Introduction
Breast cancer is the most common type of cancer and
the second leading cause of cancer death in women (1). There are approximately 12–20% of
triple-negative phenotypes (estrogen and progesterone receptors,
Her2 negative) among breast cancer (2). Hormonal or Her2-trageted therapy
treatment is not beneficial for patients with triple-negative
breast cancer due to loss of these target receptors (3). Surgery and chemotherapy are the only
available approaches for patients. However, the response of
chemotherapy in triple-negative breast cancer is relatively poor
(4). Therefore, development of
effective treatment is still a critical issue for these breast
cancers. The natural compound from traditional Chinese medicine is
a potential source for identifying novel treatment.
The root of Lindera aggregata (SIMS) KOSTERM
(synonym: Lindera strychnifolia) is a traditional herbal
medicine in China (Wu Yao) and Japan (Uyaku) (5,6).
Various bioactivities including cytoxicity, improvement of insulin
sensitivity, and slowing down the progression of diabetic
nephropathy in diabetes mice were shown (5,7,8).
Isolinderalactone is one of known sesquiterpenes extracted from
root tubers of L. aggregata (9). Wong et al observed iNOS
inhibitory activity and anti-inflammatory activity of
isolinderalactone among the secondary metabolites from roots of
Neolitsea daibuensis (10).
In addition, treatment of isolinderalactone induces apoptosis and
inhibits proliferation in non-small cell lung cancer cell through
Fas/Fas ligand pathway and cell cycle arrest (11). Above all, isolinderalactone revealed
anti-inflammatory and anticancer capacity.
Recent evidence indicates that tumor development is
associated with some inflammatory signaling pathways, such as
signal transducer and activator of transcription (STAT) and Janus
kinase (JAK) pathways (12).
Furthermore, many types of natural compounds which showed
anticancer capacity usually induced apoptosis (13). Since isolinderalactone could both
suppress inflammation and induce apoptosis, we hypothesized
isolinderalactone may be a potential treatment for triple-negative
breast cancer.
In the present study, we determined whether
isolinderalactone-induced apoptosis and inhibited growth in
triple-negative breast tumor in vitro and in vivo.
The mechanism of isolinderalactone-induced apoptosis and regulation
of inflammatory signaling pathways were investigated.
Materials and methods
Cell lines and cell culture
The human breast cancer cells MDA-MB-231 and the
human kidney epithelial cells HEK293T/17 were obtained from
American Type Culture Collection (ATCC; Rockville, MD, USA).
MDA-MB-231 was cultured in Leibovitz's L-15 medium and HEK293T/17
was cultured in Dulbecco's modified Eagle's medium. Both media were
supplemented with 10% fetal bovine serum (FBS) and
penicillin/streptomycin (100 U/0.1 mg/ml). All materials for cell
culture were obtained from Invitrogen (Carlsbad, CA, USA).
Materials and antibodies
Isolinderalactone was purchased from ChemFaces
(Wuhan, China) and was dissolved in dimethylsulfoxide (DMSO).
Antibodies of Jak, phospho-Jak (Y1022/1023), cleaved PARP, signal
transducer and activator of transcription 3 (STAT3), phospho-STAT3
(S727 and Y705), AIF, XIAP, EndoG, BCL-xL, SOCS3, Bax and α-tubulin
were obtained from Cell Signaling Technology. Anti-GAPDH antibody
and anti-Lamin A/C antibody were obtained from Millipore and BD
Transduction Laboratories, respectively.
Growth inhibition and clonogenic
assay
Cell growth was evaluated by WST-1 assay (Clontech)
according to the manufacturer's instructions. MDA-MB-231 was seeded
into 96-well plates (1×105 cells/well) and incubated
overnight for attachment. Cells were treated by vehicle (0.1% DMSO)
or several concentrations of isolinderalactone in 100 µl
medium. Forty-eight hours after isolinderalactone treatment, the
medium was replaced by WST-1 containing medium for 2 h incubation.
The plate was read at a wavelength 450 nm using a PowerWave
X340 microplate spectrophotometer (Bio-Tek Instruments Inc.,
Winooski, VT, USA). The percentage of growth inhibition was
calculated using the following formula: [100 −
(ODisolinderalactone treatment/ODvehicle
control) × 100%]. OD is the abbreviation of optical density.
To determine the long-term effect of isolinderalactone, 1,000
MDA-MB-231 cells were seeded into 6 cm dish and incubated overnight
to attach. Cells were treated with isolinderalactone and vehicle
control for 6 h and then incubated in fresh medium for 14 days
(medium was replaced every 3–4 days). Colonies were stained with
crystal violet (0.4 g/l; Sigma) and the number of colonies were
counted.
LDH assay
MDA-MB-231 cells were seeded into 96-well plates
(1×105 cells/well) and treated with isolinderalactone
and vehicle control in 100 µl medium. Medium was collected
at 48 h after treatment and then was performed by LDH cytotoxicity
detection kit (Clontech) according to the manufacturer's
instruction. The plate was read at wavelength 492 nm using a
PowerWave X340 microplate spectrophotometer. The percentage
of cytotoxicity is calculated by following formula: [100% ×
(ODisolinderalactone treatment − ODblank) −
(ODvehicle − ODblank)/ODvehicle −
ODblank].
Annexin V staining
MDA-MB-231 was seeded into 6-well plates
(5×105 cells/well) and treated with isolinderalactone
and vehicle control for 48 h. Cells were harvested and analyzed by
Annexin V-FITC apoptosis detection kit (BD Biosciences). Stained
cells were determined on a BD Accuri C6 flow cytometer and the
results were analyzed by Accuri C6 software (both from BD
Biosciences).
Fluorescent caspase activity assay
The caspase 9/6 and caspase 8 were measured using
the ApoAlert caspase fluorescent assay kit (Clontech) according to
the manufacturer's instruction. In brief, after treatment of
MDA-MB-231 cells with isolinderalactone, cells were scraped and
lysed in cell lysis buffer. Equivalent amount of cell lysates were
mixed with reaction buffer and incubated at 37°C for 1 h. The
caspases 9/6 catalyzed release fluorescence with an excitation
wavelength of 380 nm and an emission wavelength of 460 nm; the
caspase 8 catalyzed release fluorescence with an excitation
wavelength of 400 nm and an emission wavelength of 505 nm. The
results were monitored using a microplate reader (FLx800 Microplate
Fluorescence Readers; Bio-Tek Instruments Inc.)
Fas ELISA assay
After isolinderalactone treatment, the cell lysate
were collected and the level of Fas was measured by Human
APO-1/FAS/CD95 ELISA kit (Novex, Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. The results were
monitored the absorbance at 450 nm using a microplate reader
(PowerWave X340).
Western blotting
After appropriate treatment in each experiment,
cells were lysed in radioimmunoprecipitation assay buffer (RIPA)
(Millipore) on ice for 30 min. The total cell lysate was then
collected after centrifugation at 4°C, 12,000 × g for 15 min. The
nuclear protein was extracted by Nuclear Extract kit (Active Motif)
according to the manufacturer's instructions. Equivalent amount of
protein was loaded and separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (6–12%) and
transferred to polyvinylidene difluoride membranes. The membrane
was blocked in 5% non-fat dry milk for 1 h and then incubated with
each primary antibody overnight and peroxidase-conjugated secondary
antibody for 1 h. The results were detected using an enhanced
chemiluminescence substrate (Millipore) on a imaging capture system
(Alpha Innovation).
