Introduction
Breast cancer is one of the most common malignancies
among females (1), which can occur
in humans and other mammals, and most cases are women (2). Over 1 million persons are diagnosed
with breast cancer each year (3).
Similarly to other cancers, carcinogenesis of breast cancer is a
complex process. Although mortality rate of breast cancer has been
observably reduced, metastatic breast cancer still remains puzzling
(4). The mechanisms of
carcinogenesis and metastasis need to be better clarified.
MicroRNA (miRNA) is a class endogenous non-coding
single-stranded RNA. As circulating marker, miRNA is being studied
extensively (5,6). miRNAs are able to downregulate
approximately 1/3 human genes by binding to 3′-untranslated region
(3′-UTR) of target mRNA (5,7). miRNAs are involved in many biological
processes, such as cell proliferation, apoptosis, migration and
carcinogenesis (8–11). Many miRNAs are also involved in
carcinogenesis and development of breast cancer (9,10). It
has been shown that miR-200c, miR-206, miR-335, miR-494 and
miR-125b are downregulated in breast cancer tissues, suggesting
that they may play tumor suppressor roles (12–16).
As oncogenes of breast cancer, miR-155, miR-21, miR-210, miR-373
and miR-10b are upregulated in patients with breast cancer
(17–20). The miR-214 is decreased in breast
cancer (21), and miR-218 is also
known as a tumor suppressor in prostate cancer, hepatocellular
carcinoma and glioblastoma (22–24),
but the mechanisms of both miR-214 and miR-218 are unclear.
In the present study, we investigate the expression
of miR-214 and miR-218 in breast cancer and adjacent tissues, and
analyzed the correlations in miR-214 and miR-218 expression and the
clinicopathological characteristics. The effects of miR-214 or
miR-218 on cell proliferation, apoptosis and cell cycle were also
determined in vitro. Our results may provide new biomarkers
for diagnosis, prognosis and therapy, and be helpful to clarify the
mechanisms of post-transcription regulation in breast cancer.
Materials and methods
Clinical samples
Forty-nine breast cancer tissues and their paired
adjacent tissue samples, which were diagnosed by pathological
surgical resection, were collected between 2013 and 2015 at The
First Hospital of Hebei Medical University (Shijiazhuang, China).
The tissues were frozen in liquid nitrogen at −80°C immediately
until use. Breast cancer patients who had undergone chemotherapy or
radiation therapy before surgery were excluded.
Ethics statements
Permission to use human tissue samples for research
purposes was approved by the Biomedical Ethics Committee of Hebei
Medical University, Shijiazhuang, Hebei, China. All patients were
female and consented to participate in the present study.
Cell line and transfection
Breast cancer cell line MCF-7 was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA),
cultured in RPMI-1640 containing 10% fetal bovine serum (FBS), 100
U/ml of penicillin, and 100 mg/ml of streptomycin (Gibco, Grand
Island, NY, USA) in a humidified atmosphere containing 5%
CO2 at 37°C. For transfection, cells were seeded and
cultured for 24 h in 12-well plates. According to the
manufacturer's instructions, cells were transfected with miR-214
mimic, miR-218 mimic or negative control, respectively, by
Lipofectamine 2000 (Invitrogen Life Technologies, Grand Island, NY,
USA) in serum-free medium. Six hours after the transfection, the
complete medium was changed and maintained for 48 h at 37°C in 5%
CO2. The mimics of miR-214, miR-218 and negative control
were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou,
China).
Real-time reverse transcription
polymerase chain reaction (real-time RT-PCR)
Total RNA was extracted from breast cancer tissues,
adjacent tissues and MCF-7 cells by TRIzol reagent (Invitrogen),
according to the manufacturer's instructions. Reverse transcription
PCR and real-time PCR were performed with TaqMan microRNA Reverse
Transcription kit and TaqMan Universal Master Mix (Applied
Biosystems, Foster City, CA, USA, respectively) following standard
protocol. U6 was used as an endogenous control to normalize
variance. The primers of miR-214, miR-218 and U6 were purchased
from Applied Biosystems. The fold changes were calculated via
relative quantification (2−ΔCT).
Cell proliferation assay
Cell proliferation was determined by the Cell
Counting kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc.,
Beijing, China). MCF-7 cells (1,000 cells/well) were cultured in
96-well plates. After incubation, 10 µl of the CCK-8
solution was added to each well of the plate, and incubated for 1 h
in the incubator, and the absorbance was measured at 450 nm using a
microplate reader according to the CCK-8 kit manufacturer's
instructions.
Flow cytometry
The effects of miR-214 and miR-218 on breast cancer
cell apoptosis and cell cycle were examined by flow cytometry (BD
Biosciences, Mansfield, MA, USA). In brief, MCF-7 cells were
transfected with miR-214 mimic, miR-218 mimic or negative control
for 48 h, the cells were completely collected including apoptotic
cells in culture medium, and washed twice with cold PBS. The cell
apoptosis were analyzed by Annexin V-FITC detection kit
(Neobioscience Technology, Shenzhen, China) according to the
manufacturer's protocol. For determining the cell cycle, the cells
were transfected for 48 h, washed twice with PBS, treated with
trypsin, and fixed with 75% ethanol overnight at −20°C, and
incubated with 100 mg/ml RNase A and 50 mg/ml propidium iodide (PI)
at room temperature for 30 min. The percentage of the G0/G1, S and
G2/M populations were evaluated in each group. The data were
analyzed by CellQuest Pro software. Each experiment was performed
in triplicate.
