Introduction
Cancer testis antigens (CTAs) are proteins expressed
primarily in the testes. These antigens are expressed at very low
levels in other normal tissues but have enhanced expression in
cancerous tissues (1,2). CTAs do not cause an immune reaction in
the testes, but when expressed in cancerous tissue these antigens
can induce a specific immune response (3). These characteristics of CTAs make
these proteins potential diagnostic markers. Sperm-associated
antigen 9 (SPAG9) is a member of the CTA family that is highly
expressed in many types of cancers and that causes a strong immune
response (4–8). Studies of SPAG9 suggest that it
promotes proliferation and invasion (9–11), but
whether SPAG9 has clinical value as a therapeutic target or as a
diagnostic marker remains to be clarified. At present, there is no
early detection test or screening method for lung cancer that
accurately and reliably detects the disease in the early stages. In
this study, we analyzed expression of SPAG9 in lung cancer tissues
and autoantibodies in serum in order to determine the diagnostic
value of SPAG9 as a marker of lung cancer.
Materials and methods
Patients and sample collection
Patients were treated at the Tumor Hospital of Hunan
Province. Diagnoses were confirmed by pathobiology. Patients
consented to specimen collection, and the study was approved by the
Ethics Committee of the Second People's Hospital of Hunan Province.
Age, gender, tumor size and histological diagnoses of the 20
patients who participated in the tissue arm of the study are shown
in Table I. Adjacent non-cancerous
tissue specimens were also collected from these patients. It is
important to point out that these tissues cannot be regarded as
healthy and normal.
 | Table ITissue evaluation: Characteristics of
the 20 lung cancer patients. |
Table I
Tissue evaluation: Characteristics of
the 20 lung cancer patients.
| Sample | Age (years) | Gender | Tumor size (cm) | Histological
diagnosis |
|---|
| 1 | 55 | Male | 4.5×3.0×3.0 | Moderately and poorly
differentiated squamous cell carcinoma |
| 2 | 45 | Male | 3.5×2.0×2.0 | Moderately and poorly
differentiated squamous cell carcinoma |
| 3 | 70 | Male | 3.5×3.0×1.5 | Moderately
differentiated, adenocarcinoma with regional differentiation |
| 4 | 60 | Female | 4.5×3.5×3.0 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 5 | 65 | Male | 4.0×3.5×3.0 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 6 | 55 | Male | 5.0×4.5×2.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 7 | 40 | Male | 7.5×5.0×4.0 | Moderately and poorly
differentiated squamous cell carcinoma, lymph nodes |
| 8 | 52 | Male | 5.0×3.5×3.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 9 | 66 | Male | 5.0×4.0×3.0 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 10 | 55 | Male | 5.5×4.0×3.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 11 | 51 | Female | 4.5×3.0×3.0 | Moderately and poorly
differentiated adenocarcinoma |
| 12 | 62 | Male | 5.0×4.0×3.0 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 13 | 65 | Male | 5.0×4.5×3.0 | Poorly differentiated
squamous cell carcinoma, lymph node |
| 14 | 54 | Male | 4.5×3.0×2.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 15 | 65 | Male | 4.0×3.0×1.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 16 | 55 | Male | 5.5×4.0×2.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 17 | 51 | Male | 3.5×3.0×2.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 18 | 61 | Male | 5.0×4.5×4.0 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 19 | 53 | Male | 5.0×5.0×4.5 | Moderately
differentiated squamous cell carcinoma, lymph nodes |
| 20 | 43 | Female | 3.0×2.5×2.0 | Poorly
differentiated adenocarcinoma |
Serum samples were obtained from 92 lung cancer
patients and 35 healthy subjects. Age and gender of the patients
are shown in Table II; there were
no significant differences in age or gender among the groups. All
cases were diagnosed by CT, MRI, fiber-optic bronchoscopy and
percutaneous lung biopsy. Forty-one patients who had not received
any treatment were classified as the untreated group. The 51
patients who had received radiotherapy and chemotherapy were
classified as the treated group; the characteristics of these
patients are shown in Table III.
Forty-five of the above 92 lung cancer patients were followed up
for 2 years; 25 were deceased and 20 were alive. The age and gender
of the patients are shown in Table
IV. Peripheral blood was collected, and serum was separated by
centrifugation of the blood at 1,000 × g for 10 min. Serum was
stored at −80°C until analysis.
 | Table IISerum evaluation: Characteristics of
the 92 patients and 35 controls. |
Table II
Serum evaluation: Characteristics of
the 92 patients and 35 controls.
| Characteristic | Untreated group
(n=41) | Treated group
(n=51) | Healthy controls
(n=35) |
|---|
| Age (years) |
| Mean ± SD | 60±11 | 56±11 | 53±10 |
| Range | 36–79 | 32–75 | 26–71 |
| Gender |
| No. of males | 35/41 | 39/51 | 26/35 |
| % males | 85.4 | 76.5 | 74.3 |
 | Table IIISerum evaluation: Clinicopathology
and SPAG9 humoral immune reactions of the untreated and treated
patientsa. |
Table III
Serum evaluation: Clinicopathology
and SPAG9 humoral immune reactions of the untreated and treated
patientsa.
