Introduction
Chronic myeloid leukemia (CML) is a clonal
hematopoietic stem cell disorder characterized by the increased
growth of predominantly myeloid cells in the bone marrow and the
accumulation of these cells in the blood (1). Multidrug resistance (MDR) is a major
challenge to the successful treatment of CML. The mechanisms which
generate the MDR phenotype of CML cells are complex, including
increase of drug excretion, antiapoptosis, activity changes in drug
metabolizing enzymes, enhancement of DNA repair following damage,
changes in signaling pathway. Research has recently been devoted to
understanding the interaction of glycan alterations and resistance
to chemotherapy of cancer cells.
Glycans attached to proteins are ubiquitous in
biological systems, and many proteins in eukaryotes are
glycosylated (2). Most of these are
N-linked and/or O-linked glycan chains that are synthesized by
various glycosyltransferases (3).
The fucosyltransferase gene family encodes enzymes that transfer
fucose from α(1,2), α(1,3/4) and α(1,6) linkages to various
glycans. Fucosyltransferase 1 (FUT1) and FUT2 are
α(1,2)-fucosyltransferases responsible for synthesis of the H blood
group antigen (4,5). The expression of cancer-associated
carbohydrate antigens is modified by abnormal control by
glycosyltransferases. In human colon adenocarcinoma, expression of
the fucosyltransferase gene FUT1 has been found to correlate with
malignant progression (6).
Downregulation of microRNA-15b by hepatitis B virus X enhanced
hepatocellular carcinoma proliferation via fucosyltransferase
2-induced Globo H expression (7).
In addition, forced FUT1 and FUT2 expression in human ovarian
carcinoma-derived RMG-I cells promoted cell proliferation and
resistance against anticancer drugs, such as 5-fluorouracil and
carboplatin (8,9). However, little information is
available on the reversal effects of FUT1 and FUT2
glycosyltransferases and corresponding glycogenes on multi-drug
resistance in human CML cells.
Epidermal growth factor receptor (EGFR) has
attracted much attention for its potency in regulating cell
activation. Binding of ligands such as EGF, the tyrosine specific
protein kinase intrinsic to EGFR, results in activation, and is
followed by transactivation of mitogen activated protein kinase
(MAPK) and other downstream signal pathways (10). In addition, several reports
highlight that aberrant activation of EGFR/MAPK pathway contributes
to the drug resistance of different types of human cancer cells.
Non-small cell lung cancer cells with acquired resistance to
cetuximab manifested strong activation of EGFR and MAPK (11). Blockade of the EGF receptor
(EGFR)/MAPK pathway caused more marked inhibition of growth of
tamoxifen-resistant MCF-7 breast cancer cells (12). The uncoupling of EGFR with mitogenic
pathways caused resistance to EGFR inhibition in bladder cancer
(13). A potential association of
FUT1 with EGFR signaling pathway has been explored as well.
Knocking down FUT1 expression inhibited human epidermoid carcinoma
A431 cell proliferation through decreasing the EGFR signaling
pathway (19).
In the present study, we investigated the mRNA
expression levels of α(1,2)-fucosyltransferase genes in three pairs
of parental and chemoresistant CML cell lines and in BMMC isolated
from the diagnostic CML patients. We further determined the
functional role of FUT1 in CML MDR, as well as the possible
mechanisms via EGFR/MAPK pathway.
Materials and methods
Parental CML cell culture
Three CML cell lines, K562, KCL22 and KU812, were
purchased from the Nanjing KeyGen Biotech (Co., Ltd., Nanjing,
China). All cell lines were cultured in RPMI-1640 medium (Gibco,
Grand Island, NY, USA) supplemented with 1% penicillin-streptomycin
(Gibco) and 10% heat inactivated fetal bovine serum (FBS; Gibco).
Cells were kept a humidified incubator at 37°C with 5%
CO2. Adriamycin (Sigma) was added to parental cell
cultures in stepwise increasing concentrations from 0.1 to 5
µg/ml for 4 months to develop an adriamycin-resistant (ADR)
clone. Once ADR-resistant clones became resistant, the complete
medium of the resistant cell clones were supplemented with 1.0 mg/l
adriamycin. Over 90% of ADR cells were susceptible to subsequent
treatments if they were maintained in complete medium without
adriamycin for one week.
Samples from leukemia patients and
primary CML peripheral blood mononuclear cells (PBMCs)
Thirty-nine previously untreated CML patients and 9
healthy donors were included in this study. The diagnosis of CML
was based on cytomorphology, cytochemistry, multiparameter flow
cytometry, immunology, molecular genetics and cytogenetics. There
were 21 males and 18 females with age ranging from 19 to 71 years
(median age, 43 years). P-gp (+) was observed in 22 of 39 CML
patients. All the participants recruited from Jan 2012 to Dec 2014
at the Second Affiliated Hospital of Dalian Medical University
(Dalian, China) provided written informed consent. The
investigation project and the informed consent were examined and
certified by the Ethics Committee of the Second Affiliated Hospital
of Dalian Medical University.
PBMCs from CML patients were separated by
Ficoll-Hypaque density gradient centrifugation and were further
cultured in plastic dishes to remove adherent cells at 37°C for 24
h. Fresh separated non-adherent cells were maintained in modified
Dulbecco's medium containing 10% FBS, 10 mM β-mercaptoethanol, 2 mM
L-glutamine, 50 ng/ml human stem cell factor, 10 ng/ml human
interleukin-3 and 10 ng/ml human interleukin-6. Cells were then
harvested and real-time PCR analysis was completed.
Real-time PCR analysis
Total RNA was isolated with TRIzol reagents
(Gibco-BRL, Rockville, MD, USA), and cDNA was synthesized using
QuantiTect reverse transcription kit (Qiagen, Valencia, CA, USA)
according to the manufacturer's instruction. Quantitative PCR with
QuantiTect SYBR-Green (Qiagen) was performed for each transcript.
The following primers were used: 5′-AAAGCGGACTGTGGATCT-3′ and
5′-GGACACA G GATCGACAGG-3′ for FUT1; 5′-CTGCCCAACCACTCTGTC-3′ and
5′-CCGTAAAGACAAAGAGGATG-3′ for FUT2; 5′-CTCCTCCACCTTTGACGCTG-3′ and
5′-TCCTCTTGTGCTCTTGCTGG-3′ for GAPDH. Finally expression of the
transgenes was determined quantitatively by the relative Ct method.
