Introduction
Osteosarcoma (OS) is the most common form of
non-hematopoietic primary bone tumor occurring mostly in young
adults and adolescents. Currently, the favored treatment for OS
involves neoadjuvant chemotherapy, followed by surgery and
chemotherapy again, which has led to a significant improvement in
the 5-year survival rate (60–70%) in patients without metastases
(1). However, 35–55% of OS patients
with initial localized disease subsequently experience recurrence.
Moreover, the induction of drug resistance and the unwanted
side-effects involved in chemotherapy result in inadequate
treatment of the disease (2). Thus,
there is an urgent need to discover new natural or synthetic
compounds with the potential to prevent OS progression and improve
patient survival rates.
Diallyl trisulfide (DATS) is a natural garlic
extract with pungent odor and evaporability. DAT is one of the main
active compounds which is a sulfide with an allyl group. Scientific
investigations have shown that DATS can reduce the risk of
cardiovascular disease and diabetes, stimulate the immune system
and protect against infections. Meanwhile, considerable research
and epidemiologic studies have revealed that DATS has
broad-spectrum anti-neoplastic activity. Epidemiologic studies
continue to support the premise that dietary intake of
Allium vegetables, such as garlic, may be protective against
the risk of certain types of cancers (3,4). It
can induce apoptosis of multiple cancer cells, such as those of
human gastric, colon, breast and prostate cancer (5–7). It
has been reported that DATS enhanced the expression of the p38
mitogen-activated protein kinase/caspase-3 signaling pathway and
induced apoptosis in gastric carcinoma cell lines (5). DATS also inhibited the growth of
transplanted tumor xenografts by inducing apoptosis and/or by
blocking abnormal cell cycle phase (8–10), and
inhibited cell migration and invasion via the downregulation of
matrix metalloproteinases (MMPs) (6,11,12).
In addition, several studies found that reactive oxygen species
(ROS) play an important role in DATS-induced death of cancer cells
(13–15). However, recently, various studies
have shown that DATS-induced apoptosis involves the
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways
(16,17). As is known, the PI3K/Akt signaling
pathway is one of the most important oncogenic pathways in cancers,
and is deregulated in the vast majority of localized disease and
100% of advanced-stage disease in OS (18). This implies that alterations in this
pathway may be a prerequisite for OS progression. Thus, numerous
small-molecule compounds, particularly various natural compounds or
derivatives, targeting the PI3K/Akt signaling pathway, have been
developed and show promise for improving the survival of OS
patients. Our previous studies demonstrated that DATS suppressed OS
cell proliferation and reversed the drug resistance and lowered the
ratio of CD133+ cells in conjunction with methotrexate
(19,20). However, it is unclear whether the
apoptosis induced by DATS in OS cells is related to the PI3K/Akt
signaling pathway. There is little research concerning the effect
of DATS on human OS cells as well as the molecular mechanism. In
the present study, the effect of DATS and the possible molecular
mechanism were further studied in human OS cells. Our data
demonstrated that DATS induced apoptosis through the ROS-mediated
down-regulation of the PI3K/Akt pathway, thereby demonstrating DATS
as a promising therapeutic agent for the treatment of OS.
Materials and methods
Drugs and antibodies
DATS was purchased from LKT Laboratories (St. Paul,
MN, USA) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich
Chemical Co., St. Louis, MO, USA), and then diluted with the medium
to the desired concentration prior to use. The final DMSO
concentration was <0.1% and has been verified not to interfere
with the test system employed. N-acetylcysteine (NAC) and
JC-1 were purchased from Sigma-Aldrich Chemical Co. The Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies Inc. (Kumamoto, Japan). Annexin V-FITC and propidium
iodide (PI) double staining kit was obtained from Nanjing Kaiji
Biotechnology Co., Ltd. (Nanjing, China).
2,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was obtained
from Jiancheng Bio-Company (Nanjing, China). Rabbit anti-human
antibodies against PI3Kp110β, PI3Kp85α, Bad, Bax, Bcl-xL,
cytochrome c, caspase-9 and -3, cleaved PARP, p21, p27 and
GAPDH were purchased from Abcam (Cambridge, UK). Rabbit anti-human
antibodies against Akt, p-Akt (Ser473), Bcl-2, cyclin D1 and
LY294002 (the PI3K inhibitor) were purchased from Cell Signaling
Technology (Beverly, MA, USA). Horseradish peroxidase
(HRP)-conjugated secondary antibodies against rabbit IgG were
obtained from Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing,
China). The enhanced chemiluminescence (ECL) detection kit
(Immobilon Western Chemiluminescent HRP Substrate) was obtained
from Merck Millipore (Billerica, MA, USA). All other chemicals and
reagents were commercially available and of standard biochemical
quality.
Cell lines and culture
Human OS cell lines MG-63 and MNNG/HOS were obtained
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (both from Invitrogen,
Carlsbad, CA, USA) in a humidified incubator with 5% CO2
at 37°C.
Cell viability assay and morphological
observation
Cell viability was determined using the CCK-8 assay.
The MG-63 and MNNG/HOS cells were seeded in 96-well plates at
1×104 cells/well in 100 µl culture medium. After
overnight incubation, the cells were treated with different
concentrations (5, 10, 20, 40, 80, 100 and 120 µM) of DATS
for 24, 48 and 72 h. CCK-8 (10 µl) was added into the
culture well and the cells were incubated for 2 h at 37°C with 5%
CO2 in a humidified incubator. The viability of the
cells was measured by absorption at 490 nm using an ELISA reader
(BioTek, Winooski, VT, USA). Inhibitory ratio (%) = (OD control −
OD treated)/OD control × 100%. For assessment of cell morphology
after exposure to DATS, a total of 4×105 cells/well of
MG63 and MNNG/HOS cells was cultured into 6-well plates at 37°C
overnight, and then each well was treated with 0, 20, 40 and 80
µM DATS for 48 h. The cells in each well were examined under
a phase-contrast microscope and then were photographed (Olympus,
Melville, NY, USA).
Flow cytometric analysis of apoptosis and
cell cycle distribution
The apoptosis of the MG63 and MNNG/HOS cells was
examined by flow cytometry using Annexin V-FITC/PI staining.
Briefly, the cells were cultured in 6-well plates (2×105
cells/well) overnight and then were treated with the indicated
concentrations (0, 20, 40 and 80 µM) of DATS for 24 or 48 h.
Both attached and floating cells were accumulated and washed twice
with ice-cold phosphate-buffered saline (PBS; resus-pended in 500
µl binding buffer). The samples were treated with 5
µl Annexin V-FITC and 5 µl PI, and incubated at room
temperature for 15 min in the dark. Then the cells were determined
by flow cytometry (BD Calibur). For cell cycle analysis, after
treatment with different concentrations (0, 20 and 40 µM) of
DATS for 48 h and fixation with 70% ice-cold ethanol overnight at
4°C, the cells were centrifuged and treatment with RNase A (20
µl in 500 µl PBS) for 30 min at 37°C. Subsequently,
the cells were exposed to 400 µl PI and incubated at room
temperature for 30 min in the dark and measured by flow cytometry
(BD Calibur). In some groups for analysis of apoptosis and cell
cycle distribution, the cells were pretreated with 5 mM NAC for 2 h
and then co-treated with the indicated concentration of DATS for a
specific time. All the data concerning apoptosis and cell cycle
distribution were calculated and analyzed using FlowJo
software.
Measurement of ROS
The levels of intracellular ROS in the human OS
MG-63 and MNNG/HOS cells was examined using the fluorescent probe
DCFH-DA. Briefly, the MG-63 and MNNG/HOS cells were seeded in
6-well plates (2×104 cells/well) overnight and treated
with DATS (0, 20, 40 and 80 µM) and incubated for 4, 8, 16
and 24 h, respectively. Cells were washed twice with PBS and loaded
with 10 µM DCFH-DA for 30 min in the dark. In some groups,
the cells were pretreated with 5 mM NAC for 2 h and then co-treated
with DATS. The total level of ROS was measured by the changes in
the mean fluorescence intensity (MFI) under a fluorescence
microscope (Olympus) and by flow cytometric analyses (BD Calibur),
respectively, (excitation wavelength, 488 nm; emission wavelength,
530 nm).
Determination of mitochondrial membrane
potential (Δψm)
The MG-63 and MNNG/HOS cells were seeded into 6-well
plates (2×105 cells/well) and incubated overnight, and
then the cells were treated with 40 µM DATS for 24 h. The
other cell groups were pretreated with 5 mM NAC for 2 h and then
co-treated with 40 µM DATS for 24 h. After treatment, the
cells were harvested and incubated with 1 µl JC-1 which was
diluted in 500 µl 1X incubation buffer. Then, the stained
cells were washed twice with 1X incubation buffer and resuspended
in 500 µl 1X incubation buffer. The fluorescent intensity
was measured using flow cytometric analyses (BD Calibur). The JC-1
dye has an excitation of 488 nm and an emission of 530/590 nm. In
non-apoptotic cells, JC-1 enters the negatively charged
mitochondria where it aggregates and turns red. However, in cells
undergoing apoptosis, where Δψm has collapsed, JC-1
exists as monomers in the cytosol and turns green (21). The level of Δψm can be
measured by the ratio of red/green fluorescence intensity of JC-1.
