Introduction
Pancreatic cancer is the most devastating of human
digestive tract malignant tumors. The high lethality of pancreatic
cancer is primarily due to several reasons, including inapparent
early symptoms and the absence of effective screening tests,
thereby leading to a late presentation of disease and the poor
responsiveness of the tumor to existing therapies (1). To date, surgical resection is the only
potentially curative therapeutic option. However, the majority of
patients present with metastatic disease, rendering their
malignancy inoperable (2).
Conventional chemotherapy is regarded as a widely applied strategy
for the non-surgical treatment of pancreatic cancer. However, it is
still rarely curative for patients with advanced stages of the
disease, particularly in cases of metastatic pancreatic cancer
(3). Therefore, novel and effective
regimens against pancreatic cancer are urgently needed.
Chinese herbal medicine has been proven to be a
valuable resource of potential anticancer agents with minimal
toxicity (4,5). Sedum sarmentosum Bunge (SSB) is
a perennial herb widely distributed on the mountain slopes in Asian
countries and is traditionally used for the treatment of various
inflammatory diseases (6,7). The extract of SSB (SSBE) contains
multiple active chemical components, including quercetin and
isorhamnetin (8–10). Quercetin is a plant-derived
flavonoid that has potential antiviral (11), anticancer (12,13)
and anti-inflammatory properties (14). Isorhamnetin is an
O-methylated flavonol, a type of chemical compound that
exhibits biological activity such as anti-oxidant, anticancer and
improvement of cardiovascular function (15). Thus, these findings support the
concept that SSBE may have potent anti-cancer activity in various
tissues, including the pancreas.
However, a study on the role of the pancreatic
anticancer effects of SSBE has not been performed. Therefore, in
the present study, for the first time, we investigated the effects
of SSBE on pancreatic cancer cells (PCCs) in vitro by
evaluating cellular proliferation and apoptosis, cell cycle and
epithelial-mesenchymal transition (EMT), and in vivo by
observing the tumor development of animal xenograft models of
pancreatic cancer. Furthermore, we also examined the expression
levels of cytokines involved in cell cycle regulation in cultured
PCCs, evaluating the involvement of the Hedgehog signaling
pathway.
Materials and methods
Cell culture
The human pancreatic cancer PANC-1 cell line was
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). PANC-1 cells were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 5% fetal bovine
serum (FBS), 100 µg/ml streptomycin and 100 U/ml penicillin
(all from Invitrogen, Carlsbad, CA, USA). The PANC-1 cells were
seeded on 6-well culture plates to 50–70% confluency in complete
medium containing 5% FBS for 24 h, and then changed to serum-free
medium for 24 h before treatment with SSBE (Lot no. 20101017;
Xuancheng Baicao Plant Industry and Trade Co., Ltd., Anhui, China).
The extraction protocol of SSB was carried out according to a
previous study (16).
CCK-8 assay
Viability of the PANC-1 cells treated with SSBE were
measured using the CCK-8 cell proliferation and cytotoxicity assay
kit (Dojindo, Shanghai, China) according to the manufacturer's
instructions. At the indicated time points, 10 µl of this
reagent at different concentrations was added to each well
containing 100 µl of the cell suspension (5×103
cells) and incubated for an additional 1 h. The absorbance at 450
nm was monitored, and the percent viability of the cells was
calculated by comparison to that of the untreated control cells.
All the experiments were repeated at least three times.
Flow cytometric analysis
The PANC-1 cells treated with SSBE were seeded for
24 h, and then were collected by centrifugation. For apoptosis
analysis, resuspended cells were incubated with Annexin V-FITC and
propidium iodide (PI) at room temperature for 5 min in the dark.
Analysis of Annexin V-FITC binding by flow cytometery (Ex, 488 nm;
Em, 530 nm; BD FACSVerse™) (BD Biosciences, Frankin Lakes, NJ, USA)
was accomplished using FITC signal detector and PI staining by the
phycoerythrin emission signal detector. For cell cycle analysis,
resuspended cells were fixed in 70% ethanol at 4°C overnight and
permeated in 0.1% Triton X-100 at 4°C for 30 min. After incubating
with PI at 4°C for 30 min, PCCs were analyzed using flow cytometry
and the proportion of nuclei in each phase of the cell cycle was
determined using ModFit LT 3.2 software (Verity Software House,
Topsham, ME, USA).
Immunofluorescence staining
The PANC-1 cells were cultured with SSBE on 6-well
plates containing glass slides, and were washed in
phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde
(Sigma-Aldrich, St. Louis, MO, USA) at 4°C for 30 min. After
permeabilization in 0.1% Triton X-100 for 10 min, specimens were
washed in PBS, and then the substrate was blocked with 10% FBS to
eliminate the nonspecific fluorescence. Immunofluorescence staining
was performed according to a previous study (17) using antibodies against proliferating
cell nuclear antigen (PCNA; 1:200; Santa Cruz Biotechnology, Santa
Cruz, CA, USA), c-Myc (1:100; Biogot Technology, Shanghai, China),
α-SMA (1:200), p21 (1:200), Ptch1 (1:200) and Smo (1:200) (all from
Santa Cruz Biotechnology) as the primary antibodies at 4°C
overnight. After washing in PBS three times, the cell preparations
were incubated with DyLight 488 (green)/594 (red)-labeled secondary
antibodies (Sigma-Aldrich) for 1 h at room temperature. After being
washed in PBS, the cell preparations were dropped in appropriate
acacia and covered with a slide. Immunocytochemical studies were
semi-quantitatively or quantitatively assessed by two independent
investigators in a blinded manner.
Western blot analysis
Western blot analyses were examined according to a
previous study (18). Whole
proteins from PANC-1 cells were collected and the protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Beyotime Biotechnology). Whole proteins (20 µg)
from each sample were separated by SDS-PAGE and transferred to a
polyvinylidene difluoride membrane (Solarbio, Beijing, China).
After treatment with 5% skim milk at 4°C overnight, the membranes
were incubated with various antibodies for 1 h, and then incubated
with the appropriate horseradish peroxidase-conjugated secondary
antibody (Beyotime). Bound antibodies were visualized using
chemiluminescence detection on autoradiographic film. Primary
antibodies were as follows: polyclonal anti-Bcl-2 (1:400), Bax
(1:400), Bcl-2 (1:400), caspase-3 (1:400), caspase-8 (1:400) (all
from Beyotime), p53 (1:500; Biogot), p21 (1:200; Biotechnology) and
c-Myc (1:500; Biogot). Quantification was performed by measuring
the intensity of signals using Image-Pro Plus (version 6.0), and
normalized to that for GAPDH (1:10,000; Cell Signaling Technology,
Danvers, MA, USA).
