Introduction
As one of the major global public health issues,
cancer greatly threatens human survival and has become the leading
cause of mortality of humankind in this century (1). Clinically, during the rapid
development of anticancer strategies, chemotherapy has consistently
played an important role in cancer treatment. To date, classical
botanical agents, as represented by paclitaxel or vincristine, are
still first-line drugs and are widely applied as the primary choice
against multiple malignancies (2).
Benefitting from these agents, treatment effectiveness and the
survival rate of cancer patients have greatly improved in recent
years. However, in spite of the satisfactory tumor-suppressive
activities, with their extensive application worldwide, growing
evidence reveals that side-effects and drug resistance are still
the leading obstacles largely limiting the clinical use of classic
tumor-toxic agents (3).
In recent years, liver cancer has become one of the
most prevalent types of cancer and is the third most common cause
of cancer-related mortality (4,5).
Notably, its incidence and mortality rank second among Chinese
cancer patients (6) and liver
cancer patients in China account for more than half of all cases
worldwide (7).
In current clinical practice, surgical resection and
liver transplantation are the most recommended treatment strategies
against hepatocarcinoma (8).
However, the statistics show that only a small portion of patients
are suitable for these therapeutics (9), and even for those qualified patients,
the recurrence rate is over 50% (10). This situation indicates that
chemotherapy is one of the few remaining options for the vast
majority of patients (11).
Unfortunately, effective chemotherapeutic strategies for liver
cancer patients are still not well established and are under
extensive investigation. Therefore, the search for novel potential
anticancer compounds with high specificity and sensitivity for
liver cancer patients is urgently needed. During such research,
natural products provide a precious reservoir with high-level
chemical diversity. Thus, the efficacy-based high-throughput
screening of natural products serves as an efficient approach to
drug discovery (12).
Stellera chamaejasme L
(SCL), a wide-spread perennial plant in northwest
China (13), was firstly recorded
in Chinese medical ancient classic Sheng Nong's Herbal Classic.
Historically, SCL has been applied to a variety of diseases
including edema, tuberculosis, carbuncle, hemorrhoids, scrofula,
scabies, and tumors over centuries (14,15).
In modern times, characterized by high therapeutic efficacy and low
cost, SCL shows great benefit in anticancer drug research and
development and such has attracted increasing attention.
Recent pharmacologic studies have revealed that
extracts from SCL, eluted with water, petroleum ether, ethyl
acetate, acetone, ethanol, methanol and other solvents, possess
clear antitumor effects in a wide-spectrum of malignancies
(16,17). They have been confirmed to be strong
inhibitors of tumor progression mainly due to unselected cytotoxic
effects. Additionally, some of the extracts show higher efficiency
in terms of cell death induction compared with commonly used
chemotherapeutic agents. Although this promising evidence has
greatly revealed its feasibility for clinical application, its
material basis and the detailed pharmacological mechanism are still
obscure. Particularly, limited to the isolation procedures and the
establishment of disease models, current studies for SCL are merely
restricted to crude extracts with little disease specificity. The
targeted efficacy identification and optimization are still
lacking. More importantly, most of the known extracts from SCL
unexpectedly exhibit multi-organ damaging effects, mainly
represented by hematological and immunological toxicities, which
adds another obstacle for its clinical application.
Under such circumstances, the systematic efficacy
screening, verification and the detailed pharmacological analysis
for SCL are needed and will be beneficial for the comprehensive
understanding of SCL during clinical application.
Based on the above analysis, our study was designed
to screen and identify a novel extract from SCL with explicit
material basis, high activity and low systemic toxicity. In
addition, we also aimed to clarify its detailed molecular mechanism
in hepatocarcinoma. Here, we report that, by screening dozens of
Stellera chamaejasme extracts with a different polarity and
optimizing the extraction process, a new fraction from SCL, named
ESC, was identified with potent activities against the
proliferation of multiple types of cancer cell lines, particularly
SK-HEP-1 and HepG2 liver cancer cell lines. Inspired by this, we
further confirmed the growth inhibitory capacity of ESC as well as
its low systemic toxicity on hepatocarcinoma in vivo, and
finally confirmed the cyclin-dependent mechanism in the regulation
of cell cycle distribution. Taken together, our study
experimentally identified a novel cell cycle blocker for
hepatocarcinoma and provides more convincing evidence of
Stellera chamaejasme L. as a valuable resource in the
research and development of anticancer agents.
Materials and methods
Cell culture and reagents
The cancer cell lines A549, NCI-H157, NCI-H460,
HepG2 and SK-HEP-1 were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in RPMI-1640 or
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum and 1% Pen/Strep. The H22 cell line was kindly donated
by Dr Lanfang Li from the Institute of Chinese Materia Medica.
RPMI-1640, DMEM and fetal bovine serum were purchased from
Invitrogen (Carlsbad, CA, USA). ESC was extracted and prepared by
the Dalian Institute of Chemical Physics, Chinese Academy of
Sciences. An HPD-100 macroporous absorbent column was produced by
Cangzhou Bon Absorber Technology Co., Ltd. Sulforhodamine B (SRB)
was purchased from Sigma (USA). A cell cycle and apoptosis
detection kit was purchased from CWBio (Beijing, China). Primary
antibodies against cyclin B1 and total-CDK1 were purchased from
Boster (Wuhan, China); the phosphor-CDK1 (Tyr15) antibody was
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA);
β-actin antibody was purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
SCL extraction and ESC preparation
Stellera chamaejasme L. (2 kg) was decocted
thrice with ethanol at 70°C (15 l×5 h, 7.5 l×3 h, 7.5 l×3 h). The
liquids were merged and concentrated to extracts (230 g) on a
rotary vacuum evaporator at 60°C. The further purification of the
extracts (222 g) was performed by means of HPD-100 macroporous
absorbent column chromatography eluted with different
concentrations of alcohol (40–100%). Furthermore, the fractions
were concentrated on a rotary vacuum evaporator at 60°C, dried to
constant weight in vacuo and crushed into powder (ESC) before the
experiments. The yield of the ESC was 32.4% (72 g).