Quantitative real-time PCR analysis
Total RNA of isolinderalactone-treated MDA-MB-231
cells was extracted from TRIzol reagent (Invitrogen). Complementary
DNA (cDNA) of mRNA was reverse transcribed by PrimeScript RT
reagent kit and cDNA of microRNA (miRNA) by Mir-X miRNA
First-Strand Synthesis kit (both from Clontech). The level of miRNA
was determined on StepOne Plus Real-Time PCT System using Fast
SYBR-Green Master Mix (both from Applied Biosystems). Primer
sequences are listed in Table
I.
 | Table IPrimers. |
Table I
Primers.
| Gene | Primer
sequences |
|---|
| GAPDH |
5′-GAGTCAACGGATTTGGTCGT-3′ |
|
5′-TTGATTTTGGAGGGATCTCG-3′ |
| SOCS3 |
5′-AGTCTGGGACCAAGAACCTG-3′ |
|
5′-CGGAGGAGGGTTCAGTAGGT-3′ |
| hsa-miR-19a-3p |
5′-dTGTGCAAATCTATGCAAAACTGA-3′ |
| hsa-miR-19b-3p |
5′-dTGTGCAAATCCCATGCAAAACTGA-3′ |
| hsa-miR-30b-5p |
5′-dTGTAAACATCCTACACTCAGCT-3′ |
| hsa-miR-30c-5p |
5′-dTGTAAACATCCTACACTCTCAGA-3′ |
| hsa-miR-218-5p |
5′-dTTGTGCTTGATCTAACCATGT-3′ |
| hsa-miR-203-3p |
5′-dGTGAAATGTTTAGGACCACTAG-3′ |
| hsa-miR-650 |
5′-dAGGAGGCAGCGCTCTCAGGAC-3′ |
miRNA transfection
The control miRNA mimic and hsa-miR-30c-5p miRNA
mimic were synthesized by Dharmacon. HEK-293T cells were seeded in
a 6 cm dish and transfected with miRNA mimic by DharmaFECT
transfection reagent (Dharmacon). Cells were harvested at 24 or 48
h for mRNA analysis and protein analysis, respectively.
Luciferase assay
The complete sequence of SOCS3 3′ UTR is shown in
Table II and was constructed into
the pMirTarget vector (Origene). The plasmid containing the
putative binding sequence of miR-30c 'TATTTACA' is named as 'SOCS3
3′UTR'. The mutant miR-30c binding sequence 'TGCGCGCA' (SOCS3 3′UTR
mutant) was achieved by QuikChange Lightning Site-Directed
Mutagenesis kit (Agilent Technologies). For luciferase assay, 400
nM of control miRNA or miR-30c mimics was transfected into HEK-293T
cells in 24-well plates for 12 h and then the 0.8 µg of
pMirTarget vector, SOCS3 3′UTR or SOCS3 3′UTR mutant and 0.08
µg of pRL Renilla Luciferase Control Reporter Vector
(Promega) were co-transfected for 24 h. Cells were harvested and
analyzed using the Dual-Luciferase Reporter Assay (Promega)
according to the manufacturer's directions.
 | Table IIThe sequence of SOCS3 3′UTR (from
UCSC genome Bioinformatics, https://genome.ucsc.edu/) and predictive binding site
(underline). |
Table II
The sequence of SOCS3 3′UTR (from
UCSC genome Bioinformatics, https://genome.ucsc.edu/) and predictive binding site
(underline).
| Sequence of SOCS3
3′UTR |
|---|
GGGGTAAAGGGCGCAAAGGGCATGGGTCGGGAGAGGGGACGCAGGCCCCTCTC
CTCCGTGGCACATGGCACAAGCACAAGAAGCCAACCAGGAGAGAGTCCTGTAGC
TCTGGGGGGAAAGAGGGCGGACAGGCCCCTCCCTCTGCCCTCTCCCTGCAGAAT
GTGGCAGGCGGACCTGGAATGTGTTGGAGGGAAGGGGGAGTACCACCTGAGTCT
CCAGCTTCTCCGGAGGAGCCAGCTGTCCTGGTGGGACGATAGCAACCACAAGTG
GATTCTCCTTCAATTCCTCAGCTTCCCCTCTGCCTCCAAACAGGGGACACTTCGGG
AATGCTGAACTAATGAGAACTGCCAGGGAATCTTCAAACTTTCCAACGGAACTTG
TTTGCTCTTTGATTTGGTTTAAACCTGAGCTGGTTGTGGAGCCTGGGAAAGGTGG
AAGAGAGAGAGGTCCTGAGGGCCCCAGGGCTGCGGGCTGGCGAAGGAAATGGT
CACACCCCCCGCCCACCCCAGGCGAGGATCCTGGTGACATGCTCCTCTCCCTGGC
TCCGGGGAGAAGGGCTTGGGGTGACCTGAAGGGAACCATCCTGGTACCCCACAT
CCTCTCCTCCGGGACAGTCACCGAAAACACAGGTTCCAAAGTCTACCTGGTGCCT
GAGAGCCCAGGGCCCTTCCTCCGTTTTAAGGGGGAAGCAACATTTGGAGGGGAT
GGATGGGCTGGTCAGCTGGTCTCCTTTTCCTACTCATACTATACCTTCCTGTACCT
GGGTGGATGGAGCGGGAGGATGGAGGAGACGGGACATCTTTCACCTCAGGCTCC
TGGTAGAGAAGACAGGGGATTCTACTCTGTGCCTCCTGACTATGTCTGGCTAAGA
GATTCGCCTTAAATGCTCCCTGTCCCATGGAGAGGGACCCAGCATAGGAAAGCC
ACATACTCAGCCTGGATGGGTGGAGAGGCTGAGGGACTCACTGGAGGGCACCAA
GCCAGCCCACAGCCAGGGAAGTGGGGAGGGGGGGCGGAAACCCATGCCTCCCA
GCTGAGCACTGGGAATGTCAGCCCAGTAAGTATTGGCCAGTCAGGCGCCTCGTG
GTCAGAGCAGAGCCACCAGGTCCCACTGCCCCGAGCCCTGCACAGCCCTCCCTCC
TGCCTGGGTGGGGGAGGCTGGAGGTCATTGGAGAGGCTGGACTGCTGCCACCCC
GGGTGCTCCCGCTCTGCCATAGCACTGATCAGTGACAATTTACAGGAATGTAGCA
GCGATGGAATTACCTGGAACAGTTTTTTGTTTTTGTTTTTGTTTTTGTTTTTGTGGG
GGGGGGCAACTAAACAAACACAAAGTATTCTGTGTCAGGTATTGGGCTGGACAG
GGCAGTTGTGTGTTGGGGTGGTTTTTTTCTCTATTTTTTTGTTTGTTTCTTGTTTTTT
AATAATGTTTACAATCTGCCTCAATCACTCTGTCTTTTATAAAGATTCCACCTCCA
GTCCTCTCTCCTCCCCCCTACTCAGGCCCTTGAGGCTATTAGGAGATGCTTGAAG
AACTCAACAAAATCCCAATCCAAGTCAAACTTTGCACATATTTATATTTATATTC
AGAAAAGAAACATTTCAGTAATTTATAATAAAGAGCACTATTTTTTAATGAAAAA |
Animal experiments
The use of all the animals in the present study was
approved by the Animal Care and Use Committee of the Kaohsiung
Medical University. Six-weeks old male nude mice
(BALB/cAnN.Cg-Foxn1nu/CrlNarl) were obtained from the
National Laboratory Animal Center (Taiwan) and maintained in
pathogen-free conditions. Mice were subcutaneously injected with
2×106 MDA-MB-231 cells in 200 microliter mixture of
FBS-free L-15 medium/Matrigel at a ratio of 4/1. Treatment was
initiated when the mean tumor volume reached 75 mm3. A
total of 11 mice were randomly divided into two groups. The mice in
the isolinderalactone-treated group were intraperitoneally daily
injected with isolinderalactone at a dose of 10 mg/kg from an
isolinderalactone solution (2 mg/ml) containing ~40% polyethylene
glycerol, 60% PBS and 0.1% of DMSO. The control group was
intraperitoneally injected daily with equal volume of PBS. Tumor
volume was measured using calipers and tumor volume was calculated
according to the formula: Length (mm) × width (mm) × width (mm) ×
1/2 = tumor volume (mm3). All mice were sacrificed when
the mean tumor volume reached 1,000 mm3. The liver,
kidney, and tumor were embedded in OCT for 5 µm frozen
section. General toxicity of liver and kidney was evaluated by
hematoxylin and eosin (H&E) staining. The apoptotic cells in
tumors were detected by ApoAlert DNA Fragmentation Assay kit
(Clontech) according to the manufacturer's instruction.
