Introduction
Breast cancer is the most common invasive cancer and
is the leading cause of death among females worldwide (1). In patients who are younger than 50
years, chemotherapy increases the survival rate up to 15 years
(10%), but in older women the increase is only 3% (2). However, the long-term side-effects of
chemotherapy substantially affect the quality of life of these
patients (3).
Apoptosis or programmed cell death is an essential
physiological process that plays a critical role in development,
tissue homeostasis and elimination of damaged cells. The
morphological changes of apoptosis are due to the action of
caspases (4). Apoptosis was
initially described by its morphological characteristics, including
cell shrinkage, membrane blebbing, chromatin condensation and
nuclear fragmentation (5).
Biochemical features associated with apoptosis include
internucleosomal cleavage of DNA, phosphatidylserine (PS)
externalization and plasma membrane changes (6).
Three types of cell death have been identified based
on morphological criteria, including type I (apoptosis), type II
(autophagic cell death) and type III (necrosis) cell death. The
autophagic pathway involves the degradation of subcellular
components. This process includes the formation of cytoplasmic
double membrane-bound vacuoles (autophagosomes), which sequester
cytosolic cargo for delivery to the lysosomes (7). The autophagy-related (Atg) proteins
and microtubule-associated protein 1 light chain 3 (LC3) are major
proteins involved in the processes of autophagy (8). The overexpression of the autophagic
signal has been reported in various forms of cell death under
certain experimental conditions resulting in autophagy-dependent
cell death (9).
Goniothalamin, a plant bioactive styrly-lactone
found in the family Annonaceae, has been mainly isolated from the
genus Goniothalamus (10).
In the present study, we used goniothalamin extracted from
Goniothalamus macrophyllus that is found in the Southern
part of Thailand and is known by the local name, 'Ching Dok Diao'
or 'Rajchakru' (11). Goniothalamin
has been shown to exhibit antimicrobial, antifungal (12) and insecticidal activity (13). Indeed, it was reported that
goniothalamin inhibited cell proliferation and induced cytotoxicity
in a variety of cancer cells such as cervical (14), gastric, kidney (15), leukemia (16), ovarian, melanoma, colon (17), liver (18), lung (19) and breast (20) cancer cells. Moreover, goniothalamin
has been shown to possess anticancer and apoptosis-inducing
properties in several types of cancer. However, the effects of
goniothalamin on human HER2-overexpressing breast cancer, which
grows and spreads more rapidly than other breast cancer types, have
not yet been studied. Therefore, we aimed to verify the hypothesis
that goniothalamin could inhibit the growth of the human breast
cancer SK-BR-3 cell line through induction of apoptosis.
Our study demonstrated that goniothalamin increased
the levels of cleaved-caspase-7 and -9 and cleaved PARP, decreased
Bcl-2 expression and increased the Bax/Bcl-2 ratio. JC-1 staining
revealed that goniothalamin induced mitochondrial transmembrane
dysfunction. The results also showed that goniothalamin
downregulated levels of phosphorylated ERK1/2 and phosphorylated
Akt. Moreover, goniothalamin induced apoptosis through upregulation
of phosphorylated JNK1/2 and p38 in the SK-BR-3 cells. In addition,
goniothalmin induced autophagy through upregulation of Atg7,
Atg12-5 conjugation and LC3II in the SK-BR-3 cells. Our results
demonstrated that goniothalamin induced apoptosis through MAPK
signaling associated with autophagy induction in the SK-BR-3
cells.
Materials and methods
Materials
RPMI-1640 medium was purchased from Gibco (Grand
Island, NY, USA). Hoechst 33342, fetal bovine serum (FBS),
3-(4,5-dimethylthaiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT),
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imida-carbocyanine
iodide (JC-1) and phenylmethylsulphonyl fluoride (PMSF) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl
sulfoxide (DMSO) was purchased from Merck Calbiochem (San Diego,
CA, USA). Guava Cell Cycle® reagent for cell cycle
analysis was purchased from Merck Millipore Corp., Merck KGaA
(Darmstadt, Germany). MEK1/2 inhibitor (U0126) was purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Goniothalamin
was obtained from Associate Professor Wilawan Mahabusarakam,
Faculty of Science, Prince of Songkla University, Thailand in
purified powder form. It was extracted from the stems of
Goniothalamus macrophyllus which was collected from Songkhla
Province Thailand in September, 2007. Identification was made by Mr
Ponlawat Pattarakulpisutti, Department of Biology, Faculty of
Science, Prince of Songkla University. Goniothalamin was dissolved
and diluted in DMSO at the desired concentration for the
assays.
Cell culture
Breast cancer cell line SK-BR-3 was obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA). The
cells were maintained as a monolayer in RPMI-1640 medium
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin (PAA Laboratories, Pasching, Austria). The cells were
cultured in 5% CO2 at 37°C, and after reaching ~90%
confluency, the cells were subcultured and the medium was replaced
2–3 times/week.
Cell proliferation and cell viability
assays
The cytotoxicity of goniothalamin was determined by
cell proliferation analysis using MTT assay. The cells were seeded
in a 96-well plate (5×103 cells/well) and allowed to
grow for 24 h. The cells were then treated with goniothalamin at
various concentrations, whereas the control group was treated with
DMSO. After incubation for 24 h, 100 μl of 0.5 mg/ml MTT
solution was added to each well, and the plate was further
incubated for 2 h at 37°C. The supernatant was removed, and 100
μl of DMSO was added to each well to solubilize the water
insoluble purple formazan crystals. The absorbance was measured
using a microplate reader at 570 nm (Multiskan EX; Thermo Electron
Corp., Vantaa, Finland), and the IC50 value was
calculated using the software GraphPad Prism 3.03 (GraphPad
Software, Inc., San Diego, CA, USA).
The effect of goniothalamin on cell viability at
different times and doses was determined. The cells were treated
with goniothalamin at various concentrations of 5, 10, 15 and 20
μg/ml, whereas the control group was treated with DMSO.
After incubation for 3, 6, 9, 12 and 24 h, cell viability was
determined by the MTT assay. Survival percentage (%) of the cells
was calculated relative to the control. Cell viability was assessed
in three independent experiments.
Nuclear morphological staining with
Hoechst 33342
SK-BR-3 cells were seeded at 3×105/35-mm
dish for 24 h. The cells were treated with 20 μg/ml
goniothalamin for 3, 6, 9 and 12 h. As control, the cells were
treated with 0.02% DMSO for 24 h. Subsequently, the cells were
stained with 10 μM Hoechst 33342 for 30 min at 37°C and
examined under a fluorescence microscope (IX73; Olympus, Tokyo,
Japan).
Cell cycle analysis
To examine apoptosis, the SK-BR-3 cells were treated
with 20 μg/ml goniothalamin. The cells were harvested after
drug treatment and washed with phosphate-buffered saline (PBS). The
cells were fixed with 70% ethanol at 4°C for >1 h. Then, the
cells were stained according to the manufacturer's instructions
(Guava Cell Cycle® reagent from Merck Millipore Corp.,
Merck KGaA. The DNA content was observed using the Guava EasyCyte™
flow cytometer and GuavaSoft™ software (Merck Millipore Corp.,
Merck KGaA).
