Introduction
Glioblastoma (GBM) is the most common and lethal
primary brain tumor widely invading surrounding brain tissues. GBM
patients almost always suffer from tumor recurrence and have a less
optimistic median survival period even after they receive
conventional treatments involving surgery, radiation, as well as
adjuvant chemotherapy. Studies demonstrate that gliomas and other
primary brain tumors contain cancer stem cells (CSCs) which have
the ability to self-renew and give rise to differentiated progeny
(1,2). These multipotent stem cells
participating in the development and progressive growth of tumors
have also been shown to be resistant to radiation and chemotherapy
(3,4). It has been reported that glioma stem
cells (GSCs) contribute to the malignant biological behaviors of
gliomas such as infiltrating growth, therapeutic resistance and
high recurrence rates. Many therapeutic strategies for GSCs have
been developed for GBM and other brain tumors (5). GSCs are key drivers to promote tumor
growth by several complicated molecular mechanism including
genetics, epigenetics, metabolism as well as various extrinsic
regulatory factors. Recently, many investigators have found that a
variety of microRNAs (miRNAs) present altered expression and play
an important oncogenic role or tumor-suppressive function in CSCs
(6).
MicroRNAs (miRNAs) are a type of small,
single-stranded endogenous RNA molecules with a length of ~21 to 25
nucleotides present in eukaryotic cells, which regulate target gene
expression at the post-transcriptional level by degrading target
mRNAs or inhibiting translation. Studies have shown that miRNAs,
acting as tumor promoters or suppressors, may modulate tumor
initiation, development and progressive growth in a wide variety of
tumors. miR-608 is one of the newly discovered microRNAs, and its
biological functions are not clearly understood. One study showed
that miR-608 acts as a tumor-suppressive microRNA regulating
malignancy in chordoma (7). In the
present study, we found that miR-608 expression was significantly
downregulated in glioblastoma tissues and GSCs using real-time PCR,
and the overexpression of miR-608 attenuated the invasion of GSCs;
no literature has reported this finding thus far. Therefore, we
investigated the role and detailed mechanisms of miR-608 in
glioma.
In the present study, our results of the
bioinformatic analysis predicted that the 3′UTR of human macrophage
migration inhibitory factor (MIF) contained a region (nucleotides
73–80) with perfect complementarity to miR-608. Luciferase reporter
assay revealed that miR-608 negatively regulated the gene
expression of MIF at the post-transcriptional level by direct
interaction with the MIF 3′UTR target sites. Furthermore, we
verified that the expression of the MIF gene and protein was
significantly increased in GSCs using real-time PCR and western
blotting. Studies suggest that MIF has a prominent role in
regulating cell survival, proliferation, differentiation, and
angiogenesis as well as promoting the occurrence and development of
tumors. It has been reported that elevated expression of MIF
correlates with tumor recurrence and poor prognosis of patients
with gliomas (8,9), but the association between MIF and
GSCs remains unknown. We hypothesized that low expression of
miR-608 in GSCs may prohibit its suppressive effect on the
potential oncogene MIF at the transcriptional level and the
expression of the MIF gene is consequently upregulated.
It has been suggested that the phosphatidylinositol
3-kinase (PI3K)/Akt signaling pathway may facilitate the
proliferation and invasion of GBM cells, and blockage of
PI3K-mediated signaling has a potent anti-proliferative and
anti-invasive effect on GBM (10).
In addition, Jun N-terminal kinase (JNK) belongs to one of three
major mitogen-activated protein (MAP) kinase pathways, and it is
clear that activation of the JNK pathway is involved in the
induction of cell apoptosis (11).
However, investigators have uncovered that the JNK pathway has dual
roles in the malignant biological behavior of cancers. Activation
of JNK may favor the cell migration of bladder cancer cells. It has
been recently reported that ERK and JNK inhibitors effectively
inhibited the migration of glioma cells (12,13).
Therefore, the PI3K/AKT and JNK signaling pathways are critical in
the therapeutic approach to gliomas. Moreover, it was reported that
MIF may activate the JNK signaling pathway in T cells and
fibroblasts (14). MIF induced the
expression of thrombomodulin and intercellular adhesion molecule-1
via JNK and PI3K pathways in human monocytoid (THP-1) cells
(15). We questioned whether MIF
may regulate the biological behaviors of GSCs via the PI3K/AKT or
JNK signaling pathways.
In the present study, we aimed to prove the direct
interaction between the seed sequence of miR-608 and MIF 3′UTR
target sites. Then, we establish GSC models with overexpression and
knockdown of miR-608 to demonstrate the regulatory actions of
miR-608 on the expression and function of the MIF gene and the
biological behavior of GSCs. The results of the study demonstrated
the molecular mechanisms of miR-608-mediated regulation of the
biological behavior of GSCs by downregulating the expression of the
MIF gene. These findings will help us to reveal the pathogenesis of
brain glioma and provide new therapeutic targets for malignant
glioma.
Materials and methods
Clinical specimens
Glioblastoma specimens were obtained from the
Department of Neurosurgery, Shengjing Hospital of China Medical
University. Fresh human brain tissues obtained by surgery for
malignant intracranial hypertension were used as control.
Immediately after surgical resection, the specimens were frozen and
preserved in liquid nitrogen. According to WHO 2007 classification,
the glioma specimens were classified into four grades, and then
they were divided into a low-grade glioma group (WHO I–II, n=3) and
a high-grade glioma group (WHO III-IV, n=3). All procedures were
approved by the Ethics Committee of the China Medical University.
All the specimens were obtained with the consent of the patients
before surgery and written informed consent was received from all
participants.
Cell culture and GSC isolation
Human GBM cell lines (U87 and U251) and human
embryonic kidney (HEK) 293T cells were purchased from Shanghai
Institutes for Biological Sciences Cell Resource Center. The cells
were cultured in high-glucose Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS; Life Technologies
Corporation, Paisley, UK). When the U87 and U251 cells reached the
logarithmic growth phase, they were resuspended in serum-free
DMEM/F12 supplemented with 20 ng/ml of basic fibroblast growth
factor (bFGF), 20 ng/ml of epidermal growth factor (EGF) and B27
serum-free supplement (all from Life Technologies Corporation). All
cells were incubated at 37°C in a 5% CO2 humidified
incubator. The isolation and identification of GSCs from the U87
and U251 cells were carried out as described previously (16,17).
The GSCs successfully isolated from U87 and U251 were used for the
subsequent study.
Qualitative real-time PCR (qRT-PCR)
Total RNA was extracted from the non-GSCs and GSCs
with TRIzol reagent (Life Technologies Corporation) according to
the manufacturer's protocol. The expression level of miR-608 mRNA
was determined using TaqMan MicroRNA Reverse Transcription kit and
TaqMan Universal Master Mix II (Applied Biosystems Carlsbad, CA,
USA). The level of MIF mRNA was detected using
PrimeScript® RT Master Mix Perfect Real-Time and
SYBR® Premix Ex Taq™ (Takara, Dalian, Liaoning, China).
The mRNA levels of miR-608 and MIF were calculated by the relative
quantification (2−ΔΔCt) method using U6 and GAPDH as
endogenous controls.
