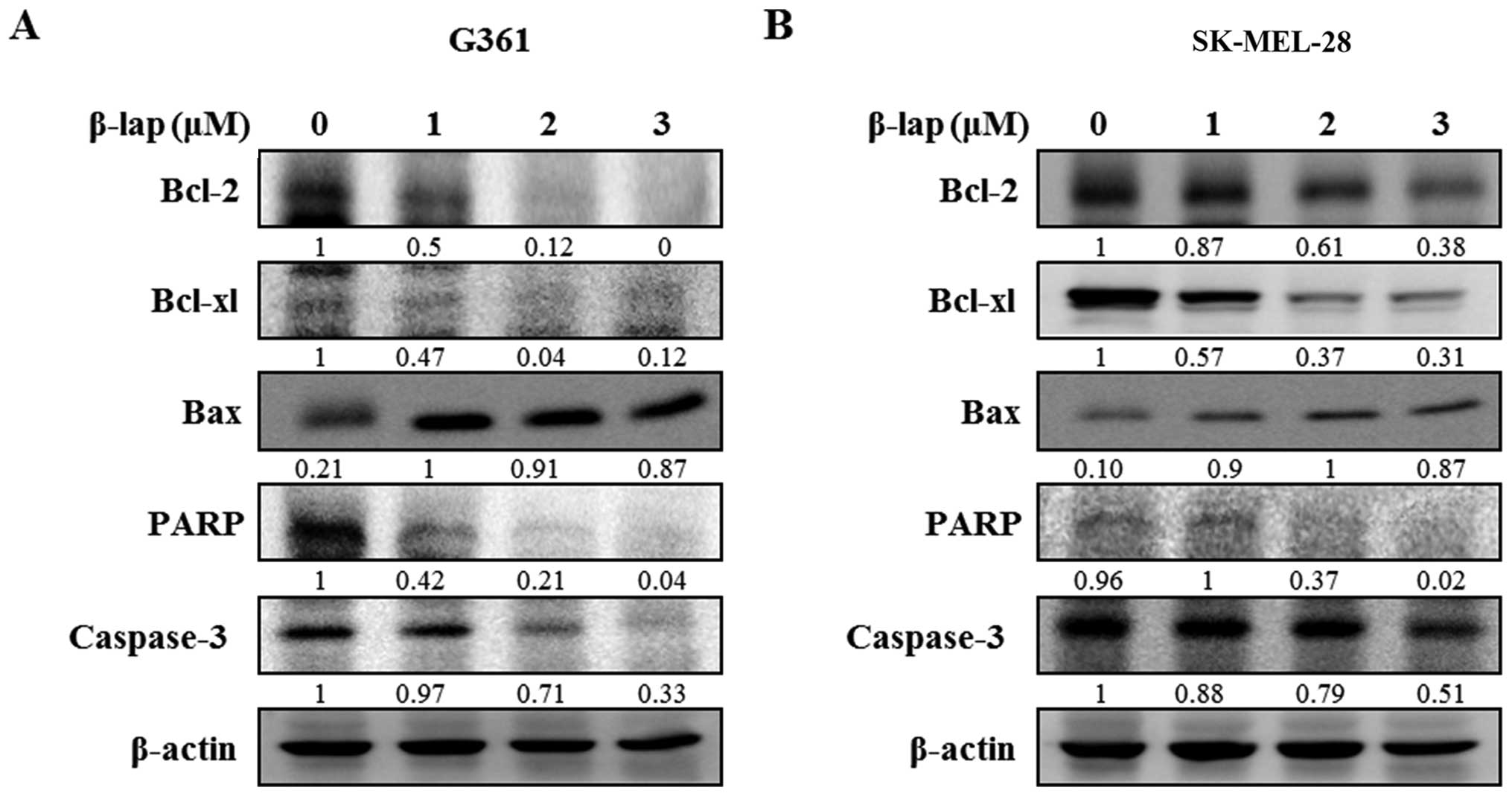
Fig. 5, β-lap reduced the
expression of Bcl-2 and Bcl-xl, and increased the expression of Bax
to induce apoptotic cell death. β-lap also
concentration-dependently caused activation of caspase-3 and PARP
in the HMM cells. Our results showed that β-lap induced the
downregulation of Sp1, resulting in cell cycle arrest and induction
of apoptosis in the HMM cells.