Nude mouse tumorigenicity assay
Male nude mice (BALB/c) weighing 18–22 g and 6–8
weeks old were purchased from the Experimental Animal Centre of
Wenzhou Medical University (Wenzhou, China). Mice were housed in a
temperature-, humidity- and light-controlled environment, and fed a
standard mouse chow and water. Mice were fasted on the day prior to
the experiments being conducted. Before the experiments, all mice
were anesthetized by an intraperitoneal injection with 0.2%
pentobarbital natrium. The left neck of the experimental mice
(n=12) were subcutaneously injected with 5×106 PANC-1
cells in 10 µl of PBS and then received daily intragastric
administration of SSBE (10 and 100 mg/kg·day) for 30 days. Model
mice (n=12) received an injection of 5×106 PANC-1 cells
and daily gastric tube of solvent (PBS), and the healthy group
(n=6) was treated with PBS only. Tumors were monitored daily until
they became cumbersome or necrotic. Tumor volume (V) was measured
every other day based on the formula: V = length2 ×
width, where length was always the longest dimension. After the
experiments, all mice were euthanized by immersion in an ice-water
bath and then burned. The animal study protocols including the
method involving animal euthanasia were approved by the
Institutional Animal Care and use Committee of Wenzhou Medical
university, China. The methods were also performed according to the
guidelines approved by the Institutional Review Board of Wenzhou
Key Laboratory of Surgery, China.
Histopathological examination
The tumor specimens were fixed in formalin and
embedded in paraffin and then cut into 4-µm sections and
stained with hematoxylin and eosin (H&E; Yuanye Biotechnology,
Shanghai, China). Slides were examined and images were captured
using a DM4000 B LED microscope system and a DFC420C 5M digital
microscope camera (both from Leica Microsystems, Wetzlar,
Germany).
Quantitative reverse transcriptase-PCR
(qRT-PCR)
Total RNA was extracted from the PANC-1 cells or
tumor tissues using TRIzol reagent (Invitrogen), and
reverse-transcribed to cDNA templates using a ReverTra Ace qPCR RT
kit (Toyobo, Osaka, Japan). qRT-PCR was performed using SYBR-Green
Real-Time PCR Master Mix Plus (Toyobo). Quality was analyzed on
agarose gels, and quantities were measured using a Varioskan Flash
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Sequence-specific primers for TP53, c-Myc, E-cadherin, α-SMA,
Bcl-2, Bax, Bad, survivin, CCND1, Smo, Ptch1 and Gli1 (Table I) were synthesized by Invitrogen and
GAPDH was used as an endogenous reference gene. Samples were
analyzed in triplicate, and the melting curve was examined to
verify that a single product was amplified. For quantitative
analysis, all samples were analyzed using the ΔΔCT value
method.
 | Table ITwo-step real-time RT-PCR primers for
analysis. |
Table I
Two-step real-time RT-PCR primers for
analysis.
| Gene | Forward sequence
(5′→3′) | Reverse sequence
(5′→3′) | Product size
(bp) |
|---|
| Smo |
AGTTACATCGCAGCCTTC |
CACACTACTCCAGCCATC | 299 |
| Gli1 |
TGCTGACACTCTGGGATA |
CAGGGCCATAGTTGGTT | 132 |
| Ptch1 |
TGGTCACACGAACAATGG |
TGAACTGGGCAGCTATGAAGTC | 202 |
| Survivin |
CACCGCATCTCTACATTCAAG |
CGGACGAATGCTTTTTATGT | 268 |
| TP53 |
TCACCATCATCACACTGGAAGACTC |
TTGGGCAGTGCTCGCTTAGT | 175 |
| Bax |
TTTCTGACGGCAACTTCAACTG |
CGGAGGAAGTCCAATGTCCAG | 136 |
| Bad |
CTCCGGCAAGCATCATCG |
CCCATCCCTTCGTCGTCCT | 162 |
| Bcl-2 |
CAACACAGACCCACCCAGA |
TGGCTTCATACCACAGGTTTC | 134 |
| c-Myc |
CCTCCACTCGGAAGGACTATC |
TTCGCCTCTTGACATTCTCC | 135 |
| CCND1 |
CCTGTCCTACTACCGCCTCA |
TCCTCCTCTTCCTCCTCCTC | 165 |
| E-cadherin |
TGGACCGAGAGAGTTTCC |
AATATGGTGTATACAGCCTC | 250 |
| α-SMA |
CGTGGCTACTCCTTCGTG |
TGATGACCTGCCCGTCT | 160 |
| GAPDH |
TCCCATCACCATCTTCCAGG |
GATGACCCTTTTGGCTCCC | 145 |
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All statistical analyses were performed using a
Statistical Package for Social Sciences (version 16.0; SPSS, Inc.,
Chicago, IL, USA). A two-sided Student's t-test was used to analyze
differences between the two groups. A one-way analysis of variance
was used when >2 groups were present. A P-value of <0.05 was
considered to indicate a statistically significant result.
Results
Effect of SSBE on the proliferation and
apoptosis of PCCs
To investigate whether SSBE exerts a protective
effect on pancreatic cancer, we first evaluated cell proliferation
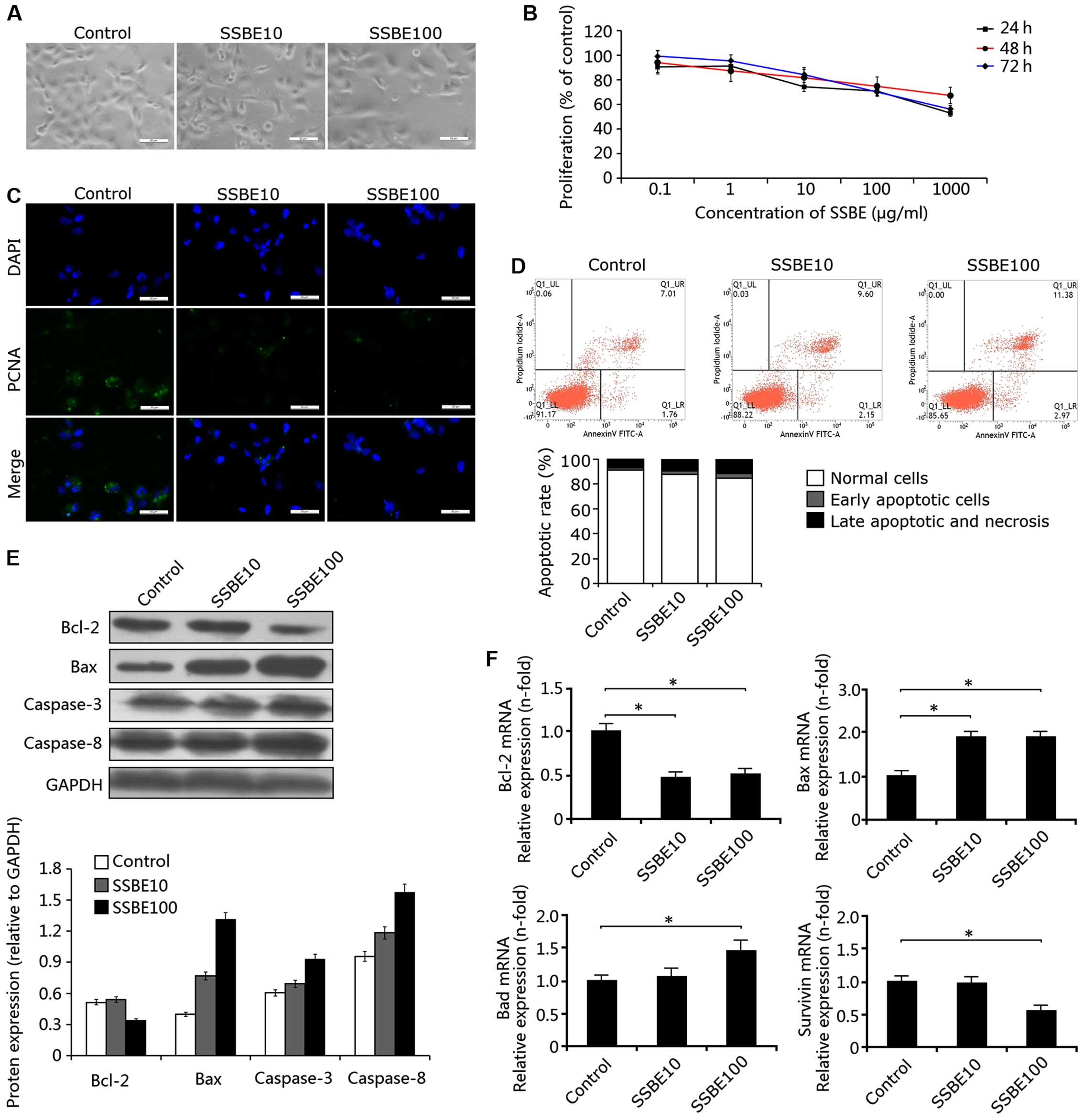
in the SSBE-treated PCCs. As shown in Fig. 1A, evidence from phase contrast
microscopy showed that the injury of PANC-1 cells induced by SSBE
was significantly aggravated, and cell number was decreased. CCK-8
assay revealed that this inhibitory effect of SSBE on cell
proliferation was concentration-dependent, but not time-dependent
(Fig. 1B). In addition,
immunofluorescence staining indicated that SSBE treatment
significantly downregulated the expression of PCNA (Fig. 1C). Thus, these findings suggested
that SSBE has a marked inhibitory effect on the proliferation of
PCCs.
