Introduction
HCC is the fifth most common cancer and the third
leading cause of cancer-related mortality globally (1,2). The
5-year survival rate of HCC patients is poor due to tumor
recurrence and metastasis (3). The
high incidence of metastasis continues to be the main obstacle of
the treatment efficacy of HCC. However, the molecular mechanisms of
hepatocarcinogenesis and metastasis remain unclear. Elucidation of
the detailed mechanisms of HCC cell growth and metastasis is
crucial to improve HCC therapeutic intervention.
Epithelial-mesenchymal transition (EMT), usually occurring in the
critical phases of embryonic development, is the differentiation
switch from adherent epithelial cells into contractile and motile
mesenchymal cells (4,5). Currently, EMT of cancer cells is
thought to attribute much to cancer invasion and metastasis, making
it a hallmark of tumor progression (6). Although the molecular mechanism
underlying tumor metastasis is still not well elucidated,
investigations into this process have led to the hypothesis that
many molecules are involved in EMT and play pivotal roles in tumor
invasion and metastasis (7).
GP73 is a resident Golgi-specific membrane protein
which is highly expressed in HCC patients. Actually, in viral and
non-viral liver diseases, expression of GP73 was found to be
obviously upregulated in hepatocytes (8–10). In
addition, there are reports describing how GP73 is secreted to
serum (11). Recent studies suggest
that GP73 is a reliable biomarker for the early diagnosis of HCC,
and the sensitivity and specificity may be superior to currently
used biomarker α fetoprotein (AFP) (12–14).
Yet, this remains controversial, as other studies found that serum
GP73 levels in patients with liver cirrhosis (LC) were
significantly higher than those in patients with HCC; having a
lower diagnostic value for HCC (15,16).
The decreased survival and severe epithelial abnormalities in the
liver and kidneys of a GP73 C-terminal truncated transgenic mouse
model helped to determine the physical role of GP73 in epithelial
cell function in these organs (17). Sun et al showed that an
elevated level of GP73 protein is strongly associated with
augmented tumor invasion and metastasis, while the exact mechanism
of elevated GP73 and tumor metastasis remains largely unknown
(18). GP73 was also found to be
negatively correlated with E-cadherin and positively correlated
with vimentin in tissues, thus it may be associated with EMT in HCC
(19).
In the present study, we demonstrated that GP73
enhanced HCC cell invasion by inducing EMT and this may promote the
metastasis of HCC. High expression of GP73 was also found in HCC
tissues with metastasis. Kaplan-Meier survival analysis showed that
the survival of patients with high GP73 expression was
significantly poorer than the survival of those with low GP73
expression, indicating it is a candidate target for HCC
therapy.
Materials and methods
Cell culture
Immortalized normal human liver cell line L02 and
HCC cell lines (Hep3B, HepG2, Huh7, SMMC7721, MHCC97L, MHCC97H,
HCCLM3) were used in our study. L02, Hep3B, HepG2, SMMC7721 and
Huh7 cells were purchased from the Chinese Academy of Sciences
(Shanghai, China). MHCC97L, MHCC97H and HCCLM3 cells were
established in the Liver Cancer Institute, Zhongshan Hospital. The
cells were all cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C with
5% CO2.
Lentivirus construction and
transfection
The GP73-RNA interference lentiviral vector was
constructed by GeneChem Co., Ltd. (Shanghai, China). The
double-stranded oligonucleotides targeted to GP73 mRNA targeting
coding sequence (5′-AGGGAATGACAGAAACATA-3′) was annealed and
inserted into the shRNA expression vector pGV115-GFP. The cDNA
encoding GP73 was amplified by reverse transcription polymerase
chain reaction (RT-PCR) and cloned into the pGV218-GFP vector (the
cells stably expressed GP73 shRNA/GP73 proteins). Cells only
expressing vectors (Mock cells) were used for the negative control.
The lentivirus was generated and harvested (Shanghai GeneChem Co.,
Ltd.). Then, the lentivirus was transfected into targeted cells
with a multiplicity of infection (MOI) of 10 to 50 (optimal MOI is
20).
Transwell migration and invasion
assays
Cell migration and invasion assays were performed
using a 24-well Transwell (8.0-µm pore size; Millipore, USA)
precoated without or with Matrigel (BD Biosciences, USA). SMMC7721,
MHCC97H or HCCLM3 cells (5×104) were suspended in 1.5 ml
serum-free DMEM and transferred into the inside chamber of a
24-well cell culture insert with a 8.0-µm pore size. An
amount of 600 µl media with 20% FBS was added into the
outside well. After incubation for 24 h, the cells remaining on the
upper side of the filters were cleaned with cotton-tipped swabs.
Cells on the lower surface of the membrane were fixed with methanol
and subjected to Giemsa staining. The cells on the underside of the
filters were counted in five randomly selected fields (at ×200
magnification), and the average cell number per view was
calculated. All experiments were performed in triplicate.
Western blot analysis
Twenty-two HCC tissues and their paired adjacent
non-tumor tissues were collected from patients undergoing resection
at the Liver Cancer Institute, Zhongshan Hospital. The tissues were
used for western blot analysis. General characteristics regarding
these 22 HCC patients are described in Table I. The study was approved by the
Research Ethics Committee of Zhongshan Hospital, and informed
consent was obtained from each patient.
 | Table IGeneral characteristics of the HCC
patients whose tissues were used for western blot analysis. |
Table I
General characteristics of the HCC
patients whose tissues were used for western blot analysis.
|
Characteristics | HCCc |
|---|
| No. of
individuals | 22 |
| Gender [male n
(%)/female n (%)] | 16 (72.7%)/6
(27.3%) |
| Mean age
(years) | 52±11 |
| Edmondson-Steiner
grade | I (n=10), II
(n=12) |
| HBV DNA
(copy)a [mean (range)] | 2.3×104
(1.3×103–1.7×105) |
| AFP (ng/ml)b [mean (range)] | 12,354.6
(3.2–70,321) |
| HbsAg+
(%) | 100 |
| AST (U/l) [mean
(range)] | 130.4
(16–1,630) |
| ALT (U/l) [mean
(range)] | 110.3
(14–1,120) |
Protein concentrations were determined using the
bicinchoninic acid (BCA) method. Aliquots (20 µg) of
proteins were loaded and resolved by 10% SDS-PAGE and transferred
to PVDF membranes using a Bio-Rad SemiDry apparatus. After being
blocked for non-specific binding sites, the membranes were
incubated with the indicated primary antibodies: anti-GP73 (1:200
dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
E-cadherin (1:200 dilution; Abcam, Hong Kong), N-cadherin (1:100
dilution; Invitrogen, Carlsbad, CA, USA) and GAPDH (1:10,000
dilution; Kang-Cheng, Shanghai, China) overnight at 4°C, followed
by HRP-conjugated secondary antibodies for 1 h at room temperature.
After washing three times in Tris-buffered saline with 0.1%
Tween-20 (TBST), immunoreactive protein bands were visualized using
an enhanced chemiluminescence (ECL) detection system (GE
Healthcare, Piscataway, NJ, USA).
