Introduction
Lung cancer, the leading cause of cancer morbidity,
accounts for 13% (1.6 million) of total carcinoma cases and 18%
(1.4 million) of the deaths in 2008 (1). Current treatments include surgery,
radiotherapy, chemotherapy and immunotherapy. Multiple therapies
improve the survival rate of non-small cell lung carcinoma (NSCLC)
patients, but the median 5-year survival rate is still only 15%
(2). The outcome of patients with
NSCLC remains poor, and distant lymph node metastasis is one of the
most important prognostic variables. Recent studies have suggested
that lymphangiogenesis, the formation of new lymphatic vessels
induced by tumors, was directly correlated with the extent of lymph
node metastasis of solid tumors. The degree of lymphatic vessel
density (LVD) could quantify tumor lymphangiogenesis. High LVD was
correlative with poor outcome (3,4).
Blood and lymphatic vessels are essential for the
transport of fluids, gases, macromolecules and cells within the
large and complex bodies of vertebrates (5). New blood and lymphatic vessels in
tumors can grow by sprouting from pre-existing vessels or by
recruitment of rare, circulating, bone marrow-derived endothelial
progenitor cells (6,7). The molecular basis of
lymphangiogenesis is only partially understood due to an overlap
between markers of endothelial cells (ECs) and lymphatic
endothelial cells (LECs), the lack of specific lymphatic molecular
markers, and the unavailability of good experimental models. The
most studied mediators of lymphangiogenesis are the members of the
vascular endothelial growth factor (VEGF) family, which are crucial
players in the regulation of lymphangiogenesis as well as
angiogenesis.
High expression of VEGF-C and VEGF-D has been
confirmed in clinicopathological studies, such as NSCLC, breast and
colon cancer, malignant melanoma, head and neck and prostate
cancer. Furthermore, LVD and lymphatic vessel invasion (LVI) are
closely related to lymph node metastasis. With LEC isolation and
establishment of lymphangiogenesis in an animal model, combined
with some known molecular marker of LECs, such as VEGF receptor-3
(VEGFR-3), Prox-1, LYVE-1 and podoplanin, lymphangiogenesis study
becomes a hot spot in tumor research (8). Our recent study showed that tumor
cells overexpressing VEGF-C could induce lymphangiogenesis in
surrounding tumor cells and promote invasion of lymphatic vessels,
which is a key step in the metastasis of primary tumors to draining
lymph nodes (9).
Insulin-like growth factor binding protein 7
(IGFBP7), a secreted 31-kDa protein, is a related member of the
IGFBP family (10). Unlike the
other family members (IGFBP1-6), it exhibits a low affinity for
IGF, but a high and specific affinity for insulin (11), and also confers a level of
regulation to the IGF signaling system. It is also seen in
endometrial glands expressing high levels of IGFBP7 during the
mid-secretory phase of the menstrual cycle (12). Varied IGFBP7 expression patterns
have been reported in different tumor types (13). Transient expression of the IGFBP7
protein in IGFBP7-deficient cells blocks cell proliferation by
causing senescence, apoptosis or delay of the cell cycle (14). A previous study provided evidence
for pro-angiogenic function of IGFBP7 in human brain endothelial
cells (HBECs) (15). In other
studies, IGFBP7 was reported to act as a tumor-suppressor through
the regulation of cell proliferation, cell adhesion, apoptosis,
cellular senescence and angiogenesis (16). IGFBP7 accumulates in the
capillary-like tubes of vascular endothelial cells in vitro
(17). According to bioinformatics
and biological function classification, IGFBP7 was included into
the superfamily members of the extracellular matrix
metalloproteases (3). In our study,
to screen genes related to lymphangiogenesis in lung adenocarcinoma
by Human Genome U133 Plus 2.0 Array, we established a
differentially expressed cDNA library based on lymphatic vessel
density. IGFBP7 was selected for further experiments in vivo
and in vitro, based on the bioinformatics analysis and
literatures review.
Materials and methods
Patients and tissues
The present study included 97 patients with NSCLC
who underwent either lobectomy or pneumonectomy at Southwest
Hospital, Xinqiao and Daping Hospitals, between February 2005 and
February 2008. Among 97 samples, 34 lung adenocarcinoma samples
were sliced into two parts, and one part was placed into the liquid
nitrogen immediately, this subset was preserved for extracting mRNA
and differential gene screening. The other part was formalin-fixed,
paraffin-embedded NSCLC tissues that were retrieved from the files
of our pathology department. Pathological stage was re-evaluated
and determined with the present TNM classification as revised in
the WHO 2004 classification criteria. Tissue blocks containing a
representative fraction of the tumor and the tumor-lung parenchyma
interface were used. Operative tissues were also embedded with
paraffin from the 97 patients with NSCLC. The study was approved by
the Ethics Committee (Faculty of Medicine, Third Military Medical
University).
Animals
Specific pathogen-free female C57BL/6 mice (4–6
weeks old) and female BALB/c mice (6 weeks old) were purchased from
the Institute of Experimental Animal of Third Military Medical
University (Chongqing, China). All animals had free access to
standard laboratory mouse feed and water. The present study was
conducted in accordance with the national and regional guidelines
for the care and use of laboratory animals.
Cell lines
Murine Lewis Lung Cancer (LLC) and L929 (murine
fibroblast) cells were purchased from American Type Culture
Collection (ATCC; Manassas, VA, USA), and were maintained in
Dulbecco's modified Eagle's medium containing 10% fetal bovine
serum (FBS) (both from Invitrogen, Carlsbad, CA, USA). Isolation
and culture of LECs was performed as previously described (18). Briefly, female BALB/c mice were
intraperitoneally injected with emulsified incomplete Freund's
adjuvant (Sigma-Aldrich, St. Louis, MO, USA) to induce
lymphangiomas. After 2 months of induction, tumors in the
peritoneal cavity were removed and mechanically disrupted. LECs
were isolated and resuspended in endothelial cell growth supplement
(EBM-2; Cambrex BioScience, Wokingham, UK) with 20% FBS, and 50
ng/ml endothelial cell growth supplement (Cambrex BioScience) at
37°C in a humidified atmosphere of 5% CO2. LECs were
used in appropriate experiments or cultivated till the fourth
passage/phase.
Co-cultivation and tube-like structure
formation assay
LECs (6×104 cells/well) were put into a
24-well plate that had been pre-coated with 100 Matrigel (10 mg/ml;
Clontech, Palo Alto, CA, USA) and cultured for 24 h. Transwell
upper inserts were then placed into the co-cultivation system. The
L929, LLC and Si-IGFBP7 LLC cells, respectively, were seeded into
the Transwell upper inserts of chambers consisting of polycarbonate
membranes (0.4-mm pore size; Millipore, Billerica, MA, USA). The
cells in the upper inserts were LECs at 1×104
cells/well, and were co-cultured for 48 h. Cells that were cultured
in basal medium were used as a control. Formation of tube-like
structures was monitored by microscopic observation at a
magnification of ×100 over 6 different fields of each well. They
were then photographed to measure the length of tube-like
structures as previously described (19).
Immunohistochemistry and LMVD
Serial 5-µm thick sections prepared from
formalin-fixed, paraffin-embedded tissues from radical
prostatectomy specimens were used for the study. Tissue blocks that
contained the maximum amount of tumor and highest Gleason score
were selected for each case, in order to ensure that the
representative blocks contained cancer of the same Gleason score as
the overall score of the case, but recognizing the limitation of
sample variation. Slides from these representative blocks were
analyzed. Slides were deparaffinized in xylene twice for 5 min and
rehydrated through graded ethanol solutions to distilled water.