The untreated cells were the 100% MMP control.
Western blot analysis
For the preparation of cytosolic extracts, the MG-63
and MNNG/HOS cells were lysed in radioimmunoprecipitation (RIPA)
buffer with 1 mM phenylmethylsulphonyl fluoride (PMSF) for 30 min
on ice. The mixture was centrifuged at 14,000 × g for 5 min and the
precipitate was discarded. Protein concentration was measured with
the BCA protein assay kit (Beyotime, Haimen, China). Samples
containing equal amount of protein were separated by SDS-PAGE, and
then transferred to polyvinylidene fluoride (PVDF) membranes (Merck
Millipore) using a standard procedure. The PVDF membrane was
blocked in 5% (w/v) skim milk powder in Tris-buffered saline
containing 0.1% Tween-20 for 2 h at room temperature. The primary
antibodies against GAPDH (ab9485), PI3Kp110β (ab32569), PI3Kp85α
(ab22653) (all from Abcam), Akt (#9272), p-Akt (Ser473) (#9271),
Bcl-2 (#2870) (all from Cell Signaling Technology), Bcl-xL
(ab2568), Bad (ab62465), Bax (ab32503), cytochrome c
(ab53056), caspase-9 (ab2014), caspase-3 (ab44976), cleaved PARP
(ab32064) (all from Abcam), cyclin D1 (#2978; Cell Signaling
Technology), p21 (ab7960), p27 (ab7961) (both from Abcam) were
diluted according to the instructions of the antibodies and
incubated overnight at 4°C. Then, the HRP-conjugated secondary
antibodies were added at a dilution ratio of 1:5,000 and incubated
at room temperature for 2 h. The blots were visualized using an
enhanced chemiluminescence (ECL) detection kit (WBKL S0100; Merck
Millipore) according to the manufacturer's instructions. The
relative protein expression levels were then determined using
ChemiDoc Touch Imaging System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and quantified by Image Lab and ImageJ software.
In order to quantify changes in protein expression, the target
protein was normalized against GAPDH.
Statistical analysis
All the experiments were performed three times
independently. All the results are expressed as the mean ± SD. The
Student's t-test by GraphPad InStat software (GraphPad Software,
Inc., San Diego, CA, USA) was used to compare the difference among
different groups. A p<0.05 was considered to indicate a
statistically significant result.
Results
DATS induces inhibition of cell viability
and cell morphological changes
Our data showed that DATS clearly inhibited MG63 and
MNNG/HOS cell viability at the concentrations of 10–120 µM
following exposure for 24 h (Fig. 1A
and B; p<0.01), and 5–120 µM for 48 and 72 h
(Fig. 1A and B; p<0.01) compared
with the control groups. The results indicated that DATS
significantly inhibited MNNG/HOS and MG63 cell viability in a dose-
and time-dependent manner. The IC50 values of DATS
inhibition of MG63 cell growth at 24, 48 and 72 h were 51.52±5.88,
32.20±6.99 and 22.02±2.33 µM, respectively, while the
IC50 values of DATS for MNNG/HOS cell growth at 24, 48
and 72 h were 67.17±3.69, 52.34±5.67 and 35.57±4.50 µM,
respectively. The 48- and 72-h treatment groups had apparent
differences compared with the 24-h treatment groups (Fig. 1A and B; p<0.05). DATS had less of
an influence on MNNG/HOS cells than MG63 cells (p<0.05).
Regarding the morphological changes of MG63 and MNNG/HOS cells
after incubation with DATS, as shown in Fig. 1C (magnification, ×100), the control
group cells showed a typical polygonal and intact appearance,
whereas the DATS-treated cells displayed dose-dependent changes in
cell shape, such as membrane blebbing, cell rounding and shrinkage,
poor adherence and floating shapes.
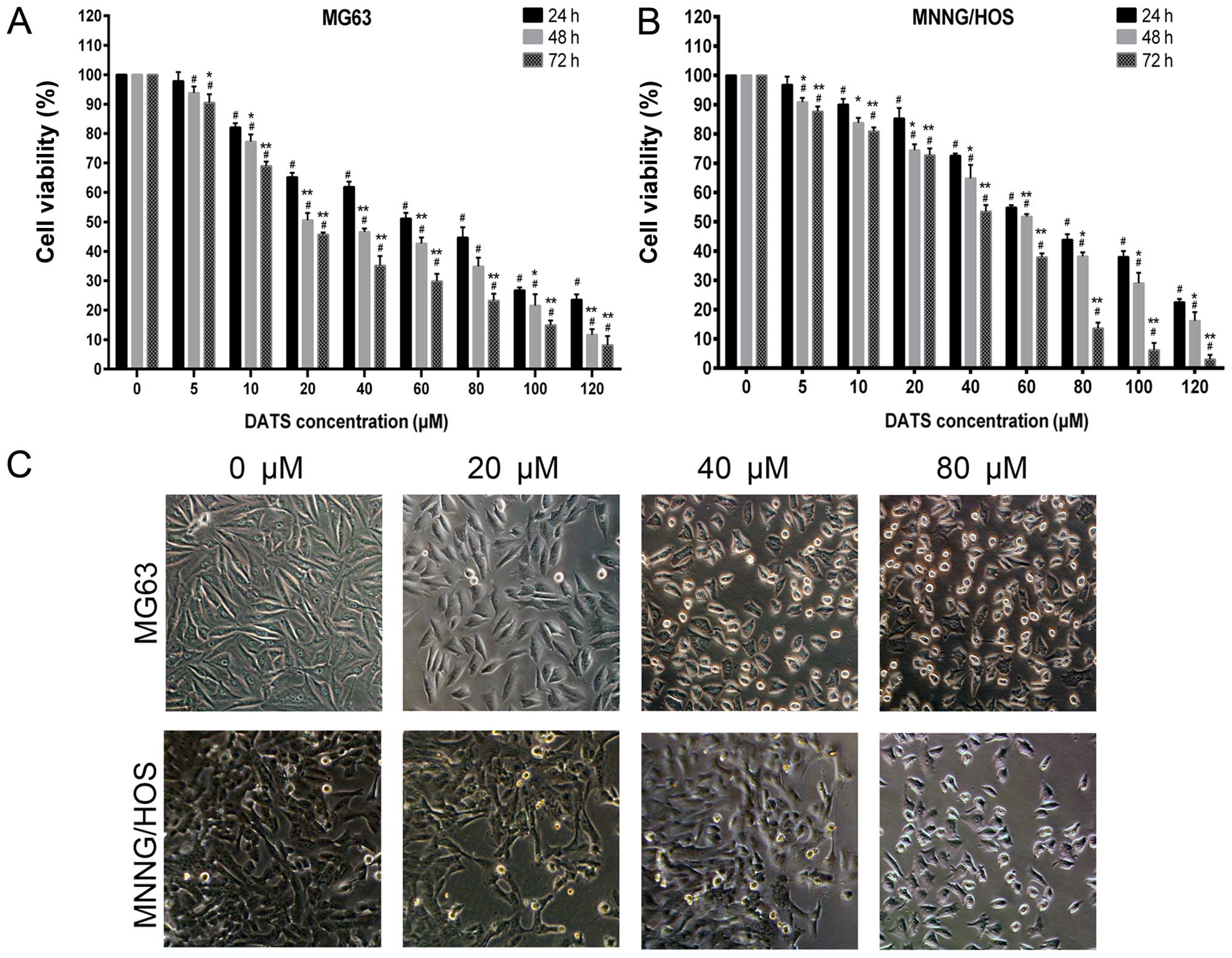 | Figure 1Effects of DATS on the cell growth
inhibition of MG63 and MNNG/HOS cells. (A) MG63 and (B) MNNG/HOS
cells were treated with 0, 5, 10, 20, 40, 60, 80, 100 and 120
µM of DATS for 24, 48 and 72 h. The cell growth inhibitory
rate was measured using the CCK-8 assay. The results from three
independent experiments are expressed as the means ± SD.
#p<0.01 compared with the control group.