Cell proliferation assay
The cell proliferation intensity was analyzed using
the SRB assay. Cells were seeded onto 96-well plates (4,000
cells/well) and treated with ESC at different concentrations for
24, 48, 72 h. At the indicated time-points, the cells were fixed
with 50% trichloroacetic acid (TCA) for 1 h at 4°C. Then the wells
were washed with deionized water for 5 times. After drying, 100
µl SRB was added to each well and reacted for 10 min, the
unbound SRB was washed out with 1% acetic acid and then dried
completely. After being dissolved in 10 mmol/l unbuffered
Tris-base, the OD490 values were detected and calculated
to assess the cell proliferation level in response to drug
treatment.
H22 xenograft model
H22 cells were passaged in the peritoneal cavity of
ICR mouse. After 10 days, the mouse was sacrificed by cervical
dislocation, the ascites was collected and diluted with cold
sterile saline. The cell suspension was planted subcutaneously at
the axillary fossa, 2×106 cells/mouse. On the next day,
the mice were randomly divided into 4 groups (10 mice/group), and
then received an intragastric administration of ESC (135.85, 271.7,
543.4 mg/kg) and the control group was gastric transfused with an
equivalent volume of distilled water for 10 days. The mice were
weighed every day, and the volume of the tumors was measured every
2 days. On the 11th day of drug administration, the blood was
collected and the mice were sacrificed. The tumors, spleens and
thymus were dissected and weighed to calculate the tumor volume,
spleen index and thymus index according to the following equations:
Tumor volume = (length × width2)/2 (18). The spleen (thymus) index (mg/g) =
spleen (thymus) weight/body weight after tumor removal.
The number of white blood cells was detected with an
automatic blood cell analyzer (Siemens Ltd., Japan).
Histological analysis
All of the tumor tissue was paraffin-fixed, and
H&E staining was performed according to a standard protocol.
The results were scored by a pathologist in a blinded manner.
Tumors were classified using the WHO classification (19). Two standard morphological features
of malignancy: cellular heteromorphism and apoptosis were assessed,
each symbolized with −, +, ++ and +++.
Apoptosis analysis
HepG2 and SK-HEP-1 cells were plated onto a 6-well
plate and exposed to ESC at concentrations of 25, 50 and 100
µg/ml for 24 h. Then the apoptotic rate was detected using
the Annexin v-FITC/PI apoptosis detection kit. The protocol was
strictly designed according to the manufacturer's instructions.
Briefly, the cells were harvested and washed with PBS for 3 times
and then re-suspended with 150 µl binding buffer containing
10 µl Annexin V-FITC and 5 µl PI. After incubation
for 20 min in the dark, the apoptotic rates were analyzed by flow
cytometry (BD FACSCalibur).
Cell cycle analysis
HepG2and SK-HEP-1 cells were plated into a 6-well
plate and treated with 25, 50 and 100 µg/ml ESC. After 24 h,
the cells were washed with PBS and trypsinized. The cell suspension
of each group was centrifuged for 5 min, at 1,000 × g, and then the
cells were fixed with 70% cold ethanol overnight. The fixative
solution was discarded, and the cells were washed with PBS twice.
Cells were re-suspended with 500 µl PBS containing 50
µg/ml PI and 50 µg/ml RNase A, incubated at 37°C for
30 min, and then the samples were analyzed with a flow cytometer.
The detailed method was previously described (20).
Western blot analysis
SK-HEP-1 and HepG2 cells were seeded onto 6-well
plates (1×105 cells/well) and treated with different
concentrations of ESC (25, 50, 100 µg/ml). The cells were
next harvested after 24 h and lysed with the appropriate volume of
lysis buffer (including 20 mM Tris-HCl, pH, 7.4, 150 mM NaCl, 1 mM
EDTA, 1 mM PMSF and 1% Triton X-100). Tumor tissues were collected
from three randomly chosen mice in each group, and the tumor lysate
samples were prepared followed the steps as previously described
(21). The protein concentration
for each lysate was determined using a BCA protein quantification
kit (Pierce, USA). Then the denatured protein samples were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and the gels were electrically blotted
onto NC membranes (Pall, USA). The membranes were blocked with 5%
albumin from bovine serum (BSA) and then incubated with the primary
antibodies at 4°C for 8 h. After that, the membranes were incubated
with the relevant secondary antibody conjugated with HRP. The
target bands were visualized using chemiluminescence detection
reagents (Thermo fisher Scientific Inc., USA). β-actin was detected
as the internal control for each experiment.
Statistical analysis
All the quantitative data shown in this manuscript
are presented as arithmetic means ± standard errors. Statistical
analysis was performed with SPSS 17.0 and one-way analysis of
variance (one-way ANOVA) was used to analyze the quantitation.
