Introduction
Cervical cancer is one of the most common
gynecological tumors and is the fourth leading cause of
tumor-related deaths in women worldwide (1). Although the screening of cervical
cancer has been widely utilized, there are still large numbers of
patients diagnosed at an advanced stage, particularly in China,
with a 5-year survival rate of less than 40% (2). Moreover, ~30% of patients suffer from
lymph node recurrence and distant metastasis in the period
following treatment (3). The
underlying molecular pathogenesis of cervical tumorigenesis is
complicated and poorly understood.
MicroRNAs (miRNAs) are a group of small RNAs (18–25
nucleotides), which regulate gene expression through
sequence-specific pairing of miRNAs with 3′UTR of target mRNAs
(4,5). It has been estimated that miRNAs
regulate up to one-third of the total human genes, indicating that
miRNAs have critical roles in carcinogenesis (6). Dysregulation of miRNAs appears
frequently in human malignancies and has been found to correlate
with cancer cell proliferation, apoptosis and invasion (7). Several groups have reported that
miR-27b is involved in cancer progression as an oncogene or
tumor-suppressor gene (8–10). However, the expression and mechanism
by which miR-27b exerts its functions in cervical cancer cells
remain unclear.
In the present study, it was revealed that miR-27b
was overexpressed in cervical cancer cells and tissue specimens,
and that downregulation of miR-27b inhibited cervical cancer cell
growth and invasion. CDH11 is a direct and functional target of
miR-27b and was found to be involved in the functional effect of
miR-27b on cell proliferation and invasion. In addition, CDH11 was
involved in miR-27b-induced epithelial-mesenchymal transition (EMT)
by regulating expression of E-cadherin, vimentin, and N-cadherin.
Our findings for the first time suggest that miR-27b may act as an
oncogene in cervical carcinogenesis by regulating CDH11 and
EMT.
Materials and methods
Samples and cells
The samples of cancer tissue and adjacent
non-cancerous tissue were obtained from 37 patients with cervical
squamous cell carcinoma who underwent resection surgery between
2010 and 2011 at the Department of Gynecology, The First Hospital
of China Medical University. Informed consent from all participants
was obtained before surgery, and the present study was approved by
the Institutional Review Boards of our hospital. None of the cases
received radiotherapy or chemotherapy before the tissues were
collected. The Federation of Gynecology and Obstetrics (FIGO) stage
of tumors and non-tumor tissues was evaluated and confirmed by two
pathologists independently. The obtained tissue specimens were
immediately snap-frozen in liquid nitrogen and stored at −80°C
until analysis.
The human cervical carcinoma cells (HeLa, SiHa,
CasKi, and C33A) and human immortalized keratinocytes (HaCaT) were
obtained from the Cell Resource Center of the Chinese Academy of
Sciences (Shanghai, China) and cultured under standard conditions
as recommended by the American Type Culture Collection (ATCC;
Manassas, VA, USA). All the cell lines were cultured at 37°C in a
humidified atmosphere with 5% CO2.
Detection of miRNA and mRNA
Total RNA from the cervical cancer cells and tissue
specimens was extracted using the TRIzol reagent (Invitrogen,
Carlsbad, CA, USA), and 1 µg of each total RNA sample was
used to synthesize cDNA using the PrimeScript RT reagent kit
Perfect Real-Time (Takara, Japan). Total miRNA was extracted using
the mirVana miRNA Isolation kit (Ambion, Austin, TX, USA), and cDNA
was subsequently synthesized using the SuperScript II Reverse
Transcriptase kit (Invitrogen).
Stem-loop RT-PCR was employed for measuring miR-27b
expression in the cell lines and tissues as previously described
(11). U6 small nuclear RNA was
applied as the endogenous control to determine relative miR-27b
expression, which was quantified by the 2−ΔΔCt method.
Real-time quantitative PCR (RT-qPCR) was conducted using a
FastStart Universal SYBR Green Master kit (Roche, Germany) and
amplified with an ABI 7500 Real-Time PCR system (Applied
Biosystems, Foster City, CA, USA). β-actin was used as an internal
control for CDH11. Each sample was assayed in triplicate.
Dual-luciferase assay
For the luciferase reporter assay, C33A cells in a
96-well plate were transfected with 50 nM miR-27b mimics or a mimic
negative control (mimic NC). Then, the cells were co-transfected
with 0.2 mg/ml of vector with the mutant or wild-type 3′UTR of
CDH11. Forty-eight hours later, luciferase activity was assayed
using the Dual-Luciferase reporter assay system (Promega, Madison,
WI, USA).
Transfection and plasmid
construction
miR-27b mimics and miR-27b inhibitor were
synthesized by GenePharma (Shanghai, China). Lipofectamine 2000
(Invitrogen) was used for the transfection of the mimics or
inhibitor. Cells were seeded in 6-well plates at a concentration of
1×105 and maintained in medium without antibiotics for
24 h before transfection. The miR-27b mimics or inhibitor were
transiently transfected into the cells. The medium was replaced
with fresh culture medium after 6 h.
CDH11-specific siRNA (Silencer™ Predesigned siRNA)
was purchased from Ambion (Shanghai, China). The CDH11 cDNA was
cloned into pcDNA3.1 to construct the CDH11 expression plasmid.