Cell transfection
GSCs were transfected with miR-608 mimic, miR-608
inhibitor or their respective negative control (NC, non-targeting
sequence) which were synthesized by Suzhou GenePharma Co., Ltd.
Opti-MEM® I and Lipofectamine 2000 reagent (Life
Technologies Corporation) were used for transfection. The cells
were harvested after a 48-h transfection, and the levels of miR-608
overexpression and inhibition in the transfected cells were
determined by qRT-PCR. For investigating the roles of miR-608 on
GSCs, the transfected cells were divided into five groups as
follows: control group, miR-608 mimic NC group, miR-608 mimic
group, miR-608 inhibitor NC group, miR-608 inhibitor group. MIF
overexpression plasmid was obtained by ligating the human
full-length MIF gene with its 3′-untranslated region (UTR) into the
pEX-2 plasmid vector (GenePharma, Suzhou, China). In order to
knockdown MIF, short hairpin RNA was directed against the human MIF
gene (GenePharma). The MIF overexpression(+) or inhibition(−)
plasmid was steadily transfected into GSCs, and screened out using
G418 after 48 h of transfection. The transfection efficiency was
verified by qRT-PCR. For determining the role of PI3K/AKT and JNK
pathways in this experiment, the co-transfected GSCs were divided
into nine groups: control group, miR-608 mimic NC + MIF(+) NC
group, miR-608 mimic + MIF(+) group, miR-608 mimic NC + MIF(−) NC
group, miR-608 mimic + MIF(−) group, miR-608 inhibitor NC + MIF(+)
NC group, miR-608 inhibitor + MIF(+) group, miR-608 inhibitor NC +
MIF(−) NC group, and miR-608 inhibitor + MIF(−) group.
MTT assay
GSCs in logarithmic growth phase were collected with
serum-free DMEM/F12 medium, and seeded in 96-well plates. After
transfection for 48 h, 20 µl of methyl thiazolyl tetrazolium
(MTT; Sigma, USA) was added to each well and the cells were
incubated at 37°C for 4 h. Then 150 µl of dimethyl sulfoxide
(DMSO; Genview, USA) was added to each well. The optical density
value was measured at 490 nm.
Flow cytometry
ApoScreen Annexin V FITC/PI Apoptosis detection kit
(Baosai, Beijing) was used to detect cell apoptosis. After
transfected for 48 h, the cells were collected, washed, and then
resuspended in 200 µl of Annexin V binding buffer. The cells
were incubated with 10 µl of Annexin V FITC at 4°C. After
incubation for 15 min, 300 µl of cold binding buffer and 10
µl of propidium iodide (PI) solution were added. Cells were
analyzed with flow cytometry (FACScan; BD Biosciences).
Cell migration and invasion assays
The 24-well chambers with a polycarbonic membrane
(8-µm pore size) were used for the cell migration assay. The
cells were added to the upper chamber, incubated for 24 h with 100
µl serum-free medium at the density of 5×105
cells/ml, and 600 µl of medium with 10% FBS was added to the
lower chamber. After incubation for 24 h at 37°C, the chambers were
removed and the cells on the upper surface of the chambers were
wiped off. Cells that had migrated to the lower surface of the
membrane were fixed with methanol and then stained with 20% Giemsa.
The stained cells were observed and counted in five randomly
selected fields using a microscope. For the cell invasion assay,
the procedure was similar to the migration assay, but the upper
chamber was precoated with 500 ng/µl Matrigel solution (BD
Biosciences).
Luciferase assay
A Dual-luciferase reporter assay was performed to
determine the effect of miR-608 on MIF 3′UTR and the functional
binding sites in the MIF 3′UTR. MIF-3′UTR-Wt and MIF-3′UTR-Mut
(GenePharma) were produced by subcloning the full-length 3′UTR
fragments of the MIF gene and its mutant into the miR-608 binding
sites into a pmirGlo Dual-Luciferase miRNA target expression vector
(Promega, Madison, WI, USA). HEK 293T cells were seeded in 96-well
plates. After incubation for 24 h at 37°C, the cells were
co-transfected with MIF-3′UTR-Wt (MIF-3′UTR-Mut) and miR-608
(miR-608 NC). HEK 293T cells were divided into five groups as
follows: empty vector group, miR-608 NC + MIF-3′UTR-Wt group,
miR-608 + MIF-3′UTR-Wt group, miR-608 NC + MIF-3′UTR-Mut group,
miR-608 + MIF-3′UTR-Mut group. After transfection for 48 h,
luciferase assays were performed using the Dual-Luciferase Reporter
Assay system (Promega) and the firefly luciferase activity was
normalized to Renilla luciferase activity.
Western blot assessment
Western blot analysis was used to detect the protein
expression of MIF, PI3K, p-PI3K, AKT, p-AKT, JNK, and p-JNK in the
GSCs. Total protein was extracted using RIPA buffer supplemented
with protease inhibitors and phosphatase inhibitor. The protein
concentration was determined using the BCA protein assay kit. Equal
amounts of proteins were separated on 10 to 15% sodium dodecyl
sulphate-polyacrylamide gels. The separated proteins were
electrophoretically transferred to nitrocellulose, and then the
membranes were blocked with 5% nonfat milk in Tris-buffered
saline-Tween-20 (TBST) overnight. The membranes were then incubated
with the primary antibody for 2 h, respectively as follows: MIF
(rabbit, polyclonal, 1:500; BoAoSen, Beijing, China); JNK, p-JNK,
PI3K, p-PI3K, AKT, p-AKT (rabbit, monoclonal, 1:1,000; Cell
Signaling Technology, Danvers, MA, USA). After the protein was
incubated with the secondary antibody conjugated with horseradish
peroxidase for 2 h at room temperature, the immunoreactive bands
were visualized by enhanced chemiluminescence (ECL kit; Santa Cruz
Biotechnology, USA) and scanned with Chemi Imager 5500 V2.03
software. The relative integrated density values (IDVs) were
calculated using a computerized image analysis system (Fluor Chen
2.0) and normalized with that of GAPDH.
Statistical analysis
All data are presented as the means ± standard
deviation (SD). Differences among multiple groups were analyzed
using one-way ANOVA. Two sample t-tests were applied to test
differences between groups. Statistical significance was assumed at
a p-value <0.05.