*p<0.05, **p<0.01 compared with the
24-h group. (C) Representative morphology of the MG63 and MNNG/HOS
cells, respectively, under phase contrast microscopy
(magnification, ×100). |
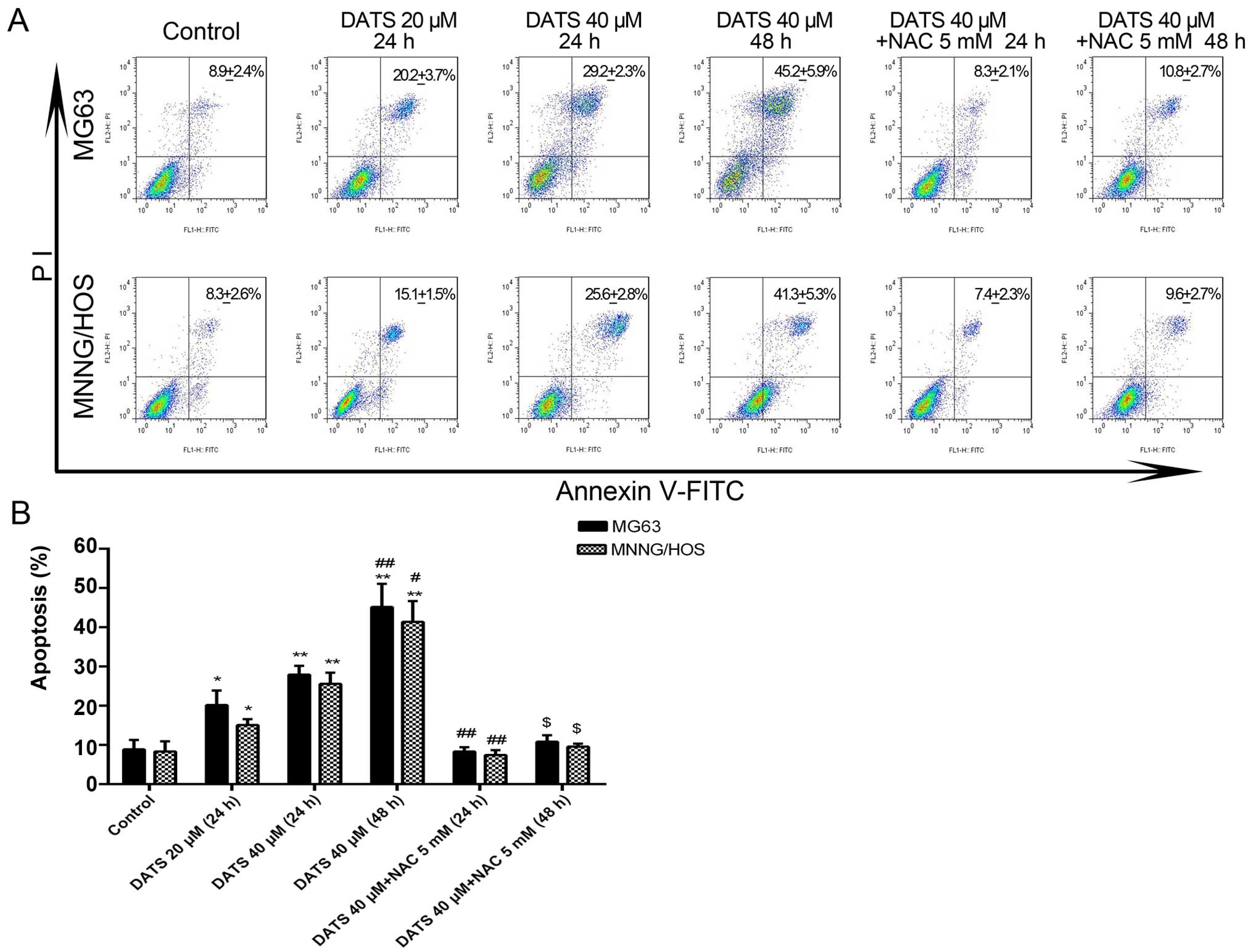
DATS induces cell apoptosis
The results from the flow cytometry showed that the
percentage of apoptotic cells was significantly increased in the
DATS treatment groups compared with that noted in the untreated
MG63 and MNNG/HOS cells (Fig. 2A).
After treatment with DATS at 20 and 40 µM for 24 h, and 40
µM for 48 h, the corresponding apoptotic ratios of the MG63
cells were 20.2±2.1, 27.9±1.3 and 45.2±3.4%, compared with the
control group (8.9±1.1%), while the corresponding apoptotic ratios
of the MNNG/HOS cells were 15.1±1.8, 25.6±1.6 and 41.4±3.1%,
respectively, compared with the control group (8.3±1.5%) (Fig. 2B; p<0.05). The apoptotic ratios
of both cell lines treated with DATS increased in a dose- and
time-dependent manner. However, after the MG63 and MNNG/HOS cells
were co-incubated with DATS 40 µM and NAC 5 mM for 24 or 48
h, the apoptotic ratios of both cell lines were significantly
decreased when compared with the corresponding DATS-treated groups
(Fig. 2B; p<0.01). Co-treatment
with NAC, a general ROS scavenger, completely blocked the
DATS-induced apoptosis. This suggested that DATS-induced apoptosis
of the MG63 and MNNG/HOS cells may be involved with the generation
of ROS.
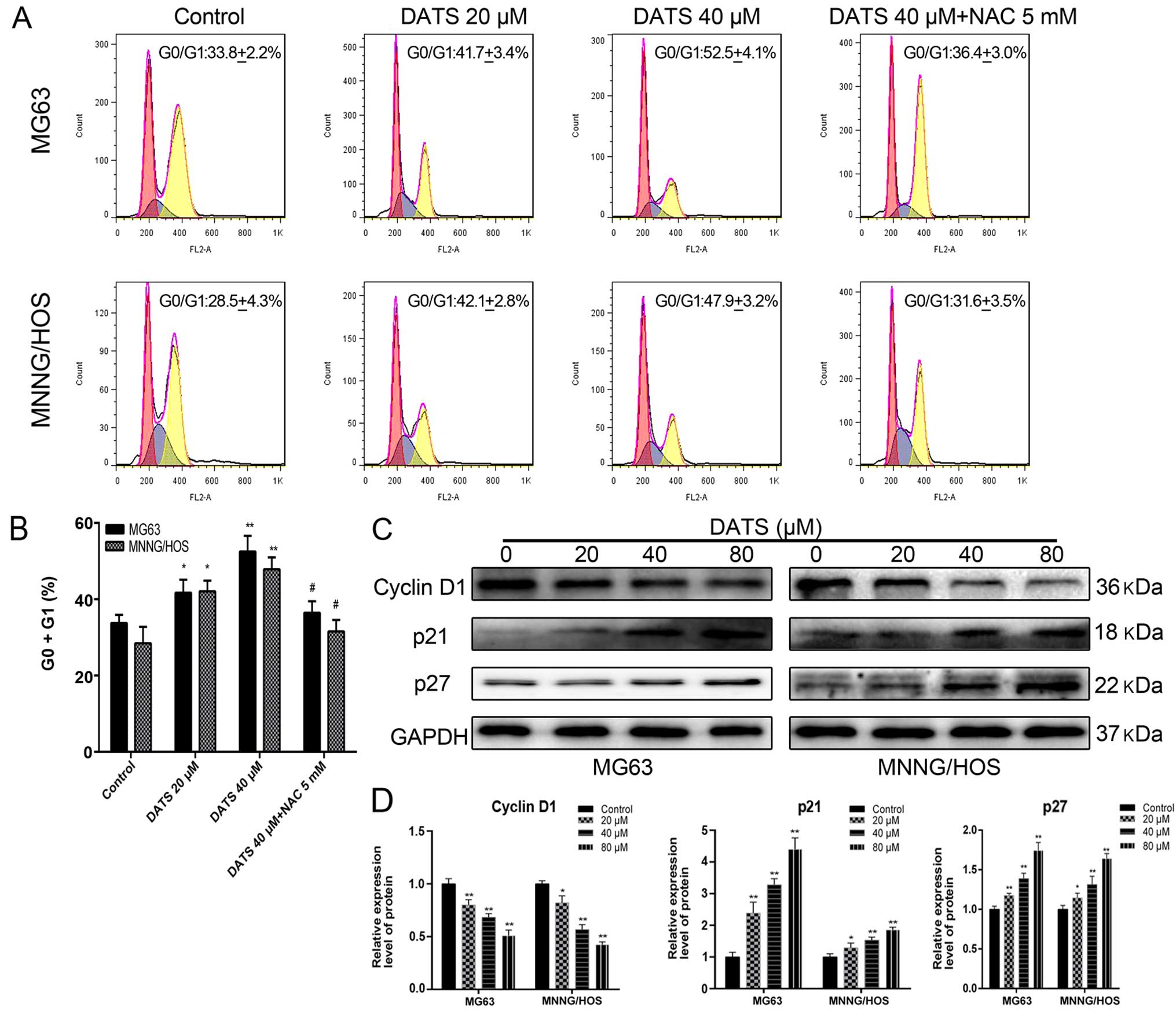
DATS induces G0/G1 phase cell cycle
arrest
Results from the flow cytometry showed that the
percentage of cells in the DATS-treated groups was significantly
increased at the G0/G1 phase in a dose-dependent manner compared
with the control groups (Fig. 3A and
B; p<0.05). The percentage of G0/G1 phase cells following
DATS treatment (20 and 40 µM) in the MG63 and MNNG/HOS cells
increased from 41.7±3.4 and 42.1±2.8 to 52.5±4.1 and 47.9±3.1%,
compared with the corresponding control groups (33.8±2.2 and
28.5±4.3%), respectively (Fig. 3A).
These results suggested that DATS-induced G0/G1 cell cycle arrest
may be one of the reasons for the inhibition of viability and the
induction of apoptosis in the MG63 and MNNG/HOS cells. Furthermore,
following co-treatment with DATS (40 µM) and NAC, the
DATS-induced G0/G1 phase arrest was completely reversed compared
with the DATS-treated (40 µM) groups (Fig. 3A and B; p<0.05). This suggested
that DATS exerted its anticancer cytotoxicity through an
ROS-dependent G0/G1 phase cell cycle arrest in the MG63 and
MNNG/HOS cells.
To elucidate the molecular mechanism of DATS-induced
G0/G1 phase arrest, we examined the expression of related proteins
by western blotting. As shown in Fig.