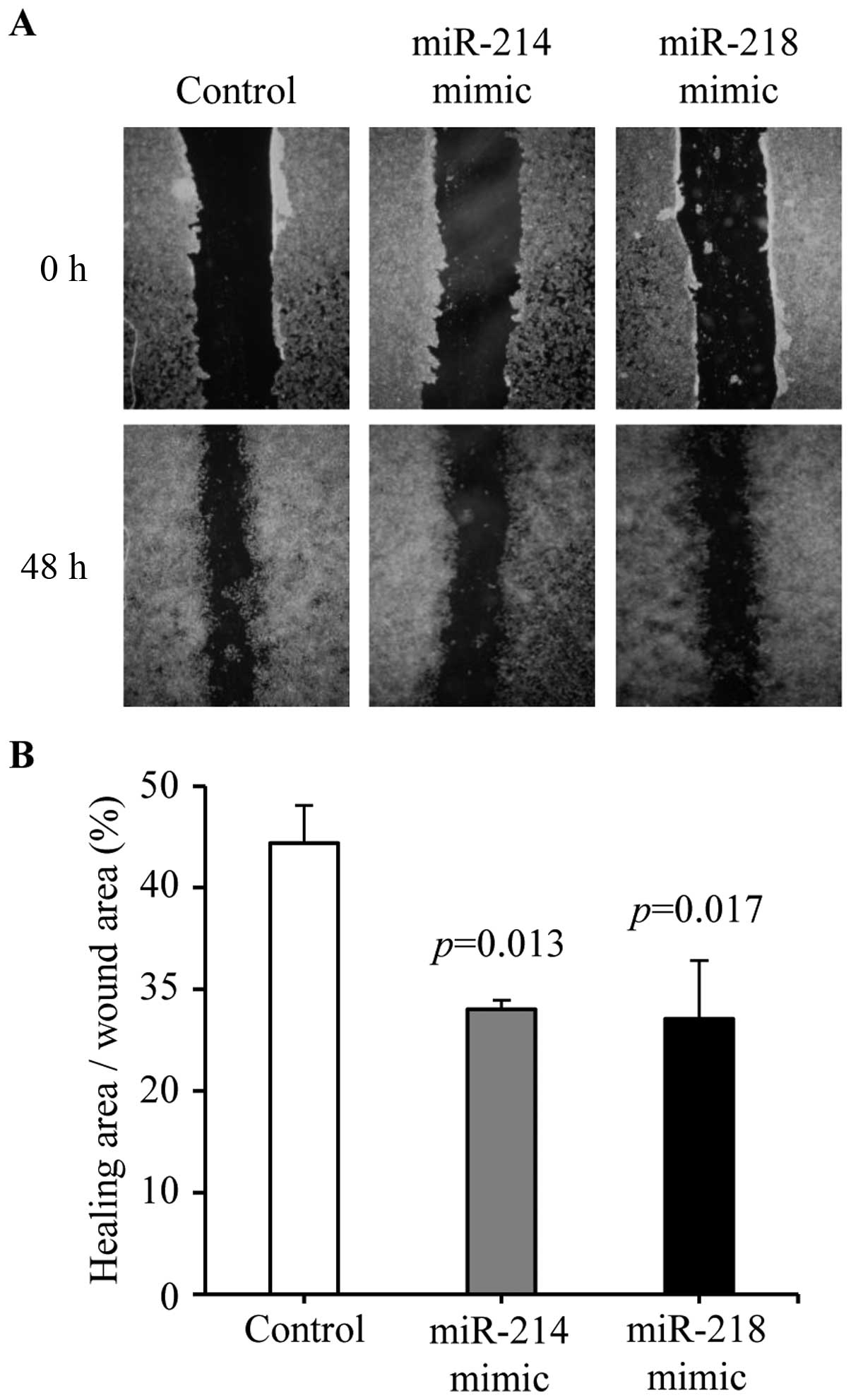
Wound-healing assay
MCF-7 cells were grown to confluence. A wound was
made by scraping with a conventional pipette tip across the
confluence cell layer, and washed with PBS twice. Forty-eight hours
after scraping, migration was determined, using the ImageJ, as a
percentage of healing area relative to the initial wound area.
Statistical analysis
Data were processed by the SPSS Graduate Pack 13.0,
GraphPad 5 software or the Student's t-test. P<0.05 was
considered to be statistically significant.
Results
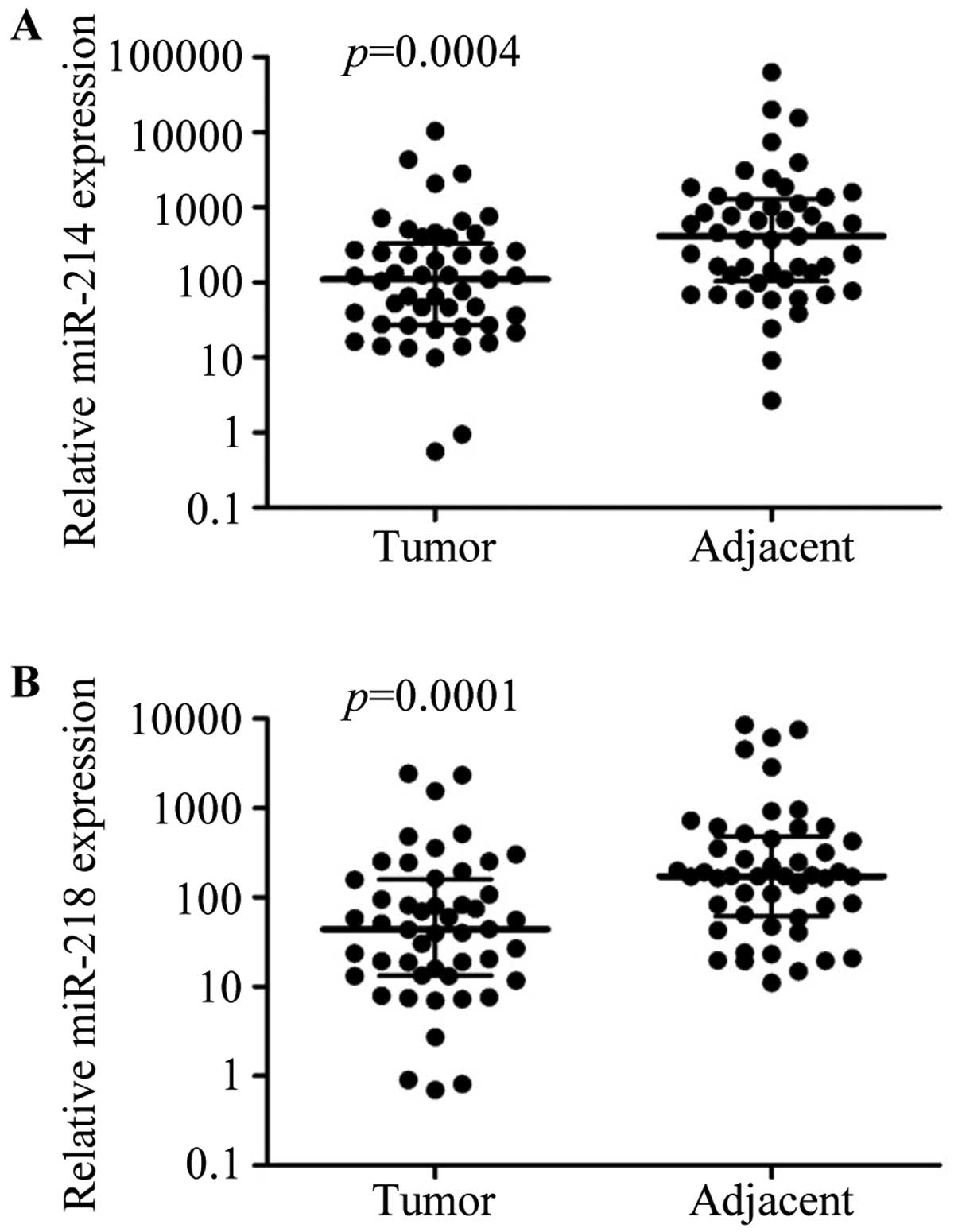
miR-214 and miR-218 are downregulated in
breast cancer tissues
To assess the effects of miR-214 and miR-218 on
breast cancer development, we first evaluated miR-214 and miR-218
expression levels in breast cancer and adjacent cancer tissues by
real-time RT-PCR. The expression of miR-214 and miR-218 was
significantly downregulated in breast cancer tissues compared with
matching adjacent tissues (P<0.01, respectively; Fig. 1).