Secondly, we examined the effect of SSBE on cellular
apoptosis. In the SSBE-treated PANC-1 cells, marked cellular
apoptosis was observed. As shown in Fig. 1D, the number of apoptotic cells
(including early and late apoptotic and necrotic cells) in the
controls was 8.77%, but reached 14.35% in the SSBE (100
µg/ml)-treated PANC-1 cells, suggesting that SSBE
significantly increased the number of apoptotic cells in a
concentration-dependent manner. In addition, western blot analyses
showed that SSBE treatment increased the protein expression of Bax,
caspase-3 and caspase-8, and decreased the protein expression of
Bcl-2 (Fig. 1E). Moreover,
upregulated gene expression of Bax and Bad, and downregulated gene
expression of Bcl-2 were observed in the PANC-1 cells treated with
SSBE (Fig. 1F). Thus, these data
indicated that SSBE-induced apoptosis of PCCs may be through a
mitochondrial-mediated pathway. This induction of apoptosis of PCCs
after SSBE treatment was also identified by the downregulation of
mRNA expression of survivin (also called baculoviral inhibitor of
apoptosis repeat-containing 5), a member of the inhibitor of
apoptosis (IAP) family (Fig. 1F)
(19).
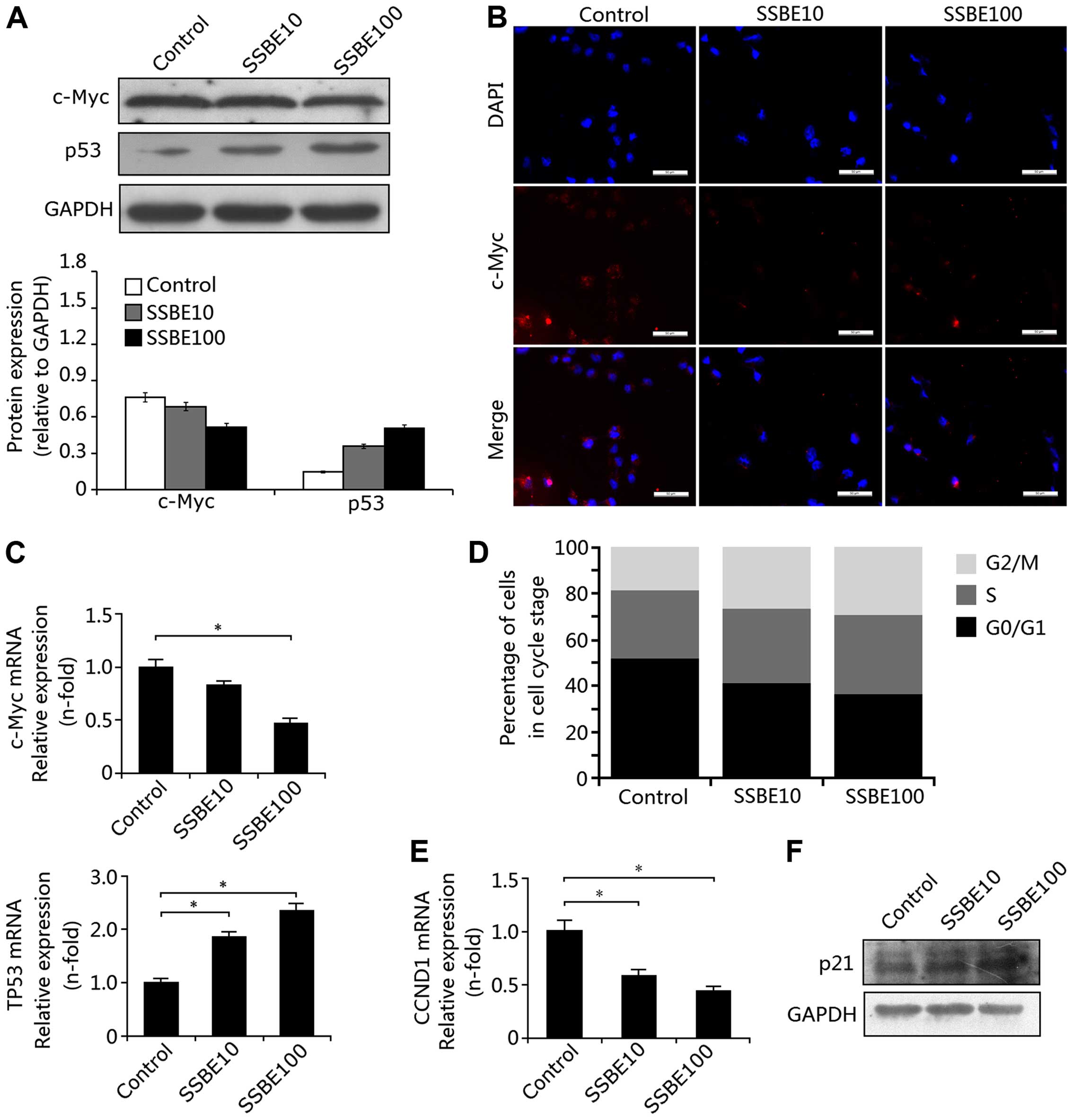
Effect of SSBE on the expression of c-Myc
and p53 in PCCs
In SSBE-treated PANC-1 cells, the protein expression
of c-Myc, determined by western blotting was significantly
decreased and the expression of p53 was increased (Fig. 2A). Reduction in c-Myc expression was
also confirmed by immunofluorescence staining (Fig. 2B). In addition, downregulated mRNA
expression of Myc and upregulated expression of TP53 were also
observed in the PANC-1 cells following SSBE treatment (Fig. 2C). These findings indicated that
SSBE inhibits c-Myc expression and promotes p53 expression in PCCs.