The relative gene expression was normalized to that of respective
GAPDH and calculated as 2-(CtTarget gene −
CtGAPDH).
Western blot analysis
Whole cell lysates were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinyledene fluoride (PVDF) membranes (Bio-Rad Laboratories,
Hercules, CA, USA). After blocking with 5% skimmed milk in PBS
containing 0.1% Tween-20 (PBST), the membrane was incubated with
antibody (1/200 diluted; Santa Cruz Biotechnology) and then with
peroxidase-conjugated anti-rabbit IgG (1/10,000 diluted; GE
Healthcare UK, Little Chalfont, UK). A GAPDH antibody (1/200
diluted; Santa Cruz Biotechnology) was used as a control. The
protein bands on the membrane were visualized using the Western
Lightning chemiluminescence reagent (Perkin-Elmer, Waltham, MA,
USA). The bands were analyzed with LabWorksTM ver4.6;
UVP, BioImaging systems).
shRNA-mediated FUT1 gene silencing
K562/ADR cells were incubated in appropriate
antibiotic-free medium with 10% fetal bovine serum, and were
transferred to a 6-well tissue culture and incubated at 37°C, in a
CO2 incubator to obtain 60–80% confluence. The cell
cultures were transfected with FUT1-specific shRNA, and scrambled
shRNA used as the negative control. FUT1 shRNA was mixed with
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Transfected
cells were cultured and incubated at 37°C for 6 h, followed by
incubation with complete medium for additional 24 h. Then, the
cells were harvested for further study. The cell transfection
efficiency was 73% by fluorescent microscopy and the cell viability
was 85% by trypan blue dye exclusion assay.
Overexpression of FUT1
The human FUT1 coding sequences obtained from Takara
Co. (Dalian, China) were inserted into the pEGFP-N2 vector
(Invitrogen) at the sites of EcoRI and XhoI. K562
cells were transfected with 5 µg of target gene expression
vector or empty vector (EV) in 100-mm dishes using PolyFect
transfection reagent (Qiagen) according to the manufacturer's
instruction. After 4 weeks of screening, the cell lines stably
expressing FUT1 (K562/FUT1), and cells with empty vector
(HL60/mock) were established. The cell transfection efficiency was
75% and the survival rate was 87%.
In vitro drug sensitivity assay
Cell drug sensitivity was measured using an MTT
assay. The K562/ADR, K562/ADR-control shRNA, K562/ADR-FUT1 shRNA,
K562, K562/mock, K562/FUT1 and K562/ADR with DMSO, PD153035,
control siRNA or EGFR siRNA treatment cells (1×104) were
grown in 96-well plates and incubated with different anticancer
drugs adriamycin, vincristine and paclitaxel (Sigma, St. Louis, MO,
USA) for 48 h, respectively. Then, the cells were treated with 100
µl MTT (5 mg/ml; Sigma). After 4-h incubation at 37°C in 5%
CO2, 100 µl dimethyl sulfoxide (DMSO; Gibco) was
added to dissolve formazan crystals that formed and the absorbance
was measured at 490 nm using microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The drug resistance was estimated by
comparing the IC50 values (the drug concentration that
inhibits cell growth by 50%) from growth inhibition curves.
In vivo chemosensitivity assay
Approval for animal studies was obtained from the
Dalian Medical University Institutional Animal Care and Use
Committees. Five-week-old male athymic nude mice were obtained from
the Animal Facility of Dalian Medical University, and were fed with
sterilized food and water. Approximately, 1×107 cells
were injected subcutaneously into the right flank of each nude
mouse, respectively. One week after tumor cell injection,
tumor-bearing mice were randomly divided into control and treatment
groups (n=6 animals per group). The treatment groups received 7
mg/kg adriamycin i.p. three times a week for 3 weeks, and the
control groups received physiological saline alone. The mice were
sacrificed and their tumors were isolated, weighed and
photographed. The tumor volume was calculated by the following
formula: Tumor volume = 1/2 (length × width2).
Immunohistochemical (IHC) staining
analysis
Visible tumors were removed from the mice and
immunohistochemistry was performed on paraffin-embedded tissue
sections using the fully automated Dako immunohistochemistry
staining system (Autostainer Link 48; Dako, Glostrup, Denmark). The
slides were dried, deparaffinized and rehydrated. After
deparaffinization and blocking of endogenous peroxidase, the slides
were labeled with antibodies (Abcam, Cambridge, UK) at a dilution
of 1:200 at 4°C overnight. The secondary
streptavidin-HRP-conjugated antibody staining (Santa Cruz
Biotechnology) was performed at room temperature for 60 min.
Finally, the sections were counterstained with hematoxylin and
coverslipped.
Inhibition of the EGFR/MAPK
signaling
PD153035 (Sigma) or EGFR siRNA was applied to
suppress the activity of the EGFR/MAPK signaling in K562/ADR cells.
Briefly, the leukemia cells (1×104 cells/well) were
incubated in DMSO supplemented with the EGFR inhibitor PD153035 (10
µM). EGFR control siRNA and EGFR siRNA cells were collected
after 24 h.
Flow cytometric analysis
Expression of α-(1,2)
fucosylation at the cell surface was analyzed by flow cytometry
using FITC-UEA-1 lectin (Sigma). Expression of P-gp at the cell
surface was incubated with anti-P-gp antibody. After repeated
centrifugation at 1,000 r/min, labeled cells were resuspended in
0.2 ml PBS and were analyzed with FACSCalibur (BD Biosciences, San
Jose, CA, USA). For mean fluorescence intensity, each value of the
geometric mean was calculated by CellQuest software.
Statistical analysis
The data from the triple tests of each group were
expressed as the mean ± SD and analyzed by the SPSS 16.0
statistical software to evaluate the statistical difference. The
Student's t-tests were used to compare the significance of
differences among the examined groups. A statistically significant
difference was considered at P<0.05.