Discussion
HMM is one of the most deadly types of skin cancer
and its incidence has increased over the last 50 years in the
fair-skinned population (2).
Surgery, thermotherapy, chemotherapy and radiotherapy are used to
treat HMM although these methods cause side effects, such as
cytotoxicity to normal cells and host immune reaction (32). Hence, the objective of studies has
concentrated upon natural compounds derived or originating from
Mother Nature (33). Moreover, the
value of natural compounds with anti-proliferative properties has
been considered to play an important role in cancer therapy. In the
present study, we concentrated on the anti-proliferative effect of
β-lap, a novel natural quinone derived from the bark of the Pink
trumpet tree (Tabebuia avellanedae) (34). β-lap has been reported as being a
topoisomerase I and II inhibitor (35,36),
and was found to inhibit lymphocyte-(37), neutrophil-(37) and macrophage-related joint
inflammatory (37). Thus, β-lap may
be a potential agent against anti-proliferation. In this regard,
several studies have reported that β-lap has an anti-proliferation
effect on various cancer-derived cells, including breast carcinoma
(18,19) prostate carcinoma (10,11,17),
lung carcinoma (20) and gastric
carcinoma (21). Despite numerous
studies on cancer cells, the anti-proliferative properties of β-lap
on HMM cells are not well understood.
In the present study, we demonstrated that β-lap
induced apoptosis in HMM G361 and SK-MEL-28 cells. The apoptosis
was accompanied by morphological alterations, such as chromatin
condensation and rounded cells. We observed an increase in nuclear
condensation and perinuclear apoptotic bodies though DAPI staining,
and determined changes in the percentages of apoptotic and
depolarized cells by Annexin V and Dead Cell assay and MMP assay
following β-lap treatment. These results suggest that β-lap induces
apoptosis in HMM cells.
The transcription factor, Sp1, is responsible for a
variety of cellular processes, including transcription initiation,
survival, cell growth, differentiation, metabolism and angiogenesis
(38,39). Previous studies have reported that
Sp1 is overexpressed in cancer cells and contributes to the
formation of cancer (40) as well
as regulates cell cycle- and apoptosis-associated proteins
(25,41,42).
In addition, several studies have shown that inhibition of Sp1
plays a role in growth inhibition and induction of apoptosis in
malignant pleural mesothelioma and that Sp1 is activated in
melanoma cells (43,44). For these reasons, the regulation of
Sp1 has been suggested to enhance the efficacy of cancer therapy
and improve poor prognosis. Our data showed that the expression
level of Sp1 was significantly deceased in the β-lap-treated HMM
cells.
p21 and p27 are well known as negative regulators of
cell cycle progression (45). The
functions of p21 and p27 are responsible for sub-G1 phase arrest by
interaction between cyclins and cyclin-dependent kinase (CDK)
complexes (46,47). Cyclin D1 performs functions,
including proto-oncogene (48),
tumorigenesis (49), cell
maintenance (49) and integrator of
extracellular signals of cells in early to mid-G1 phase (50). Survivin, an inhibitor of apoptosis
(IAP) (51), plays an important
role in mitosis and cell cycle arrest and apoptosis (52) and is also associated with
Sp1-related oncogenes (53). The
apoptotic caspases appear to be activated in a protease cascade and
activated apical caspases respond to apoptotic stimuli and directly
activate the effector caspases in a precisely controlled process
(54). Among these caspases,
caspase-3 plays a central role in the execution phase of both the
intrinsic and extrinsic pathways of apoptosis by cleaving key
cellular proteins, such as PARP (55). These cleavages regulate disassembly
of the cell into the typical apoptotic morphological alterations,
including cell shrinkage, chromatin condensation and DNA
fragmentation (56). PARP is
activated by binding DNA strand breaks produced by various DNA
damaging agents, including hydrogen peroxide (57). Bcl-2 and Bcl-xl, blockers of cell
death, are anti-apoptotic proteins and Bax, which promotes cell
death, is a pro-apoptotic protein (58,59).
The results showed that β-lap-induced apoptosis was accompanied by
the upregulation of p27, p21 and Bax and downregulation of cyclin
D1, survivin, Bcl2, Bcl-xl, PARP and caspase-3, strongly suggesting
that β-lap is a potential apoptosis-inducing agent by regulating
Sp1 protein to exert anti-proliferation activity in HMM cells.