Previous studies have shown that c-Myc (encoded by the gene Myc)
has an important role in apoptosis, cell cycle progression and
cellular transformation (20).
Enhanced expression of c-Myc induces the unregulated expression of
many genes, some of which are involved in cell proliferation.
Similarly, as a tumor-suppressor protein, p53 (encoded by the gene
Tp53) plays a crucial role in cellular apoptosis, genomic stability
and inhibition of angiogenesis (21). Low expression of p53 induces G2/M
transition in various cancer cells. In the present study, SSBE
inhibited gene and protein expression of c-Myc and promoted the
expression of p53, thereby inducing the cellular apoptosis of PCCs
and inhibiting proliferation.
Effect of SSBE on cell cycle phase
distribution in PCCs
The induction of cellular apoptosis of PCCs and
inhibition of proliferation may be a result of cell cycle arrest.
Thus, we examined the effect of SSBE on the cell cycle. Flow
cytometric analysis showed that the percentage of G0/G1 phase PCCs
in the SSBE-treated PCCs was significantly decreased compared with
this percentage in the controls, and the percentage of G2/M-phase
cells was increased (Fig. 2D),
indicating that SSBE treatment induced excessive accumulation and
cell cycle arrest of PCCs at the G2/M phase. Further study showed
that the SSBE-induced cell cycle arrest of PCCs was through the
downregulation of the expression of cyclin D1 (encoded by the gene
CCND1) (Fig. 2E) and upregulation
of the expression of cell cycle inhibitor p21 (Fig. 2F). Cyclin D1 is a protein required
for progression through the G1 phase of the cell cycle (22). As a regulatory subunit of
cyclin-dependent kinases CDK4, the cyclin D1-CDK4 complex promotes
passage through the G1 phase by inhibiting the retinoblastoma
protein (pRb), and enables the activation of cyclin E-CDK2 complex
by sequestering CDK inhibitory protein p21 (23). SSBE-induced changes in gene and
protein expression of PCCs drive cell cycle arrest at the G2/M
phase.
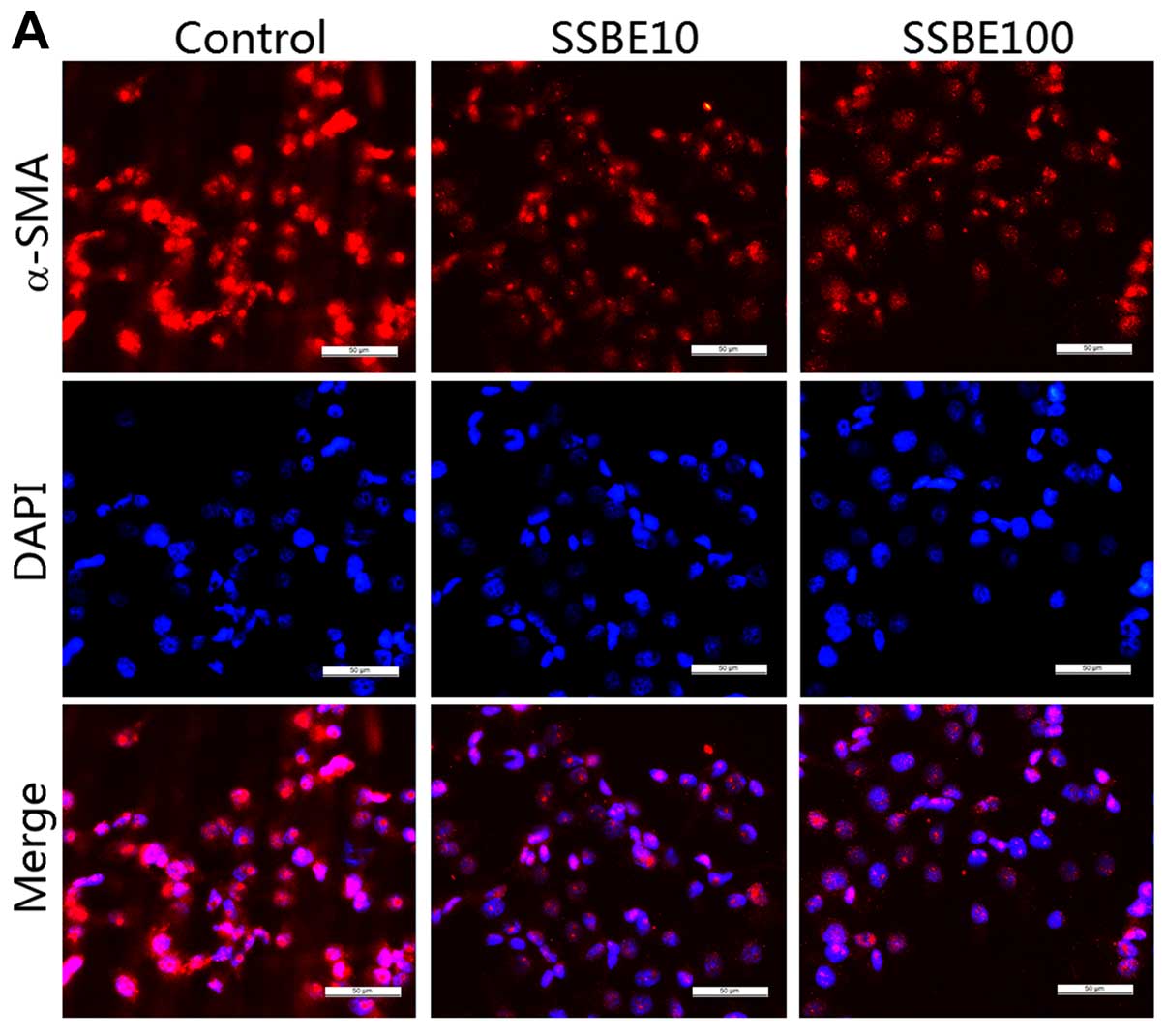
Effect of SSBE on EMT in PCCs
EMT is a process whereby epithelial cells lose their
epithelial phenotypes such as E-cadherin, and regain new
characteristic features of mesenchymal molecules including α-smooth
muscle actin (α-SMA) (24). EMT is
critical for tumor invasion and metastasis (25). In the present study, the gene and
protein expression levels of α-SMA in PANC-1 cells were
downregulated by SSBE treatment (Fig.
3A and B). Additionally, the expression of E-cadherin was
upregulated in the SSBE-treated cells (Fig. 3B). Thus, SSBE inhibited the EMT
process of PCCs, resulting in the reduction in the ability for
invasion and metastasis.