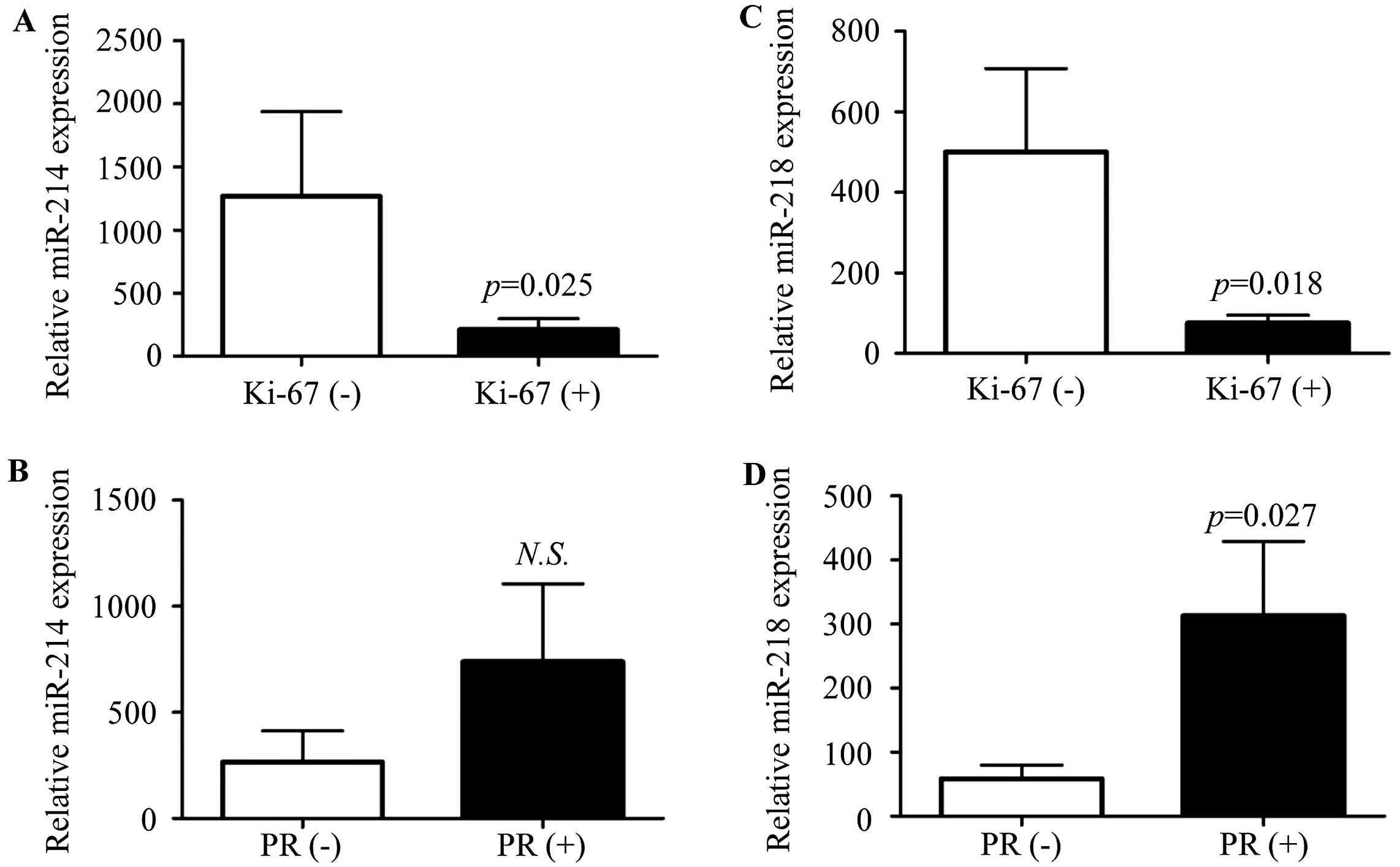
Correlation of miR-214 or miR-218
expression with clinicopathological data
We further evaluated the correlation of miR-214 or
miR-218 expression and the clinicopathological factors, including
age, tumor size, lymph node metastasis, clinical stage, estrogen
receptor (ER), progesterone receptor (PR), epidermal factor
receptor-2 (HER-2), p53 and Ki-67. ER, PR, HER-2, p53 and Ki-67
were detected by immunohistochemistry, respectively. When the
number of stained cells was >10% in one field, it was defined as
positive. We found that miR-214 and miR-218 expression was
negatively associated with Ki-67 expression (Fig. 2A and C; P=0.025, 0.018,
respectively), and the expression of PR was positively associated
with miR-218 (Fig. 2D; P=0.027),
but not miR-214 (Fig. 2B; P=0.4).
There was no correlation between the miR-214 or miR-218 expression
level with age, tumor size, lymph node metastasis, stage, ER, HER-2
and p53 (Table I).
 | Table ICorrelations of miR-214 and miR-218
expression and the clinicopathological characteristics of breast
cancer patients. |
Table I
Correlations of miR-214 and miR-218
expression and the clinicopathological characteristics of breast
cancer patients.