Cell immunofluorescence assay
For immunofluorescence staining, cells grown on
glass coverslip were fixed in 4% paraformaldehyde and permeabilized
using 0.5% Triton X-100. Non-specific binding sites were blocked
with normal goat or rabbit serum. The cells were then incubated
with the primary antibodies against GP73 (1:50 dilution),
E-cadherin (1:100 dilution), N-cadherin (1:100 dilution) overnight
at 4°C. After thorough washing, the cells were then incubated with
Alexa-Fluor 555 anti-mouse IgG (1:1,000 dilution; Cell Signaling
Technology, Danvers, MA, USA) or anti-goat IgG (1:1,000 dilution;
Abcam). Finally, the cells were washed and stained with DAPI.
Images were captured using a Leica fluorescence microscope.
Immunohistochemical analysis of tissue
microarrays
Tissue samples of 48 HCC patients with or without
metastasis were obtained from the Department of Hepatobiliary
Surgery, The First Affiliated Hospital of Guangxi Medical
University (Nanning, China). HCC diagnosis was based on World
Health Organization criteria. Ethical approval was obtained from
the Research Ethics Committee of The First Affiliated Hospital of
Guangxi Medical University, and written informed consent was
obtained from each patient. Tissue microarrays were constructed
using formalin-fixed, paraffin-embedded tissue samples. Primary
antibody against GP73 (1:50 dilution) and donkey anti-goat
secondary antibody was used for immunohistochemical staining. Then,
the integrated optical density of the tissue microarray derived
from 48 HCC patients was evaluated by IPP software (Image-Pro Plus
5.1).
Staining for GP73 in the tissue microarray of 90 HCC
patients was assessed using a previously described scoring method
(20). The 90 HCC samples were
obtained from the Liver Cancer Institute, Zhongshan Hospital.
Ethical approval was obtained from the Research Ethics Committee of
Zhongshan Hospital, and written informed consent was obtained from
each patient. The staining intensity was scored on a scale of 0 to
3 as negative, weak, medium and strong, respectively. The stained
area, which was calculated as the percentage of positively stained
cells relative to the total cells, was scored on a scale of 0 to 4:
0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). The
overall score was calculated by multiplying the intensity score and
the staining area score. Samples were categorized into four grades:
an overall score equal to 0 was graded as '−'; an overall score
equal to 1, 2, 3 or 4 was graded as '+'; an overall score equal to