4′,6-Diamidino-2-phenylindol (DAPI) was used as counter-stain. The
images were collected using a confocal microscope, LSM 700 (Carl
Zeiss MicroImaging).
Statistical analysis
Difference between two independent groups were
analyzed by the Student's t-test. Comparisons between three groups
were performed using ANOVA with Dunnett's test. Significant
difference (p<0.05) between each group was considered. All
calculations were carried out using the program GraphPad Prism
version 5.03 (GraphPad Software, San Diego, CA, USA).
Results
Isolinderalactone treatment inhibits
breast cancer cell growth and colony formation
A recent report indicates that isolinderalactone
treatment inhibits cell proliferation and induces apoptosis in
human non-small cell lung cancer cells (11). To further investigate the anti-tumor
effect on triple-negative breast cancer, the triple-negative breast
cancer cell line MDA-MB-231 was treated with different doses of
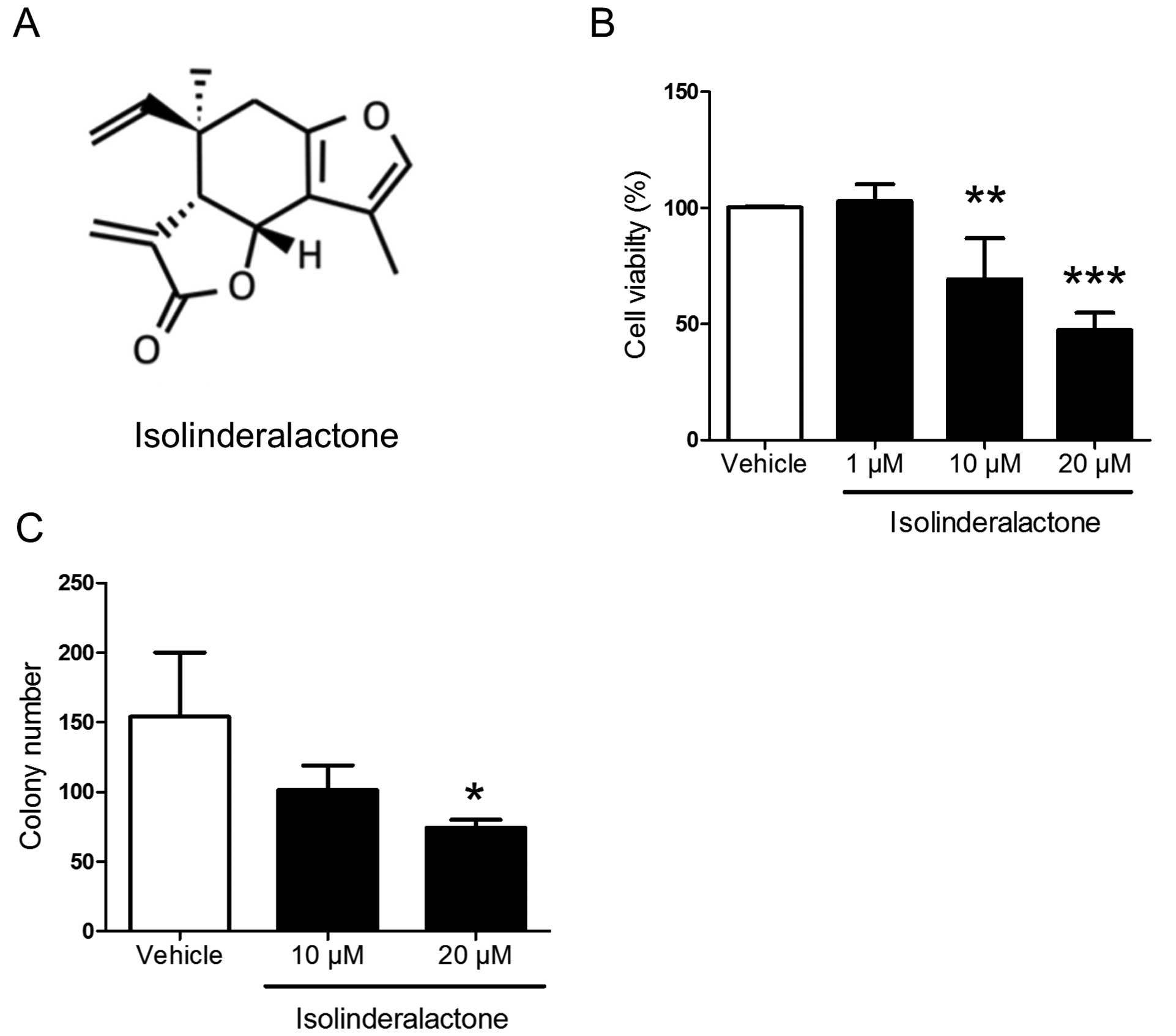
isolinderalactone. The structure of isolinderalactone is shown in
Fig. 1A. Isolinderalactone
significantly inhibited cell viability and colony formation at the
dose of 20 µM (Fig. 1). The
results indicated that isolinderalactone may serve as a novel
treatment for triple-negative breast cancer.
