Introduction
Lung cancer is one of the malignant tumors with very
high global incidence and mortality (1). In addition to smoking cessation and
early detection measures, chemoprevention has become an important
means of prevention and control of lung cancer (2). Green tea is widely consumed for its
characteristic flavor and potential health benefits. Many studies
have provided evidence that green tea and its components reduce the
risk of cancers, including lung, prostate, and breast cancers
(3–6).
A typical cup of green tea contains 100–150 mg of
tea polyphenols; the major green tea polyphenol is
(−)-epigallocatechin-3-gallate (EGCG), which comprises more than
50% of total tea polyphenols (7).
Research has shown that EGCG is a potential chemopreventive and
therapeutic agent for various tumors (8–10).
EGCG has been demonstrated to act on multiple key elements in
signal transduction pathways related to inhibition of
carcinogen-induced mutagenesis (11,12),
induction of cell cycle arrest (13), induction of apoptosis (14), inhibition of growth factor-mediated
proliferation (15), inhibition of
transformation (9), inhibition of
angiogenesis (16) and inhibition
of telomerase activity (17). It
has been shown that EGCG can effectively regulate various key
molecules in cell mitochondrial apoptosis pathways in other tumors
as a potential antitumor substance (18,19).
However, the molecular mechanisms of EGCG inducing apoptosis have
not been completely elucidated in lung cancer.
Ku70 was first characterized as part of the
Ku70/Ku80 heterodimer that is essential as a DNA binding component
of the non-homologous end joining (NHEJ) double-strand break (DSB)
repair (20). Although Ku70 was
originally found in the nucleus, its cytoplasmic function has been
investigated as a regulatory factor of cell death through
interaction with an apoptotic protein Bax (21). Dissociating Bax from Ku70, either by
pharmacological means or by agents that block the interaction
between Ku70 and Bax, may result in cell death (21). Our previous study revealed that EGCG
could effectively inhibit the growth of lung adenocarcinoma A549
cell line transplanted tumors and that the general mechanism
involved interference with the interaction between Ku70 and Bax
(22). Therefore, the specific
mechanism of the EGCG-regulated interaction of Ku70-Bax that
induces apoptosis in lung cancer A549 cells is further explored in
the present study.
Materials and methods
Cell lines, strains and plasmid
vector
The human lung adenocarcinoma A549 cell line was
purchased from the Cell Bank of Xiangya School of Medicine, Central
South University. E. Coli DH5α was provided by the Cancer
Research Institute of Xiangya School of Medicine, Central South
University. The pMD 18-T vector kit was purchased from Takara
(Otsu, Japan). The pCDNA3.1 (+) plasmid was supplied by the Cancer
Research Institute of Xiangya School of Medicine, Central South
University.
Drugs and reagents
EGCG (purity 98%), dimethyl sulfoxide (DMSO) (both
from Sigma, St. Louis, MO, USA), Annexin V and PI double staining
flow method cell apoptosis detection kit (Invitrogen, Carlsbad, CA,
USA), RT-PCR kit (two-step method), real-time PCR kit (both from
Fermentas, Vilnius, Lithuania), rabbit anti-human Bcl-xL antibody
(Proteintech, Chicago, IL, USA), rabbit anti-human Bax antibody
(Cell Signaling Technology, Boston, MA, USA), rabbit anti-human
caspase-3 antibody (Auragene, Changsha, China), and rabbit
anti-human Ku70 antibody (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA).
Construction of gene point mutation
plasmid
Ku70-pcDNA3.1 recombinant plasmid was used as the
template. Two mutation primers were designed in the locus to be
mutated (the primers were: Mut-Ku-F, AAGGGAGAGTTACCAGGAGAAAA
CACGATAATGAAGGTTCTGGAA and Mut-Ku-R, TTCTC
CTGGTAACTCTCCCTTCAGGATTGTAATCTGGTGGG TAAAC, respectively) for PCR
amplification. The conditions of reaction system were
pre-denaturation at 94°C for 4 min, 94°C for 30 sec, 56°C for 30
sec and 72°C for 7 min, for a total of 30 amplification cycles. The
Notch mutant was amplified. A total of 1 µl of DpnI
restriction endonuclease was added to the thermal cycling product.
The template plasmid without mutation was digested. A total of 5
µl of digested thermal cycle product was used to transform
competent bacteria. Then, 5 ml of bacteria were agitated overnight.
The plasmid was extracted from the bacterial suspension. The
nucleic acid electrophoresis confirmed plasmid bands. A small
amount of bacterial suspension was used for plasmid extraction and
sequencing. The residual bacterial suspension was frozen.
Apoptosis analysis
The cells in each group were treated with different
concentrations of EGCG for given times. Thereafter, the cells were
divided into two groups; one was used for detecting the effect of
EGCG on human lung cancer A549 cell survival rates in vitro
by the MTT method, whereas the other was used for detecting cell
apoptosis by Annexin V/PI double staining flow cytometry. The
effects of EGCG with different concentrations and different action
times on cell apoptosis in each group were comprehensively
analyzed.
Western blotting
To detect protein expression in the cells of each
group, total protein was extracted from the treated cells. The
protein concentration was measured, and western blot analysis was
conducted to analyze the expression of various proteins following
the manufacturer's instructions. Aliquots of equal amounts of
protein (40 µg) from the cell lysates were subjected to
SDS-PAGE electrophoresis and transferred to polyvinylidene
difluoride (PVDF) membranes. After blocking the non-specific
binding sites, the membranes were incubated overnight with the
desired primary antibody at 4°C. The membranes were then incubated
with the respective HRP-conjugated secondary antibody for 1 h at
room temperature and the immunoreactive bands were detected by
enhanced chemiluminescence detection systems (Thermo Scientific,
Waltham, MA, USA).
Co-immunoprecipitation
To detect the interaction of Ku70-Bax and the
acetylation status of Ku70, the treated cell protein was extracted.
The protein concentration was determined. A total of 1,000
µg of cell lysate was divided into two portions. One portion
was 100 µg. An equal volume of 2X SDS sample buffer was
added, mixed, degenerated at 100°C in boiling water for 10 min and
centrifuged. A total of 50 µg of supernatant was extracted.
The protein level in whole cell lysates was analyzed by western
blotting. The remaining portion of the cell lysate was 900
µg. A total of 5 µl of protein A/G agarose beads and
a given amount of antibody (1–2 µg) were added. Lysis buffer
was added to a final volume of 1 ml. The sample was fixed in a
vertical mixer and slowly rotated for 3 h. The beads were washed
three times with 1 ml of lysis buffer and centrifuged in a
refrigerated centrifuge at 3,000 rpm at 4°C for 3 min. The
supernatant was absorbed after the final washing. A total of 50
µl of 1X SDS sample buffer was added, mixed evenly, boiled
at 100°C for 10 min and centrifuged. A 15-µl sample was
extracted. The interaction between the proteins was analyzed by
western blotting.
Statistical analysis
All of the data were processed by the statistical
software SPSS10.0. The data are shown as the mean and standard
deviation (mean ± SD). Student's t-test was used for comparisons
between the two groups. The SNK-q test was used for comparisons
among multiple groups. P<0.05 indicates statistical
significance.
