Introduction
Ovarian cancer is the most lethal gynecologic
malignancy (1), and more than 90%
is classified as epithelial ovarian cancer (EOC) (2). The majority of EOC patients are
diagnosed at advanced stages and the overall 5-year survival rate
is approximately 40% (3). It has
been reported that invasion and metastasis process remains the
leading cause of recurrence and death from EOC and the molecular
mechanisms of invasiveness and the metastatic property are not
clearly understood.
Accumulating evidence reveals that epithelial to
mesenchymal transition (EMT), a well-recognized process by which
cells from a differentiated epithelial state could convert into a
dedifferentiated migratory mesenchymal phenotype, which plays an
essential role in the regulation of embryogenesis, organ fibrosis,
wound healing and cancer metastasis (4). The crucial events of EMT include
decreasing the cell-cell adhesion molecule E-cadherin; increasing
more plastic mesenchymal proteins such as vimentin, N-cadherin, and
deregulating the canonical WNT/β-catenin signaling pathway
(5). Emerging evidence indicates
that EMT is of vital importance in obtaining invasive and migratory
ability in EOC (6–8). A number of transcription factors
(TFs), such as Twist, Snail, Slug, and zinc finger E-box binding
homeobox 1 (ZEB1)/2, are considered as the major inducers of EMT
that repress E-cadherin directly or indirectly in EOC cells
(9–13). Thus, EOC patients may benefit from
targeted therapies that inhibit EMT.
Emodin (1, 3, 8-trihydroxy-6-methylanthraquinone;
EMO) is a natural anthraquinone derivative in the roots and
rhizomes of Polygonum cuspidatum and Rheum palmatum,
possessing various biological activities such as anti-inflammatory,
anti-bacterial, antioxidant and anticancer properties (14–17).
EMO displays anticancer activities in several types of cancers,
including EOC. In one study it was elucidated that EMO exerted
antiproliferative and apoptosis-inducing effects in A2780
(paclitaxel-sensitive) and A2780/taxol (paclitaxel-resistant) cells
by reducing the expression of anti-apoptotic molecules, such as
survivin and X-linked inhibitor of apoptosis (XIAP) (18), whereas, EMO had potential to inhibit
tumor invasion and metastasis of HO-8910PM cells via repression of
the production of MMP-9 (19).
Studies in vitro also demonstrated that EMO was effective on
restraining SKOV3 and HO8910 cell invasion (20). However, the molecular mechanisms of
the anti-invasive and antimetastatic functions are unknown. In
previous studies, EMO was shown to exert inhibitory effects on cell
invasion and migration of colorectal and cervical cancer, and head
and neck squamous cell carcinoma (HNSCC), which is associated with
the inhibition of EMT and deregulation of WNT/β-catenin signaling
pathway (21–23). Therefore, we speculated that EMO may
inhibit the EOC cells to undergo the EMT progress by regulation of
WNT/β-catenin signaling pathway, consequently weakening the
invasiveness and meta-static ability.
We investigated the effects of EMO on EOC cells
in vitro. In the present study, we demonstrated that EMO
could inhibit EOC cells invasion and migration by suppressing EMT,
demonstrated by increased epithelial markers E-cadherin and keratin
and decreased mesenchymal markers vimentin and N-cadherin. One of
the molecular mechanisms was that EMO was able to activate glycogen
synthase kinase-3β (GSK-3β), which may targets β-catenin and then
decreases its target gene ZEB1 expression.
Materials and methods
Cell culture and treatment
A2780, OVCAR-3 and SK-OV-3 cells, and human EOC cell
lines were purchased from the Conservation Genetics Chinese Academy
of Sciences (CAS) Cell Bank (Shanghai, China). A2780 cells were
maintained in RPMI-1640 medium (Gibco by Life Technologies, Grand
Island, NY, USA) supplemented with 2.0 g/l NaHCO3 and
10% fetal bovine serum (FBS) (Gibco by Life Technologies,
Australia). OVCAR-3 cells were cultured in RPMI-1640 medium
supplemented with 2.0 g/l NaHCO3 and 20% FBS. SK-OV-3
cells were maintained in McCoy's 5A medium (Sigma, St. Louis, MO,
USA) with 2.2 g/l NaHCO3 and 10% FBS. All cell culture
supplements contained 100 U/ml penicillin and 0.1 mg/ml
streptomycin (Beijing Solarbio Science and Technology Co., Ltd.).
Cells were kept at 37°C with subconfluence in 95% air and 5%
CO2 in a humidified incubator. EMO and inhibitor
SB216763, dissolved in 100% dimethylsulfoxide (DMSO; Sigma) at 50
mM stock solution respectively, were purchased from Sigma. For EMO
or SB216763 treatment, stock solution was added into culture medium
at a final concentration of <0.1% DMSO (v/v). DMSO solution
alone was used as a control for all experiments.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay (BestBio,
Shanghai, China) was used to study the influence of EMO on cell
proliferation. Cells were seeded at a density of 2.5×103
cells/well in 100 µl medium into 96-well plates. After
treatment with 20 µM EMO for 48 h, 10 µl of CCK-8 was
added to each well, followed with incubation for 2 h at 37°C. Cell
viability was determined by the absorbance at 450 nm in each well
measured by a plate reader (Bio-Rad, Hercules, CA, USA). Each
experiment was performed in quintuplicate.