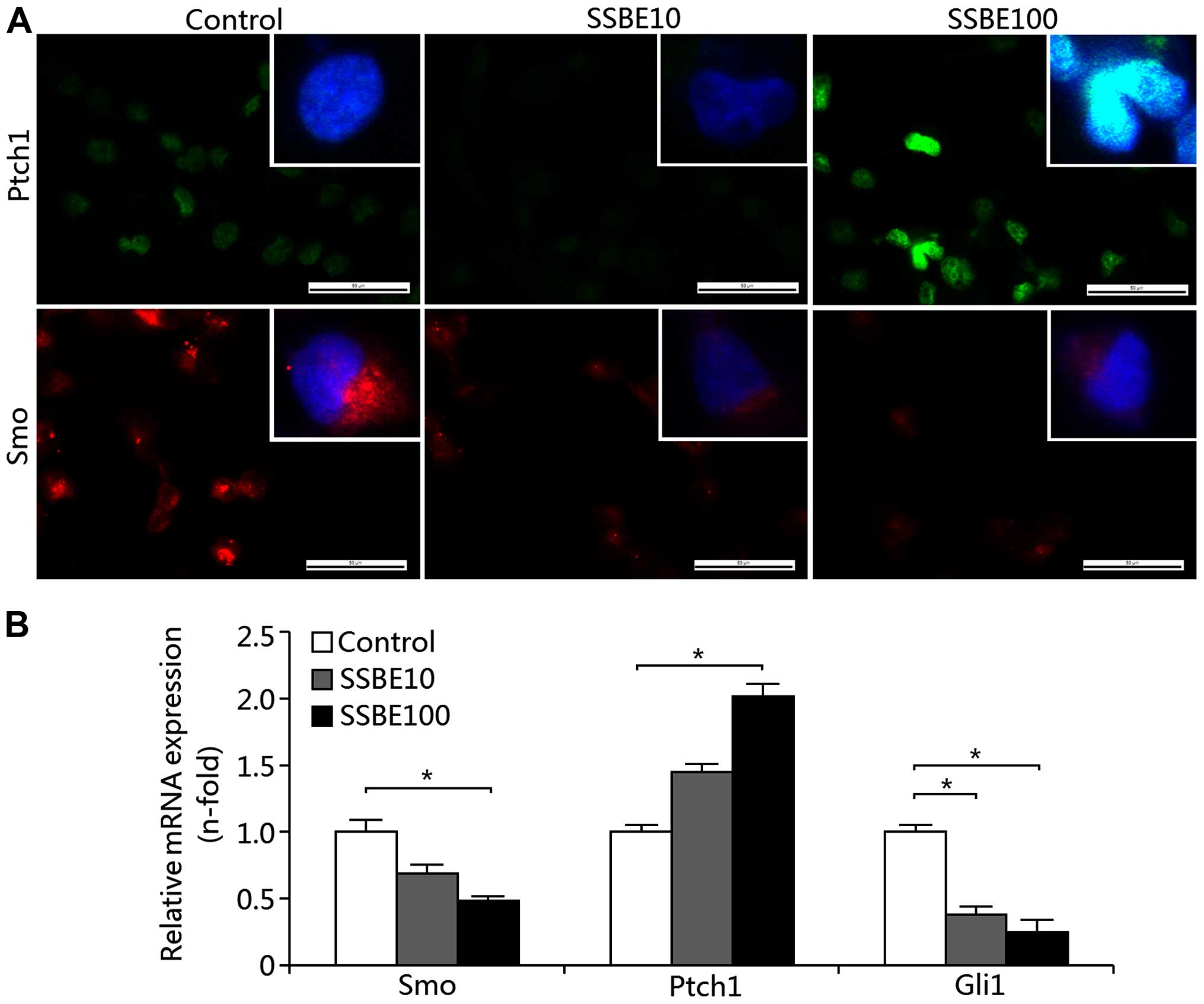
Effect of SSBE on the activity of
Hedgehog signaling in PCCs
As mentioned above, SSBE has an inhibitory effect on
the proliferation of PCCs. This inhibition of proliferation may be
associated with the activation of proliferation-related signaling.
Thus, we examined the activity of the Hedgehog signaling pathway in
the SSBE-treated PANC-1 cells. Our results showed that SSBE
treatment increased the protein expression of Ptch1 as determined
by immunofluorescence staining (Fig.
4A) and the gene expression as determined by qRT-PCR (Fig. 4B). As a crucial receptor of
canonical Hedgehog signaling, enhanced Ptch1 expression inhibits
the activation of signaling. In addition to these, SSBE also
decreased the expression of Smo and Gli1 (Fig. 4A and B), which are targets of
activated Hedgehog signaling, suggesting that activity of the
Hedgehog pathway in PCCs was reduced by SSBE treatment. As a
result, SSBE inhibits the proliferation of PCCs.
Effect of activated Hedgehog signaling on
SSBE-mediated inhibition of PCC proliferation
Since SSBE treatment exerts its inhibitory effect on
the activation of the Hedgehog signaling pathway in PCCs, we
ascertained whether regulation of Hedgehog signaling is involved in