Antigen retrieval was carried out by heating sections in 0.1 mol/l
citrate buffer, pH 6.0, in a pressure steamer for 20 min.
Endogenous peroxidase activity was inactivated by incubation in 3%
H2O2 for 15 min. Non-specific binding sites
were blocked using protein block (Dako Corp., Carpinteria, CA, USA)
for 20 min. The slides were then incubated sequentially with
primary antibody (clone podoplanin, prediluted antibody; Abcam
Inc., Cambridge, MA, USA), biotinylated secondary antibody,
avidin-peroxidase complex and chromogenic substrate
diaminobenzidine. Positive and negative controls were run in
parallel with each batch and demonstrated that the procedure
functioned properly (20).
At least 6 random fields per cross-section were
visualized at a magnification of ×20 and used for image analysis
that was performed with the NIS-Elements Advanced Research 2.3
imaging software (image pro-plus), which identifies signals by
threshold key intensity values. Furthermore, it permits imposing
restrictions to the measurements by excluding false positive
signals. Briefly, the number of positive cells expressing a
particular antibody was calculated as a percent of the region of
interest (ROI), as indicated in the individual figure legend.
Co-localization of two antibodies was calculated by converting the
area occupied by cells positive for the first antibody into a ROI.
Then the percent of cells that were positive for the second
antibody was calculated within the ROI (21).
Total RNA isolation and DNA microarray
analysis
Total RNA was isolated from cultured cells using a
single-step procedure with the TRIzol reagent (Invitrogen,
Gaithersburg, MD, USA). The cells were lysed to extract the total
RNA. The quality of total RNA was excellent, as deemed by measuring
the 260/280 nm ratio (>2.0) and was then used for further cDNA
synthesis and a chip hybridization procedure according to the
manufacturer's instructions (HU133 Plus 2; Affymetrix, Inc., Santa
Clara, CA, USA). The microarray data were analyzed at the NetAffx
Analysis Center within the Affymetrix website, http://www.affymetrix.com/analysis/index.affx. cDNA
was synthesized from 5 µg of total RNA with 200 U of MMLV-RT
(Promega, Madison, WI, USA) and 500 ng of a 16-mer oligo(dT). All
the primer sequences for the PCR reactions are listed in Table I and the cycling conditions for all
of the PCRs were 30 cycles of a denaturation period (94°C/30 sec)
that was followed by an annealing period (55°C/30 sec), and an
extension period (72°C/40 sec), then a final extension period
(72°C/10 min), except for β-actin which contained an annealing
period of 60°C/30 sec in a thermal cycler (Bio-Rad MJ Research PCR;
Bio-Rad, Waltham, MA, USA). PCR products were separated on a 1.5%
agarose gel in a 0.5X TBE buffer and were stained with ethidium
bromide and visualized under UV light.
 | Table IAssociation of LVD with
clinicopathological features in lung adenocarcinoma. |
Table I
Association of LVD with
clinicopathological features in lung adenocarcinoma.
| Clinicopathological
features | Cases | ptLVD | itLVD |
|---|
| Age (years) | | | |
| ≥54.5 | 17 | 23.1±7.9 | 10.3±5.0 |
| <54.5 | 17 | 21.7±8.1 | 14.2±4.9 |
| Gender | | | |
| Male | 21 | 21.5±7.5 | 10.2±4.7 |
| Female | 13 | 21.3±7.7 | 9.2±4.5 |
| Tumor
differentiation | | | |
| Well-moderate | 25 | 22.3±8.1 | 10.2±6.4 |
| Poor | 9 | 21.7±8.1 | 10.7±5.4 |
| Pathologic N
stage | | | |
| N1-2 | 16 | 24.3±8.7b | 11.3±5.5 |
| N0 | 18 | 18.4±6.3 | 11.7±4.3 |
| ptLVDa | | | |
| High (≥18.7) | 17 | – | 12.6±6.1 |
| Low
(<18.7) | 17 | – | 12.1±4.3 |
| itLVDa | | | |
| High (≥10.5) | 17 | 23.9±7.1 | – |
| Low
(<10.5) | 17 | 21.3±6.5 | – |
| Pathological
stage | | | |
| I−II | 19 | 18.4±5.6 | 10.2±4.4 |
| III−IV | 15 | 24.1±8.2b | 10.3±3.4 |
Microarray hybridization
Purified total RNA (5 Ag) was labeled and hybridized
onto the Affymetrix U133 Plus 2.0 GeneChip oligonucleotide arrays
(Affymetrix, Inc.) according to the manufacturer's instructions.
Briefly, hybridization signals were scaled in Affymetrix GCOS
software (version 1.1.1) using a scaling factor determined by
adjusting the global trimmed mean signal intensity value to 500 for
each array and importing them into GeneSpring version 6.2 (Silicon
Genetics, Redwood City, CA, USA). Signal intensities were then
centered to the 50th percentile of each chip, and for each
individual gene, to the median intensity of each specific subset
first to minimize the possible technical bias and then to the whole
sample set. The intensities of any replicate hybridizations were
averaged subsequent to further analysis.
Real-time PCR confirmation of a selection
of differentially expressed genes
Relative gene copy numbers and gene expression was
determined by quantitative real-time PCR using a PRISM 7500
Sequence Detection system (Applied Biosystems) and a QuantiTect
SYBR-Green PCR kit (Qiagen, Inc. Valencia, CA, USA). The standard
curve method was used to calculate target gene copy number in the
tumor cDNA sample normalzed to a repetitive element line-1 and
normal reference cDNA. The comparative threshold cycle method was
used to calculate gene expression normalized to β-actin as a gene
reference and normal human lung RNA was used as an RNA reference.
Primers were designed using Primer3 (http://frodo.wi.mit.edu/cgibin/primer3/primer3_www.cgi)
and were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
Primers (Invitrogen, Shanghai, China) used for RT-PCR were: IGFBP7
5′-CTGGGTGCTGGTATCTCCTC-3′ (sense), and 5′-TATAGCTCGGCACCTTCACC-3′
(antisense); VEGF-C 5′-TGTAAAACGACGGCCAGT-3′ (sense), and
5′-CAGGAAACAGCTATGACC-3′ (antisense); GAPDH
5′-GCACCGTCAAGGCTGAGAAC-3′ (sense), and 5′-TGGTGGTGAAGACGCCAGT-3′
(antisense).
Protein pathway analysis
Gene Ontology (GO) annotation of the identified
proteins was obtained using DAVID (version 6.7); (see http://david.abcc.ncifcrf.gov/summary.jsp.)
Differentially expressed proteins were analyzed using Ingenuity
Pathway Analysis (IPA; Ingenuity Systems; see www.ingenuity.com). Cell Death, Cellular, Development,
Hematological Systems Development and Function, Endocrine System,
Disorders, Metabolic Disease, Cell-To-Cell Signaling interactions
were generated based on information contained in the Ingenuity
Pathways Knowledge Base.
Western blotting
Biological replicates were used for western blot
analysis of astroglial differentiation. In brief, lysates from
whole cell extracts or membrane pellets containing 20 µg of
proteins were subjected to gel electrophoresis. Proteins were then
transferred to PVDF membranes (Millipore). The blots were blocked
in 4% BSA in TBST solution for 30 min at room temperature and were
then incubated at 4°C overnight with the primary antibody.
Anti-β-actin (1:4,000) and IGFBP7 (1:1,000) were purchased from
Abcam, Inc. After incubation with secondary antibodies (1:2,000,
Millipore) at room temperature for 1 h, the blot was visualized by
ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA, USA).