Results
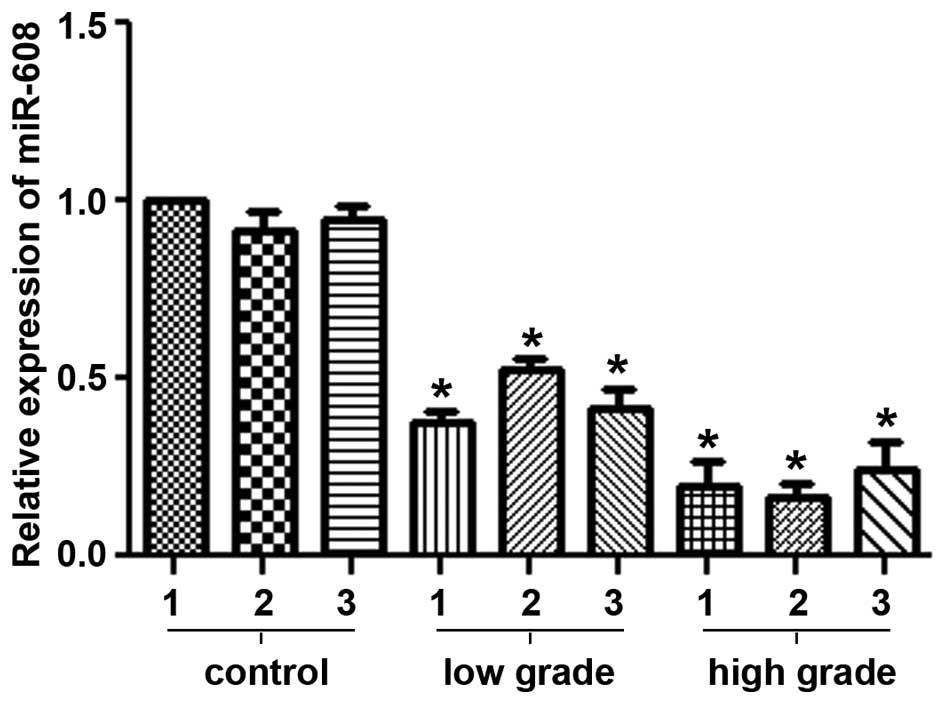
Downregulation of miR-608 expression is
detected in glioblastoma tissues
The expression levels of miR-608 mRNA were analyzed
in low-grade and high-grade glioma tissues by qRT-PCR. As shown in
Fig. 1, miR-608 mRNA expression was
significantly downregulated in glioblastoma tissues compared with
the average expression levels in the control brain tissues
(P<0.05). Moreover, the expression levels declined with the
increasing pathological grades of the glioblastoma samples.
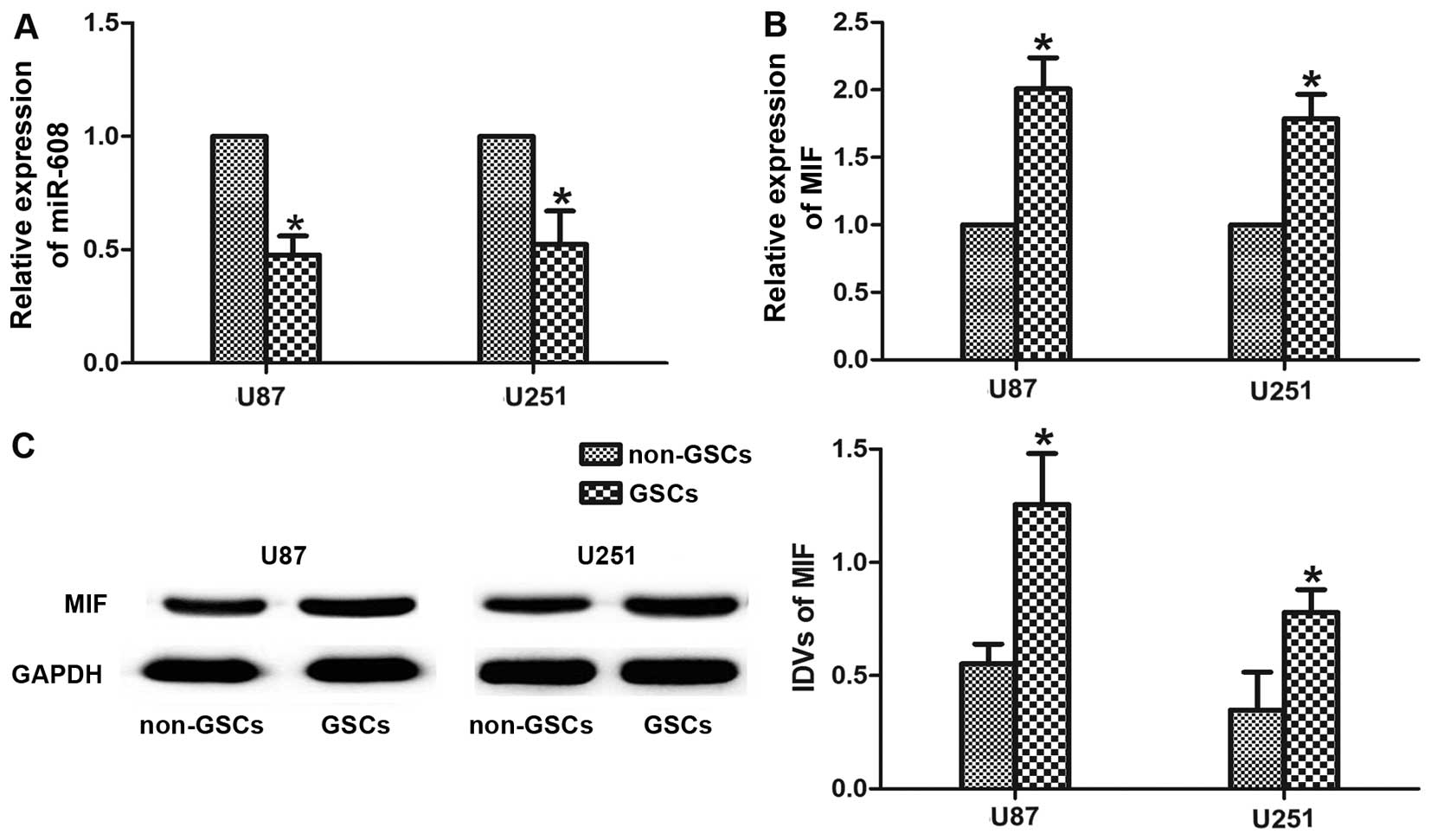
Low expression of miR-608 and high
expression of MIF are verified in GSCs
The endogenous expression levels of miR-608 and MIF
were evaluated in GSCs-U87 and GSCs-U251 using real-time PCR
analysis. As shown in Fig. 2A and
B, both miR-608 and MIF were expressed in malignant cells from
the two types of glioma. The expression levels of miR-608 were
significantly lower in the GSCs-U87 and GSCs-U251 than levels in
non-GSCs-U87 and non-GSCs-U251 (P<0.05). By contrast, the
expression level of the MIF gene was signifi-cantly increased in
the GSCs (P<0.05). Western blot analysis revealed that the
expression level of MIF protein also showed an increase in the
GSCs-U87 and GSCs-U251 compared with the level in the non-GSCs
(P<0.05, Fig. 2C).