Measurement of mitochondrial membrane
potential (ΔΨm)
The ΔΨm was determined using the potential sensitive
dye JC-1, which is a lipophilic cation that is incorporated into
the mitochondrial membrane. The cells were seeded at
3×105/35-mm dish for 24 h and treated with 20
μg/ml goniothalamin for 3, 6 and 9 h, and the control cells
were treated with 0.02% DMSO. The cells were then stained with 5
μg/ml of JC-1 in the dark at 37°C for 15 min before analysis
by fluorescence microscopy (IX73).
Western blot analysis
The SK-BR-3 cells were seeded at
3×105/35-mm dish for 24 h, and treated with 20
μg/ml goniothalamin, and harvested at designated time
points. Then, the cells were lysed with RIPA lysis buffer (50 mM
Tris-HCL, pH 7.5, 5 mM EDTA, 250 mM NaCl, 0.5% Triton X-100)
supplemented with 10 mM PMSF and Complete Mini Protease Inhibitor
Cocktail (Roche Diagnostics GmbH, Mannheim, Germany). The
supernatants were prepared by centrifugation, and the protein
content was determined using a protein assay kit (Bio-Rad
Laboratories, USA). The total protein extracts were separated by
8–12% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF)
membranes (GE Healthcare, Buckinghamshire, UK) for 1–2 h at 100 V
using a Mini Trans-Blot Cell (Bio-Rad Laboratories). The membranes
were blocked with 5% non-fat dry milk in Tris-buffered saline and
Tween-20 (TBST) (10 mM Tris, pH 7.5, 150 mM NaCl and 0.1% Tween-20)
for 1 h at room temperature and incubated overnight at 4°C with the
primary antibody (Cell Signaling Technology, Beverly, MA, USA).
Membranes were washed three times in TBST buffer, followed by
incubation for 1 h at room temperature with the corresponding
HRP-linked secondary antibodies. The specific protein bands were
detected by chemiluminescent HRP substrate (Merck Millipore Corp.,
Merck KGaA) and detected under a chemiluminescent imaging system
(GeneGnome Gel Documentation; Synoptics Ltd., Cambridge, UK).
Statistical analysis
All data presented were obtained from at least three
independent experiments and are presented as mean ± standard
deviation (SD). Statistical significance was assessed by one-way
analysis of variance (ANOVA). Statistical analysis was performed
using SPSS statistical software package (version 11.5) also carried
out using the software GraphPad Prism 3.03 (GraphPad Software,
Inc.). The western blotting band intensity was quantified by ImageJ
densitometer. An asterisk indicates that the experimental values
are significantly different from those of the control
(p<0.05).
Results
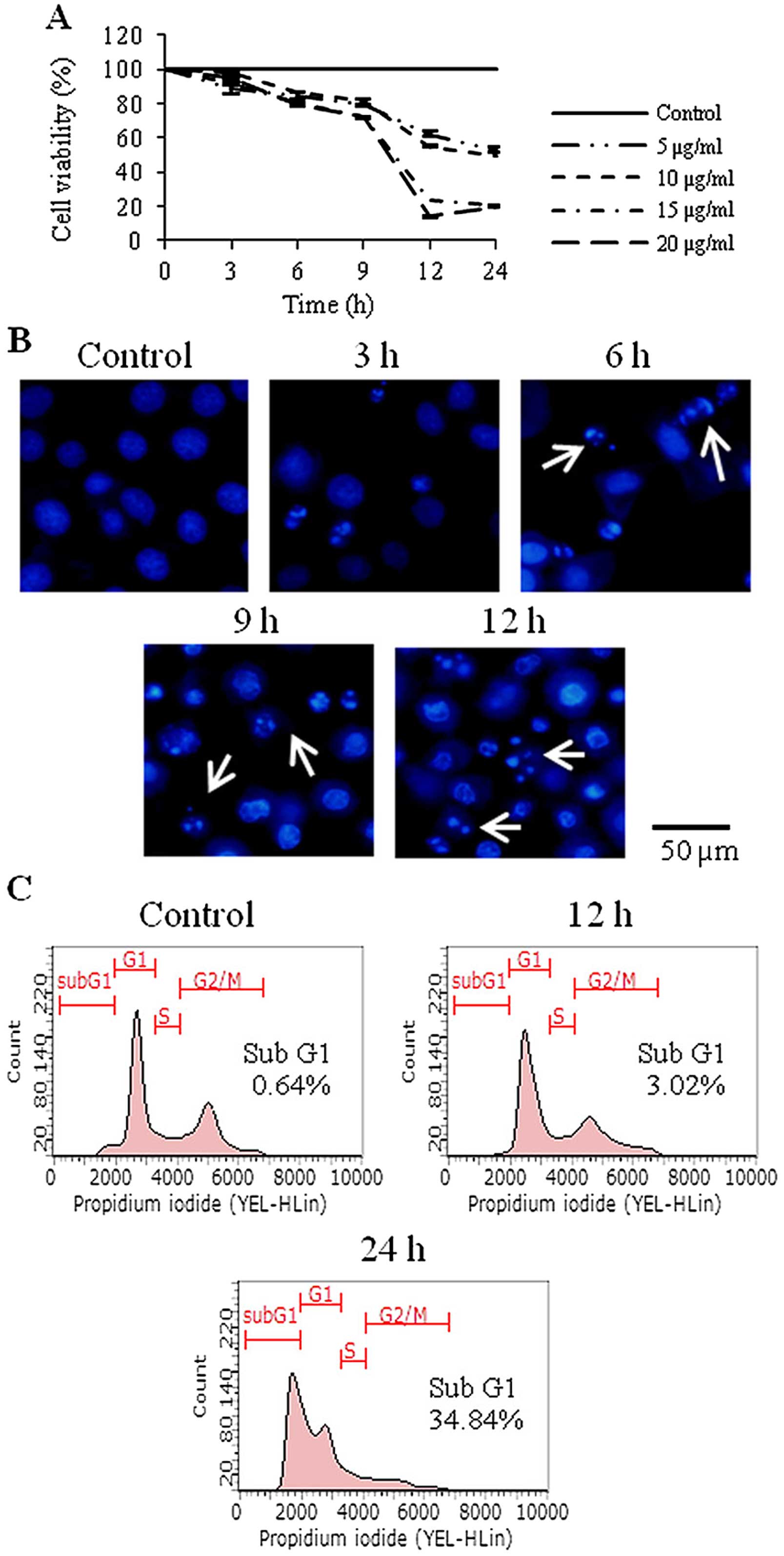
Goniothalamin inhibits cell viability and
induces apoptosis in SK-BR-3 human breast cancer cells
The antiproliferative activity of goniothalamin in
the SK-BR-3 cells was determined by MTT assay. The IC50
value was 10±0.45 μg/ml. Goniothalamin inhibited cell
viability in a dose- and time-dependent manner. Treatment of
SK-BR-3 cells with 20 μg/ml goniothalamin for 12 h reduced
cell viability to ~20% comparing with that noted in the control
cells (Fig. 1A).