Cell proliferation and cell cycle
analysis
Transfected cells were seeded into 96-well plates
(2,000 cells/well) and then MTT (5 mg/ml) was added to each well
for 4 h. The reaction was then terminated by removal of the
supernatant followed by the addition of 200 µl of DMSO, and
absorbance readings at 490 nm were obtained in triplicate. For the
cell cycle analysis, the cells were harvested by trypsinization,
washed twice and fixed in 70% ethanol overnight at 4°C. Then the
cells were subsequently incubated with 20 mg/ml propidium iodide
(Sigma) for 20 min, and cell cycle analysis was performed using
FACS flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Wound-healing and invasion assays
HeLa cells (4×105/well) and C33A cells
(4×105/well) were seeded in 12-well plates, cultured
overnight, and transfected with the miR-27b inhibitor or mimics,
respectively. When the culture had reached nearly 80% confluency,
the cell layer was scratched with a sterile plastic tip and then
was immediately washed with growth medium twice and cultured again
in Dulbecco's modified Eagle's medium (DMEM) at 37°C for 48 h.
Wound healing was measured at 0 and 48 h. The invasion assay of the
HeLa and C33A cells was analyzed in Transwell chambers with
membranes coated with Cultrex Basement Membrane Extract (R&D
Systems, Minneapolis, MN, USA). For this purpose, 2×104
cells transfected with the miR-27b mimic or inhibitor were
suspended in 200 µl DMEM without serum and seeded on the
upper chamber. Forty-eight hours later, cells on the lower side of
the membrane were fixed, stained and counted.
Western blot analysis
Total proteins in the transfected HeLa and C33A
cells were extracted and then separated using SDS-PAGE. β-actin was
used as a loading control. Specific antibodies for E-cadherin
(1:1,500, DECMA-1, sc-59778), vimentin (1:1,500, RV202, sc-32322),
and N-cadherin (1:1,500, D-4, sc-8424) (all from Santa Cruz
Biotechnology, Santa Cruz, CA, USA) were employed for the immune