Transwell Matrigel invasion assays
Cell invasion assay with a Matrigel-coated membrane
(1:4 dilution in serum-free medium; BD Biosciences, San Jose, CA,
USA) was performed using 24-well Transwell inserts with a pore
diameter of 8-µm (Corning Costar, Cambridge, MA, USA). When
cells were seeded into 6-well plates until treatment with EMO for
48 h when reaching confluence of 70–80%, 3×104 cells
were re-suspended in 200 µl serum-free medium and then
seeded into the upper chamber. Medium (750 µl) containing
10% FBS was added into the lower chamber. After incubation for 24
h, the cells on the upper surface of the filter were removed by
wiping with a cotton swab. The invaded cells on the lower surface
of the filter were fixed in methanol solution for 5 min and 3.7%
formaldehyde solution for 5 min, and then stained with Giemsa
(Beijing Solarbio Science and Technology Co., Ltd.). Images were
captured by an Olympus IX51 inverted microscope (Olympus Optical,
Melville, NY, USA) and five visual fields (magnification, ×100)
were counted.
Western blot analysis
The total cellular proteins were extracted by adding
appropriate RIPA lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS and sodium
orthovanadate, sodium fluoride, EDTA, leupeptin] complemented a
protease inhibitor phenylmethanesulfonyl fluoride (PMSF) (all from
Beyotime Biotechnology) for western blot analysis. After
centrifugation with 15,000 rpm at 4°C for 15 min, the supernatants
were collected, then protein concentration was determined using BCA
protein assay kit according to the manufacturer's instructions and
whole lysates were mixed with 5X SDS-PAGE sample loading buffer
(both from Beyotime Biotechnology) at a ratio of 1:4. Samples were
heated at 95°C in metal bath for 5 min and were separated on
SDS-polyacrylamide gels. The separated proteins were then
transferred to a pure nitrocellulose blotting membrane (Pall Life
Sciences, Mexico). The membrane blots were probed with the
appropriate primary antibody at 4°C overnight and then incubation
with horseradish peroxidase-conjugated second antibody. Signals
from the bound antibodies were detected with enhanced
chemiluminescent substrate (Thermo Fisher Scientific Inc., Waltham,
MA, USA). The results were quantified by densitometry, using ImageJ
software (NIH, Bethesda, MD, USA). Primary antibodies against
matrix metallopro-teinase-9 (MMP-9), matrix metalloproteinase-2
(MMP-2), E-cadherin, keratin, N-cadherin, vimentin, ZEB1, GSK-3β,
β-catenin and glyceraldehyde-phosphate dehydrogenase (GAPDH) were
purchased from Cell Signaling Technology (Danvers, MA, USA).
Primary antibodies against phospho-Ser9-GSK-3β
(p-GSK-3βSer9) was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG), goat
anti-mouse IgG and rabbit anti-goat IgG were all obtained from
Zhongshan Jinqiao Biological Technology (Beijing, China).
Small interfering RNA (siRNA)
experiments
The sequence for ZEB1 specific siRNA (Shanghai
GenePharma Co., Ltd., China) was: 5′-UGAUCAGCCUCAAUCUGCAAAUGCA-3′
and non-targeting scrambled siRNA was the negative control. Cells
were transfected with 50 nM of siRNA using NanoFectin transfection
reagent (Excell Biotechnology, Shanghai, China) according to the
manufacturer's instructions. The transfection cocktail mixing 5
µl of NanoFectin transfection reagent and 245 µl
Opti-MEM medium (Gibco, Auckland, New Zealand) with 5 µl
siRNA and 245 µl Opti-MEM medium were incubated for 20 min
at room temperature. Then, the above mixtures were added to the
6-well plates with cells that had been seeded overnight to reach
50–60% confluence prior to transfection process. Transfection was
performed for 6 h at 37°C in a 5% CO2 incubator and the
old medium was changed with fresh complete medium for a further 12
h followed by EMO 20 µM treatment for 48 h. Then the cells
were harvested for Transwell assay and western blot analysis.
Statistical analysis
All statistical analyses were performed by
two-tailed Student's t-test using GraphPad Prism version 6.02
(GraphPad Software, Inc., La Jolla, CA, USA) and P<0.05 were
considered as significant. The data are shown as the mean ±
standard deviation (SD) from three independent assays.