Results
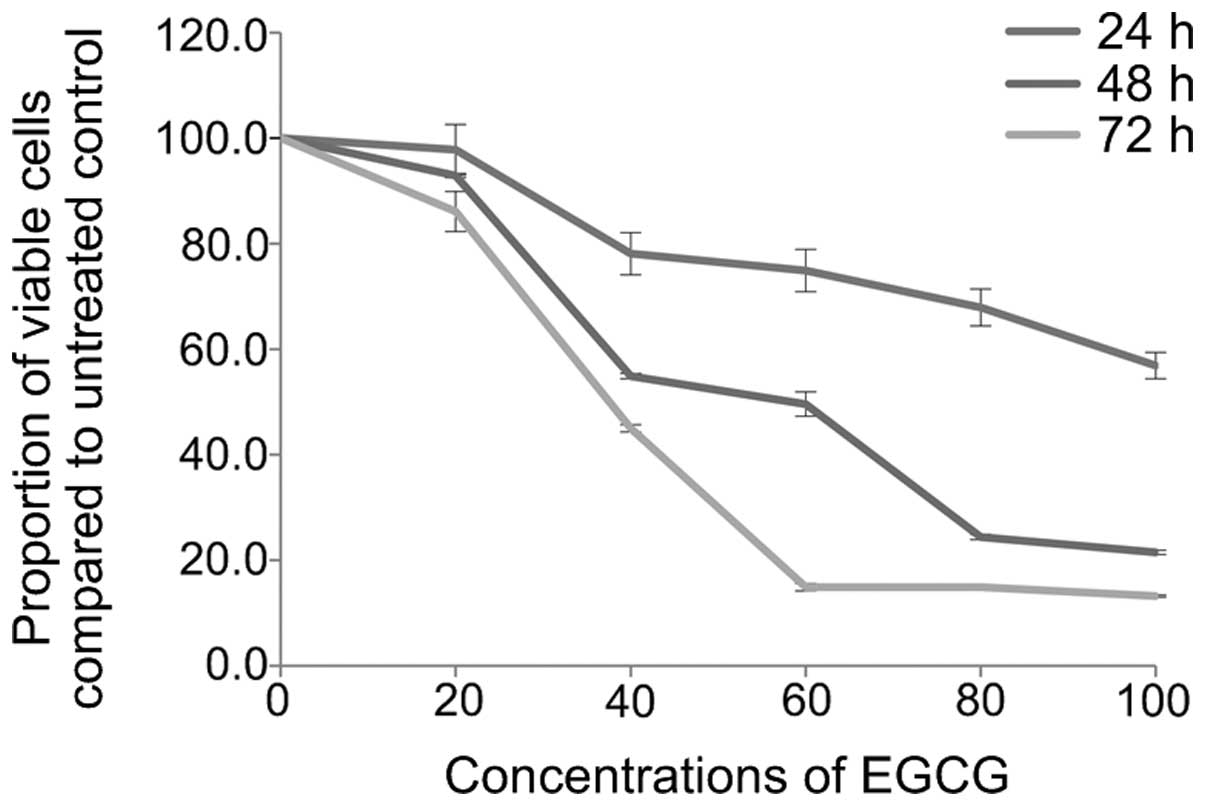
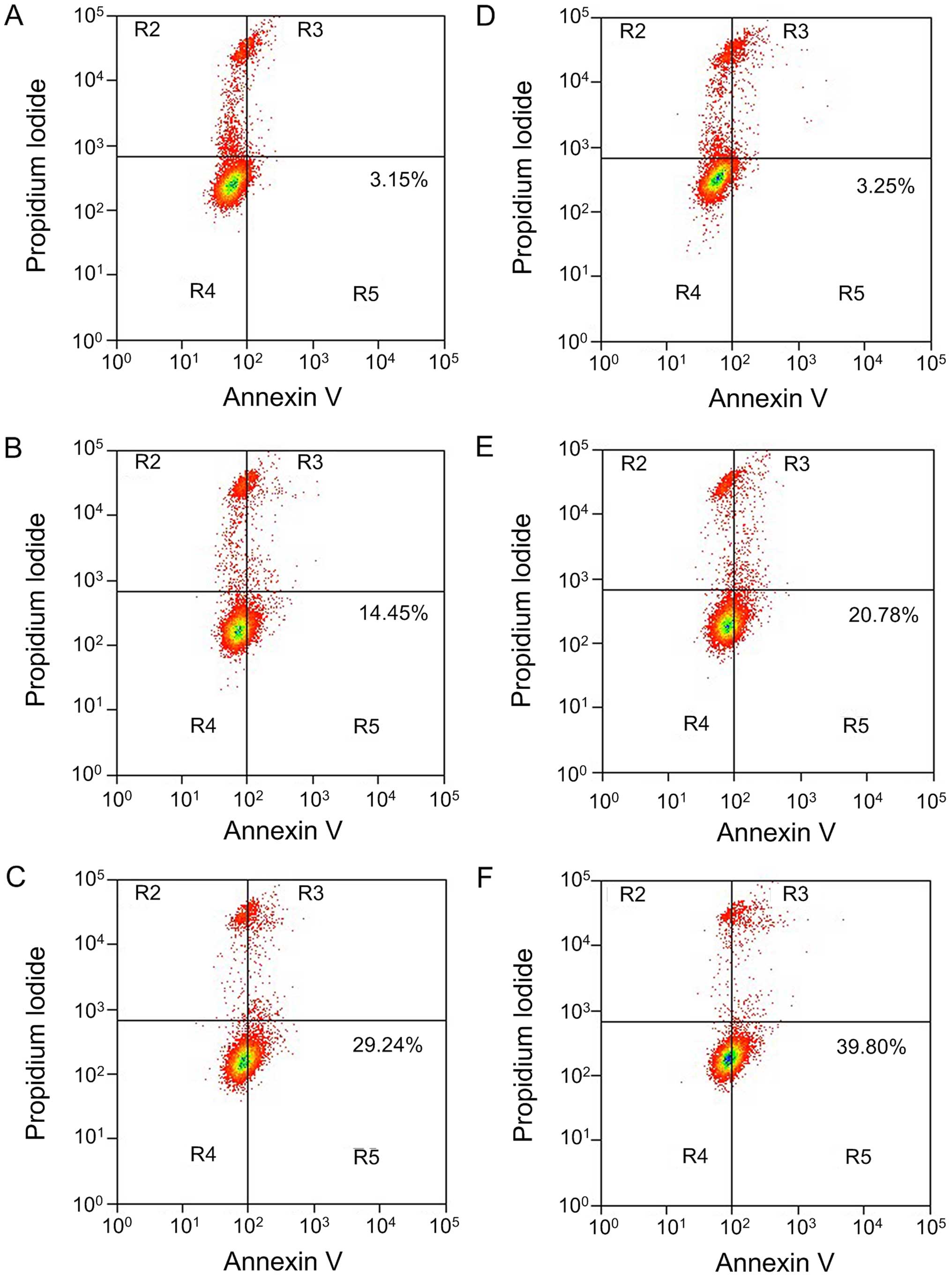
EGCG induces A549 cell apoptosis
After treating A549 cells with different
concentrations of EGCG (0, 20, 40, 60, 80 and 100 µmol/l)
for 24, 48 and 72 h, A549 cell survival rates were detected by the
MTT assay (Fig. 1), and apoptosis
was detected by Annexin V/PI double staining flow cytometry
(Fig. 2). After treating A549 cells
with 40 µmol/l EGCG for 24 h, cell growth was significantly
inhibited (P=0.017). The inhibition was stronger after 48 h
(P<0.01) and even stronger again after 72 h (P<0.01). The
inhibitory rate in the other dosage groups also showed an
increasing trend with prolongation of drug action time, displaying
an obvious time-effect relationship (pair comparison P<0.05).
Furthermore, the cell survival rate in each group also showed a
declining trend along with the increase of EGCG concentration,
displaying a significant dosage-effect relationship (P<0.05). In
addition, with the increase of EGCG concentration, the early cell
apoptosis rate in each group showed a increasing trend along with
the increase of EGCG concentration, displaying a dose-dependent
relationship (P<0.05). When the action time of EGCG was
prolonged to 48 h, the apoptosis rate also increased, and the
apoptosis inducement ability showed a time-dependent relationship
(P<0.05).