5, 6, 7 or 8 was graded as '++'; an overall score equal to 9, 10,
11 or 12 was graded as '+++'. The stained tissue sections were
analyzed by two pathologists without any knowledge regarding the
patient clinical information. Based on the immunohistochemical
grades, the patients were divided into two groups: the high
expression group, which included patients with grades '++' or
'+++', and the low expression group, including patients graded as
'−' or '+'. General characteristics of these HCC patients for the
tissue microarray are described in Table II.
 | Table IIGeneral characteristics of the HCC
patient whose tissues were used for the immunohistochemical tissue
microarray. |
Table II
General characteristics of the HCC
patient whose tissues were used for the immunohistochemical tissue
microarray.
|
Characteristics | HCCc |
|---|
| No. of
individuals | 138 |
| Gender [male n
(%)/female n (%)] | 103 (74.6%)/35
(25.4%) |
| Mean age
(years) | 55±13 |
| Edmondson-Steiner
grade | I (n=78), II
(n=60) |
| HBV DNA
(copy)a [mean (range)] | 1.8×104
(1.0×103–1.3×105) |
| AFP (ng/ml)b [mean (range)] | 10,345.6
(3.2–70,321) |
| HbsAg+
(%) | 100 |
| AST (U/l) [mean
(range)] | 134.1
(18–1,567) |
| ALT (U/l) [mean
(range)] | 120.7
(16–1,256) |
Statistical analysis
Statistical analysis was performed with SPSS 15.0
for Windows (SPSS, Chicago, IL, USA). Data are presented as the
mean ± SD unless otherwise indicated. The Student's t-test
(two-tailed) was used to compare two groups of parametric variants,
and Spearman's Rho test or Chi-square test was used to analyze
non-parametric variants. p<0.05 was considered to indicate a
statistically significant result.
Results
GP73 is correlated with cellular EMT
Expression levels of GP73 in various human cell
lines (L02, Hep3B, HepG2, Huh7, SMMC7721, MHCC97L, MHCC97H and
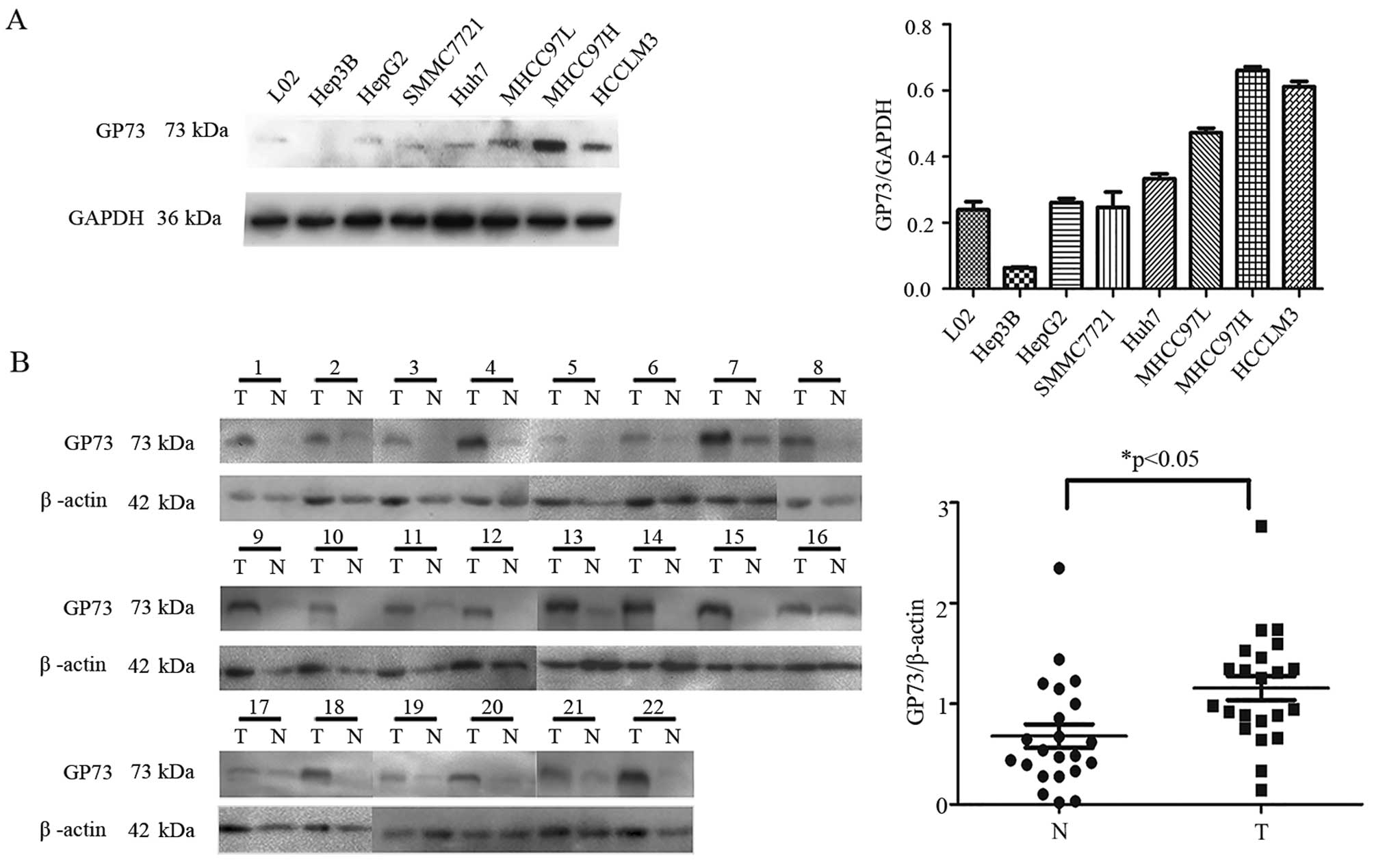
HCCLM3) were investigated. As shown in Fig. 1A, increased expression of GP73 was
observed in HCC cell lines with high metastatic potential (MHCC97L,
MHCC97H, HCCLM3) compared with the levels in the low or
non-metastatic cell lines (L02, Hep3B, HepG2, Huh7, SMMC7721). To
elucidate the effects of GP73 knockdown and overexpression on HCC
cell behavior, lentiviral-mediated shRNA was used to knock down the
expression of GP73 in the MHCC97H and HCCLM3 cells, while a GP73
cDNA expression vector was introduced into the SMMC7721 cells for
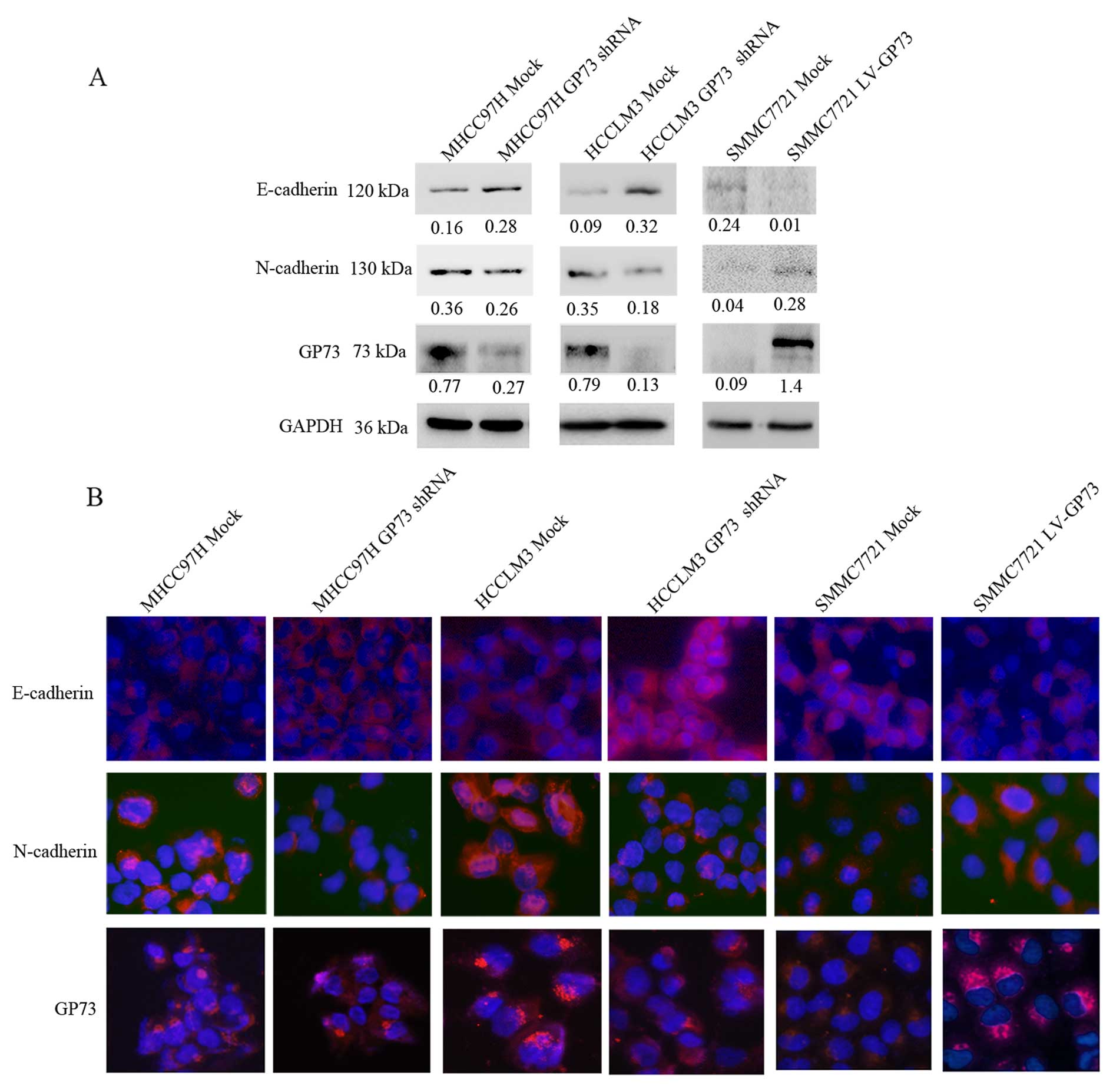
overexpression of GP73. GP73 expression in the transfected cells
was confirmed by western blotting (Fig.