To determine the antiproliferation and cell death
induction mediated by goniothalamin, Hoechst 33342 staining was
carried out. The results showed that goniothalamin induced
chromatin condensation and DNA fragmentation, characteristics of
apoptotic cells (Fig. 1B).
Effect of goniothalamin on cell cycle
distribution
The effect of goniothalamin on the cell cycle showed
that goniothalamin alone did not increase the sub-G1 population. In
contrast, the goniothalamin-treated cells showed an increase in the
sub-G1 population to 3.02 and 34.84% after treatment with 20
μg/ml goniothalamin for 12 and 24 h, respectively (Fig. 1C).
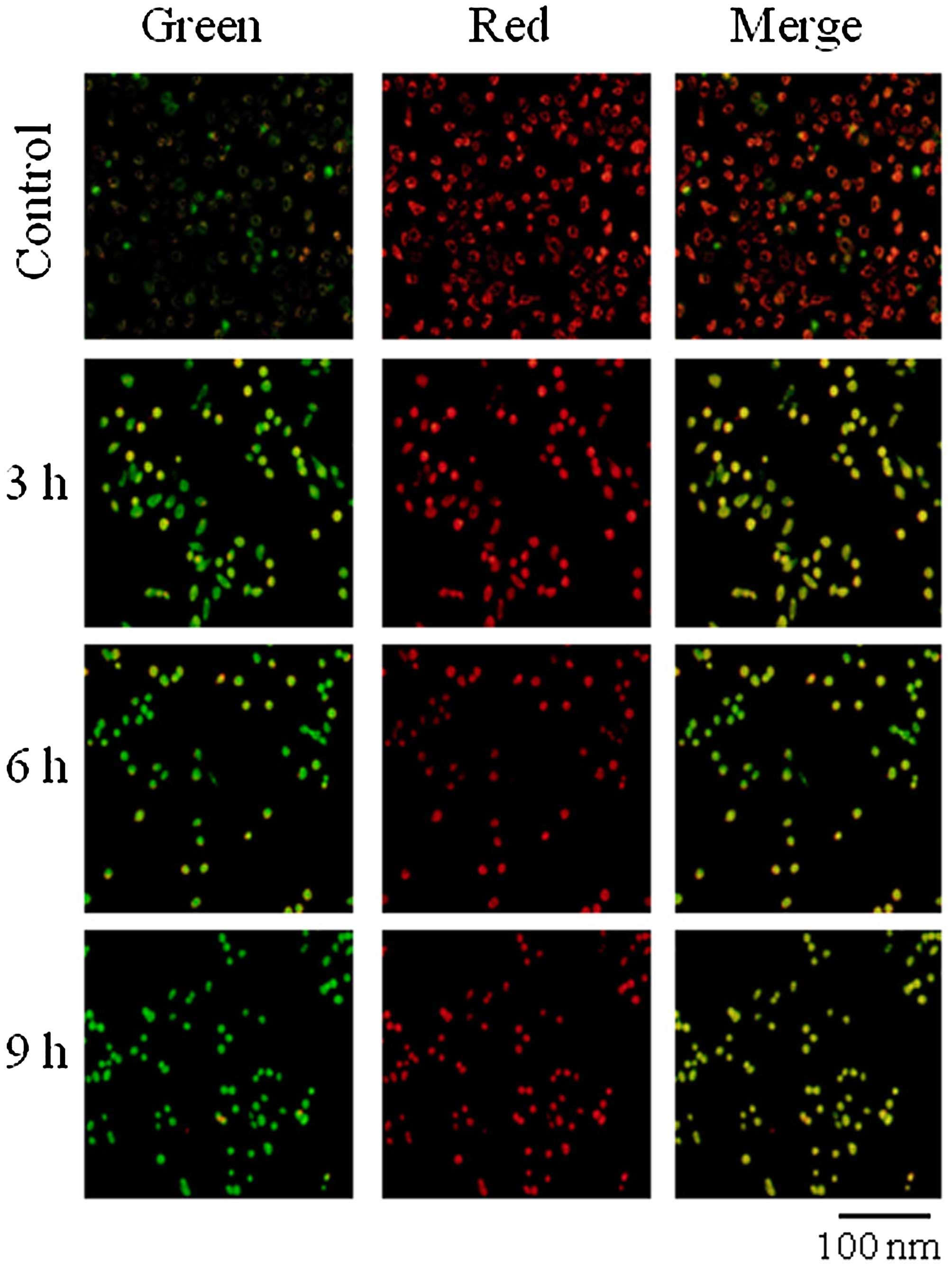
Effect of goniothalamin on ΔΨm
During apoptosis, several key events occur in the
mitochondria. Bax forms oligomers on the mitochondrial membrane
leading to changes in electron transport and loss of ΔΨm. JC-1 is a
cytofluorimetric, lipophilic cationic dye that can selectively
enter into mitochondria and reversibly change color from green to
red as the membrane potential increases. In healthy cells with high
ΔΨm, JC-1 spontaneously forms complexes known as J-aggregates with
intense red fluorescence. In contrast, in apoptotic or unhealthy
cells with low ΔΨm, JC-1 remains in the monomeric form, which shows
only green fluorescence. The ratio of green to red fluorescence is
dependent only on the ΔΨm. The results showed that SK-BR-3 cells
treated with goniothalamin for 3, 6 and 9 h had an increased
green/red ratio, while the control cells showed red fluorescence
(Fig. 2) indicating that
goniothalamin induced the loss of ΔΨm in the SK-BR-3 cells.