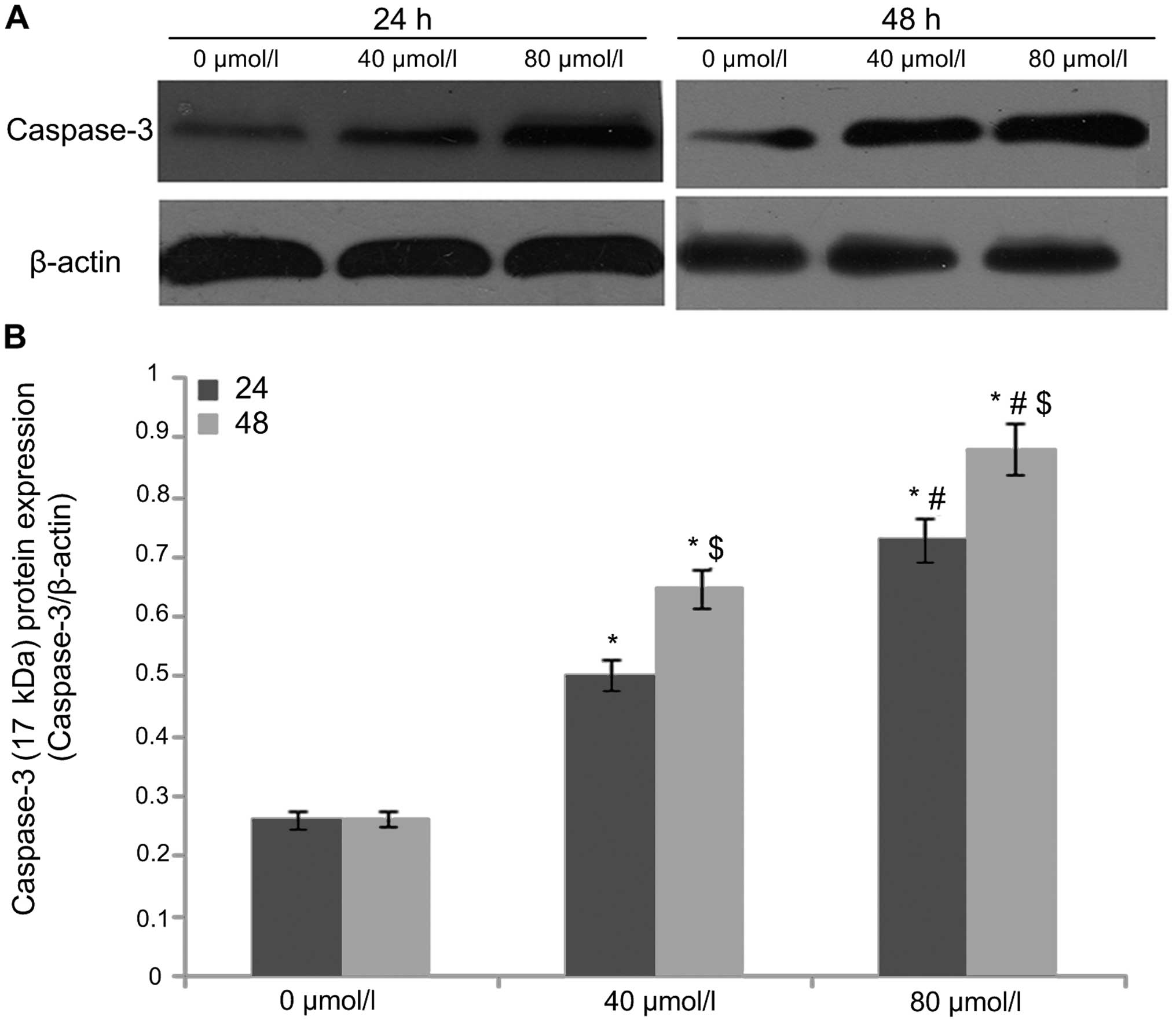
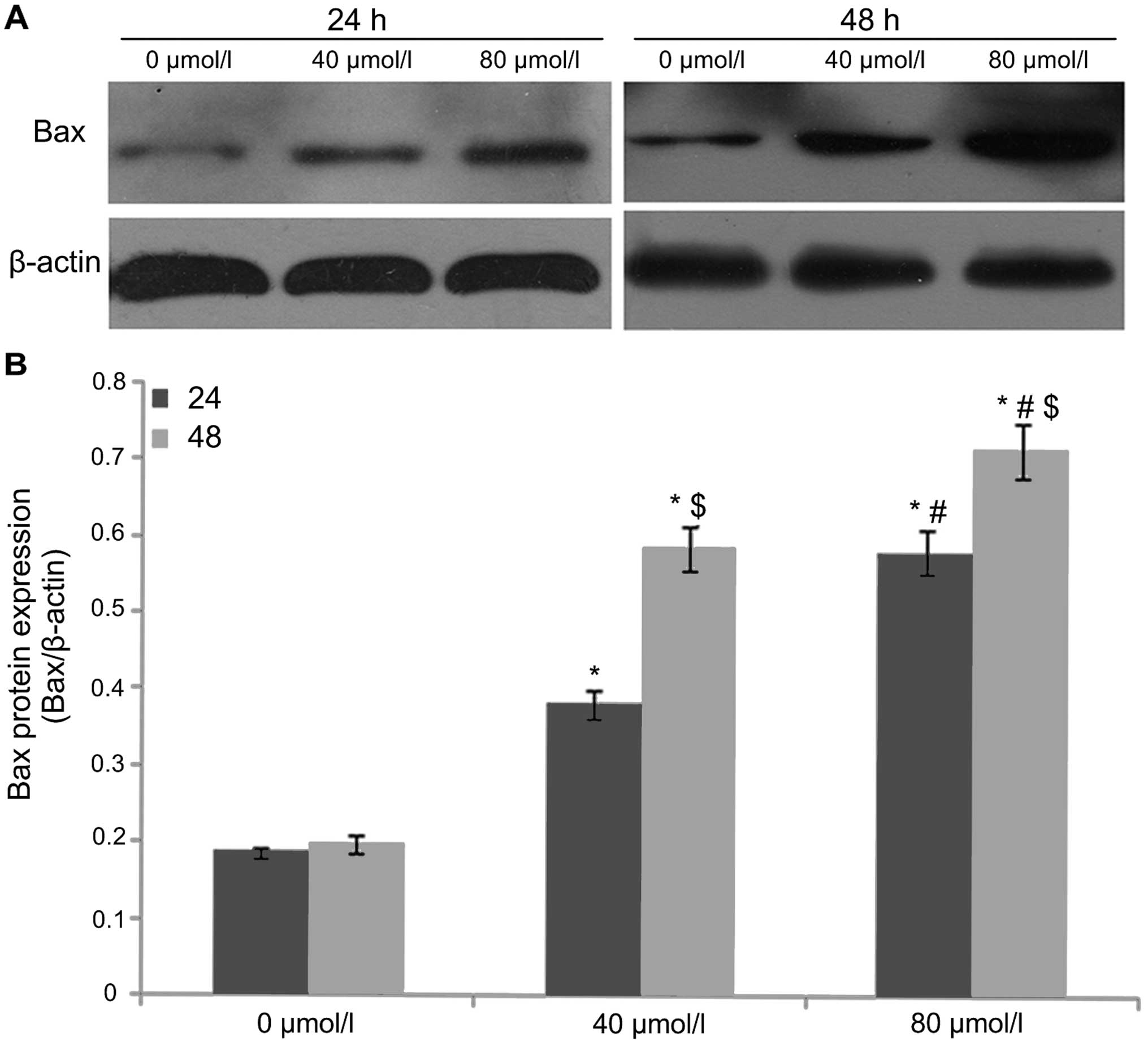
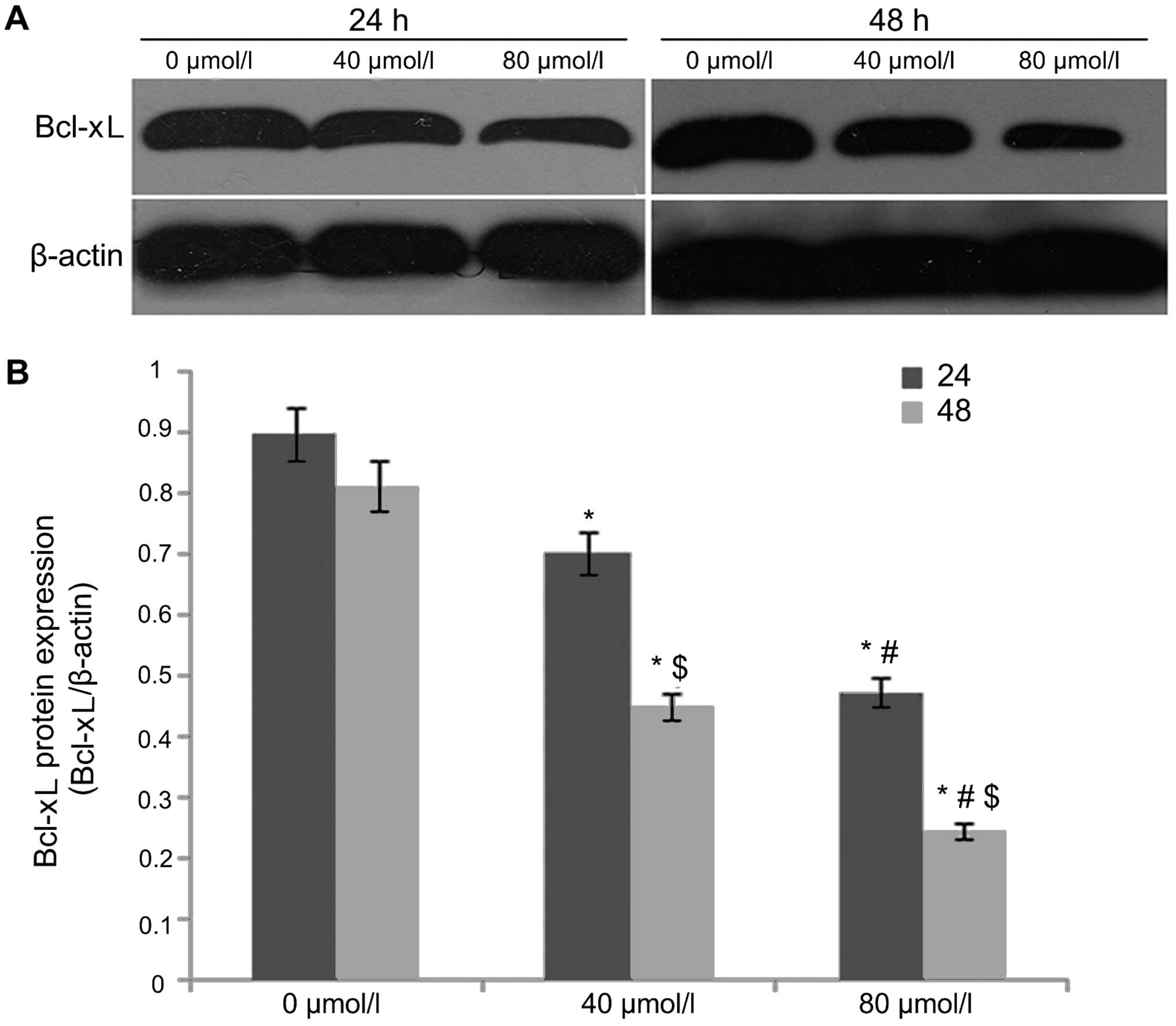
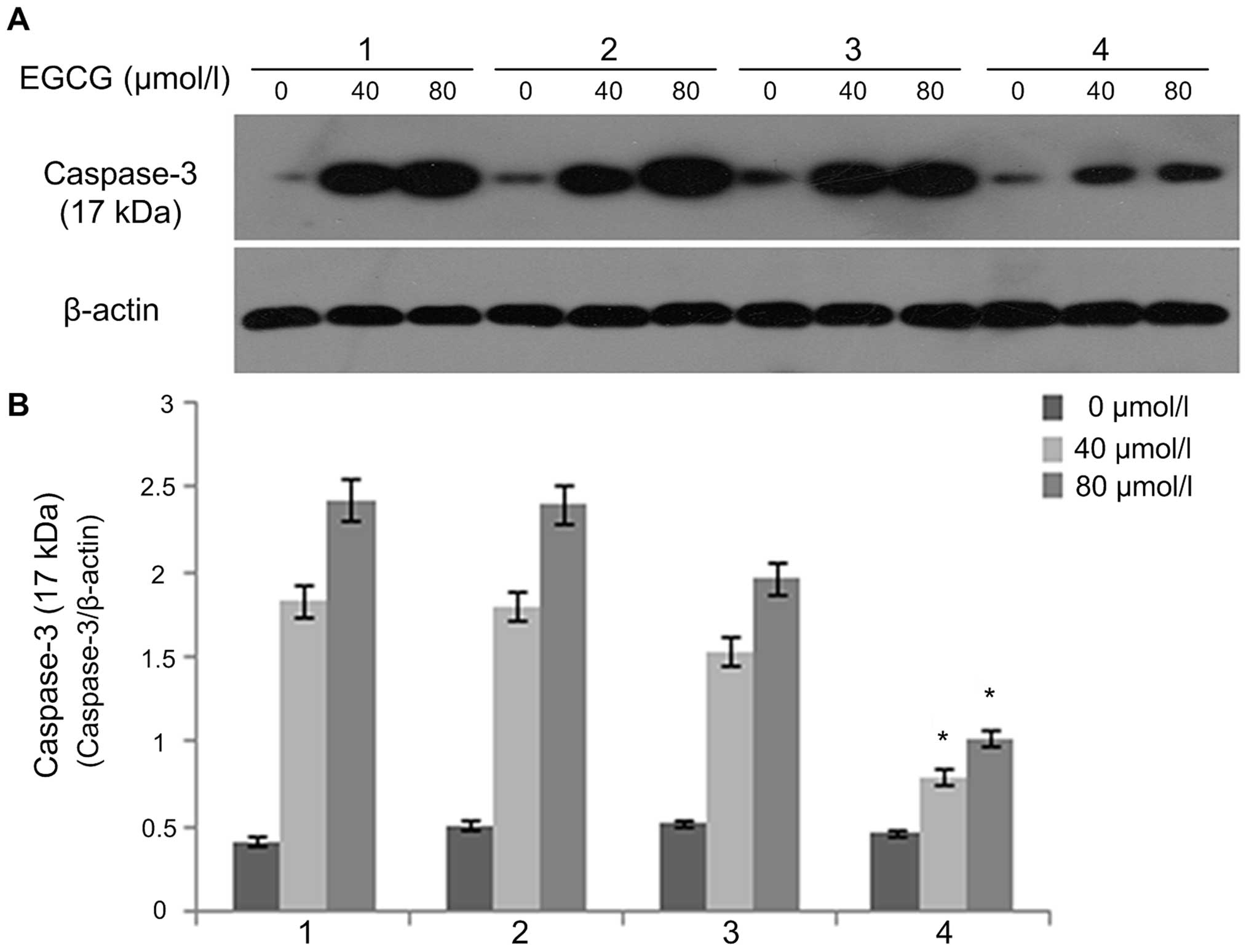
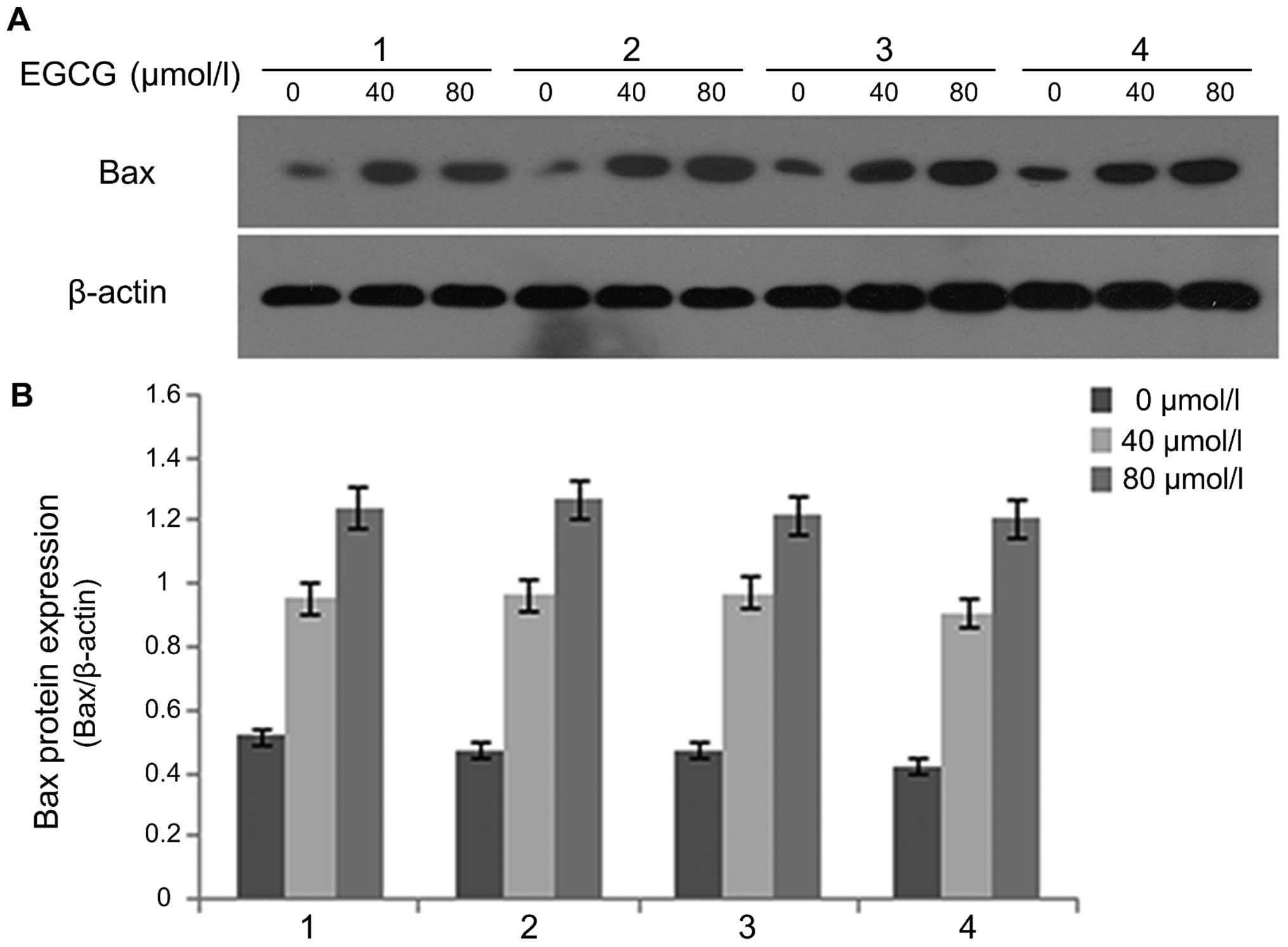
EGCG regulates cleaved caspase-3, Bax and
Bcl-xL expression
After A549 cells were treated with EGCG
concentrations of 0, 40 and 80 µmol/l for 24 and 48 h,
western blot analysis was used to detect the protein expression of
cleaved caspase-3 (Fig. 3), Bax
(Fig. 4) and Bcl-xL (Fig. 5). The results showed that the
protein expression of caspase-3 (17 kDa) and Bax in the 0
µmol/l group was relatively low after 24 h of treatment.