In the present study, we investigated the
anti-proliferative effect of β-lap on HMM cells. The results of our
study showed that β-lap inhibited cell growth and induced apoptosis
through regulation of anti- and pro-apoptotic proteins by Sp1.
Therefore, these results suggest that the anti-proliferative and
apoptotic effects of β-lap are modulated by the regulation of
Sp1-mediated gene products.
Acknowledgments
This study was supported by the Next-Generation
BioGreen 21 Program (PJ01116401), Rural Development Administration,
Republic of Korea.
References
|
1
|
Bandarchi B, Ma L, Navab R, Seth A and
Rasty G: From melanocyte to metastatic malignant melanoma. Dermatol
Res Pract. 2010:5837482010.PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erdmann F, Lortet-Tieulent J, Schüz J,
Zeeb H, Greinert R, Breitbart EW and Bray F: International trends
in the incidence of malignant melanoma 1953–2008 - are recent
generations at higher or lower risk? Int J Cancer. 132:385–400.
2013. View Article : Google Scholar
|
|
4
|
Kesmodel SB and Spitz FR: Gene therapy for
cancer and metastatic disease. Expert Rev Mol Med. 5:1–18. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatia S, Tykodi SS and Thompson JA:
Treatment of metastatic melanoma: An overview. Oncology (Williston
Park). 23:488–496. 2009.
|
|
6
|
Moon DO, Kang CH, Kim MO, Jeon YJ, Lee JD,
Choi YH and Kim GY: Beta-lapachone (LAPA) decreases cell viability
and telomerase activity in leukemia cells: Suppression of
telomerase activity by LAPA. J Med Food. 13:481–488. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guiraud P, Steiman R, Campos-Takaki GM,
Seigle-Murandi F and Simeon de Buochberg M: Comparison of
antibacterial and antifungal activities of lapachol and
beta-lapachone. Planta Med. 60:373–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Almeida ER, da Silva Filho AA, dos
Santos ER and Lopes CA: Antiinflammatory action of lapachol. J
Ethnopharmacol. 29:239–241. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li CJ, Zhang LJ, Dezube BJ, Crumpacker CS
and Pardee AB: Three inhibitors of type 1 human immunodeficiency
virus long terminal repeat-directed gene expression and virus
replication. Proc Natl Acad Sci USA. 90:1839–1842. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Planchon SM, Wuerzberger S, Frydman B,
Witiak DT, Hutson P, Church DR, Wilding G and Boothman DA:
Beta-lapachone-mediated apoptosis in human promyelocytic leukemia
(HL-60) and human prostate cancer cells: A p53-independent
response. Cancer Res. 55:3706–3711. 1995.PubMed/NCBI
|
|
11
|
Planchon SM, Pink JJ, Tagliarino C,
Bornmann WG, Varnes ME and Boothman DA: Beta-lapachone-induced
apoptosis in human prostate cancer cells: Involvement of NQO1/xip3.
Exp Cell Res. 267:95–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller K, Sellmer A and Wiegrebe W:
Potential antipsoriatic agents: Lapacho compounds as potent
inhibitors of HaCaT cell growth. J Nat Prod. 62:1134–1136. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sitônio MM, Carvalho Júnior CH, Campos IA,
Silva JB, Lima MC, Góes AJ, Maia MB, Rolim Neto PJ and Silva TG:
Anti-inflammatory and anti-arthritic activities of
3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione
(β-lapachone). Inflamm Res. 62:107–113. 2013. View Article : Google Scholar
|
|
14
|
Frydman B, Marton LJ, Sun JS, Neder K,
Witiak DT, Liu AA, Wang HM, Mao Y, Wu HY, Sanders MM, et al:
Induction of DNA topoisomerase II-mediated DNA cleavage by
beta-lapachone and related naphthoquinones. Cancer Res. 57:620–627.
1997.PubMed/NCBI
|
|
15
|
Chau YP, Shiah SG, Don MJ and Kuo ML:
Involvement of hydrogen peroxide in topoisomerase inhibitor
beta-lapachone-induced apoptosis and differentiation in human
leukemia cells. Free Radic Biol Med. 24:660–670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiah SG, Chuang SE, Chau YP, Shen SC and
Kuo ML: Activation of c-Jun NH2-terminal kinase and subsequent
CPP32/Yama during topoisomerase inhibitor beta-lapachone-induced
apoptosis through an oxidation-dependent pathway. Cancer Res.