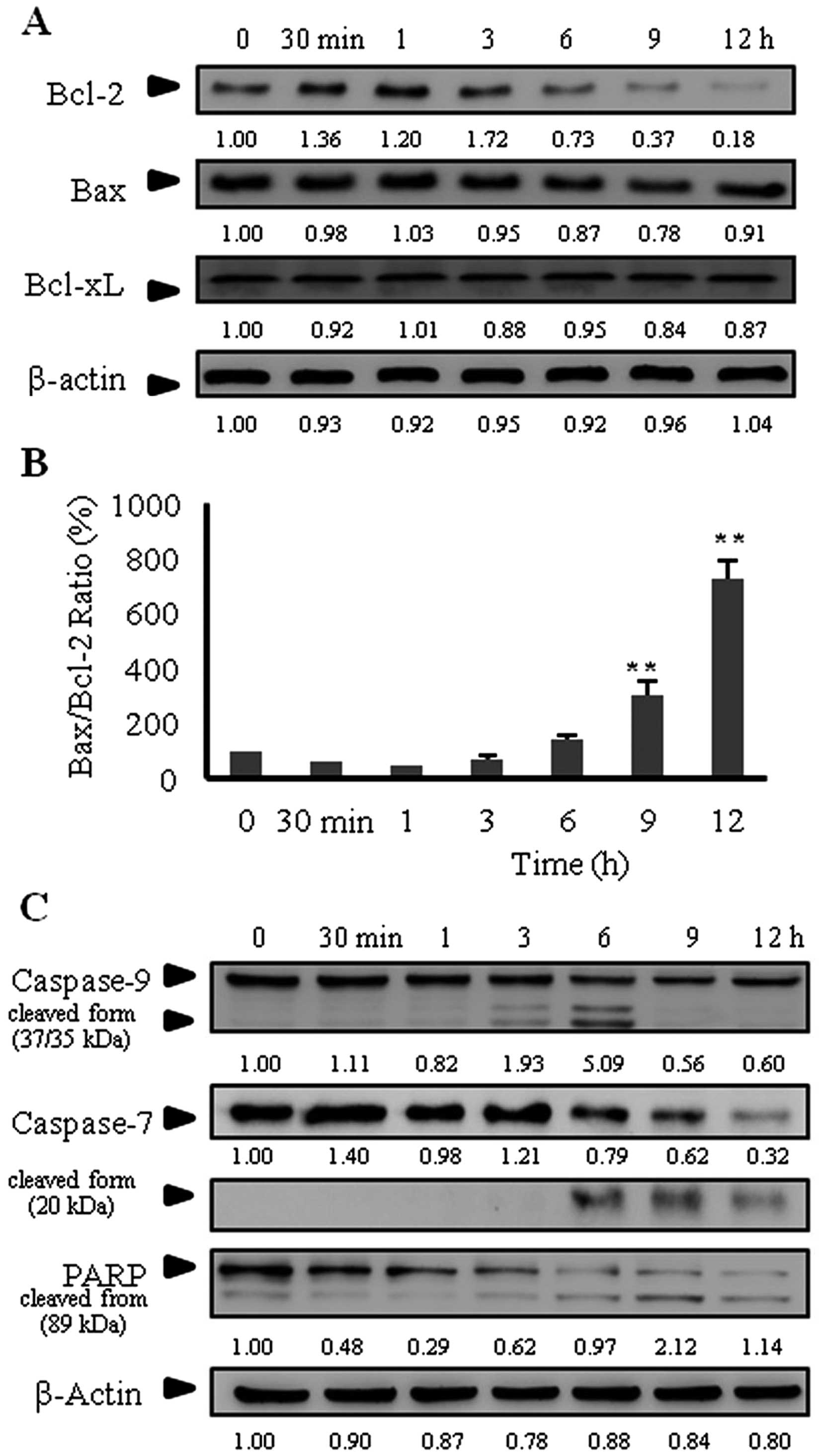
Effect of goniothalamin on the expression
of Bcl-2 family proteins, caspase-7 and -9, and cleaved PARP
activation
The Bcl-2 family proteins have expanded
significantly and are composed of both pro-apoptotic and
anti-apoptotic molecules. To determine whether goniothalamin
induces apoptosis through the mitochondrial apoptotic pathway, we
examined the expression of the Bcl-2 family proteins. As shown in
Fig. 3A, goniothalamin decreased
Bcl-2 expression at 6 h, while it increased the Bax/Bcl-2 ratio at
9 h (Fig. 3B). These results
indicate that goniothalamin induced apoptosis through the
mitochondrial pathway.
Caspase expression was also determined by western
blot analysis. The results showed that goniothalamin induced
caspase-9 and -7 cleavage after 3 and 6 h of treatment (Fig. 3C). The maximal induction of cleaved
caspase-9 was at 6 h, and then was markedly decreased at 9 and 12
h. In addition, cleaved caspase-7 was maximally activated at 6 h
and was decreased at 9 and 12 h, which was correlated with the
expression of the proform. Ferguson et al showed that
apoptosis induction in MCF-7 was independent of caspase-9
expression. Caspase-7 expression was not correlated with caspase-9
expression for apoptosis induction in MCF-7 cells (21). Hakem et al also showed that
caspase-9 deficiency could not protect cells from apoptosis induced
by α-CD95 and could not protect caspase-3 activation in vivo
(22). Moreover, goniothalamin
induced cleaved PARP activation downstream of caspase. The results
indicated that goniothalamin induced apoptosis mediated by caspases
and PARP through the intrinsic apoptosis pathway (via
caspase-9).
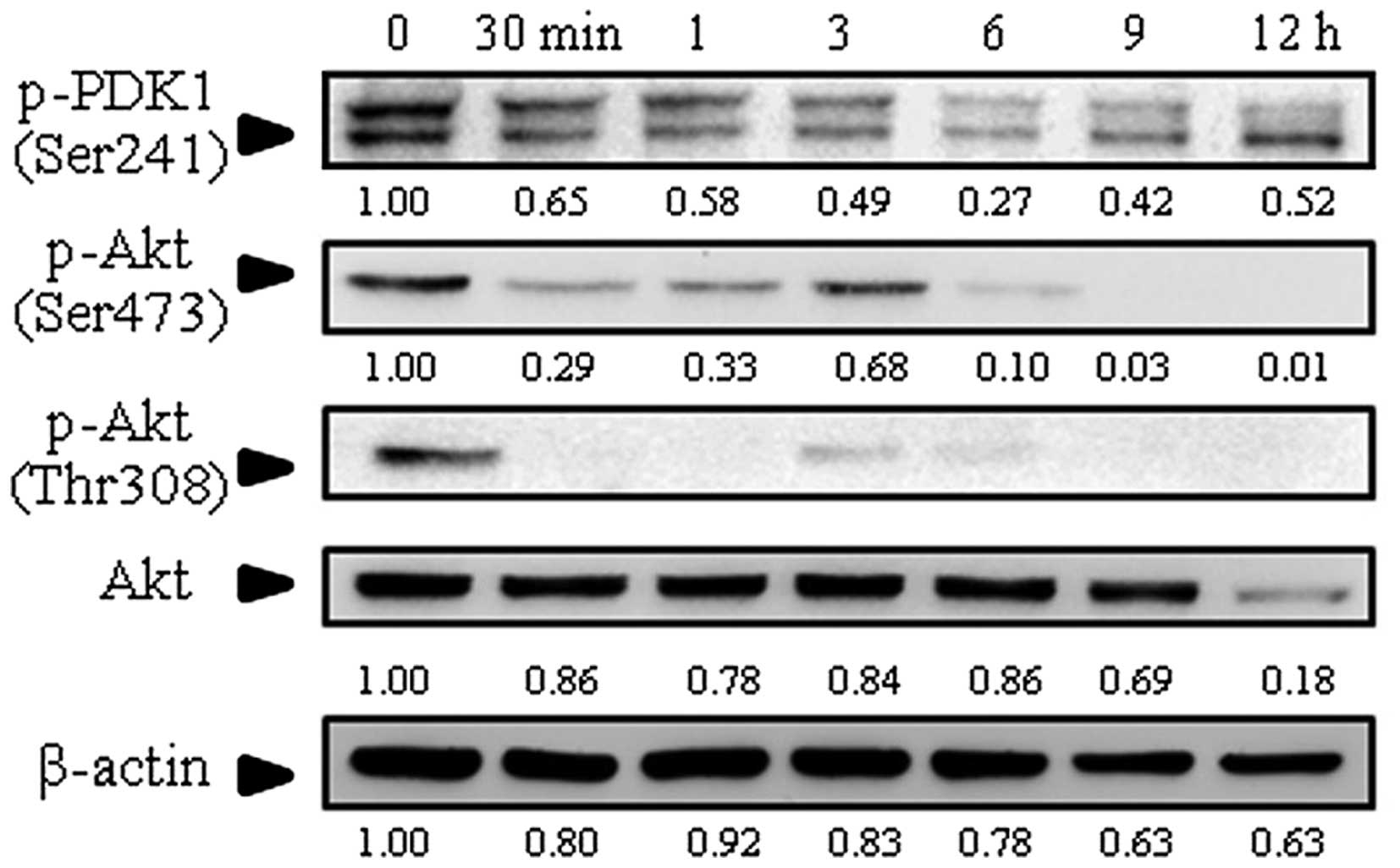
Effect of goniothalamin on expression of
Akt
Akt plays a key role in multiple cellular processes
such as cell growth, cell proliferation, transcription and cell
migration. PDK activates Akt by phosphorylation at Thr308 and
Ser473. The results showed that levels of phosphorylated Akt
(p-Akt) at Thr308 and Ser473 were decreased as well as
phosphorylated PDK1 (p-PDK1) at Ser241 (Fig. 4), suggesting that goniothalamin
inhibited cell survival by downregulation of p-Akt expression in
the SK-BR-3 cells.