Isolinderalactone treatment induces
apoptosis in breast cancer cells
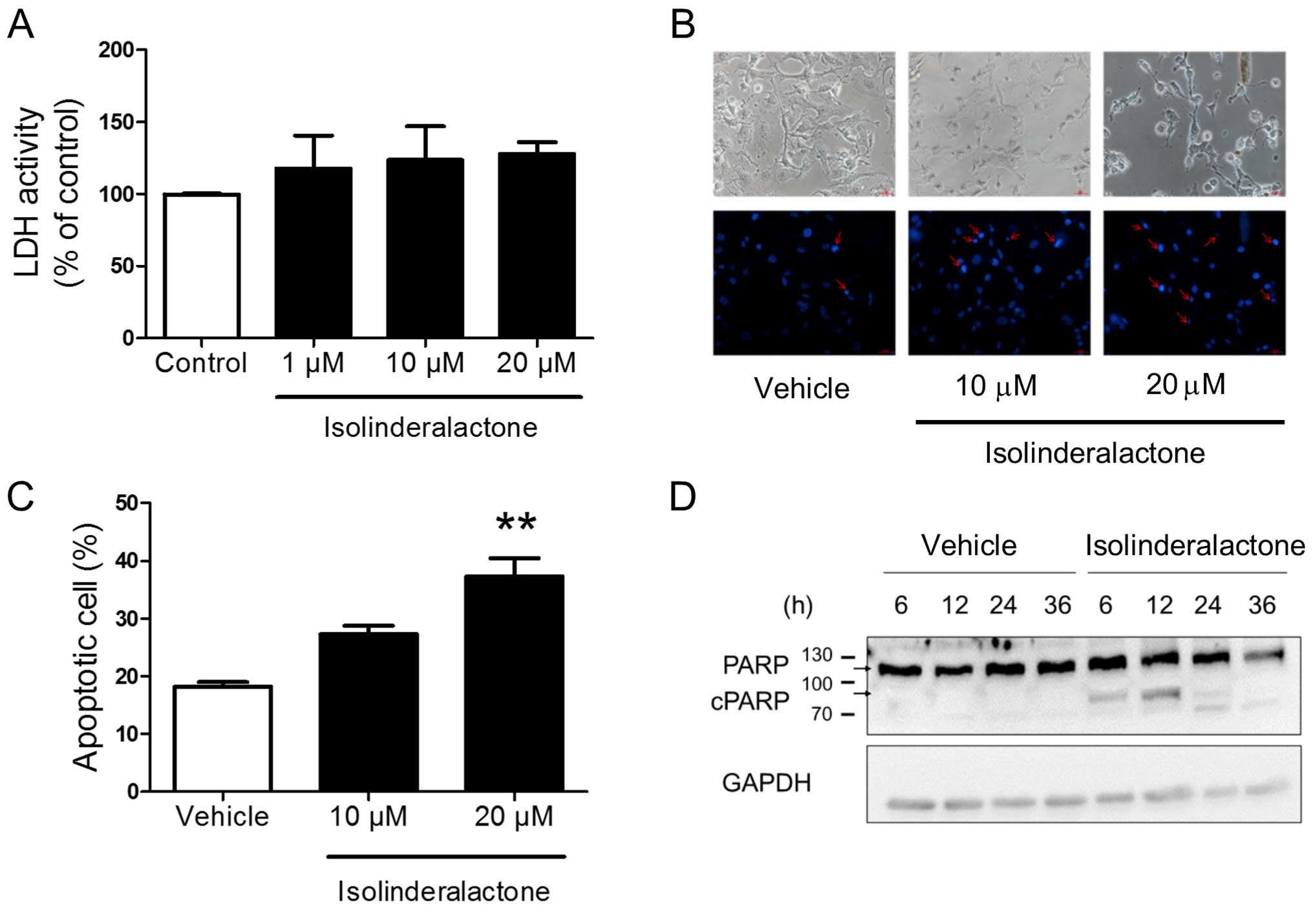
The result of lactate dehydrogenase (LDH) assay
showed that the isolinderalactone treatment did not induce necrosis
in MDA-MB-231 cells (Fig. 2A). The
morphology of isolinderalactone-treated cells revealed the
condensation of nuclear chromatin (Fig.
2B). In addition, isolinderalactone treatment increased the
Annexin V- and PI-positive population and induced poly(ADP-ribose)
polymerase (PARP) cleavage (Fig. 2C and
D). The results indicated that isolinderalactone-induced
apoptosis, but not necrosis in MDA-MB-231 cells.
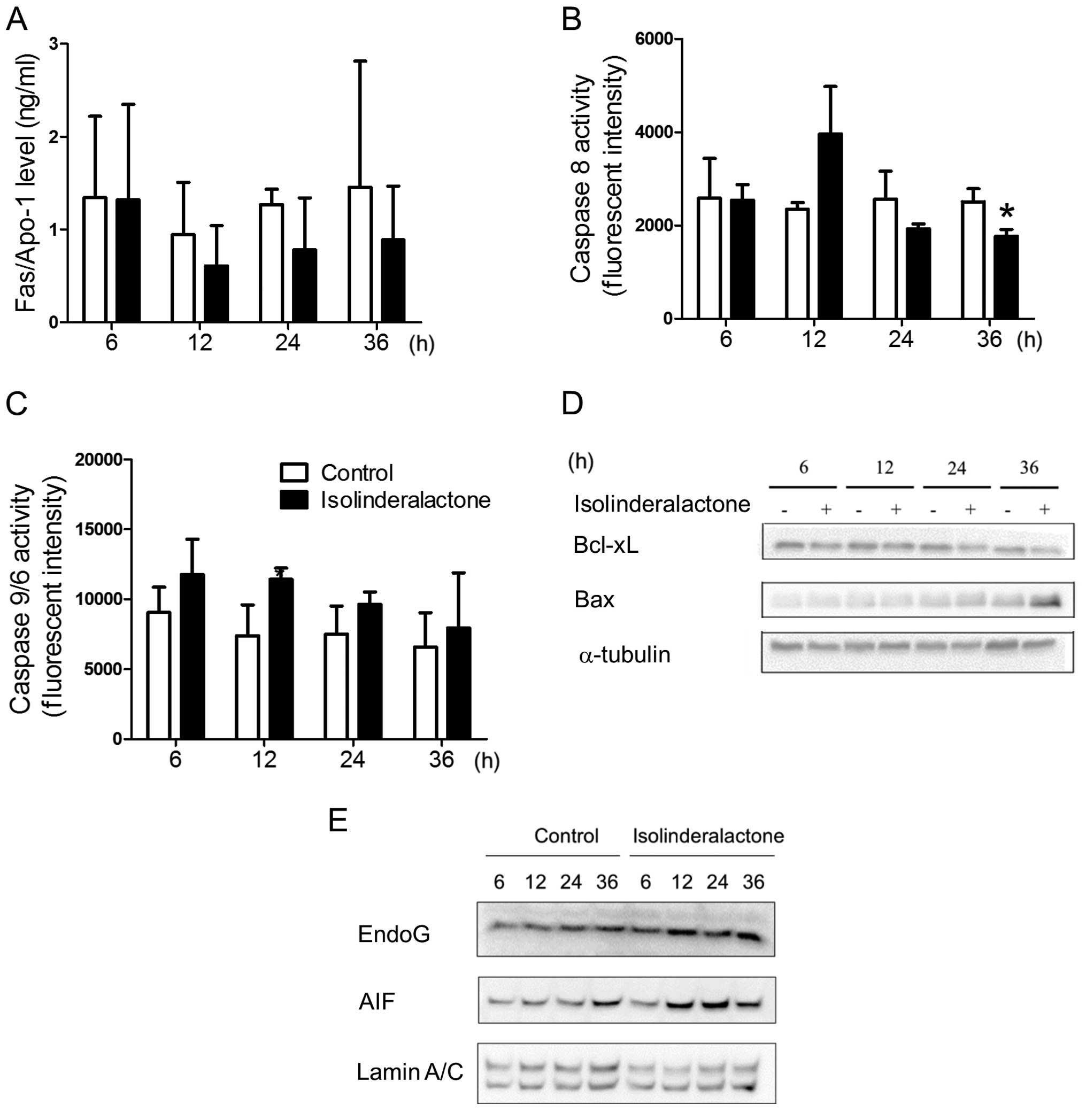
Isolinderalactone treatment induces
apoptosis in breast cancer cells through intrinsic
mitochondria-mediated and caspase-independent cell death
Isolinderalactone treatment induced apoptosis in
non-small cell lung cancer cells through Fas-mediated pathways
(11). However, the Fas level was
not affected by isolinderalactone treatment (Fig. 3A). We further determined the
isolinderalactone-induced apoptosis pathways in breast cancer
cells. Isolinderalactone treatment did not significantly induce
either caspase 8 or 9 activity (Fig. 3B
and C). Furthermore, reduced level of Bcl-xL and increased
level of Bax were detected after isolinderalactone treatment
(Fig. 3D). In addition, increased
level of apoptosis-inducing factor (AIF) and endonuclease G (EndoG)
were observed in the nucleus (Fig.