| Pathological and
clinical features | SPAG9 antibody (OD)
| Positive/tested (%)
|
|---|
| Untreated | Treated | Untreated | Treated |
|---|
| All tumors | 0.612±0.482b | 0.342±0.213 | 26/41
(63.4)b | 10/51 (19.6) |
| Stage of non-small
cell lung cancer |
| Early (T1 and
T2) | 0.664±0.642 | 0.392±0.228 | 7/13 (53.8) | 6/21 (28.6) |
| Late (T3 and
T4) | 0.550±0.334 | 0.365±0.226 | 10/16
(62.5)b | 2/17 (11.8) |
|
Indeterminated | 0.476±0.254b | 0.200±0.09 | 7/12 (58.3)b | 2/13 (15.4) |
| Grade of non-small
cell lung cancer |
| Low (G1 and
G2) | 0.542±0.281 | 0.413±0.188 | 8/13 (61.5) | 3/13 (23.1) |
| High (G3 and
G4) | 0.614±0.587 | 0.378±0.244 | 9/18 (50.0)b | 5/24 (20.8) |
|
Indeterminated | 0.510±0.276b | 0.224±0.127 | 7/10 (70.0)b | 2/14 (14.3) |
| Type of tumor |
| Squamous cell
carcinoma | 0.626±0.540 | 0.431±0.247c | 12/23 (47.1) | 8/26 (30.8)c |
|
Adenocarcinoma | 0.477±0.208b | 0.264±0.101 | 5/7 (71.4)b | 0/13 (0.0) |
| Small cell lung
cancer | 0.844±0.661b | 0.235±0.152 | 4/5 (80.0)b | 1/8 (12.5) |
 | Table IVSerum evaluation: Characteristics of
the 45 patients who survived 2-years post-diagnosis and those who
did not. |
Table IV
Serum evaluation: Characteristics of
the 45 patients who survived 2-years post-diagnosis and those who
did not.
| Characteristic | Survival group
(n=25) | Non-survival group
(n=20) | Healthy controls
(n=35) |
|---|
| Age (years) |
| Mean ± SD | 59±11 | 56±10 | 53±10 |
| Range | 39–75 | 47–75 | 26–71 |
| Gender |
| No. of males | 19/25 | 16/20 | 26/35 |
| % males | 76.0 | 80.0 | 74.3 |
RT-PCR testing
mRNA expressed from the SPAG9 gene in tissues was
quantified as previously described (12). Total RNA was isolated and cDNA was
synthesized using a RevertAid M-MulV First Strand cDNA Synthesis
kit (Thermo Fisher) in accordance with the supplier's protocol.
RT-PCR was performed using SPAG9-specific primers. Primers were
designed using software Primer 3.0 and were synthesized by China
Yuantai Company. Primers were: homo-SPAG9 forward,
5′-AGCCGACTTTTCAGCTCCTC-3′ and reverse, 5′-AAAGCCTGCACTCTACCGTC-3′.
Expected fragment length was 114 bp, and the predicted melting
temperature was 59°C. The GAPDH mRNA was amplified as an
internal control with primers homo-GAPDH forward,
5′-caatgaccccttcatt-gacc-3′ and reverse,
5′-gacaagcttcccgttctcag-3′. The expected fragment length was 106
bp. The real-time PCR results were analyzed with SDS 7900 software
(ABI).
Western blot analysis
Total protein was extracted from tissue, separated
by SDS-PAGE, and transferred to supported nitrocellulose membranes.
The protein blots were blocked with 5% milk in PBS overnight at
4°C. Each blot was then incubated with the anti-SPAG9 antibody
(PAB8794; Abnova) and mouse GAPDH antibody (sc-166574; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at dilutions of 1:500 in 5%
milk prepared in PBS for 2 h at 4°C with gentle shaking. After four
5–10 min washes with PBS-T (PBS with 0.05% Tween-20), each blot was
incubated with the secondary antibody (goat anti-rabbit IgG/HRP at
a dilution of 1:40,000 or goat anti-mouse IgG/HRP at a dilution of
1:50,000) for 1 h. After four washes with PBS-T, the SPAG9 proteins
were detected with an Amersham enhanced chemiluminescence detection
kit according to the manufacturer-supplied protocol. After
exposure, the X-ray film was analyzed with an ImageQuant LAS-4000
(Fuji). The bands were analyzed using Automated Digitizing System
Gel Pro 4.0. The relative expression levels (fold) were calculated
by dividing the integrated optical density (IOD) for the band
corresponding to SPAG9 by the IOD of the GAPDH band.
Immunohistochemistry
Sections (3-µm) were prepared from the
paraffin-embedded tissues. Immunostaining was performed using the
two-step EliVision Plus kit (KIT-5020; Maixin). The sections were
deparaffinized in xylene, rehydrated with graded alcohol, and then
boiled in citrate buffer (pH 6.0) for 2 min in an autoclave. Next,
0.3% hydrogen peroxide was applied to block the endogenous
peroxidase activity, and the sections were incubated with normal
animal serum to reduce non-specific binding. Tissue sections were
incubated with SPAG9 rabbit polyclonal antibody (1:150 dilution;
Abcam) for 2 h at room temperature. Rabbit immunoglobulin (at the
same concentration as used for the antigen-specific antibody) was
used as a negative control. The staining was followed by incubation
with polymer secondary antibodies. Color reaction was developed by
using 3,3′-diaminobenzidine tetrachloride (DAB) chromogen solution.