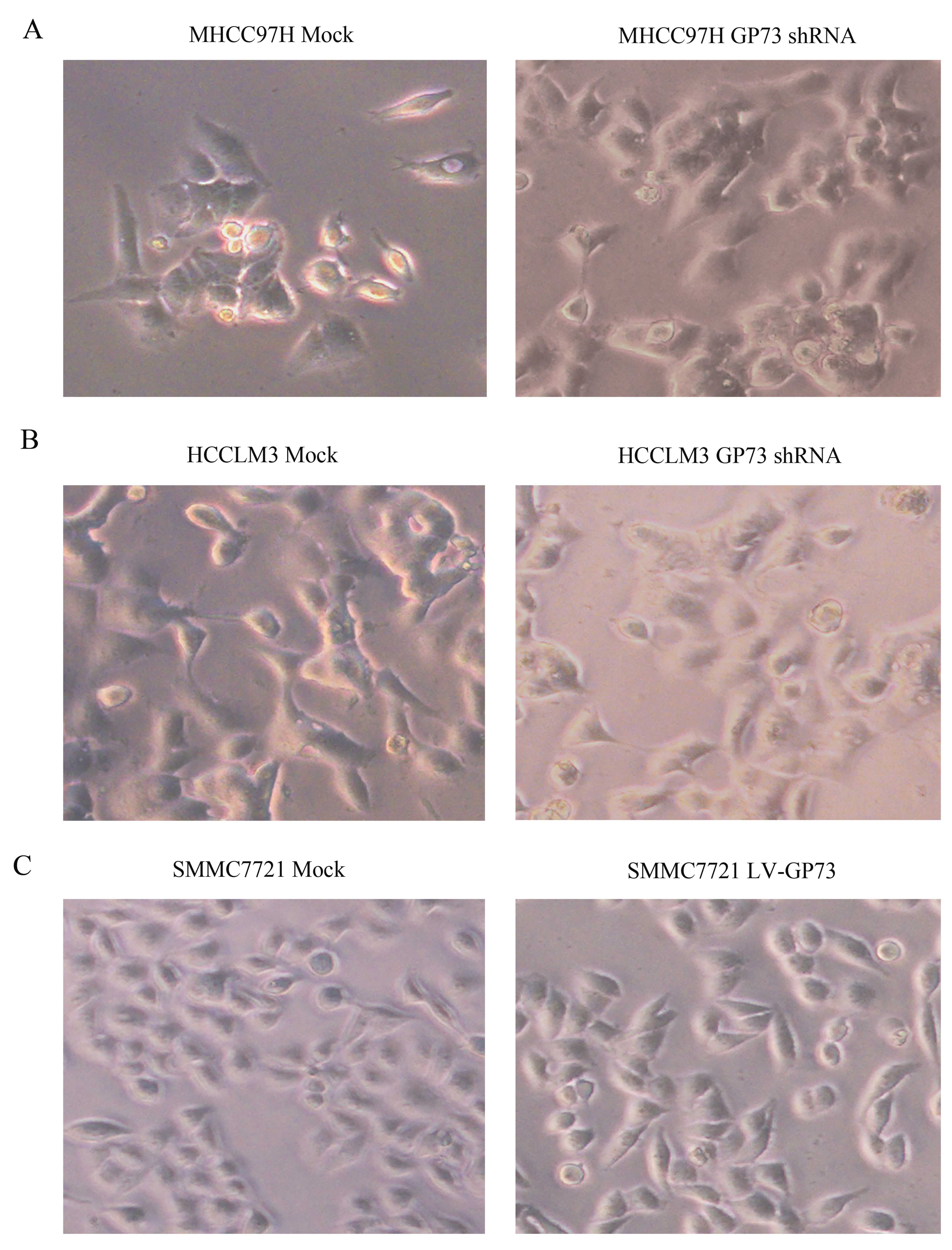
2A). To examine whether GP73 promotes EMT, cellular morphology
was observed. Knockdown of GP73 led to marked morphological changes
from a mesenchymal-like phenotype to an epithelial-like phenotype
in the MHCC97H and HCCLM3 cells (Fig.
3A and B). While in the SMMC7721 cells, overexpression of GP73
altered cells from an epithelial-like phenotype to a
mesenchymal-like phenotype (Fig.
3C). Western blot and cell immunofluorescence analyses were
used to detect expression of EMT markers in the GP73-shRNA-treated
MHCC97H and HCCLM3 cells and the GP73-overexpressing SMMC7721
cells. In the GP73-shRNA-treated cells, the expression of
E-cadherin (an epithelial marker) was increased and the expression
of N-cadherin (a mesenchymal marker) was decreased. In contrast,
the protein level of E-cadherin was downregulated and N-cadherin
was upregulated in the GP73-overexpressing cells (Fig. 2). These findings indicated that GP73
was involved in EMT in HCC cell lines.
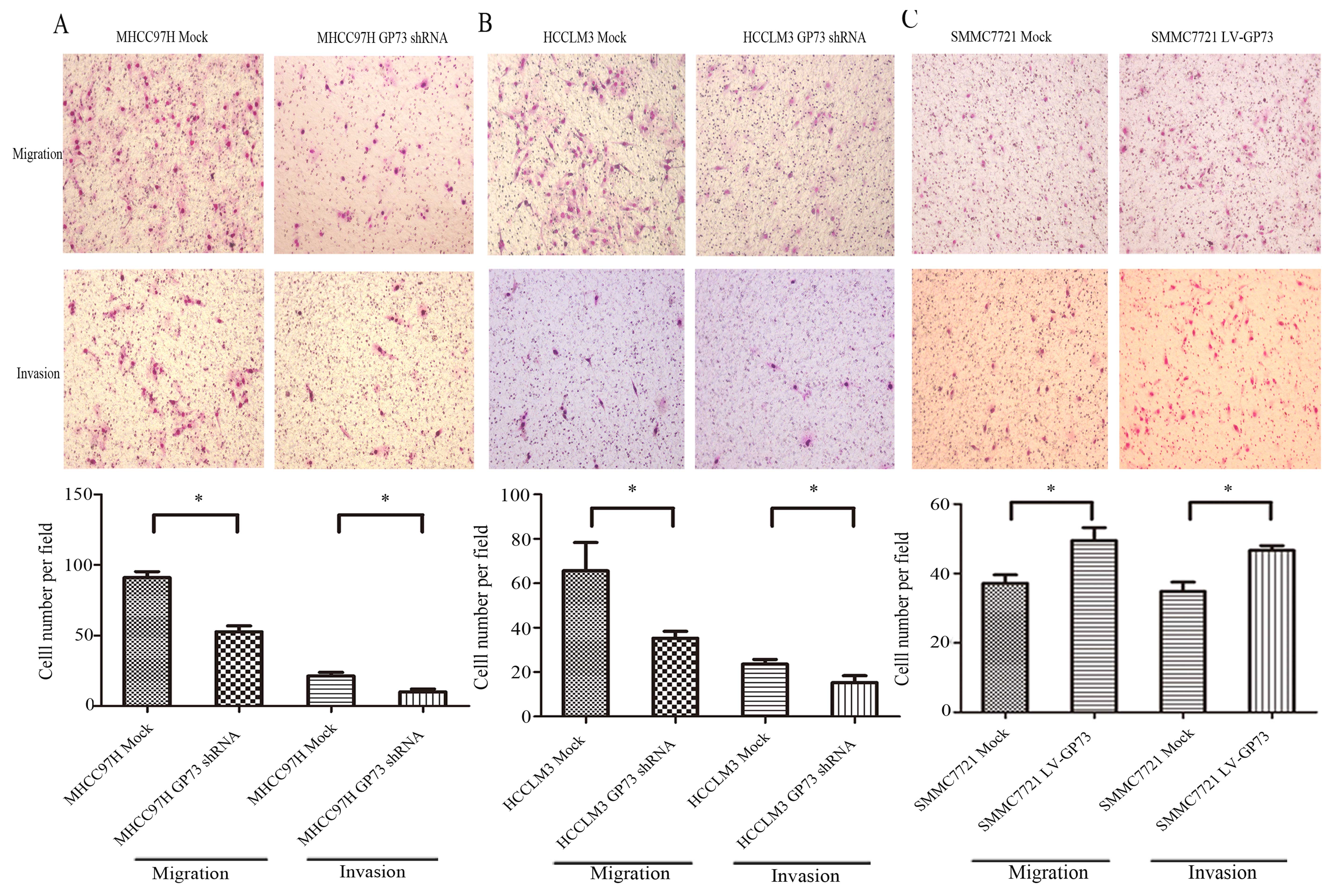
Knockdown of GP73 expression inhibits
cell motility and invasion
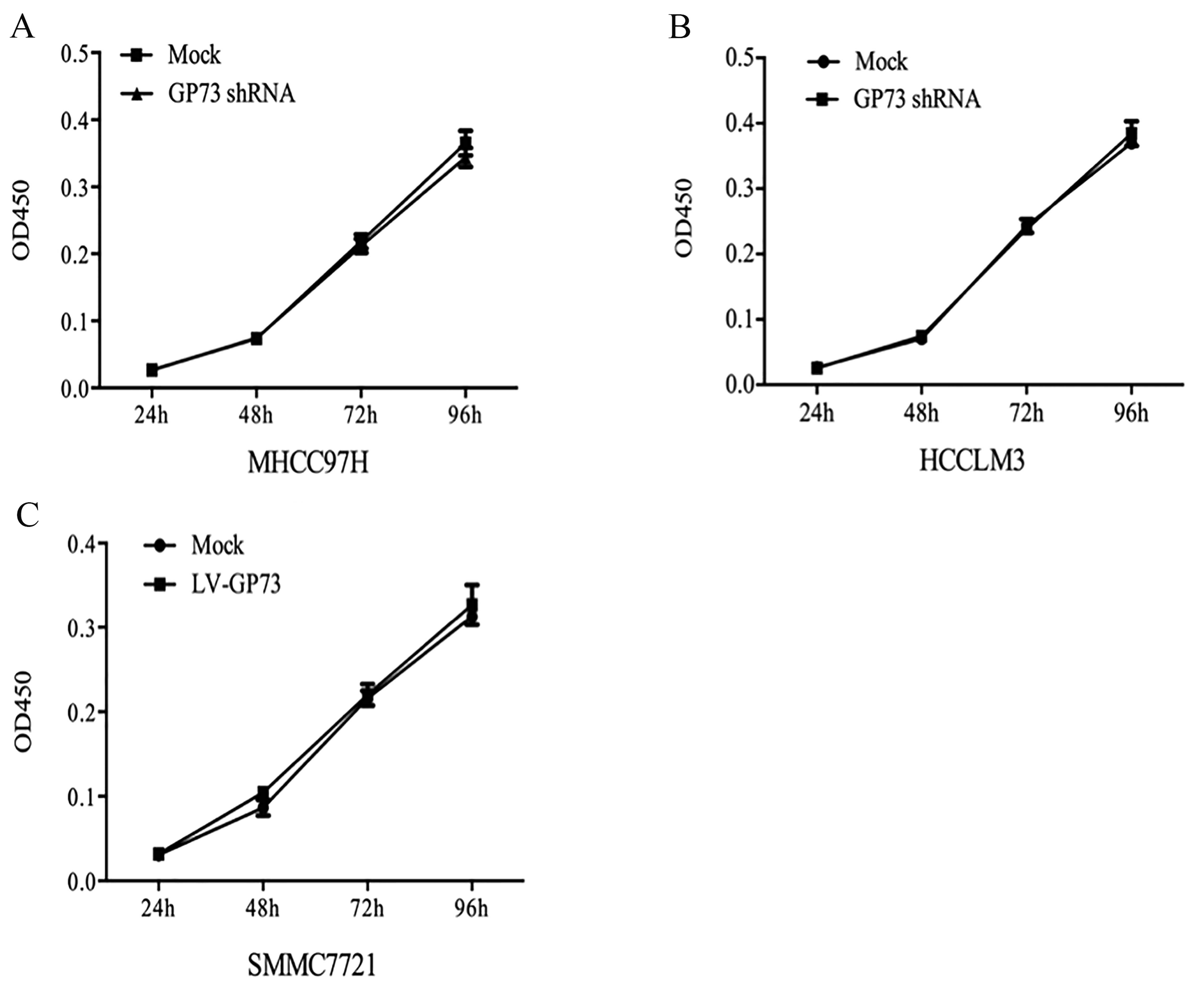
We also examined whether the change in GP73
expression affects the proliferation, migration and invasion of the
HCC cells using the transfected (knockdown or overexpression of
GP73) cell lines. MHCC97H Mock and HCCLM3 Mock cells (cells
transfected with scrambled shRNA) and MHCC97H GP73 shRNA and HCCLM3
GP73 shRNA cells (cells transfected with GP73 shRNA), grew at
similar rates (Fig. 4A and B). The