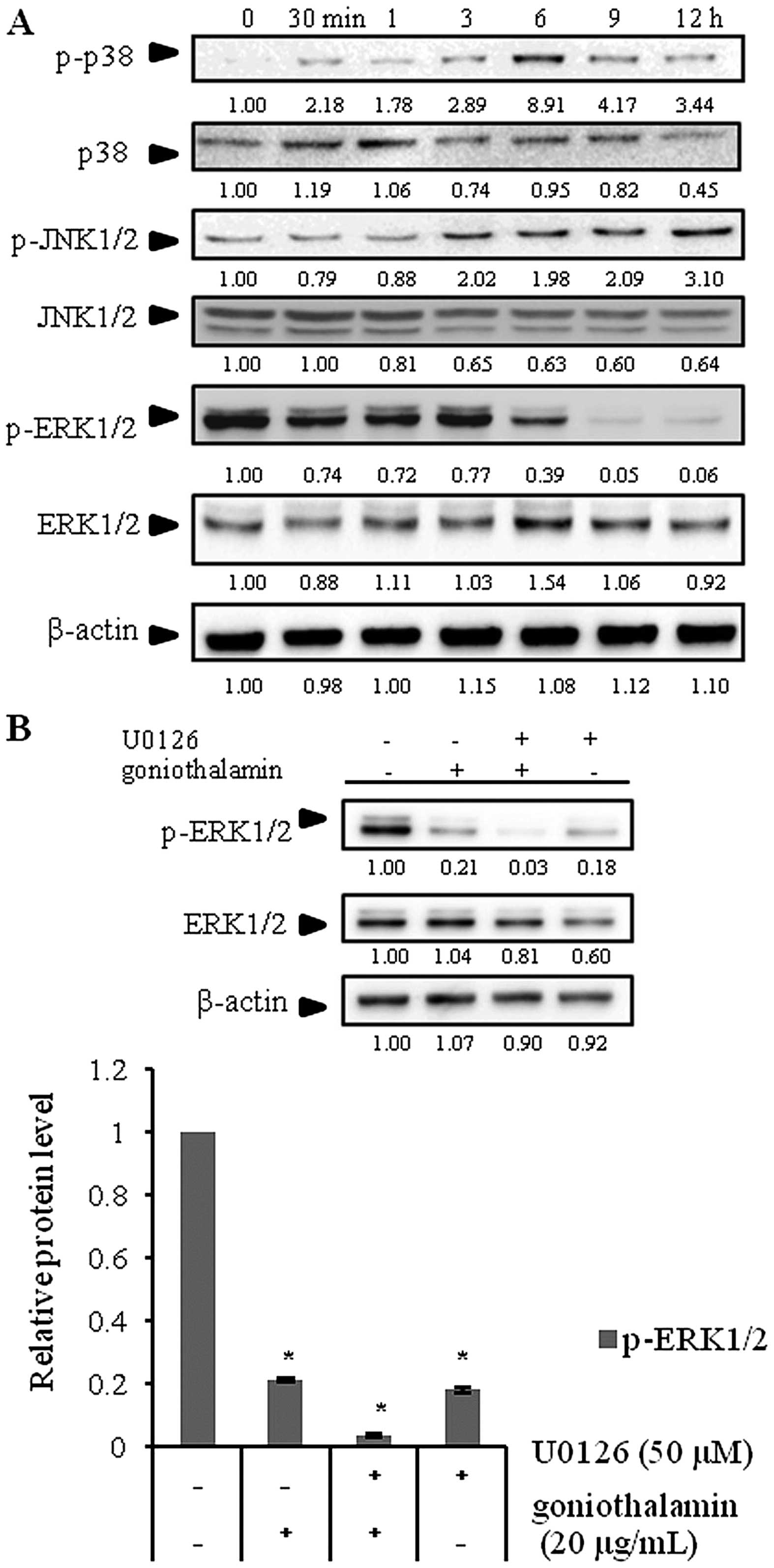
Effect of goniothalamin on expression of
MAPKs
MAPK pathways are evolutionarily conserved kinases,
which link extracellular signals to the machinery that controls
fundamental cellular processes such as growth, proliferation,
differentiation, migration and apoptosis. There is a three-step
kinase module in which a MAPK is activated upon phosphorylation by
a MAPKK, which in turn is activated when phosphorylated by a
MAPKKK. Our results showed an increase in phosphorylated JNK1/2
(p-JNK1/2) and phosphorylated p38 (p-p38) expression, but a
decrease in phosphorylated ERK1/2 (p-ERK1/2) expression in the
SK-BR-3 cells following goniothalamin treatment (Fig. 5A). In addition, a MEK1/2 inhibitor
(U0126) simultaneously blocked p-ERK1/2 indicating that
goniothalamin inhibited cell survival through ERK signaling
(Fig. 5B). These results
demonstrated that goniothalamin induced apoptosis by inhibiting
cell survival through p-ERK1/2 activation.