Results
Differential expression of FUT1, FUT2 in
three pairs of parental and chemoresistant human CML cell lines and
CML patients
To increase our understanding of the regulation of
α(1,2)-fucosylation, we determined the mRNA levels of FUT1 and FUT2
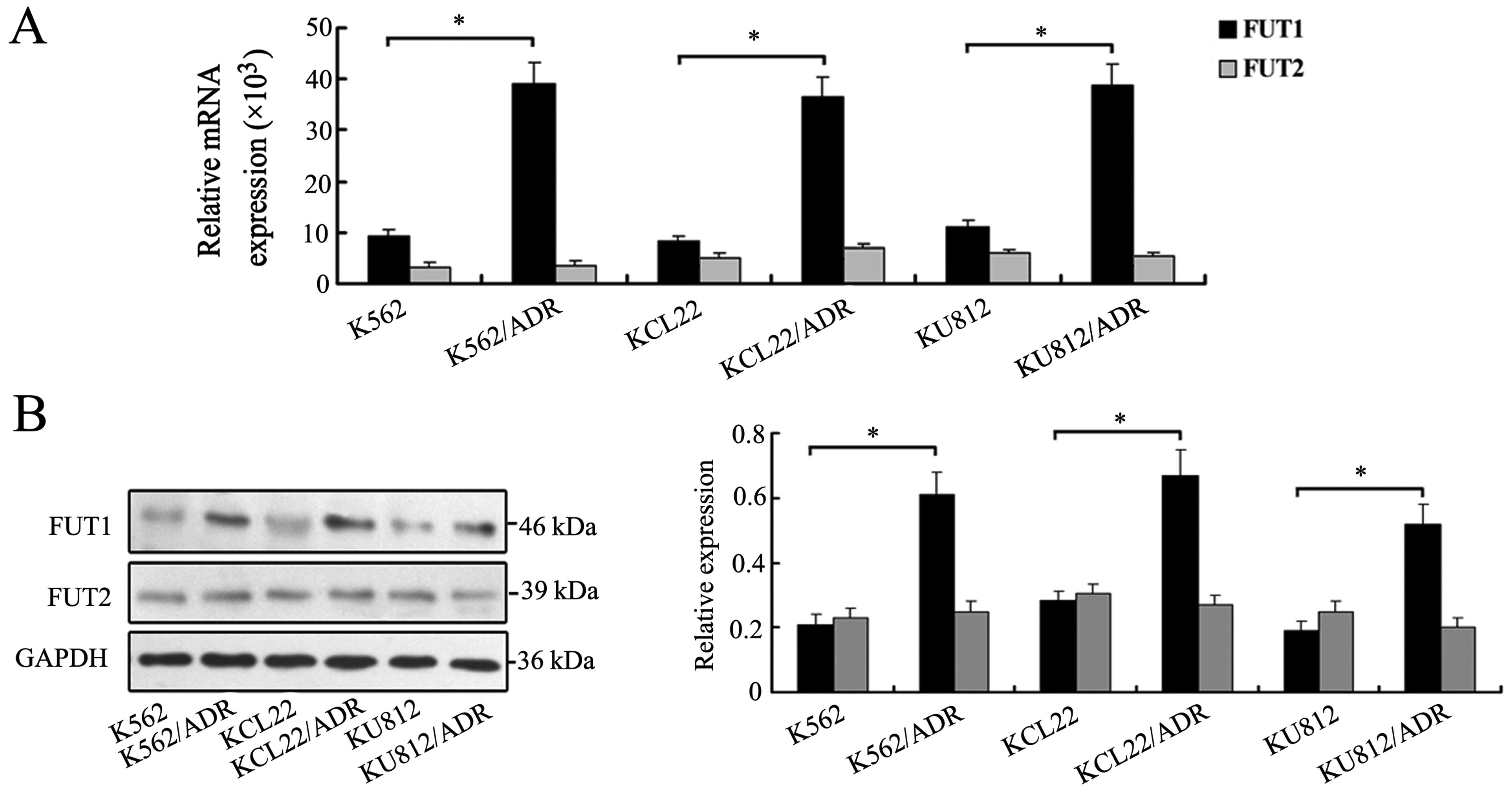
in drug sensitive and MDR cells. As shown in Fig. 1A, three MDR cell lines showed
elevated levels of FUT1 expression compared with the three
drug-sensitive parental cell lines. By contrast, no significant
change of FUT2 was observed (Fig.
1A). Western blot analysis further confirmed the protein
expression levels of FUT1 and FUT2 in drug sensitive and MDR cells
(Fig. 1B).
Expression of MDR-related marker, FUT1, FUT2 present
in peripheral blood mononuclear cells of CML patients is summarized
in Table I. The frequency of P-gp
positivity was 56.4% (22 of 39) in the CML patients. The mRNA
expression levels of FUT1 and FUT2 were measured in the PBMC of CML
without MDR and CML/MDR by real-time PCR. The group of CML/MDR
showed significantly higher level of FUT1 (P=0.004) mRNA expression
than one of the chemo-sensitive groups. Expression of FUT2 showed
no difference in expression levels between the two groups. These
observations indicated that the differential expression of FUT1
might contribute to MDR of CML.
 | Table IExpressional profiles of FUT1 and
FUT2 in CML and CML/MDR patients. |
Table I
Expressional profiles of FUT1 and
FUT2 in CML and CML/MDR patients.