Results
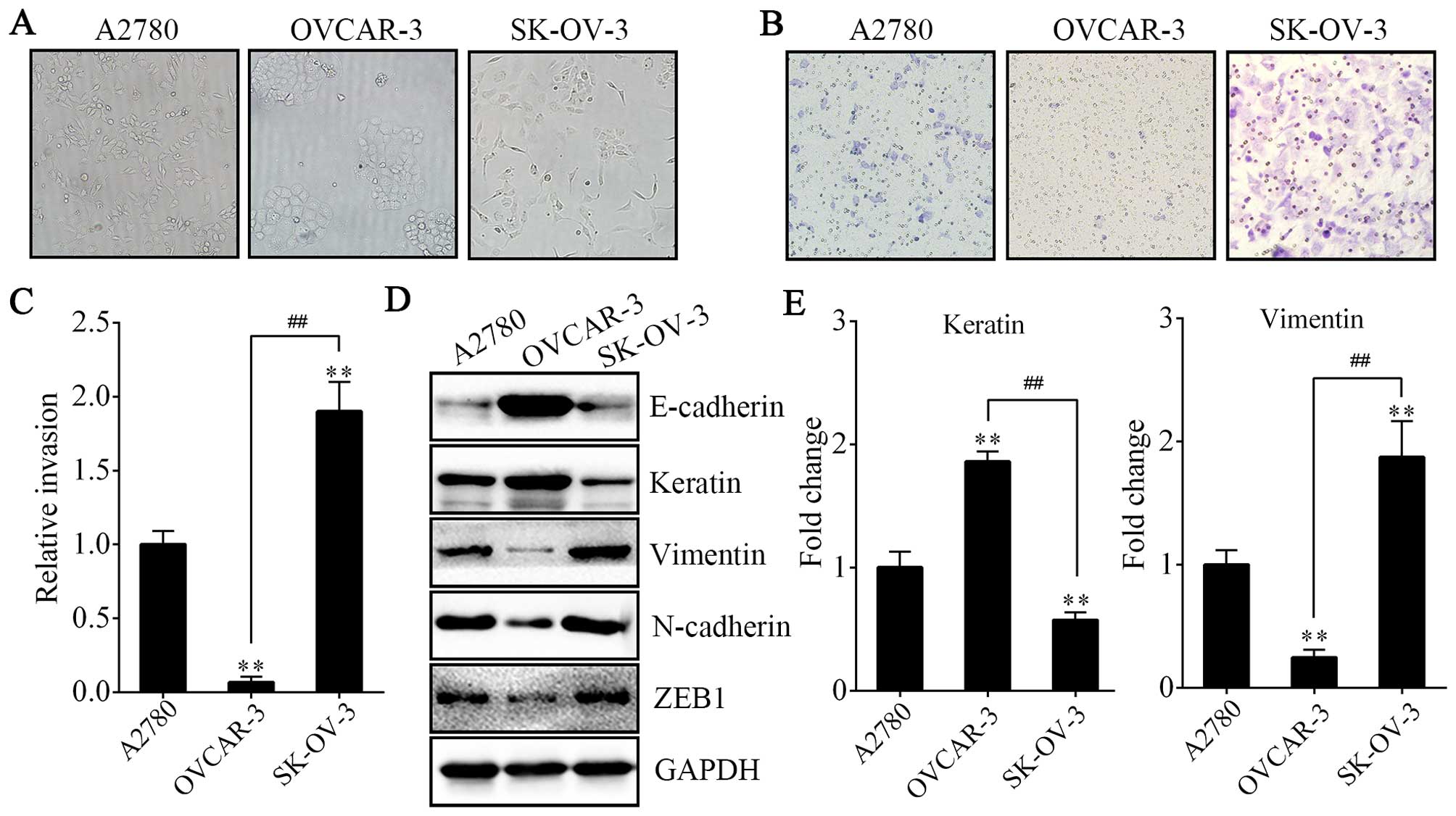
A2780 and SK-OV-3 cells exhibit a
spindle-like mesenchymal phenotype with more invasive ability than
OVCAR-3 cells
Before conducting the following experiment, we chose
cells with more invasive potential and having undergone EMT. First,
we compared the morphological characteristics of the human EOC cell
lines A2780, OVCAR-3 and SK-OV-3. We found that the SK-OV-3 cells
displayed elongated fibroblastoid morphology while the OVCAR-3
cells showed a rounded shape, typical of an epithelial
cobblestone-like appearance, growing in clusters. Morphology
feature of A2780 cells was between epithelial round-shaped and
spindle-shaped mesenchymal form (Fig.
1A). The results above were in accordance with their relative
invasive properties and the expression of markers of epithelial and
mesenchymal phenotype (Fig. 1B–E).
The weakest invasive activity and highest expression of E-cadherin
and keratin (epithelial markers) were shown in OVCAR-3 cells;
however, vimentin, N-cadherin and ZEB1 (mesenchymal markers) were
rarely expressed. In contrast, the A2780 and SK-OV-3 cells
exhibited more invasive ability and expressed significantly lower
levels of E-cadherin and keratin and higher levels of vimentin,
N-cadherin and ZEB1. Thus, we used A2780 and SK-OV-3 cells to
undergo EMO treatment.