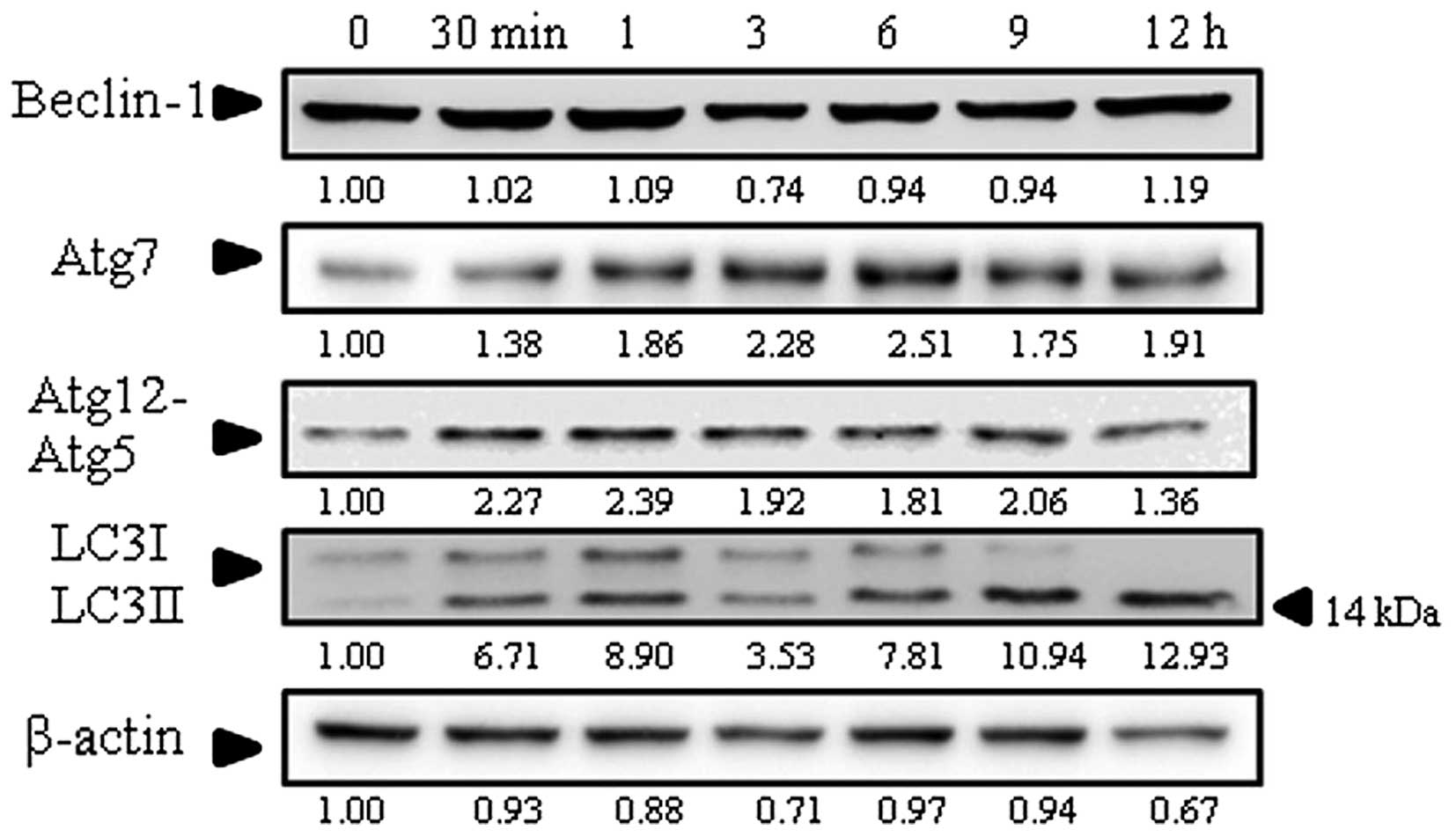
Effect of goniothalamin on autophagy
induction
For autophagy induction, the protein level of LC3II,
which is a protein marker of autophagy, was determined. As shown in
Fig. 6, the LC3II level was
increased in the SK-BR-3 cells treated with goniothalamin. In
addition, the levels of Atg7 and Atg12-Atg5 conjugation were
upregulated while no effect was noted on Beclin-1. These results
demonstrated that goniothalmin induced autophagy in the SK-BR-3
cells through the upregulation of LC3II, Atg7 and Atg12-Atg5
conjugation.
Discussion
Recent research has demonstrated the cytotoxicity
and antitumor properties of goniothalamin against various human
tumor cell lines such as A-549 (lung carcinoma), HL-60
(promyelocytic leukemia) and SGC-7901 (stomach cancer) (23). It also induced apoptosis in cancer
cells such as HeLa (cervical cancer), HT29 (colon cancer) (24), Ca9-22 (oral cancer) (25), HepG2 (hepatoblastoma), and invasive
breast carcinoma MDA-MB-231 cell lines (26). However, the mechanisms of apoptosis
induction in breast cancer SK-BR-3 cells have not yet been
reported.
The results showed that goniothalamin inhibited
SK-BR-3 cell growth in a time- and dose-dependent manner with an
IC50 value of 10±0.45 μg/ml (Fig. 1A). To confirm apoptosis induction,
we investigated characteristic morphological changes including cell
shrinkage, membrane blebbing, chromatin condensation and formation
of apoptotic bodies. Hoechst 33342 staining revealed condensed
chromatin and apoptotic bodies in the SK-BR-3 cells following
treatment with goniothalamin (Fig.
1B). The population of sub-G1 cells indicated apoptotic cell
death (Fig. 1C). Furthermore, the
effect of goniothalamin on the mitochondrial membrane potential
(ΔΨm) dysfunction in SK-BR-3 cells was detected by increased
green/red fluorescence ratio at 3 h (Fig. 2). Loss of the ΔΨm and release of
sequestered pro-apoptotic proteins from the intermembranous space
into the cytosol stimulates apoptosome formation followed by
activation of caspase-9 (27).
It is well-known that the apoptosis signaling
pathway consists of two main pathways: extrinsic and intrinsic
pathways. Our results showed that goniothalamin decreased Bcl-2
expression (Fig. 3A) and increased
the Bax/Bcl-2 ratio (Fig. 3B) in
SK-BR-3 cells. These events suggested that goniothalamin induced
apoptosis through the intrinsic pathway. Inayat-Hussain et
al reported that goniothamin did not alter the level of Bcl-2
expression in Jurkat cells. Then, Bcl-2-overexpressed Jurkat cells
were used to demonstrate the effects of Bcl-2 on cell death by MTT
assay. They found that Bcl-2 overexpression did not protect the
cells from the cytotoxic effects of goniothalamin (28). This discrepancy is likely due to the
different cell types examined, as Jurkat cells (T-lymphocyte) are
suspension cells whereas SK-BR-3 (breast cancer) are adherent
cells. In addition, they possess different receptors on the cell
surface; thus they responded differently. Another study showed that
goniothalamin induced apoptosis via Bcl-2 inactivation by JNK1/2.
JNK1/2 phosphorylated Bcl-2 (Ser70, Ser87 and Thr69) leading to
inactivation of the anti-apoptotic function (29).
The control and regulation of these apoptotic
mitochondrial events occurs through members of the Bcl-2 family
proteins. Anti-apoptotic Bcl-2 proteins exert their activity by
binding to the pro-apoptotic members Bax and Bak, preventing
mitochondrial damage (30). Thus,
decreased Bcl-2 was associated with mitochondrial dysfunction and
led to apoptosis. To confirm whether the apoptosis induction by
goniothalamin was caspase-dependent, we examined the active forms
of caspases by western blot analysis. The effect of goniothalamin
on PARP activity was also determined. The results indicated that
treatment with 20 μg/ml goniothalamin induced cleaved
caspase-9, and -7, and cleaved PARP expression in the SK-BR-3 cells
(Fig. 3C). The initiator caspase-9
causes the activation of the effector caspases (-3, -6 and -7),
which cleave vital substrates including PARP, resulting in cellular
death. PARP plays an important role in DNA repair and the
activation of cellular defense mechanisms against DNA damage. PARP
is inactivated by caspase cleavage via caspase-3 or -7. The size of
the cleaved fragments (89 kDa) can provide insight into which
enzyme is responsible for the cleavage, and can be useful in
determining which cell death pathway has been activated (31). In HeLa cells, goniothalamin was
reported to induce apoptosis through caspase-9 activity (32). Moreover, goniothalamin induced
caspase-3 and cleaved PARP expression in breast cancer MDA-MB-231
cells treated with 30 μM goniothalamin for 48 h (26).