| Parameters | No. of
patients | Relative miR-214
expression Median (interquartile range) | P-value | Relative miR-218
expression Median (interquartile range) | P-value |
|---|
| Age (years) | | | 0.27 | | 0.81 |
| ≤52 | 25 | 104.02
(26.96–689.51) | | 43.73
(10.42–179.10) | |
| >52 | 24 | 116.64
(26.30–221.89) | | 47.39
(13.84–144.50) | |
| Tumor size
(cm) | | | 0.82 | | 0.63 |
| ≤2 | 17 | 122.86
(25.15–427.12) | | 30.29
(5.10–248.76) | |
| >2 | 32 | 107.23
(29.92–246.91) | | 47.38
(18.90–100.79) | |
| Lymph node
metastasis | | | 0.90 | | 0.94 |
| Negative | 23 | 110.43
(26.69–406.72) | | 50.74
(15.88–94.53) | |
| Positive | 26 | 94.33
(26.87–294.63) | | 43.88
(12.79–170.83) | |
| Clinical
stagea | | | 0.77 | | 0.72 |
| I | 11 | 124.65
(23.62–447.52) | | 81.16
(7.47–303.16) | |
| II, III | 38 | 107.23
(27.63–253.54) | | 43.88
(15.25–119.50) | |
| ER | | | 0.77 | | 0.32 |
| Negative | 13 | 122.86
(37.19–160.50) | | 26.69
(10.39–160.97) | |
| Positive | 36 | 90.27
(26.04–437.32) | | 57.13
(18.77–161.17) | |
| Her-2 | | | 0.82 | | 0.68 |
| Negative | 20 | 65.41
(17.21–698.63) | | 35.18
(9.21–161.17) | |
| Positive | 29 | 123.76
(38.05–255.90) | | 50.74
(16.05–163.83) | |
| p53 | | | 0.29 | | 0.16 |
| Negative | 33 | 65.81
(22.50–333.40) | | 40.06
(9.82–132.00) | |
| Positive | 16 | 124.21
(48.80–398.44) | | 58.11
(24.42–329.89) | |
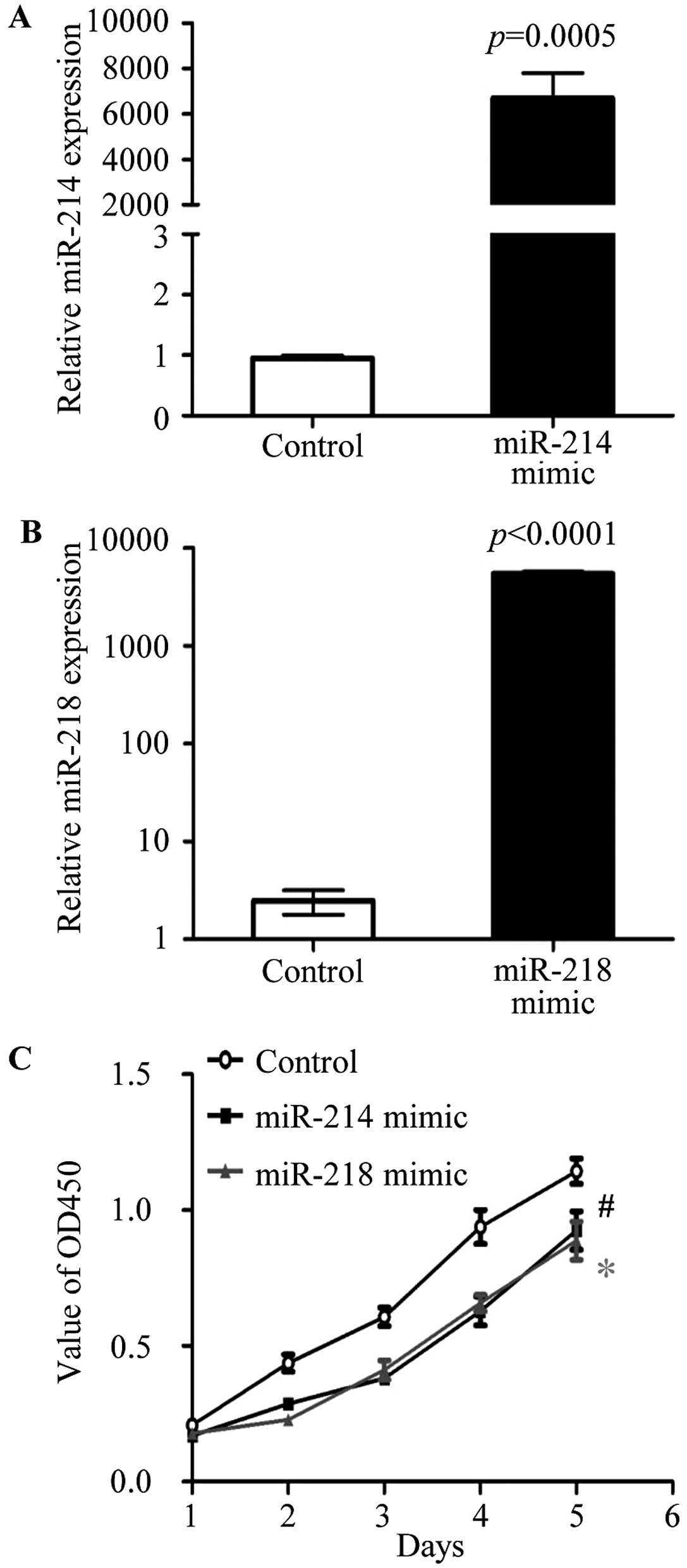
Overexpression of miR-214 and miR-218
inhibits the breast cancer cell proliferation
To test the biological function of miR-214 and
miR-218, the miR-214 mimic, miR-218 mimic and negative controls
were purchase from Applied Biosystems. The overexpression of
miR-214 or miR-218 was carried out in MCF-7 cells by transfection
with miR-214 mimic or miR-218 mimic, respectively. The expression
of miR-214 (Fig. 3A) and miR-218
(Fig. 3B) were detected by
real-time RT PCR in transfected cells. To investigate the effect of
miR-214 and miR-218 on cell proliferation, cell proliferation assay
were performed by the Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies), according to the manufacturer's instructions. After
transfection with miR-214 and miR-218 mimic, respectively, the
cells proliferation was significantly reduced compared to negative
control transfected MCF-7 cells (Fig.
3C). The data showed that miR-214 and miR-218 were able to
regulate cell proliferation of breast cancer.
Overexpression of miR-214 and miR-218
perturbs breast cancer cell cycle
The cell cycles were analyzed by flow cytometry (BD
Biosciences) after staining with propidium iodide (PI). The
percentage of cells in the G0/G1, S and G2/M phases was,
respectively, evaluated under the negative control (Fig. 4A), miR-214 (Fig. 4B) or miR-218 (Fig. 4C) mimic transfection. As shown in
Fig. 4D, the percentage of S phase
cells was significantly decreased in miR-214 overexpressed MCF-7
cells comparing with control cells (13.21±1.38 vs. 17.20±0.39,
P=0.0298), accompanied by an increase of G0/G1 phase cell ratio
(79.78±2.29 vs. 76.08±0.02, P=0.11, no-statistical significance).
The percentage of G0/G1 phase cells was significantly reduced in
miR-218 overexpressed MCF-7 cells compared with control cells
(65.74±1.40 vs. 76.08±0.02, P=0.006; Fig. 4D), and a marked increase of the cell
ratio of S phase was observed in the miR-218 mimic transfected
cells, compared to control cells (27.62±0.98 vs. 17.20±0.39,
P=0.0009; Fig. 4D). The results
confirmed that miR-214 and miR-218 disturbed the breast cancer cell
cycle.