Small interfering RNA transfection
Transfection with IGFBP7 siRNA (10 pmole) and
irrelevant scrambled control siRNA was performed using
Lipofectamine 2000 (Invitrogen) according to the manufacturer's
protocol. LLCs at 50% confluency were treated with siRNA for 24 h
in medium lacking 20% serum and antibiotics. Inhibition of IGFBP7
protein expression was observed within 24 h and this knockdown was
maintained for at least 72 h after removal of the medium containing
the siRNA.
Statistical analysis
Three statistical methods were used to identify the
optimal prognostic gene signature: Cox proportional-hazard
regression modeling, bootstrapping, and a 2-fold cross-validation.
Hierarchical clustering was done to identify major clusters of gene
activation and investigate their associations with patient
covariates. The method for developing a risk index is similar to
that previously described. Pathway analysis was carried out by
first mapping genes to the biological process categories of GO and
then calculating the significance of overrepresented categories in
the selected gene list. Correlations between podoplanin and the
vessel numbers as continuous variables were used to determine
positive vessel counts with the Spearman rank correlation test.
Categorical data was compared by the χ2 or Fishers'
exact probability test. Distribution was normal or within the Mann
Whitney U test if the sample distribution was asymmetrical. The
relationship between lymph vessel variables and lymph node status
was analyzed by one-way ANOVA, followed by the Neuman Keuls
test.
Results
Morphology of lymphatic vessels in lung
adenocarcinoma tissue
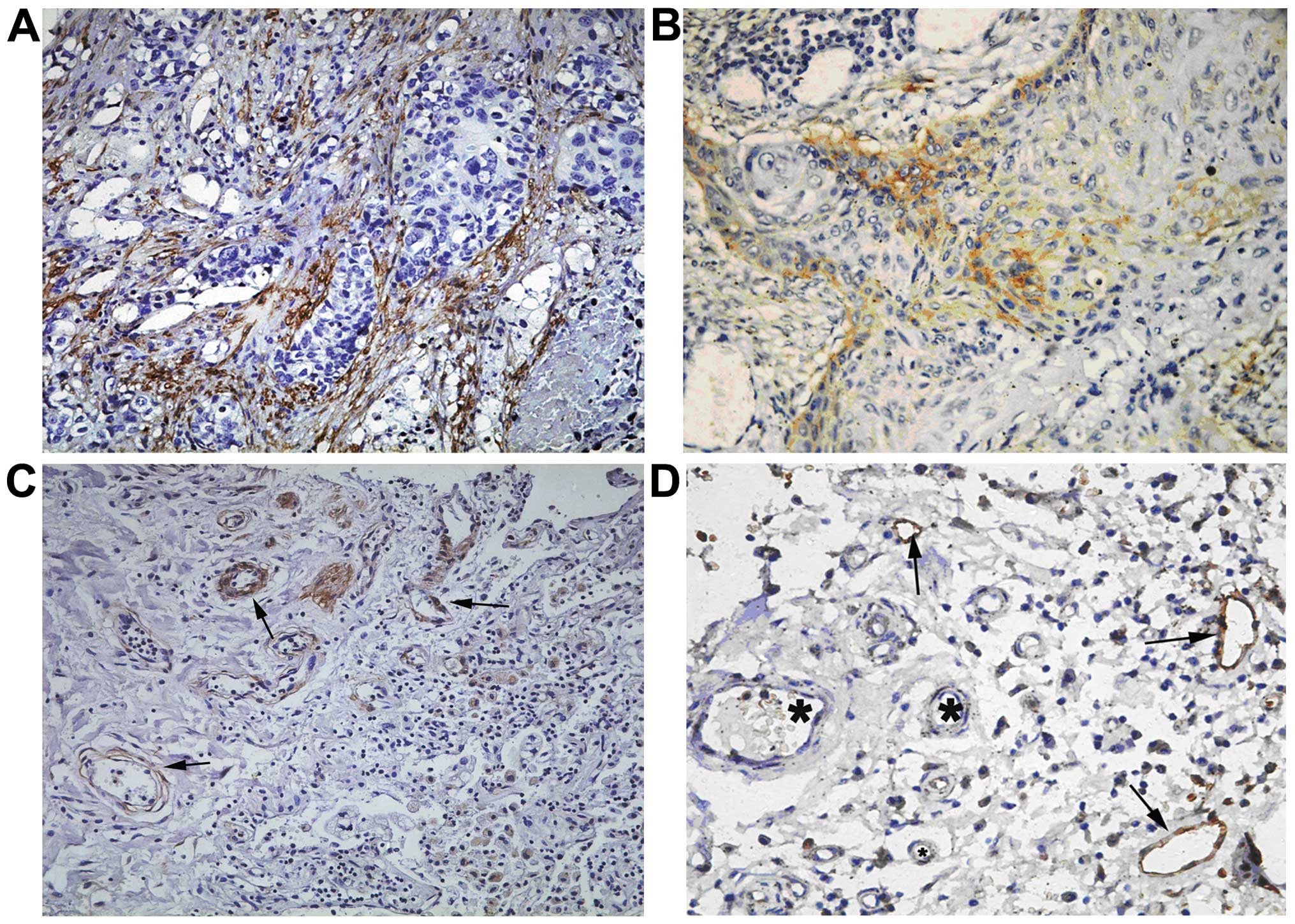
Podoplanin expression mostly presented in the
thin-walled structures. Podoplanin was positive in endothelial cell
plasma, in thin-walled lymph vessel, as indicated by the
brown-yellow color. Podoplanin positive lymph vessels were both
located at the adenocarcinoma interstitium (Fig. 1A) and at the tumor boundary
(Fig. 1B). In some cases, low
density lymphatic vessels (LVD-low) appear around tumor
cells (Fig. 1C). Podoplanin
positive stained cells only appear in thin-walled structures like
the lymphatic vessel, but does not stain blood vessels, which are
indicated by the presence of blood cells (Fig. 1D).
Association of LVD with
clinicopathological features
Table I depicts
pathologic N1-2 as significantly different with No patients in
assessing peritumoral lymphatic vessel density, but not
intratumoral lymphatic vessel density (P<0.05). PtLVD was
24.1±8.2/field in clinic pathlogical stage III–IV, which was
significantly different with stage I–II (P<0.05), the mean of
ptLVD is 18.4±5.6/field. The median ptLVD was 18.7/field, which is
separated by LVD-high and LVD-low group. The
clinicopathological features show that for ptLVD there was no
significant difference with age, gender or tumor differentiation.
No significant association was found with ptLVD or any of the
clinicopathological criteria.