All slides were counterstained with hematoxylin. Positive control
slides were included in every experiment in addition to the
internal positive controls. The specificity of the antibody was
determined with matched IgG isotype antibody as a negative control
(13).
Sections were evaluated by two investigators in a
blinded manner in an effort to provide a consensus on staining
patterns by light microscopy (Olympus). Each case was rated
according to a score that added a scale of intensity of staining to
the area of staining. At least 10 high-power fields were chosen
randomly, and >1,000 cells were counted for each section. The
intensity of staining was graded on the following scale: 0, no
staining; 1+, mild staining; 2+, moderate staining; 3+, intense
staining. The area of staining was evaluated as follows: 0, no
staining of cells in any microscopic fields; 1+, <30% of tissue
stained positive; 2+, between 30 and 60% stained positive; 3+,
>60% stained positive. The minimum score when summed (extension
+ intensity) was, therefore, 0, and the maximum, 6. A combined
staining score (extension + intensity) of ≤2 was considered to be
negative staining (low staining); a score between 3 and 4 was
considered to be moderate staining; whereas a score between 5 and 6
was considered to be strong staining. An optimal cut-off level was
identified as follows: a staining index score of 0–2 was used to
define tumors with negative expression and 3–7 indicated positive
expression of these two proteins. Agreement between the two
evaluators was 95%, and all scoring discrepancies were resolved
through discussion between the two evaluators.
ELISA
Recombinant human SPAG9 protein (r-hSPAG9; Abnova)
was used as antigen in an ELISA to detect serum anti-SPAG9 IgG
antibody levels (6). Basal levels
in the ELISA were established using serum from 35 healthy donors,
and the cut-off signal intensity (mean ± 1.96 SD) was an OD of
0.416 (0.187±0.229). The absorbance was read at 450 with 630 nm as
reference filter, and the intra-assay and inter-assay coefficients
of variation were 2.3 and 8.6%, respectively.
In order to evaluate the response of the immune
system to SPAG9, we quantified the serum SPAG9 IgG antibody in
peripheral blood of 92 lung cancer patients and 35 healthy
subjects; 41 lung cancer patients who had not received any
treatment as the untreated group, 51 patients who had received
radiotherapy and chemotherapy as the treated group.
Statistical analyses
The Pearson's Chi-square test, Fisher's exact test,
Student's t-test for unpaired data, Wilcoxon signed-rank test,
Mann-Whitney U test, and Kruskal-Wallis test were performed using
the SPSS 16.0 statistical software. Results are expressed as mean ±
standard deviation (SD). All p-values were two-sided, and a p-value
<0.05 was considered to indicate a statistically significant
result.
Results
SPAG9 expression in lung tumors
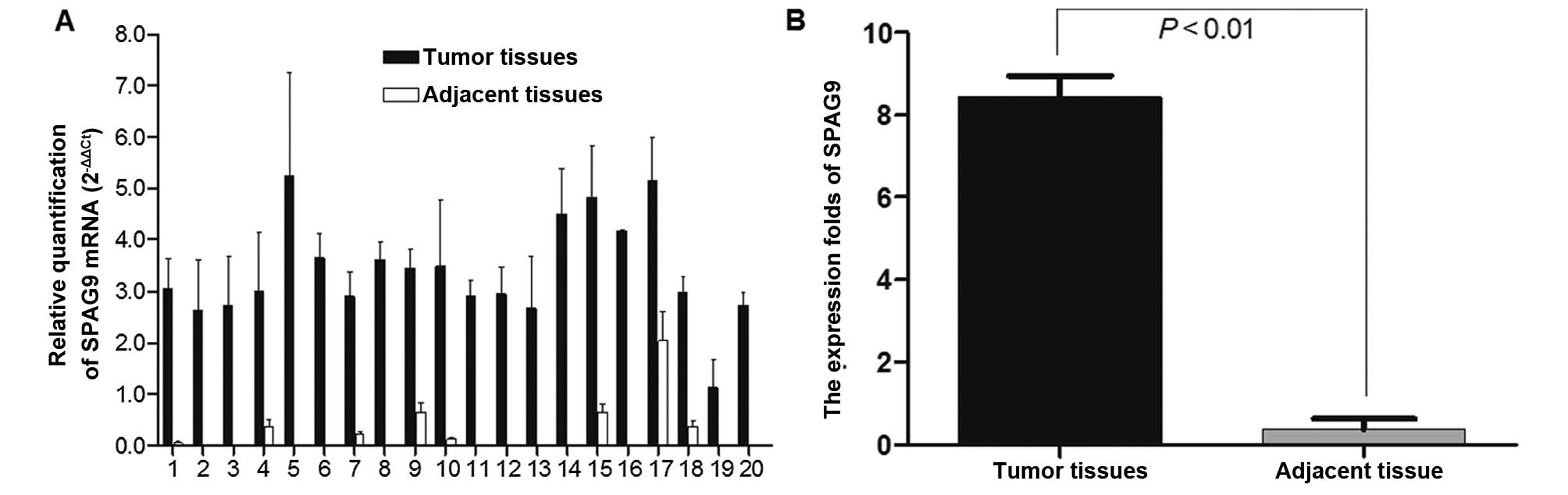
SPAG9 mRNA levels were quantified by RT-PCR in lung
cancer tissues and in adjacent tissues from 20 patients; GAPDH was
used as internal reference. The expression of the SPAG9 gene was
higher in the lung cancer tissue samples compared with that in the
adjacent non-cancerous tissues (Fig.