3E). The results suggest that isolinderalactone treatment
induced intrinsic mitochondria-mediated and caspase-independent
cell death.
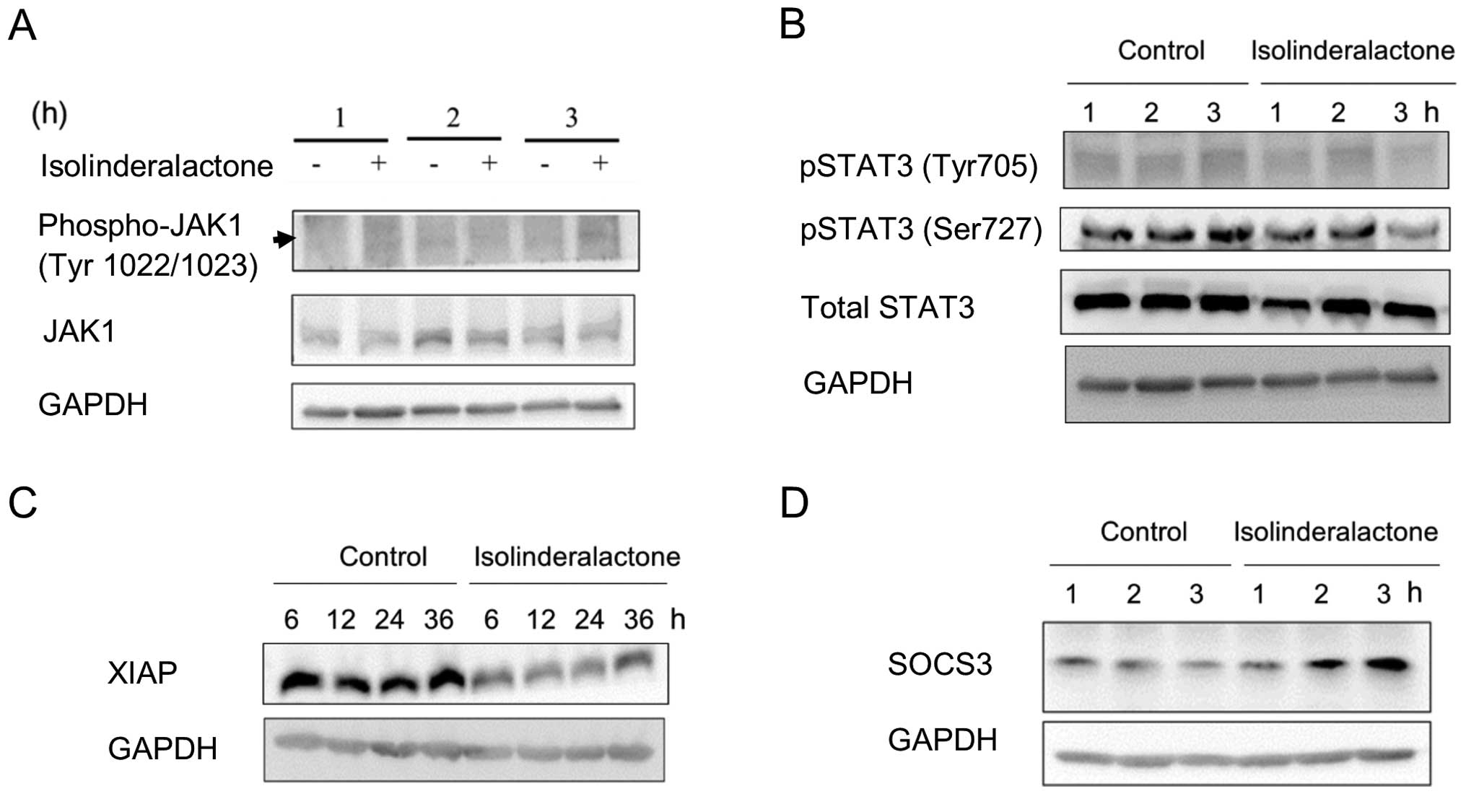
Isolinderalactone treatment suppresses
phosphorylation of STAT3 through SOCS3-dependent pathway
JAK-STAT3 signaling pathways are associated with
cell differentiation, apoptosis and inflammation. Furthermore,
activation of STAT3 signaling induces surviving gene expression and
resists apoptosis in human breast cancer cells (14,15).
Fig. 4A and B, shows that the level
of JAK1, phosphorylated-JAK1 and STAT3 was not affected by
isolinderalactone. However, the level of phospho-STAT3 (Ser727) was
suppressed in 3 h treatment. Decreased level of the molecule X
chromosome-linked inhibitor of apoptosis (XIAP) was detected after
treatment (Fig. 4C). The results
indicated that STAT3 signaling was suppressed by isolinderalactone
treatment. Suppressor of the cytokine signaling 3 (SOCS3) is a
repressor of STAT3 signaling pathway (16). The induction of SOCS3 was observed
at similar time point with downregulation of STAT3 phosphorylation
after treatment (Fig. 4D). It may
imply that the STAT3 repression is mediated through SOCS3
induction. In addition, the result may suggest isolinderalactone
induces SOCS3 expression.