| Gene | Relative mRNA
expression (×103)
| P-value |
|---|
| CML | CML/MDR |
|---|
| FUT1 | 15.466±1.094 | 32.101±7.382 | 0.004a |
| FUT2 | 1.088±0.245 | 1.236±0.519 | 0.699 |
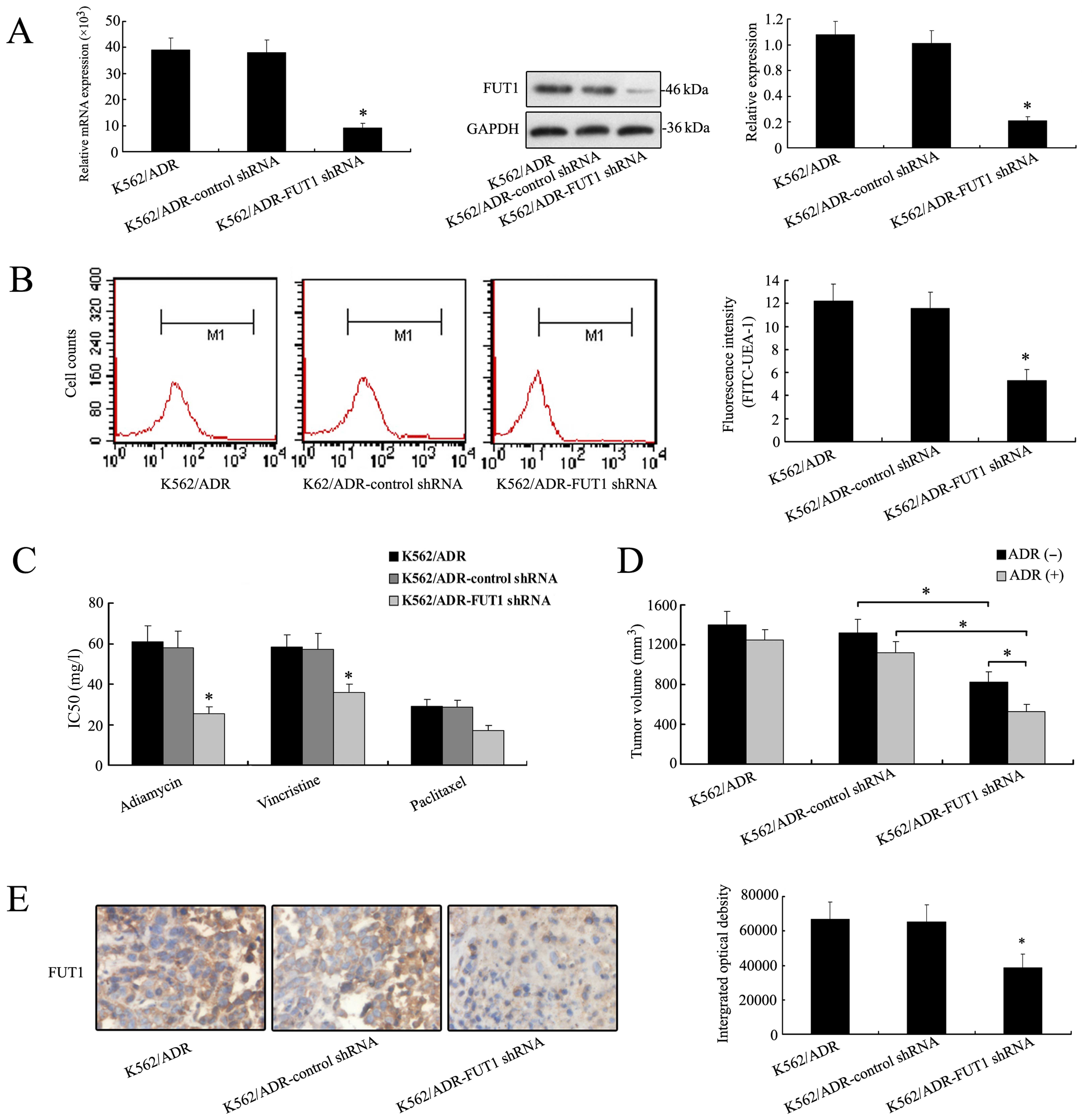
Downregulation of FUT1 gene enhances
chemosensitivity of K562/ADR cells in vitro and in vivo
Due to the significant increase of FUT1 mRNA and
protein expression in K562/ADR cells, we silenced FUT1 with shRNA
to elucidate the direct implication of FUT1 in the chemosensitivity
of K562/ADR cells. As shown in Fig.
2A, the expression level of FUT1 was significantly decreased in
FUT1 shRNA transfectants compared to those in control. Furthermore,
flow cytometric analysis was performed to study α-(1,2)
fucosylation level on cell surface by using FITC-UEA-1. A typical
image is shown in Fig. 2B. Results
from three independent experiments demonstrated that the mean
fluorescence intensity was reduced in K562/ADR-FUT1 shRNA cells.
Significant differences were seen across cell lines (K562/ADR-FUT1
shRNA cells to control shRNA cells, P<0.05). These results
clearly showed that FUT1 was responsible for the overcoming of
tumor cell MDR resistance via regulating fucosylation profile in
terms of α-1, 2 branched structures in CML cells.
After FUT1 shRNA transfection, the ability of
adriamycin, paclitaxel and vincristine to inhibit the growth of
K562/ADR was evaluated by MTT assay. The results showed that
IC50 values were significantly decreased in
K562/ADR-FUT1 shRNA group compared to the control, suggesting that
cell proliferation was inhibited by therapeutic drug and
chemosensitivity was remarkably restored when FUT1 gene was
suppressed (Fig. 2C).
Nude mice bearing K562/ADR, K562/ADR-control shRNA,
and K562/ADR-FUT1 shRNA xenografts were used to determine the
treatment efficacy of adriamycin by measuring tumor volumes.
Fig. 2D showed that a significant
reduction of mean tumor volume of K562/ADR-FUT1 shRNA tumor (536±71
mm3) was observed, as compared with control shRNA group
(1121±125 mm3), and the effect of concomitant
application of adriamycin. These data were consistent with the
results of in vitro chemosensitivity analysis. IHC staining
analysis of the tumor sections revealed that the expression of FUT1
protein was decreased in the mouse group treated with FUT1 shRNA
compared to that in the untreated group (Fig. 2E).