1A). The normalized SPAG9 gene expression in lung cancer
tissues was upregulated by 8.29-fold (P=0.000<0.01) (Fig. 1B).
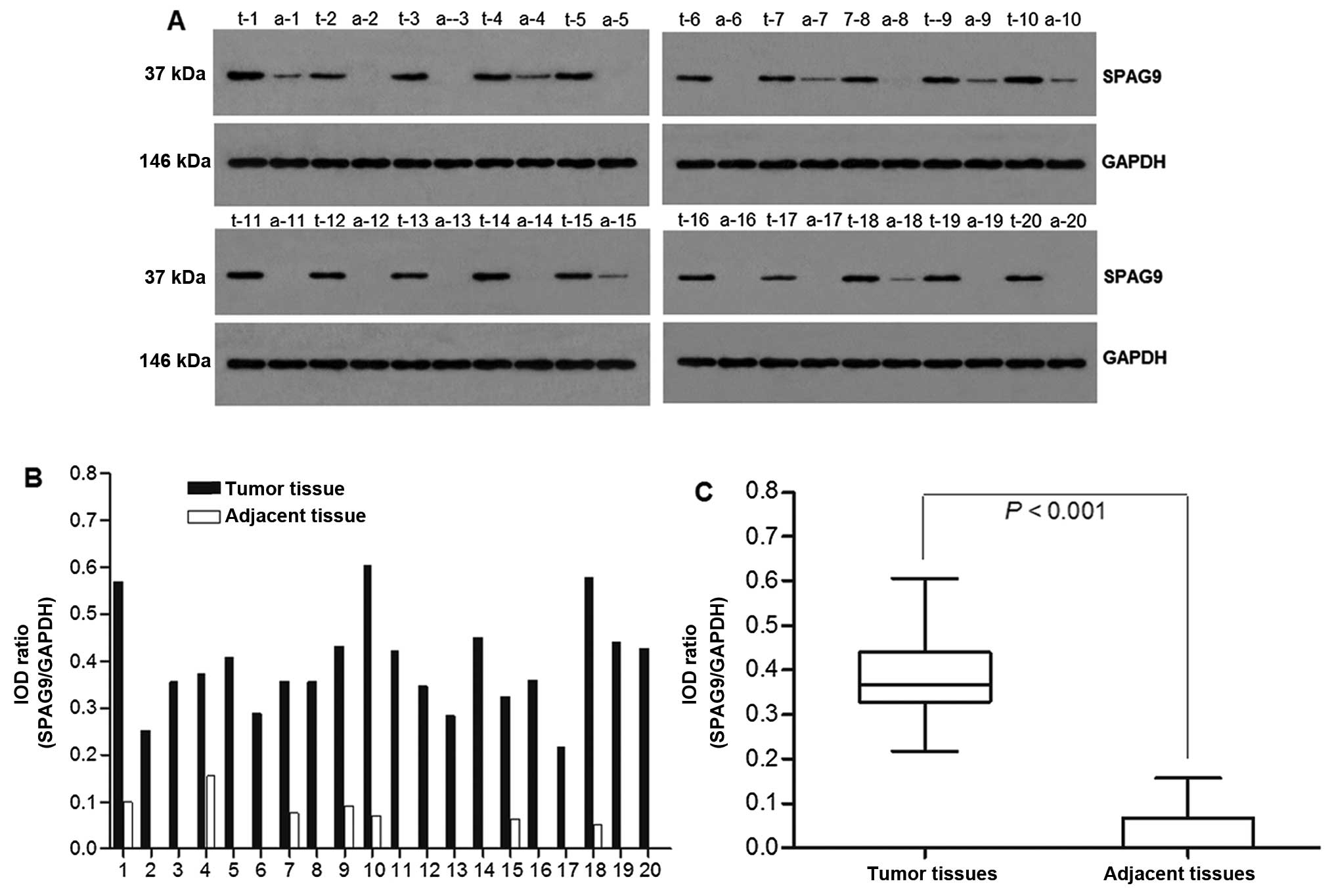
SPAG9 protein expression in the lung tissues was
investigated by western blot analysis using anti-SPAG9 antibodies.
GAPDH was used as a control (Fig.
2A). SPAG9 expression was significantly higher in the tumor
tissues than that in the adjacent non-cancerous tissue (IOD ratio
was 0.392±0.104) and was also detected in some adjacent
non-cancerous tissues, but with low expression (IOD ratio was
0.03±0.047) (P<0.001) (Fig. 2B and
C).