detection of the corresponding proteins. After blocking with 5%
free-fat milk in Tris-buffered saline/0.05% Tween-20 (TBST), the
membrane was incubated with a specific primary antibody and then
the secondary antibody. Proteins were visualized using ECL reagents
(Pierce, Rockford, IL, USA).
Statistical analysis
Data are presented as means ± SD from at least three
independent experiments. The Student's t-test was used to analyze
differences in experiments with cell lines. Expression of miR-27b
and CDH11 in tissues was compared by using a paired-sample t-test.
Correlation between expression levels of miR-27b and CDH11 in the
cervical cancer tissues was analyzed using Pearson's correlation
coefficient. All statistical tests were two-sided, and p<0.05
was considered to indicate a statistically significant
difference.
Results
miRNA-27 directly targets CDH11 in
cervical cancer cells and tissues
miR-27b has been reported as a tumor-suppressor or
an oncogene based on different malignancies. However, its role in
cervical carcinogenesis remains unclear. Therefore, we initially
verified the level of miR-27b in cervical cancer and HaCaT cells.
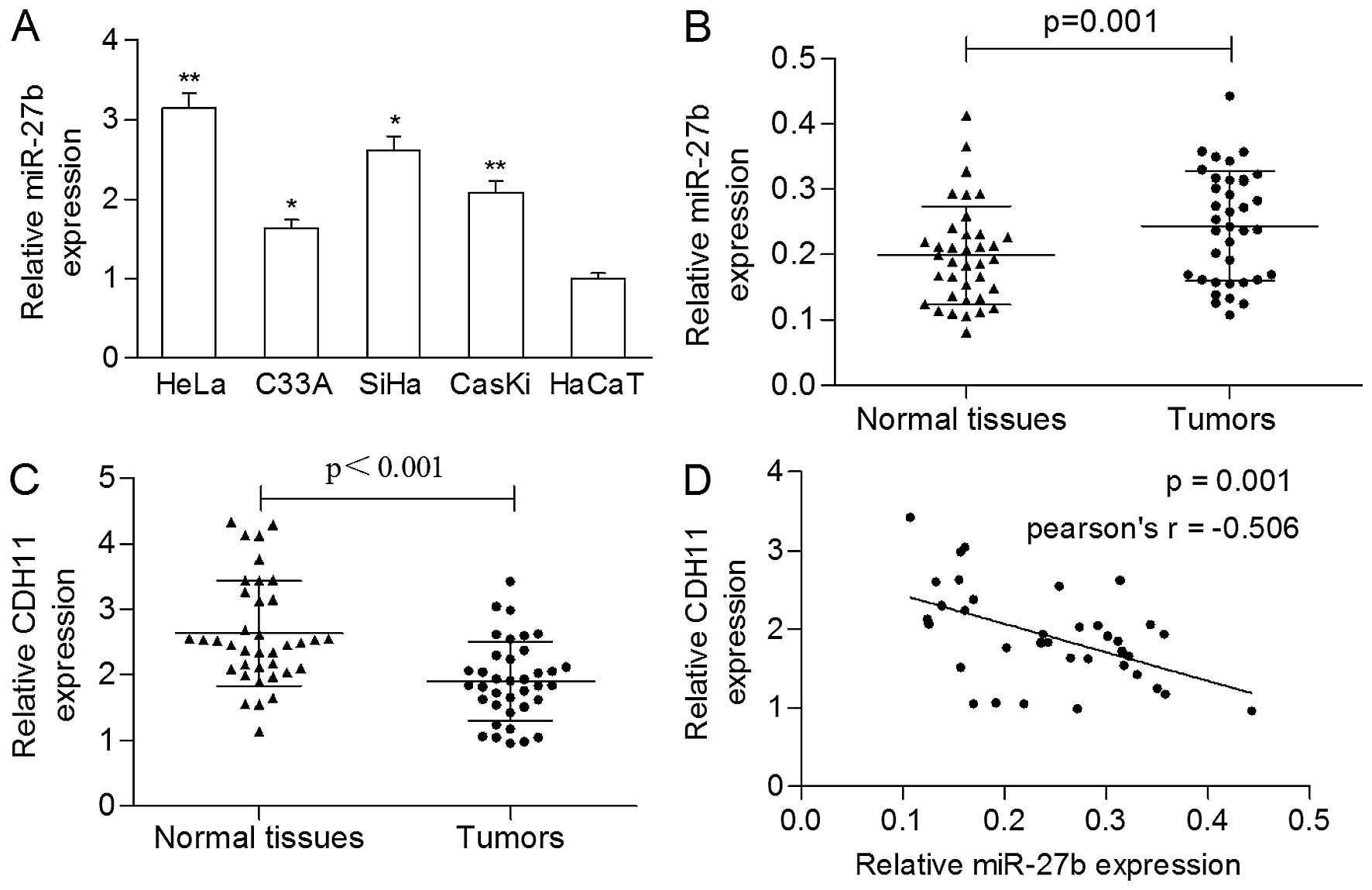
As shown in Fig. 1A, expression of
miR-27b was significantly increased in the cervical cancer cells
compared with the HaCaT cells. The expression of miR-27b in the
tumors was markedly higher in comparison to the adjacent normal
tissues (0.244±0.084 vs. 0.199±0.075, p=0.001; Fig. 1B).
Three publically available bioinformatic databases,
TargetScan, PicTar, and miRanda, were used to predict the potential
targets of miR-27b. All three databases predicted CDH11 as a
putative target of miR-27b. Thus, we detected the mRNA level of
CDH11 in clinical samples and found that the expression of CDH11
was significantly reduced in the primary tumors compared to that in
the matched nonmalignant tissues (1.907±0.600 vs. 2.631±0.800,
p<0.001; Fig. 1C). A
significantly negative correlation was found between the expression