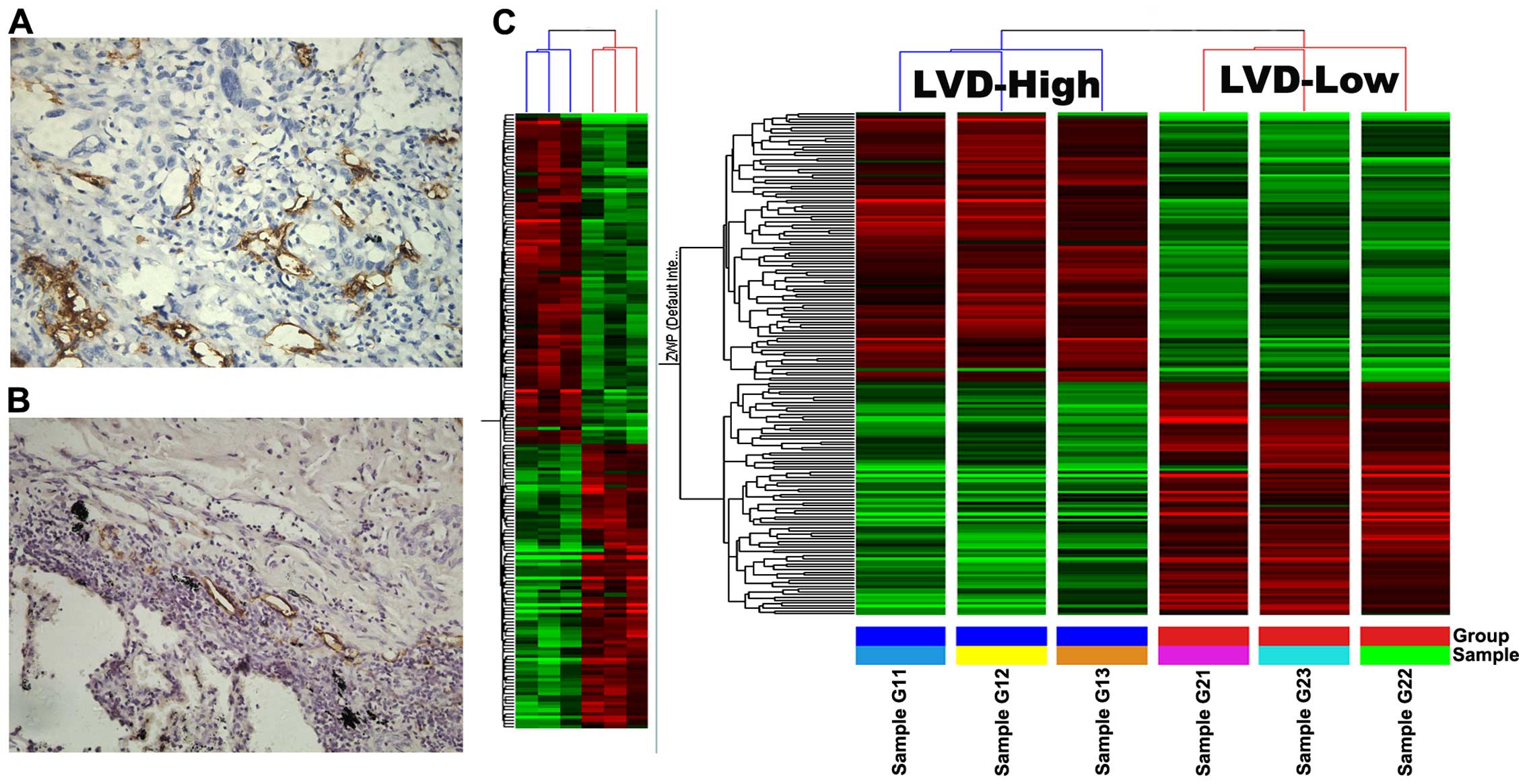
Genes differentially expressed between
high ptLVD and low ptLVD lung carcinoma tissue
With analyzing association of LVD with
clinicopathological feature, the three high ptLVD and low ptLVD
lung carcinoma tissues were chosen for microarray screening. Using
the criteria described in Materials and methods for microarray data
analysis, we found 181 genes which showed a 2-fold difference.
Among these, 97 genes are upregulated in high lymphatic vessels and
84 were downregulated. Upregulated and downregulated genes are
reported in Table II. For each
gene symbol, GenBank ID, fold-change and description are reported.
Based on bioinformatics analysis and their respective functions
reported in the literature, we selected 10 differentially expressed
genes of interest and determined the expression levels by
quantitative real-time RT-PCR. RT-PCR among these 10 genes
demonstrated IGFBP7 to be the most differentially expressed. Thus,
we chose to focus on IGFBP7 in the present study, whereas other
candidates are being investigated in additional studies (Fig. 2).
 | Table II
|
Table II
| Gene symbol | GenBank | Fold-change | Description |
|---|
| Top upregulated
genes | | | |
| LOC387601 | AK091990 | 38.6 | Putative UST1-like
organic anion transporter |
| ANXA8 | NM_001630 | 14.89 | Annexin A8 |
| SLC1A2 | AV722518 | 11.7 | Solute carrier
family 1 (glial high affinity glutamate transporter), member 2 |
| PAPSS2 | AI821404 | 9.784 | 3′-Phosphoadenosine
5′-phosphosulfate synthase 2 |
| IGFBP7 | AU144916 | 8.95 | Insulin-like growth
factor binding protein 7 |
| DOCK1 | AK000789 | 5.441 | Dedicator of
cytokinesis 1 |
| ATP5S | BE968806 | 5.223 | ATP synthase,
H+ transporting, mitochondrial F0 complex, subunits
(factor B) |
| RPL23 | AK025200 | 4.651 | Ribosomal protein
L23 |
| ATP1B3 | AI928218 | 4.508 | ATPase,
Na+/K+ transporting, β3 polypeptide |
| LOC152573 | AI735586 | 3.805 | Hypothetical
protein BC012029 |
| CD1E | AA309511 | 3.568 | CD1E antigen, e
polypeptide |
| CCNA1 | NM_003914 | 3.528 | Cyclin A1 |
| STXBP1 | NM_003165 | 3.506 | Syntaxin binding
protein 1 |
| C9orf3 | AI147867 | 3.44 | Chromosome 9 open
reading frame 3 |
| ZDHHC21 | BE467787 | 3.289 | Zinc finger,
DHHC-type containing 21 |
| Top downregulated
genes |
| GMDS | AK000788 | 0.227 | GDP-mannose
4,6-dehydratase |
| CDH1 | L08599 | 0.224 | Cadherin 1, type 1,
E-cadherin (epithelial) |
| CCL28 | AF266504 | 0.199 | Chemokine (C-C
motif) ligand 28 |
| TNFRSF21 | NM_016629 | 0.196 | Tumor necrosis