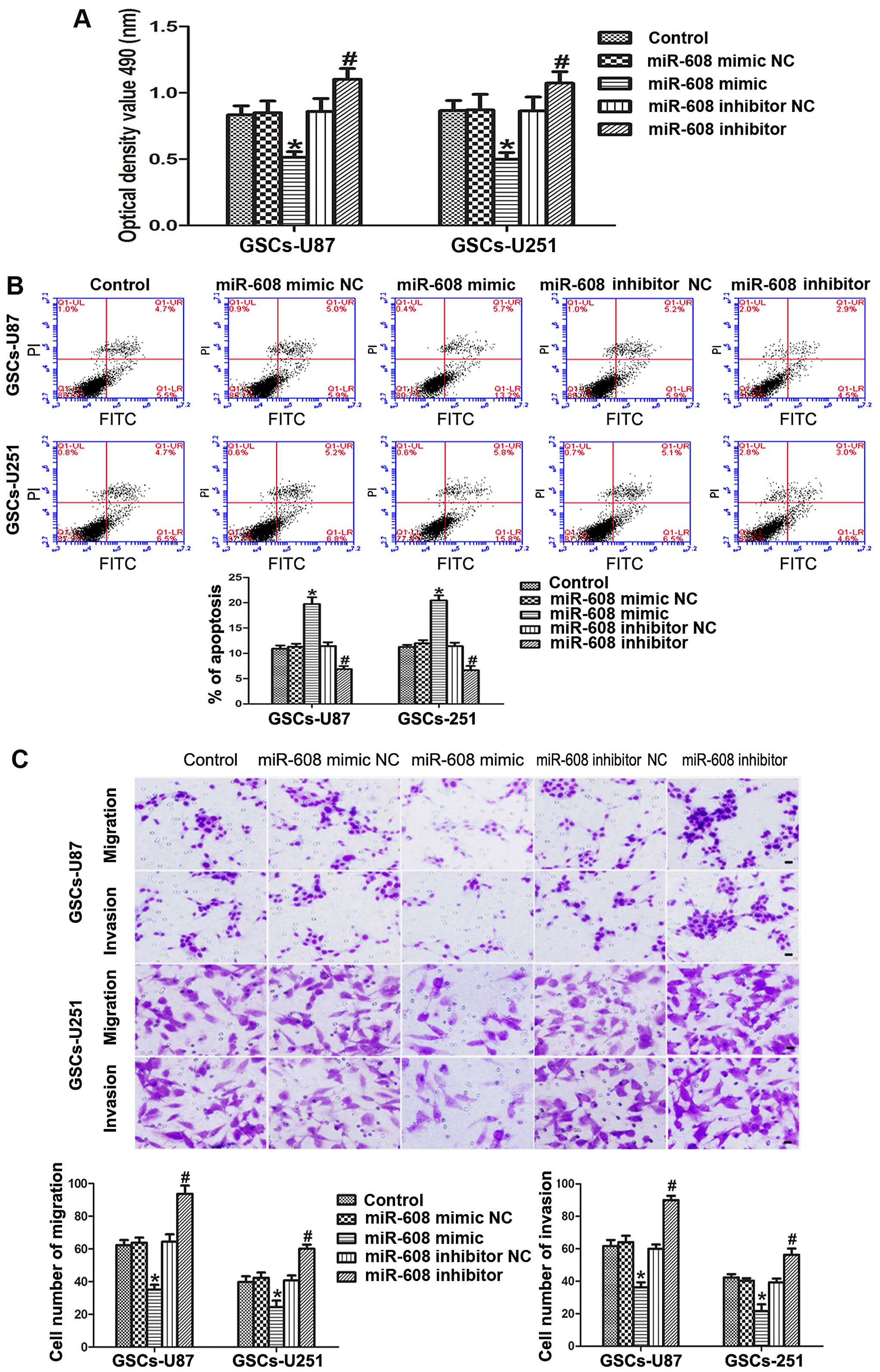
miR-608 overexpression inhibits the
proliferation, migration and invasion, and induces the apoptosis of
the GSCs
We explored the role of miR-608 in the malignant
biological behaviors of human GSCs isolated from U87 and U251 cell
lines via diversified functional analysis methods. MTT assays
showed that the cell proliferation ability of the GSCs-U87 and
GSCs-U251 transfected with the miR-608 mimic was signifi-cantly
decreased compared with that noted in the miR-608 mimic NC group
(P<0.05). In contrast, miR-608 inhibition increased the
proliferation of the GSCs compared with that noted in the miR-608
inhibitor NC group (P<0.05, Fig.
3A).
As shown in Fig. 3B,
the overexpression of miR-608 induced a prominent increased
apoptosis in the GSCs, and the cells transfected with the miR-608
inhibitor showed a decreased apoptosis ability compared with that
noted in the miR-608 inhibitor NC group (P<0.05). Furthermore,
miR-608 to a large extent altered the migration and invasion
abilities of the GSCs. The results of the cell migration and
invasion assays revealed that miR-608 overexpression significantly
attenuated the migration and invasion of the GSCs-U87 or GSCs-U251
compared with these abilities noted in the miR-608 mimic NC group,
thus the migration and invasion abilities were strengthened in the
GSCs-U87 and GSCs-U251 cells transfected with the miR-608 inhibitor
(P<0.05, Fig. 3C). All these
results indicate that miR-608 is a type of miRNA that inhibits the
malignant progression of GSCs.
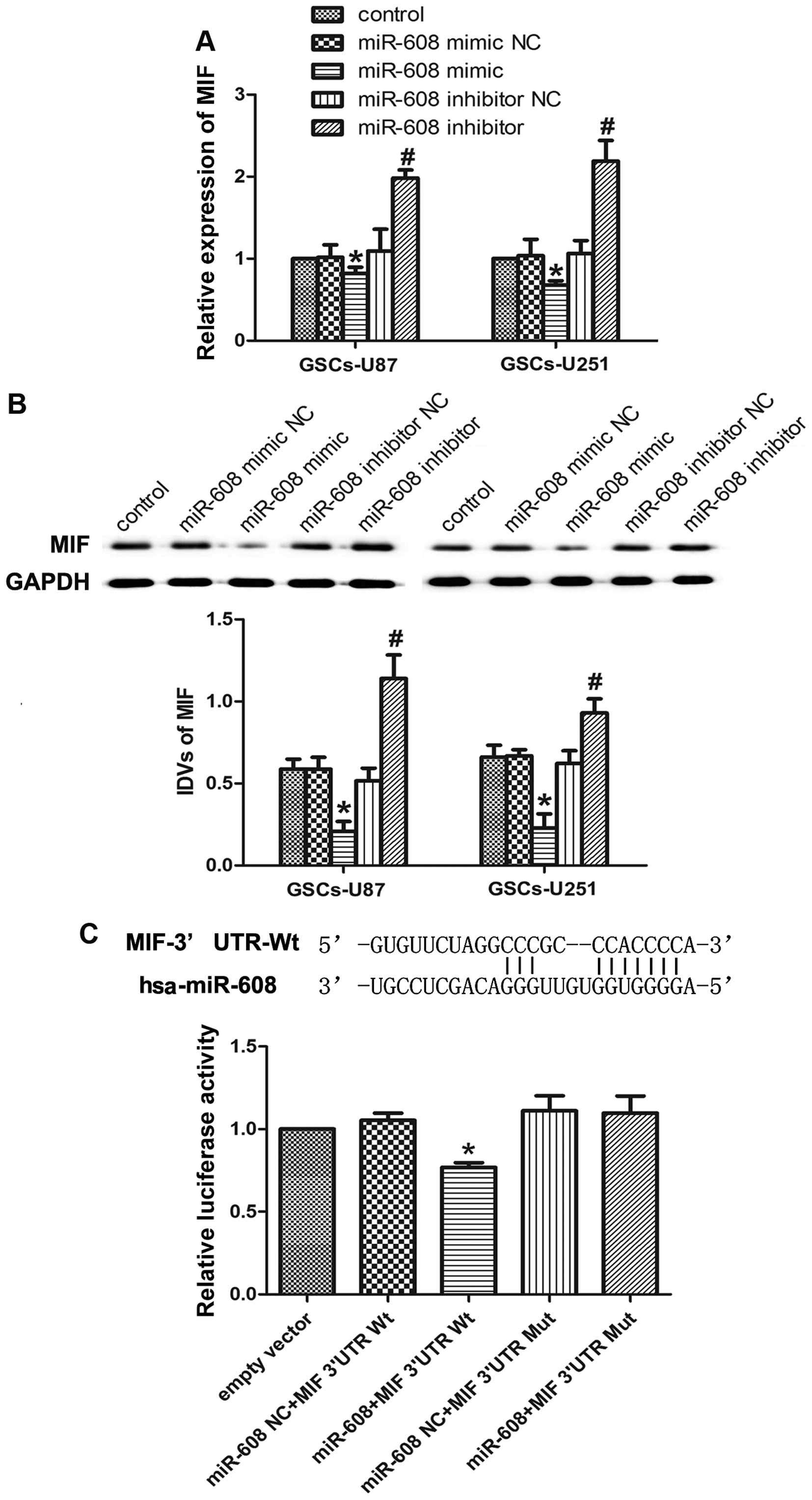
Overexpression of miR-608 inhibits MIF
expression by targeting its 3′UTR
The bioinformatic databases (TargetScan, PicTar,
RNAhybrid) predicted that MIF may be one of the potential mRNA
targets of miR-608. We assessed the effects of miR-608 on the
regulation of MIF in GSCs by transfecting pre-miR-608 or
anti-miR-608. Real-time PCR results showed that the MIF mRNA
expression was significantly decreased in the GSCs-U87 and
GSCs-U251 cells transfected with the miR-608 mimic as compared to
the respective NC, while miR-608 inhibition resulted in a
significant increase in MIF expression in the GSCs (Fig. 4A). The effects of miR-608
overexpression or inhibition on the protein expression levels of
MIF in GSCs were consistent with those of MIF mRNA (Fig. 4B).
MIF was predicted to have one miR-608 binding site
in the 3′UTR using TargetScan 6.2. A dual-luciferase reporter
system was used to determine whether MIF 3′UTR is a direct target
of miR-608. miR-608 mimic and MIF 3′UTR-Wt reporter were
co-transfected into HEK 293T cells and luciferase activity was
measured. The relative luciferase activity in the cells