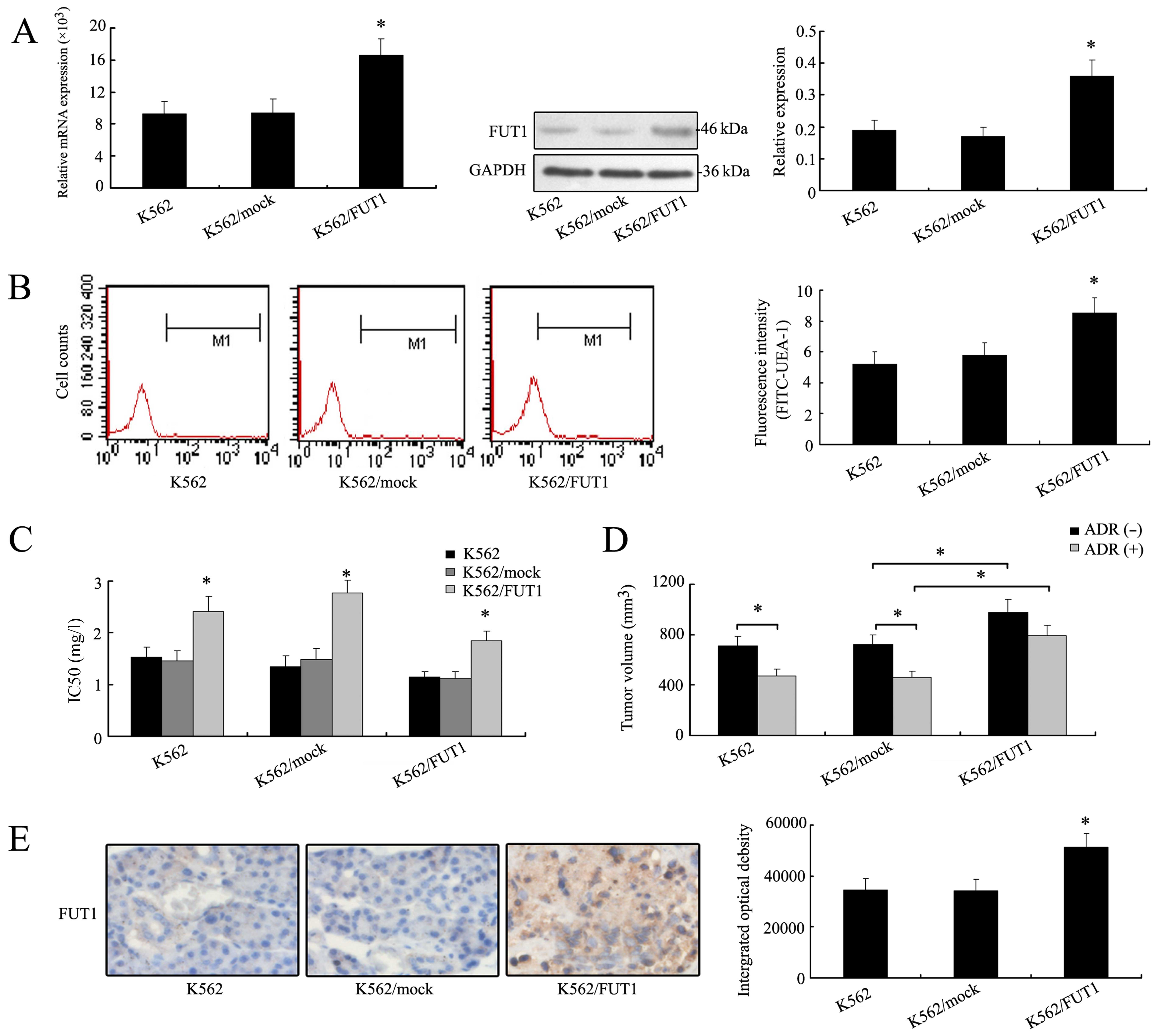
Overexpression of FUT1 gene enhances the
chemoresistance of K562 cells in vitro and in vivo
After verifying the effect of FUT1 gene
downregulation on tumor cell chemosensitivity, we transfected K562
cells with FUT1 expression vector to determine the effect of
overexpression of FUT1 on chemoresistance of K562 cells. Notably,
the levels of mRNA and protein of FUT1 increased in FUT1
transfectant (Fig. 3A). Fig. 3B also showed that the FUT1
overexpression resulted in an increase of fluorescence intensity
(cell surface α-1,2 fucose) compared with the K562/mock cells. MTT
assays revealed that IC50 values of three drugs were
higher in K562/FUT1 group than those in the K562/mock groups,
suggesting a positive association between the FUT1 gene expression
and chemoresistance of human CML cells (Fig. 3C).
The nude mice inoculated with tumor cells K562,
K562/mock and K562/FUT1 were used to measure and compare tumor
volumes with or without adriamycin treatment. Fig. 3D showed that in the group of mice
bearing K562 tumors, tumor volume was reduced after adriamycin
treatment (475±59 mm3) compared to those without (711±68
mm3). In the group of mice bearing K562/FUT1 (985±87
mm3) tumors, tumor volume increased significantly
compared to those of the K562/mock group (718±62 mm3)
even after adriamycin treatment (794±78 vs. 462±53 mm3).
High expression level of FUT1 protein was illustrated in the tumor
cells of K562/FUT1 by IHC staining, as shown in Fig. 3E. Thus, overexpression of FUT1 gene
in K562 cells led to increasing resistance to adriamycin
chemotherapy.
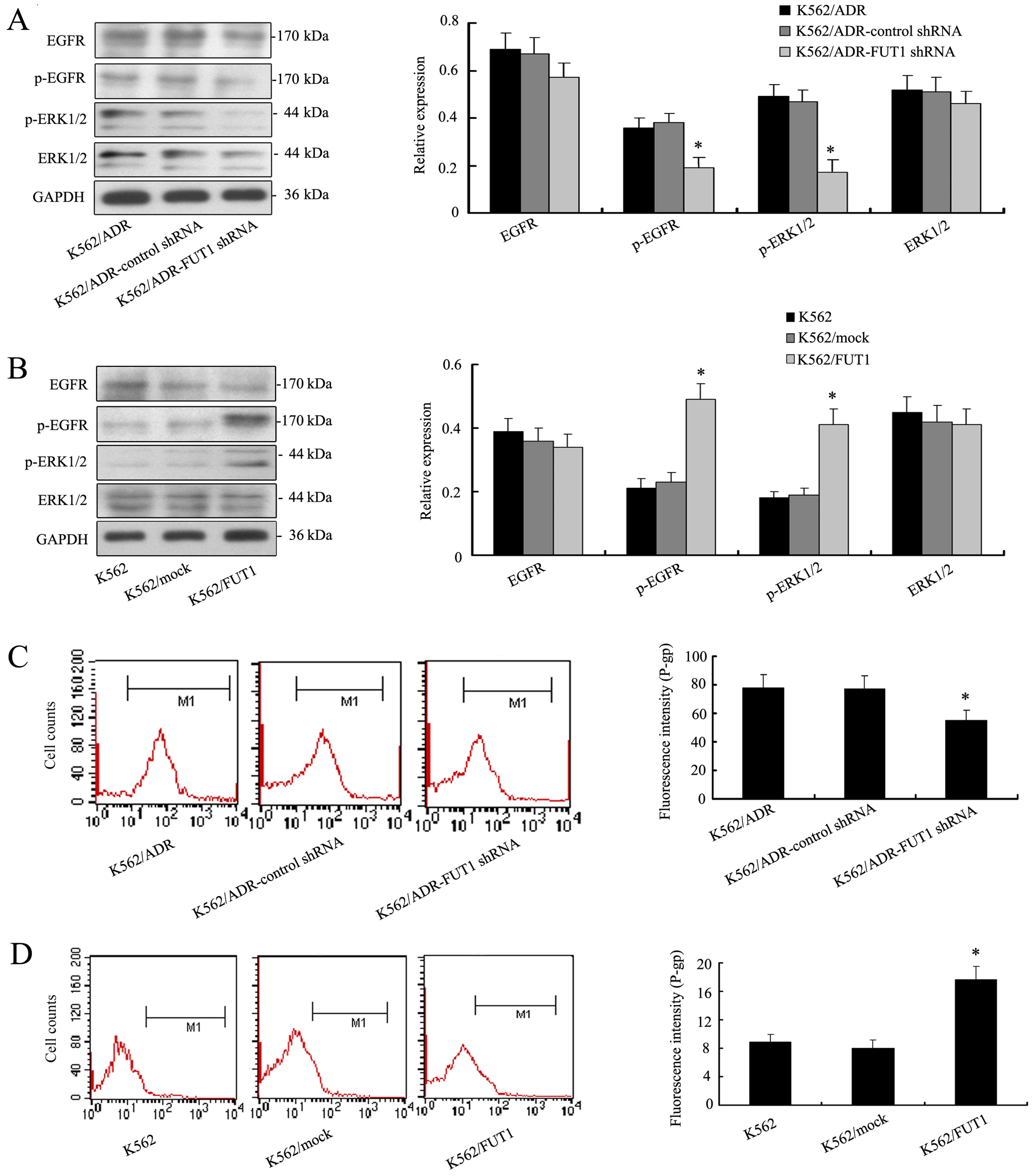
FUT1-induced chemoresistance to CML cell
lines is through EGFR/MAPK and P-gp expression
Given the critical role of EGFR/MAPK pathway in
controlling cell chemosensitivity, we analyzed whether FUT1
activated the EGFR/MAPK pathway and whether this pathway was
involved in FUT1-mediated cell chemosensitivity. Fig. 4A showed that the levels of the
p-EGFR and p-ERK1/2 were significantly decreased in low
K562/ADR-FUT1 shRNA cells. But the total amount of EGFR and ERK1/2
remained unchanged. On the contrary, overexpression of FUT1 in K562
cells significantly enhanced proteins expression of p-EGFR and
p-ERK1/2 as illustrated in Fig.
