Introduction
ABCE1, a member of the ATP-binding cassette (ABC)
family (1), was initially
identified as an RNaseL inhibitor (RLI) which is involved in the
pathway of antiviral defense mediated by interferon (2,3). To
date, several studies have revealed that ABCE1 also functions in
translation initiation and termination, ribosome recycling and
human immunodeficiency virus capsid assembly (4–6).
In addition, ABCE1 also plays important roles in
malignant tumors. ABCE1 is highly expressed in melanoma (7), retinoblastoma (8), colorectal cancer (9), breast cancer (10,11)
and esophageal cancer (12). It may
possibly become a therapeutic target in colon cancer (13), hepatocellular carcinoma (14) and prostate cancer (15,16).
However, the effect of ABCE1 on malignant carcinoma warrants
further investigation.
In our previous study, we demonstrated that
suppression of ABCE1 inhibited the proliferation and invasion of
lung cancer cells in vitro. In addition, ABCE1 was
overexpressed at both the mRNA and protein levels in lung carcinoma
tissues and metastatic lymph nodes and was found to be
significantly associated with advanced clinical stages (17,18).
Previous studies suggest that overexpression of ABCE1 may be
related to cancer metastasis [reviewed in Tian et al
(19)]. Therefore, we aimed to
ascertain whether ABCE1 is involved in lung cancer progression and
metastasis. In the present study, we demonstrated that ABCE1
increases cell migration and invasion and rearranges the
cytoskeleton by binding to β-actin.
Materials and methods
Cell culture and transfection
Cell culture, LTEP-a-2 was obtained from the Chinese
Academy of Science Cell Bank (CAS; Shanghai, China). Cells were
grown in RPMI-1640 medium supplemented with 10% (v/v)
heat-inactivated fetal bovine serum at 37°C, in a humidified
atmosphere of 95% air and 5% CO2. All culture medium and
reagents were obtained from Hyclone (USA). Cell counting was
performed using a hemocytometer and Beckman Coulter Cell counter,
according to the manufacturer's instructions. The plasmid of
pEGFP-C1-ABCE1 was constructed by YeTian.
LTEP-a-2 cells were seeded (2×105
cells/well) in 6-well plates. After 24 h of incubation, they were
transfected with pEGFP-C1-ABCE1 (3 µg) or pEGFP-C1 (3
µg), in serum-free medium using Lipofectamine 2000
(Invitrogen, USA) mixed and incubated for 30 min at room
temperature. The mixture was then added to the LTEP-a-2 cells.
After 6 h of incubation, the mixture was replaced with full
medium.
Immunoblotting
Western blotting was performed as previously
described (17). Briefly, proteins
were separated by 10% SDS-polyacrylamide gel electrophoresis and
electrotransferred onto a polyvinylidene difluoride (PVDF) membrane
for immunoblotting using a Mini-Protean Tetra system (Bio-Rad,
USA). The membranes were then incubated with the respective
antibodies, and developed using SuperSignal West Pico (Thermo
Scientific, USA). Image analysis was carried out with ImageJ
software (NIH) by calculating the mean intensity.
Expression of protein GST-ABCE1
Expression plasmid pGEX-4T-1-ABCE1 was a gift from
Dr J.R. Lingappa (20).
pGEX-4T-1-ABCE1 and pGEX-4T-1 were transformed into Competent BL21
(DE3) Escherichia coli cells (Takara, China). The cells were
induced with 0.4 mM isopropyl 1-thio-β-D-galactopyranoside
(Tiangen, China) overnight at 20°C on a rotating wheel. E.
coli culture was centrifuged at 5,000 x g for 5 min, and
resuspended in 1 ml of cold ProFound™ lysis buffer (Thermo
Scientific) and protease inhibitors. Cells were homogenized using a
sonicator (8 pulses, 10 sec each) and then centrifuged at 14,000 ×
g for 10 min.
GST pull-down assays
The pull-down assay was performed according to the
protocol of the ProFound™ Pull-Down GST Protein:Protein Interaction
kit (21516; Pierce). Bacterial lysate was clarified by
centrifugation in a 12124 rotor (Sigma) at 14,000 × g for 10 min,
and 800 µl of the resulting supernatant was incubated with
50 µl of settled immobilized glutathione resin for 2 h at
4°C. LTEP-a-2 cell lysates were harvested in ProFound™ lysis buffer
and incubated with washed GST-ABCE1 glutathione-Sepharose columns
for 2 h at 4°C on a rotating wheel. After incubation with LTEP-a-2
cell lysate, glutathione-Sepharose was washed five times with 400
µl 1:1 wash solution of TBS:ProFound™ lysis buffer.
Fifty-microliter elution was carried out using buffer containing
100 mM glutathione and boiled in SDS sample buffer, and loaded onto
an SDS-PAGE gel. Gels were stained using Coomassie Brilliant Blue
R350 (GE Healthcare, USA) and protein bands were excised and
collected in 96-cell plates.
In-gel digestion
Gel slices were incubated in destaining buffer (25
mM NH4HCO3, 50% CH3CN) at 37°C for
20 min. Destaining was repeated with fresh buffer until the gel
turned colorless. Gel slices were dehydrated in 100 µl
acetonitrile until the gel turned white. Then the gels were reduced
in a buffer containing 10 mM DTT soluble in 25 mM ammonium
bicarbonate at 37°C for 1 h. Protein alkylation was performed by
incubation of the gel slices in 25 mM ammonium bicarbonate for 45
min in darkness at room temperature. Afterwards, the gel slices
were washed using 100 µl of 50% CH3CN and
dehydrated by acetonitrile. Three microliters of 10 ng/l trypsin
(Promega, USA) was added to each gel slice and incubated at 4°C for
30 min. Ammonium bicarbonate (10 µl 25 mM) was added to the
gels at 37°C overnight.