co-transfected with the miR-608 mimic and MIF 3′UTR-Wt was
significantly attenuated compared with that in miR-608 NC and MIF
3′UTR-Wt group, while co-transfection of the miR-608-NC and MIF
3′UTR-Mut did not change the luciferase activity (Fig. 4C). All these results suggest that
MIF is a direct target of miR-608 with the specific binding site,
and miR-608 negatively regulates the expression of MIF gene and
protein.
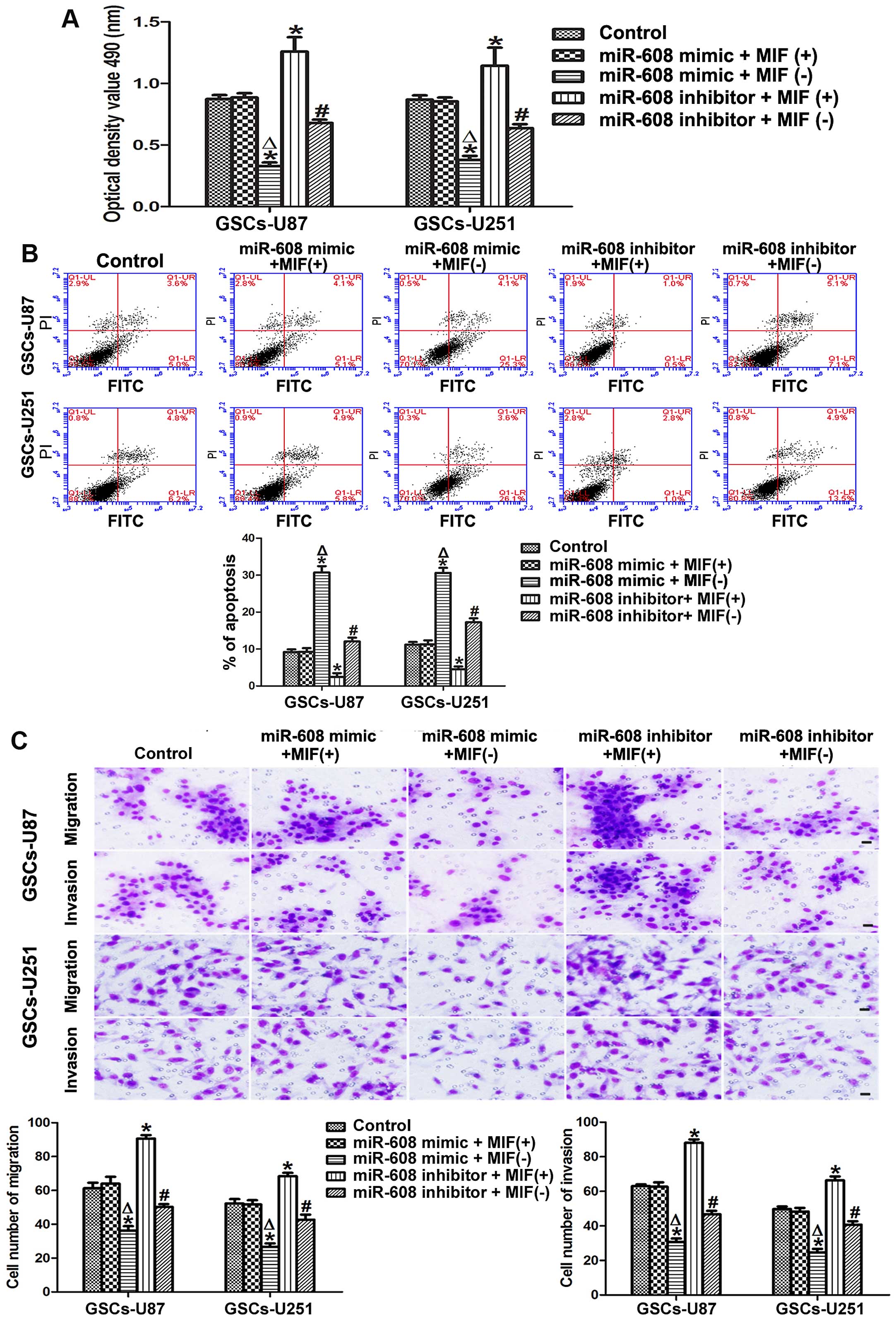
miR-608 overexpression inhibits the cell
proliferation, migration and invasion, and promotes the apoptosis
of GSCs by downregulating MIF
To explore whether miR-608 plays a tumor-suppressive
role by regulating MIF, we assessed the ability of proliferation,
migration, invasion and apoptosis after expression levels of
miR-608 and MIF in the GSCs-U87 and GSCs-U251 cells were altered
simultaneously. miR-608 overexpression combined with MIF inhibition
significantly suppressed the proliferation of the GSCs, while the
miR-608 inhibitor combined with MIF overexpression resulted in
increased proliferation of GSCs in comparison with the control
group. Moreover, the proliferation ability of GSCs with miR-608
overexpression combined with MIF inhibition was significantly
attenuated compared with miR-608 overexpression combined with MIF
overexpression. Similarly, miR-608 inhibition combined with MIF
inhibition resulted in decreased proliferation compared with
miR-608 inhibition combined with MIF overexpression (Fig. 5A). Furthermore, MTT assay data
showed that miR-608 overexpression combined with MIF inhibition led
to a significant increase in the apoptosis rate, while miR-608
inhibition combined with MIF overexpression induced a significant
reduction in apoptosis compared with the control in the GSCs-U87
and GSCs-U251. In addition, MIF inhibition combined with miR-608
overexpression or inhibition resulted in increased apoptosis
compared with MIF overexpression combined with miR-608
overexpression or inhibition groups (Fig. 5B). The migration and invasion data
showed a similar tendency with the MTT results. miR-608
overexpression combined with MIF inhibition attenuated the
migration and invasion of the GSCs-U87 and GSCs-U251, and miR-608
inhibition combined with MIF overexpression increased the migration
and invasion abilities of the GSCs compared with these abilities
noted in the control. Furthermore, MIF inhibition combined with
miR-608 overexpression or inhibition significantly decreased the
migration and invasion abilities of the GSCs compared with these
abilities in the MIF overexpression combined with miR-608
overexpression or inhibition groups (Fig. 5C). These results revealed that
miR-608 plays a tumor-suppressive role by downregulating MIF.