P<0.05 indicates statistical significance
(*P<0.05, **P<0.01,
***P<0.001).
Results
Screening of the tumor-suppressive
efficacy of ESC in different tumor cell lines
Based on the historical clinical record of SCL, lung
and liver cancer have been proven to be the most sensitive disease
models. Based on this, we firstly examined the inhibitory effect of
ESC on cell proliferation in lung cancer (A549, NCI-H157, NCI-H460)
and liver cancer cell lines (HepG2, SK-HEP-1), respectively. During
the efficacy screening, cells were treated with different
concentrations of ESC for 24, 48 and 72 h. At each time-point, the
tumor proliferative intensity was quantified by the SRB
incorporative assay, and the inhibitory rates were calculated in
the different groups. As expected, the results showed that ESC
exerted clear but different levels of suppressive influence on all
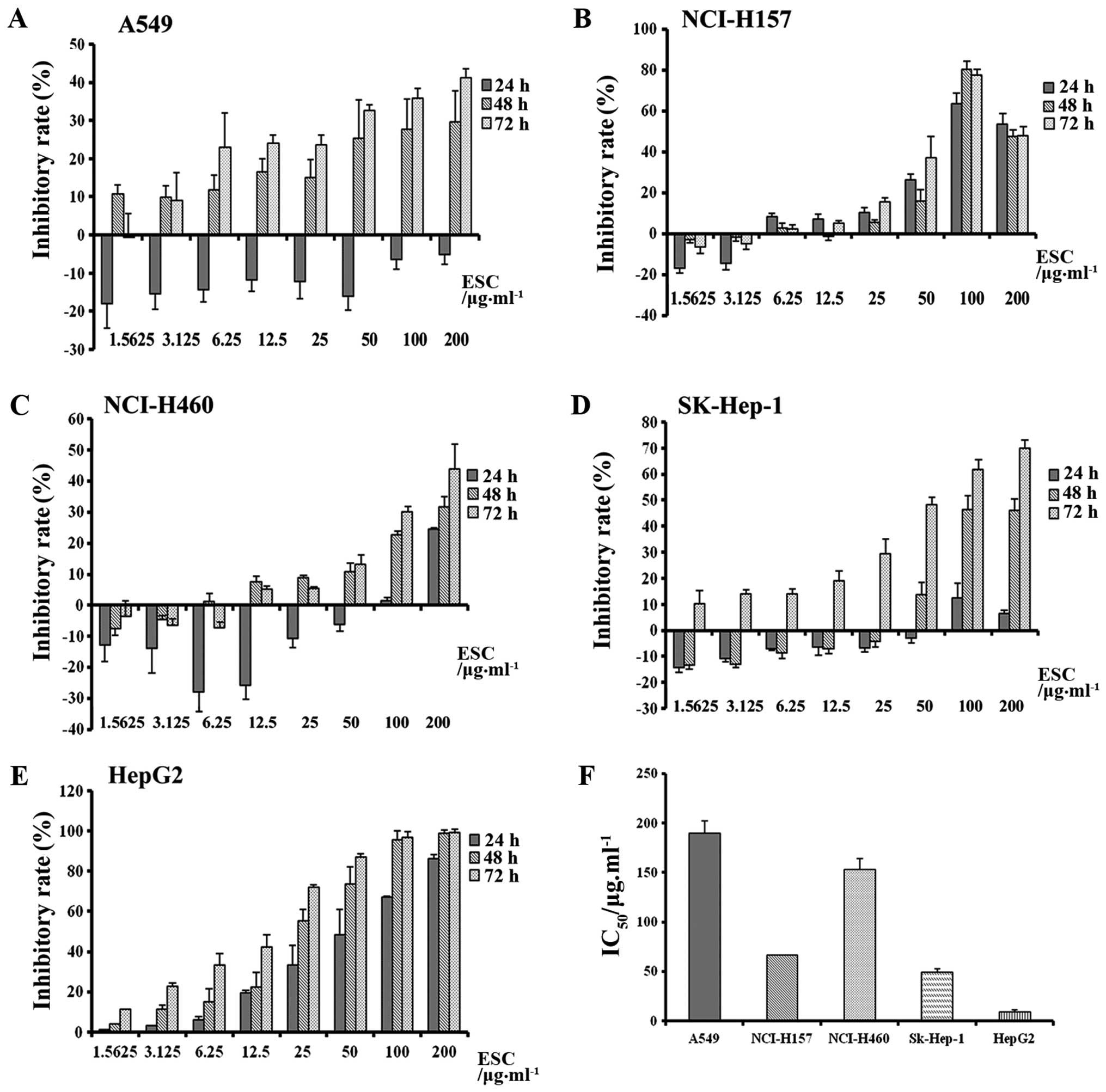
5 types of cell lines in a time-and dose-dependent manner (Fig. 1A–E).
Additionally, the inhibitory sensitivity toward
different cancers was further analyzed through calculation of the
IC50 value. The minimum IC50 was 49.00 and
9.50 µg/ml in the SK-HEP-1 and HepG2 cells, respectively,
which clearly suggested that ESC exhibited more powerful
anti-proliferation activity in the liver cancer cell lines and
hepatocarcinoma was determined to be the targeted cancer model for
further study.
Safety assessment of ESC application in
vivo
As mentioned previously, the multi-organ-involved
toxic effect of SCL was the widely existed and severely restrictive
factor for SCL application. To exclude this in vivo, we
conducted a safety test on the liver cancer xenograft mice, which
was confirmed to be the most sensitive model for ESC.
According to the results, 135.85, 271.7 and 543.4
mg/kg (equivalent to 1/10, 1/5, 1/2.5 of the LD50 value,
respectively) were chosen as a low, medium and high dose for ESC
oral application. The tumor-bearing mice were randomly grouped,