59:391–398. 1999.PubMed/NCBI
|
|
17
|
Choi YH, Kang HS and Yoo MA: Suppression
of human prostate cancer cell growth by beta-lapachone via
down-regulation of pRB phosphorylation and induction of Cdk
inhibitor p21(WAF1/CIP1). J Biochem Mol Biol. 36:223–229. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pink JJ, Wuerzberger-Davis S, Tagliarino
C, Planchon SM, Yang X, Froelich CJ and Boothman DA: Activation of
a cysteine protease in MCF-7 and T47D breast cancer cells during
beta-lapachone-mediated apoptosis. Exp Cell Res. 255:144–155. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wuerzberger SM, Pink JJ, Planchon SM,
Byers KL, Bornmann WG and Boothman DA: Induction of apoptosis in
MCF-7:WS8 breast cancer cells by beta-lapachone. Cancer Res.
58:1876–1885. 1998.PubMed/NCBI
|
|
20
|
Dong GZ, Oh ET, Lee H, Park MT, Song CW
and Park HJ: Beta-lapachone suppresses radiation-induced activation
of nuclear factor-kappaB. Exp Mol Med. 42:327–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu HY, Kim SO, Jin CY, Kim GY, Kim WJ, Yoo
YH and Choi YH: β-lapachone-induced apoptosis of human gastric
carcinoma AGS cells is caspase-dependent and regulated by the
PI3K/Akt pathway. Biomol Ther (Seoul). 22:184–192. 2014. View Article : Google Scholar
|
|
22
|
Wierstra I: Sp1: Emerging roles - beyond
constitutive activation of TATA-less housekeeping genes. Biochem
Biophys Res Commun. 372:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong LM, Liao CG, Fei F, Guo X, Xing JL
and Chen ZN: Transcription factor Sp1 regulates expression of
cancer-associated molecule CD147 in human lung cancer. Cancer Sci.
101:1463–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting Sp1 transcription factors in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Wang L, Gong W, Wei D, Le X, Yao
J, Ajani J, Abbruzzese JL, Huang S and Xie K: A high expression
level of insulin-like growth factor I receptor is associated with
increased expression of transcription factor Sp1 and regional lymph
node metastasis of human gastric cancer. Clin Exp Metastasis.
21:755–764. 2004. View Article : Google Scholar
|
|
27
|
Kim DW, Ko SM, Jeon YJ, Noh YW, Choi NJ,
Cho SD, Moon HS, Cho YS, Shin JC, Park SM, et al:
Anti-proliferative effect of honokiol in oral squamous cancer
through the regulation of specificity protein 1. Int J Oncol.
43:1103–1110. 2013.PubMed/NCBI
|
|
28
|
Zhang R, Luo H, Wang S, Chen W, Chen Z,
Wang HW, Chen Y, Yang J, Zhang X, Wu W, et al: MicroRNA-377
inhibited proliferation and invasion of human glioblastoma cells by
directly targeting specificity protein 1. Neuro Oncol.
16:1510–1522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdelrahim M, Smith R III, Burghardt R and
Safe S: Role of Sp proteins in regulation of vascular endothelial
growth factor expression and proliferation of pancreatic cancer
cells. Cancer Res. 64:6740–6749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin JA, Han G, Kim HJ, Kim HM and Cho SD:
Chemopreventive and chemotherapeutic effect of a novel histone
deacetylase inhibitor, by specificity protein 1 in MDA-MB-231 human
breast cancer cells. Eur J Cancer Prev. 23:277–285. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho JH, Shin JC, Cho JJ, Choi YH, Shim JH
and Chae JI: Esculetin (6,7-dihydroxycoumarin): A potential cancer
chemo-preventive agent through suppression of Sp1 in oral squamous
cancer cells. Int J Oncol. 46:265–271. 2015.
|
|
32
|
Safdie FM, Dorff T, Quinn D, Fontana L,
Wei M, Lee C, Cohen P and Longo VD: Fasting and cancer treatment in
humans: A case series report. Aging (Albany NY). 1:988–1007.