SSBE-mediated inhibition. In the present study, exogenous
recombinant protein sonic Hedgehog (Shh) was used to activate
Hedgehog signaling in the SSBE-treated PANC-1 cells. We found that
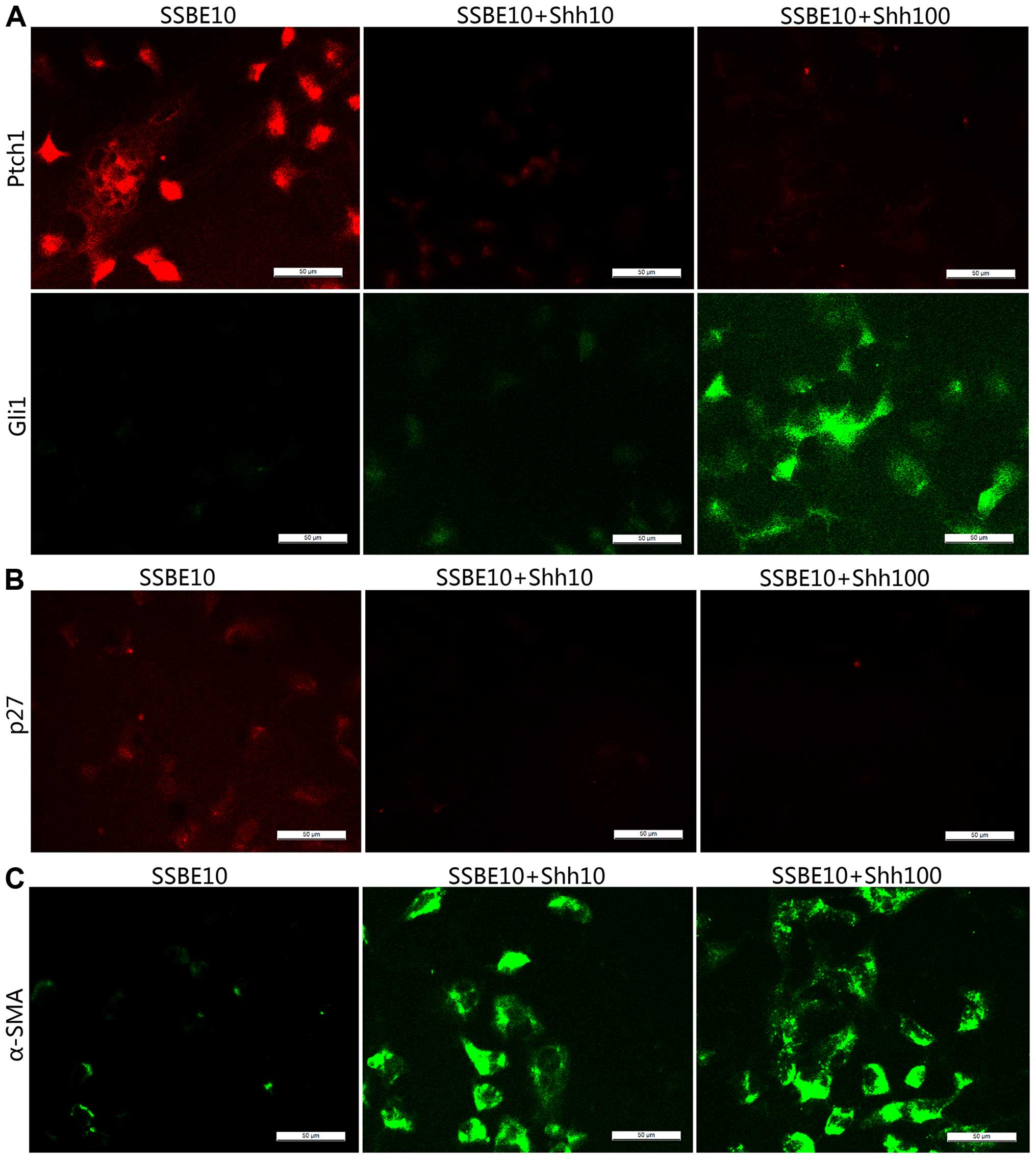
Shh abolished SSBE-mediated upregulated expression of Ptch1 and
downregulated expression of Gli1 (Fig.
5A), indicating that Hedgehog signaling was reactivated,
resulting in the reduction of p27 and α-SMA (Fig. 5B and C). p27, also called Kip1,
regulates the cell cycle by inhibiting the checkpoint kinase
cdk2/cyclin E and blocking cell cycle progression through G1/S
transition. Downregulated expression of p27 abolishes cell cycle
arrest, and thereby promotes proliferation and inhibits apoptosis.
Thus, our in vitro experiment indicated that the Hedgehog
signaling pathway plays an important role in SSBE-mediated
inhibition of PCC proliferation and EMT.
Effect of SSBE on Hedgehog signaling
activity in animal xenograft models of pancreatic cancer
To assess whether similar anticancer effects also
occur in vivo, SSBE (10 and 100 mg/kg·day) was administered
continuously for 30 days to mice subjected to injection of PCCs.
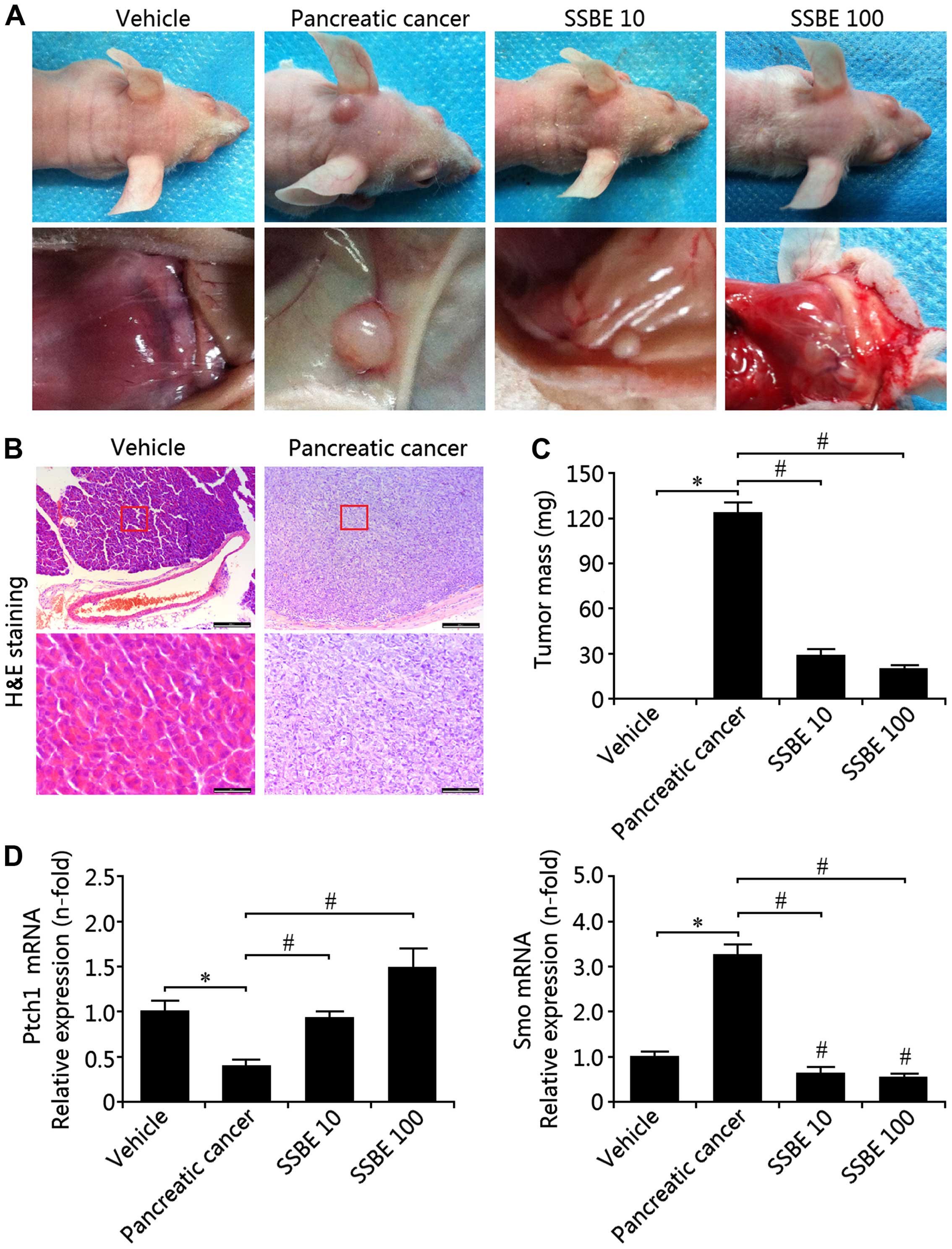
Fig. 6A shows the morphological
changes in the experimental groups as a result of SSBE treatment.