regularly treated and weighed. The most susceptible tissues,
including peripheral blood, thymus gland and spleen, were collected
after 10 days of drug administration and prepared for further
detailed analysis.
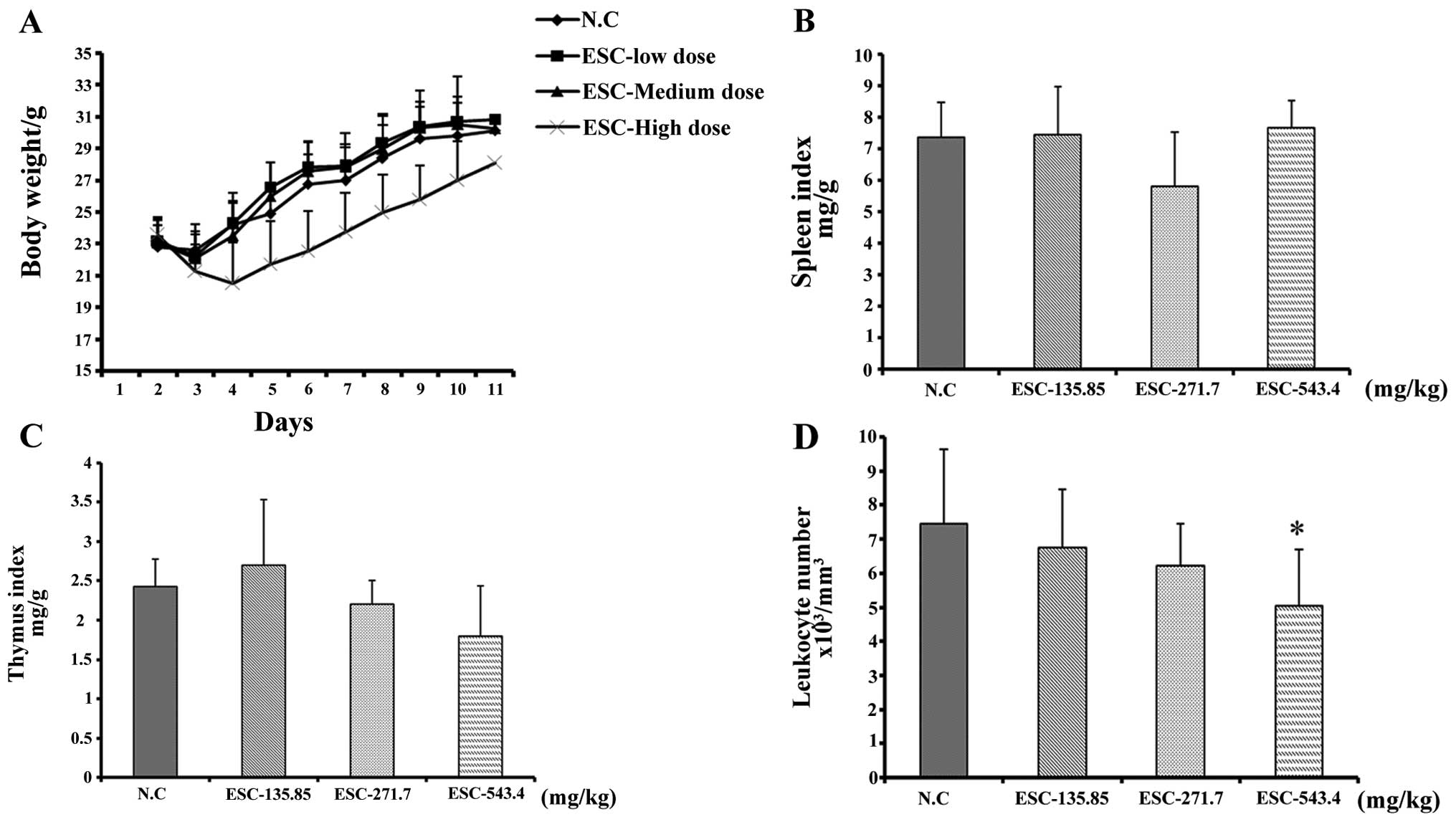
The results showed that intragastric administration
of a low and medium dose of ESC had little impact on body weight
(Fig. 2A), and the spleen and
thymus indices (fig. 2B and C).
Moreover, whole blood analysis revealed that the quantity of
leukocytes was also maintained at a similar level when compared
with the negative control mice (Fig.
2D). It is notable that, although the leukocyte count in the
high dose group decreased to
5.05±1.66×103/mm3, its value was maintained
within the physiological level, which is
4.0–12.0×103/mm3. The spleen and thymus
indices were not obviously affected by ESC treatment. Collectively,
our test clearly indicated that, different from the known extracts
from SCL, ESC exhibited minimal hematotoxicity and had little
influence on the immune system following oral application, which
fully guaranteed the application safety and met the requirement for
further study.
Identification of the tumor inhibitory
effect of ESC in vivo
Based on the above results, we further aimed to
ascertain whether the anti-proliferative effects of ESC in
vitro functionally contribute to the suppression of tumor
burden in vivo.
To accomplish this, an H22 hepatocarcinoma model was
established and tumor morphological observation, volume calculation
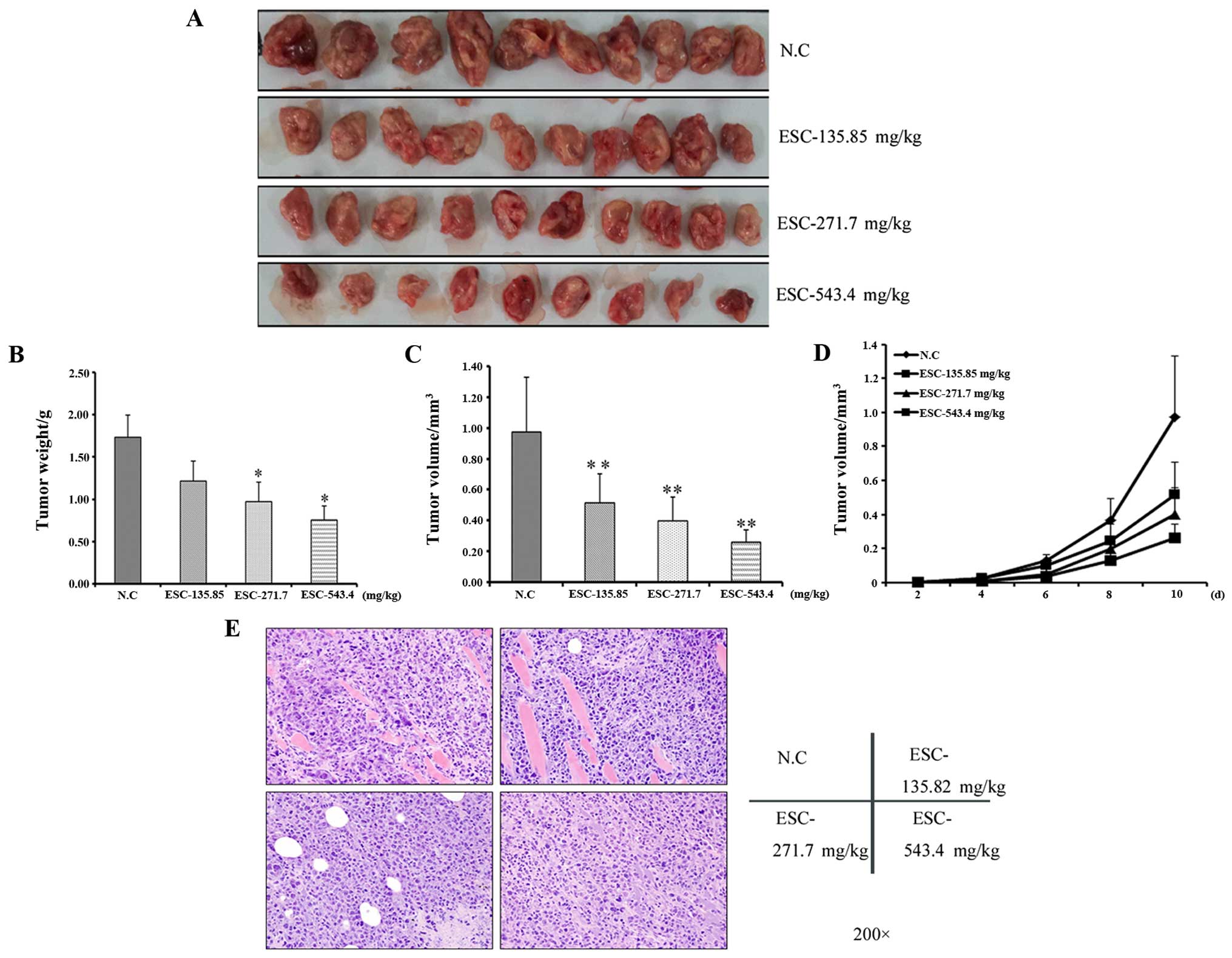
and pathological analysis were performed. Firstly, through general
observation, tumors in the negative control group were formed by
the red and crisp tissues and characterized by their incomplete
pseudomembrane and bleeding tendency. Importantly, at the margin of
the tumors, part of the cancer cells invaded into the surrounding
tissue, which is the morphological marker for an aggressive growth
pattern and high malignancy.