With an increase in EGCG concentration to 40 and 80 µmol/l,
the protein expression of caspase-3 (17 kDa) and Bax in 40 and 80
µmol/l EGCG treatment groups showed an increasing trend that
was statistically significant (P<0.05), displaying a dose- and
time-dependent relationship. In contrast, Bcl-xL protein expression
was higher in the 0 µmol/l group after 24 h of treatment.
With the increase of EGCG concentration to 40 and 80 µmol/l,
Bcl-xL protein expression gradually decreased and the difference
was statistically significant (P<0.05).
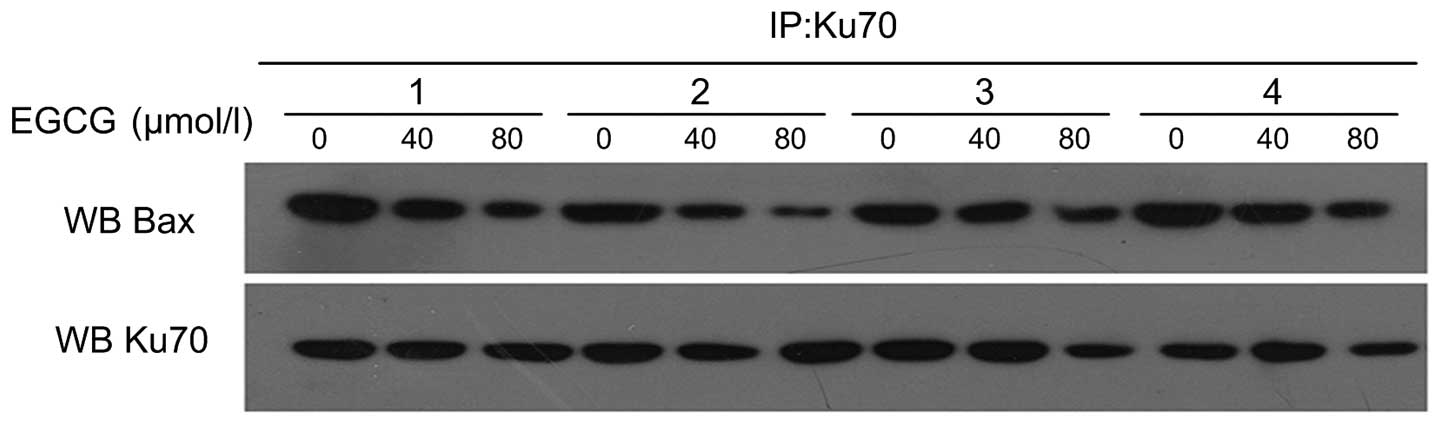
Regulation of Ku70 acetylation and
interference with Ku70-Bax interaction by EGCG
Following A549 cell treatment with 0, 40 and 80
µmol/l EGCG for 24 and 48 h, co-immunoprecipitation was used
to detect the acetylation status of Ku70 and the interaction
between Ku70-Bax (Fig. 6). The
results showed that in the 0 µmol/l group after treatment
for 24 h, the Ku70 acetylation status was weak, but the interaction
of Ku70-Bax was strong. With an increase of EGCG concentration to
40 and 80 µmol/l, Ku70 acetylation status was strengthened,
but the interaction of Ku70-Bax showed a decreasing trend. After
treatment with the same concentration of EGCG for a different time,
the acetylation status of Ku70 was positively related to the length
of treatment. However, the interaction of Ku70-Bax gradually
decreased with the prolongation of treatment.
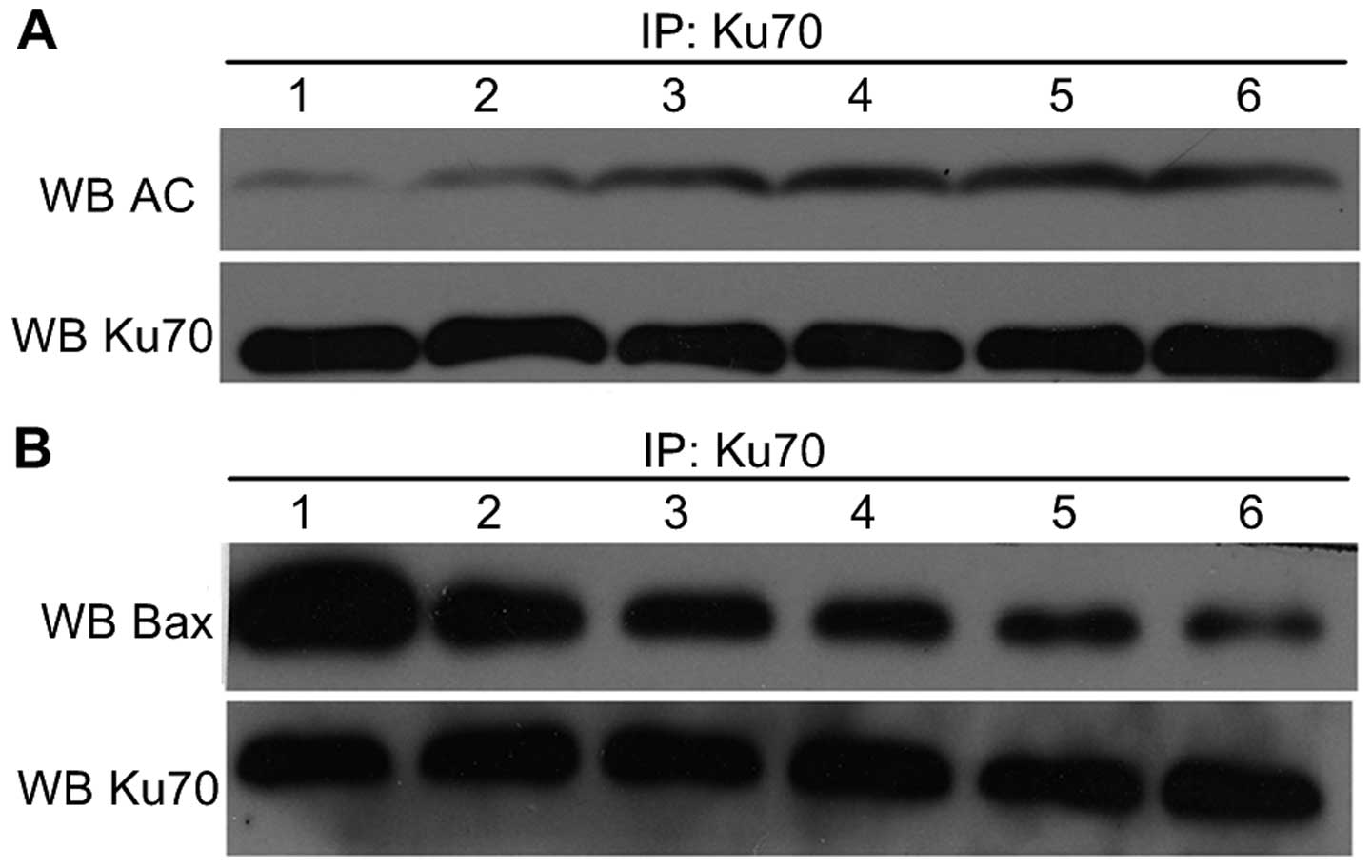 | Figure 6Ku70 acetylation level and Bax-Ku70
protein interaction level in A549 cells treated with EGCG. (A) Ku70
acetylation level in A549 cells treated with different
concentrations of EGCG for various lengths of time (1, 0