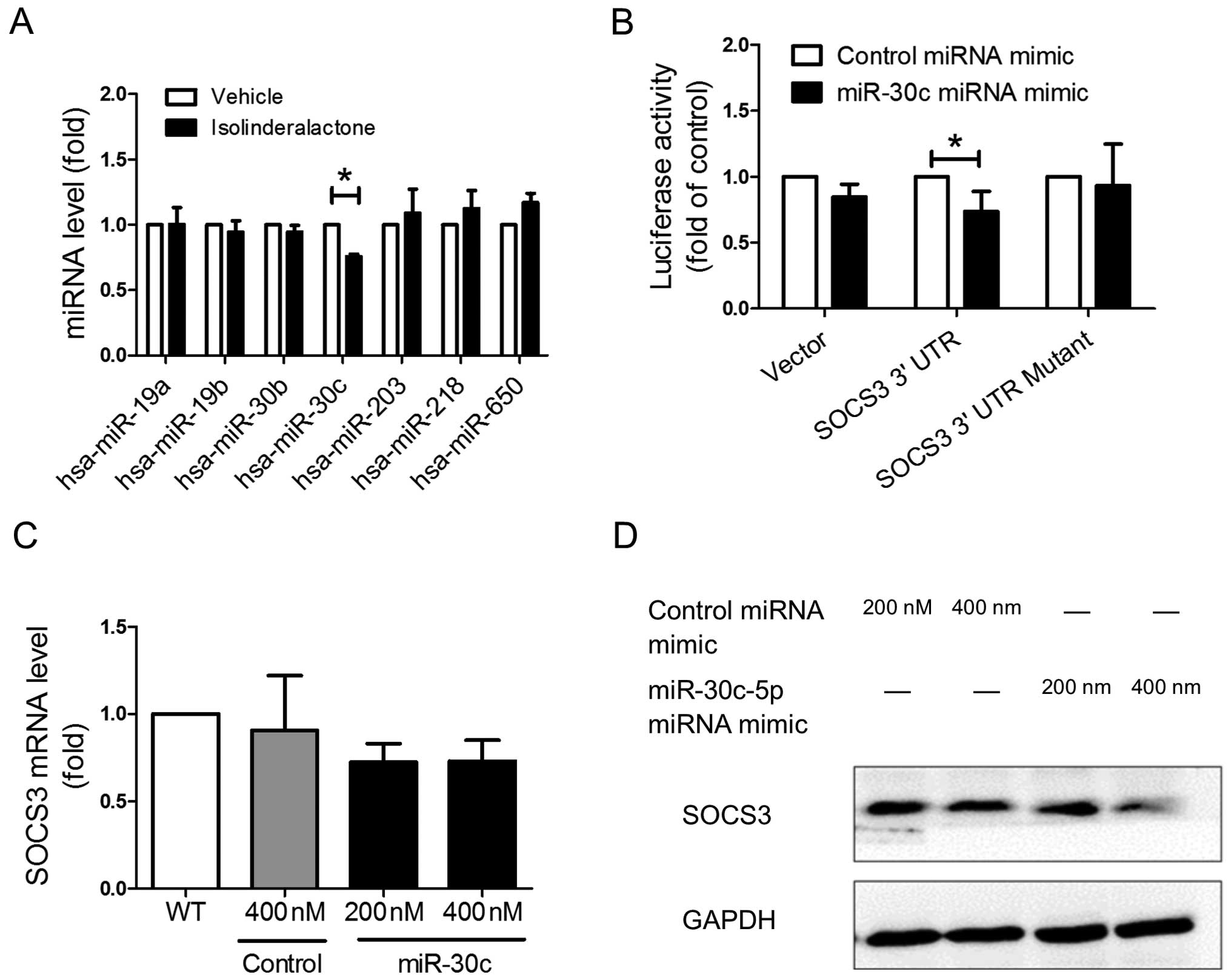
Isolinderalactone treatment enhances
SOCS3 expression through inhibition of miR-30c expression
In various types of cancer, several kinds of
micro-RNA (miRNA) regulate STAT3 signaling pathway through
inhibition of SOCS3 expression (17–19).
In order to investigate whether isolinderalactone treatment induce
SOCS3 through regulation of miRNA, the miRNAs which targeted the 3′
untranslated region (3′UTR) of SOCS3 were predicted by TargetScan
website (v6.2; http://targetscan.org) (20,21).
The level of miRNAs which may target on SOCS3 was analyzed by Q-PCR
after treatment. Isolinderalactone treatment significantly
suppressed the level of the hsa-miR30c-5p (miR-30c), but did not
affect other miRNAs (Fig. 5A). The
reporter plasmids which contain wild-type SOCS3 3′UTR, or putative
miR-30c binding site mutated SOCS3 3′UTR were constructed for
determining the binding capacity of miR-30c. The reporter assay
demonstrated that miR-30c could bind the 3′UTR region of SOCS3
(Fig. 5B). Overexpression of
miR-30c decrease the protein level of SOCS3 but did not affect the
mRNA level of SOCS3 (Fig. 5C and
D). The results suggested that regulation of SOCS3 by miR-30c
was mainly through suppression of translation efficiency.
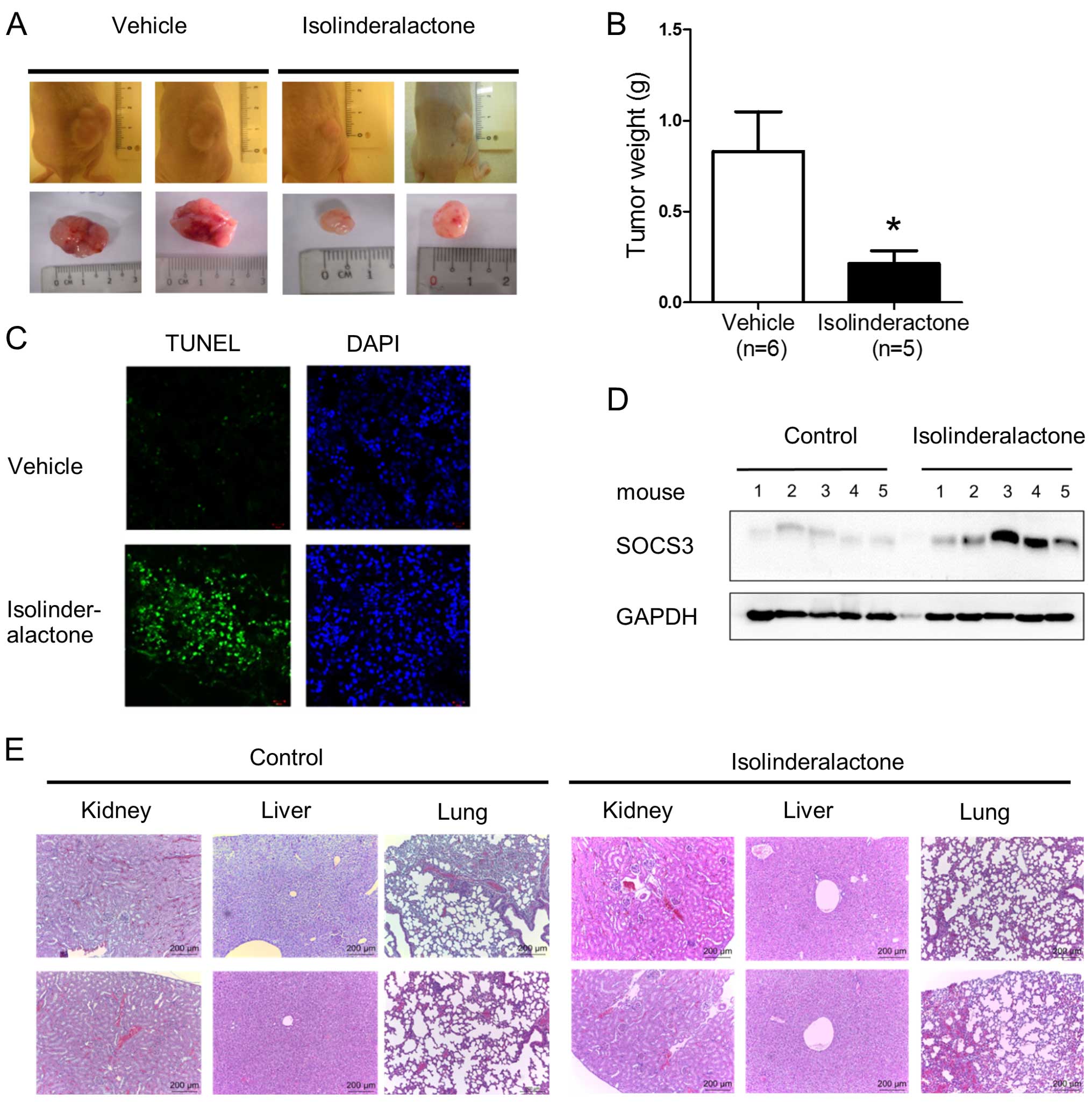
Isolinderalactone treatment inhibits
tumor growth and induces apoptosis in a xenograft breast tumor
model
We further investigated whether isolinderalactone
treatment inhibited tumor growth in the animal tumor model. In the
xenograft tumor model, the tumor size and tumor weight of the
isolinderalactone treated-mice is smaller than that of
vehicle-treated mice (Fig. 6A and
B). Isolinderalactone treatment also induced apoptosis and
SOCS3 expression in the tumor (Fig. 6C
and D). Finally, we examined whether isolinderalactone induced
toxicity on liver, kidney and lung of mice. No significant
histology between vehicle and isolinderalactone treatment groups
was noted (Fig. 6E).