In contrast, the drug-exposed tumors were
characterized by a rounded shape with a low perfusion level and
firm texture (Fig. 3A). In line
with this observation, the average tumor weight and volume in the
ESC-treated groups were obviously and dose dependently decreased in
response to ESC treatment (fig. 3B and
C). Moreover, the tumor growth curve clearly showed that ESC
exerted a tumor growth suppressive effect since the 4th day after
modeling in a dose-dependent manner (fig. 3D), which further statistically
confirms that ESC can efficiently and negatively regulate primary
hepatocarcinoma growth.
Accordingly, the pathological analysis further
supported our results. As noted from the microscopic observation
and pathological scoring, in the negative control mice, 80% of
tumors showed high heteromorphism (Table I). In addition, cell death was
detected at a low level (Table
II). By H&E staining, the surrounding muscles and
connective tissue were extensively and widely infiltrated by tumor
cells. In contrast from the above results, the cell heteromorphism
was gradually alleviated along with the elevated level of cell
death following ESC treatment (Fig.
3E and Tables I and II). Taken together, our results clearly
revealed the potent activity of ESC on the regulation of malignant
growth behavior, which finally resulted in the inhibition of
proliferation of hepatocarcinoma in vivo.
 | Table ITumor cell heteromophism in each
group. |
Table I
Tumor cell heteromophism in each
group.
| Group | No. of animals | Heteromorphism
| P-value |
|---|
| − | + | ++ | +++ |
|---|
| N.C | 10 | 0 | 0 | 2 | 8 | |
| ESC - low dose | 9 | 0 | 0 | 6 | 3 | >0.05 |
| ESC - medium
dose | 10 | 0 | 0 | 7 | 3 | <0.05 |
| ESC - high
dose | 8 | 0 | 0 | 7 | 1 | <0.01 |
 | Table IIThe apoptosis of tumor cells in each
group. |
Table II
The apoptosis of tumor cells in each
group.
| Group | No. of animals | Apoptosis
| P-value |
|---|
| − | + | ++ |
|---|
| N.C | 10 | 9 | 1 | 0 | |
| ESC - low dose | 9 | 6 | 3 | 0 | >0.05 |
| ESC - medium
dose | 10 | 4 | 4 | 0 | <0.10 |
| ESC - high
dose | 8 | 3 | 5 | 0 | <0.05 |
ESC induces G2/M arrest in
hepatocarcinoma cells
Indicated by the above evidence, we next
investigated the underlying mechanism of ESC in the regulation of
tumor cell growth and proliferation.
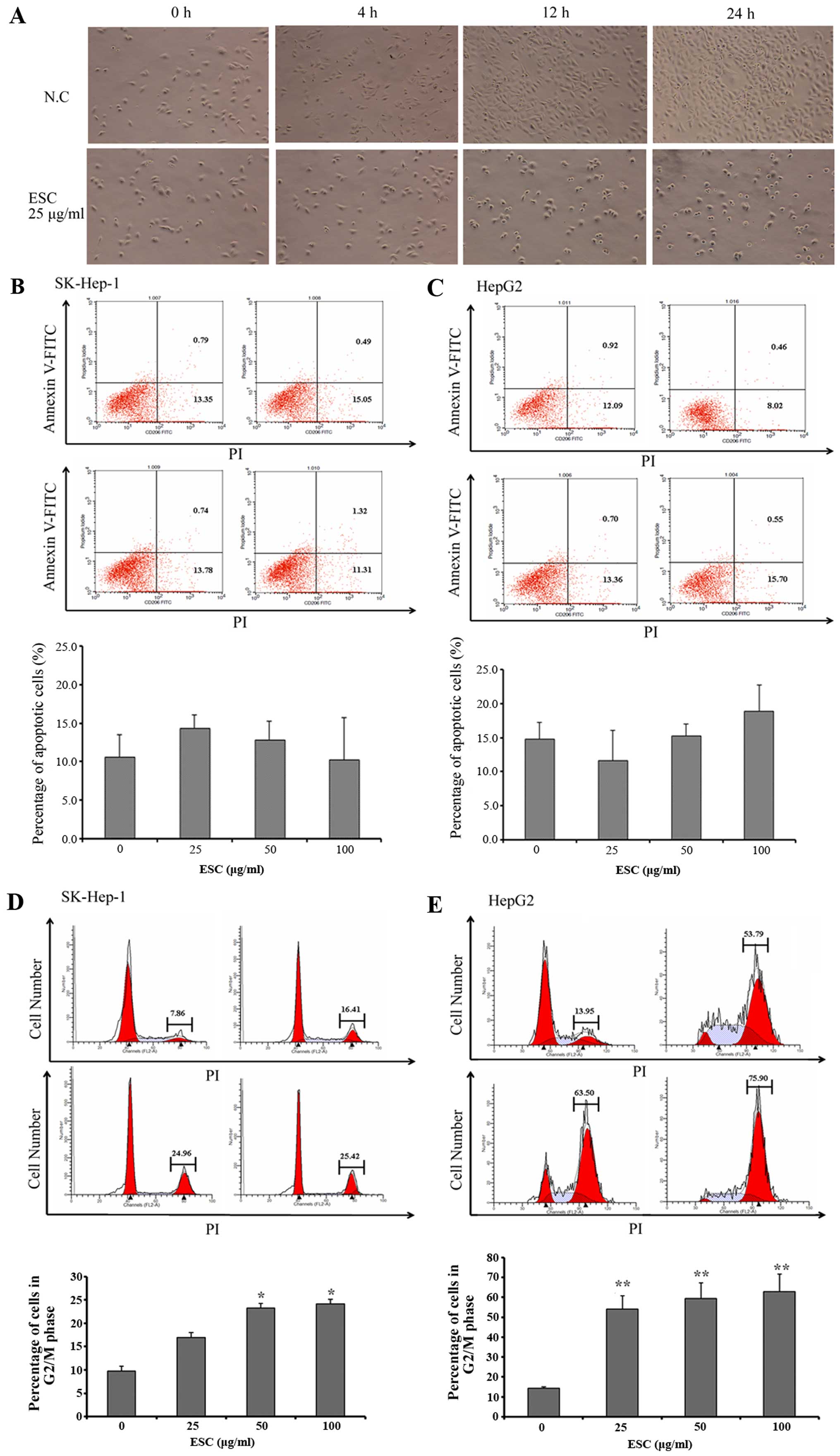
In the present study, we firstly noted that, with
the same initial cell density, the cultural confluency was
significantly and time-dependently lower in the ESC groups than
that in the negative control group (Fig. 4A). Initially, we speculated this
phenomenon was closely associated with apoptosis induction.