Mass spectrometry analysis
Mass analysis was performed by using a MALDI TOF/TOF
analyzer (Bruker Daltonic, Germany). Data were searched in
AutoFlex3 against SwissProt databases. Mascot software was used to
analyze Mass data. Mascot search parameters were set as follow:
taxonomy, Homo sapiens; fixed modification, carbamidomethyl
(C); variable modification, oxidation (M); MS/MS fragment
tolerance, 0.7 Da; precursor tolerance, 100 ppm; peptide charge,
+1, monoisotopic. Proteins were accepted when scored greater than
56 (P<0.05)
Co-immunoprecipitation assays
LTEP-a-2 cell lysate was harvested in a
immunoprecipitation lysis buffer (Thermo) with Halt™ Protease
Inhibitor Cocktail (Thermo Scientific). After sonication, the
lysates were clarified by centrifugation at 14,000 × g for 10 min.
Co-immunoprecipitation (co-IP) was conducted following the
manufacturer's protocol (co-IP Kit, 26149; Thermo Scientific
Pierce). Briefly, the ABCE1 antibody was first immobilized for 2 h
using AminoLink Plus coupling resin. After washing, the resin was
incubated with the LTEP-a-2 lysate overnight at 4°C. After
incubation, the resin was again washed and the protein was eluted
using elution buffer. A negative control resin that was provided
with the IP kit to assess nonspecific binding received the same
treatment as the co-IP samples, including the ABCE1 antibody. In
this control, the coupling resin was not amine-reactive preventing
covalent immobilization of the primary antibody onto the resin.
Another control was used coupling resin without the ABCE1 antibody.
Antibodies used were: rabbit monoclonal anti-ABCE1 (Abcam, USA) and
rabbit polyclonal anti-β-actin (Santa Cruz Biotechnology, USA).
Immunofluorescence microscopy
The cells were grown on glass coverslips for 36 h
before treatment. The cells were washed in PBS and fixed in 4%
paraformaldehyde for 20 min. The cells were rinsed for 5 min in
0.5% Triton X-100 three times, and then incubated with the primary
antibodies at 4°C overnight in a wet box. The coverslips were
rinsed three times in PBS. The following steps were operated in
darkness. The cells were incubated with the secondary antibodies
for 2 h at 37°C. The coverslips were rinsed three times in PBS and
mounted on glass slides. Antibodies used were: rabbit monoclonal
anti-ABCE1 (Abcam), mouse polyclonal anti-β-actin (Santa Cruz
Biotechnology), and conjugated secondary antibodies (EarthOx, USA),
and Hoechst 33342 (Sigma, USA). ABCE1 was detected using
anti-rabbit IgG, Rhodamine Fluor (red fluorescence), and actin was
detected using anti-mouse IgG Dylight 649 (yellow fluorescence).
Nuclei were observed by Hoechst 33342 staining. Images were
obtained utilizing the Olympus FV1000S-SIM/IX81 confocal
system.
F-actin staining
Cells were grown on glasses and fixed with 4%
paraformaldehyde solution. The cells were stained with 100
µM Rhodamine Phalloidin (cytoskeleton) for 30 min before the
addition of Hoechst 33342. Images were also obtained on the Olympus
FV1000S-SIM/IX81 confocal system. Microspikes were counted in 3
different fields; 5 cells were chosen from each field. The
quantification was repeated by three individuals.
Transwell cell migration assay
Cell migration assay was performed using a 24-well
Transwell chamber (8.0-µm pore size; Corning). The cells
(4×104) were seeded in the upper chamber which was
inserted into a 24-well plate and cultured for another 48 h. Then,
the cells were allowed to migrate forward to DMEM containing 15%
FBS in the bottom chamber. The non-migratory cells on the upper
membrane surface were removed with a cotton tip, and the migratory
cells that had attached to the lower membrane surface were fixed
with 4% paraformaldehyde and stained with crystal violet. The
number of migrated cells was counted in five randomly selected high
power fields under a microscope. Data presented are representative
of three individual wells.
Statistical analysis
All the statistical analyses were performed with
SPSS13.0 using one-way ANOVA test. P<0.05 is indicative of a
significant difference.