of miR-27b and the mRNA level of CDH11 in the cancerous tissues
(Pearson r=−0.506, p=0.001; Fig.
1D).
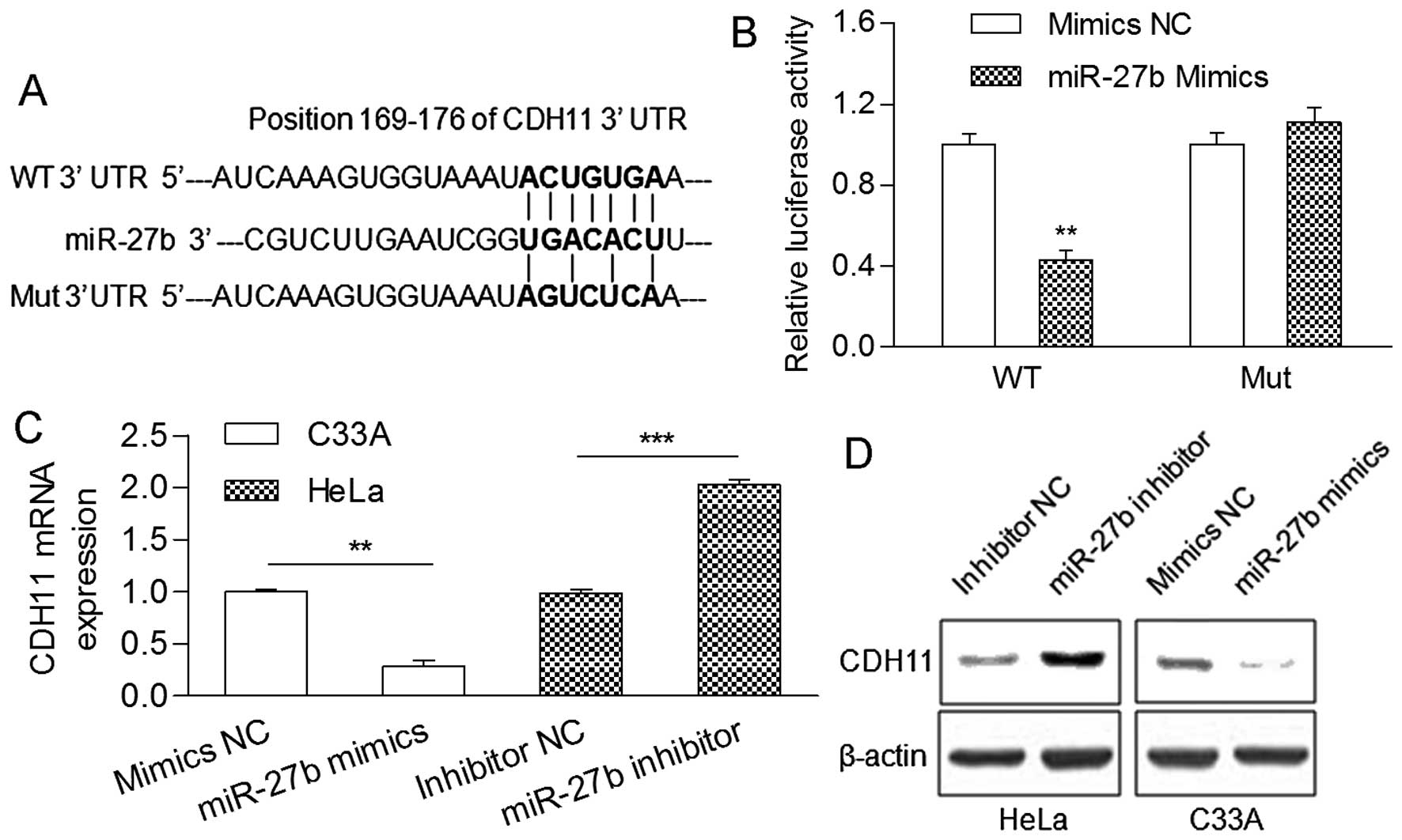
As shown in Fig. 2A,
a highly conserved position (169–176 bp) in the CDH11 3′UTR was
predicted to be a binding site of miR-27b. Then, a luciferase
reporter assay showed that the relative luciferase activity of the
reporter gene with the wild-type CDH11 3′UTR was significantly
decreased in the presence of the miR-27b mimic (Fig. 2B). Moreover, upregulation of miR-27b
by transfection with mimics in the HeLa cells reduced the mRNA and
protein levels of CDH11, while downregulation of miR-27b by
transfection with an inhibitor in the C33A cells increased mRNA and
protein levels (Fig. 2C and D),
indicating that miR-27b directly targets the CDH11 3′UTR.
Effect of miR-27b on cervical cancer cell
proliferation and invasion
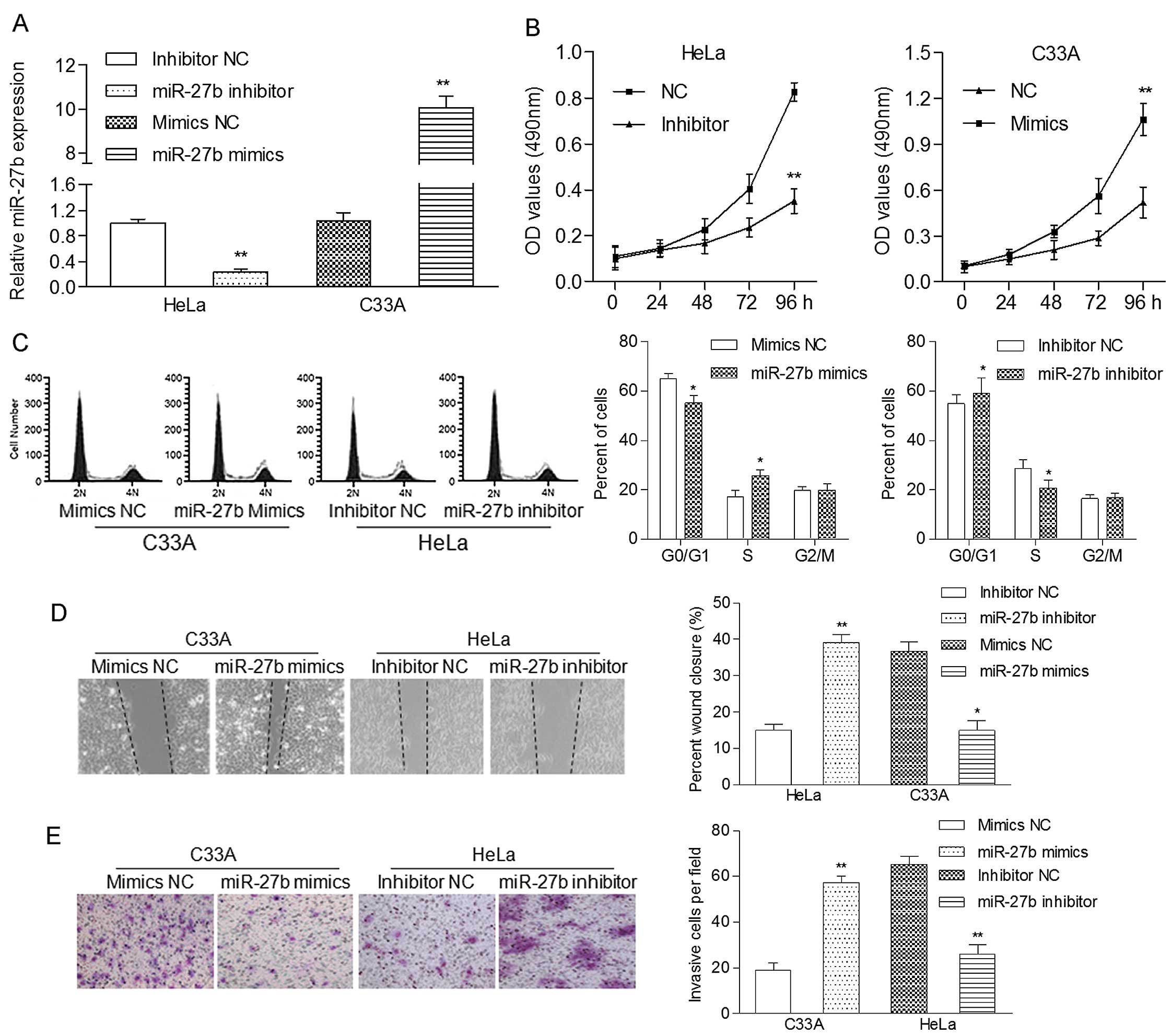
To analysis the function of miR-27b in cervical
cancer cell growth, HeLa cells expressing a relatively high level
of endogenous miR-27b and C33A cells expressing a relatively low
level of endogenous miR-27b were transfected with the miR-27b
inhibitor and miR-27b mimics, respectively. The level of miR-27b
was significantly decreased in the HeLa cells and increased in the
C33A cells when compared to the negative control (Fig. 3A). MTT analysis showed that
upregulation of miR-27b significantly promoted the proliferation
rate of the C33A cells, while downregulation of miR-27b suppressed
the proliferation of the HeLa cells (Fig. 3B). To further understand the
mechanism of how miR-27b accelerated cell proliferation, we
subsequently studied whether miR-27b plays a role in cell cycle
progression. We found that upregulation of miR-27b in the C33A
cells led to a significant reduction in the cellular population in
the G0/G1 phase but a sharp increase in the cell population in the
S phase, while suppression of miR-27b in HeLa cells obviously
induced G1 phase arrest (Fig. 3C).