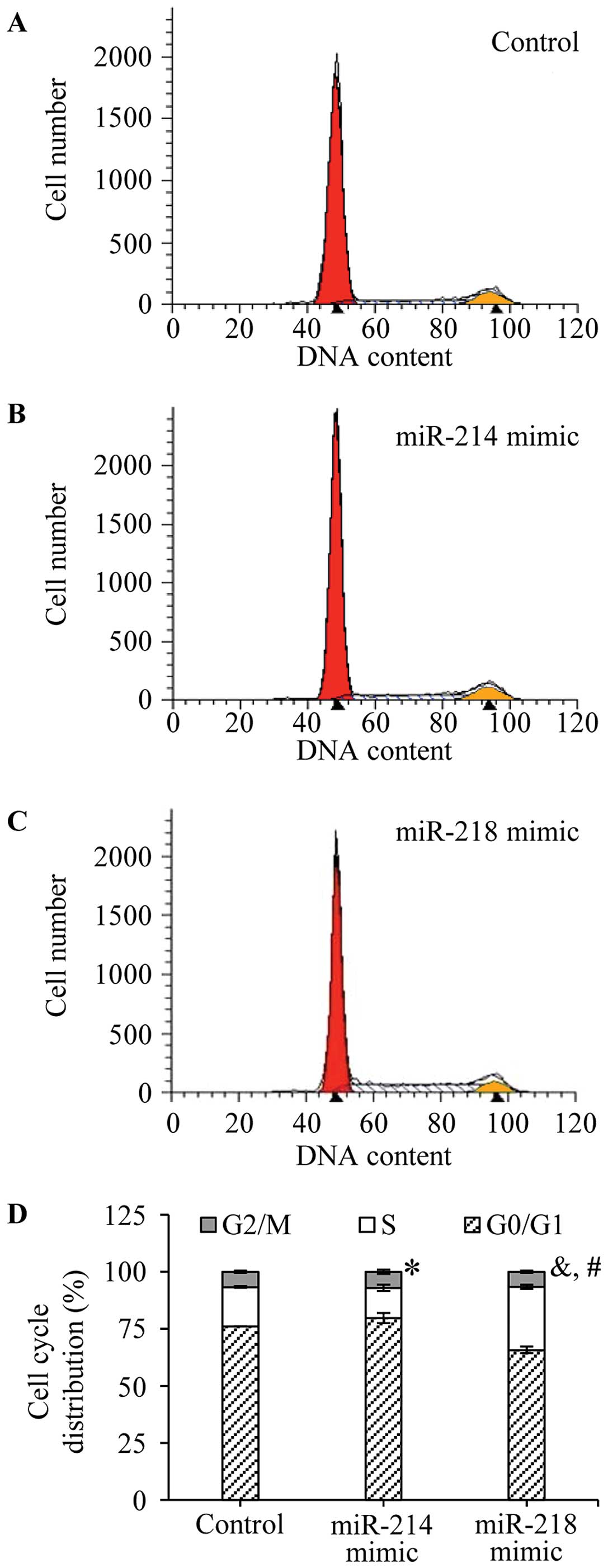 | Figure 4Effects of miR-214 and miR-218 on the
cell cycle of breast cancer. After transfection, the cells were
washed with PBS twice, and treated with trypsin. The surviving
cells were collected, excepting apoptotic cells, in culture medium.
The cell cycles were analyzed by flow cytometry after staining with
PI. The representative results of cell cycles are shown in (A)
negative control, (B) miR-214 mimic and (C) miR-218 mimic
transfected cells. (D) In addition, the percentage of cells in the
G0/G1, S and G2/M phases was, respectively, evaluated. The
experiments were performed in triplicate. *P=0.0298 (the
percentage of S phase cells, miR-214 mimic transfected cells vs.
negative control cells). &P=0.0009 (the percentage
of S phase cells, miR-218 mimic transfected cells vs. negative
control cells). #P=0.006 (the percentage of G0/G1 phase
cells, miR-218 mimic transfected cells vs. negative control
cells). |
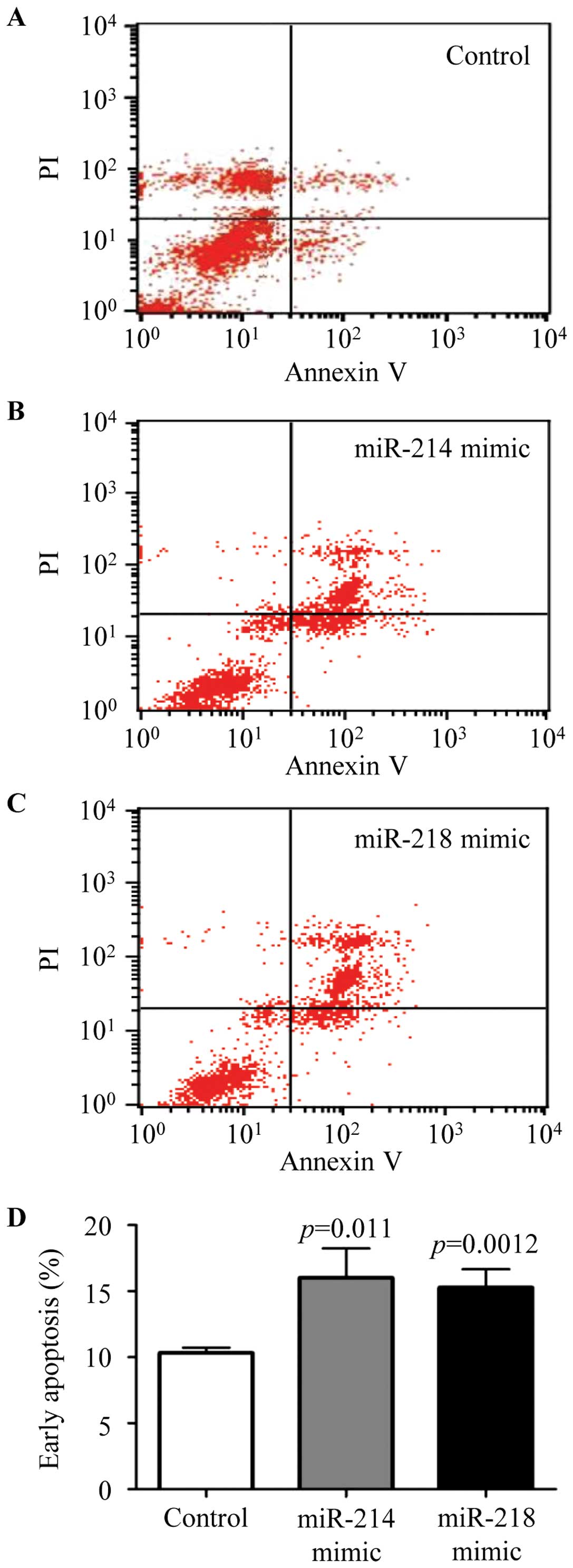
Overexpression of miR-214 and miR-218
induced early breast cancer cells apoptosis
The cells were seeded into 6-well plates and
transfected with negative control, miR-214 or miR-218 mimic, and
cell apoptosis were analyzed by Annexin V-FITC detection kit
according to the manufacturer's protocol. Representative results of
negative control (Fig. 5A), miR-214
mimic (Fig. 5B), and miR-218 mimic
(Fig. 5C) are shown, respectively.