3C and D, when MG63 and MNNG/HOS cells were treated with DATS
at 20, 40 and 80 µM for 48 h, a concentration-dependent
decrease in levels of cyclin D1 was noted, whereas the protein
levels of p21 and p27 were upregulated in a dose-dependent manner,
compared with the control groups (p<0.05). These results
suggested that DATS-induced G0/G1 phase arrest of the MG63 and
MNNG/HOS cells involved the generation of ROS and the
downregulation of cyclin D1 and upregulation of p21 and p27.
DATS increases the generation of ROS
To explore the induction of intracellular ROS
generation by DATS, we quantified the MFI of cells stained with
DCFH-DA by FlowJo and ImageJ software in the MG63 and MNNG/HOS
cells. Our results from fluorescence microscopy revealed that the
levels of ROS in the DATS treatment cells for 4 and 8 h were not
significantly altered, whereas the green fluorescence was markedly
elevated following 16 h of treatment with DATS compared with those
of the control groups (Fig. 4A;
magnification, ×100). Sixteen hours of treatment of DATS at 20, 40
and 80 µM enhanced the ROS level by 2.7-, 4.4- and 5.4-fold
of the control groups, respectively, in the MG63 cells, and 5.0-,
8.1- and 11.4-fold of the control groups, respectively, in the
MNNG/HOS cells (Fig. 4C;
p<0.01). When the cells were co-treated with DATS at 40 or 80
µM and NAC 5 mM for 16 h, the intracellular ROS generation
was reversed compared with that noted in the DATS-treated groups
(Fig. 4A and C; p<0.01). The
intracellular ROS generation increased in a dose-dependent manner
in the DATS-treated cells. Furthermore, we examined the
intracellular ROS level by flow cytometric analyses in the
DATS-treated cells at 40 µM for 16 h, 20 µM for 24 h
and 40 µM for 24 h, respectively. As shown in Fig. 4B and D, the intracellular ROS
generation in the DATS-treated cells increased in a dose- and
time-dependent manner compared to the control groups (p<0.05).
However, co-treatment with DATS (40 µM) and NAC (5 mM)
completely blocked the increase in intracellular ROS compared with
the DATS (40 µM)-treated groups in the MG63 and MNNG/HOS
cells (Fig. 4B and D;
p<0.01).
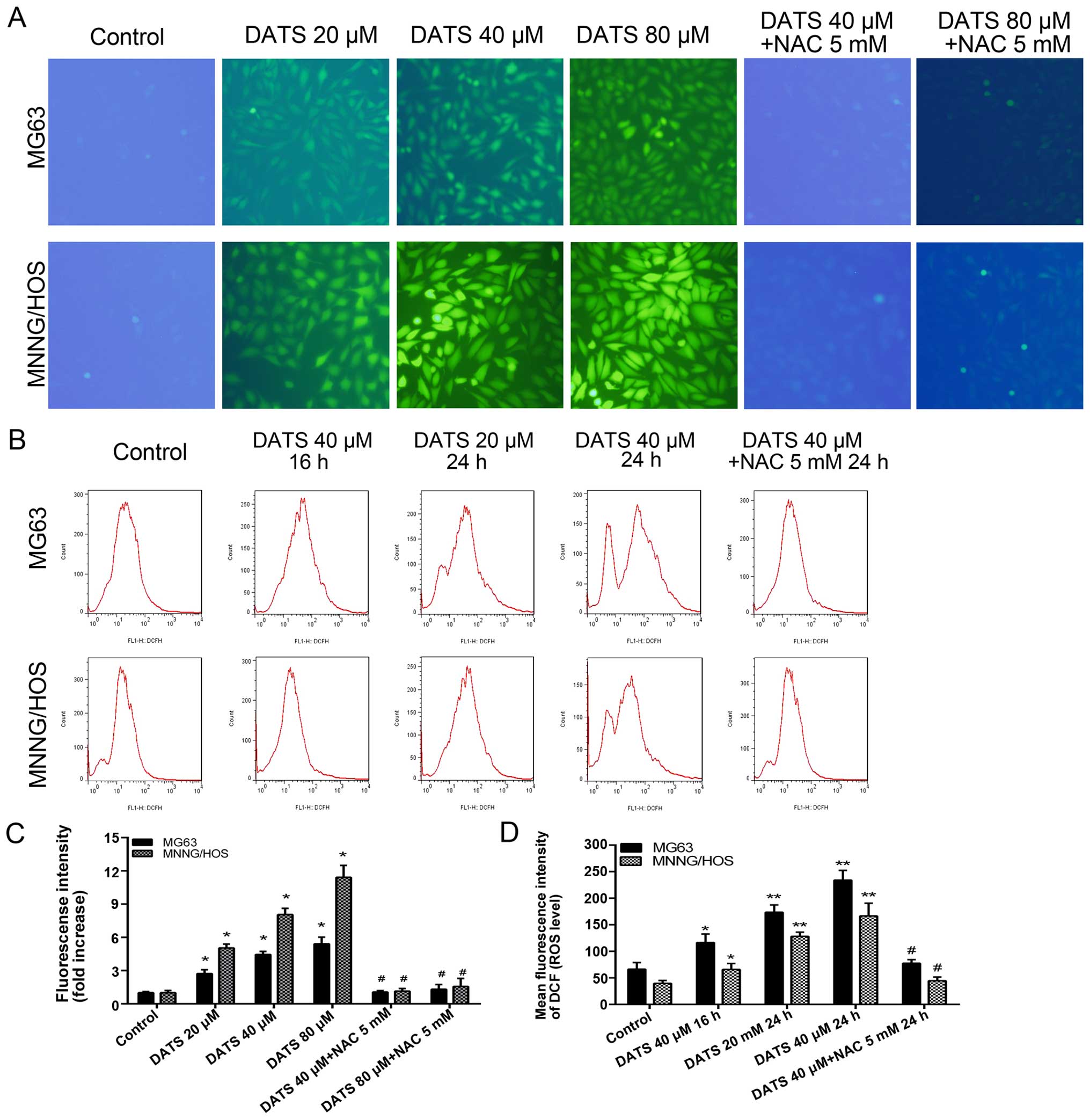 | Figure 4DATS increases the generation of
intracellular ROS in the MG63 and MNNG/HOS cells. (A) After
treatment with DATS at 20, 40 and 80 µM, 40 µM + NAC
5 mM, 80 µM + NAC 5 mM for 16 h, respectively, the cells
were loaded with 10 µM DCFH-DA for 30 min and examined by a
fluorescence microscope (magnification, ×100). (B) After treatment
with DATS at 40 µM for 16 h, 20, 40 and 40 µM + NAC 5
mM for 24 h, respectively, the cells were loaded with 10 µM
DCFH-DA for flow cytometric analysis. (C) The fluorescence
intensity (ROS level) from fluorescence microscopy was quantified
by ImageJ software. (D) The mean fluorescence intensity (ROS level)
from flow cytometric analysis. Data are expressed as the means ± SD
from three independent experiments. *p<0.05,
**p<0.01 vs. the control group. #p<0.01
vs. the corresponding DATS treatment groups. |
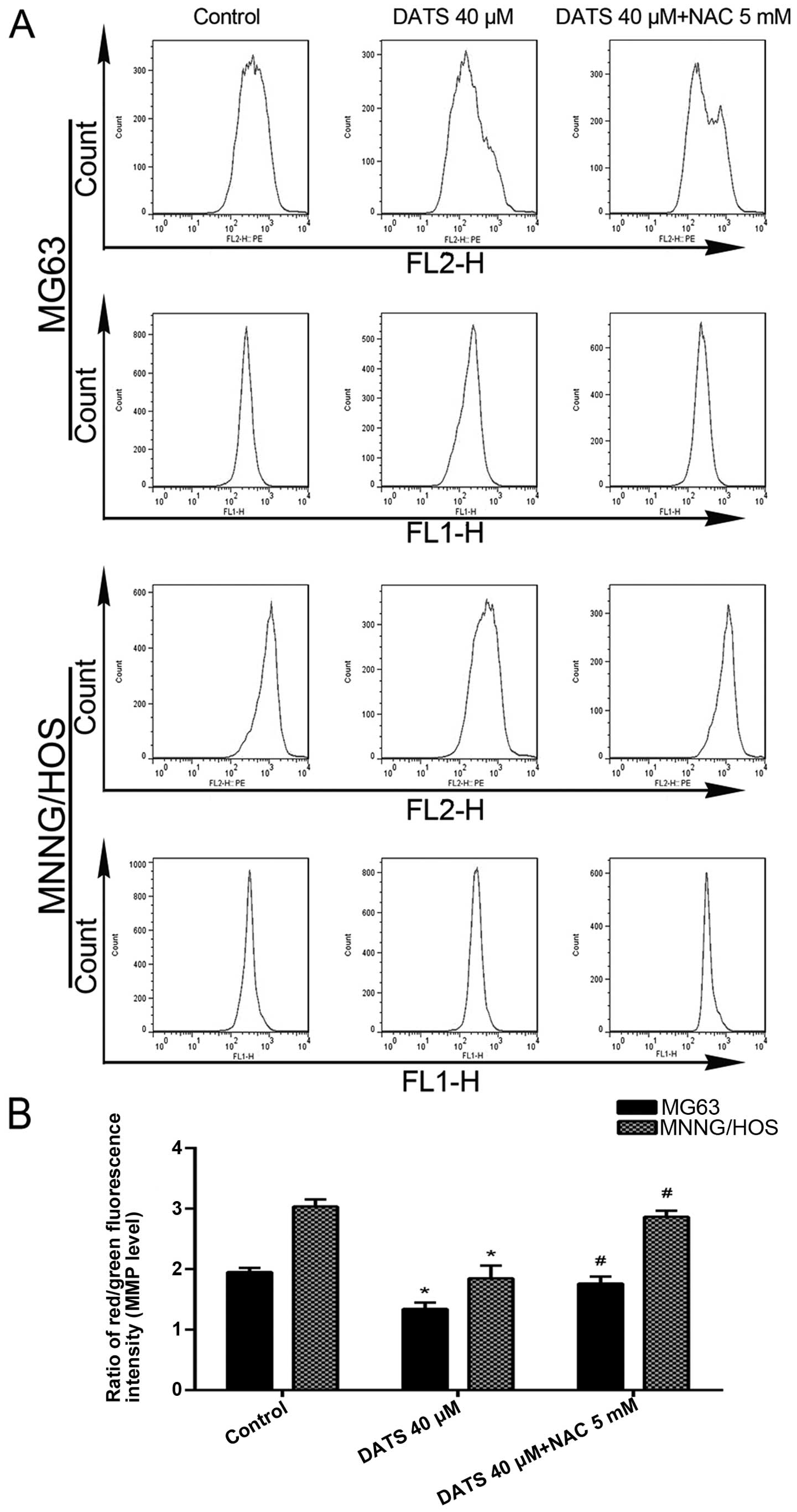
DATS induces disruption of
Δψm
As can be seen in Fig.