Akt upregulation is likely to be viewed as a
compensatory protective mechanism of cells for escaping death, and
the fully active Akt mediates numerous cellular functions leading
to cell survival (33). Our results
showed that goniothalamin inhibited cell survival via p-Akt
downregulation (Fig. 4), which
corresponded with a previous study that the anticancer mechanism of
RA-V was related to the blockage of PDK1 and Akt interaction
leading to apoptosis induction (34).
The MAPK kinase family plays a critical role in cell
survival and cell death. Conventional MAPKs in mammalian cells
include ERK1/2, JNK1/2 and p38, which are activated through a
specific phosphorylation cascade. Active ERK1/2 phosphorylates
numerous cytoplasmic and nuclear targets, including kinases,
phosphatases, transcription factors and cytoskeletal proteins
(35). It is well-known that ERK1/2
promotes cell survival, while JNK1/2 and p38 induce apoptosis. Dunn
et al reported that the presence of the ERK signaling
pathway depends on the particular cell type, and progresses to
regulate proliferation, differentiation, survival, migration,
angiogenesis and chromatin remodeling (36). ERK1/2 can also promote cell survival
by upregulation of anti-apoptotic molecules via enhancement of
their activities or activation of their transcription. JNK1/2 can
activate transcriptional factors including c-Jun and AP1, whereas
their tumor-suppressive functions are probably related to their
pro-apoptotic activity (37). In
the present study, goniothalamin downregulated p-ERK1/2 at 6 h, but
upregulated p-JNK1/2 and p-p38 at 3 and 6 h, respectively (Fig. 5A). Indeed, we also confirmed that
goniothalamin in combination with U0126, an ERK inhibitor,
suppressed p-ERK1/2 activation (Fig.
5B). These results demonstrated that goniothalamin induced
apoptosis via p-JNK1/2 and p-p38 upregulation and inhibited cell
survival via p-ERK1/2 downregulation.
Notably, goniothalamin showed autophagy induction
through upregulation of Atg7, Atg12-Atg5 conjugation and LC3II
(Fig. 6) in a time- and
dose-dependent manner indicating autophagic cell death associated
with the elevation of autophagosome formation. These results were
supported by a previous study that Atg12-Atg5 conjugation and the
conversion of LC3I to LC3II indicate autophagic activity (38). In addition, another function of
Atg12 is to stimulate mitochondrial apoptosis by inactivating Bcl-2
and Mcl-1 (39). Therefore, our
findings showed that goniothalamin induced apoptosis and autophagy
which are processes of programmed cell death and interplay with
each other (40). The Atg family
and LC3 are key proteins involved in the autophagy signaling
pathway. The conversion of LC3I (16 kDa) to LC3II (14 kDa) is used
as a common indicator of autophagy detection (41). In addition, our results showed that
goniothalamin induced p-p38 and p-JNK1/2 upregulation and p-Akt
downregulation in the SK-BR-3 cells. These results correlated with
previous reseach that found that baicalin induced autophagy
accompanied by downregulation of the p-Akt (Ser473) level leading
to increased Atg5, Atg7 and Atg12 and then the conversion of LC3I
to LC3II and finally autophagy induction (42). Increased p-p38 leading to inhibition
or induction of autophagy depends on the cellular context and cell
type (43). Alisertib was reported
to increase the p-p38 level correlated with highly accumulated
LC3II in MCF-7 and MDA-MB-231 cells (44). Furthermore, graphene quantum dots
(GQDs) significantly increased p-p38 which was correlated with
increased Beclin-1 and LC3II (43).
In addition, increased p-JNK1/2 activation occurred upstream of the
autophagy induction when cells were starved (45). Furthermore, p-JNK1/2 activation led
to Bcl-2 phosphorylation at Thr69, Ser70 and Ser87 which
dissociated Bcl-2 from Beclin-1 leading to autophagy progression.
Activation of p-JNK1/2 induces FoxOs nuclear localization and
increases ATG gene transcription (46). Thus, goniothalamin induced apoptosis
through an increase in cleaved caspase-9, cleaved caspase-7,
p-JNK1/2 and p-p38 activation as well as p-ERK1/2 and p-Akt
suppression. In addition, p-p38 and p-JNK1/2 upregulation and Akt
downregulation may lead to autophagy.
In conclusion, goniothalamin induced apoptosis
associated with autophagy through p-p38 and p-JNK1/2 upregulation
and Akt downregulation in SK-BR-3 cells. Our findings imply that
goniothalamin may be used as an anticancer drug candidate for the
treatment of human breast cancer.
Acknowledgments
We would like to thank the Royal Golden Jubilee
Ph.D. Program (grant no. PHD/0218/2552), the Thailand Research
Fund, the Strategic Wisdom and Research Institute, the
Srinakharinwirot University and Research Division, and the Faculty
of Medicine, Srinakharinwirot University for their support.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eifel P, Axelson JA, Costa J, Crowley J,
Curran WJ Jr, Deshler A, Fulton S, Hendricks CB, Kemeny M,
Kornblith AB, et al: National Institutes of Health Consensus
Development Conference Statement: Adjuvant therapy for breast
cancer, November 1–3, 2000. J Natl Cancer Inst. 93:979–989. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: Key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lowe SW and Lin AW: Apoptosis in cancer.
21:485–495. 2000.
|
|
6
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizushima N: The role of the Atg1/ULK1
complex in autophagy regulation. Curr Opin Cell Biol. 22:132–139.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blázquez MA, Bermejo A, Zafra-Polo MC and
Cortes D: Styryl-lactones from Goniothalamus species - A review.
Phytochem Anal. 10:161–170. 1999. View Article : Google Scholar
|
|
11
|
Wattanapiromsakul C, Wangsintaweekul B,
Sangprapan P, Itharat A and Keawpradub N: Goniothalamin, a
cytotoxic compound, isolated from Goniothalamus macrophyllus
(Blume) Hook. f. & Thomson var macrophyllus. Warasan Songkhla
Nakharin. 27:479–487. 2005.
|
|
12
|
Barcelos RC, Pastre JC, Caixeta V,
Vendramini-Costa DB, de Carvalho JE and Pilli RA: Synthesis of
methoxylated goniothalamin, aza-goniothalamin and γ-pyrones and
their in vitro evaluation against human cancer cells. Bioorg Med
Chem. 20:3635–3651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bruder M, Vendramini-Costa DB, de Carvalho
JE and Pilli RA: Design, synthesis and in vitro evaluation against
human cancer cells of 5-methyl-5-styryl-2,5-dihydrofuran-2-ones, a
new series of goniothalamin analogues. Bioorg Med Chem.