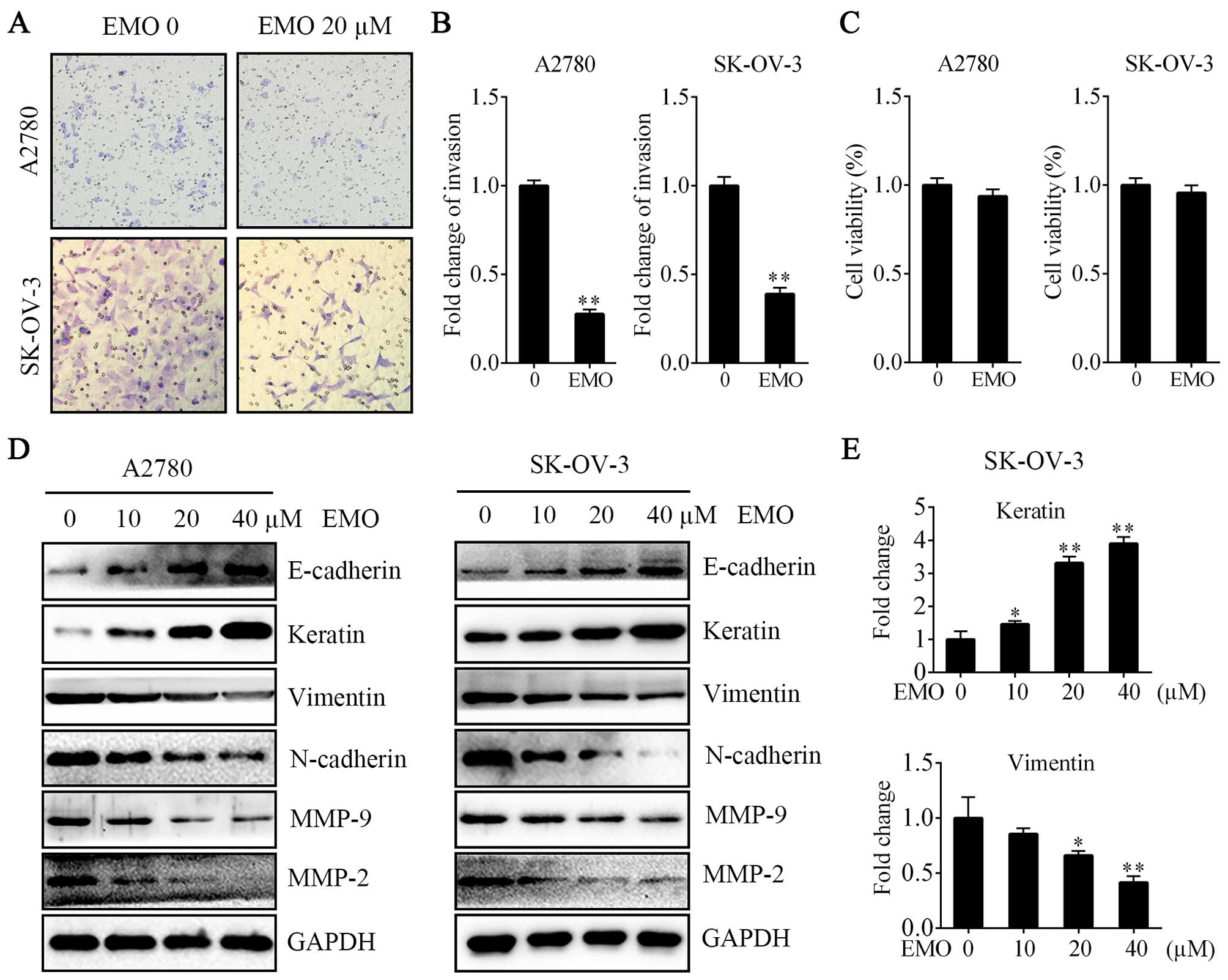
EMO suppresses the invasion property of
A2780 and SK-OV-3 cells by inhibiting EMT
To confirm that EMO inhibited the invasion of
ovarian cancer cells as previously reported, we performed Transwell
Matrigel invasion assays. We found that the invasion activities
were significantly decreased compared with the normal control group
in both A2780 and SK-OV-3 cells (Fig.
2A and B). Moreover, EMO 20 µM failed to regulate cell
proliferation ability (Fig. 2C). We
further studied whether EMO could change the EMT phenotype. After
the treatment of cells with various concentrations of EMO for 48 h,
upregulation of E-cadherin and keratin and downregulation of
vimentin, N-cadherin, MMP-9 and MMP-2 in a concentration-dependent
manner were observed (Fig. 2D and
E).