The ratio of early apoptosis was analyzed by flow cytometry
(Fig. 5D). The results confirmed
that cell apoptosis was significantly augmented in the miR-214 or
miR-218 mimic transfected cells. These results verified that
miR-214 and miR-218 were able to mediate breast cancer cell
apoptosis.
Overexpression of miR-214 and miR-218
reduced breast cancer cell migration
Cell migration was tested by wound-healing assay.
Cell migration was decreased in miR-214 mimic (28.02±0.89 vs.
44.38±3.71%, P=0.013) and miR-218 (27.07±5.75 vs. 44.38±3.71%,
P=0.017) mimic treated cells compared with negative control
transfected cells (Fig. 6A and B).
These results demonstrated that overexpression of miR-214 or
miR-218 could inhibit breast cancer cell migration.
Discussion
The expression of miR-214 has been verified
downregulated in human cervical cancer (25–27),
pancreatic cancer (28),
hepatocellular carcinoma (29,30)
and breast cancer (28) and miR-218
expression is also reduced in oral squamous cell carcinoma,
nasopharyngeal carcinoma, and bladder cancer cells (31–34).
However, the molecular biological functions of miR-214 and miR-218
have not clarified in breast cancer. In the present study, we
comprehensively evaluated the biological functions of miR-214 and
miR-218 in breast cancer. The results indicated that miR-214 and
miR-218 function as tumor suppressor genes in breast cancer.
In particular, expression of miR-214 and miR-218 was
reduced in human breast cancer tissues, and miR-214 or miR-218
expression is negatively associated with Ki-67, a cellular marker
of tumor cells proliferation (Fig.
2). In vitro, we also validated that miR-214 and miR-218
negatively mediated MCF-7 cell proliferation and interfered with
cell cycle (Figs. 3 and 4). Previous studies have already
demonstrated that the miR-214 could suppress oncogenesis of bladder
cancer by targeting the p53 and DNA damage-regulated protein 1
(PDRG1) (35), inhibit the
progression of hepatocellular carcinoma by regulating β-catenin
(36,37), also mediating cell proliferation by
targeting ERK (38), mitochondrial
transcription factor A (TFAM) (39)
and ADP-ribosylation factor-like protein 2 (ARL2) (40). The miR-218 suppresses lung cancer
and cardiac myxoma cell growth by reducing myocyte enhancer factor
2D (MEF2D) (41,42), inhibits glioma cells proliferation
by inactivation of cyclin D1 (43)
pathway and directly target E2F2 (24), reduces bladder cancer and esophageal
squamous cells proliferation by targeting BMI-1 (34,44).
The above evidence supports our conclusion.
Our results also prove that the miR-214 and miR-218
promote early apoptosis of breast cancer cells (Fig. 5). The effects of miR-214 are
inconsistent, but some studies above confirm that overexpression of
miR-214 can promote apoptosis and the sensitivity of cisplatin
(45,46). In addition, miR-218 promoted
prostate cancer cells apoptosis by repression tumor protein D52
(22), enhanced chemotherapy
sensitivity by targeting BIRC5 and breast cancer 1 (47,48),
sensitized glioma cells to apoptosis by regulating NF-κB (49). Our results, and the previous results
imply that miR-214 and miR-218 could protect against tumorigenesis
by inducing apoptosis.
The effects of miR-214 and miR-218 on breast cancer
cell migration are shown in Fig. 6.
Some previous research supports our results, there is evidence to
prove that miR-214 could directly bind to the 3′-UTR of vascular
endothelial growth factor (VEGF) (50), the 3′-UTR of polypeptide GalNAc
transferase 7 (26) and the 3′-UTR
of PTEN (51). The miR-218 also
suppresses the cancer cell migration or invasion by downregulating
high mobility group box 1, LIM and SH3 protein 1, T-cell lymphoma
invasion and metastasis 1 (TIAM1), matrix metalloproteinase 2 and 9
(52–54). These genes induced cell migration or
invasion. Other targets of miR-214 and miR-218 will be analyzed in
the following study. Interestingly, the expression of miR-214 and
miR-218 are positively associated with PR (55), although there is no statistic
difference between miR-214 and PR expression. Many investigators
consider that PR promotes cell migration, but some investigators
still assert that progesterone or PR could exert cell migration
inhibitory action (56–59). Our results suggest that PR may, via
mediating miR-214 or miR-218, inhibit breast cancer migration.
In conclusion, our results show that miR-214 and
miR-218 expression are decreased in breast cancer tissues.
Overexpression of miR-214 or miR-218 could suppress cell
proliferation and migration, disturb the cell cycle and induce cell
apoptosis in vitro. Our findings suggest that miR-214 and
miR-218, as tumor suppressors, could play important roles in
development of breast cancer, and may be potential therapeutic
targets for breast cancer. Therefore, further research on miR-214
and miR-218 functional mechanisms in breast cancer is required.
Acknowledgments
The present study was supported by the Science and
Technology Support Project of Hebei, China (no. 14277755D).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fentiman IS, Fourquet A and Hortobagyi GN:
Male breast cancer. Lancet. 367:595–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kroemer G, Senovilla L, Galluzzi L, André
F and Zitvogel L: Natural and therapy-induced immunosurveillance in
breast cancer. Nat Med. 21:1128–1138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spanheimer PM, Carr JC, Thomas A, Sugg SL,
Scott-Conner CE, Liao J and Weigel RJ: The response to neoadjuvant
chemotherapy predicts clinical outcome and increases breast
conservation in advanced breast cancer. Am J Surg. 206:2–7. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang X, Kanda T, Wu S, Nakamura M,
Miyamura T, Nakamoto S, Banerjee A and Yokosuka O: Regulation of
microRNA by hepatitis B virus infection and their possible
association with control of innate immunity. World J Gastroenterol.
20:7197–7206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Day E and Lal A: MicroRNAs and their
target gene networks in breast cancer. Breast Cancer Res.
12:2012010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakurai M, Masuda M, Miki Y, Hirakawa H,
Suzuki T and Sasano H: Correlation of miRNA expression profiling in
surgical pathology materials, with Ki-67, HER2, ER and PR in breast
cancer patients. Int J Biol Markers. 30:e190–e199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antolín S, Calvo L, Blanco-Calvo M,
Santiago MP, Lorenzo-Patiño MJ, Haz-Conde M, Santamarina I,
Figueroa A, Antón-Aparicio LM and Valladares-Ayerbes M: Circulating
miR-200c and miR-141 and outcomes in patients with breast cancer.