H&E staining identified the pathological results of pancreatic
cancer in tissues of the model group (Fig. 6B) and the tissue mass of the model
group was significantly increased when compared with the vehicle
group (Fig. 6C). Following SSBE
treatment, the mass of the tumor tissues was significantly
decreased in a concentration-dependent manner (Fig. 6C). Thus, these findings suggest that
SSBE exerts its inhibitory effect on tumor growth. Further study
showed that the mRNA expression of Ptch1 was decreased in the model
group, and the expression of Smo was increased, indicating that
Hedgehog signaling was activated in the occurrence and development
of pancreatic cancer (Fig. 6D).
This activation of Hedgehog signaling in the model group was
inhibited by SSBE administration. Therefore, these findings
reconfirmed that SSBE-mediated antitumor activity may be through
suppression of Hedgehog signaling.
Discussion
In the present study, we investigated the effects of
SSBE on pancreatic cancer using a cell culture system and an
experimental mouse model. In cultured PCCs, SSBE exerted its
protective effects by inhibiting cell proliferation and inducing
marked apoptosis. This SSBE-mediated apoptosis appeared to be
through a mitochondrial-dependent pathway. In addition, SSBE
treatment induced cell cycle arrest at the G2/M phase by
upregulating the expression of p21. Our further study showed that
cell proliferation of PCCs was triggered by the activation of
Hedgehog signaling, which was inhibited by SSBE treatment. In the
animal xenograft models of pancreatic cancer, the activity of
Hedgehog signaling was increased, but was inhibited by SSBE
administration. Thus, our results provide a rationale for the use
of SSBE as a potential supplemental treatment to attenuate
pancreatic cancer.
Hedgehog signaling is a stem-related pathway that
plays a key role during embryogenesis and tissue regeneration
(26). Aberrant activation of the
Hedgehog signaling pathway results in pathological consequences,
including a variety of human tumors, such as pancreatic cancer
(27). In pancreatic tissues after
injury, activation of the Hedgehog pathway is triggered through
binding of the ligands, including Shh, to its membrane receptor
patched1 (Ptch1). The binding of ligands to Ptch1 relieves signal
transducer Smoothened (Smo) and activates a cascade that leads to
translocation of the transcription factor Gli1 to the nucleus
(28). As a result, activated
Hedgehog signaling promotes cell proliferation and tissue
regeneration by upregulating the expression of downstream target
genes, such as c-Myc and cyclin D1 (29). Thus, the Hedgehog signaling pathway
is important for the occurrence and development of pancreatic
cancer.
In patients with pancreatic cancer, the level of
plasma Shh was found to be higher than that in healthy individuals
(30). Enhanced levels of plasma
Shh were also found in various PCCs (31). In addition, abnormal expression of
Ptch1 and Smo are identified to be associated with tumor size,
tumor differentiation, lymph node metastasis and clinical stage
(32). Furthermore, aberrant
methylation of the human Hedgehog interacting protein (HHIP) gene
contributes to increased Hedgehog signaling and is closely related
to pancreatic neoplasms (33).
Thus, these findings elucidate the crucial role of the Hedgehog
signaling pathway in pancreatic cancer. In the present study,
upregulated gene and protein expression of Smo and Gli1, and
downregulated expression of Ptch1 in PCCs were observed.
Additionally, activated Hedgehog signaling was also confirmed in
the animal xenograft models of pancreatic cancer. Further study
showed that this activation of Hedgehog signaling may result in
abnormal expression of downstream target genes. For instance,
enhanced c-Myc expression and reduced p53 expression were observed
in pancreatic cancer cells. The target genes subsequently regulated
the p21 expression, which controls the cell cycle. As a result,
Hedgehog signaling-mediated hyperproliferation of pancreatic cells
results in carcinogenesis. These findings reconfirmed the
importance of Hedgehog signaling in pancreatic cancer, and blockade
of this pathway may be a strategy for the treatment of pancreatic
cancer (34,35).
In the present study, we revealed that SSBE, a
traditional Chinese herbal medicine, has a marked anti-pancreatic
cancer activity. To date, few studies have investigated the
anticancer activity of SSBE. A study by Huang et al reported
that SSBE has potential for preventing and inhibiting
hepatocellular carcinoma growth, which is associated with the
apoptosis of cancer cells (36). In
addition, the mRNA and protein expressions of vascular endothelial
growth factor (VEGF) and the protein level of p-STAT3 were
significantly decreased after SSBE treatment. Thus, SSBE may have
an anti-angiogenesis and apoptotic induction effect on PCCs. Our
findings also confirmed the above conclusion that SSBE inhibited
the proliferation and induced marked apoptosis of PCCs. Our further
study revealed that this inhibitory effect of SSBE on PCCs may be
associated with cell cycle arrest at the G2/M phase by upregulating
p21 expression. SSBE-mediated overexpression of p21 was negatively
correlated with c-Myc expression, but positively correlated with
p53 expression. In addition, SSBE decreased the gene expression of
cyclin D1, aggravating the inhibitory effect on the cell cycle.
Moreover, SSBE also suppressed the EMT response of PCCs, thereby
reducing the ability of invasion and metastasis of tumors. In
animal xenograft models of pancreatic cancer, SSBE markedly
inhibited tumor growth. Thus, SSBE may be an effective therapeutic
drug for pancreatic cancer.
Further study revealed that SSBE exerted its
anticancer effects by antagonizing the activation of Hedgehog
signaling. Like other studies, our study also identified activated
Hedgehog signaling in PCCs and in animal xenograft models of
pancreatic cancer. With the treatment of SSBE, the activation of
Hedgehog signaling was inhibited in a concentration-dependent
manner. In cultured PCCs, exogenous recombinant protein Shh was
used to induce the activation of Hedgehog signaling, and resulted
in the abolishment of apoptotic induction and EMT response. In
pancreatic cancer models, SSBE administration also suppressed the
activity of Hedgehog signaling. Thus, Hedgehog signaling may be a
crucial mechanism for the SSBE-mediated anticancer effects. Our
study also supported the conclusion that inhibition of Hedgehog
signaling has a protective effect on pancreatic cancer (34). However, it is necessary to note that
the clinical application of SSBE needs more investigation,
including safety and effectiveness. In addition, as a Chinese
herbal medicine, SSBE contains many complicated and uncertain
chemical components, and thereby may exhibit unpredictable and
complex effects, thus the drug dosage and molecular mechanisms of
SSBE must be elucidated.
In conclusion, SSBE exerts its pancreatic anticancer
activity by suppressing the Hedgehog pathway in vitro and
in vivo, resulting in the inhibition of proliferation and
induction of apoptosis of PCCs. Thus, SSBE has potential for the
treatment of pancreatic cancer.
Acknowledgments
The present study was supported in part by research
grants from the Natural Science Foundation of China (30772548), and
the Foundation from the Excellent Master Dissertation of the
College of Laboratory Medicine at Chongqing Medical University
(201202).