5A and B, after the MG63 and MNNG/HOS cells were treated with
DATS at 40 µM for 24 h, the ratio of red/green fluorescence
intensity (Δψm level) decreased 31.3 and 39.1%,
respectively, compared with control groups (p<0.01). To
investigate whether the increase in ROS induced by DATS mediated
mitochondrial damage, the cells were co-treated with DATS and NAC.
The results showed that NAC significantly restored the
Δψm level compared with DATS-treated cells (Fig. 5; p<0.05). This suggested that the
disruption of Δψm induced by DATS was ROS-dependent.
DATS induces apoptosis through
ROS-mediated downregulation of the PI3K/Akt and mitochondrial
apoptotic pathways
To assess whether DATS-induced apoptosis was
affected by PI3K/Akt pathway inactivation, MG63 and MNNG/HOS cells
were treated with various concentrations of DATS (0, 20, 40 and 80
µM) for 48 h and analyzed by western blotting. As shown in
Fig. 6A–C, DATS reduced the
expression levels of PI3Kp110β, PI3Kp85α, Akt and p-Akt in a
concentration-dependent manner compared with these levels in the
untreated cells (p<0.05). In addition, we investigated the
downstream target involvement of the PI3K/Akt pathway. As shown in
Fig. 6A, D and E, the levels of
Bcl-2 and Bcl-xL proteins were decreased, whereas the expression
levels of Bad and Bax were increased in response to DATS treatment
in a concentration-dependent manner when compared with these levels
in the control groups (p<0.05). These results are consistent
with previously reported studies (16,22).
Our present study showed that DATS-induced apoptosis involved an
increase in intracellular ROS and mitochondrial damage, which led
to loss of Δψm level. This suggests that the
mitochondrial apoptotic pathway may play a pivotal role in
DATS-induced apoptosis. To reveal the mechanisms underlying the
apoptotic effect of DATS on MG63 and MNNG/HOS cells, we further
investigated the expression levels of cytochrome c,
caspase-9 and -3 in the DATS-treated cells. As shown in Fig. 6A, F and G, after treatment with DATS
at 20, 40 and 80 µM for 48 h, the levels of cytochrome
c, caspase-9 and -3 were upregulated in a dose-dependent
manner, respectively, compared with these levels in the control
groups (p<0.05). In addition, DATS treatment led to progressive
proteolytic cleavage of poly(ADP-ribose) polymerase (PARP), a
well-known substrate protein of activated caspase-3. Taken
together, caspase-9 upregulation by DATS demonstrated an
association of DATS-induced apoptosis with the intrinsic or
mitochondrial-dependent pathway.
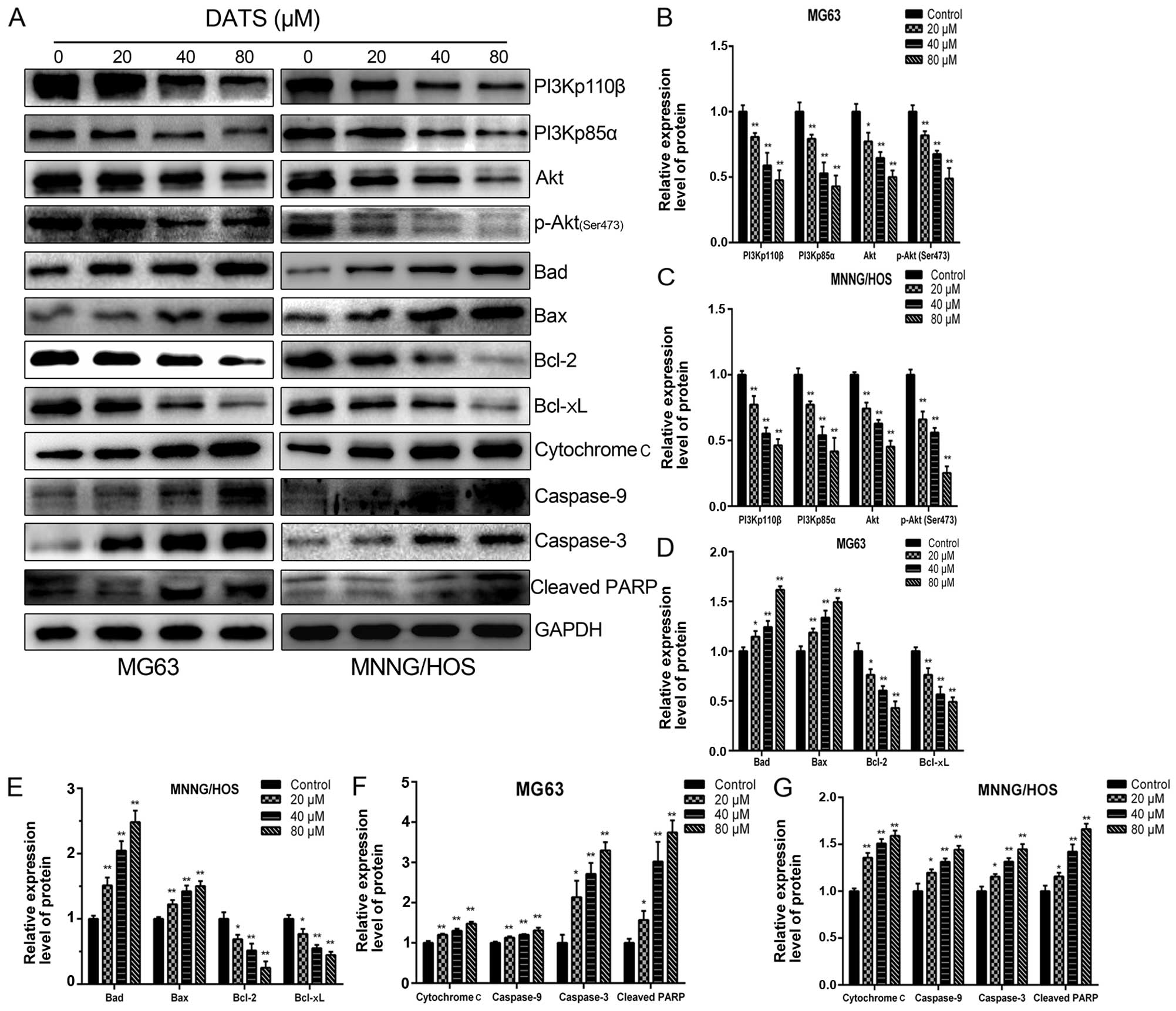 | Figure 6DATS induces apoptosis via inhibition
of the PI3K/Akt signaling axis and through the mitochondrial
apoptotic pathway in the MG63 and MNNG/HOS cells. The cells were
treated with DATS at 0, 20, 40 and 80 µM for 48 h, and
protein expression levels were determined by western blotting and
quantified by densitometric analysis. (A) Representative blots.