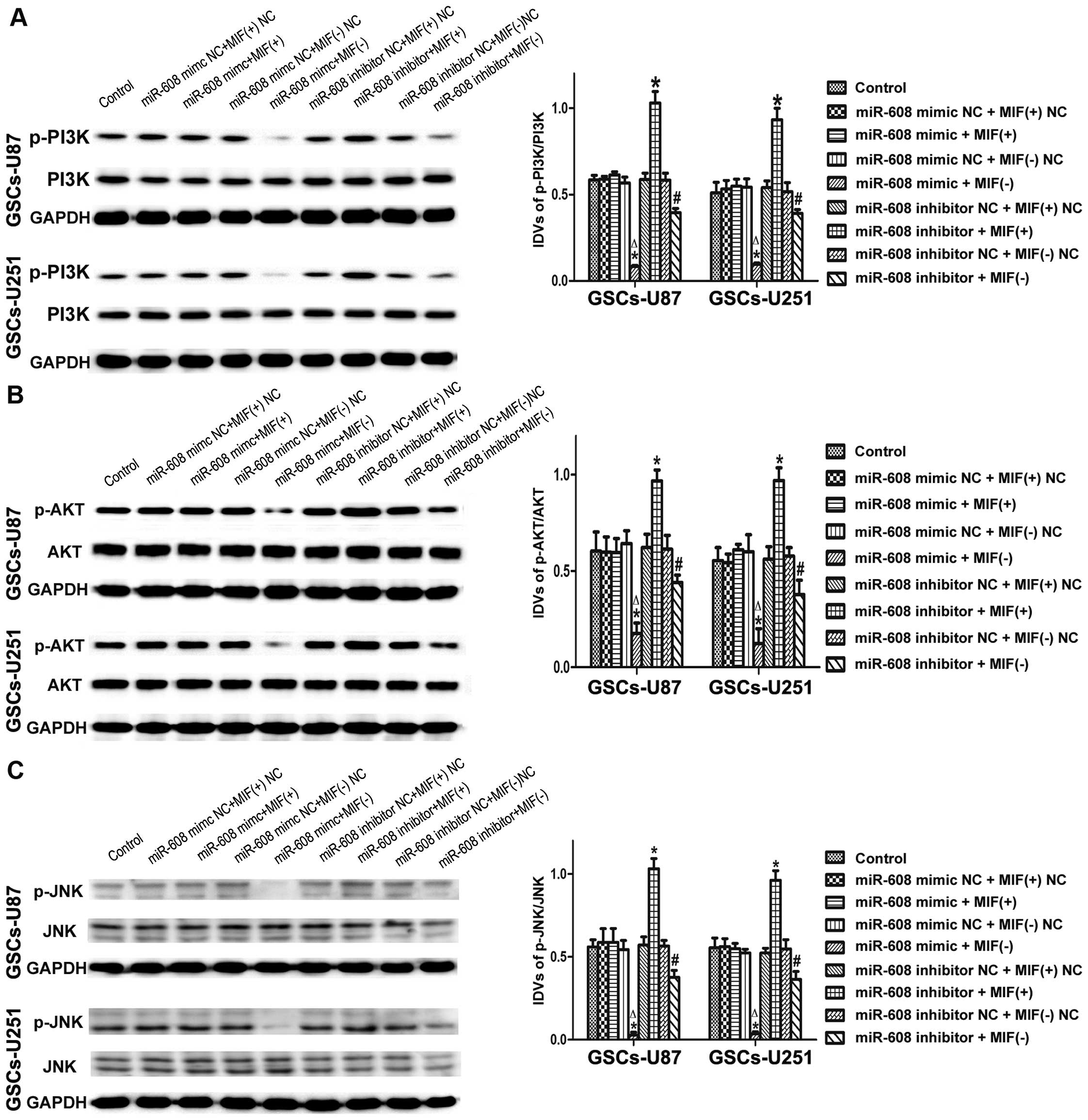
miR-608 overexpression inhibits the
PI3K/AKT and JNK pathways by downregulating MIF in GSCs
To determine whether the PI3K/AKT and JNK pathways
are involved in the tumor-suppressive effects of miR-608 in GSCs,
we detected the protein expression levels of p-PI3K, p-AKT and
p-JNK after the expression levels of miR-608 and MIF in the
GSCs-U87 or GSCs-U251 cells were altered at the same time. As shown
in Fig. 6A, the expression level of
the p-PI3K protein was significantly lower in the miR-608
overexpression combined with MIF inhibition group, and highest in
the miR-608 inhibition combined with MIF overexpression group
compared with the control group. Furthermore, MIF inhibition
combined with miR-608 overexpression or inhibition significantly
inhibited the activation of p-PI3K compared with the MIF
overexpression combined with miR-608 overexpression or inhibition
groups. The expression levels of phosphorylated AKT and JNK were
consistent with the results of p-PI3K in the GSCs-U87 and GSCs-U251
cells as determined by western blotting (Fig. 6B and C). These results suggest that
the PI3K/AKT and JNK pathways may be involved in the
tumor-suppressive effects of miR-608 in GSCs.