Results
Expression and identification of
recombinant GST-ABCE1
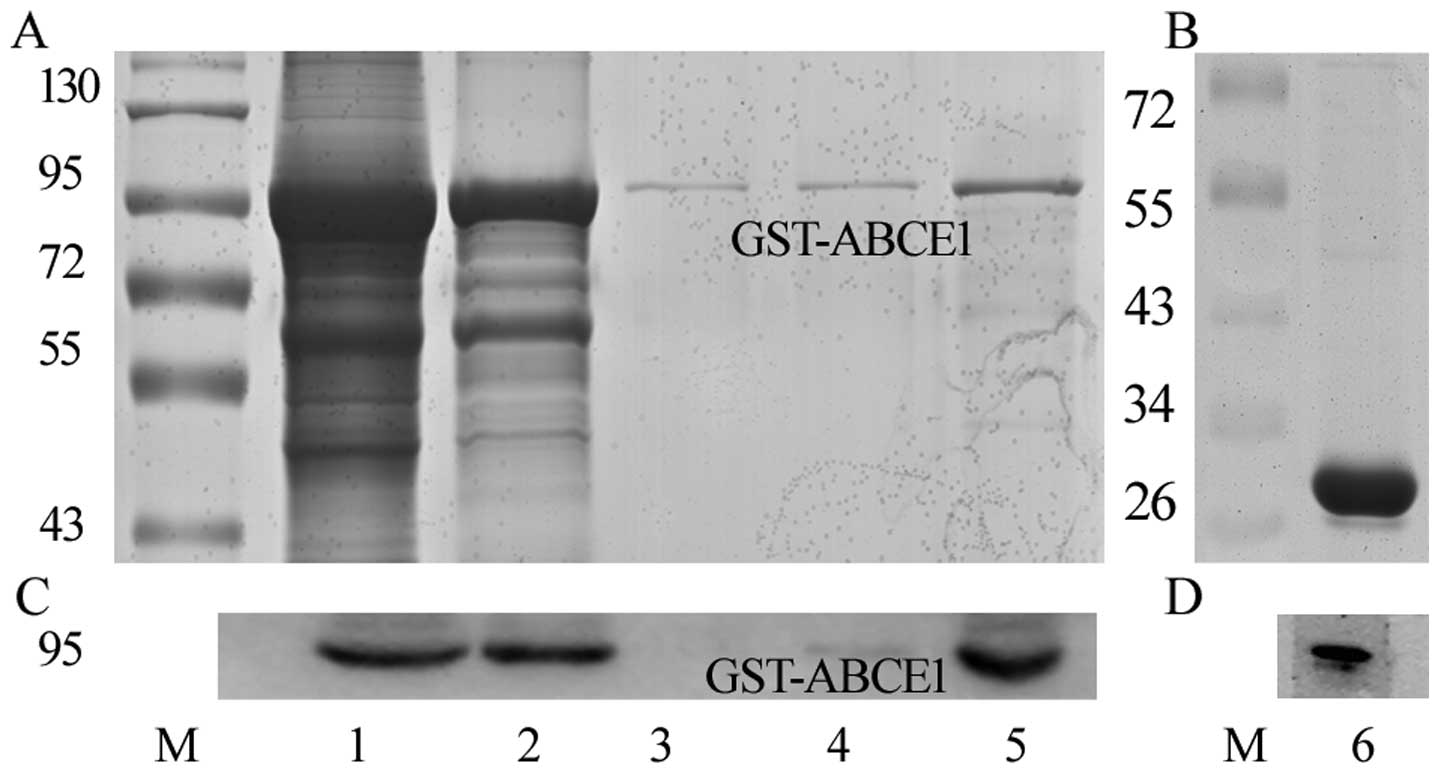
SDS-PAGE analysis with Coomassie staining showed
that recombinant GST-ABCE1 was expressed with a molecular mass of
~94 kDa (Fig. 1A, lane 1) which was
consistent with the ABCE1 predicted size as the increased 26 kDa is
related to GST tag. The purified GST-tagged fusion protein appeared
as nearly one single band, indicating the high purity of the
preparation (Fig. 1A, lane 5). The
protein was confirmed by western blot analysis (Fig. 1C) using the anti-ABCE1 antibody. The
purified GST protein is shown in Fig.
1B, lane 6. It was also confirmed by western blot analysis
(Fig. 1D) using the anti-GST
antibody.
ABCE1 interacts with β-actin
GST pull-down assays were used to screen the target
protein interacting with ABCE1. To eliminate unspecific binding of
non-target proteins, the LTEP-a-2 cell lysate was also incubated
with GST and agarose beads as the control (Pierce protocol).
Several protein bands that interacted with the GST-ABCE1 protein
were found after Coomassie Blue staining. But the most distinct
specific peptide that was confirmed by mass spectrometry was
β-actin (Fig. 2A). The peptide and
the score of β-actin and ABCE1 are shown in Fig. 2B and D. As a result, GST pull-down
interaction screening provided the evidence of the original
possible interaction between ABCE1 and β-actin.