Therefore, the enhancement of cell cycle progression at the G1/S
transition may be responsible for the growth-promotion role of
miR-27b in cervical cancer cells.
The migratory capability of the HeLa and C33A cells
after transfection was measured using a wound-healing assay. We
observed that miR-27b inhibitor-transfected HeLa cells had a
notably depressed migratory ability in comparison with the control
cells. miR-27b mimic-transfected C33A cells displayed an enhanced
migratory capability when compared with the control cells (Fig. 3D). Moreover, the Matrigel Transwell
assay showed that miR-27b mimics obviously increased the invasive
capability of the C33A cells, whereas the miR-27b inhibitor
suppressed this action in the HeLa cells (Fig. 3E). These results strongly indicate
that miR-27b exerts an oncogenic effect on cervical
carcinogenesis.
CDH11 is involved in miR-27b-induced
effects on cervical cancer proliferation and invasion
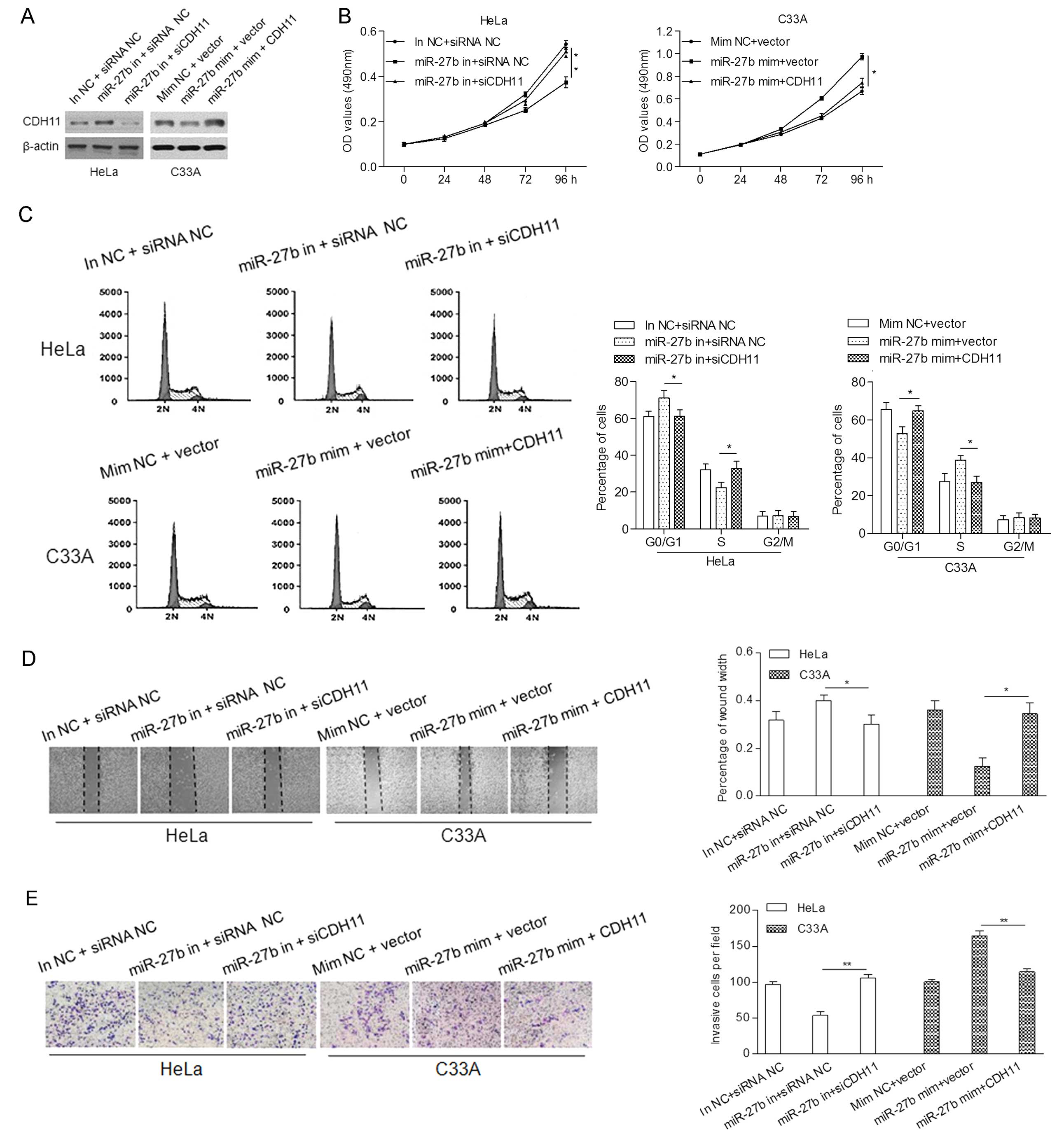
To further explore that miR-27b may be involved in
the promotion of cell proliferation, migration and invasion by
modulating CDH11, we co-transfected the HeLa cells with an miR-27b
inhibitor and siCDH11, and the C33A cells with miR-27b mimics and
CDH11 cDNA, in which the expression of CDH11 was determined by
western blot analysis (Fig.