Relative expression levels of (B and C) PI3Kp110β, PI3Kp85α, Akt,
p-Akt (Ser473), (D and E) Bad, Bax, Bcl-2, Bcl-xL (F and G)
cytochrome c, caspase-9 and caspase-3, cleaved PARP in the
DATS-treated MG63 and MNNG/HOS cells. GAPDH was used as a loading
control. Data are expressed as the means ± SD from three
independent experiments. *p<0.05,
**p<0.01 vs. the control group. |
To further test the contribution of the PI3K/Akt
pathway in DATS-induced apoptosis, LY294002, a specific PI3K
inhibitor, was used. The MG63 and MNNG/HOS cells were treated with
DATS (40 µM) or LY294002 (20 µM) or co-treated with
DATS (40 mM) and LY294002 (20 µM) for 48 h. As shown in
Fig. 7, the levels of PI3Kp110β,
PI3Kp85α, Akt and p-Akt were significantly decreased with respect
to those in the presence of either DATS or LY294002, and the
combined treatment groups showed a more powerful synergistic effect
to trigger apoptosis compared with either treatment alone in the
MG63 and MNNG/HOS cells (p<0.05). Meanwhile, the downstream
proteins, Bad and Bax were upregulated whereas Bcl-2 and Bcl-xL
were downregulated in the treated alone groups or the combined
treatment groups compared with the control groups (p<0.01). In
addition, as expected, the expression changes in proteins of the
Bcl-2 family in the combined treatment groups were enhanced
compared with the single factor treatment groups. We found that
DATS served as an inhibitor of the PI3K/Akt pathway in DATS-induced
apoptosis.
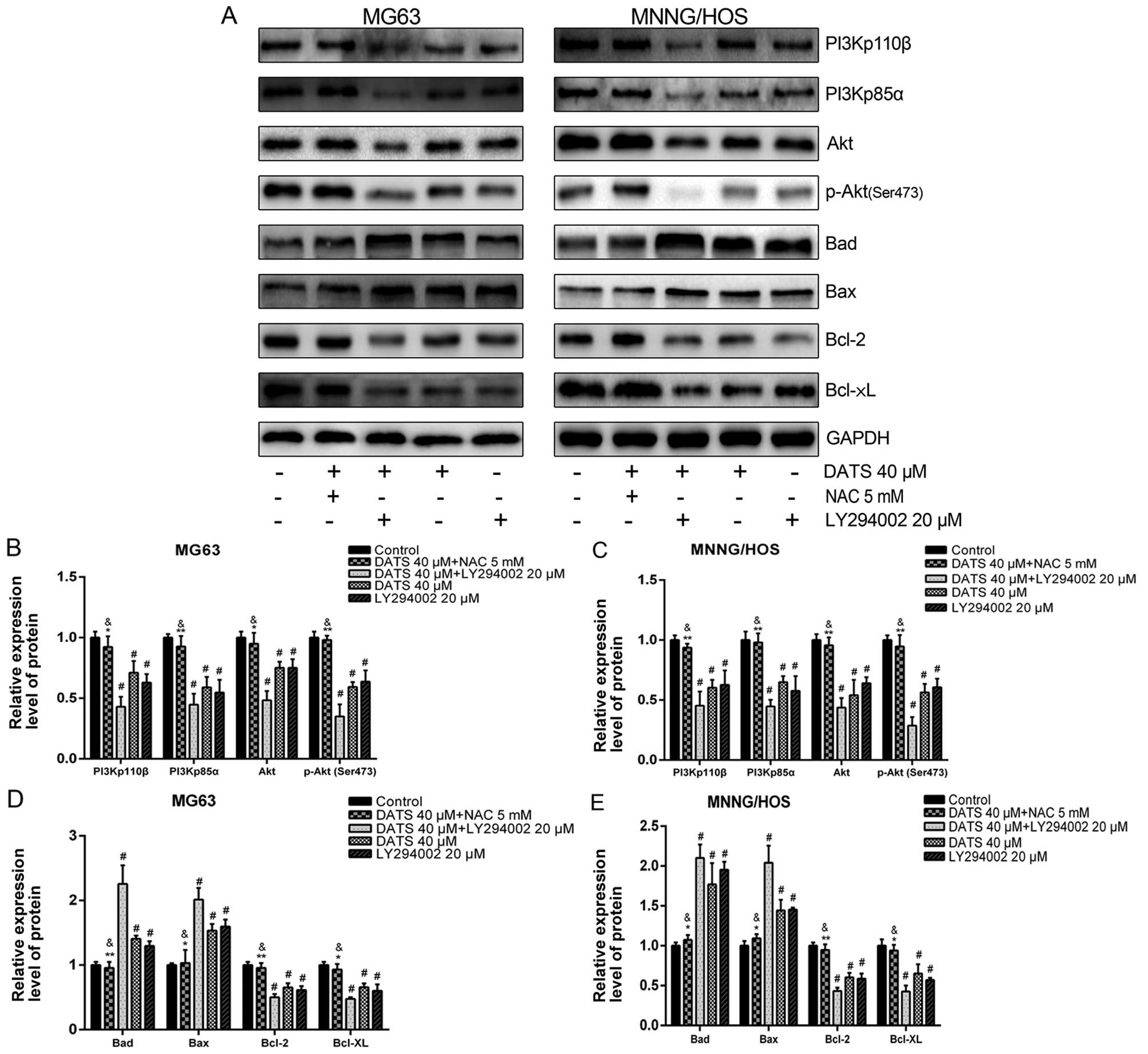 | Figure 7ROS-dependent inhibition of the
PI3K/Akt signaling pathway is involved in DATS-induced apoptosis.
The MG63 and MNNG/HOS cells were pretreated with 5 mM NAC for 2 h,
and then co-treated with 40 µM DATS for 48 h; other groups
were treated with 40 µM DATS or 20 µM LY294002, or
co-treated with 40 µM DATS + 20 µM LY294002 for 48 h.
Protein expression levels were determined by western blotting and
quantified by densitometric analysis. (A) Representative blots.
Relative expression levels of (B and C) PI3Kp110β, PI3Kp85α, Akt,
p-Akt (Ser473), (D and E) Bad, Bax, Bcl-2, Bcl-xL in the
DATS-treated MG63 and MNNG/HOS cells. GAPDH was used as a loading
control. Data are expressed as the means ± SD from three
independent experiments. #p<0.01 vs. the control
group. *p<0.05, **p<0.01 vs. the DATS
40 µM group, &p>0.05 vs. the control
group. |
To investigate the relationship between the increase
of ROS and inhibition of the PI3K/Akt pathway in DATS-induced
apoptosis, the MG63 and MNNG/HOS cells were treated with DATS (40
µM) for 48 h, in the absence or presence of NAC (5 mM).
Previous studies have shown that NAC, a common ROS scavenger, has
no effect on cell viability and apoptosis induction at the
concentration of 10 mM (14). The
western blot data (Fig. 7A–C),
showed that the presence of NAC almost completely blocked
DATS-induced downregulation of the PI3K/Akt pathway (p<0.05). In
addition, the changes in the protein expression of the Bcl-2 family
(upregulation of Bad and Bax, downregulation of Bcl-2 and Bcl-xL)
were completely abrogated by NAC compared with the DATS (40
µM)-treated groups (Fig. 7A, D
and E; p<0.05).
Discussion
In previous studies, the anticancer effect of
diallyl trisulfide (DATS) was mainly restricted to malignant tumor
cells of the digestive, reproductive or hematological system, while
research on the effect and mechanism of DATS against osteosarcoma
(OS) is rare. In the present study, to the best of our knowledge,
this is the first study to report that DATS induced MG63 and
MNNG/HOS cell proliferation inhibition, increased apoptosis, G0/G1
phase arrest and mitochondrial damage. The possible molecular
mechanism by which DATS induced apoptosis involved inhibition of
the PI3K/Akt signaling pathway, which was dependent on the
generation of ROS.
In the present study, our experiments revealed that
DATS inhibited the viability and proliferation of MG63 and MNNG/HOS
cells in a dose- and time-dependent manner, which was in agreement
with previous research (5,16,23).
Furthermore, our results showed that DATS is capable of triggering
apoptosis in MG63 and MNNG/HOS cells in a dose- and time-dependent
manner, which was consistent with previous research (9,16,23).
Apoptosis is a highly regulated physiologic process, which is
carried out mainly through two key pathways: the death
receptor-mediated pathway (extrinsic pathway) and the
mitochondrial-initiated apoptotic pathway (intrinsic pathway). The
mitochondria play a central role in apoptosis regulation by
releasing cytochrome c into the cytoplasm, leading to the
activation of the caspase-cascade system (24). We also investigated the effect of
DATS on the cell cycle by flow cytometry, and the results showed
that DATS induced G0/G1 phase cell cycle arrest in a dose-dependent
manner in the MG63 and MNNG/HOS cells, which is consistent with
previous research (25). Notably,
various previous studies have reported that DATS induced G2/M phase
cell cycle arrest (lung, skin and pancreas cancer cells) (26–28),
and these contradictory results appear to indicate that DATS exerts
a differential effect in a cell type-specific manner. Furthermore,
we examined the cell cycle-related protein expression to elucidate
the underlying mechanism of DATS-induced G0/G1 phase arrest. Cyclin
D1, which belongs to the cyclin D family, is required for cell
cycle G1/S transition. Overexpression of cyclin D1 is known to
correlate with the risk of tumor progression (29). The p21 and p27 genes have recently
been discovered to be important cyclin-dependent kinase inhibitors
(CDKIs) and regulate the cell cycle as well as DNA replication and
repair (30). Our results found
that the expression of cyclin D1 was decreased whereas levels of
p21 and p27 were upregulated. This is the first study to report the
possible mechanism of DATS-induced G0/G1 phase arrest to date.
However, surprisingly, co-treatment with antioxidant NAC completely
blocked the DATS-induced apoptosis and G0/G1 phase arrest, which
suggests that DATS exerted its anticancer cytotoxicity through an
ROS-dependent manner in the MG63 and MNNG/HOS cells.