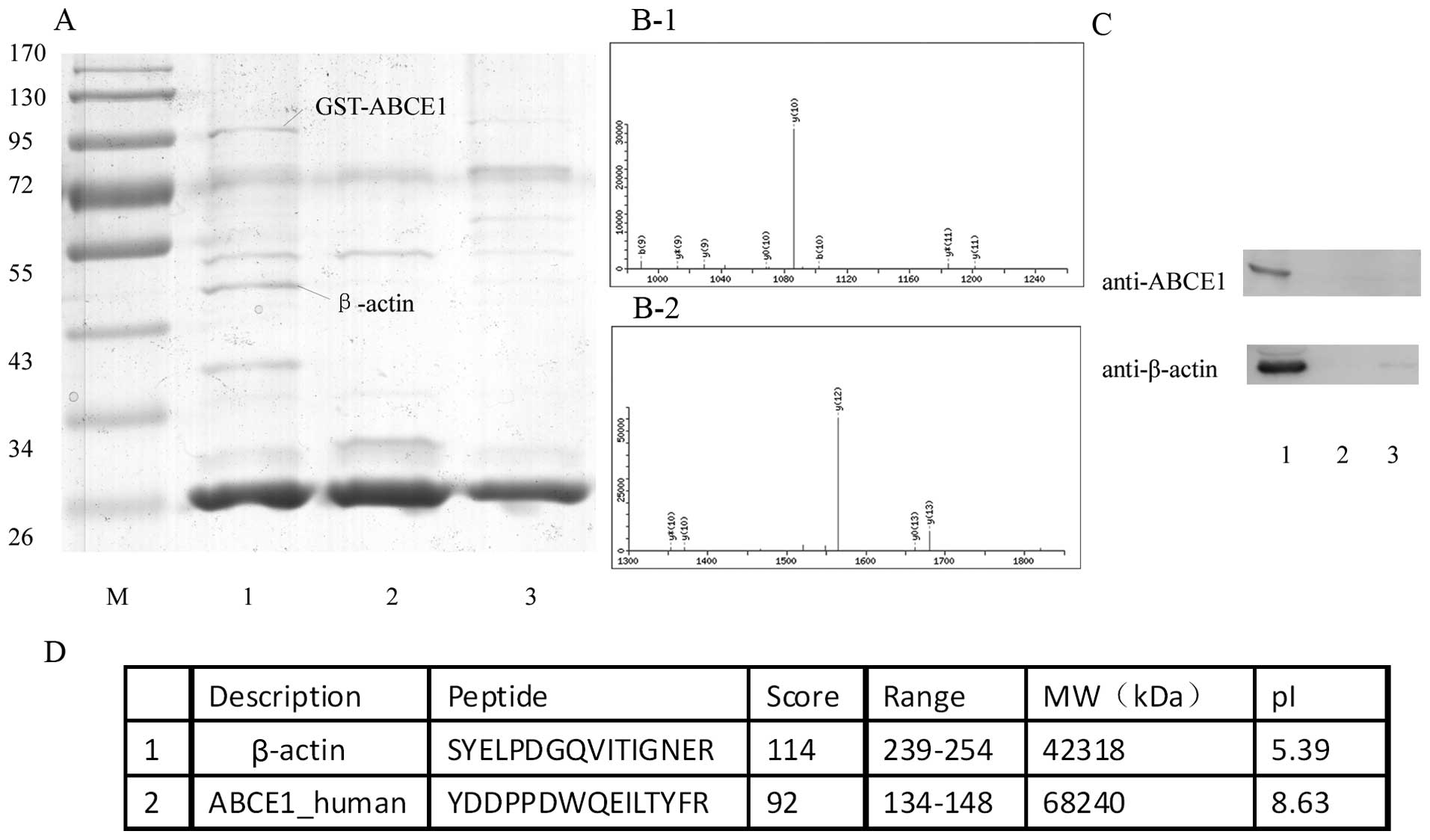 | Figure 2ABCE1 interacts with β-actin. (A)
ABCE1 interacts with β-actin in the GST pull-down assay. GST-ABCE1
was used to probe LTEP-a-2 cell lysate. The β-actin band only
appeared in lane 1. M, standard protein marker; lane 1, purified
GST-ABCE1 as bait protein, β-actin was detected; lane 2, purified
GST as a bait protein; lane 3, control agarose beads. (B-1) MS/MS
spectrum identified the peptide unique to ABCE1. (B-2) The MS/MS
spectrum identified the peptide unique to β-actin. (C) ABCE1
co-precipitates with β-actin. Lane 1, resin coupling the anti-ABCE1
antibody, lane 2, control resin coupling anti-ABCE1 antibody, lane
3, resin without any antibody. (D) Characteristics of the
identified proteins by using mass spectrometry. |
ABCE1 co-immunoprecipitates with
β-actin
Co-IP using ABCE1 antibody was used to determine the
interaction between ABCE1 and β-actin; control resin and resin
without antibodies were used as negative controls. ABCE1 was
immunoprecipitated from the LTEP-a-2 cell lysates, and β-actin was
detected in the precipitates with ABCE1 by Western blot analysis
(Fig. 2C). This finding indicated
that the interaction between ABCE1 and β-actin may happen at
endogenous protein levels.
ABCE1 overexpression leads to aggregation
of β-actin
After transfection with pEGFP-C1-ABCE1, ABCE1 (red)
was overexpressed in both the cytoplasm and membrane-proximal areas
(Fig. 3A compared with Fig. 3E and I). As a result, the β-actin
(yellow) obviously aggregated into plaque (Fig. 3B compared with Fig. 3F and J). Western blot analysis
(Fig. 3M) also showed that both
ABCE1 (Fig. 3N) and β-actin
(Fig. 3O) were more strongly
expressed in transfected group 1 than levels in group 2 and group
3. Moreover, they presented in roughly equal amounts in group 2 and
group 3.