4A).
MTT assay showed that knockdown of CDH11
significantly reversed the inhibitory effect of the miR-27b
inhibitor on cell proliferation of the HeLa cells. Overexpression
of CDH11 markedly inhibited the miR-27b-induced proliferation of
the C33A cells (Fig. 4B).
Furthermore, upregulation of CDH11 markedly abrogated the
enhancement of cell cycle progression at the G1/S transition
induced by miR-27b in the C33A cells, whereas knockdown of CDH11
rescued the G1 phase arrest induced by the miR-27b inhibitor in the
HeLa cells (Fig. 4C).
In addition, wound-healing and invasion assays
indicated that the upregulation of CDH11 attenuated miR-27b-induced
migration and invasion of the C33A cells, while silencing of CDH11
impaired the tumor suppressive role of the miR-27b inhibitor on the
HeLa cells (Fig. 4D and E).
Altogether, these data suggest that CDH11 is a functional target of
miR-27b, involved in miR-27b-induced proliferation and invasion of
cervical cancer cells.
miR-27b induces EMT
For exploring the underlying mechanism of the
promotion effect of miR-27b on migration and invasion, we further
analyzed classical markers of EMT in HeLa and C33A cells. As shown
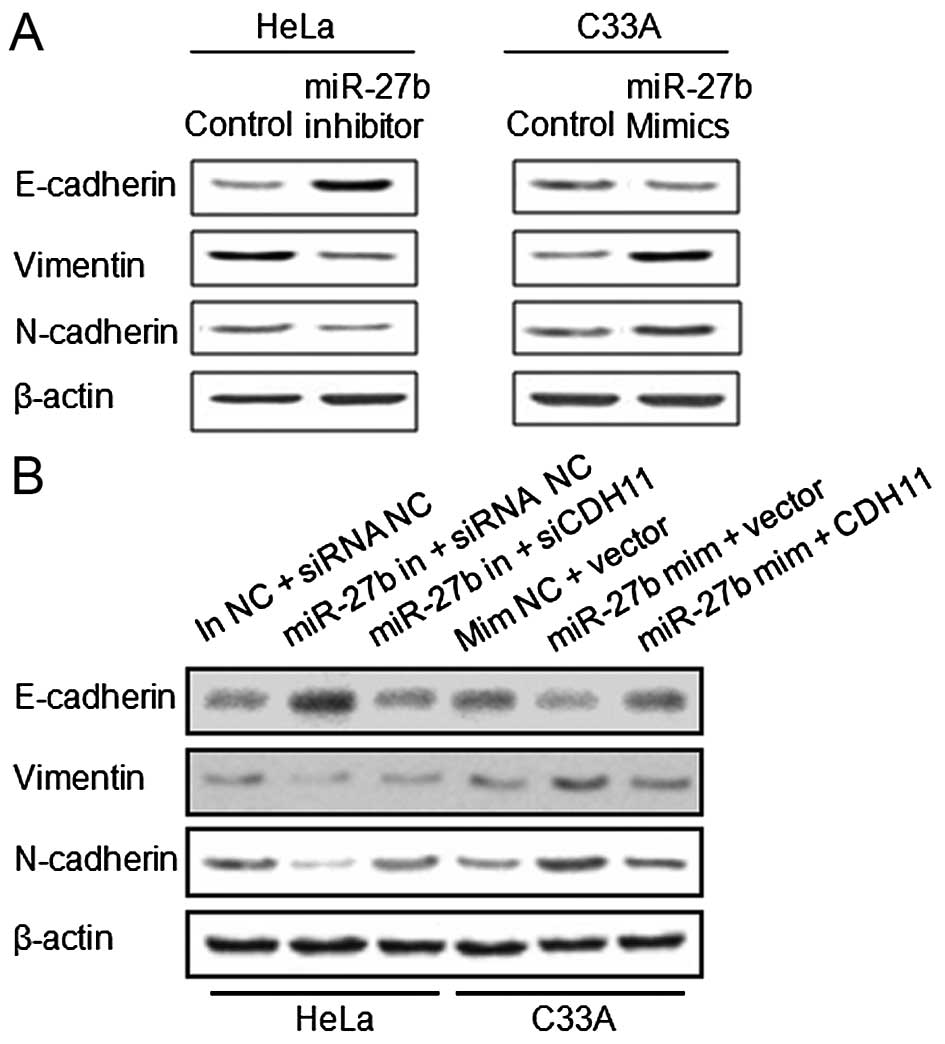
in Fig. 5A, the miR-27b inhibitor
led to elevated expression of E-cadherin and reduced expression of
vimentin and N-cadherin in the HeLa cells when compared with levels
in the control cells. In the transfected miR-27b mimic C33A cells,
we observed a decreased expression of E-cadherin and enhanced
expression of vimentin and N-cadherin in the C33A cells when
compared with these levels in the control cells. These data suggest
that activation of EMT at least partially explains the
miR-27b-induced migration and invasion of cervical cancer
cells.