ROS, various small, short-lived and highly reactive
molecules, are well known mediators of intracellular cascade
signaling. It has been reported that ROS generation plays a crucial
role in the pro-apoptotic activities of various anticancer agents
(31–33). The oxidative stress damage leads to
mitochondrial dysfunction, disruption of Δψm, ultimately
resulting in cell apoptosis. The present study showed that the
level of ROS increased whereas the Δψm collapsed
significantly in the DATS-treated cells. However, co-treatment of
NAC significantly restored the level of ROS and Δψm
compared with the DATS-treated cells, suggesting that the
disruption of Δψm induced by DATS was ROS-dependent.
Several studies have reported the critical roles of ROS in
DATS-induced cell apoptosis, such as ROS-mediated activation of JNK
and AP-1 in breast cancer cells (34), the ROS-dependent caspase pathway in
leukemia cells (14), and
ROS-dependent activation of the ASK1-JNK-Bim signaling transduction
pathway in human breast carcinoma cells (35). These controversial mechanisms again
raise the possibility that DATS may affect different signaling
pathways according to cell type or culture condition. In the
present study, we demonstrated that DATS-stimulated ROS generation
may play a significant upstream role by targeting various
cancer-associated proteins and may contribute to DATS-induced
apoptosis in OS cells. We found the downregulation of PI3K, Akt,
p-Akt in a dose-dependent manner in the DATS-treated MG63 and
MNNG/HOS cells, a finding corroborated by several previous studies
(16,17). The PI3K/Akt signaling pathway, which
plays a critical role in controlling the balance between cell
survival and apoptosis, is abnormally activated in a wide variety
of cancers and results in enhanced resistance to apoptosis through
multiple mechanisms, and therefore, they are prime targets for
cancer therapy (36). Cells
overexpressing constitutively active Akt show a much higher
resistance to drug-induced cell death (37). In addition, activated Akt protects
cells against apoptosis by increasing the phosphorylation of Bad, a
pro-apoptotic Bcl-2 family member which promotes cell death by
competing with Bcl-2/Bcl-xL in binding to Bax (38). A decrease in Δψm level
and an unbalance between pro-apoptotic (Bad and Bax) and
anti-apoptotic (Bcl-2 and Bcl-xL) members of the Bcl-2 family cause
mitochondrial permeability transition and contribute to the release
of cytochrome c, which in turn activates caspase-9 and
downstream caspase-3. Active capase-3 promotes a cascade reaction
including degradation of PARP finally leading to apoptosis. In the
present study, DATS induced the downregulation of Bcl-2 and Bcl-xL,
as well as the upregulation of Bad, Bax, cytochrome c,
caspase-9, and -3 and cleaved PARP, which provided evidence for a
direct contribution of mitochondria in DATS-induced apoptosis.
Furthermore, to investigate the effect of ROS
induced by DATS on the PI3K/Akt pathway and contribution of the
PI3K/Akt pathway in DATS-induced apoptosis, a specific
pharmacological inhibitor of PI3K/Akt, LY294002, and an ROS
scavenger, NAC, were used in the MG63 and MNNG/HOS cells. The
results revealed that DATS effectively inhibited the PI3K/Akt
pathway, and the efficiency of DATS basically approached the
efficacy of LY294002. However, complete blockage of DATS-induced
apoptosis and inhibition of the PI3K/Akt pathway by NAC treatment
highlighted a possible mechanism - an increase in ROS induced by
DATS was a key step required for inhibition of the PI3K/Akt pathway
in the MG63 and MNNG/HOS cells.
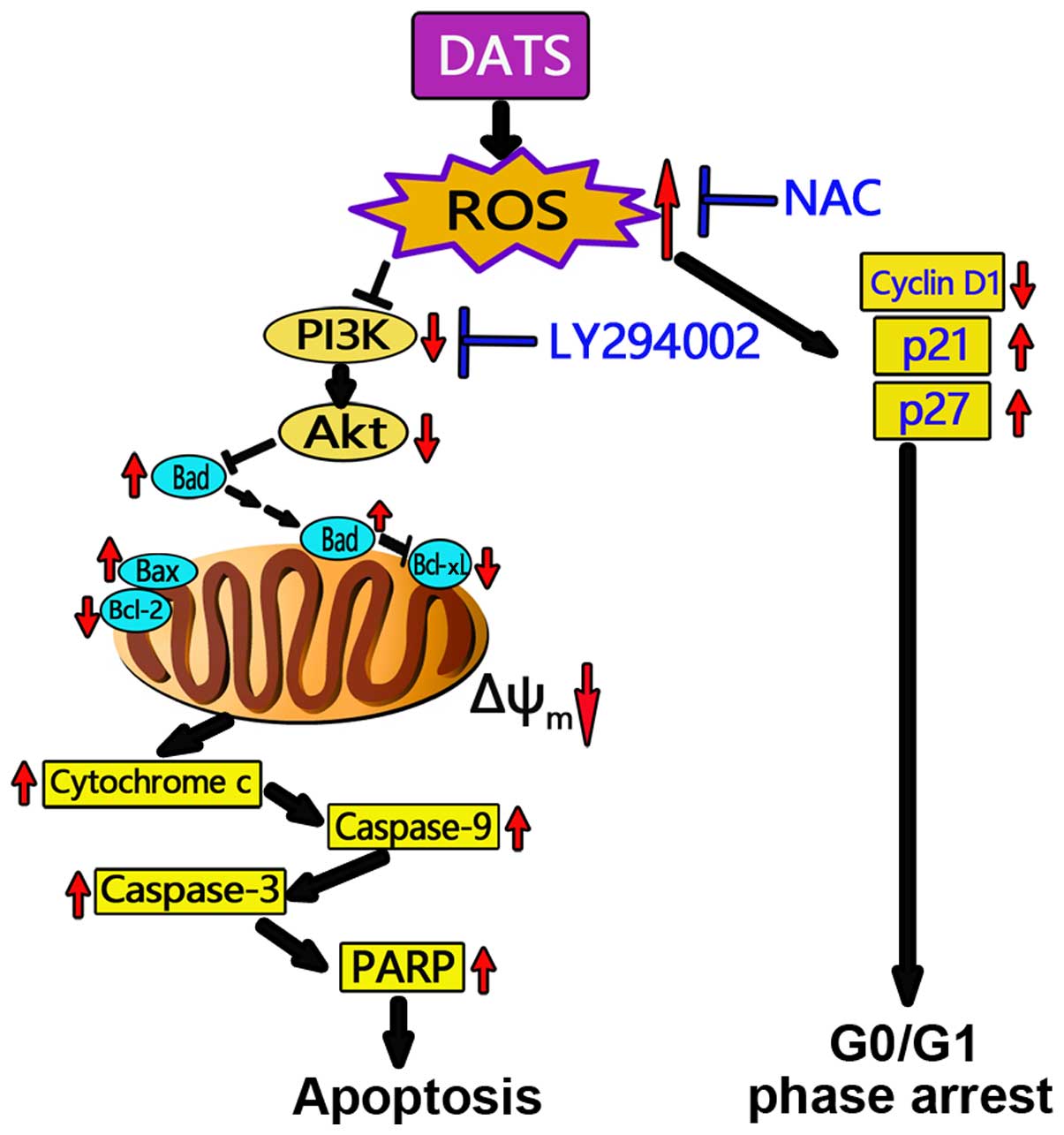
In conclusion, the present study demonstrated that
DATS exerted cytotoxic and antiproliferative effects on human OS
MG63 and MNNG/HOS cells. DATS induced an increase in intracellular
ROS and collapse of Δψm, thus further inducing MG63 and
MNNG/HOS cell apoptosis and G0/G1 phase cell cycle arrest.
DATS-induced apoptosis in the MG63 and MNNG/HOS cells was mediated
by inactivation of the PI3K/Akt signaling axis and through the
mitochondrial apoptotic pathway, which was ROS-dependent (Fig. 8). Our data emphasize the key role of
ROS in the apoptosis induced by DATS and indicates that a positive
correlation exists between ROS and the PI3K/Akt signaling pathway
as well as mitochondrial events leading to apoptosis in the MG63
and MNNG/HOS cells. Our novel findings shed new light on the
molecular mechanisms of the inhibitory effects of DATS on the
growth of cancer cells, and raise the possibility of DATS as a
potential anticancer and/or cancer preventive agent.
Acknowledgments
The present study was supported by the National
Natural Scientific Foundation of China (81172551) and the Shandong
Technological Development Project (ZR2011HM037).
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosar-coma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boos G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fleischauer AT, Poole C and Arab L: Garlic
consumption and cancer prevention: Meta-analyses of colorectal and
stomach cancers. Am J Clin Nutr. 72:1047–1052. 2000.PubMed/NCBI
|
|
4
|
Hsing AW, Chokkalingam AP, Gao YT, Madigan
MP, Deng J, Gridley G and Fraumeni JF Jr: Allium vegetables and
risk of prostate cancer: A population-based study. J Natl Cancer
Inst. 94:1648–1651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Zhu Y, Duan W, Feng C and He X:
Allicin induces apoptosis of the MGC-803 human gastric carcinoma
cell line through the p38 mitogen-activated protein
kinase/caspase-3 signaling pathway. Mol Med Rep. 11:2755–2760.