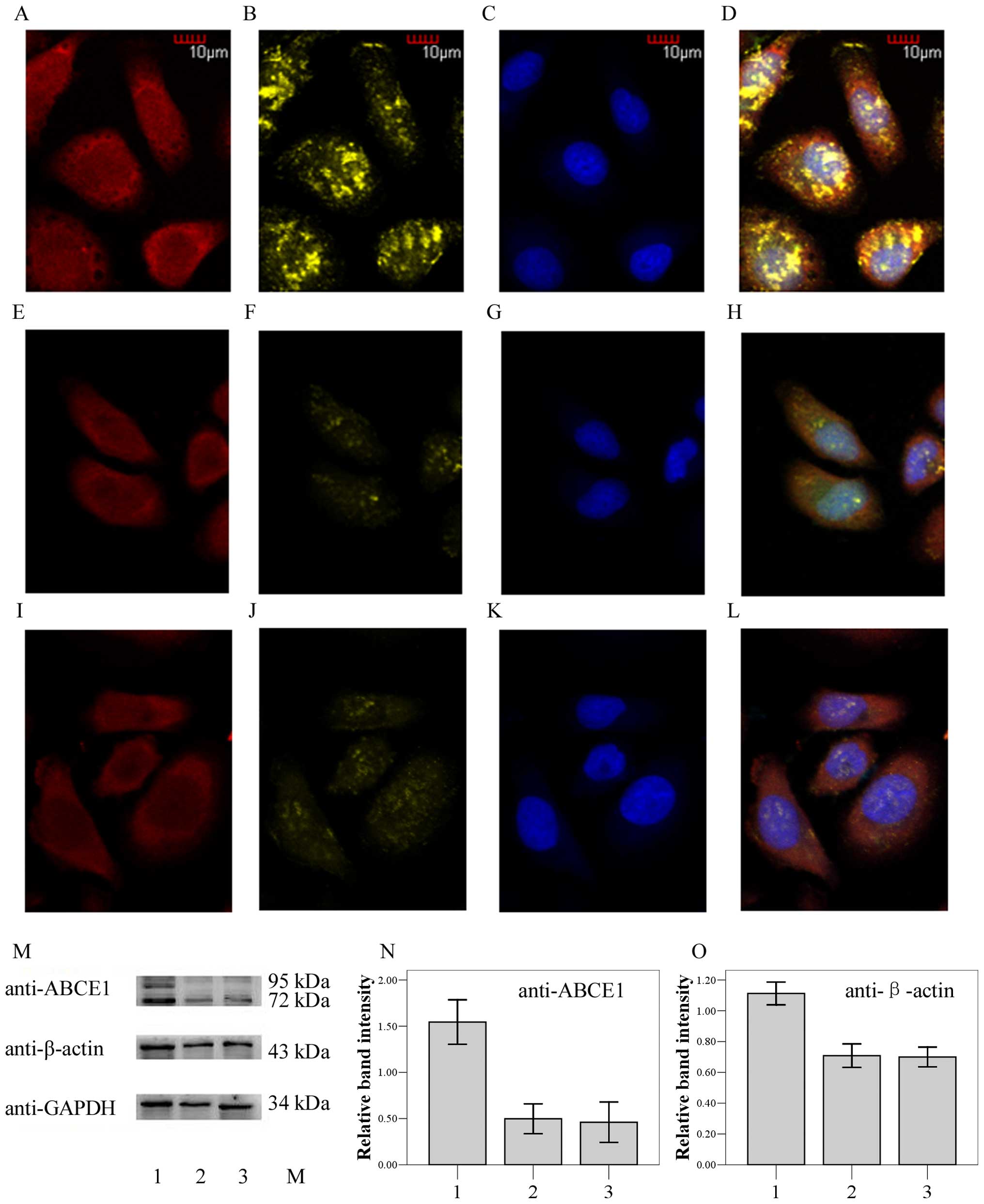 | Figure 3Expression and localization of ABCE1
and β-actin in the LTEP-a-2 cells. Column 1 (A, E and I), ABCE1
staining red. Column 2 (B, F and J), β-actin staining yellow.
Column 3 (C, G and K), cell nuclei staining blue. Column 4 (D, H
and L), merged image. Row 1 (A–D), LTEP-a-2 cells transfected with
pEGFP-ABCE1 (group 1). Row 2 (E–H), LTEP-a-2 cells transfected with
pEGFP (group 2). Row 3 (I–L), non-transfected LTEP-a-2 cells (group
3). (M–O), Western blot analysis (M) also showed that both ABCE1
(N, the relative band intensity is the ratio of GST-ABCE1 plus
ABCE1 to GAPDH) and β-actin (O, the relative band intensity is the
ratio of β-actin to GAPDH) were more strongly expressed in the
transfected group 1 than group 2 and group 3. |
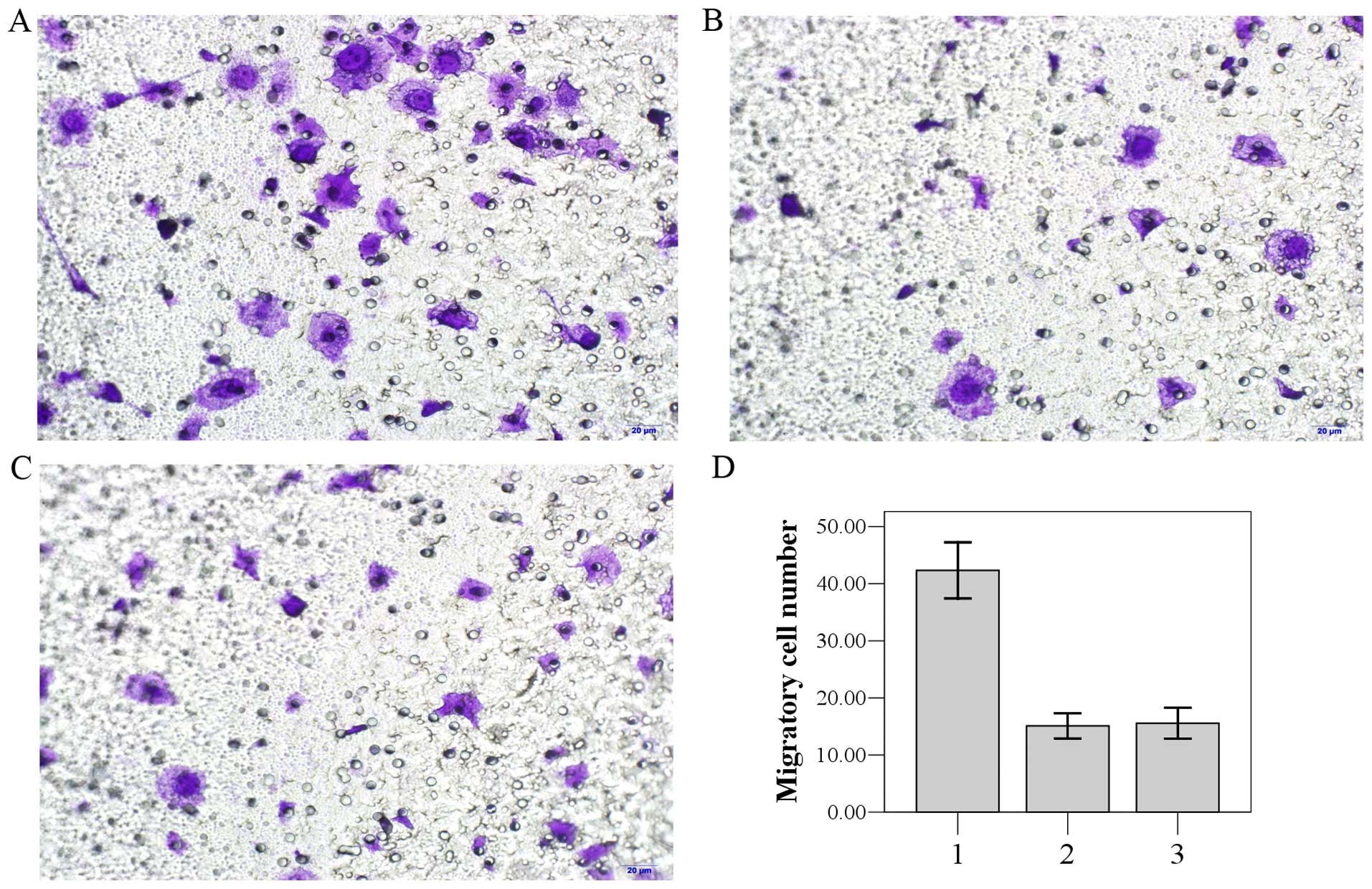
ABCE1 promotes cell migration
Transwell invasion assay was used to investigate the
role of ABCE1 in the invasion of lung cancer cells. LTEP-a-2 cells
transfected with the pEGFP-C1-ABCE1 and pEGFP-C1 plasmids and
non-transfected cells were plated, respectively, on Matrigel-coated
filters. After incubation for 48 h, the filters were stained with