BMC Cancer. 15:2972015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J, Tian Y, Li J, Lu B, Sun M, Zou Y,
Kong R, Luo Y, Shi Y, Wang K, et al: miR-206 is down-regulated in
breast cancer and inhibits cell proliferation through the
up-regulation of cyclinD2. Biochem Biophys Res Commun. 433:207–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Zeng F, Wu JY, Li HY, Fan JJ, Mai
L, Zhang J, Ma DM, Li Y and Song FZ: MiR-335 inhibits migration of
breast cancer cells through targeting oncoprotein c-Met. Tumour
Biol. 36:2875–2883. 2015. View Article : Google Scholar
|
|
15
|
Song L, Liu D, Wang B, He J, Zhang S, Dai
Z, Ma X and Wang X: miR-494 suppresses the progression of breast
cancer in vitro by targeting CXCR4 through the Wnt/β-catenin
signaling pathway. Oncol Rep. 34:525–531. 2015.PubMed/NCBI
|
|
16
|
Xie X, Hu Y, Xu L, Fu Y, Tu J, Zhao H,
Zhang S, Hong R and Gu X: The role of
miR-125b-mitochondria-caspase-3 pathway in doxorubicin resistance
and therapy in human breast cancer. Tumour Biol. 36:7185–7194.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Mao Q, Liu Y, Hao X, Zhang S and
Zhang J: Analysis of miR-205 and miR-155 expression in the blood of
breast cancer patients. Chin J Cancer Res. 25:46–54.
2013.PubMed/NCBI
|
|
18
|
Müller V, Gade S, Steinbach B, Loibl S,
von Minckwitz G, Untch M, Schwedler K, Lübbe K, Schem C, Fasching
PA, et al: Changes in serum levels of miR-21, miR-210, and miR-373
in HER2-positive breast cancer patients undergoing neoadjuvant
therapy: A translational research project within the Geparquinto
trial. Breast Cancer Res Treat. 147:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Wang X, Shen H, Deng R and Xue K:
Combination of mir-21 with circulating tumor cells markers improve
diagnostic specificity of metastatic breast cancer. Cell Biochem
Biophys. 73:87–91. 2015. View Article : Google Scholar
|
|
20
|
Han X, Yan S, Weijie Z, Feng W, Liuxing W,
Mengquan L and Qingxia F: Critical role of miR-10b in transforming
growth factor-β1-induced epithelial-mesenchymal transition in
breast cancer. Cancer Gene Ther. 21:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Derfoul A, Juan AH, Difilippantonio MJ,
Palanisamy N, Ried T and Sartorelli V: Decreased microRNA-214
levels in breast cancer cells coincides with increased cell
proliferation, invasion and accumulation of the Polycomb Ezh2
methyltransferase. Carcinogenesis. 32:1607–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han G, Fan M and Zhang X: microRNA-218
inhibits prostate cancer cell growth and promotes apoptosis by
repressing TPD52 expression. Biochem Biophys Res Commun.
456:804–809. 2015. View Article : Google Scholar
|
|
23
|
Dong Y, Zou J, Su S, Huang H, Deng Y, Wang
B and Li W: MicroRNA-218 and microRNA-520a inhibit cell
proliferation by downregulating E2F2 in hepatocellular carcinoma.
Mol Med Rep. 12:1016–1022. 2015.PubMed/NCBI
|
|
24
|
Zhang Y, Han D, Wei W, Cao W, Zhang R,
Dong Q, Zhang J, Wang Y and Liu N: MiR-218 inhibited growth and
metabolism of human glioblastoma cells by directly targeting E2F2.
Cell Mol Neurobiol. 35:1165–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Chen S, Luan X, Li Y, Liu M, Li X,
Liu T and Tang H: MicroRNA-214 is aberrantly expressed in cervical
cancers and inhibits the growth of HeLa cells. IUBMB Life.
61:1075–1082. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng RQ, Wan HY, Li HF, Liu M, Li X and
Tang H: MicroRNA-214 suppresses growth and invasiveness of cervical
cancer cells by targeting
UDP-N-acetyl-α-D-galactosamine:polypeptide
N-acetylgalactosaminyltransferase 7. J Biol Chem. 287:14301–14309.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiang R, Wang F, Shi LY, Liu M, Chen S,
Wan HY, Li YX, Li X, Gao SY and Sun BC: Plexin-B1 is a target of
miR-214 in cervical cancer and promotes the growth and invasion of
HeLa cells. Int J Biochem Cell Biol. 43:632–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XJ, Ye H, Zeng CW, He B, Zhang H and
Chen YQ: Dysregulation of miR-15a and miR-214 in human pancreatic
cancer. J Hematol Oncol. 3:462010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan Q, Wang X, Gong W, Ni L, Chen C, He
X, Chen F, Yang L, Wang P and Wang DW: ER stress negatively
modulates the expression of the miR-199a/214 cluster to regulates
tumor survival and progression in human hepatocellular cancer. PLoS
One. 7:e315182012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC,
Huang CH, Lee YS, Yen TC and Hsieh SY: MicroRNA-214 downregulation
contributes to tumor angiogenesis by inducing secretion of the
hepatoma-derived growth factor in human hepatoma. J Hepatol.
57:584–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uesugi A, Kozaki K, Tsuruta T, Furuta M,
Morita K, Imoto I, Omura K and Inazawa J: The tumor suppressive
microRNA miR-218 targets the mTOR component Rictor and inhibits AKT
phosphorylation in oral cancer. Cancer Res. 71:5765–5778. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alajez NM, Lenarduzzi M, Ito E, Hui AB,
Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al: MiR-218
suppresses nasopharyngeal cancer progression through downregulation
of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 71:2381–2391.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tatarano S, Chiyomaru T, Kawakami K,
Enokida H, Yoshino H, Hidaka H, Yamasaki T, Kawahara K, Nishiyama
K, Seki N, et al: miR-218 on the genomic loss region of chromosome
4p15.31 functions as a tumor suppressor in bladder cancer. Int J
Oncol. 39:13–21. 2011.PubMed/NCBI
|
|
34
|
Cheng Y, Yang X, Deng X, Zhang X, Li P,
Tao J and Lu Q: MicroRNA-218 inhibits bladder cancer cell
proliferation, migration, and invasion by targeting BMI-1. Tumour
Biol. 36:8015–8023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Zhang X, Wang L, Yang Y, Dong Z,
Wang H, Du L and Wang C: MicroRNA-214 suppresses oncogenesis and
exerts impact on prognosis by targeting PDRG1 in bladder cancer.