2015.
|
|
6
|
Lai KC, Hsu SC, Yang JS, Yu CC, Lein JC
and Chung JG: Diallyl trisulfide inhibits migration, invasion and
angiogenesis of human colon cancer HT-29 cells and umbilical vein
endothelial cells, and suppresses murine xenograft tumour growth. J
Cell Mol Med. 19:474–484. 2015. View Article : Google Scholar :
|
|
7
|
Chandra-Kuntal K and Singh SV: Diallyl
trisulfide inhibits activation of signal transducer and activator
of transcription 3 in prostate cancer cells in culture and in vivo.
Cancer Prev Res. 3:1473–1483. 2010. View Article : Google Scholar
|
|
8
|
Wu PP, Liu KC, Huang WW, Chueh FS, Ko YC,
Chiu TH, Lin JP, Kuo JH, Yang JS and Chung JG: Diallyl trisulfide
(DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft
model in vivo. Phytomedicine. 18:672–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Tian H, Li L, Li S, Yue W, Chen Z,
Qi L, Hu W, Zhu Y, Hao B, et al: Diallyl trisulfide induces
apoptosis and inhibits proliferation of A549 cells in vitro and in
vivo. Acta Biochim Biophys Sin. 44:577–583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH, Bommareddy A and Singh SV: Garlic
constituent diallyl trisulfide suppresses x-linked inhibitor of
apoptosis protein in prostate cancer cells in culture and in vivo.
Cancer Prev Res. 4:897–906. 2011. View Article : Google Scholar
|
|
11
|
Liu Y, Zhu P, Wang Y, Wei Z, Tao L, Zhu Z,
Sheng X, Wang S, Ruan J, Liu Z, et al: Antimetastatic therapies of
the polysulfide diallyl trisulfide against triple-negative breast
cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK
signaling pathways. PLoS One. 10:e01237812015. View Article : Google Scholar
|
|
12
|
Shin DY, Cha HJ, Kim GY, Kim WJ and Choi
YH: Inhibiting invasion into human bladder carcinoma 5637 cells
with diallyl trisulfide by inhibiting matrix metalloproteinase
activities and tightening tight junctions. Int J Mol Sci.
14:19911–19922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chandra-Kuntal K, Lee J and Singh SV:
Critical role for reactive oxygen species in apoptosis induction
and cell migration inhibition by diallyl trisulfide, a cancer
chemopreventive component of garlic. Breast Cancer Res Treat.
138:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi YH and Park HS: Apoptosis induction
of U937 human leukemia cells by diallyl trisulfide induces through
generation of reactive oxygen species. J Biomed Sci. 19:502012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YA, Xiao D, Xiao H, Powolny AA, Lew
KL, Reilly ML, Zeng Y, Wang Z and Singh SV: Mitochondria-mediated
apoptosis by diallyl trisulfide in human prostate cancer cells is
associated with generation of reactive oxygen species and regulated
by Bax/Bak. Mol Cancer Ther. 6:1599–1609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin DY, Kim GY, Hwang HJ, Kim WJ and Choi
YH: Diallyl trisulfide-induced apoptosis of bladder cancer cells is
caspase-dependent and regulated by PI3K/Akt and JNK pathways.
Environ Toxicol Pharmacol. 37:74–83. 2014. View Article : Google Scholar
|
|
17
|
Wang YB, Qin J, Zheng XY, Bai Y, Yang K
and Xie LP: Diallyl trisulfide induces Bcl-2 and
caspase-3-dependent apoptosis via downregulation of Akt
phosphorylation in human T24 bladder cancer cells. Phytomedicine.
17:363–368. 2010. View Article : Google Scholar
|
|
18
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
19
|
Zhang YK, Zhang XH, Li JM, Sun S, Yang Q
and Diao DM: A proteomic study on a human osteosarcoma cell line
Saos-2 treated with diallyl trisulfide. Anticancer Drugs.
20:702–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Liu W, Zhao K, Zhang Y, Li X, Yang
Q, Li Z and Li J: Diallyl trisulfide reverses drug resistance and
lowers the ratio of CD133+ cells in conjunction with
methotrexate in a human osteosarcoma drug-resistant cell subline.
Mol Med Rep. 2:245–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salvioli S, Ardizzoni A, Franceschi C and
Cossarizza A: JC-1, but not DiOC6 (3) or rhodamine 123,
is a reliable fluorescent probe to assess ΔΨ changes in intact
cells: Implications for studies on mitochondrial functionality
during apoptosis. FEBS Lett. 411:77–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou C, Mao XP, Guo Q and Zeng FQ: Diallyl
trisulphide-induced apoptosis in human melanoma cells involves
downregulation of Bcl-2 and Bcl-xL expression and activation of
caspases. Clin Exp Dermatol. 34:e537–e543. 2009. View Article : Google Scholar
|
|
23
|
Xu L, Yu J, Zhai D, Zhang D, Shen W, Bai
L, Cai Z and Yu C: Role of JNK activation and mitochondrial Bax
translocation in allicin-induced apoptosis in human ovarian cancer
SKOV3 cells. Evid Based Complement Alternat Med. 2014:3786842014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antico Arciuch VG, Elguero ME, Poderoso JJ
and Carreras MC: Mitochondrial regulation of cell cycle and
proliferation. Antioxid Redox Signal. 16:1150–1180. 2012.
View Article : Google Scholar :
|
|
25
|
Li Y, Zhang J, Zhang L, Si M, Yin H and Li
J: Diallyl trisulfide inhibits proliferation, invasion and
angiogenesis of osteosarcoma cells by switching on suppressor
microRNAs and inactivating of Notch-1 signaling. Carcinogenesis.
34:1601–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma HB, Huang S, Yin XR, Zhang Y and Di ZL:
Apoptotic pathway induced by diallyl trisulfide in pancreatic
cancer cells. World J Gastroenterol. 20:193–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang HC, Yang JH, Hsieh SC and Sheen LY:
Allyl sulfides inhibit cell growth of skin cancer cells through
induction of DNA damage mediated G2/M arrest and apoptosis. J Agric
Food Chem. 58:7096–7103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao D, Zeng Y, Hahm ER, Kim YA,
Ramalingam S and Singh SV: Diallyl trisulfide selectively causes
Bax- and Bak-mediated apoptosis in human lung cancer cells. Environ
Mol Mutagen. 50:201–212. 2009. View Article : Google Scholar :
|
|
29
|
Kyomoto R, Kumazawa H, Toda Y, Sakaida N,
Okamura A, Iwanaga M, Shintaku M, Yamashita T, Hiai H and Fukumoto
M: Cyclin-D1-gene amplification is a more potent prognostic factor
than its protein overexpression in human head-and-neck
squamous-cell carcinoma. Int J Cancer. 74:576–581. 1997. View Article : Google Scholar
|
|
30
|
Karimian H, Moghadamtousi SZ, Fadaeinasab
M, Golbabapour S, Razavi M, Hajrezaie M, Arya A, Abdulla MA, Mohan
S, Ali HM, et al: Ferulago angulata activates intrinsic pathway of
apoptosis in MCF-7 cells associated with G1 cell cycle
arrest via involvement of p21/p27. Drug Des Devel Ther.
8:1481–1497. 2014. View Article : Google Scholar
|
|
31
|
Park HS, Han MH, Kim GY, Moon SK, Kim WJ,
Hwang HJ, Park KY and Choi YH: Sulforaphane induces reactive oxygen
species-mediated mitotic arrest and subsequent apoptosis in human
bladder cancer 5637 cells. Food Chem Toxicol. 64:157–165. 2014.
View Article : Google Scholar
|
|
32
|
Jeong JB, Choi J, Baek SJ and Lee SH:
Reactive oxygen species mediate tolfenamic acid-induced apoptosis
in human colorectal cancer cells. Arch Biochem Biophys.
537:168–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rasul A, Di J, Millimouno FM, Malhi M,
Tsuji I, Ali M, Li J and Li X: Reactive oxygen species mediate
isoalantolactone-induced apoptosis in human prostate cancer cells.
Molecules. 18:9382–9396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Na HK, Kim EH, Choi MA, Park JM, Kim DH
and Surh YJ: Diallyl trisulfide induces apoptosis in human breast
cancer cells through ROS-mediated activation of JNK and AP-1.
Biochem Pharmacol. 84:1241–1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee BC, Park BH, Kim SY and Lee YJ: Role
of Bim in diallyl trisulfide-induced cytotoxicity in human cancer
cells. J Cell Biochem. 112:118–127. 2011. View Article : Google Scholar
|
|
36
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hahne JC, Honig A, Meyer SR, Gambaryan S,
Walter U, Wischhusen J, Häussler SF, Segerer SE, Fujita N, Dietl J,
et al: Downregulation of AKT reverses platinum resistance of human
ovarian cancers in vitro. Oncol Rep. 28:2023–2028. 2012.PubMed/NCBI
|
|
38
|
Peng SF, Lee CY, Hour MJ, Tsai SC, Kuo DH,
Chen FA, Shieh PC and Yang JS: Curcumin-loaded nanoparticles
enhance apoptotic cell death of U2OS human osteosarcoma cells
through the Akt-Bad signaling pathway. Int J Oncol. 44:238–246.
2014.
|