crystal violet and inspected under a microscope. The number of
LTEP-a-2 cells transfected with pEGFP-C1-ABCE1 found in the filter
(group 1, 42.11±3.14) was higher than the number in group 2
(16.22±1.72) and group 3 (18.67±2.12) (P<0.05) (Fig. 4). There was no significant
difference between group 2 and group 3 (P>0.05).
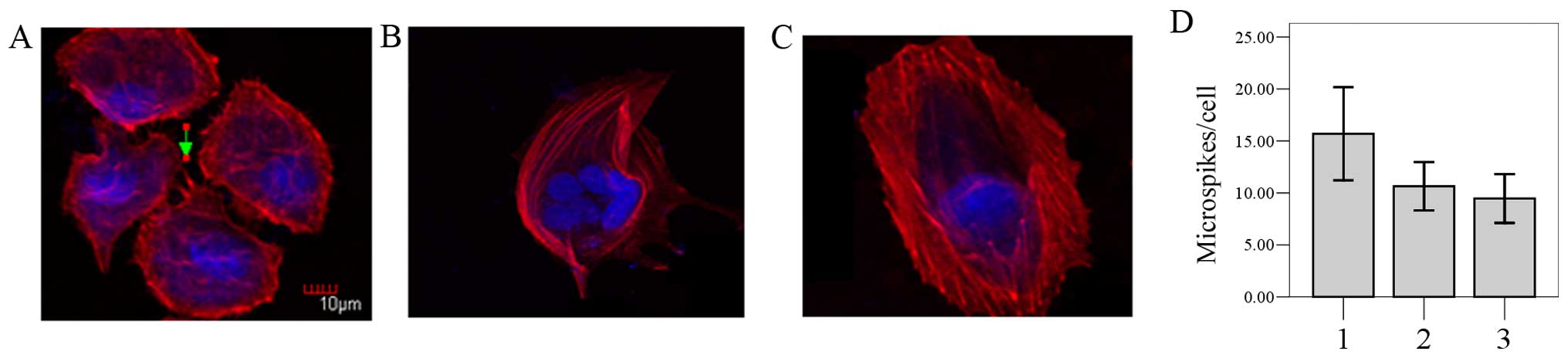
Overexpression of ABCE1 influences actin
cytoskeletal organization and the morphology of LTEP-a-2 cells
We showed that ABCE1 can bind β-actin. To
investigate the cellular function of ABCE1 binding to β-actin, we
analyzed the effect of the overexpression of ABCE1 on cell
morphology. Phalloidin staining of F-actin showed that there were
irregular edges and numerous irregular stress fibers in the
LTEP-a-2 cells transfected with pEGFP-C1-ABCE1 (Fig. 5A). In contrast, cells in group 2
(Fig. 5B) and group 3 (Fig. 5C) appeared to have a rounded
morphology with few regular stress fibers. Quantitative analysis
confirmed that the number of filopodia-like microspikes in group 1
(15.69±4.47) was more than that in group 2 (10.64±2.33) and group 3
(9.47±2.35) (Fig. 5D, P<0.05).