Moreover, we detected the expression of EMT-related
proteins in the knockdown and overexpressing CDH11 cells,
respectively. The expression of E-cadherin was notably reduced,
while the expression of vimentin and N-cadherin was increased in
the HeLa cells co-transfected with siCDH11 and the miR-27b
inhibitor when compared to the cells transfected with the inhibitor
alone. E-cadherin was recovered, and vimentin and N-cadherin were
suppressed in the C33A cells co-transfected with the miR-27b mimic
and CDH11 in comparison with levels in the control cells (Fig. 5B). These data suggest that CDH11 is
involved in miR-27b-regulated EMT.
Discussion
Exploration of novel miRNAs involved in
carcinogenesis would provide new insights for the diagnostic
application and therapeutic advances to benefit cervical cancer
patients. Our study demonstrated for the first time to the best of
our knowledge that miR-27b plays an oncogenic role in cervical
tumorigenesis.
In the present study, we detected the expression
level of miR-27b in cervical cancer cells and tissues, and found
that miR-27b was significantly upregulated in the tumors,
suggesting an oncogenic role of miR-27b in the development of
cervical cancer. Notably, miR-27b expression patterns differ among
human cancers. miR-27b was found to be upregulated in clinical
samples of glioma and hepatocellular carcinoma (8,12),
whereas its downregulation occurred in clear cell renal cell
carcinoma (10). More importantly,
the level of miR-27b was significantly increased in metastatic
breast cancer cases and invasive endometrioid endometrial
adenocarcinomas (13,14). Thus, we hypothesized that miR-27b
may be an invasion-related gene in cancer. The relationship between
upregulation of miR-27b and clinicopathological features, such as
lymph node metastasis and distant metastasis, require further
research in a large cohort of cervical cancer cases.
Functionally, the results of the MTT assay showed
that miR-27b significantly promoted the proliferation of C33A
cells. miR-27b overexpression led to an accelerated rate of cell
migration and invasion in the C33A cells. We observed a
tumor-suppressive role of the miR-27b inhibitor on the
proliferation and migration of HeLa cells, suggesting that
downregulation of miR-27b may be used as a novel therapeutic
approach for the treatment of cervical cancer. In accordance with
our data, growth suppression and inhibition of invasion were
induced by loss of function of miR-27b in glioma cells (8). Wang et al reported that
anti-miR-27b inhibited cell invasion in MDA-MB-231 cells, while
pre-miR-27b promoted invasion (9).
However, it cannot be ignored that miR-27b has been reported as a
tumor-suppressor due to its inhibitory effect on cell proliferation
and migration (10,15,16).
These controversial studies suggest that miR-27b functions as a
tumor-suppressor or oncogene depending on tumor type and its target
in different tumors. Our data provide initial evidence that miR-27b
is an oncogene in cervical carcinogenesis by accelerating
proliferation and invasion.
EMT phenotypes have been reported to occur in
chemo-resistant human cancer cells, and the gain of the EMT
phenotype is correlated with the migratory ability of cancer cells
(17). In the present study, we
revealed for the first time that miR-27b induced EMT of cervical
cancer cells through downregulation of E-cadherin and upregulation
of vimentin and N-cadherin expression. These data suggest that
miR-27b may play a part in EMT of cervical carcinogenesis, which is
consistent with previous studies (18,19).
Moreover, many studies have found that overexpression of miR-27b is
correlated with drug-resistance in different types of cancer
(12,20,21).
Further analysis regarding the relationship between miR-27b and
chemoresistance may provide a valuable approach for the
chemotherapy of cervical cancer.
Furthermore, we identified CDH11 as a direct target
of miR-27b, showing a negative correlation between the levels of
miR-27b and CDH11 mRNA in primary tumor tissues. CDH11 a member of
the cadherin superfamily, is an integral membrane protein that
regulates cell-cell adhesion. The expression of CDH11 is
downregulated in osteosarcoma (22)
and breast cancer (23). More
importantly, several molecular studies have confirmed that CDH11 is
a novel invasion-related gene in many types of cancers (23–25).
Our data provide evidence that CDH11 is involved in invasion
probably through the regulation of EMT, and counteracts the
oncogenic effects of miR-27b in cervical cancer cells. Taken
together, our data suggest that CDH11 is an invasion-related gene
and participates in miR-27b-regulated carcinogenesis.