factor receptor superfamily, member 21 |
| BMP5 | AK021486 | 0.193 | Bone morphogenetic
protein 5 |
| AP1S3 | AI474433 | 0.174 | Adaptor-related
protein complex 1, sigma 3 subunit |
| HSPC105 | AI914083 | 0.154 | NAD(P) dependent
steroid dehydrogenase-like |
| PRLR | S78505 | 0.154 | Prolactin
receptor |
| FLJ14503 | AW237462 | 0.126 | Hypothetical
protein FLJ14503 |
| OPRK1 | AU153412 | 0.0987 | Opioid receptor,
κ1 |
| C18orf2 | AF295726 | 0.0783 | Chromosome 18 open
reading frame 2 |
| KIAA1324 | AI672868 | 0.0556 | KIAA1324 |
| CRISP2 | M25532 | 0.0233 | Cysteine-rich
secretory protein 2 |
| MAGEA6 | U10691 | 0.0118 | Melanoma antigen
family A, 6 |
| CRISP3 | NM_006061 | 0.00807 | Cysteine-rich
secretory protein 3 |
| MAGEA3 | BC000340 | 0.0047 | Melanoma antigen
family A, 3 |
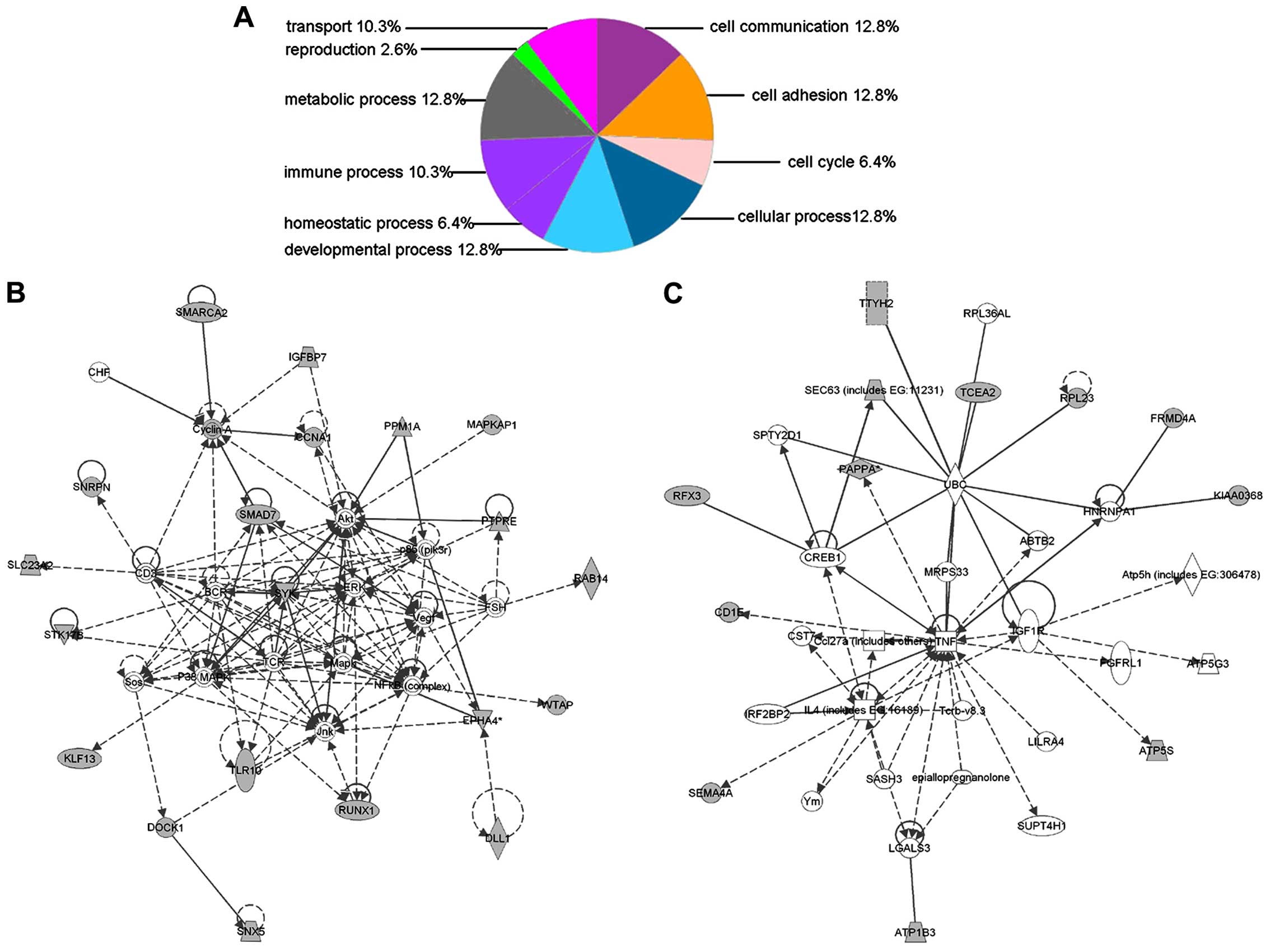
Bioinformatics analysis of the
differentially expressed membrane proteins
The DAVID Bioinformatics Resource 6.7 was used for
annotation of the cellular component. IPA was used to assign
identified proteins into different functional groups based on the
Ingenuity Pathways Analysis literature database. All the
differentially expressed proteins were uploaded to the IPA server.
For molecular and cellular functions, the data indicated that many
proteins were involved in top 10 biological processes (Fig. 3A). Pathway analysis was also used to
analyze different cellular functional networks to determine which
were altered in the lymphangiogenesis procedure. In the present
study, several networks were grouped by IPA, i.e. there are 20
proteins involved in cell death, cellular, development,
hematological system development and function were grouped as the
top network (network 1), which had the highest score (47) (Fig. 3B). Additionally, network 2 grouped
by IPA has 12 related genes involved in endocrine system,
disorders, metabolic disease, cell-to-cell signaling and
interaction, and had a score of 25 (Fig. 3C).
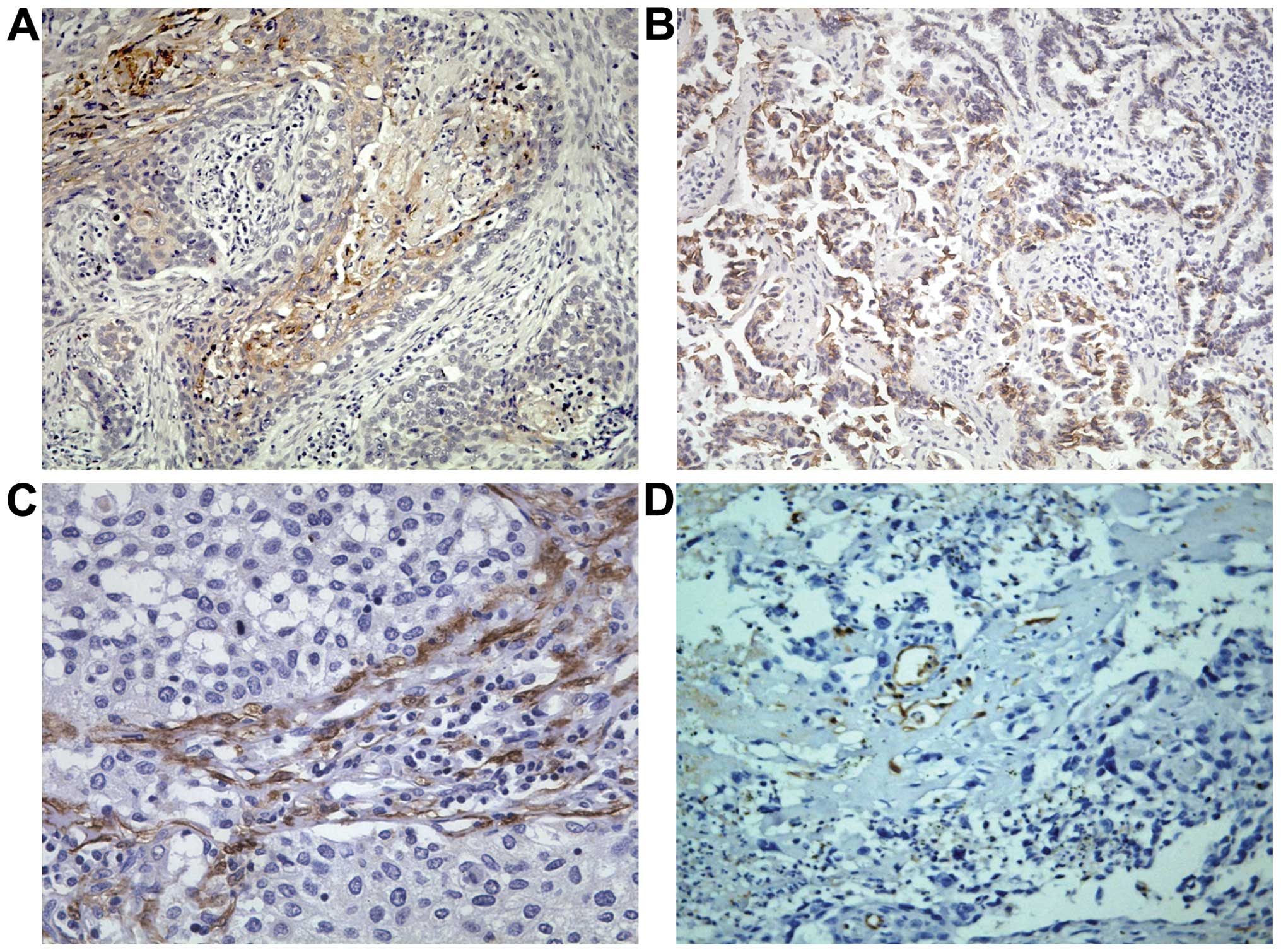
Association of IGFBP7 with
clinicopathological variables
In the NSCLC tissue, moderate IGFBPP7
immunoreactivity was present in the cytoplasm of lung cancer cells;
a weak immunoreactivity was seen in some ductal cells within the
small ductules. IGFBP7 positive substances that were in the shape
of brownish-yellow fine particles were mainly located in the
cytoplasm of the cancer cells. There was expression of various
degrees on the cell membrane, presenting pale-yellow to
brownish-yellow (Fig. 4).