These results indicated that overexpression of ABCE1 induced
morphological and actin-related cytoskeletal changes in the
LTEP-a-2 cells.
Discussion
In our previous study, Ren et al (17) confirmed that downregulation of ABCE1
significantly inhibited the growth of lung cancer cells, and
accompanied by downregulation of ABCE1, it was determined that
expression of several lung cancer-related genes was also changed.
Huang et al (18)
demonstrated that downregulation of ABCE1 may inhibit the
proliferation and invasiveness of lung cancer cells. Therefore, we
speculated that the ABCE1 gene is involved in lung cancer
metastasis.
In this present study, β-actin was firstly screened
as an interacting protein of ABCE1 by GST-pull-down assay. Although
there are several other candidates such as IFNA1-66, AKAP9, CD63,
and elongin (data not shown), we aimed to ascertain how the ABCE1
protein promotes the movement of cancer cells by identifying
β-actin in vitro and in vivo. The complex was next
testified by co-immunoprecipitation assays. We were interested in
researching how the ABCE1 protein in vitro relates to the
movement of cancer cells in living tumor tissue.
In the immunofluorescence analysis, we found that
β-actin expression was significantly increased and aggregated into
plaque when ABCE1 was overexpressed. More exactly, more G-actin was
detected when ABCE was overexpressed.
Transwell invasion assay next demonstrated that
upregulation of ABCE1 promoted the invasiveness of lung cancer
cells in vitro. After labeling F-actin, overexpression of
ABCE1 induced actin-related cytoskeletal changes in the LTEP-a-2
cells.
Obviously, our results showed that overexpression of
ABCE1 in the LTEP-a-2 cells led to upregulation of G-actin
expression and aggregation, increased cell migration and increased
invasiveness of lung cancer cells.
The concept of a multi-stage process of cancer
metastasis, involves invasion into surrounding tissue,
intravasation, transit in the blood or lymph, extravasation, and
growth at a new site (21). There
is little doubt that invasion into surrounding tissue is the
prerequisite of cancer metastasis. Dove et al (22) reported that cell motility is driven
by cycles of actin polymerization, cell adhesion and acto-myosin
contraction. The actin polymerization acts as the initial forces
for translocation (23,24). In addition, the protrusive
structures from the cell membrane such as lamellipodia and
filopodia act as morphologic markers for cell motility. In fact,
the actin polymerization requires energy. Notable, ABCE1 belongs to
a protein family which can transfer ATP.
There are several classical pathways regulating
actin polymerization: FH protein and Ena/WASP directly promote
actin binding and extension in the positive terminal (25–27),
ARP2/3 can promote the extension of existing actin filaments
(28); p38 MAPK can also promote
actin polymerization and actin formation (29). Cofilin can cut off existing
filaments, increasing barbed (plus end) side for polymerization
(30). Yet, we believe that ABCE1
can influence the cytoskeleton by its own mechanism in lung cancer
cells.
Karcher et al found that ABCE1 has an
N-terminal iron-sulfur (FeS) domain in contrast to all other ABC
enzymes (31). Thus, further
investigation should focus on the function of the FeS domain in the
interaction of ABCE1 and β-actin.
In summary, ABCE1 may be a new interaction protein
of β-actin, and it can increase the motility of lung cancer cells
through cytoskeleton rearrangement. ABCE1 localizes to the cytosol,
and is enriched at the cell periphery. Upregulation of ABCE1
stimulates β-actin polymerization and promotes migration. In
summary, our findings offer the first insight on the biological
role of ABCE1 in lung cancer biology. Increased expression of
ABCE1, which is clinically correlated to aggressive tumor growth
and invasion, may increase the G-actin pool to increase the
formation of protrusions in lung cancer cells and increased their
invasive ability.
Acknowledgments
The authors thank Qin Li, Yuhua Chen, Liying Hao for
discussions and advice. We thank Dr J.R. Lingappa for providing the
pGEX-4T-1-ABCE1 expression plasmid. The study was funded by the
National Natural Science Foundation of China (30973502, 30170914)
and the Department of Education of Liaoning Province
(L2013302).
References
|
1
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
2
|
Bisbal C, Martinand C, Silhol M, Lebleu B
and Salehzada T: Cloning and characterization of a RNAse L
inhibitor. A new component of the interferon-regulated 2–5A
pathway. J Biol Chem. 270:13308–13317. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassel BA, Zhou A, Sotomayor C, Maran A
and Silverman RH: A dominant negative mutant of 2–5A-dependent
RNase suppresses antiproliferative and antiviral effects of
interferon. EMBO J. 12:3297–3304. 1993.PubMed/NCBI
|
|
4
|
Zimmerman C, Klein KC, Kiser PK, Singh AR,
Firestein BL, Riba SC and Lingappa JR: Identification of a host
protein essential for assembly of immature HIV-1 capsids. Nature.