µmol/l, 24 h; 2, 40 µmol/l, 24 h; 3, 80
µmol/l, 24 h; 4, 0 µmol/l, 48 h; 5, 40 µmol/l,
48 h; and 6, 80 µmol/l, 48 h). (B) Bax-Ku70 protein
interaction level in A549 cells treated with different
concentrations of EGCG for various lengths of time (1, 0
µmol/l, 24 h; 2, 40 µmol/l, 24 h; 3, 80
µmol/l, 24 h; 4, 0 µmol/l, 48 h; 5, 40 µmol/l,
48 h; and 6, 80 µmol/l, 48 h). Co-immunoprecipitation showed
Ku70 acetylation status was strengthened, but the interaction of
Ku70-Bax was decreased with the EGCG treatment, dose- and
time-dependently. |
EGCG induces A549 cell apoptosis with
different plasmid transfections
Following A549 cell treatment with different
concentrations of EGCG (0, 40 and 80 µM) and undergoing
plasmid transfections [pCDNA3.1(+), and pCDNA3.1(+)-Ku70 and
pCDNA3.1(+)-Ku70539/542R plasmid transfection groups]
for 48 h, Annexin V/PI double staining was used to detect the
apoptosis rate of the cells (Fig.
7). The results showed that the early apoptosis rate of A549
cells increased with an increasing concentration of EGCG in the
pCDNA3.1(+) plasmid transfection group. However, the early cell
apoptosis rate was similar with or without EGCG treatment in the
pCDNA3.1(+)-Ku70 and in the pCDNA3.1(+)-Ku70539/542R
plasmid transfection groups. In contrast, for cells treated with
EGCG, the early apoptosis rate in the pCDNA3.1(+)-Ku70 and in the
pCDNA3.1(+)-Ku70539/542R plasmid transfection groups was
lower than that in the control group and in the pCDNA3.1(+) plasmid
transfection group (P<0.05).
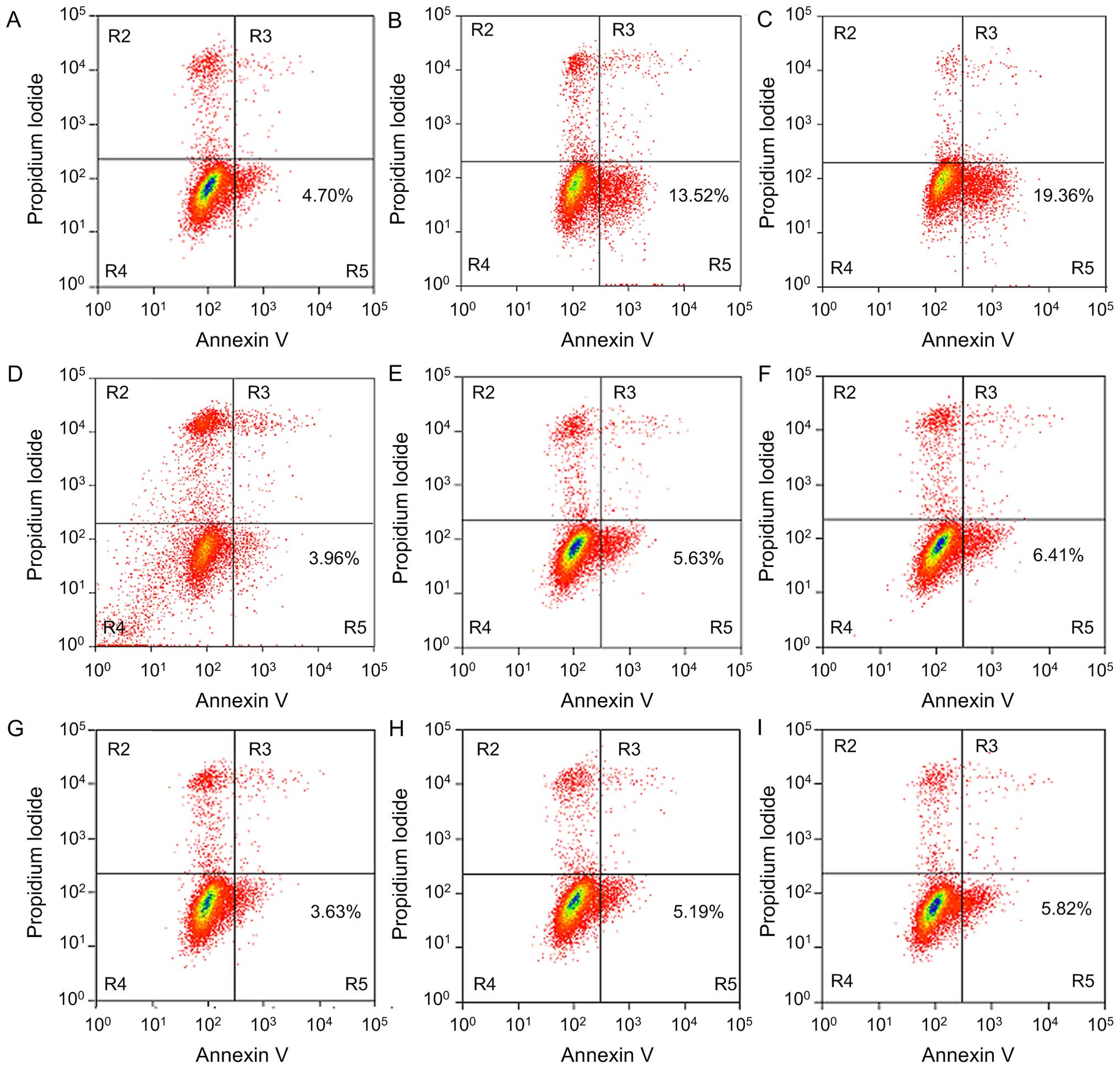 | Figure 7Apoptosis inducing effect of A549
cells treated with EGCG, after undergoing different plasmid
transfections for 48 h, detected by Annexin V/PI double staining.