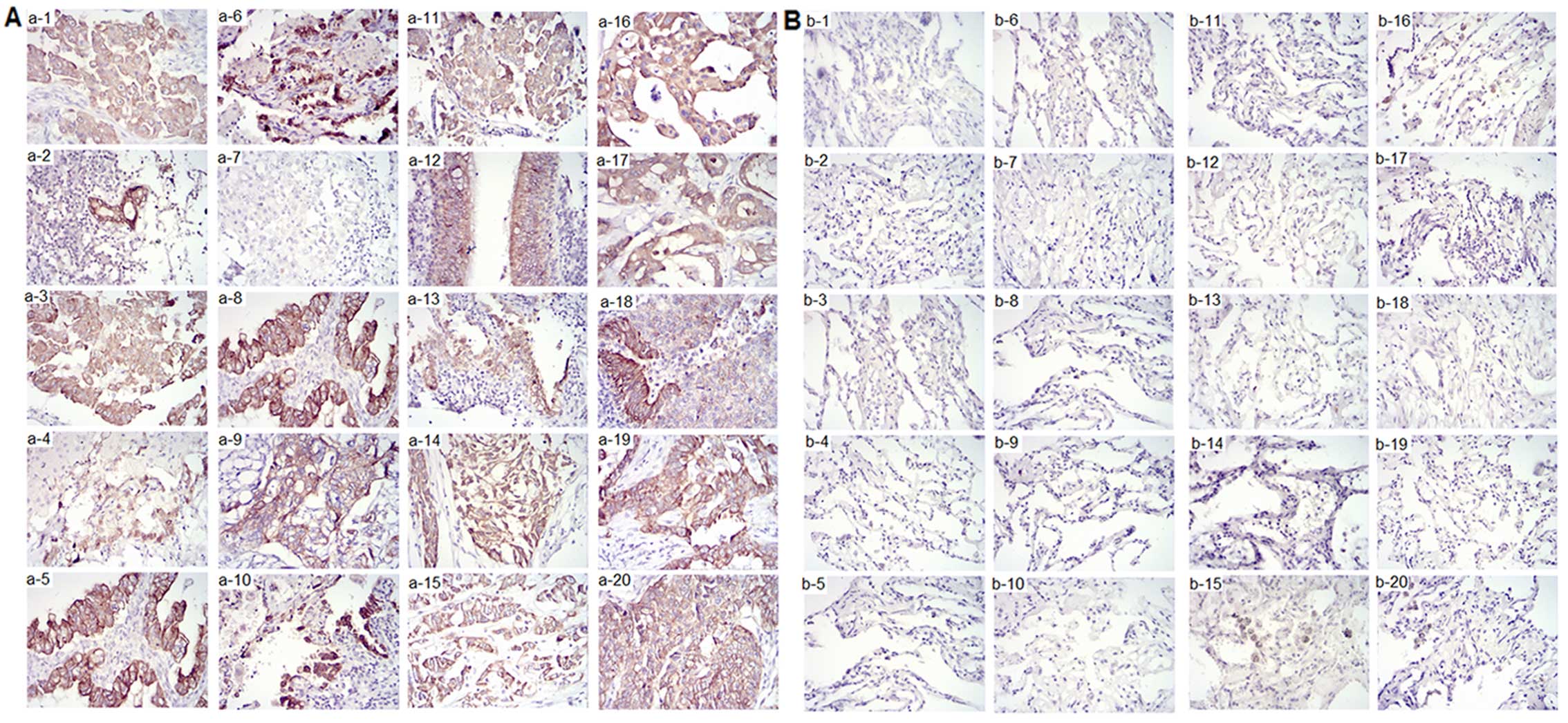
SPAG9 protein expression in lung tissues was
investigated by immunohistochemical staining. The samples from the
20 patients were also evaluated by immunohistochemical staining; 20
cases of non-small cell lung cancer tissue (without consideration
of the type and stage of cancer) and 20 cases of adjacent
non-cancerous tissue specimens were analyzed. Positive staining for
SPAG9 was observed in 16 out of the 20 (80%) lung cancer tissue
samples. In these cases, SPAG9 protein was localized in the
cytoplasmic compartments. No SPAG9 expression was detected in any
of the samples of the adjacent non-cancerous tissue (Table V). Representative images of stained
tissues from the 20 patients with lung cancer are shown in Fig. 3A and B.
 | Table VTissue evaluation:
Immunohistochemistry of the 20 lung cancer and adjacent
non-cancerous tissues. |
Table V
Tissue evaluation:
Immunohistochemistry of the 20 lung cancer and adjacent
non-cancerous tissues.