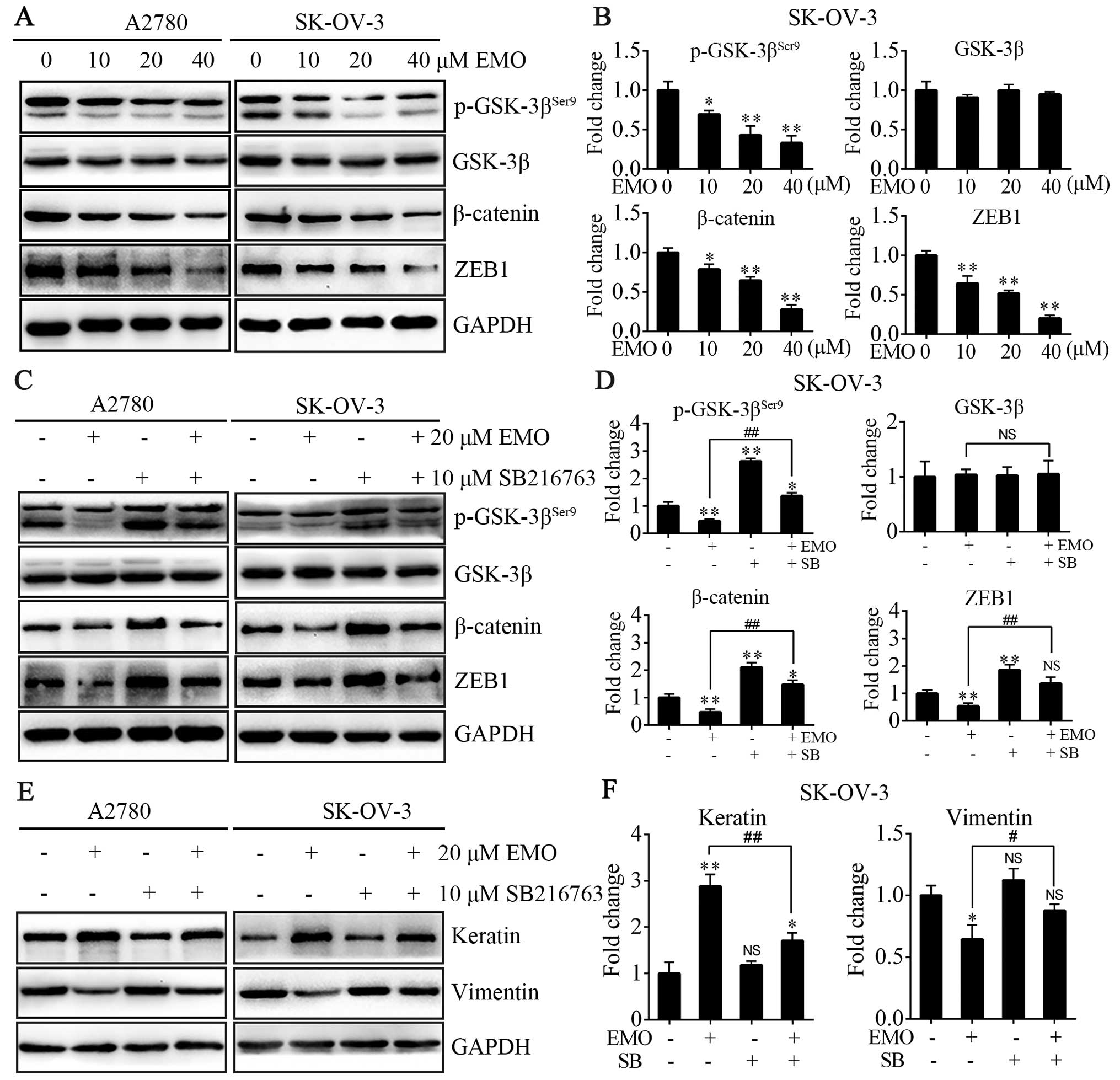
EMO inhibits WNT/β-catenin signaling
pathway and the expression of ZEB1 via GSK-3β activation
To investigate the functional mechanism of EMO
inhibition of EMT on A2780 and SK-OV-3 cells, we tested
WNT/β-catenin signaling pathway and transcription factor ZEB1, both
closely connected with EMT. The cells were treated with EMO
concentrations ranging from 0 to 40 µM for 48 h. The data
showed that EMO treatment dose-dependently increased levels of
p-GSK-3βSer9, one of negative regulatory sites, led to
the increase of GSK-3β kinase activity accordingly, occurred in the
absence of any changes in total GSK-3β levels and decreased total
β-catenin protein levels (Fig. 3A and
B). EMO treatment also significantly decreased ZEB1 protein
levels in a concentration-dependent manner (Fig. 3A and B). To further clarify that EMO
regulated the GSK-3β/β-catenin/ZEB1 pathways, cells were pretreated
with SB216763, a selective GSK-3β kinase inhibitor, and we found
that the effects of EMO were blocked (Fig. 3C and D). SB216763 treatment restored
EMO-induced decrease of p-GSK-3βSer9, β-catenin and ZEB1
protein expression. Furthermore, EMO treatment did not decrease
mesenchymal phenotype such as vimentin and slightly increased
epithelial phenotype such as keratin protein levels in the presence
of SB216763 compared to its absence (Fig. 3E and F).