same results (Fig. 4C) were
obtained for the GP73-overexpressing SMMC7721 cells, indicating
that GP73 was not required for the proliferation of HCC cells. Cell
migration using a Transwell assay chamber was also assessed. Cells
that migrated into the lower compartment of the migration chamber
were fixed and then stained with Giemsa. Effective knockdown of
GP73 in both MHCC97H and HCCLM3 cells markedly decreased cellular
motility, as the number of migrated cells was significantly less
than that for the MHCC97H Mock and HCCLM3 Mock cells. The invasive
activity caused by knockdown of GP73 was determined using invasion
chamber assays with Matrigel. Consistent with the cell migration
results, knockdown of GP73 significantly decreased cell invasion
capacity compared with the mock cells (Fig. 5A and B). Taken together, these
results indicated that knockdown of GP73 in MHCC97H and HCCLM3
cells inhibited cell migratory and invasive abilities.
Overexpression of GP73 enhances cell
motility and invasion
Cell migration and invasion abilities in the
SMMC7721 LV-GP73 cells (cells overexpressing GP73) were assessed.
The results showed that overexpression of GP73 markedly increased
cell migratory and invasive abilities, as the numbers of migrated
SMMC7721 LV-GP73 cells were significantly higher compared to the
SMMC7721 Mock cells (cells trans-fected with an empty vector)
(Fig. 5C). Consistent with the
results of GP73 knockdown in the MHCC97H and HCCLM3 cells, GP73
overexpression in SMMC7721 cells enhanced cell invasion and
metastasis.