2009.
|
|
33
|
Wilson RM and Danishefsky SJ: Small
molecule natural products in the discovery of therapeutic agents:
The synthesis connection. J Org Chem. 71:8329–8351. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tobin DD, Menon M, Menon M, Spatta BC,
Hodges EV and Perry DG: The intrapsychics of gender: A model of
self-socialization. Psychol Rev. 117:601–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li CJ, Averboukh L and Pardee AB:
Beta-lapachone, a novel DNA topoisomerase I inhibitor with a mode
of action different from camptothecin. J Biol Chem.
268:22463–22468. 1993.PubMed/NCBI
|
|
36
|
Jackson JK, Higo T, Hunter WL and Burt HM:
Topoisomerase inhibitors as anti-arthritic agents. Inflamm Res.
57:126–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tudan C, Jackson JK, Higo TT and Burt HM:
The effect of inhibiting topoisomerase I and II on the
anti-apoptotic response associated with pro-inflammatory crystals
of calcium pyrophosphate dihydrate in human neutrophils. Inflamm
Res. 52:8–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu TI, Wang MC, Chen SY, Yeh YM, Su WC,
Chang WC and Hung JJ: Sp1 expression regulates lung tumor
progression. Oncogene. 31:3973–3988. 2012. View Article : Google Scholar :
|
|
41
|
Kavurma MM and Khachigian LM: Sp1 inhibits
proliferation and induces apoptosis in vascular smooth muscle cells
by repressing p21WAF1/Cip1 transcription and cyclin
D1-Cdk4-p21WAF1/Cip1 complex formation. J Biol Chem.
278:32537–32543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu R, Zhang P, Huang J, Ge S, Lu J and
Qian G: Sp1 and Sp3 regulate basal transcription of the survivin
gene. Biochem Biophys Res Commun. 356:286–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chae JI, Jeon YJ and Shim JH:
Downregulation of Sp1 is involved in honokiol-induced cell cycle
arrest and apoptosis in human malignant pleural mesothelioma cells.
Oncol Rep. 29:2318–2324. 2013.PubMed/NCBI
|
|
44
|
Hong IK, Byun HJ, Lee J, Jin YJ, Wang SJ,
Jeoung DI, Kim YM and Lee H: The tetraspanin CD81 protein increases
melanoma cell motility by up-regulating metalloproteinase MT1-MMP
expression through the pro-oncogenic Akt-dependent Sp1 activation
signaling pathways. J Biol Chem. 289:15691–15704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Yang WT and Zheng PS: Msi1 promotes
tumor growth and cell proliferation by targeting cell cycle
checkpoint proteins p21, p27 and p53 in cervical carcinomas.
Oncotarget. 5:10870–10885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ewen ME and Lamb J: The activities of
cyclin D1 that drive tumorigenesis. Trends Mol Med. 10:158–162.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weinstein IB: Relevance of cyclin D1 and
other molecular markers to cancer chemoprevention. J Cell Biochem
Suppl. 25:23–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jarrin M, Mansergh FC, Boulton ME, Gunhaga
L and Wride MA: Survivin expression is associated with lens
epithelial cell proliferation and fiber cell differentiation. Mol
Vis. 18:2758–2769. 2012.PubMed/NCBI
|
|
53
|
Xu Q, Liu M, Xu N and Zhu H: Variation in
Sp1 binding sites correlates with expression of survivin in breast
cancer. Mol Med Rep. 10:1395–1399. 2014.PubMed/NCBI
|
|
54
|
Grütter MG: Caspases: Key players in
programmed cell death. Curr Opin Struct Biol. 10:649–655. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vaziri H, West MD, Allsopp RC, Davison TS,
Wu YS, Arrowsmith CH, Poirier GG and Benchimol S: ATM-dependent
telomere loss in aging human diploid fibroblasts and DNA damage
lead to the post-translational activation of p53 protein involving
poly(ADP-ribose) polymerase. EMBO J. 16:6018–6033. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zauli G, Gibellini D, Caputo A, Bassini A,
Negrini M, Monne M, Mazzoni M and Capitani S: The human
immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene
expression in Jurkat T-cell lines and primary peripheral blood
mononuclear cells. Blood. 86:3823–3834. 1995.PubMed/NCBI
|
|
59
|
Yang E, Zha J, Jockel J, Boise LH,
Thompson CB and Korsmeyer SJ: Bad, a heterodimeric partner for
Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell.
80:285–291. 1995. View Article : Google Scholar : PubMed/NCBI
|