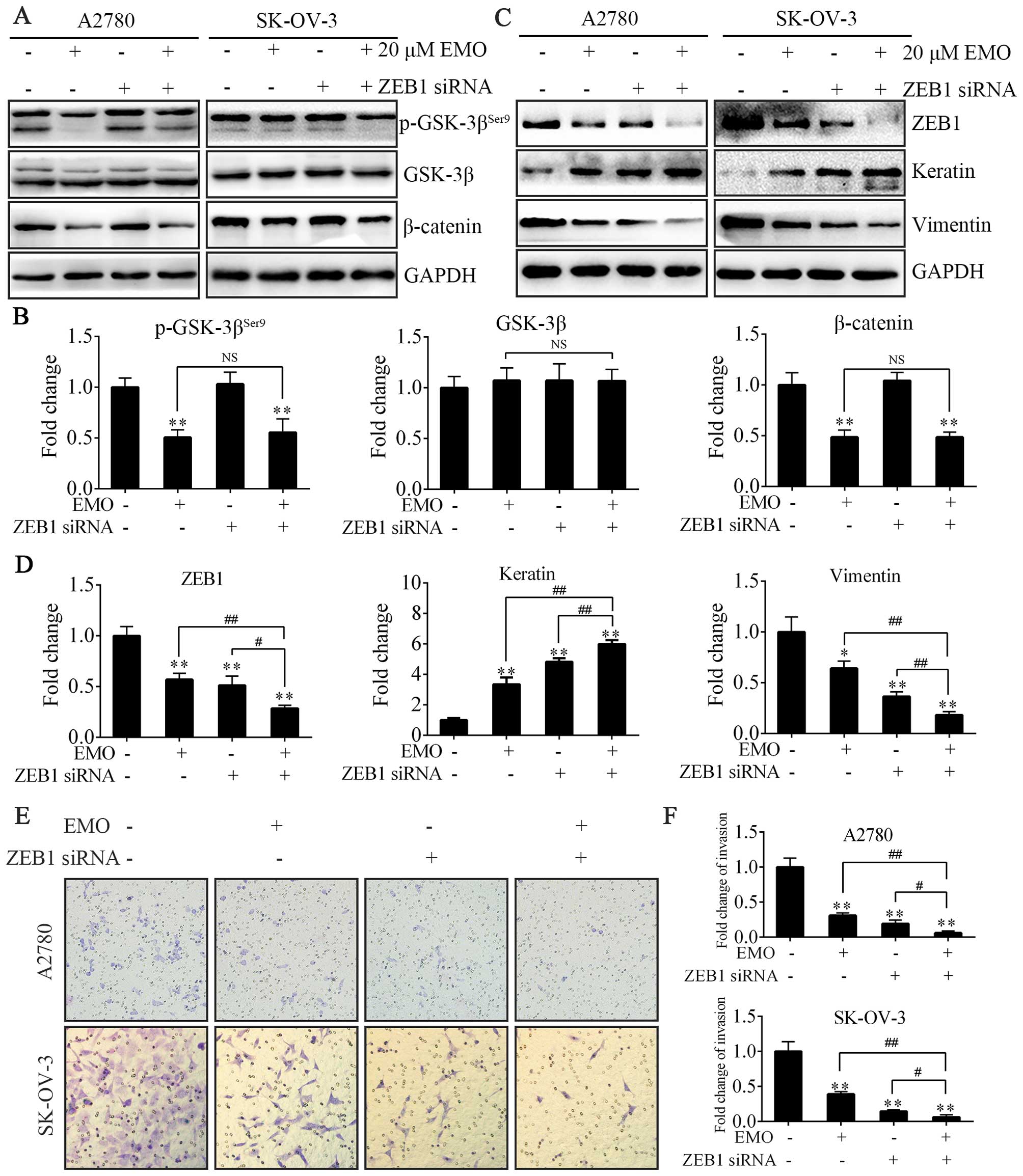
EMO represses EMT via ZEB1
inhibition
To further study the mechanism of inhibition of EMT,
ZEB1 siRNA was designed to evaluate the relationship between
WNT/β-catenin signaling pathway and ZEB1. Either EMO treatment
alone or treatment of ZEB1-knockdown cells with EMO almost equally
played the same role in regulating the activity of GSK-3β and the
expression of β-catenin (Fig. 4A and
B). Actually, ZEB1 siRNA failed to participate in the above
process. Furthermore, we investigated the effects of EMO on cell
invasion followed by ZEB1 knockdown. Upon ZEB1 knockdown, A2780 and
SK-OV-3 cells treated with EMO exhibited more decreased invasion
capacity (Fig. 4E and F), decreased
vimentin protein levels and consistently more increased keratin
expression levels (Fig. 4C and D)
compared to ZEB1 knockdown or EMO treatment alone. Accordingly,
ZEB1 protein levels were lowest in the EMO-treated ZEB1 knockdown
cells (Fig. 4C and D).
Discussion
We have validated that A2780 and SK-OV-3 cells
displayed a spindle-like mesenchymal phenotype with higher invasive
property than OVCAR-3 cells. Then, we showed for the first time
that the inhibition of invasion and EMT by treating A2780 and
SK-OV-3 cells with different concentrations of EMO. No previous
studies had found the influence of EMO on
GSK-3β/β-catenin-regulated EMT markers, including ZEB1 in EOC.
Therefore, the present study provides the first report to explore
the role of GSK-3β/β-catenin/ZEB1 pathways in repressing EMT by
EMO.
We confirmed that A2780 and SK-OV-3 cells had
obviously lower protein levels of E-cadherin and keratin and higher
protein levels of vimentin, N-cadherin and transcription factor
ZEB1. We also showed that A2780 and SK-OV-3 cells had elongated
fibroblastoid mesenchymal phenotype with more invasive ability than
OVCAR-3 cells, which displayed epithelial cobblestone-like
morphology in line with earlier reports (24).
In agreement with previous studies showing that
exposure to EMO could inhibit EOC cells invasion and metastasis
(19,20), we further demonstrated the molecular
mechanism in the present study. We showed that EMO could target the
EMT process in A2780 and SK-OV-3 cells, as manifested by decreased
invasive capacities and alterations in the expression of
EMT-related markers, including upregulation of the expression of
E-cadherin and keratin, and downregulation of the expression of
vimentin, N-cadherin, MMP-9 and MMP-2. These results demonstrated
that EMO alters correlation of the gene products with EMT induction
or maintenance by repression of the mesenchymal phenotype of EOC
cells.