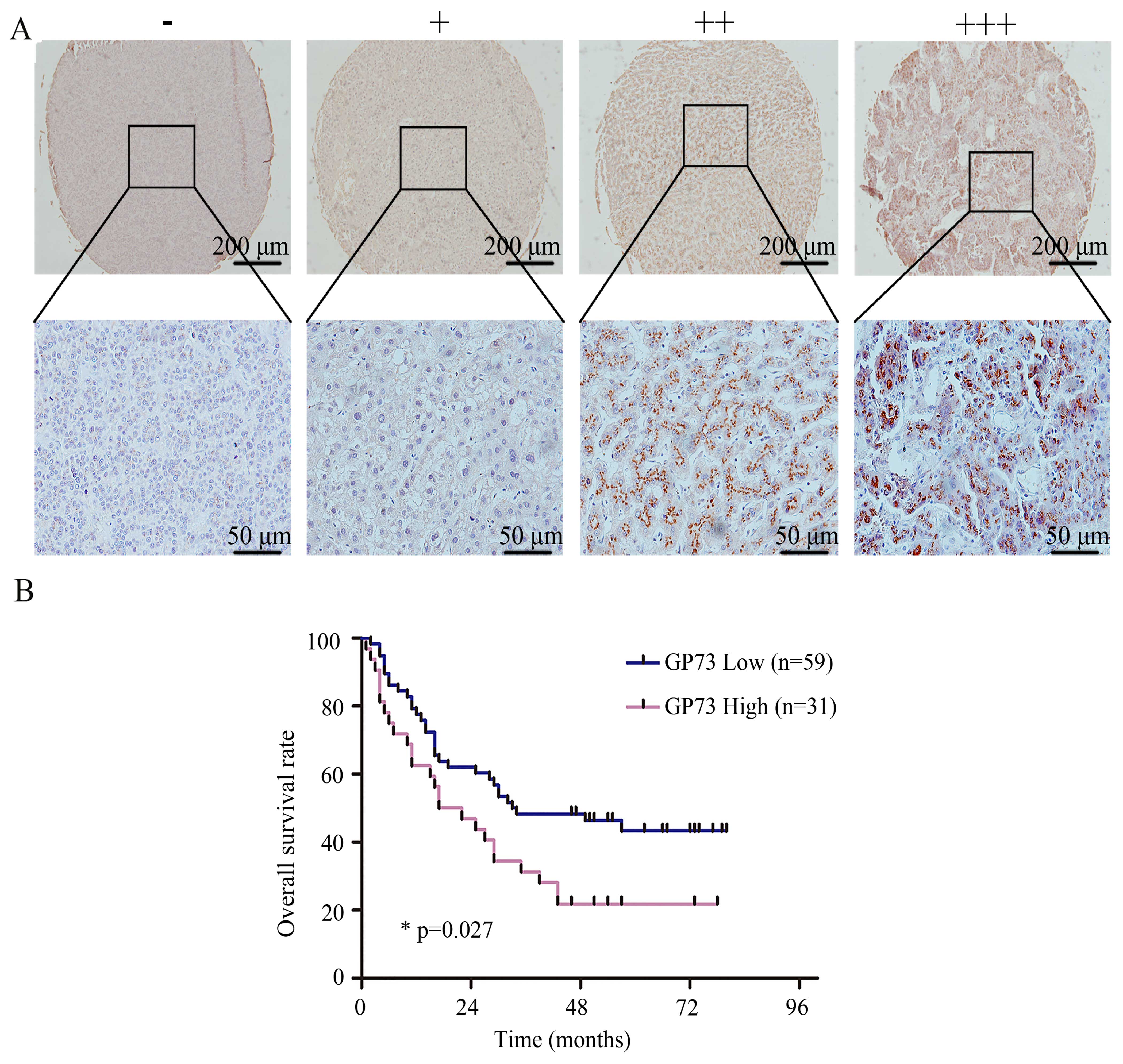
High expression of GP73 is correlated
with poor survival and metastasis of HCC patients
GP73 was significantly increased in the HCC tissues
compared with the paired non-cancerous tissues in 22 patients by
western blot analysis (Fig. 1B).
Furthermore, HCC tissues from 90 patients with survival information
from an 80-month follow-up period were collected for production of
a tissue microarray. The typical images of negative and positive
staining of GP73 are shown in Fig.
6A. Kaplan-Meier survival analysis showed that the survival of
patients with high GP73 expression was significantly poorer than
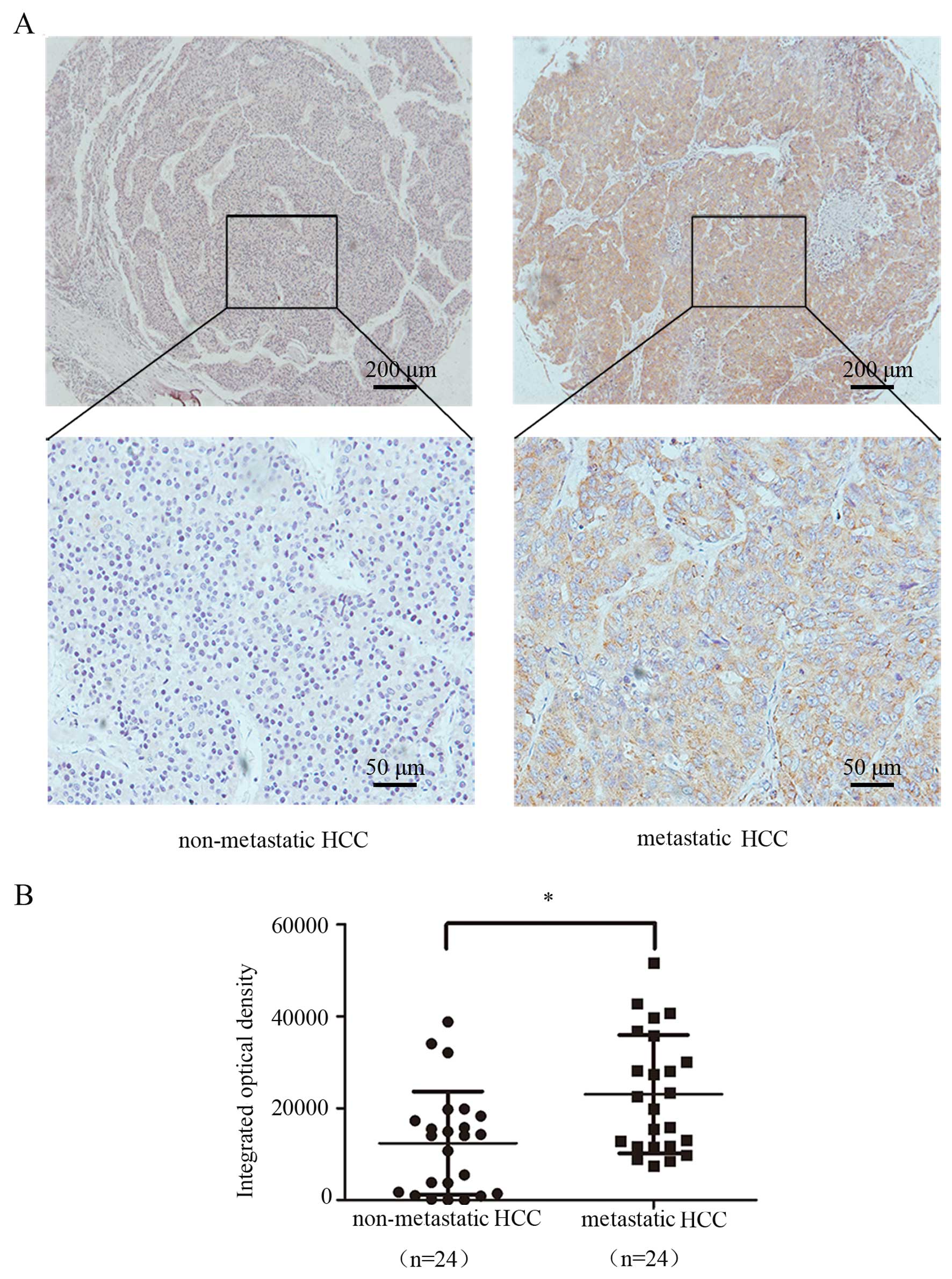
the survival of those with low GP73 expression (Fig. 6B). The GP73 level in the HCC tissues
derived from the patients with metastasis was obviously increased
in comparison with that in the HCC tissues from patients without
metastasis by immunohistochemical analysis, indicating the possible
role of GP73 in HCC metastasis and its aberrant expression was
indicative of poor outcomes in HCC (Fig. 7).