(A) pCDNA3.1(+) plasmid transfection group, 0 µmol/l; (B)
pCDNA3.1(+) plasmid transfection group, 40 µmol/l; (C)
pCDNA3.1(+) plasmid transfection group, 80 µmol/l; (D)
pCDNA3.1(+)-Ku70 plasmid transfection group, 0 µmol/l; (E)
pCDNA3.1(+)-Ku70 plasmid transfection group, 40 µmol/l; (F)
pCDNA3.1(+)-Ku70 plasmid transfection group, 80 µmol/l; (G)
pCDNA3.1 (+)-Ku70539/542R plasmid transfection group, 0
µmol/l; (H) pCDNA3.1 (+)-Ku70539/542R plasmid
transfection group, 40 µmol/l; (I) pCDNA3.1
(+)-Ku70539/542R plasmid transfection group, 80
µmol/l. For cells treated with EGCG, the early apoptosis
rate in the pCDNA3.1(+)-Ku70 and in the
pCDNA3.1(+)-Ku70539/542R plasmid transfection groups was
lower than that in the control and in the pCDNA3.1(+) plasmid
transfection groups. |
Effects of EGCG on cleaved caspase-3 and
Bax expression in A549 cells with different plasmid
transfections
A549 cells were treated with different
concentrations of EGCG (0, 40 and 80 µmol/l) and undergwent
different plasmid transfections [control, pCDNA3.1(+),
pCDNA3.1(+)-Ku70 and pCDNA3.1(+)-Ku70539/542R plasmid
transfection groups] for 48 h, western blot analysis was used to
detect the protein expression of cleaved caspase-3 (Fig. 8) and Bax (Fig. 9). Caspase-3 (17 kDa) expression in
the pCDNA3.1(+)-Ku70 plasmid transfection group and in the
pCDNA3.1(+)-Ku70539/542R plasmid transfection group was
similar to that of the control group and the pCDNA3.1(+) plasmid
transfection group without EGCG treatment. In contrast, for cells
treated with EGCG, caspase-3 (17 kDa) expression in the
pCDNA3.1(+)-Ku70 and in the pCDNA3.1(+)-Ku70539/542R
plasmid transfection groups was lower than that of the control and
the pCDNA3.1(+) plasmid transfection groups. In particular,
caspase-3 expression in the pCDNA3.1(+)-Ku70539/542R
plasmid transfection group was lower, and the difference was
statistically significant (P<0.05). However, the Bax protein
expression in A549 cells showed no significant difference after
intervention by different plasmid transfections with the same
concentration of EGCG.
Effect of EGCG on the Bax-Ku70
interaction in A549 cells with different plasmid transfections
A549 cells were treated with different
concentrations of EGCG (0, 40 and 80 µmol/l) then underwent
different plasmid transfections [control, pCDNA3.1(+),
pCDNA3.1(+)-Ku70 and pCDNA3.1 (+)-Ku70539/542R plasmid
transfection groups] for 48 h, co-immunoprecipitation was used to
detect the interaction of Ku70-Bax (Fig. 10). The interaction of Bax-Ku70 in
the pCDNA3.1(+)-Ku70 and in the pCDNA3.1(+)-Ku70539/542R
plasmid transfection groups was similar to that of the control
group and the pCDNA3.1(+) plasmid transfection group without EGCG
treatment. In contrast, for cells treated with EGCG, the
interaction of Bax-Ku70 in the pCDNA3.1(+)-Ku70 and in the
pCDNA3.1(+)-Ku70539/542R plasmid transfection groups was
significantly stronger than that of the control and the pCDNA3.1(+)
plasmid transfection groups. In particular, the interaction of
Bax-Ku70 was stronger in the pCDNA3.1(+)-Ku70539/542R
plasmid transfection group, and the difference was statistically
significant (P<0.05).
Discussion
Green tea is one of the most consumed beverages
worldwide, particularly in Asian countries. EGCG is the main
monomer component of green tea polyphenols. Many research studies
have shown that EGCG has an inhibitory effect on the occurrence and
development of malignant tumors. The present study showed that EGCG
could effectively induce apoptosis of human lung adenocarcinoma
A549 cells, further confirming our previous results. The
outstanding advantage of EGCG is the milder effect on normal cells
while killing tumor cells. Kang et al showed that the
killing effect of EGCG on Ewing's sarcoma cells of children was
stronger than that on normal cells (23). In their study, 25 and 50
µmol/l EGCG showed obvious cell growth inhibition on Ewing's
sarcoma cell lines TC32 and TC71, respectively, but only mild
damage on the normal human microvascular endothelial cell line
HBMEC, up to an EGCG concentration of 100 µmol/l. A similar
drug action was also shown by EGCG on the adrenal carcinoma cell
line NCI-H295 and normal primary human embryonic skin cells
(24).
Apoptosis is a complex process regulated by several
molecules that function as either promoters, including Bax, Bak and
caspases, or inhibitors of the cell death process such as Bcl-2,
Bcl-xL and the IAP proteins (25).
Studies have shown that EGCG can effectively regulate various key
molecules in cell mitochondrial apoptosis pathways in other tumors
as a potential antitumor substance (18,19).
Our previous study showed that the long-term ingestion of green tea
could effectively prevent lung cancer, in rats, induced by the
carcinogen 3,4-benzopyrene (B[a]P), a process that may be related
to the EGCG-mediated upregulation of P53 expression and
down-regulation of Bcl-2 expression (26). The present study showed that on one
hand, EGCG upregulated the expression of the apoptosis-promoting
factor Bax, whereas on the other hand, it downregulated the
expression of the apoptosis inhibitor Bcl-xL. EGCG also activated a
member of the caspase-3 family to achieve anticancer effects,
suggesting that EGCG may regulate human lung adenocarcinoma A549
cells through the mitochondrial apoptosis pathway. Hastak et
al knocked down Bax, and then the wild-type group and the Bax
interference group were treated with placebo and EGCG. The results
showed that the apoptosis rate in the Bax interference group was
significantly higher than that of the wild-type group after
treatment with EGCG. However, there was no obvious difference in
the apoptosis rate between the Bax interference group and the
wild-type group after treatment with placebo, suggesting that
EGCG-induced cell apoptosis required the interference of Bax
(27).
Sawada et al screened functional inhibitory
protein Ku70 combined with Bax by the functional screening method
based on yeast and confirmed that Ku70 had a direct inhibitory
effect on the Bax-mediated mitochondrial apoptosis pathway
(28). Further studies showed that
the inhibitory effect was related to the direct binding of BIP and
Bax on the Ku70 protein C terminal (29). The present study also showed that
Ku70 and its acetylation status may play a critical role in the
mitochondrial apoptosis pathway mediated by Bax. Our study showed
that for A549 cells under the influence of EGCG, the interaction of
Bax-Ku70 decreased with an increasing concentration of EGCG. In
addition, their interaction showed a decreasing trend with
prolongation of the action time. To further understand whether the
EGCG-mediated interaction of Ku70-Bax was related to Ku70
acetylation, this study further detected the regulation of EGCG on
the Ku70 acetylation status of A549 cells. The results showed that
EGCG could also effectively upregulate the acetylation status of
A549 cells and showed that this effect was concentration- and
time-dependent.
Cohen et al (30) compared the Ku70 amino acid sequence
and the amino acid sequence of other factors regulated by
acetylation, including P53, FEN1, GATA1 and EFIILβ. The results
showed that the 530–583 sequence of Ku70 was similar to the amino
acid sequence in the acetylation area of the above factors. A point
mutation of several lysine residues in the 530–583 sequence of Ku70
was introduced. The results revealed that if lysine was mutated
into arginine, the loci would lose the acetylation function but
would not affect the entire Ku70 protein function. The final
results showed that two loci, K539 and K542, played a crucial role
in Ku70 acetylation (31). Therefore, in this study, the
pCDNA3.1(+)-Ku70 and pCDNA3.1(+)-Ku70539/542R plasmids
were constructed and successfully transferred into A549 cells. The
two acetylation loci of Ku70, K539 and K542, were lost after
pCDNA3.1(+)-Ku70539/542R transfection, and therefore,
the acetylation function was lost. Thereafter, we determined that
the apoptosis-promoting effect of EGCG on A549 cells was obviously
weakened, concurrent with strengthening of the Bax-Ku70 interaction
and a decline in cleaved caspase-3 expression, after
pCDNA3.1(+)-Ku70 plasmid and pCDNA3.1(+)-Ku70539/542R
plasmid transfection. This result verified that EGCG may induce
apoptosis of human lung adenocarcinoma A549 cells through the K539
and K542 acetylation loci of Ku70.