However, the results of Annexin V-FITC/PI staining explicitly
showed that the percentage of apoptotic cells was maintained at the
approximate level in each group in both cell lines (Fig. 4B and C), which excluded an
apoptosis-inducing effect of ESC. Therefore, we naturally
hypothesized that ESC treatment may lead to cell cycle arrest.
In order to clarify this, flow cytometry assay was
carried out to detect changes in the cell cycle distribution of the
SK-HEP-1 and HepG2 cells.
As expected, in the HepG2 and SK-HEP-1 negative
control groups, the percentages of cells in the G2/M phase were
7.86 and 13.95%, respectively. In contrast, ESC treatment induced a
3- to 6-fold increase in G2/M-phase cells; the percentages at most
reached 25.42% in the HepG2 and 75.90% in the SK-HEP-1 cells
(Fig. 4D and E). In addition, these
data further revealed the typical dose-dependent characteristic of
ESC in the regulation of cell cycle distribution in hepatocarcinoma
cells. This result clearly proved our hypothesis and identified
that ESC functions as a potent cell cycle regulator which results
in arrest of proliferation and tumor growth inhibition in
vivo.
Regulatory effects of ESC on cell
cycle-associated molecules
Based on the above results, we further analyzed the
influence of ESC on cell cycle regulation at the molecular level.
It is widely accepted that mitosis is strictly controlled by a
molecular complex at different checkpoints consisting of
phase-specific cyclins and cyclin-dependent kinases (CDKs).
Sequential activation of such a complex is necessary for motivating
the cell cycle physiologically. In contrast, any prolonged or
excessive signals will lead to uncontrolled mitosis and
pathological cell cycle progression, which functionally causes the
malignant proliferation in transformed cells. Led by this theory,
we detected the expression levels of CDK1 and cyclin B1, the
essential regulatory components specifically for the G2/M
phase.
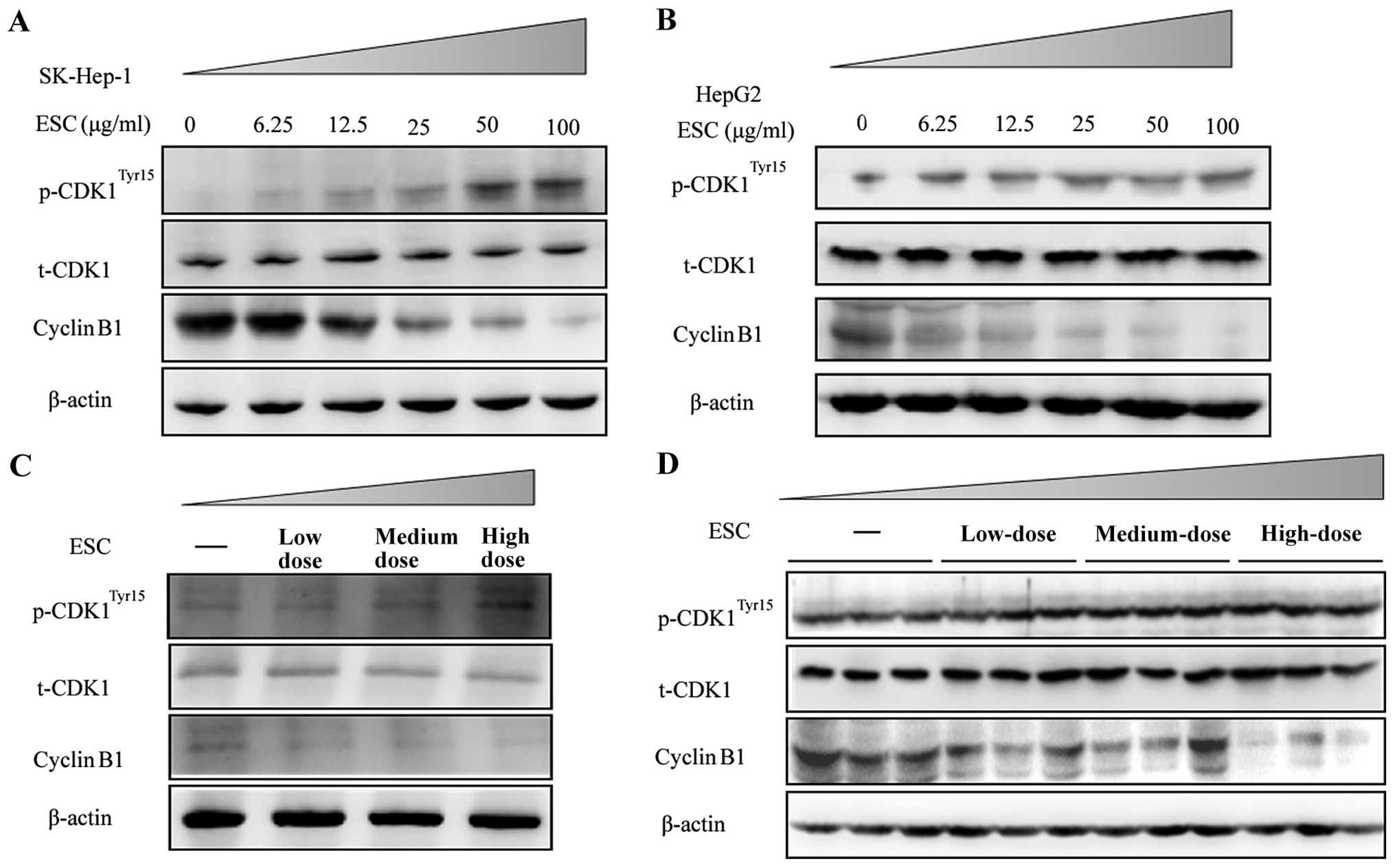
By immune blot analysis, the results clearly
revealed that ESC treatment at 25, 50, 100 µg/ml
dose-dependently decreased the expression level of cyclin B1.