Previous reviews summarized that the combined action
of various signaling pathways, including WNT/β-catenin signaling
pathway, make a greater contribution to the initiation and
morphogenic process of the EMT (25). In particular, WNTs and their
downstream effectors could regulate the important processes for
tumor initiation, tumor growth, cell senescence and apoptosis,
differentiation and metastasis (26). Besides, WNT signaling pathway is
activated in EOC. One way of the upregulated signaling transduction
is directly through activating ligand, another is by a
ligand-independent increase in nuclear β-catenin via crosstalk with
other pathways. In addition, WNT signaling pathway was investigated
as a potential target in the development of new drugs for ovarian
cancer (27). Previous studies
showed that EMO possessed the capability of inhibiting the WNT
signaling pathway by downregulating T-cell factor (TCF)/lymphocyte
enhancer factor (LEF) transcriptional activity and inducing
morphological changes indicating MET accompanied by the increase in
E-cadherin expression in human colorectal cancer cells (SW480 and
SW620) (21). Besides, EMO
inhibited cell population and migration in cervical cancer cells
(SiHa and HeLa cells) and downregulated the WNT/β-catenin signaling
pathway activated by TGF-β through inhibiting β-catenin in HeLa
cells (22). Furthermore, in head
and neck squamous cell carcinoma (HNSCC), EMO inhibited
Twist1-induced invasion and EMT by inhibiting the β-catenin and Akt
pathways in vitro and in vivo (23). In the present study, we demonstrated
for the first time that EMO had anti-invasive effect in A2780 and
SK-OV-3 cells, evidenced by the repression of EMT in vitro
and these effects were mediated by decreased phosphorylation of
GSK-3β on Ser9 site and total β-catenin levels and inhibition of
subsequent ZEB1 expression.
It is well known that reduction of the expression of
p-GSK-3βSer9 led to the increase of GSK-3β kinase
activity, followed by phosphorylation of β-catenin, a
multifunctional protein in WNT-mediated signal transduction,
leading to ubiquitylation or proteasomal degradation. Accordingly,
the non-phosphoform of β-catenin binds to TCF bind elements
(TBE)-specific DNA sequences that interact with TCF/LEF TFs after
it translocates into the nucleus, leading to the transcription of
WNT-responsive genes (28).
Moreover, ZEB1 is a direct target of β-catenin/TCF4 and acts also
as an effector of this signaling pathway in regulating genes
associated with tumor invasiveness in intestinal primary tumors and
colorectal cancer cell lines with mutant APC (29). In addition, PI3K/Akt targeting
GSK3β/β-catenin pathway could dynamically control the ZEB1 gene
transcription in bladder cancer cells (T24-L, lung metastasis)
(30). In our study, western blot
analysis showed that EMO decreased the expression of
p-GSK-3βSer9, total β-catenin and ZEB1, and these
effects were rescued by the selective GSK-3β inhibitor, SB216763.
Besides, cells treated with SB216763 alone expressed elevated
β-catenin and ZEB1 protein. These results suggest that EMO inhibits
EMT in EOC cells via activating GSK-3β followed by targeting
β-catenin and regulating its downstream target ZEB1.
Studies have demonstrated that a number of TFs were
shown to be key regulators of E-cadherin expression, including
Twist, Snail, Slug, ZEB1 and ZEB2 (31–34).
Among them, the ZEB1, encoded by the TCF8 gene, contributes to
malignant progression of various epithelial tumors. ZEB1 plays
crucial effects on induction of EMT by inhibiting expression of
E-cadherin and microRNAs, which induce an epithelial phenotype
(35). The downregulation of the
ZEB1 was found to be associated with the decreased colony-forming
ability and the cell migration ability in the human ovarian cancer
SK-OV-3 and HO8910 cells in vitro and also with the
attenuated tumorigenesis of the shZEB1-SKOV3 cells in nude mice by
blocking the EMT process (36). So
ZEB1 may be a potential therapeutic target in future clinical
trials for preventing and treating EOC metastases. In the present
study, concomitant knockdown of ZEB1 expression and EMO treatment
synergistically suppressed cell invasion and EMT at greater levels.
These results indicate that ZEB1 is a key regulator of cell
invasion and EMT in EMO-treated EOC cells. Besides, ZEB1 knockdown
alone did not affect GSK-3β activity and β-catenin levels.
Similarly, EMO treatment alone or treatment of ZEB1-knockdown cells
with EMO almost equally decreased p-GSK-3βSer9 and
β-catenin levels. The results suggest that ZEB1 is downstream of
β-catenin.
In conclusion, the present study showed that the
anti-invasive efficacy of EMO on EOC cells was associated with
inhibition of EMT. The mechanism may be that GSK-3β activation by
EMO treatment decreased expression of total β-catenin, leading to
repressed WNT/β-catenin signaling pathway and sequentially the
decreased ZEB1 levels. Based on our findings, we provide additional
scientific evidence for the use of EMO in the treatment of EOC.