| Sample | Grade of staining
intensity | Area of
staining | Summed score | Result |
|---|
| 1 | 2+ | 3+ | 4 | Moderate
staining |
| 2 | 1+ | 1+ | 1 | Negative |
| 3 | 2+ | 3+ | 4 | Moderate
staining |
| 4 | 2+ | 1+ | 2 | Negative |
| 5 | 3+ | 2+ | 4 | Moderate
staining |
| 6 | 3+ | 2+ | 4 | Moderate
staining |
| 7 | 0 | 0 | 0 | Negative |
| 8 | 3+ | 2+ | 4 | Moderate
staining |
| 9 | 3+ | 2+ | 4 | Moderate
staining |
| 10 | 3+ | 1+ | 3 | Moderate
staining |
| 11 | 3+ | 3+ | 6 | Strong
staining |
| 12 | 2+ | 2+ | 3 | Moderate
staining |
| 13 | 1+ | 2+ | 2 | Negative |
| 14 | 2+ | 3+ | 5 | Strong
staining |
| 15 | 2+ | 2+ | 4 | Moderate
staining |
| 16 | 3+ | 3+ | 6 | Strong
staining |
| 17 | 2+ | 2+ | 4 | Moderate
staining |
| 18 | 3+ | 2+ | 5 | Strong
staining |
| 19 | 3+ | 2+ | 5 | Strong
staining |
| 20 | 2+ | 3+ | 5 | Strong
staining |
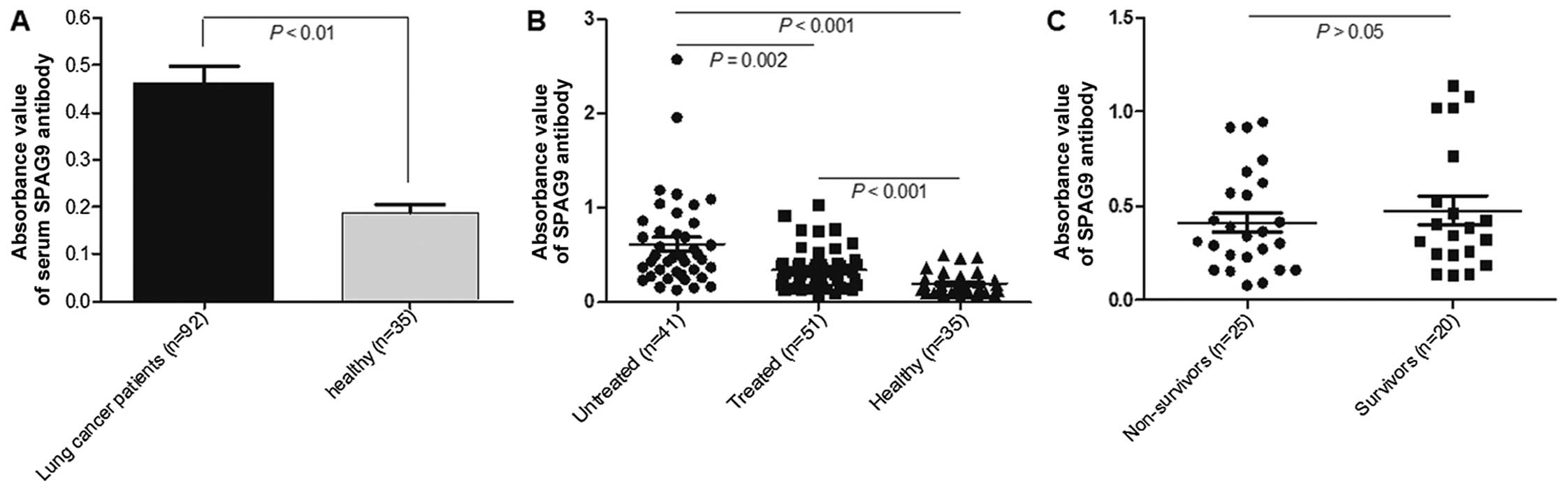
Humoral immune response induced by
SPAG9
The serum level of SPAG9 IgG was significantly
higher in the 92 lung cancer patients than that in the healthy
subjects (0.187±0.117, P<0.001) (Fig. 4A). The serum level of SPAG9 IgG was
significantly higher in the untreated patients (0.612±0.482) than
that in the treated lung cancer patients (0.342±0.213; P=0.002)
(Fig. 4B), irrespective of the
disease stage. There were no statistical differences between levels
of SPAG9 IgG in the 2-year survivors and the non-survivors
(Fig. 4C). No significant
differences were observed in serum SPAG9 IgG levels between early
stage (T1 and T2) and late stage (T3 and T4) cancer patients or
between patients with differentiation grade G1 and G2 and those
with low differentiation grades (G3 and G4) irrespective of whether
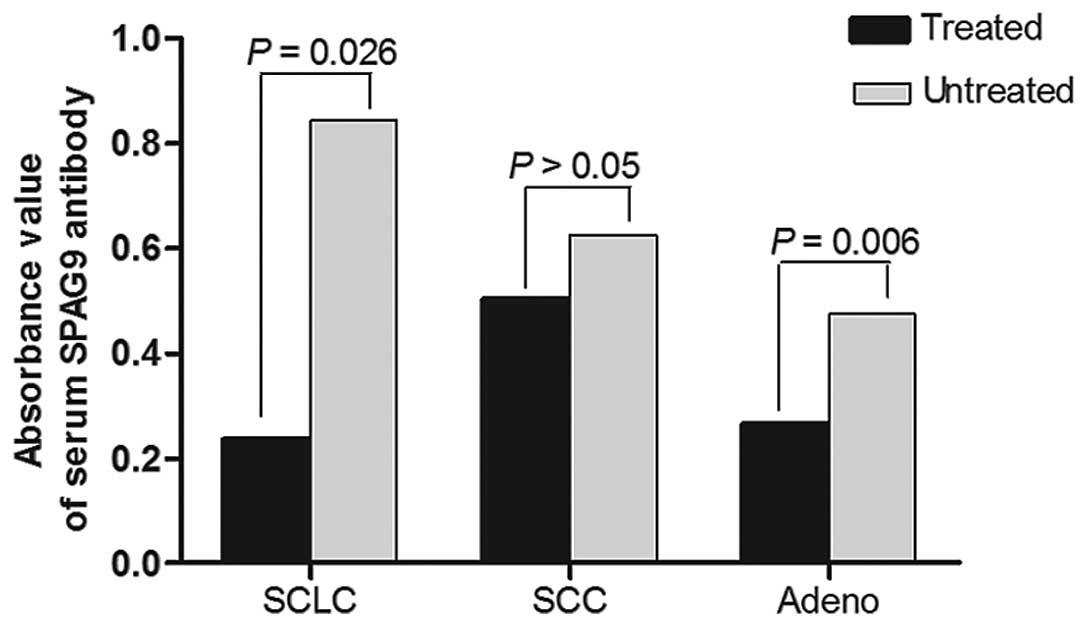
patients had been treated or not. SPAG9 IgG antibody levels were
significantly lower in treated adenocarcinoma and small-cell lung
cancer (SCLC) patients than levels in the untreated patients
(P=0.006, P=0.026, respectively), but no statistical difference was
found between treated and untreated squamous cell carcinoma
patients (Fig. 5).