Isolinderalactone induced only slight hypokalemic nephropathy. The
results indicated isolinderalactone treatment suppressed tumor
growth, induced apoptosis and SOCS3 expression without significant
toxicity in mice.
Discussion
Isolinderalactone treatment inhibited proliferation
and colony formation in MDA-MB-231 cells (Fig. 1). When the cells were treated with a
dose of 20 µM, apoptosis was significantly induced (Fig. 2). Induction of apoptosis could be
through intrinsic and extrinsic pathways (22). In extrinsic pathway, the level of
Fas was not affected after isolinderalactone treatment. Besides,
caspase 8 activity was not significantly induced. The results
suggested that the apoptosis was via the intrinsic pathway.
Although isolinderalactone treatment did not increase the activity
of caspase 9, it increased the level of Bcl-xL and decreased the
level of Bax. Therefore, the isolinderalactone-induced apoptosis
may be through caspase 9-independent intrinsic apoptosis pathway
(23). AIF and EndoG are
mitochondrial pro-apoptotic proteins and translocate to the nucleus
during mitochondria-mediated apoptosis (24,25).
The level of AIF and EndoG was significantly induced in the nucleus
after treatment. The results suggest that isolinderalactone
treatment cause intrinsic mitochondria-mediated and
caspase-independent cell death in MDA-MB-231 cells.
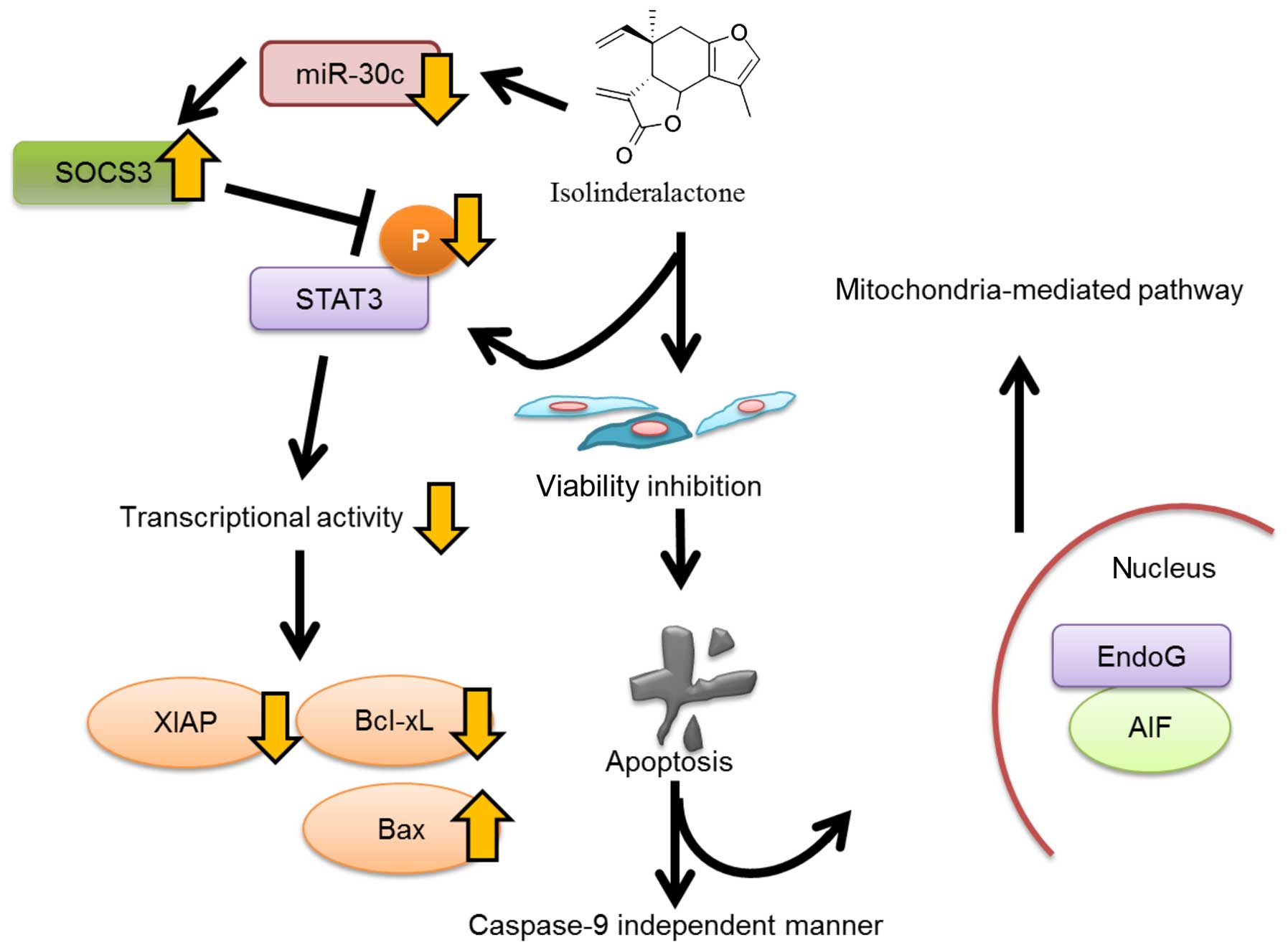
We demonstrated that isolinderalactone treatment
inhibited the phosphorylation of STAT3 and STAT3 signaling pathway.