Discussion
Previous studies have demonstrated the upregulation
of GP73 in liver diseases (21,22).
In addition, upregulation of GP73 has been reported in Alzheimer's
disease (23), Wilson's disease
(24), prostate cancer (25–27),
renal cell cancer (28) and lung
cancer (29). A number of studies
show that serum GP73 levels in patients with liver disease are
markedly upregulated (13,14,30),
suggesting that GP73 may play an important role in liver disease.
Hu et al showed that the transmembrane domain with a
positively charged residue in the cytoplasmic N-terminal tail was
necessary to support its Golgi localization (31). However, the function of GP73 remains
unclear.
A previous study showed that expression of GP73 may
be associated with enhanced tumor invasion and metastasis (18). In contrast, it was reported that
GP73 expression had no relation with HCC metastasis (33). Different reagents used in various
studies may have led to such discrepancy (34). Whether GP73 really plays a role in
HCC metastasis is of great significance. Recently, GP73 was found
to be associated with EMT markers in HCC (19). EMT plays a pivotal role in tumor
metastasis. Evidence suggests that EMT could give rise to carcinoma
cell metastasis (35–38). Although numerous factors have been
identified to participate in EMT (4,39),
whether GP73 promotes cancer metastasis via EMT remains unclear. To
investigate whether GP73 is related with EMT in HCC progression,
stable cell lines were established by recombinant lentiviruses for
knockdown and overexpression of GP73. The results showed that the
mesenchymal marker, N-cadherin, was upregulated in the SMMC7721
LV-GP73 cells, whereas the epithelial marker, E-cadherin, was
decreased. These results were further confirmed by
immunofluorescence staining analysis of the cultured cells.
Opposite results were obtained for the MHCC97H GP73 shRNA and
HCCLM3 GP73 shRNA cells. A significant regression of EMT features
was observed; a gain in the expression of epithelial marker,
E-cadherin and a loss in the expression of mesenchymal marker,
N-cadherin. Thus, GP73 was found to be involved in EMT in the HCC
cell lines.
As EMT is a process involved during cancer
metastasis, cell migratory and invasive abilities were also
explored. The results showed that MHCC97H GP73 shRNA and HCCLM3
GP73 shRNA cells had significantly reduced cell migration and
invasion capabilities, while SMMC7721 LV-GP73 cells exhibited
increased cell migratory and invasive abilities. The proliferation
ability of these cells was not affected. Thus, we conclude that
GP73 was responsible for HCC invasion. This finding may be in
agreement with the results of Sun et al which showed that a
higher GP73 expression level was detected in tumors with a larger
load or stronger invasiveness, indicating that the overexpression
of GP73 protein may be involved in the progression of HCC (18). These data indicated that GP73 may
promote HCC metastasis via, at least partially, induction of EMT in
HCC cell lines. However, the mechanisms of how GP73 influences the
progression of HCC and EMT remain unclear. Since GP73 is a Golgi
transmembrane protein, it is unlikely whether GP73 is directly
involved in the signaling pathways inducing EMT. We suspect that
GP73 interacted with other important proteins directly in the EMT
pathways to play its role in the process. Related reports are
largely limited. Further studies should focus on this issue in
order to explain the detailed mechanism of GP73 in HCC EMT.
In the present study, GP73 expression in the HCC
tissues was higher than the level in the paired non-cancerous
tissues. The survival of patients with high GP73 expression was
significantly poorer than the survival of those with low GP73
expression, which was in accord with a study of Chen et al
(32). GP73 expression in the HCC
tissues with metastasis and the primary tumors was then detected.
HCC tissues with metastasis had higher GP73 expression, and thus
strong expression of GP73 in tumor tissues was correlated with
metastasis and poor survival. Hence, GP73 may be a potential
biomarker for HCC prognosis and a candidate target for HCC therapy.
However, the sample size for this study was limited and more
stratified samples should be considered for further study.
In conclusion, the present data indicated that GP73
may play an important role in HCC metastasis by EMT induction. High
expression of GP73 was also found in HCC tissues with metastasis,
and the survival of patients with high GP73 expression was
significantly poorer than the survival of those with low GP73
expression. Taken together, the function of GP73 is of potential
value for understanding tumor metastasis and it is a candidate
target for HCC therapy.
Abbreviations:
|
GP73
|
Golgi protein 73
|
|
HCC
|
hepatocellular carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
LC
|
liver cirrhosis
|
|
AFP
|
α-fetoprotein
|
Acknowledgments
The present study was financially supported by the
China National Key Projects for Infectious Diseases (nos.
2012ZX10002009-002, 2012ZX10002009-007 and 2012ZX10002012-002), the
National High Tech Program (863 program: 2012AA020204), and the
National Natural Science Foundation of China (21505022). All
procedures performed in the studies involving human participants
were in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun C, Sun L, Jiang K, Gao DM, Kang XN,
Wang C, Zhang S, Huang S, Qin X, Li Y, et al: NANOG promotes liver
cancer cell invasion by inducing epithelial-mesenchymal transition
through NODAL/SMAD3 signaling pathway. Int J Biochem Cell Biol.
45:1099–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen W, Ding J, Sun W, Fu J, Chen Y, Wu K,
Ning B, Han T, Huang L, Chen C, et al: Cyclin G1-mediated
epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt
signaling facilitates liver cancer progression. Hepatology.
55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iftikhar R, Kladney RD, Havlioglu N,
Schmitt-Gräff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR and
Fimmel CJ: Disease- and cell-specific expression of GP73 in human
liver disease. Am J Gastroenterol. 99:1087–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kladney RD, Bulla GA, Guo L, Mason AL,
Tollefson AE, Simon DJ, Koutoubi Z and Fimmel CJ: GP73, a novel
Golgi-localized protein upregulated by viral infection. Gene.
249:53–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kladney RD, Cui X, Bulla GA, Brunt EM and
Fimmel CJ: Expression of GP73, a resident Golgi membrane protein,
in viral and nonviral liver disease. Hepatology. 35:1431–1440.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachert C, Fimmel C and Linstedt AD:
Endosomal trafficking and proprotein convertase cleavage of cis
Golgi protein GP73 produces marker for hepatocellular carcinoma.
Traffic. 8:1415–1423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giannelli G and Antonaci S: New frontiers
in biomarkers for hepatocellular carcinoma. Dig Liver Dis.