The associations of high expression levels of IGFBP7
with clinicopathological parameters are shown in Table III. The positive expression of
IGFBP7 is 55.67% (54/97). We observed a significant association
between IGFBP7 expression in NSCLC associated with increased lymph
node metastasis (P=0.002). Furthermore, ptLVD of the positive
IGFBP7 is 23.1±8.5/field, statistically significantly higher than
the negative group (16.9±6.0). There were no statistically
significant differences with regard to patient age, gender or
histological types (Table
III).
 | Table IIICorrelation of clinicopathologic
features and expression of IGFBP7 in patients with non-small cell
lung carcinoma. |
Table III
Correlation of clinicopathologic
features and expression of IGFBP7 in patients with non-small cell
lung carcinoma.
| Clinicopathological
factors | Expression of
IGFBP7 |
|---|
| Negative | Positive | P-value |
|---|
| Age (years) | | | |
| ≤65 | 25 | 34 | 0.679 |
| >65 | 18 | 20 | |
| Gender | | | |
| Male | 30 | 32 | 0.298 |
| Female | 13 | 22 | |
| Histologic
type | | | |
| Squamous
carcinoma | 27 | 30 | 0.536 |
|
Adenocarcinoma | 16 | 24 | |
|
Differentiation | | | |
| Well | 35 | 37 | 0.169 |
| Poorly | 8 | 17 | |
| Pathological
stages | | | |
| I+II | 29 | 39 | 0.659 |
| III+IV | 14 | 15 | |
| Pathologic N
factor | | | |
| N0 | 9 | 26 | 0.002a |
| N1+N2+N3 | 34 | 28 | |
| ptLVD | 16.9±6.0 | 23.1±8.5 | 0.001b |
The effect of IGFBP7 on tube-like
structure formation of LECs
In the co-culture system, different cells were
placed in the upper chambers, while LECs were in the lower
chambers. After 48 h of LLC-LECs co-culture, LECs start
establishing a complex tube system. After 72 h of culture in
conditioned medium, the number of LECs permeating septum in the LLC
group was the highest among the four groups. LLCs more strongly
enhanced the formation of extensive capillary-like structures in
vitro than medium alone or the L929 cells group (P<0.05).
Furthermore, compared to the LLC group, the LLC-Si-IGFBP7 group
decreased LEC formation of capillary-like structures in
vitro (P<0.05) (Fig. 5).
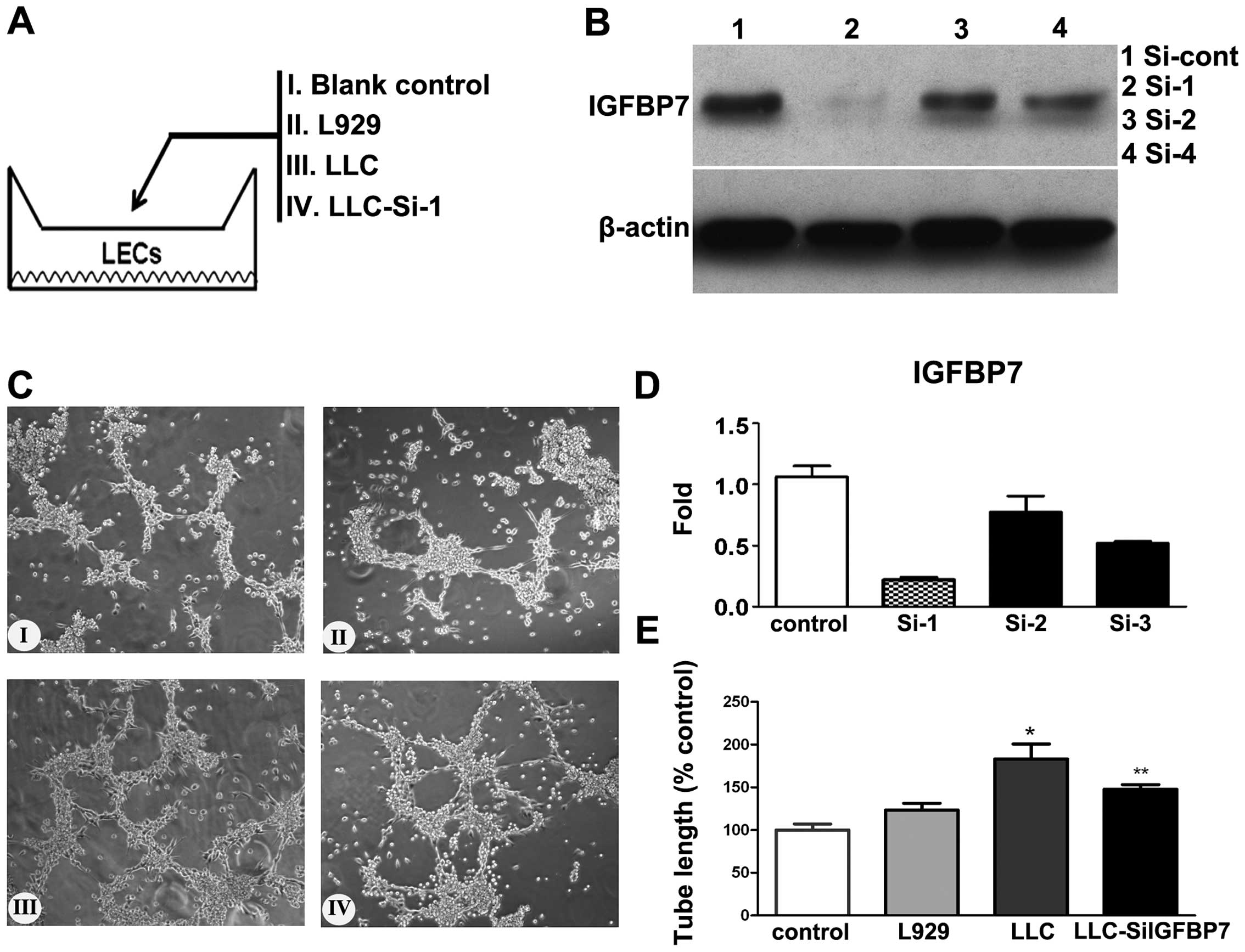 | Figure 5The effect of IGFBP7 on tube-like
structure formation of LECs. (A) I, no cells in upper chamber; II,
L929 cells in upper chamber; III, Lewis lung cancer cells in upper
chamber; IV, LLC with IGFBP7 knocked down with LECs co-culture. (B)
LLCs with IGFBP7-Si-control, Si-1, Si-2 and Si-3 were qualified by
IGFBP7 western blotting. (D) RT-PCR confirmed IGFBP7-Si-control,
Si-1, Si-2 and Si-3 expression. (C and E) Among the different cells
co-cultured, the number of the intersecting branches of assembled
endothelial cell networks was observed at a magnification of ×200.
Branch point numbers were calculated as the mean ± SD.