21:5107–5117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ali AM, Mackeen MM, Hamid M, Aun QB,
Zauyah Y, Azimahtol HL and Kawazu K: Cytotoxicity and electron
microscopy of cell death induced by goniothalamin. Planta Med.
63:81–83. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fátima A, Kohn LK, Carvalho JE and Pilli
RA: Cytotoxic activity of (S)-goniothalamin and analogues against
human cancer cells. Bioorg Med Chem. 14:622–631. 2006. View Article : Google Scholar
|
|
16
|
Inayat-Hussain SH, Wong LT, Chan KM, Rajab
NF, Din LB, Harun R, Kizilors A, Saxena N, Mourtada-Maarabouni M,
Farzaneh F, et al: RACK-1 overexpression protects against
goniothalamin-induced cell death. Toxicol Lett. 191:118–122. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Fátima A, Kohn LK, Antônio MA, de
Carvalho JE and Pilli RA: (R)-Goniothalamin: Total syntheses and
cytotoxic activity against cancer cell lines. Bioorg Med Chem.
13:2927–2933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Qubaisi M, Rozita R, Yeap SK, Omar AR,
Ali AM and Alitheen NB: Selective cytotoxicity of goniothalamin
against hepatoblastoma HepG2 cells. Molecules. 16:2944–2959. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiu CC, Liu PL, Huang KJ, Wang HM, Chang
KF, Chou CK, Chang FR, Chong IW, Fang K, Chen JS, et al:
Goniothalamin inhibits growth of human lung cancer cells through
DNA damage, apoptosis, and reduced migration ability. J Agric Food
Chem. 59:4288–4293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pihie AH, Stanslas J and Din LB:
Non-steroid receptor-mediated antiproliferative activity of
styrylpyrone derivative in human breast cancer cell lines.
Anticancer Res. 18:1739–1743. 1998.PubMed/NCBI
|
|
21
|
Ferguson HA, Marietta PM and Van Den Berg
CL: UV-induced apoptosis is mediated independent of caspase-9 in
MCF-7 cells: A model for cytochrome c resistance. J Biol Chem.
278:45793–45800. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hakem R, Hakem A, Duncan GS, Henderson JT,
Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, et al:
Differential requirement for caspase 9 in apoptotic pathways in
vivo. Cell. 94:339–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou FS, Tang WD, Mu Q, Yang GX, Wang Y,
Liang GL and Lou LG: Semisynthesis and antitumor activities of new
styryl-lactone derivatives. Chem Pharm Bull (Tokyo). 53:1387–1391.
2015. View Article : Google Scholar
|
|
24
|
Alabsi AM, Ali R, Ali AM, Al-Dubai SA,
Harun H, Abu Kasim NH and Alsalahi A: Apoptosis induction, cell
cycle arrest and in vitro anticancer activity of gonothalamin in a
cancer cell lines. Asian Pac J Cancer Prev. 13:5131–5136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yen CY, Chiu CC, Haung RW, Yeh CC, Huang
KJ, Chang KF, Hseu YC, Chang FR, Chang HW and Wu YC:
Antiproliferative effects of goniothalamin on Ca9–22 oral cancer
cells through apoptosis, DNA damage and ROS induction. Mutat Res.
747:253–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen WY, Wu CC, Lan YH, Chang FR, Teng CM
and Wu YC: Goniothalamin induces cell cycle-specific apoptosis by
modulating the redox status in MDA-MB-231 cells. Eur J Pharmacol.
522:20–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inayat-Hussain SH, Chan KM, Rajab NF, Din
LB, Chow SC, Kizilors A, Farzaneh F and Williams GT:
Goniothalamin-induced oxidative stress, DNA damage and apoptosis
via caspase-2 independent and Bcl-2 independent pathways in Jurkat
T-cells. Toxicol Lett. 193:108–114. 2010. View Article : Google Scholar :
|
|
29
|
Yamamoto K, Ichijo H and Korsmeyer SJ:
BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal
protein kinase pathway normally activated at G2/M. Mol
Cell Biol. 19:8469–8478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu SW, Wang H, Poitras MF, Coombs C,
Bowers WJ, Federoff HJ, Poirier GG, Dawson TM and Dawson VL:
Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by
apoptosis-inducing factor. Science. 297:259–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alabsi AM, Ali R, Ali AM, Harun H,
Al-Dubai SA, Ganasegeran K, Alshagga MA, Salem SD and Abu Kasim NH:
Induction of caspase-9, biochemical assessment and morphological
changes caused by apoptosis in cancer cells treated with
goniothalamin extracted from Goniothalamus macrophyllus. Asian Pac
J Cancer Prev. 14:6273–6280. 2013. View Article : Google Scholar
|
|
33
|
Stokoe D, Stephens LR, Copeland T, Gaffney
PR, Reese CB, Painter GF, Holmes AB, McCormick F and Hawkins PT:
Dual role of phosphatidylinositol-3,4,5-trisphosphate in the
activation of protein kinase B. Science. 277:567–570. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang XY, Chen W, Fan JT, Song R, Wang L,
Gu YH, Zeng GZ, Shen Y, Wu XF, Tan NH, et al: Plant cyclopeptide
RA-V kills human breast cancer cells by inducing
mitochondria-mediated apoptosis through blocking PDK1-AKT
interaction. Toxicol Appl Pharmacol. 267:95–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dunn KL, Espino PS, Drobic B, He S and
Davie JR: The Ras-MAPK signal transduction pathway, cancer and
chromatin remodeling. Biochem Cell Biol. 83:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eferl R and Wagner EF: AP-1: A
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohsumi Y: Molecular dissection of
autophagy: Two ubiquitin-like systems. Nat Rev Mol Cell Biol.
2:211–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: Where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC,
Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013.PubMed/NCBI
|
|
43
|
Qin Y, Zhou ZW, Pan ST, He ZX, Zhang X,
Qiu JX, Duan W, Yang T and Zhou SF: Graphene quantum dots induce
apoptosis, autophagy, and inflammatory response via p38
mitogen-activated protein kinase and nuclear factor-κB mediated
signaling pathways in activated THP-1 macrophages. Toxicology.
327:62–76. 2015. View Article : Google Scholar
|
|
44
|
Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST,
He ZX, Edelman JL, Wang D, Yang YX, Zhang X, et al: Alisertib, an
Aurora kinase A inhibitor, induces apoptosis and autophagy but
inhibits epithelial to mesenchymal transition in human epithelial
ovarian cancer cells. Drug Des Devel Ther. 9:425–464.
2015.PubMed/NCBI
|
|
45
|
Wei Y, Pattingre S, Sinha S, Bassik M and
Levine B: JNK1-mediated phosphorylation of Bcl-2 regulates
starvation-induced autophagy. Mol Cell. 30:678–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou YY, Li Y, Jiang WQ and Zhou LF:
MAPK/JNK signalling: A potential autophagy regulation pathway.
Biosci Rep. 35:pii: e00199. 2015. View Article : Google Scholar
|