References
|
1
|
Douglass HO Jr: Adjuvant therapy for
pancreatic cancer. World J Surg. 19:270–274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kayahara M, Funaki K, Tajima H, Takamura
H, Ninomiya I, Kitagawa H and Ohta T: Surgical implication of
micrometastasis for pancreatic cancer. Pancreas. 39:884–888. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richter J and Saif MW: Updates in adjuvant
therapy in pancreatic cancer: Gemcitabine and beyond. Highlights
from the '2010 ASCO Gastrointestinal Cancers Symposium'; Orlando,
FL, USA. January 22–24, 2010;
JOP. 11:144–147. 2010.
|
|
4
|
Woo JH, Li D, Wilsbach K, Orita H, Coulter
J, Tully E, Kwon TK, Xu S and Gabrielson E: Coix seed extract, a
commonly used treatment for cancer in China, inhibits NFkappaB and
protein kinase C signaling. Cancer Biol Ther. 6:2005–2011. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Habib SH, Makpol S, Abdul Hamid NA, Das S,
Ngah WZ and Yusof YA: Ginger extract (Zingiber officinale) has
anti-cancer and anti-inflammatory effects on ethionine-induced
hepatoma rats. Clinics. 63:807–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heo BG, Park YS, Chon SU, Lee SY, Cho JY
and Gorinstein S: Antioxidant activity and cytotoxicity of methanol
extracts from aerial parts of Korean salad plants. BiofaIctors.
30:79–89. 2007. View Article : Google Scholar
|
|
7
|
Jung HJ, Kang HJ, Song YS, Park EH, Kim YM
and Lim CJ: Anti-inflammatory, anti-angiogenic and anti-nociceptive
activities of Sedum sarmentosum extract. J Ethnopharmacol.
116:138–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morikawa T, Zhang Y, Nakamura S, Matsuda
H, Muraoka O and Yoshikawa M: Bioactive constituents from Chinese
natural medicines. XXII Absolute structures of new megastigmane
glycosides, sedumosides E1, E2,
E3, F1, F2, and G, from Sedum
sarmentosum (Crassulaceae). Chem Pharm Bull. 55:435–441. 2007.
View Article : Google Scholar
|
|
9
|
Ninomiya K, Morikawa T, Zhang Y, Nakamura
S, Matsuda H, Muraoka O and Yoshikawa M: Bioactive constituents
from Chinese natural medicines. XXIII Absolute structures of new
megastigmane glycosides, sedumosides A4, A5,
A6, H, and I, and hepatoprotective megastigmanes from
Sedum sarmentosum. Chem Pharm Bull. 55:1185–1191. 2007. View Article : Google Scholar
|
|
10
|
Oh H, Kang DG, Kwon JW, Kwon TO, Lee SY,
Lee DB and Lee HS: Isolation of angiotensin converting enzyme (ACE)
inhibitory flavonoids from Sedum sarmentosum. Biol Pharm Bull.
27:2035–2037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johari J, Kianmehr A, Mustafa MR, Abubakar
S and Zandi K: Antiviral activity of baicalein and quercetin
against the Japanese encephalitis virus. Int J Mol Sci.
13:16785–16795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Song L, Wang H and Xing N:
Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth
and inducing apoptosis in human prostate cancer cells. Oncol Rep.
30:357–363. 2013.PubMed/NCBI
|
|
13
|
Youn H, Jeong JC, Jeong YS, Kim EJ and Um
SJ: Quercetin potentiates apoptosis by inhibiting nuclear
factor-kappaB signaling in H460 lung cancer cells. Biol Pharm Bull.
36:944–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahmoud MF, Hassan NA, El Bassossy HM and
Fahmy A: Quercetin protects against diabetes-induced exaggerated
vasoconstriction in rats: Effect on low grade inflammation. PLoS
One. 8:e637842013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J, Sun G, Meng X, Wang H, Luo Y, Qin
M, Ma B, Wang M, Cai D, Guo P, et al: Isorhamnetin protects against
doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One.
8:e645262013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai Y, Lu H, Zhang G, Wu C, Lin C, Liang Y
and Chen B: Sedum sarmentosum Bunge extract exerts renal
anti-fibrotic effects in vivo and in vitro. Life Sci. 105:22–30.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai Y, Lu H, Wu C, Liang Y, Wang S, Lin C,
Chen B and Xia P: Resveratrol inhibits epithelial-mesenchymal
transition and renal fibrosis by antagonizing the hedgehog
signaling pathway. Biochem Pharmacol. 92:484–493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu F, Guo Y, Chen B, Dong P and Zheng J:
MicroRNA-17-5p activates hepatic stellate cells through targeting
of Smad7. Lab Invest. 95:781–789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Athanasoula KC, Gogas H, Polonifi K,
Vaiopoulos AG, Polyzos A and Mantzourani M: Survivin beyond
physiology: Orchestration of multistep carcinogenesis and
therapeutic potentials. Cancer Lett. 347:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMahon SB: MYC and the control of
apoptosis. Cold Spring Harb Perspect Med. 4:a0144072014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edwards PC, Ruggiero S, Fantasia J,
Burakoff R, Moorji SM, Paric E, Razzano P, Grande DA and Mason JM:
Sonic hedgehog gene-enhanced tissue engineering for bone
regeneration. Gene Ther. 12:75–86. 2005. View Article : Google Scholar
|
|
27
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varjosalo M and Taipale J: Hedgehog:
Functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katoh Y and Katoh M: Hedgehog signaling
pathway and gastric cancer. Cancer Biol Ther. 4:1050–1054. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Zaatari M, Daignault S, Tessier A,
Kelsey G, Travnikar LA, Cantu EF, Lee J, Plonka CM, Simeone DM,
Anderson MA, et al: Plasma Shh levels reduced in pancreatic cancer
patients. Pancreas. 41:1019–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W,
Lei J, Ma J, Wang X, Lv S, et al: Sonic hedgehog paracrine
signaling activates stromal cells to promote perineural invasion in
pancreatic cancer. Clin Cancer Res. 20:4326–4338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Tian X, Xie X, Zhuang Y, Wu W and
Wang W: Expression and regulation of hedgehog signaling pathway in
pancreatic cancer. Langenbecks Arch Surg. 395:515–525. 2010.
View Article : Google Scholar
|
|
33
|
Martin ST, Sato N, Dhara S, Chang R,
Hustinx SR, Abe T, Maitra A and Goggins M: Aberrant methylation of
the human Hedgehog interacting protein (HHIP) gene in pancreatic
neoplasms. Cancer Biol Ther. 4:728–733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, An Y, Wang X, Zha W and Li X:
Inhibition of the Hedgehog pathway induces autophagy in pancreatic
ductal adenocarcinoma cells. Oncol Rep. 31:707–712. 2014.
|
|
35
|
Guo J, Gao J, Li Z, Gong Y, Man X, Jin J
and Wu H: Adenovirus vector-mediated Gli1 siRNA induces growth
inhibition and apoptosis in human pancreatic cancer with
Smo-dependent or Smo-independent Hh pathway activation in vitro and
in vivo. Cancer Lett. 339:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang D, Zhang W, Huang D and Wu J:
Antitumor activity of the aqueous extract from Sedum sarmentosum
Bunge in vitro. Cancer Biother Radiopharm. 25:81–88. 2010.
View Article : Google Scholar : PubMed/NCBI
|