*P<0.05, LLC vs. L929; **P<0.05, LLC
vs. LLC-SiIGFBP7. |
Discussion
In recent years, lymphangiogenesis in pulmonary
cancer constitutes a topic of intense study. The importance of
lymph node metastases in predicting the course of neoplastic
disease focuses our attention on understanding how tumors interact
with the lymphatic vasculature (22). Our team used meta-analysis of the
literature on lymphatic microvessel density in NSCLC. The lymphatic
microvessel count or LVD, which reflects the level of
lymphangiogenesis, is a strong indicator of poor prognosis for
patient survival in surgically treated NSCLC (23).
The most extensively studied molecular system that
signals for tumor lymphangiogenesis, and is associated with
lymphatic spread from primary cancers, is the VEGF-C/VEGF-D/VEGFR-3
signaling axis (24). Although
lymph node metastasis occurs frequently in lung adenocarcinoma,
size of the primary tumor and occurrence of distant metastasis is
not parallel in many clinic cases. However, the
VEGF-C/VEGF-D/VEGFR-3 signaling axis cannot completely explain the
complicated lymphangiogenesis in tumor cell metastasis. To
investigate the related genes involved in lymphangiogenesis in
NSCLC, we first assessed both intratumoral and peritumoral
lymphatic vessel density in 34 lung adenocarcinomas. The threshold
number was 18.7 between the high ptLVD and the low ptLVD group.
Human Genome Microarray was employed for comparison of expression
profiles of LVD-high lung adenocarcinoma and
LVD-low lung adenocarcinoma. We found 181 genes which
were 2-fold differentially changed. In other studies, IGFBP7 was
screened in the microarray (25,26),
which may be related with tumor metastasis. By identification and
quantification of membrane proteins, network 2 (grouped by IPA)
shows 12-related genes involved in the endocrine system, disorders,
metabolic disease cell-to-cell signaling and interaction.
IGFBP7 expression in peripheral endothelial cells
can be modulated by cell confluence, hypoxia and cytokines
including VEGF, bFGF and TGF-b1. Secreted IGFBP7 is reported to
interact with the ECM components, and could thus participate in the
TGF-b1-induced extracellular matrix turnover and angiogenesis
(27). Assessing the
immunohistochemistry result of IGFBP7 staining, together with a
previous study (28), our data show
that IGFBP7 expression in NSCLC is associated with increased lymph
node metastasis In other studies, esophageal adenocarcinoma
exhibits the IGFBP7 expression pattern expected in an aggressive
form of cancer (29).
Overexpression of IGFBP7 was shown at the mRNA level in two
independent Sporadic Pilocytic Astrocytomas tumor series (30). Furthermore, IGFBP7 plays a positive,
contributing role in the interaction between leukemia cells and the
microenvironment, which may promote the leukemic cell adhesion,
invasion and migration (31).
Another assay with IGFBP7 knockdown and acute lymphoblastic
leukemia (ALL), showed that IGFBP7 acts as a positive regulator of
ALL and stromal cell growth, and significantly enhances in
vitro resistance of ALL to sparaginase (32). Hypomethylation of IGFBP7 is likely
to characterize an immature and a more malignant subtype of the
disease (33). In glioblastoma,
developed anti-IGFBP7-iron oxide single domain antibody-targeted
MRI contrast agent selectively binds to abnormal vessels (34), IGFBP7 antibody are novel
glioblastoma vessel-targeting moieties suitable for molecular
imaging (35), the retinal
phenotype appears to be mediated by a role in the vascular
endothelium, where IGFBP7 is highly expressed (36). In lung cancer patients, high serum
levels of IGFBP7 is correlated with positive nodal status
(P=0.008), thus is not beneficial for recurrence-free survival
(37), in vitro IGFBP7 is
only reported during cancer progression and metastasis formation
(38).
As endothelial IGFBP7 induction by the tumor
microenvironment is predominantly mediated by the ALK5/Smad-2
pathway, IGFBP7 accumulates in the basal lamina of GBM vessels
in vivo (15). We propose
that IGFBP7 may be involved in lymphangiogenesis in the context of
NSCLC. As there is currently no clear understanding of whether
lymphangiogenesis contributes to tumor progression or tumor growth
stabilization, the overall effect of IGFBP7 in the tumor milieu
cannot be easily generalized. As a secreted protein, IGFBP7 may
also modulate the function of the immune system generating an
antitumor immune response; this hypothesis remains to be
experimentally tested. The proposed angiogenic role of IGFBP7 may
appear contradictory to literature evidence of the tumor-suppressor
activity of IGFBP7 (14,39). The main explanation is that IGFBP7
could trigger differential signaling pathways in tumor and
endothelial cells.
In summary, we identified IGFBP7 as enhancer of
lymphangiogenesis in NSCLC, therefore, we believe it is a good
target for inhibition. Our preliminary findings document that
IGFBP7 efficiently enhances tube-like structure formation of LEC.
Studies are ongoing to develop targeted and effective delivery
systems for administering IGFBP7 in patients.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472183). We thank
Crystina Bronk for revising the manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Adjei AA and Jett JR: Advances
in chemotherapy of non-small cell lung cancer. Chest.
130:1211–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kadota K, Huang CL, Liu D, Nakashima N,
Yokomise H, Ueno M and Haba R: The clinical significance of the
tumor cell D2-40 immunoreactivity in non-small cell lung cancer.
Lung Cancer. 70:88–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao Y, Liu Z, Gao F and Meng XY: High
density of peritumoral lymphatic vessels is a potential prognostic
marker of endometrial carcinoma: A clinical immunohistochemical
method study. BMC Cancer. 10:1312010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Li Y, Liao Z, Lin G, Cai G, Lin K,
Zhan Q and Chen C: Active lymphangiogenesis is a major risk factor
for anastomotic leakage following sphincter-sparing resection of
rectal cancer. J Surg Oncol. 104:493–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen D, Zheng J, Li H, Wang Q and Jiao X:
Computer-assisted morphometric analysis of lymphatic vessel changes
in hamster tongue carcinogenesis. J Oral Pathol Med. 39:518–524.
2010.PubMed/NCBI
|
|
7
|
Sasahira T, Kirita T, Kurihara M, Yamamoto
K, Bhawal UK, Bosserhoff AK and Kuniyasu H: MIA-dependent
angiogenesis and lymphangiogenesis are closely associated with
progression, nodal metastasis and poor prognosis in tongue squamous
cell carcinoma. Eur J Cancer. 46:2285–2294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vittet D and Feige JJ: Lymphangiogenesis
and tumor progression]. Bull Cancer. 94:881–886. 2007.In French.
PubMed/NCBI
|
|
9
|
Sun JG, Wang Y, Chen ZT, Zhuo WL, Zhu B,
Liao RX and Zhang SX: Detection of lymphangiogenesis in non-small
cell lung cancer and its prognostic value. J Exp Clin Cancer Res.
28:212009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinzelbecker J, Kempf KM, Kurz K,
Steidler A, Weiss C, Jackson DG, Bolenz C, Haecker A and Trojan L:
Lymph vessel density in seminomatous testicular cancer assessed
with the specific lymphatic endothelium cell markers D2-40 and
LYVE-1: Correlation with pathologic parameters and clinical
outcome. Urol Oncol. 31:1386–1394. 2013. View Article : Google Scholar
|
|
11
|
Feng Y, Wang W, Hu J, Ma J, Zhang Y and
Zhang J: Expression of VEGF-C and VEGF-D as significant markers for
assessment of lymphangiogenesis and lymph node metastasis in
non-small cell lung cancer. Anat Rec. 293:802–812. 2010. View Article : Google Scholar
|
|
12
|
Tamura K, Matsushita M, Endo A, Kutsukake
M and Kogo H: Effect of insulin-like growth factor-binding protein
7 on steroidogenesis in granulosa cells derived from equine
chorionic gonadotropin-primed immature rat ovaries. Biol Reprod.