Acknowledgments
The present study was supported by the National Key
Scientific and Technology Support Program. Collaborative innovation
of Clinical Research for chronic obstructive pulmonary disease and
lung cancer, no. 2013BAI09B09.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kushi LH, Doyle C, McCullough M, Rock CL,
Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K and
Gansler T; American Cancer Society 2010 Nutrition and Physical
Activity Guidelines Advisory Committee: American Cancer Society
Guidelines on nutrition and physical activity for cancer
prevention: Reducing the risk of cancer with healthy food choices
and physical activity. CA Cancer J Clin. 62:30–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin L, Li C, Xu Y, Wang L, Liu J, Wang D,
Hong C, Jiang Z, Ma Y, Chen Q, et al: Epigallocatechin gallate
promotes p53 accumulation and activity via the inhibition of
MDM2-mediated p53 ubiquitination in human lung cancer cells. Oncol
Rep. 29:1983–1990. 2013.PubMed/NCBI
|
|
4
|
Ma YC, Li C, Gao F, Xu Y, Jiang ZB, Liu JX
and Jin LY: Epigallocatechin gallate inhibits the growth of human
lung cancer by directly targeting the EGFR signaling pathway. Oncol
Rep. 31:1343–1349. 2014.
|
|
5
|
Lee YH, Kwak J, Choi HK, Choi KC, Kim S,
Lee J, Jun W, Park HJ and Yoon HG: EGCG suppresses prostate cancer
cell growth modulating acetylation of androgen receptor by
anti-histone acetyltransferase activity. Int J Mol Med. 30:69–74.
2012.PubMed/NCBI
|
|
6
|
Tang Y, Zhao DY, Elliott S, Zhao W, Curiel
TJ, Beckman BS and Burow ME: Epigallocatechin-3 gallate induces
growth inhibition and apoptosis in human breast cancer cells
through survivin suppression. Int J Oncol. 31:705–711.
2007.PubMed/NCBI
|
|
7
|
Yang CS and Wang ZY: Tea and cancer. J
Natl Cancer Inst. 85:1038–1049. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Xin Y, Xiao Y and Zhao J: Low-dose
docetaxel combined with (−)-epigallocatechin-3-gallate inhibits
angiogenesis and tumor growth in nude mice with gastric cancer
xenografts. Cancer Biother Radiopharm. 27:204–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu
K, Wang F, Liu K, Liu Q, Yang C, et al: Anti-cancer activities of
tea epigallocatechin-3-gallate in breast cancer patients under
radiotherapy. Curr Mol Med. 12:163–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tudoran O, Soritau O, Balacescu O,
Balacescu L, Braicu C, Rus M, Gherman C, Virag P, Irimie F and
Berindan-Neagoe I: Early transcriptional pattern of angiogenesis
induced by EGCG treatment in cervical tumour cells. J Cell Mol Med.
16:520–530. 2012. View Article : Google Scholar
|
|
11
|
Lu YP, Lou YR, Xie JG, Peng QY, Liao J,
Yang CS, Huang MT and Conney AH: Topical applications of caffeine
or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and
selectively increase apoptosis in UVB-induced skin tumors in mice.
Proc Natl Acad Sci USA. 99:12455–12460. 2002. View Article : Google Scholar
|
|
12
|
Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia
N, Spiegelman VS and Mukhtar H: Green tea polyphenol EGCG
suppresses cigarette smoke condensate-induced NF-kappaB activation
in normal human bronchial epithelial cells. Oncogene. 26:673–682.
2007. View Article : Google Scholar
|
|
13
|
Lee MH, Han DW, Hyon SH and Park JC:
Apoptosis of human fibrosarcoma HT-1080 cells by
epigallocatechin-3-O-gallate via induction of p53 and caspases as
well as suppression of Bcl-2 and phosphorylated nuclear factor-κB.
Apoptosis. 16:75–85. 2011. View Article : Google Scholar
|
|
14
|
Tsukamoto S, Hirotsu K, Kumazoe M, Goto Y,
Sugihara K, Suda T, Tsurudome Y, Suzuki T, Yamashita S, Kim Y, et
al: Green tea polyphenol EGCG induces lipid-raft clustering and
apoptotic cell death by activating protein kinase Cδ and acid
sphingomyelinase through a 67 kDa laminin receptor in multiple
myeloma cells. Biochem J. 443:525–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adachi S, Nagao T, Ingolfsson HI, Maxfield
FR, Andersen OS, Kopelovich L and Weinstein IB: The inhibitory
effect of (−)-epigallocatechin gallate on activation of the
epidermal growth factor receptor is associated with altered lipid
order in HT29 colon cancer cells. Cancer Res. 67:6493–6501. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB,
Lee CN and Hsieh CY: Interleukin-6 promotes cervical tumor growth
by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene.
22:1517–1527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Hao MW, Dong K, Lin F, Ren JH and
Zhang HZ: Apoptosis induction effects of EGCG in laryngeal squamous
cell carcinoma cells through telomerase repression. Arch Pharm Res.
32:1263–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Xie Y, Feng Y, Zhang L, Huang X,
Shen X and Luo X: (−)-Epigallocatechingallate induces apoptosis in
B lymphoma cells via caspase-dependent pathway and Bcl-2 family
protein modulation. Int J Oncol. 46:1507–1515. 2015.PubMed/NCBI
|
|
19
|
Sonoda JI, Ikeda R, Baba Y, Narumi K,
Kawachi A, Tomishige E, Nishihara K, Takeda Y, Yamada K, Sato K, et
al: Green tea catechin, epigallocatechin-3-gallate, attenuates the
cell viability of human non-small-cell lung cancer A549 cells via
reducing Bcl-xL expression. Exp Ther Med. 8:59–63. 2014.PubMed/NCBI
|
|
20
|
Hurwitz JL, Stasik I, Kerr EM, Holohan C,
Redmond KM, McLaughlin KM, Busacca S, Barbone D, Broaddus VC, Gray
SG, et al: Vorinostat/SAHA-induced apoptosis in malignant
mesothelioma is FLIP/caspase 8-dependent and HR23B-independent. Eur
J Cancer. 48:1096–1107. 2012. View Article : Google Scholar
|
|
21
|
Hada M and Kwok RP: Regulation of ku70-bax
complex in cells. J Cell Death. 7:11–13. 2014.PubMed/NCBI
|
|
22
|
Li JJ, Gu QH, Li M, Yang HP, Cao LM and Hu
CP: Role of Ku70 and Bax in epigallocatechin-3-gallate-induced
apoptosis of A549 cells in vivo. Oncol Lett. 5:101–106. 2013.
|
|
23
|
Kang HG, Jenabi JM, Liu XF, Reynolds CP,
Triche TJ and Sorensen PH: Inhibition of the insulin-like growth
factor I receptor by epigallocatechin gallate blocks proliferation
and induces the death of Ewing tumor cells. Mol Cancer Ther.
9:1396–1407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu PP, Kuo SC, Huang WW, Yang JS, Lai KC,
Chen HJ, Lin KL, Chiu YJ, Huang LJ and Chung JG:
(−)-Epigallocatechin gallate induced apoptosis in human adrenal
cancer NCI-H295 cells through caspase-dependent and
caspase-independent pathway. Anticancer Res. 29:1435–1442.
2009.PubMed/NCBI
|
|
25
|
Deveraux QL, Schendel SL and Reed JC:
Antiapoptotic proteins. The bcl-2 and inhibitor of apoptosis
protein families. Cardiol Clin. 19:57–74. 2001. View Article : Google Scholar
|
|
26
|
Gu Q, Hu C, Chen Q and Xia Y: Tea
polyphenols prevent lung from preneoplastic lesions and effect p53
and bcl-2 gene expression in rat lung tissues. Int J Clin Exp
Pathol. 6:1523–1531. 2013.PubMed/NCBI
|
|
27
|
Hastak K, Agarwal MK, Mukhtar H and
Agarwal ML: Ablation of either p21 or Bax prevents p53-dependent
apoptosis induced by green tea polyphenol
epigallocatechin-3-gallate. FASEB J. 19:789–791. 2005.PubMed/NCBI
|
|
28
|
Sawada M, Sun W, Hayes P, Leskov K,
Boothman DA and Matsuyama S: Ku70 suppresses the apoptotic
translocation of Bax to mitochondria. Nat Cell Biol. 5:320–329.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawada M, Hayes P and Matsuyama S:
Cytoprotective membrane-permeable peptides designed from the
Bax-binding domain of Ku70. Nat Cell Biol. 5:352–357. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen HY, Lavu S, Bitterman KJ, Hekking B,
Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM and Sinclair
DA: Acetylation of the C terminus of Ku70 by CBP and PCAF controls
Bax-mediated apoptosis. Mol Cell. 13:627–638. 2004. View Article : Google Scholar : PubMed/NCBI
|