38:854–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marrero JA, Romano PR, Nikolaeva O, Steel
L, Mehta A, Fimmel CJ, Comunale MA, D'Amelio A, Lok AS and Block
TM: GP73, a resident Golgi glycoprotein, is a novel serum marker
for hepatocellular carcinoma. J Hepatol. 43:1007–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riener MO, Stenner F, Liewen H, Soll C,
Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N,
Hellerbrand C, Müllhaupt B, et al: Golgi phosphoprotein 2 (GOLPH2)
expression in liver tumors and its value as a serum marker in
hepatocellular carcinomas. Hepatology. 49:1602–1609. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozkan H, Erdal H, Tutkak H, Karaeren Z,
Yakut M, Yüksel O and Köklü S: Diagnostic and prognostic validity
of Golgi protein 73 in hepatocellular carcinoma. Digestion.
83:83–88. 2011. View Article : Google Scholar
|
|
16
|
Tian L, Wang Y, Xu D, Gui J, Jia X, Tong
H, Wen X, Dong Z and Tian Y: Serological AFP/Golgi protein 73 could
be a new diagnostic parameter of hepatic diseases. Int J Cancer.
129:1923–1931. 2011. View Article : Google Scholar
|
|
17
|
Wright LM, Yong S, Picken MM, Rockey D and
Fimmel CJ: Decreased survival and hepato-renal pathology in mice
with C-terminally truncated GP73 (GOLPH2). Int J Clin Exp Pathol.
2:34–47. 2009.
|
|
18
|
Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G,
Lu X, Sang X, Zhao H, Zhong S, et al: Increased Golgi protein 73
expression in hepatocellular carcinoma tissue correlates with tumor
aggression but not survival. J Gastroenterol Hepatol. 26:1207–1212.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bao YX, Cao Q, Yang Y, Mao R, Xiao L,
Zhang H, Zhao HR and Wen H: Expression and prognostic significance
of golgiglyco-protein73 (GP73) with epithelial-mesenchymal
transition (EMT) related molecules in hepatocellular carcinoma
(HCC). Diagn Pathol. 8:1972013. View Article : Google Scholar
|
|
20
|
Masunaga R1, Kohno H, Dhar DK, Ohno S,
Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H and
Nagasue N: Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.PubMed/NCBI
|
|
21
|
Mao Y, Yang H, Xu H, Lu X, Sang X, Du S,
Zhao H, Chen W, Xu Y, Chi T, et al: Golgi protein 73 (GOLPH2) is a
valuable serum marker for hepatocellular carcinoma. Gut.
59:1687–1693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei H, Li B, Zhang R, Hao X, Huang Y, Qiao
Y, Hou J and Li X and Li X: Serum GP73, a marker for evaluating
progression in patients with chronic HBV infections. PLoS One.
8:e538622013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Wetten S, Li L, St Jean PL, Upmanyu
R, Surh L, Hosford D, Barnes MR, Briley JD, Borrie M, et al:
Candidate single-nucleotide polymorphisms from a genomewide
association study of Alzheimer disease. Arch Neurol. 65:45–53.
2008.
|
|
24
|
Wright LM, Huster D, Lutsenko S, Wrba F,
Ferenci P and Fimmel CJ: Hepatocyte GP73 expression in Wilson
disease. J Hepatol. 51:557–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo JH, Yu YP, Cieply K, Lin F, Deflavia
P, Dhir R, Finkelstein S, Michalopoulos G and Becich M: Gene
expression analysis of prostate cancers. Mol Carcinog. 33:25–35.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laxman B, Morris DS, Yu J, Siddiqui J, Cao
J, Mehra R, Lonigro RJ, Tsodikov A, Wei JT, Tomlins SA, et al: A
first-generation multiplex biomarker analysis of urine for the
early detection of prostate cancer. Cancer Res. 68:645–649. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kristiansen G, Fritzsche FR, Wassermann K,
Jäger C, Tölls A, Lein M, Stephan C, Jung K, Pilarsky C, Dietel M,
et al: GOLPH2 protein expression as a novel tissue biomarker for
prostate cancer: Implications for tissue-based diagnostics. Br J
Cancer. 99:939–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fritzsche FR, Riener MO, Dietel M, Moch H,
Jung K and Kristiansen G: GOLPH2 expression in renal cell cancer.
BMC Urol. 8:152008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang F, Gu Y, Li X, Wang W, He J and Peng
T: Up-regulated Golgi phosphoprotein 2 (GOLPH2) expression in lung
adenocarcinoma tissue. Clin Biochem. 43:983–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Block TM, Comunale MA, Lowman M, Steel LF,
Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS,
et al: Use of targeted glycoproteomics to identify serum
glycoproteins that correlate with liver cancer in woodchucks and
humans. Proc Natl Acad Sci USA. 102:779–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu L, Li L, Xie H, Gu Y and Peng T: The
Golgi localization of GOLPH2 (GP73/GOLM1) is determined by the
transmembrane and cytoplamic sequences. PLoS One. 6:e282072011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen MH, Jan YH, Chang PM, Chuang YJ, Yeh
YC, Lei HJ, Hsiao M, Huang SF, Huang CY and Chau GY: Expression of
GOLM1 correlates with prognosis in human hepatocellular carcinoma.
Ann Surg Oncol. 20(Suppl 3): S616–S624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan SG, Gao YT, Xu YJ, Huang Y, Zhang Q,
Zhai DK, Li JB, Wang FM, Jing X, Du Z, et al: Gradually increased
Golgi protein 73 expression in the progression of benign liver
diseases to precancerous lesions and hepatocellular carcinoma
correlates with prognosis of patients. Hepatol Res. 43:1199–1210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schrohl AS, Holten-Andersen M, Sweep F,
Schmitt M, Harbeck N, Foekens J and Brünner N; European
Organisation for Research and Treatment of Cancer (EORTC) Receptor
and Biomarker Group: Tumor markers: From laboratory to clinical
utility. Mol Cell Proteomics. 2:378–387. 2003.PubMed/NCBI
|
|
35
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cui B, Zhang S, Chen L, Yu J, Widhopf GF
II, Fecteau JF, Rassenti LZ and Kipps TJ: Targeting ROR1 inhibits
epithelial-mesenchymal transition and metastasis. Cancer Res.
73:3649–3660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Y and Pantel K: Tumor cell
dissemination: Emerging biological insights from animal models and
cancer patients. Cancer Cell. 23:573–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang K, Corsa CA, Ponik SM, Prior JL,
Piwnica-Worms D, Eliceiri KW, Keely PJ and Longmore GD: The
collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to
facilitate breast cancer metastasis. Nat Cell Biol. 15:677–687.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang C, Jiang K, Kang X, Gao D, Sun C, Li
Y, Sun L, Zhang S, Liu X, Wu W, et al: Tumor-derived secretory
clusterin induces epithelial-mesenchymal transition and facilitates
hepatocellular carcinoma metastasis. Int J Biochem Cell Biol.
44:2308–2320. 2012. View Article : Google Scholar : PubMed/NCBI
|