Acknowledgments
The present study was supported by grants from the
Science and Technology Development Project of Shandong Province
(2008GG2NS02017) as well as National Natural Science Foundation of
China (NSFC) (81302268).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piek JM, van Diest PJ and Verheijen RH:
Ovarian carcinogenesis: An alternative hypothesis. Adv Exp Med
Biol. 622:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banerjee S and Kaye SB: New strategies in
the treatment of ovarian cancer: Current clinical perspectives and
future potential. Clin Cancer Res. 19:961–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA: Epithelial plasticity: A common
theme in embryonic and cancer cells. Science. 342:12348502013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and WNT/β-catenin signalling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar
|
|
7
|
Rosanò L, Spinella F, Di Castro V, Nicotra
MR, Dedhar S, de Herreros AG, Natali PG and Bagnato A: Endothelin-1
promotes epithelial-to-mesenchymal transition in human ovarian
cancer cells. Cancer Res. 65:11649–11657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elloul S, Vaksman O, Stavnes HT, Trope CG,
Davidson B and Reich R: Mesenchymal-to-epithelial transition
determinants as characteristics of ovarian carcinoma effusions.
Clin Exp Metastasis. 27:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nuti SV, Mor G, Li P and Yin G: TWIST and
ovarian cancer stem cells: Implications for chemoresistance and
metastasis. Oncotarget. 5:7260–7271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin G, Alvero AB, Craveiro V, Holmberg JC,
Fu HH, Montagna MK, Yang Y, Chefetz-Menaker I, Nuti S, Rossi M, et
al: Constitutive proteasomal degradation of TWIST-1 in
epithelial-ovarian cancer stem cells impacts differentiation and
metastatic potential. Oncogene. 32:39–49. 2013. View Article : Google Scholar :
|
|
11
|
Kurrey NK, Amit K and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prislei S, Martinelli E, Zannoni GF,
Petrillo M, Filippetti F, Mariani M, Mozzetti S, Raspaglio G,
Scambia G and Ferlini C: Role and prognostic significance of the
epithelial-mesenchymal transition factor ZEB2 in ovarian cancer.
Oncotarget. 6:18966–18979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian K, Zhang H, Chen X and Hu Z:
Determination of five anthraquinones in medicinal plants by
capillary zone electrophoresis with beta-cyclodextrin addition. J
Chromatogr A. 1123:134–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Srinivas G, Babykutty S, Sathiadevan PP
and Srinivas P: Molecular mechanism of emodin action: Transition
from laxative ingredient to an antitumor agent. Med Res Rev.
27:591–608. 2007. View Article : Google Scholar
|
|
16
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hei ZQ, Huang HQ, Tan HM, Liu PQ, Zhao LZ,
Chen SR, Huang WG, Chen FY and Guo FF: Emodin inhibits dietary
induced atherosclerosis by antioxidation and regulation of the
sphingomyelin pathway in rabbits. Chin Med J. 119:868–870.
2006.PubMed/NCBI
|
|
18
|
Li J, Liu P, Mao H, Wanga A and Zhang X:
Emodin sensitizes paclitaxel-resistant human ovarian cancer cells
to paclitaxel-induced apoptosis in vitro. Oncol Rep. 21:1605–1610.
2009.PubMed/NCBI
|
|
19
|
Zhu F, Liu XG and Liang NC: Effect of
emodin and apigenin on invasion of human ovarian carcinoma
HO-8910PM cells in vitro. Ai Zheng. 22:358–362. 2003.In Chinese.
PubMed/NCBI
|
|
20
|
Xue H, Chen Y, Cai X, Zhao L, He A, Guo K
and Zheng X: The combined effect of survivin-targeted shRNA and
emodin on the proliferation and invasion of ovarian cancer cells.
Anticancer Drugs. 24:937–944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pooja T and Karunagaran D: Emodin
suppresses Wnt signaling in human colorectal cancer cells SW480 and
SW620. Eur J Pharmacol. 742:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thacker PC and Karunagaran D: Curcumin and
emodin down-regulate TGF-β signaling pathway in human cervical
cancer cells. PLoS One. 10:e01200452015. View Article : Google Scholar
|
|
23
|
Way TD, Huang JT, Chou CH, Huang CH, Yang
MH and Ho CT: Emodin represses TWIST1-induced
epithelial-mesenchymal transitions in head and neck squamous cell
carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J
Cancer. 50:366–378. 2014. View Article : Google Scholar
|
|
24
|
Davidowitz RA, Selfors LM, Iwanicki MP,
Elias KM, Karst A, Piao H, Ince TA, Drage MG, Dering J, Konecny GE,
et al: Mesenchymal gene program-expressing ovarian cancer spheroids
exhibit enhanced mesothelial clearance. J Clin Invest.
124:2611–2625. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
27
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sánchez-Tilló E, de Barrios O, Siles L,
Cuatrecasas M, Castells A and Postigo A: β-catenin/TCF4 complex
induces the epithelial-to-mesenchymal transition (EMT)-activator
ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA.
108:19204–19209. 2011. View Article : Google Scholar
|
|
30
|
Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou
J, Li L, Chen Y, Zhang T, Wang X, et al: PI3K/Akt to
GSK3β/β-catenin signaling cascade coordinates cell colonization for
bladder cancer bone metastasis through regulating ZEB1
transcription. Cell Signal. 24:2273–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen D, Wang J, Zhang Y, Chen J, Yang C,
Cao W, Zhang H, Liu Y and Dou J: Effect of down-regulated
transcriptional repressor ZEB1 on the epithelial-mesenchymal
transition of ovarian cancer cells. Int J Gynecol Cancer.
23:1357–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|