Discussion
In China, lung cancer detection is often based on
chest X-ray; the disease is usually advanced at the time of
diagnosis thus few patients are cured by treatment. If lung cancer
is diagnosed early, the chances of a cure can reach 90%; therefore,
it is necessary to find methods that enable the early diagnosis of
lung cancer.
Some tumor-associated autoantibodies have been
detected in patients with lung cancer at the pre-symptomatic stage
or before radiographic detection (14–17).
CTAs elicit specific humoral immune responses (18) and play an important role in cancer
progression (19–22). Therefore, their utility as
biomarkers and their potential use in immunotherapeutic strategies
are of interest (1,18,23).
This study focused on the new CTA SPAG9. Consistent with a previous
report (24), overexpression of
SPAG9 in lung cancer tissue specimens was detected by
immunohistochemistry and SPAG9 protein was localized in the
cytoplasmic compartments of tumor cells. The positive rate in this
study was higher than that found in the previous study which may be
due to the difference in sample size and case selection, or the
staining method and the difference in the positive judgment
standard. Furthermore, we found that both SPAG9 mRNA and protein
expression were higher in lung cancer tissues than levels in the
adjacent non-cancerous tissues.
We also discovered that there is a humoral immune
response to SPAG9 in lung cancer patients. As a diagnostic marker,
serum autoantibodies to SPAG9 were previously shown to be detected
in lung cancer patients (25). In
our cohort of patients, high expression of SPAG9 IgG antibodies in
peripheral blood was observed in newly confirmed lung cancer
patients, and low expression in healthy people, indicating that the
humoral immune response promoted by SPAG9 was related to the
activity of tumor cells. The amount of SPAG9 IgG was lower in the
treated lung cancer patients than that in the untreated patients,
suggesting that various clinical therapies may reduce SPAG9
expression so as to reduce the humoral immune response caused by
SPAG9.
Our study was unable to address whether a decrease
in SPAG9 autoantibody levels indicates effectiveness of the
treatment. Dynamic observations will be necessary to answer this
question. We did find that there was no statistical difference in
SPAG9 IgG antibody expression between patients who survived for 2
years after diagnosis and those who did not; therefore, there is no
direct relationship between humoral immune response to SPAG9 and
patient prognosis. We also found no difference among different
disease stages or differentiation grades, and this may be related
to the number of samples or the standard of grading. Thus, we need
to expand the sample size and accurate staging in further
study.
Adenocarcinoma and SCLC are more likely to spread to
the lymphatic and hematogenous systems than SCC, thus easily cause
an immune response and produce antibodies. Our results showed that
expression of serum SPAG9 antibody in treated adenocarcinoma and
SCLC patients was significantly lower than that in the treated SCC
patients, but the mechanism is not clear, and the role of the
immune response in cancer development warrants further study.
However, the marker function of SPAG9 in the course of cancer
occurrence and development can undoubtedly help the early diagnosis
of cancer.
In summary, the phenomenon of SPAG9 mRNA and protein
overexpression in lung cancer tissues was observed, and the SPAG9
IgG antibody was detected in peripheral blood of lung cancer
patients indicating that it has potential as a biomarker for lung
cancer diagnosis. Whether a decrease in level of the SPAG9
autoantibody correlates with treatment effectiveness requires
further study. Our data suggest that there may be differences in
the level of expression of the SPAG9 autoantibody in various types
of lung cancer and before and after treatment, yet, a larger number
of cases need to be evaluated to confirm these results.
Acknowledgments
This study was supported in part by the Hunan
Province Science and Technology Fund (2009SK3092) and Key Clinical
Specialized Fund of Hunan Second People's Hospital.
Abbreviations:
|
CTA
|
cancer testis antigen
|
|
SPAG9
|
sperm-associated antigen 9
|
|
SCC
|
squamous cell carcinoma
|
|
SCLC
|
small cell lung cancer
|
References
|
1
|
Suri A: Cancer testis antigens - their
importance in immuno-therapy and in the early detection of cancer.