Discussion
The present study demonstrated that miR-608
exhibited a low expression level in glioblastoma tissues and GSCs
which were isolated from the U87 and U251 cell lines, and the
overexpression of miR-608 inhibited the proliferation, migration
and invasion and induced the apoptosis of the GSCs. Moreover, the
results of the dual-luciferase reporter assay and bioinformatic
databases showed that MIF is a direct target gene of miR-608, and
miR-608 downregulated the expression level of MIF in the GSCs.
Subsequently, high expression levels of MIF mRNA and protein were
verified in GSCs. Moreover, we found that miR-608 plays a
tumor-suppressive role by targeting MIF, and the PI3K/AKT and JNK
signaling pathways may be involved in this process.
Glioma is a type of prevalent central nervous system
cancer which seriously threatens human health. In particular, GBM
is a fatal malignancy characterized by a highly invasive nature,
devastating recurrence and short survival period (18,19).
To date, the current treatment for glioma can not yield the desired
long-term survival of patients due to rapid tumor growth and
limitation of the blood-brain barrier. Moreover, the high
heterogeneity of glioma in regards to growth, invasive ability,
therapeutic effect, prognosis and so on further exacerbates the
difficulty and complexity of treatment. Moreover, high-level
individualized treatment for glioma has not yet been realized.
Recently, investigators found that there exists cancer stem-like
cells in glioma, to a large extent, contributing to the
tumorigenicity and chemoresistance. These GSCs have self-renewal
capacity and multipotency of differentiation as well as the ability
to initiate tumor formation (20–22).
Elucidation of the detailed molecular mechanisms and related
molecules that regulate GSCs may offer a promising choice for
targeted therapeutic methods. In this study, the qRT-PCR data
showed that the expression of miR-608 was significantly lower in
GSCs compared with non-GSCs. Yet, miR-608 is relatively unexplored
and little is known about its function. In recent years, some
studies have reported the association between a single-nucleotide
polymorphisms (SNPs) in hsa-mir-608 and several cancers including
nasopharyngeal carcinoma, colorectal cancer, breast cancer, and
renal cell carcinoma (23–26). In addition, it has been reported
that miR-608 is downregulated in chordoma cells, acting as a tumor
suppressor (7). Our subsequent
experiments indicated that overexpression of miR-608 inhibits the
proliferation, migration and invasion and promotes the apoptosis of
GSCs, while the knockout of miR-608 led to reverse effects. The
results suggest that downregulation of miR-608 in GSCs is
implicated in the malignant development of glioma.
Zhang et al (7) revealed that EGFR and Bcl-xL are target
genes of miR-608 in chordoma cells, and miR-608 may regulate
chordoma proliferation and invasion by targeting EGFR and Bcl-xL.
To explore the target gene of miR-608 in GSCs, we predicted that
the 3′UTR of human MIF contained binding sites of base positions
with miR-608 using bioinformatic tools. Furthermore, we found that
the expression levels of MIF mRNA and protein were significantly
enhanced in GSCs in comparison to non-GSCs. Actually, it has been
proven that MIF is highly expressed in human glioma tissues and in
a series of glioma cell lines. Moreover, glioma cell-derived MIF
plays a crucial role in tumor progression and angiogenesis as well
as the immune escape of malignant gliomas (8). MIF, as a cytokine, is overexpressed in
many tumors, such as esophageal, colon, prostate cancers and GBM.
It has already been regarded as a promising target molecule for the
treatment of various types of tumors. One study showed that ISO, an
inhibitor of MIF, reduced the cell growth rate and invasive ability
of GBM (27). Our new finding was
that high expression of MIF was observed in GSCs-U87 and GSCs-U251.
The luciferase assay further confirmed that luciferase activities
of 293T cells co-transfected with miR-608 mimic and MIF 3′UTR-Wt
were significantly inhibited, suggesting that there exist binding
sites between miR-608 and MIF which coincided with the predicted
results of the bioinformatic software. Additionally, qRT-PCR and
western blot results also verified that GSCs transfected with the
miR-608 mimic had attenuated expression of MIF mRNA and protein,
while MIF presented a high expression in GSCs transfected with the
miR-608 inhibitor. Taken together, these results reinforce the
notion that MIF is involved in the molecular mechanism of miR-608
in the regulation of the malignancy of GSCs which highly elucidates
the role of MIF in glioma.
To demonstrate whether MIF also plays an oncogenic
role in GSCs, and whether miR-608 could influence the tumor
progression of GSCs via regulating MIF, we constructed a GSC model
co-transfected with miR-608 and MIF. A series of experiments proved
that MIF overexpression combined with miR-608 inhibition
significantly decreased the apoptosis and promoted the
proliferation, migration and invasion of GSCs. By contrast, miR-608
overexpression combined with MIF inhibition led to opposite
results. Therefore, we conclude that GSC-derived MIF, as a
potential target molecule of miR-608 plays an indispensible role in
the malignant progression of GSCs.
Baron et al (27) revealed that MIF may bind to the
CD74/CD44 receptor complex and promote migration, invasion, and
proliferation by activating the ERK1/2 MAPK cascade in GBM tumor
cell lines. Inhibition of PI3K signaling has been thought to be a
potential adjuvant strategy for glioma therapy (10,28).
Our laboratory team reported that endothelial-monocyte activating
polypeptide-II (EMAP-II), an anticancer agent, may inhibit the
activation of PI3K/Akt pathway in GSCs (29). In this study, we found that the
expression levels of p-PI3K and p-AKT protein in the co-transfected
GSCs with miR-608 overexpression combined with MIF inhibition were
significantly lower than those in the control group, while GSCs
with miR-608 inhibition combined with MIF overexpression
significantly activated PI3K/AKT signaling, suggesting that the
PI3K/Akt pathway was mediated by the effects of miR-608 on GSCs via
regulation of MIF. Together with the PI3K/AKT pathway, the role of
the JNK pathway was also investegated in this study. Among the
three major MAP kinase families including extracellular
signal-regulated kinases (ERK), JNK and p38, JNK is thought to be
closely associated with the maintenance of GSCs. And targeting JNK
may be a viable, clinically treatment for depletion of GSCs
(12). Our data showed that MIF
overexpression and miR-608 knockdown could elevate the expression
of p-JNK protein in GSCs, which heightened the findings that the
JNK pathway may play a cancer-promoting role in glioma.
In conclusion, we reported for the first time that
overexpression of miR-608 inhibited the cell proliferation,
migration, invasion, and promoted the apoptosis of GSCs isolated
from the U87 and U251 cell lines by targeting MIF, and the JNK and
PI3K/AKT signaling pathways may be involved in this biological
process. The results indicated that miR-608 could be used as a
potential target miRNA for the treatment of human glioblastoma.