77:485–491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruan W, Xu E, Xu F, Ma Y, Deng H, Huang Q,
Lv B, Hu H, Lin J, Cui J, et al: IGFBP7 plays a potential tumor
suppressor role in colorectal carcinogenesis. Cancer Biol Ther.
6:354–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wajapeyee N, Serra RW, Zhu X, Mahalingam M
and Green MR: Oncogenic BRAF induces senescence and apoptosis
through pathways mediated by the secreted protein IGFBP7. Cell.
132:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pen A, Moreno MJ, Durocher Y, Deb-Rinker P
and Stanimirovic DB: Glioblastoma-secreted factors induce IGFBP7
and angiogenesis by modulating Smad-2-dependent TGF-beta signaling.
Oncogene. 27:6834–6844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang YJ, Niu J, Liu Z, Wang LE, Sturgis
EM and Wei Q: The functional IGFBP7 promoter -418G>A
polymorphism and risk of head and neck cancer. Mutat Res.
702:32–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu LB, Liu C, Gao GQ, Yu XH, Zhang R and
Wang J: Nerve growth factor-beta expression is associated with
lymph node metastasis and nerve infiltration in human hilar
cholangiocarcinoma. World J Surg. 34:1039–1045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Guo Y, Zhang BC, Chen ZT and Gao
JF: Induction of apoptosis and inhibition of cell migration and
tube-like formation by dihydroartemisinin in murine lymphatic
endothelial cells. Pharmacology. 80:207–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Wang J, Gao J, Guo Y, Chen X,
Wang B, Gao J, Rao Z and Chen Z: Alternatively activated RAW264.7
macrophages enhance tumor lymphangiogenesis in mouse lung
adenocarcinoma. J Cell Biochem. 107:134–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Z, Wang T, Luo H, Lai Y, Yang X, Li
F, Lei Y, Su C, Zhang X, Lahn BT, et al: Expression of nestin in
lymph node metastasis and lymphangiogenesis in non-small cell lung
cancer patients. Hum Pathol. 41:737–744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saban MR, Towner R, Smith N, Abbott A,
Neeman M, Davis CA, Simpson C, Maier J, Mémet S, Wu XR, et al:
Lymphatic vessel density and function in experimental bladder
cancer. BMC Cancer. 7:2192007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sleeman JP and Thiele W: Tumor metastasis
and the lymphatic vasculature. Int J Cancer. 125:2747–2756. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Li K, Wang B and Bi J: Lymphatic
microvessel density as a prognostic factor in non-small cell lung
carcinoma: A meta-analysis of the literature. Mol Biol Rep.
39:5331–5338. 2012. View Article : Google Scholar
|
|
24
|
Achen MG and Stacker SA: Molecular control
of lymphatic metastasis. Ann NY Acad Sci. 1131:225–234. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bièche I, Lerebours F, Tozlu S, Espie M,
Marty M and Lidereau R: Molecular profiling of inflammatory breast
cancer: Identification of a poor-prognosis gene expression
signature. Clin Cancer Res. 10:6789–6795. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Zhang D, Zheng W, Luo J, Bai Y and
Lu Z: Multiple gene methylation of nonsmall cell lung cancers
evaluated with 3-dimensional microarray. Cancer. 112:1325–1336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramsauer M and D'Amore PA: Contextual role
for angiopoietins and TGFbeta1 in blood vessel stabilization. J
Cell Sci. 120:1810–1817. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shersher DD, Vercillo MS, Fhied C, Basu S,
Rouhi O, Mahon B, Coon JS, Warren WH, Faber LP, Hong E, et al:
Biomarkers of the insulin-like growth factor pathway predict
progression and outcome in lung cancer. Ann Thorac Surg.
92:1805–1811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nancarrow DJ, Clouston AD, Smithers BM,
Gotley DC, Drew PA, Watson DI, Tyagi S, Hayward NK and Whiteman DC;
Australian Cancer Study; Study of Digestive Health: Whole genome
expression array profiling highlights differences in mucosal
defense genes in Barrett's esophagus and esophageal adenocarcinoma.
PLoS One. 6:e225132011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacob K, Quang-Khuong DA, Jones DT, Witt
H, Lambert S, Albrecht S, Witt O, Vezina C, Shirinian M, Faury D,
et al: Genetic aberrations leading to MAPK pathway activation
mediate oncogene-induced senescence in sporadic pilocytic
astrocytomas. Clin Cancer Res. 17:4650–4660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu S, Chen R, Man X, Feng X, Cen J, Gu W,
He H, Li J, Chai Y and Chen Z: Function and expression of
insulin-like growth factor-binding protein 7 (IGFBP7) gene in
childhood acute myeloid leukemia. Pediatr Hematol Oncol.
28:279–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laranjeira AB, de Vasconcellos JF, Sodek
L, Spago MC, Fornazim MC, Tone LG, Brandalise SR, Nowill AE and
Yunes JA: IGFBP7 participates in the reciprocal interaction between
acute lymphoblastic leukemia and BM stromal cells and in leukemia
resistance to asparaginase. Leukemia. 26:1001–1011. 2012.
View Article : Google Scholar
|
|
33
|
Heesch S, Bartram I, Neumann M, Reins J,
Mossner M, Schlee C, Stroux A, Haferlach T, Goekbuget N, Hoelzer D,
et al: Expression of IGFBP7 in acute leukemia is regulated by DNA
methylation. Cancer Sci. 102:253–259. 2011. View Article : Google Scholar
|
|
34
|
Tomanek B, Iqbal U, Blasiak B, Abulrob A,
Albaghdadi H, Matyas JR, Ponjevic D and Sutherland GR: Evaluation
of brain tumor vessels specific contrast agents for glioblastoma
imaging. Neuro Oncol. 14:53–63. 2012. View Article : Google Scholar :
|
|
35
|
Iqbal U, Albaghdadi H, Luo Y, Arbabi M,
Desvaux C, Veres T, Stanimirovic D and Abulrob A: Molecular imaging
of glioblastoma multiforme using anti-insulin-like growth
factor-binding protein-7 single-domain antibodies. Br J Cancer.
103:1606–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abu-Safieh L, Abboud EB, Alkuraya H,
Shamseldin H, Al-Enzi S, Al-Abdi L, Hashem M, Colak D, Jarallah A,
Ahmad H, et al: Mutation of IGFBP7 causes upregulation of
BRAF/MEK/ERK pathway and familial retinal arterial macroaneurysms.
Am J Hum Genet. 89:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shersher DD, Vercillo MS, Fhied C, Basu S,
Rouhi O, Mahon B, Coon JS, Warren WH, Faber LP, Hong E, et al:
Biomarkers of the insulin-like growth factor pathway predict
progression and outcome in lung cancer. Ann Thorac Surg.
92:1805–1811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Georges RB, Adwan H, Hamdi H, Hielscher T,
Linnemann U and Berger MR: The insulin-like growth factor binding
proteins 3 and 7 are associated with colorectal cancer and liver
metastasis. Cancer Biol Ther. 12:69–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Yoo BK, Santhekadur PK, Gredler R,
Bhutia SK, Das SK, Fuller C, Su ZZ, Fisher PB and Sarkar D:
Insulin-like growth factor-binding protein-7 functions as a
potential tumor suppressor in hepatocellular carcinoma. Clin Cancer
Res. 17:6693–6701. 2011. View Article : Google Scholar : PubMed/NCBI
|