4B.
Furthermore, we investigated whether FUT1 could
influence the expression of P-gp. Interestingly, flow cytometric
analysis illustrated that low expression level of P-gp was detected
in K562/ADR-FUT1 shRNA cells compared to those in control cell
groups (Fig. 4C). In contrast, K562
cells expressed high level of P-gp with FUT1 overexpression
(Fig. 4D). These data indicated a
possible pathogenetic mechanism of MDR development of CML
cells.
Blocking EGFR/MAPK modulates the
chemosensitivity of K562/ADR cells both in vitro and in vivo
To further investigate what role EGFR/MAPK pathway
plays in the signal transduction of FUT1 in K562/ADR cells, the
effects of specific inhibitor of EGFR/MAPK or EFGR siRNA to silence
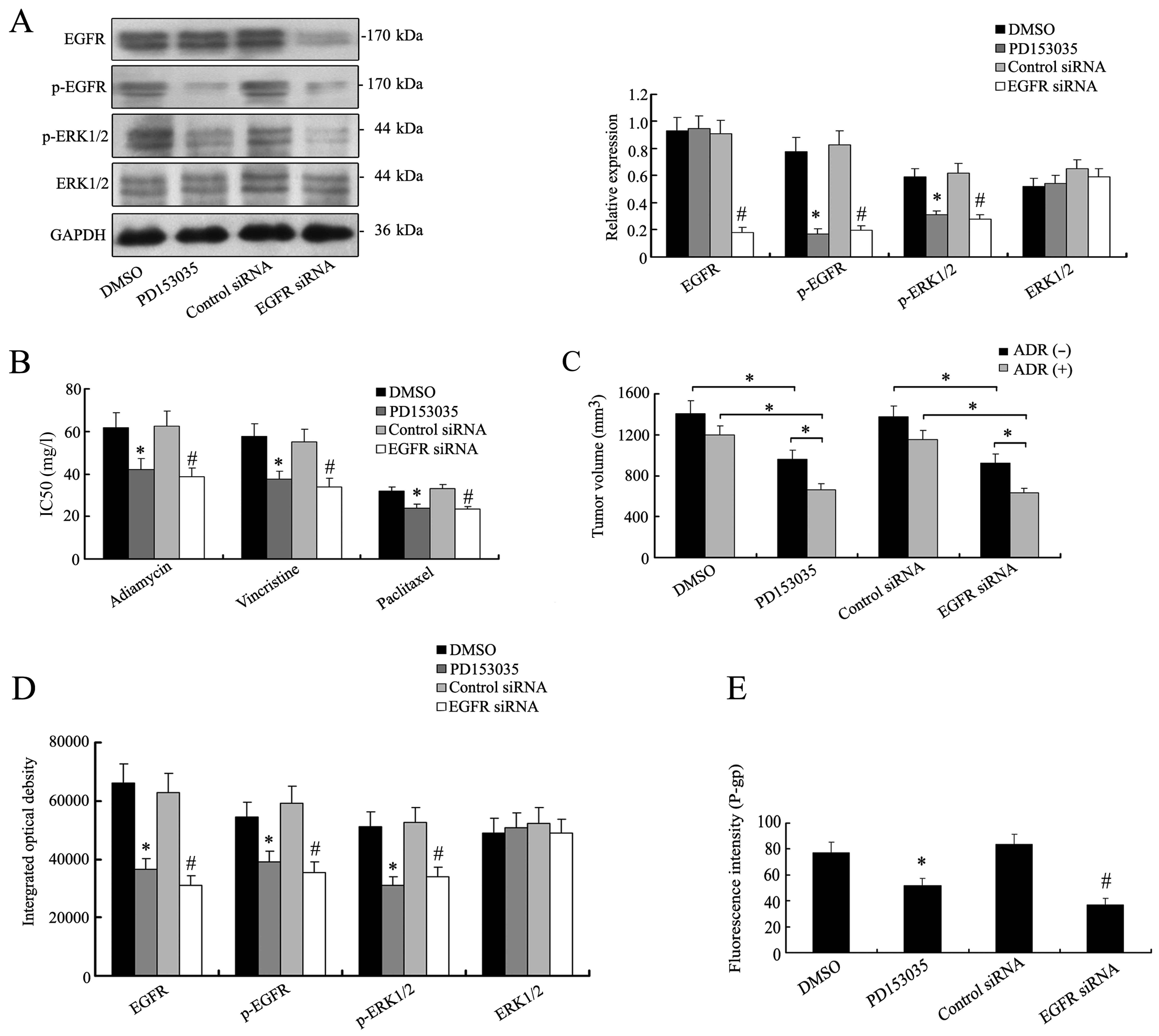
EFGR were selected to treat K562/ADR cells. By western blotting,
the expression levels of the main signal molecules of EGFR/MAPK
pathway apparently decreased in K562/ADR cells treated with
PD153035 or EGFR siRNA (Fig. 5A).