In conclusion, miR-27b was significantly upregulated
in human cervical cancer tissues, and upregulation of miR-27b
contributed to cell proliferation and invasion. In addition, CDH11
was identified as a direct target of miR-27b and is involved in
miR-27b-induced carcinogenesis and EMT, providing new clues for the
treatment of metastatic cervical cancer. Therefore, our results
indicate that miR-27 functions as an oncogene partially by
modulating CDH11 and can be utilized as a potential therapeutic
target in the treatment of cervical cancer.
Acknowledgments
This study was supported by grants from the National
Natural Scientific Foundation of China (no. 81171649).
References
|
1
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society: Cervical Cancer.
American Cancer Society; Atlanta, GA: 2013
|
|
3
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mukherji S, Ebert MS, Zheng GX, Tsang JS,
Sharp PA and van Oudenaarden A: MicroRNAs can generate thresholds
in target gene expression. Nat Genet. 43:854–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Li H, Han L, Zhang K, Wang G, Wang
Y, Liu Y, Zheng Y, Jiang T, Pu P, et al: Expression and function of
miR-27b in human glioma. Oncol Rep. 26:1617–1621. 2011.PubMed/NCBI
|
|
9
|
Wang Y, Rathinam R, Walch A and Alahari
SK: ST14 (suppression of tumorigenicity 14) gene is a target for
miR-27b, and the inhibitory effect of ST14 on cell growth is
independent of miR-27b regulation. J Biol Chem. 284:23094–23106.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishihara T, Seki N, Inoguchi S, Yoshino H,
Tatarano S, Yamada Y, Itesako T, Goto Y, Nishikawa R, Nakagawa M,
et al: Expression of the tumor suppressive miRNA-23b/27b cluster is
a good prognostic marker in clear cell renal cell carcinoma. J
Urol. 192:1822–1830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuo L, Liu J, Wang B, Gao M and Huang A:
Differential miRNA expression profiles in hepatocellular carcinoma
cells and drug-resistant sublines. Oncol Rep. 29:555–562. 2013.
|
|
13
|
Shen S, Sun Q, Liang Z, Cui X, Ren X, Chen
H, Zhang X and Zhou Y: A prognostic model of triple-negative breast
cancer based on miR-27b-3p and node status. PLoS One.
9:e1006642014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mozos A, Catasús L, D'Angelo E, Serrano E,
Espinosa I, Ferrer I, Pons C and Prat J: The FOXO1-miR27 tandem
regulates myometrial invasion in endometrioid endometrial
adenocarcinoma. Hum Pathol. 45:942–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang J, Lv X, Fan L, Huang G, Zhan Y,
Wang M and Lu H: MicroRNA-27b suppresses growth and invasion of
NSCLC cells by targeting Sp1. Tumour Biol. 35:10019–10023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Findlay VJ, Wang C, Watson DK and Camp ER:
Epithelial-to-mesenchymal transition and the cancer stem cell
phenotype: Insights from cancer biology with therapeutic
implications for colorectal cancer. Cancer Gene Ther. 21:181–187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai ZG, Zhang SM, Zhang H, Zhou YY, Wu HB
and Xu XP: Aberrant expression of microRNAs involved in
epithelial-mesenchymal transition of HT-29 cell line. Cell Biol
Int. 37:669–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Susuki D, Kimura S, Naganuma S, Tsuchiyama
K, Tanaka T, Kitamura N, Fujieda S and Itoh H: Regulation of
microRNA expression by hepatocyte growth factor in human head and
neck squamous cell carcinoma. Cancer Sci. 102:2164–2171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park YT, Jeong JY, Lee MJ, Kim KI, Kim TH,
Kwon YD, Lee C, Kim OJ and An HJ: MicroRNAs overexpressed in
ovarian ALDH1-positive cells are associated with chemoresistance. J
Ovarian Res. 6:182013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mu W, Hu C, Zhang H, Qu Z, Cen J, Qiu Z,
Li C, Ren H, Li Y, He X, et al: miR-27b synergizes with anticancer
drugs via p53 activation and CYP1B1 suppression. Cell Res.
25:477–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakajima G, Patino-Garcia A, Bruheim S, Xi
Y, San Julian M, Lecanda F, Sierrasesumaga L, Müller C, Fodstad O
and Ju J: CDH11 expression is associated with survival in patients
with osteosarcoma. Cancer Genomics Proteomics. 5:37–42.
2008.PubMed/NCBI
|
|
23
|
Marino N, Collins JW, Shen C, Caplen NJ,
Merchant AS, Gökmen-Polar Y, Goswami CP, Hoshino T, Qian Y, Sledge
GW Jr, et al: Identification and validation of genes with
expression patterns inverse to multiple metastasis suppressor genes
in breast cancer cell lines. Clin Exp Metastasis. 31:771–786. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delic S, Lottmann N, Jetschke K,
Reifenberger G and Riemenschneider MJ: Identification and
functional validation of CDH11, PCSK6 and SH3GL3 as novel glioma
invasion-associated candidate genes. Neuropathol Appl Neurobiol.
38:201–212. 2012. View Article : Google Scholar
|
|
25
|
Li L, Ying J, Li H, Zhang Y, Shu X, Fan Y,
Tan J, Cao Y, Tsao SW, Srivastava G, et al: The human cadherin 11
is a pro-apoptotic tumor suppressor modulating cell stemness
through Wnt/β-catenin signaling and silenced in common carcinomas.
Oncogene. 31:3901–3912. 2012. View Article : Google Scholar :
|