PLoS One. 10:e01180862015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang LL, Guo YJ, Zhao CN and Gao JY:
Effects and mechanism of miR-214 on hepatocellular carcinoma. Asian
Pac J Trop Med. 8:392–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Chen J, Li F, Lin Y, Zhang X, Lv Z
and Jiang J: MiR-214 inhibits cell growth in hepatocellular
carcinoma through suppression of β-catenin. Biochem Biophys Res
Commun. 428:525–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamane K, Jinnin M, Etoh T, Kobayashi Y,
Shimozono N, Fukushima S, Masuguchi S, Maruo K, Inoue Y, Ishihara
T, et al: Down-regulation of miR-124/-214 in cutaneous squamous
cell carcinoma mediates abnormal cell proliferation via the
induction of ERK. J Mol Med Berl. 91:69–81. 2013. View Article : Google Scholar
|
|
39
|
Wen Z, Lei Z, Jin-An M, Xue-Zhen L,
Xing-Nan Z and Xiu-Wen D: The inhibitory role of miR-214 in
cervical cancer cells through directly targeting mitochondrial
transcription factor A (TFAM). Eur J Gynaecol Oncol. 35:676–682.
2014.
|
|
40
|
Long LM, He BF, Huang GQ, Guo YH, Liu YS
and Huo JR: microRNA-214 functions as a tumor suppressor in human
colon cancer via the suppression of ADP-ribosylation factor-like
protein 2. Oncol Lett. 9:645–650. 2015.PubMed/NCBI
|
|
41
|
Song L, Li D, Zhao Y, Gu Y, Zhao D, Li X,
Bai X, Sun Y, Zhang X, Sun H, et al: miR-218 suppressed the growth
of lung carcinoma by reducing MEF2D expression. Tumour Biol. Sep
26–2015.Epub ahead of print.
|
|
42
|
Cao Q, Dong P and Wang Y, Zhang J, Shi X
and Wang Y: miR-218 suppresses cardiac myxoma proliferation by
targeting myocyte enhancer factor 2D. Oncol Rep. 33:2606–2612.
2015.PubMed/NCBI
|
|
43
|
Jun GJ, Zhong GG and Ming ZS: miR-218
inhibits the proliferation of glioma U87 cells through the
inactivation of the CDK6/cyclin D1/p21Cip1/Waf1 pathway.
Oncol Lett. 9:2743–2749. 2015.PubMed/NCBI
|
|
44
|
Wang T, Chen T, Niu H, Li C, Xu C, Li Y,
Huang R, Zhao J and Wu S: MicroRNA-218 inhibits the proliferation
and metastasis of esophageal squamous cell carcinoma cells by
targeting BMI1. Int J Mol Med. 36:93–102. 2015.PubMed/NCBI
|
|
45
|
Heishima K, Mori T, Sakai H, Sugito N,
Murakami M, Yamada N, Akao Y and Maruo K: MicroRNA-214 promotes
apoptosis in canine hemangiosarcoma by targeting the COP1-p53 axis.
PLoS One. 10:e01373612015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Phatak P, Byrnes KA, Mansour D, Liu L, Cao
S, Li R, Rao JN, Turner DJ, Wang JY and Donahue JM: Overexpression
of miR-214-3p in esophageal squamous cancer cells enhances
sensitivity to cisplatin by targeting survivin directly and
indirectly through CUG-BP1. Oncogene. View Article : Google Scholar : Epub ahead of
print.
|
|
47
|
Li PL, Zhang X, Wang LL, Du LT, Yang YM,
Li J and Wang CX: MicroRNA-218 is a prognostic indicator in
colorectal cancer and enhances 5-fluorouracil-induced apoptosis by
targeting BIRC5. Carcinogenesis. 36:1484–1493. 2015.PubMed/NCBI
|
|
48
|
He X, Xiao X, Dong L, Wan N, Zhou Z, Deng
H and Zhang X: MiR-218 regulates cisplatin chemosensitivity in
breast cancer by targeting BRCA1. Tumour Biol. 36:2065–2075. 2015.
View Article : Google Scholar
|
|
49
|
Xia H, Yan Y, Hu M, Wang Y, Wang Y, Dai Y,
Chen J, Di G, Chen X and Jiang X: MiR-218 sensitizes glioma cells
to apoptosis and inhibits tumorigenicity by regulating
ECOP-mediated suppression of NF-κB activity. Neuro Oncol.
15:413–422. 2013. View Article : Google Scholar :
|
|
50
|
Jin Y, Yang CJ, Xu X, Cao JN, Feng QT and
Yang J: MiR-214 regulates the pathogenesis of patients with
coronary artery disease by targeting VEGF. Mol Cell Biochem.
402:111–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Z, Xu Y, Long J, Guo K, Ge C and Du R:
microRNA-218 suppresses the proliferation, invasion and promotes
apoptosis of pancreatic cancer cells by targeting HMGB1. Chin J
Cancer Res. 27:247–257. 2015.PubMed/NCBI
|
|
53
|
Nishikawa R, Goto Y, Sakamoto S, Chiyomaru
T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya
Y, et al: Tumor-suppressive microRNA-218 inhibits cancer cell
migration and invasion via targeting of LASP1 in prostate cancer.
Cancer Sci. 105:802–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee TS, Lin JJ, Huo YN and Lee WS:
Progesterone inhibits endothelial cell migration through
suppression of the rho activity mediated by cSrc activation. J Cell
Biochem. 116:1411–1418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
van der Horst PH, Wang Y, Vandenput I,
Kühne LC, Ewing PC, van Ijcken WF, van der Zee M, Amant F, Burger
CW and Blok LJ: Progesterone inhibits epithelial-to-mesenchymal
transition in endometrial cancer. PLoS One. 7:e308402012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bokhari AA, Lee LR, Raboteau D, Hamilton
CA, Maxwell GL, Rodriguez GC and Syed V: Progesterone inhibits
endometrial cancer invasiveness by inhibiting the TGFβ pathway.
Cancer Prev Res (Phila). 7:1045–1055. 2014. View Article : Google Scholar
|
|
58
|
Xie M, Zhou L, Chen X, Gainey LO, Xiao J,
Nanes MS, Hou A, You S and Chen Q: Progesterone and Src family
inhibitor PP1 synergistically inhibit cell migration and invasion
of human basal phenotype breast cancer cells. BioMed Res Int.
2015:4264292015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu Y, Lee JS, Xie N, Li E, Hurtado-Coll A,
Fazli L, Cox M, Plymate S, Gleave M and Dong X: Prostate stromal
cells express the progesterone receptor to control cancer cell
mobility. PLoS One. 9:e927142014. View Article : Google Scholar : PubMed/NCBI
|