The inhibition of EGFR/MAPK pathway made the K562/ADR cells
susceptible to chemotherapy (Fig.
5B). Accordingly in vivo chemosensitivity analysis
revealed that reduced tumor volumes were detected in mouse group
bearing K562/ADR tumors with PD153035 (669±73 mm3) or
EGFR siRNA treatment (638±57 mm3), as compared with DMSO
(1206±98 mm3) or control siRNA group (1156±95
mm3), and the effect of concomitant application of
adriamycin (Fig. 5C). Altered
expression levels of the main signal molecules of EGFR/MAPK pathway
were also validated in mouse group bearing K562/ADR tumors treated
with PD153035 or EGFR siRNA by IHC staining, as shown in Fig. 5D. Moreover, the inhibitor of
EGFR/MAPK or silencing EGFR reduced the expression of P-gp
(Fig. 5E). The results implicated a
role of EGFR/MAPK signaling in regulating P-gp expression and
modulating the chemoresistance of K562/ADR cells.
Discussion
MDR is considered to be a major problem for clinical
therapy related to hematological malignancies. Leukemia cells give
rise to a series of biological changes during the MDR development.
In the present study, we investigated the association between
alteration of α(1,2)-fucosylation and expression of their related
glycogenes as well as the possible mechanism of FUT1 on MDR
development in human CML cell lines.
Changes in the surface fucosylation have been
detected in colorectal, hepatocellular carcinoma, head and neck and
gastric cancer (14–17). The biosynthetic pathway of
fucosylated glycans showed great importance of fucosyltransferases.
In this study, we found that the expression profiles of
α(1,2)-fucosyltransferase were remodeled in three pairs of CML cell
lines. All MDR cells were characterized by higher levels of FUT1.
The expression of FUT2 exhibited no significant difference in three
pairs of CML cell lines. In addition, a great number of CML
patients were examined and analyzed in the present study, while
>56.4% of the CML patients were found resistant to the
anticancer drugs. FUT1 was expressed at a high level in peripheral
blood mononuclear cells of CML/MDR patients. On the basis of the
above results, it implied the utilization of FUT1 as a biomarker
for clinical diagnosis and prognosis of MDR of CML.
Besides listing the gene profile, we were also
interested in the influence of FUT1 on MDR in human CML cells.
Here, we targeted FUT1, which was differentially expressed in K562
and K562/ADR cells. The altered level of FUT1 was responsible for
changed drug-resistant phenotype of K562 and K562/ADR cells both
in vitro and in vivo. FUT1 product also altered
remarkably in CML cell lines labeled with FITC-UEA-1 lectin. These
results clearly showed that the change in FUT1 expression level had
impact in the remodeling of cell surface fucosylated
oligosaccharides, which might consequently affect the biological
functions of leukemia cells such as MDR.
Several studies have explored a potential
association of FUT1 with signaling pathways. The knockdown of FUT1
gene downregulated HER2 signaling via EGFR downregulation and
attenuated cell proliferation in the HER2-overexpressing gastric
cancer cell line NCI-N87 (18).
Knocking down FUT1 expression by short interfering RNA technique
dramatically reduced the expression of FUT1 and inhibited human
epidermoid carcinoma A431 cells proliferation through decreasing
EGFR signaling pathway (19). The
EGFR/MAPK signaling pathway also controls the expression and
function of many proteins that are necessary for tumor cell
multidrug resistance (20,21). In the present study, we evaluated
the correlation of the FUT1-mediated EGFR/MAPK signaling pathway
with MDR. The resistant cell line K562/ADR, presented higher
EGFR/MAPK activity than the sensitive one, which was in accordance
with the MDR phenotype. Altered expression of FUT1 markedly
modulated the activity of EGFR/MAPK pathway in human CML cell
lines. In addition, inhibition of the EGFR/MAPK pathway with EGFR
inhibitor PD153035 or EGFR siRNA reversed the chemoresistance of
K562/ADR cells. Our results together with the previous findings,
explored a possible mechanism of MDR in CML cells that drug
resistance might develop and vary via the EGFR/MAPK pathway
activated by FUT1 expression. FUT1-modulated CML cell MDR was, at
least in part, EGFR/MAPK-dependent.
Increasing evidence indicates that the EGFR/MAPK
pathway enhances drug efflux by ATP-binding cassette (ABC)
transporters, maintaining MDR of tumor cells (22,23).
Activation of the tyrosine kinase pathway by EGF induced MDR by
upregulating the ABC protein expression and enhanced the survival
of resistant HCC cells. In contrast, EGFR inhibition restored
chemosensitivity (20). Moreover,
reports revealed that activation of the EGFR-pathway increased P-gp
expression in colorectal cancer cells and enhanced ABCC1
gene expression in MCF-7 breast cancer cells (24,25).
In addition, it has been well demonstrated that the knockdown of
FUT1 expression inhibited human epidermoid carcinoma A431 cell
proliferation through decreasing EGFR signaling pathway (19). Therefore, a close association is
indicated between the level of FUT1 and the levels of
phosphorylated EGFR, as well as P-gp expression in cancer cells. In
the present study, we found that the level of P-gp had a positive
relationship with the expression of FUT1 and the activity of
EGFR/MAPK signaling in K562 and K562/ADR cell lines. Consequently,
the MDR mediated by FUT1 was also involved in EGFR/MAPK pathway
activation and P-gp expression.
In conclusion, by analyzing the differential
expression pattern of α(1,2)-fucosyltransferase in three pairs of
CML cell lines and in peripheral blood mononuclear cells of the CML
patients, at least in this system, FUT1 regulation elucidated the
unusual property of association with CML cell MDR via modulating
the EGFR/MAPK signaling pathway and P-gp expression. Together,
these data increase our understanding of the factors that
contribute to the complex regulation of glycosylation in
cancer.