Accordingly, the phosphorylation level of CDK1 at the site of Tyr15
was significantly upregulated in both the SK-HEP-1 and HepG2 cells
(Fig. 5A and B).
To further determine whether ESC exhibits the same
function in vivo, samples from the xenograft model described
in Fig. 3 were used, and the key
cell cycle regulators were detected. Consistent with the results
in vitro, ESC dose-dependently induced a decrease in cyclin
B1 and upregulated the phosphorylation of CDK1Tyr15 in
the mixed tumor lysate (Fig. 5C) or
in single tumor samples (Fig. 5D).
Thus, we concluded that ESC played an important role in cell cycle
arrest by suppressing the monitor protein in the G2/M phase.
Discussion
During this century, with advancements in technology
and scientific research, improvements have been achieved in the
area of cancer therapy, and various types of effective drugs have
been discovered. However, to date, the incidence and mortality rate
of liver cancer remain at a high level. Even though locoregional
therapies such as hepatic artery ligation or embolization have been
developed in the past decades, they have failed to significantly
prolong the overall survival of liver cancer patients (22–24).
Despite decades of efforts made by researchers worldwide, the
investigation of anti-liver cancer drugs is stagnant (25–28).
Conventionally, the commonly used drugs for liver cancer
chemotherapy are 5-fluorouracil, mitomycin C, cisplatin,
doxorubicin and their derivatives. In recent years, etoposide
(VP-16) and paclitaxel have also been used in the clinic, but the
efficacy has not obviously improved (29,30).
Unfortunately, chemoresistance accompanies the
extensive and long-term use of chemotherapeutic drugs in clinical
liver cancer treatment (29).
Moreover, the commonly used chemical agents usually have unexpected
side-effects, such as hair loss, nausea, vomiting, and diarrhea
(31). Additionally, after
resection or transarterial chemoembolization, the impaired
physiological function of the liver and gallbladder leads to
further severe side-effects and systemic toxicity (32). Therefore, the lack of effective
chemotherapy for liver cancer contributes to the death of patients
as early as one year after diagnosis (33). It is imperative to search for novel
and effective anti-hepatocarcinoma drugs. Thus, natural extracts
and their derivatives are valuable sources for drug discovery.
SCL is a wide-spread and historical-recorded plant,
which has been used to cure cancer for over a thousand years. In
previous studies of SCL, the extraction processes were immature and
the efficiency was unstable. In addition, the unselective toxicity
was another inevitable obstacle in SCL application. In our study,
we established a novel extraction platform and made a reasonable
optimization on SCL extraction. Through high throughput screening,
we found a new extract, ESC, which exerted a specific inhibitory
effect against liver cancer both in vitro and in
vivo. Importantly, the antitumor activity showed little
toxicity for immune and hematological systems in vivo. Thus,
ESC has the potential to become an ideal candidate for clinical
liver cancer treatment.
Based on a previous study, sustaining proliferative
signaling, evading growth factors and enabling replicative
immortality are widely regarded as the most important biological
capabilities required in tumor progression (34). Whereas, the oncogenic cell cycle
regulation is the foundation of all these three hallmarks.
Therefore, therapeutics targeting the cell cycle are reliable
strategies for cancer treatment. Inspired by this, in addition to
identification of efficacy, we also carried out a preliminary but
indicative exploration on the mechanism of ESC against liver
cancer. In this study, we found that ESC induced cell cycle arrest
at the G2/M phase in both HepG2 and SK-HEP-1 liver cancer cell
lines. It is widely accepted that the cell cycle is governed by a
series of checkpoint molecular complexes consisting of cyclins and
CDKs. The sequential paired combination and separation between
specific cyclin and CDK is the foundation of a normal cell cycle.
Controlled by cyclin B-CDK1 complex activation, the G2/M checkpoint
prevents DNA-damaged cells from entering mitosis and allowing DNA
repair (35). During this process,
the cyclin B-CDK1 complex activation is dependent on expression of
cyclin B1 and dephosphorylation of CDK1Tyr15. By
analyzing the expression level of cyclin B1 and the phosphorylation
level of CDK1Tyr15, we found that the activity of the
cyclin B1-CDK1 complex was downregulated in a dose-dependent manner
by ESC.
In conclusion, ESC exhibited a potent effect against
liver cancer under a relatively safe condition via arresting tumor
cells in the G2/M phase. Thus, ESC is a promising chemotherapeutic
candidate to treat liver cancer. This may provide patients, who are
not qualified for surgery, with another option and offer a novel
comprehensive therapeutic strategy combining liver surgical
resection and hepatic artery ligation.
Encouraged by the results mentioned previously,
further experiments will be conducted to clarify the detailed and
precise mechanism in ESC-induced cell cycle arrest. The purified
chemical compound isolated from ESC would certainly be another aim
of future study.
Acknowledgments
This study has been supported by the Five-Year
National Science and Technology Major Projects (2013ZX09301307004)
and the National Natural Science Foundation of China (81303272 and
81303273).
Abbreviations:
|
CDKs
|
cyclin-dependent kinases
|
|
SCL
|
Stellera chamaejasme L.
|
|
ESC
|
extract of Stellera chamaejasme L
|
References
|
1
|
Wu F, Lin GZ and Zhang JX: An overview of
cancer incidence and trend in China. China Cancer. 81–85. 2012.
|
|
2
|
Rabindran SK, Ross DD, Doyle LA, Yang W
and Greenberger LM: Fumitremorgin C reverses multidrug resistance
in cells transfected with the breast cancer resistance protein.