In Fig. 4C, the decreased XIAP
protein level was observed in treated cells since XIAP is an
inhibitor of apoptosis and a downstream target for STAT3 activation
(26,27). In addition, the oncogenic STAT3
activation increased expression of prosurvival proteins including
survivin, Bcl-2 and Bcl-xL (15).
Thus, the decreased Bcl-xL protein level was detected in Fig. 3D. The evidence indicates that STAT3
plays an important role in isolinderalactone-mediated
apoptosis.
SOCS3 is a repressor of STAT3 signaling pathway. In
addition, SOCS3 plays a negative feedback regulator of STAT3
(16). In the present study, the
induction of SOCS3 and the repression of STAT3 phosphorylation was
observed at similar time point after isolinderalactone treatment
(Fig. 4B and D). It may suggest
upregulation of SOCS3 is not regulated by negative feedback pathway
of STAT3, but isolinderalactone treatment. Several studies
indicated miRNAs such as miR-19b and miR-221 are involved in
regulating SOCS3-mediated repression of STAT3 activation (17–19).
After screening all putative miRNA targeting SOCS3 3′UTR, miR-30c
was the only miRNA which was affected by isolinderalactone.
Micro-RNAs regulate target gene expression through messenger RNA
(mRNA) degradation and translation control (28). In the present study, our results
further suggest that miR-30c targeted SOCS3 3′UTR and interfered in
translation efficiency of SOCS3 (Fig.
5).
Isolinderalactone induced apoptosis and SOCS3
expression not only in vitro but also in vivo. In
addition, no significant toxicity was observed in lung, liver and
kidney of isolinderalactone-treated mice. In summary,
isolinderalactone inhibits STAT3 activation since SOCS3 expression
is enhanced by decreasing miR-30c expression in MDA-MB-231 cells
(Fig. 7). It shows promise in a
future treatment for triple-negative breast cancer.
Acknowledgments
The present study was supported by grants from the
National Science Council (NSC; 102-2628-B-037-002-MY3,
102-2632-B-037-001-MY3 and 102-2314-B-037-035-MY3), the Ministry of
Science and Technology (MOST; 104-2320-B-037-014-MY3,
104-2314-B-037-053-MY4 and 103-2320-B-037-006-MY3), and the
Kaohsiung Medical University 'Aim for the Top 500 Universities
Grant' (grant no. KMU-DT105010).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9(Suppl 2): S73–S81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wahba HA and El-Hadaad HA: Current
approaches in treatment of triple-negative breast cancer. Cancer
Biol Med. 12:106–116. 2015.PubMed/NCBI
|
|
4
|
Ismail-Khan R and Bui MM: A review of
triple-negative breast cancer. Cancer Control. 17:173–176.
2010.PubMed/NCBI
|
|
5
|
Ohno T, Nagatsu A, Nakagawa M, Inoue M, Li
YM, Minatoguchi S, Mizukami H and Fujiwara H: New sesquiterpene
lactones from water extract of the root of Lindera strychnifolia
with cytotoxicity against the human small cell lung cancer cell,
SBC-3. Tetrahedron Lett. 46:8657–8660. 2005. View Article : Google Scholar
|
|
6
|
Wang F, Gao Y, Zhang L, Bai B, Hu YN, Dong
ZJ, Zhai QW, Zhu HJ and Liu JK: A pair of windmill-shaped
enantiomers from Lindera aggregata with activity toward improvement
of insulin sensitivity. Org Lett. 12:3196–3199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Gao Y, Zhang L and Liu JK:
Bi-linderone, a highly modified methyl-linderone dimer from Lindera
aggregata with activity toward improvement of insulin sensitivity
in vitro. Org Lett. 12:2354–2357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohno T, Takemura G, Murata I, Kagawa T,
Akao S, Minatoguchi S, Fujiwara T and Fujiwara H: Water extract of
the root of Lindera strychnifolia slows down the progression of
diabetic nephropathy in db/db mice. Life Sci. 77:1391–1403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi W, Miyase T, Sano M, Umehara K,
Warashina T and Noguchi H: Prolyl endopeptidase inhibitors from the
roots of Lindera strychnifolia F. Vill. Biol Pharm Bull.
25:1049–1052. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong SL, Chang HS, Wang GJ, Chiang MY,
Huang HY, Chen CH, Tsai SC, Lin CH and Chen IS: Secondary
metabolites from the roots of Neolitsea daibuensis and their
anti-inflammatory activity. J Nat Prod. 74:2489–2496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang WA, Lin ES, Tsai MJ, Huang MS and
Kuo PL: Isolinderalactone inhibits proliferation of A549 human
non-small cell lung cancer cells by arresting the cell cycle at the
G0/g1 phase and inducing a Fas receptor and
soluble Fas ligand-mediated apoptotic pathway. Mol Med Rep.
9:1653–1659. 2014.PubMed/NCBI
|
|
12
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song H, Wang R, Wang S and Lin J: A
low-molecular-weight compound discovered through virtual database
screening inhibits Stat3 function in breast cancer cells. Proc Natl
Acad Sci USA. 102:4700–4705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White CA and Nicola NA: SOCS3: An
essential physiological inhibitor of signaling by interleukin-6 and
G-CSF family cytokines. JAK-STAT. 2:e250452013. View Article : Google Scholar
|
|
17
|
Collins AS, McCoy CE, Lloyd AT, O'Farrelly
C and Stevenson NJ: miR-19a: An effective regulator of SOCS3 and
enhancer of JAK-STAT signalling. PLoS One. 8:e690902013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kneitz B, Krebs M, Kalogirou C, Schubert
M, Joniau S, van Poppel H, Lerut E, Kneitz S, Scholz CJ, Ströbel P,
et al: Survival in patients with high-risk prostate cancer is
predicted by miR-221, which regulates proliferation, apoptosis, and
invasion of prostate cancer cells by inhibiting IRF2 and SOCS3.
Cancer Res. 74:2591–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel K, Kollory A, Takashima A, Sarkar S,
Faller DV and Ghosh SK: MicroRNA let-7 downregulates STAT3
phosphorylation in pancreatic cancer cells by increasing SOCS3
expression. Cancer Lett. 347:54–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:42015. View Article : Google Scholar
|
|
21
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peter ME: Programmed cell death: Apoptosis
meets necrosis. Nature. 471:310–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arnoult D, Gaume B, Karbowski M, Sharpe
JC, Cecconi F and Youle RJ: Mitochondrial release of AIF and EndoG
requires caspase activation downstream of Bax/Bak-mediated
permeabilization. EMBO J. 22:4385–4399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al:
Essential role of the mitochondrial apoptosis-inducing factor in
programmed cell death. Nature. 410:549–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LY, Luo X and Wang X: Endonuclease G is
an apoptotic DNase when released from mitochondria. Nature.
412:95–99. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahashi R, Deveraux Q, Tamm I, Welsh K,
Assa-Munt N, Salvesen GS and Reed JC: A single BIR domain of XIAP
sufficient for inhibiting caspases. J Biol Chem. 273:7787–7790.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Obexer P and Ausserlechner MJ: X-linked
inhibitor of apoptosis protein - a critical death resistance
regulator and therapeutic target for personalized cancer therapy.
Front Oncol. 4:1972014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|