Abbreviations:
|
FUT
|
fucosyltransferase gene
|
|
MDR
|
multidrug resistance
|
|
CML
|
chronic myeloid leukemia
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
siRNA
|
small interfering RNA
|
|
shRNA
|
short hairpin RNA
|
|
P-gp
|
P-glycoprotein
|
|
PBS
|
phosphate-buffered saline
|
|
PBST
|
containing 0.1% Tween-20
|
|
ADR
|
adriamycin
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
methylthiazolyl tetrazolium
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Sloma I, Jiang X, Eaves AC and Eaves CJ:
Insights into the stem cells of chronic myeloid leukemia. Leukemia.
24:1823–1833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hart GW: Glycosylation. Curr Opin Cell
Biol. 4:1017–1023. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunphy WG, Brands R and Rothman JE:
Attachment of terminal N-acetylglucosamine to asparagine-linked
oligosaccharides occurs in central cisternae of the Golgi stack.
Cell. 40:463–472. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelly RJ, Rouquier S, Giorgi D, Lennon GG
and Lowe JB: Sequence and expression of a candidate for the human
Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2).
Homozygosity for an enzyme-inactivating nonsense mutation commonly
correlates with the non-secretor phenotype. J Biol Chem.
270:4640–4649. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larsen RD, Ernst LK, Nair RP and Lowe JB:
Molecular cloning, sequence, and expression of a human
GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA
that can form the H blood group antigen. Proc Natl Acad Sci USA.
87:6674–6678. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun J, Thurin J, Cooper HS, Wang P,
Mackiewicz M, Steplewski Z and Blaszczyk-Thurin M: Elevated
expression of H type GDP-L-fucose:beta-D-galactoside
alpha-2-L-fucosyltransferase is associated with human colon
adenocarcinoma progression. Proc Natl Acad Sci USA. 92:5724–5728.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CS, Yen CJ, Chou RH, Chen JN, Huang WC,
Wu CY and Yu YL: Downregulation of microRNA-15b by hepatitis B
virus X enhances hepatocellular carcinoma proliferation via
fucosyltransferase 2-induced Globo H expression. Int J Cancer.
134:1638–1647. 2014. View Article : Google Scholar
|
|
8
|
Iwamori M, Tanaka K, Kubushiro K, Lin B,
Kiguchi K, Ishiwata I, Tsukazaki K and Nozawa S: Alterations in the
glycolipid composition and cellular properties of ovarian
carcinoma-derived RMG-1 cells on transfection of the
α1,2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hao YY, Lin B, Zhao Y, Zhang YH, Li FF,
Diao B, Ou YL and Zhang SL: alpha1,2-fucosyltransferase gene
transfection influences on biological behavior of ovarian
carcinoma-derived RMG-I cells. Fen Zi Xi Bao Sheng Wu Xue Bao.
41:435–442. 2008.In Chinese.
|
|
10
|
Fischer OM, Hart S and Ullrich A:
Dissecting the epidermal growth factor receptor signal
transactivation pathway. Methods Mol Biol. 327:85–97.
2006.PubMed/NCBI
|
|
11
|
Iida M, Brand TM, Campbell DA, Starr MM,
Luthar N, Traynor AM and Wheeler DL: Targeting AKT with the
allosteric AKT inhibitor MK-2206 in non-small cell lung cancer
cells with acquired resistance to cetuximab. Cancer Biol Ther.
14:481–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan P, Wang J, Santen RJ and Yue W:
Long-term treatment with tamoxifen facilitates translocation of
estrogen receptor alpha out of the nucleus and enhances its
interaction with EGFR in MCF-7 breast cancer cells. Cancer Res.
67:1352–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kassouf W, Dinney CP, Brown G, McConkey
DJ, Diehl AJ, Bar-Eli M and Adam L: Uncoupling between epidermal
growth factor receptor and downstream signals defines resistance to
the antiproliferative effect of Gefitinib in bladder cancer cells.
Cancer Res. 65:10524–10535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lattová E, Tomanek B, Bartusik D and
Perreault H: N-glycomic changes in human breast carcinoma MCF-7 and
T-lymphoblastoid cells after treatment with herceptin and
herceptin/Lipoplex. J Proteome Res. 9:1533–1540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shu H, Zhang S, Kang X, Li S, Qin X, Sun
C, Lu H and Liu Y: Protein expression and fucosylated glycans of
the serum haptoglobin-{beta} subunit in hepatitis B virus-based
liver diseases. Acta Biochim Biophys Sin (Shanghai). 43:528–534.
2011. View Article : Google Scholar
|
|
16
|
Mejías-Luque R, López-Ferrer A, Garrido M,
Fabra A and de Bolós C: Changes in the invasive and metastatic
capacities of HT-29/M3 cells induced by the expression of
fucosyltransferase 1. Cancer Sci. 98:1000–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vasseur JA, Goetz JA, Alley WR Jr and
Novotny MV: Smoking and lung cancer-induced changes in
N-glycosylation of blood serum proteins. Glycobiology.
22:1684–1708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawai S, Kato S, Imai H, Okada Y and
Ishioka C: Suppression of FUT1 attenuates cell proliferation in the
HER2-overexpressing cancer cell line NCI-N87. Oncol Rep. 29:13–20.
2013.
|
|
19
|
Zhang Z, Sun P, Liu J, Fu L, Yan J, Liu Y,
Yu L, Wang X and Yan Q: Suppression of FUT1/FUT4 expression by
siRNA inhibits tumor growth. Biochim Biophys Acta. 1783:287–296.
2008. View Article : Google Scholar
|
|
20
|
Hoffmann K, Xiao Z, Franz C, Mohr E, Serba
S, Büchler MW and Schemmer P: Involvement of the epidermal growth
factor receptor in the modulation of multidrug resistance in human
hepatocellular carcinoma cells in vitro. Cancer Cell Int.
11:402011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barancík M, Bohácová V, Kvackajová J,
Hudecová S, Krizanová O and Breier A: SB203580, a specific
inhibitor of p38-MAPK pathway, is a new reversal agent of
P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci.
14:29–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang JM, Vassil AD and Hait WN: Activation
of phospholipase C induces the expression of the multidrug
resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol.
60:674–680. 2001.PubMed/NCBI
|
|
24
|
Katayama K, Yoshioka S, Tsukahara S,
Mitsuhashi J and Sugimoto Y: Inhibition of the mitogen-activated
protein kinase pathway results in the down-regulation of
P-glycoprotein. Mol Cancer Ther. 6:2092–2102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia R, Franklin RA and McCubrey JA: EGF
induces cell motility and multi-drug resistance gene expression in
breast cancer cells. Cell Cycle. 5:2820–2826. 2006. View Article : Google Scholar : PubMed/NCBI
|