Abbreviations:
|
GSCs
|
glioma stem cells
|
|
miRNA
|
microRNA
|
|
MIF
|
macrophage migration inhibitory
factor
|
|
GBM
|
glioblastoma
|
|
CSCs
|
cancer stem cells
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
JNK
|
Jun N-terminal kinase
|
|
MAP
|
mitogen-activated protein
|
|
HEK
|
human embryonic kidney
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
bFGF
|
basic fibroblast growth factor
|
|
EGF
|
epidermal growth factor
|
|
qRT-PCR
|
real-time quantitative polymerase
chain reaction
|
|
UTR
|
untranslated region
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
DMSO
|
dimethyl sulfoxide
|
|
TBST
|
Tris-buffered saline-Tween-20
|
|
ECL
|
enhanced chemiluminescence
|
|
IDVs
|
integrated density values
|
|
SD
|
standard deviation
|
|
ERK
|
extracellular signal-regulated
kinases
|
Acknowledgments
The present study was funded by the following
grants: contract grant sponsor Natural Science Foundation, China,
contract grant no. 81201800; contract grant sponsor Shenyang
Science and Technology Plan Projects, China, contract grant no.
F12-277-1-68; contract grant sponsor Natural Science Foundation of
Liaoning Province, China, contract grant no. 2015020750.
References
|
1
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lathia JD, Mack SC, Mulkearns-Hubert EE,
Valentim CL and Rich JN: Cancer stem cells in glioblastoma. Genes
Dev. 29:1203–1217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Li Y, Yu TS, McKay RM, Burns DK,
Kernie SG and Parada LF: A restricted cell population propagates
glioblastoma growth after chemotherapy. Nature. 488:522–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seymour T, Nowak A and Kakulas F:
Targeting aggressive cancer stem cells in glioblastoma. Front
Oncol. 5:1592015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu S, Yin F, Zhang J, Wicha MS, Chang AE,
Fan W, Chen L, Fan M and Li Q: Regulatory roles of miRNA in the
human neural stem cell transformation to glioma stem cells. J Cell
Biochem. 115:1368–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Schiff D, Park D and Abounader R:
MicroRNA-608 and microRNA-34a regulate chordoma malignancy by
targeting EGFR, Bcl-xL and MET. PLoS One. 9:e915462014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mittelbronn M, Platten M, Zeiner P,
Dombrowski Y, Frank B, Zachskorn C, Harter PN, Weller M and
Wischhusen J: Macrophage migration inhibitory factor (MIF)
expression in human malignant gliomas contributes to immune escape
and tumour progression. Acta Neuropathol. 122:353–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XB, Tian XY, Li Y, Li B and Li Z:
Elevated expression of macrophage migration inhibitory factor
correlates with tumor recurrence and poor prognosis of patients
with gliomas. J Neurooncol. 106:43–51. 2012. View Article : Google Scholar
|
|
10
|
Ströbele S, Schneider M, Schneele L,
Siegelin MD, Nonnenmacher L, Zhou S, Karpel-Massler G, Westhoff MA,
Halatsch ME and Debatin KM: A potential role for the inhibition of
PI3K signaling in glioblastoma therapy. PLoS One. 10:e01316702015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J and Lin A: Role of JNK activation in
apoptosis: A double-edged sword. Cell Res. 15:36–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda K, Sato A, Okada M, Shibuya K,
Seino S, Suzuki K, Watanabe E, Narita Y, Shibui S, Kayama T, et al:
Targeting JNK for therapeutic depletion of stem-like glioblastoma
cells. Sci Rep. 2:5162012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alapati K, Kesanakurti D, Rao JS and
Dasari VR: uPAR and cathepsin B-mediated compartmentalization of
JNK regulates the migration of glioma-initiating cells. Stem Cell
Res (Amst). 12:716–729. 2014. View Article : Google Scholar
|
|
14
|
Lue H, Dewor M, Leng L, Bucala R and
Bernhagen J: Activation of the JNK signalling pathway by macrophage
migration inhibitory factor (MIF) and dependence on CXCR4 and CD74.
Cell Signal. 23:135–144. 2011. View Article : Google Scholar
|
|
15
|
Yeh TM, Liu SH, Lin KC, Kuo C, Kuo SY,
Huang TY, Yen YR, Wen RK, Chen LC and Fu TF: Dengue virus enhances
thrombomodulin and ICAM-1 expression through the macrophage
migration inhibitory factor induction of the MAPK and PI3K
signaling pathways. PLoS One. 8:e550182013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang FL, Wang P, Liu YH, Liu LB, Liu XB,
Li Z and Xue YX: Topoisomerase I inhibitors, shikonin and
topotecan, inhibit growth and induce apoptosis of glioma cells and
glioma stem cells. PLoS One. 8:e818152013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao Y, Xue Y, Ma J, Shang C, Wang P, Liu
L, Liu W, Li Z, Qu S, Li Z, et al: miR-330-mediated regulation of
SH3GL2 expression enhances malignant behaviors of glioblastoma stem
cells by activating ERK and PI3K/AKT signaling pathways. PLoS One.
9:e950602014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khasraw M and Lassman AB: Advances in the
treatment of malignant gliomas. Curr Oncol Rep. 12:26–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malik B and Nie D: Cancer stem cells and
resistance to chemo and radio therapy. Front Biosci (Elite Ed).
4:2142–2149. 2012. View
Article : Google Scholar
|
|
23
|
Lin J, Horikawa Y, Tamboli P, Clague J,
Wood CG and Wu X: Genetic variations in microRNA-related genes are
associated with survival and recurrence in patients with renal cell
carcinoma. Carcinogenesis. 31:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang AJ, Yu KD, Li J, Fan L and Shao ZM:
Polymorphism rs4919510:C>G in mature sequence of human
microRNA-608 contributes to the risk of HER2-positive breast cancer
but not other subtypes. PLoS One. 7:e352522012. View Article : Google Scholar :
|
|
25
|
Ryan BM, McClary AC, Valeri N, Robinson D,
Paone A, Bowman ED, Robles AI, Croce C and Harris CC: rs4919510 in
hsa-mir-608 is associated with outcome but not risk of colorectal
cancer. PLoS One. 7:e363062012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu F, Yang L, Zhang L, Yang X, Yang R,
Fang W, Wu D, Chen J, Xie C, Huang D, et al: Polymorphism in mature
microRNA-608 sequence is associated with an increased risk of
nasopharyngeal carcinoma. Gene. 565:180–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baron N, Deuster O, Noelker C, Stüer C,
Strik H, Schaller C, Dodel R, Meyer B and Bacher M: Role of
macrophage migration inhibitory factor in primary glioblastoma
multiforme cells. J Neurosci Res. 89:711–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi L, Fei X, Wang Z and You Y: PI3K
inhibitor combined with miR-125b inhibitor sensitize TMZ-induced
anti-glioma stem cancer effects through inactivation of
Wnt/β-catenin signaling pathway. In Vitro Cell Dev Biol Anim.
51:1047–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Liu L, Xue Y, Meng F, Li S, Wang P
and Liu Y: Anti-neoplastic activity of low-dose
endothelial-monocyte activating polypeptide-II results from
defective autophagy and G2/M arrest mediated by PI3K/Akt/FoxO1 axis
in human glioblastoma stem cells. Biochem Pharmacol. 89:477–489.
2014. View Article : Google Scholar : PubMed/NCBI
|