Cancer Res. 60:47–50. 2000.PubMed/NCBI
|
|
3
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J and Luo R: Advances of
biochemotherapy in hepatocellular carcinoma. J Mol Diagnostics
Therapy. 1:602009.
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasegawa K, Kokudo N and Makuuchi M:
Surgical management of hepatocellular carcinoma. Liver resection
and liver transplantation. Saudi Med J. 28:1171–1179.
2007.PubMed/NCBI
|
|
9
|
Poon RT, Fan ST, Tsang FH and Wong J:
Locoregional therapies for hepatocellular carcinoma: A critical
review from the surgeon's perspective. Ann Surg. 235:466–486. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: The need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dizon DS and Kemeny NE: Intrahepatic
arterial infusion of chemotherapy: Clinical results. Semin Oncol.
29:126–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi GL, Liu SQ, Cao H, Zhao LL, Li J and
Li SY: Acaricidal activities of extracts of Stellera chamaejasme
against Tetranychus viennensis (Acari: Tetranychidae). J Econ
Entomol. 97:1912–1916. 2004. View Article : Google Scholar
|
|
14
|
Yang BY: Inhibitory effects of Stellera
chamaejasme on the growth of a transplantable tumor in mice. Zhong
Yao Tong Bao. 11:58–59. 1986.In Chinese. PubMed/NCBI
|
|
15
|
Yoshida M, Feng W, Saijo N and Ikekawa T:
Antitumor activity of daphnane-type diterpene gnidimacrin isolated
from Stellera chamaejasme L. Int J Cancer. 66:268–273. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang G, Liao Z, Xu Z, Zhang H and Chen D:
Antimitotic and antifungal C-3/C-3″-biflavanones from Stellera
chamaejasme. Chem Pharm Bull (Tokyo). 53:776–779. 2005. View Article : Google Scholar
|
|
17
|
Yang G and Chen D: Biflavanones,
flavonoids, and coumarins from the roots of Stellera chamaejasme
and their antiviral effect on hepatitis B virus. Chem Biodivers.
5:1419–1424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
19
|
Wittekind C: The new WHO classification of
liver tumors - what is really new? Verh Dtsch Ges Pathol.
85:212–218. 2001.In German.
|
|
20
|
Pozarowski P and Darzynkiewicz Z: Analysis
of cell cycle by flow cytometry. Methods Mol Biol. 281:301–311.
2004.PubMed/NCBI
|
|
21
|
Novotny-Diermayr V, Sangthongpitag K, Hu
CY, et al: SB939, a novel potent and orally active histone
deacetylase inhibitor with high tumor exposure and efficacy in
mouse models of colorectal cancer. Mol Cancer Ther. 9:642–652.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yim HJ, Suh SJ and Um SH: Current
management of hepatocellular carcinoma: An Eastern perspective.
World J Gastroenterol. 21:3826–3842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okuda K, Ohtsuki T, Obata H, Tomimatsu M,
Okazaki N, Hasegawa H, Nakajima Y and Ohnishi K: Natural history of
hepatocellular carcinoma and prognosis in relation to treatment.
Study of 850 patients. Cancer. 56:918–928. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagasue N, Yukaya H, Hamada T, Hirose S,
Kanashima R and Inokuchi K: The natural history of hepatocellular
carcinoma. A study of 100 untreated cases. Cancer. 54:1461–1465.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SH, Lee Y, Han SH, Kwon SY, Kwon OS,
Kim SS, Kim JH, Park YH, Lee JN, Bang SM, et al: Systemic
chemotherapy with doxorubicin, cisplatin and capecitabine for
metastatic hepatocellular carcinoma. BMC Cancer. 6:32006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boucher E, Corbinais S, Brissot P,
Boudjema K and Raoul JL: Treatment of hepatocellular carcinoma
(HCC) with systemic chemotherapy combining epirubicin, cisplatinum
and infusional 5-fluorouracil (ECF regimen). Cancer Chemother
Pharmacol. 50:305–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pohl J, Zuna I, Stremmel W and Rudi J:
Systemic chemotherapy with epirubicin for treatment of advanced or
multifocal hepatocellular carcinoma. Chemotherapy. 47:359–365.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feun LG, O'Brien C, Molina E, Rodriguez M,
Jeffers L, Schiff ER, Marini A, Savaraj N and Ardalan B:
Recombinant leukocyte interferon, doxorubicin, and 5FUDR in
patients with hepatocellular carcinoma-A phase II trial. J Cancer
Res Clin Oncol. 129:17–20. 2003.PubMed/NCBI
|
|
29
|
Alexandre J, Tigaud JM, Gross-Goupil M,
Gornet JM, Romain D, Azoulay D, Misset JL and Goldwasser F:
Combination of topotecan and oxaliplatin in inoperable
hepatocellular cancer patients. Am J Clin Oncol. 25:198–203. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsu JL, Chiang PC and Guh JH: Tunicamycin
induces resistance to camptothecin and etoposide in human
hepatocellular carcinoma cells: Role of cell-cycle arrest and
GRP78. Naunyn Schmiedebergs Arch Pharmacol. 380:373–382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ueda H, Fukuchi H and Tanaka C: Toxicity
and efficacy of hepatic arterial infusion chemotherapy for advanced
hepatocellular carcinoma (Review). Oncol Lett. 3:259–263.
2012.PubMed/NCBI
|
|
32
|
Basile A, Carrafiello G, Ierardi AM,
Tsetis D and Brountzos E: Quality-improvement guidelines for
hepatic transarterial chemoembolization. Cardiovasc Intervent
Radiol. 35:765–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gerunda GE, Neri D, Merenda R, Barbazza F,
Zangrandi F, Meduri F, Bisello M, Valmasoni M, Gangemi A and
Faccioli AM: Role of transarterial chemoembolization before liver
resection for hepatocarcinoma. Liver Transpl. 6:619–626. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Ji P, Liu J, Broaddus RR, Xue F
and Zhang W: Centrosome-associated regulators of the G(2)/M
checkpoint as targets for cancer therapy. Mol Cancer. 8:82009.
View Article : Google Scholar : PubMed/NCBI
|