415:88–92. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pisareva VP, Skabkin MA, Hellen CU,
Pestova TV and Pisarev AV: Dissociation by Pelota, Hbs1 and ABCE1
of mammalian vacant 80S ribosomes and stalled elongation complexes.
EMBO J. 30:1804–1817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becker T, Franckenberg S, Wickles S,
Shoemaker CJ, Anger AM, Armache JP, Sieber H, Ungewickell C,
Berninghausen O, Daberkow I, et al: Structural basis of highly
conserved ribosome recycling in eukaryotes and archaea. Nature.
482:501–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heimerl S, Bosserhoff AK, Langmann T,
Ecker J and Schmitz G: Mapping ATP-binding cassette transporter
gene expression profiles in melanocytes and melanoma cells.
Melanoma Res. 17:265–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hendig D, Langmann T, Zarbock R, Schmitz
G, Kleesiek K and Götting C: Characterization of the ATP-binding
cassette transporter gene expression profile in Y79: A
retinoblastoma cell line. Mol Cell Biochem. 328:85–92. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hlavata I, Mohelnikova-Duchonova B,
Vaclavikova R, Liska V, Pitule P, Novak P, Bruha J, Vycital O,
Holubec L, Treska V, et al: The role of ABC transporters in
progression and clinical outcome of colorectal cancer. Mutagenesis.
27:187–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang B, Zhou H, Lang X and Liu Z:
siRNA-induced ABCE1 silencing inhibits proliferation and invasion
of breast cancer cells. Mol Med Rep. 10:1685–1690. 2014.PubMed/NCBI
|
|
11
|
Hlaváč V, Brynychová V, Václavíková R,
Ehrlichová M, Vrána D, Pecha V, Koževnikovová R, Trnková M, Gatěk
J, Kopperová D, et al: The expression profile of ATP-binding
cassette transporter genes in breast carcinoma. Pharmacogenomics.
14:515–529. 2013. View Article : Google Scholar
|
|
12
|
Huang B, Gong X, Zhou H, Xiong F and Wang
S: Depleting ABCE1 expression induces apoptosis and inhibits the
ability of proliferation and migration of human esophageal
carcinoma cells. Int J Clin Exp Pathol. 7:584–592. 2014.PubMed/NCBI
|
|
13
|
Shichijo S, Ishihara Y, Azuma K, Komatsu
N, Higashimoto N, Ito M, Nakamura T, Ueno T, Harada M and Itoh K:
ABCE1, a member of ATP-binding cassette transporter gene, encodes
peptides capable of inducing HLA-A2-restricted and tumor-reactive
cytotoxic T lymphocytes in colon cancer patients. Oncol Rep.
13:907–913. 2005.PubMed/NCBI
|
|
14
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar
|
|
15
|
Silverman RH: Implications for RNase L in
prostate cancer biology. Biochemistry. 42:1805–1812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shea PR, Ishwad CS, Bunker CH, Patrick AL,
Kuller LH and Ferrell RE: RNASEL and RNASEL-inhibitor variation and
prostate cancer risk in Afro-Caribbeans. Prostate. 68:354–359.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren Y, Li Y and Tian D: Role of the ABCE1
gene in human lung adenocarcinoma. Oncol Rep. 27:965–970.
2012.PubMed/NCBI
|
|
18
|
Huang B, Gao Y, Tian D and Zheng M: A
small interfering ABCE1-targeting RNA inhibits the proliferation
and invasiveness of small cell lung cancer. Int J Mol Med.
25:687–693. 2010.PubMed/NCBI
|
|
19
|
Tian Y, Han X and Tian DL: The biological
regulation of ABCE1. IUBMB Life. 64:795–800. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lingappa JR, Dooher JE, Newman MA, Kiser
PK and Klein KC: Basic residues in the nucleocapsid domain of Gag
are required for interaction of HIV-1 gag with ABCE1 (HP68), a
cellular protein important for HIV-1 capsid assembly. J Biol Chem.
281:3773–3784. 2006. View Article : Google Scholar
|
|
21
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar
|
|
22
|
Dove SL, Joung JK and Hochschild A:
Activation of prokaryotic transcription through arbitrary
protein-protein contacts. Nature. 386:627–630. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rafelski SM and Theriot JA: Crawling
toward a unified model of cell mobility: Spatial and temporal
regulation of actin dynamics. Annu Rev Biochem. 73:209–239. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goode BL and Eck MJ: Mechanism and
function of formins in the control of actin assembly. Annu Rev
Biochem. 76:593–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar
|
|
27
|
Krause M, Dent EW, Bear JE, Loureiro JJ
and Gertler FB: Ena/VASP proteins: Regulators of the actin
cytoskeleton and cell migration. Annu Rev Cell Dev Biol.
19:541–564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pollard TD: Regulation of actin filament
assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol
Struct. 36:451–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esfandiarei M, Yazdi SA, Gray V, Dedhar S
and van Breemen C: Integrin-linked kinase functions as a downstream
signal of platelet-derived growth factor to regulate actin
polymerization and vascular smooth muscle cell migration. BMC Cell
Biol. 11:162010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang W, Eddy R and Condeelis J: The
cofilin pathway in breast cancer invasion and metastasis. Nat Rev
Cancer. 7:429–440. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karcher A, Schele A and Hopfner KP: X-ray
structure of the complete ABC enzyme ABCE1 from Pyrococcus abyssi.
J Biol Chem. 283:7962–7971. 2008. View Article : Google Scholar
|