Expert Opin Biol Ther. 6:379–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawakami Y: New cancer therapy by
immunomanipulation: Development of immunotherapy for human melanoma
as a model system. Cornea. 19(Suppl 3): S2–S6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: Potential targets for immunotherapy. Cancer Sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garg M, Chaurasiya D, Rana R, Jagadish N,
Kanojia D, Dudha N, Kamran N, Salhan S, Bhatnagar A, Suri S, et al:
Sperm-associated antigen 9, a novel cancer testis antigen, is a
potential target for immunotherapy in epithelial ovarian cancer.
Clin Cancer Res. 13:1421–1428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garg M, Kanojia D, Khosla A, Dudha N, Sati
S, Chaurasiya D, Jagadish N, Seth A, Kumar R, Gupta S, et al:
Sperm-associated antigen 9 is associated with tumor growth,
migration, and invasion in renal cell carcinoma. Cancer Res.
68:8240–8248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9, a novel biomarker for early
detection of breast cancer. Cancer Epidemiol Biomarkers Prev.
18:630–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garg M, Kanojia D, Salhan S, Suri S, Gupta
A, Lohiya NK and Suri A: Sperm-associated antigen 9 is a biomarker
for early cervical carcinoma. Cancer. 115:2671–2683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garg M, Kanojia D, Suri S, Gupta S, Gupta
A and Suri A: Sperm-associated antigen 9: A novel diagnostic marker
for thyroid cancer. J Clin Endocrinol Metab. 94:4613–4618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Peng Y, Niu H, Wu B, Zhang Y, Zhang
Y, Bai X and He P: SPAG9 is overexpressed in human prostate cancer
and promotes cancer cell proliferation. Tumour Biol. 35:6949–6954.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi F, Ni W, Liu W, Pan X, Han X, Yang L,
Kong X, Ma R and Chang R: SPAG9 is overexpressed in human
astrocytoma and promotes cell proliferation and invasion. Tumour
Biol. 34:2849–2855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie C, Fu L, Liu N and Li Q:
Overexpression of SPAG9 correlates with poor prognosis and tumor
progression in hepatocellular carcinoma. Tumour Biol. 35:7685–7691.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Hara A and Okayasu I: Cyclooxygenase-2 and
inducible nitric oxide synthase expression in human astrocytic
gliomas: Correlation with angiogenesis and prognostic significance.
Acta Neuropathol. 108:43–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong L, Coe SP, Stromberg AJ, Khattar NH,
Jett JR and Hirschowitz EA: Profiling tumor-associated antibodies
for early detection of non-small cell lung cancer. J Thorac Oncol.
1:513–519. 2006. View Article : Google Scholar
|
|
15
|
Chapman CJ, Murray A, McElveen JE, Sahin
U, Luxemburger U, Türeci O, Wiewrodt R, Barnes AC and Robertson JF:
Auto-antibodies in lung cancer: Possibilities for early detection
and subsequent cure. Thorax. 63:228–233. 2008. View Article : Google Scholar
|
|
16
|
Qiu J, Choi G, Li L, Wang H, Pitteri SJ,
Pereira-Faca SR, Krasnoselsky AL, Randolph TW, Omenn GS, Edelstein
C, et al: Occurrence of autoantibodies to Annexin I, 14-3-3 theta
and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol.
26:5060–5066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan HT, Low J, Lim SG and Chung MC: Serum
autoantibodies as biomarkers for early cancer detection. FEBS J.
276:6880–6904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
19
|
de Visser KE, Korets LV and Coussens LM:
De novo carcinogenesis promoted by chronic inflammation is B
lymphocyte dependent. Cancer Cell. 7:411–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F, Lu Z, Deng J, Han X, Bai J, Liu Q,
Xi Y and Zheng J: SPAG9 expression is increased in human prostate
cancer and promotes cell motility, invasion and angiogenesis in
vitro. Oncol Rep. 32:2533–2540. 2014.PubMed/NCBI
|
|
21
|
Jiang J, Liu Y, Fang W and Liu F: Sperm
associated antigen 9 promotes astrocytoma cell invasion through the
upregulation of podocalyxin. Mol Med Rep. 10:417–422.
2014.PubMed/NCBI
|
|
22
|
Kanojia D, Garg M, Saini S, Agarwal S,
Parashar D, Jagadish N, Seth A, Bhatnagar A, Gupta A, Kumar R, et
al: Sperm associated antigen 9 plays an important role in bladder
transitional cell carcinoma. PLoS One. 8:e813482013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baser E, Togrul C, Ozgu E, Ayhan S, Caglar
M, Erkaya S and Gungor T: Sperm-associated antigen 9 is a promising
marker for early diagnosis of endometrial cancer. Asian Pac J
Cancer Prev. 14:7635–7638. 2013. View Article : Google Scholar
|
|
24
|
Wang Y, Dong Q, Miao Y, Fu L, Lin X and
Wang E: Clinical significance and biological roles of SPAG9
overexpression in non-small cell lung cancer. Lung Cancer.
81:266–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu J and Hanash S: Autoantibody profiling
for cancer detection. Clin Lab Med. 29:31–46. 2009. View Article : Google Scholar : PubMed/NCBI
|