Introduction
Brain glioma, the most common primary brain tumor is
thought to be one of the deadliest forms of cancer and over 2/3 of
the patients were diagnosed with malignant glioma (1). In the last twenty years, although the
surgical techniques and treatment strategy have been greatly
improved, the prognosis of patients with malignant glioma is still
poor (2,3). The one-year survival rate of glioma is
<30% (4). The main biological
characteristics of glioma is invasive growth, which results in
incomplete surgery, high rate of recurrence and short survival
period (5,6). As the understanding of tumor mechanism
has improved, the incidence and development of glioma is considered
the result of interactions among various abnormally expressed
genes. Thus, finding genes related to growth and invasiveness of
glioma and revealing the possible mechanism has significant meaning
in treatment of glioma.
SPOCK1, an oncogene, is frequently overexpressed in
various cancer tissues (7).
Increasing evidence has demonstrated that SPOCK1 plays important
roles in proliferation, migration and invasion of tumor cells.
Study by Miao et al showed that SPOCK1 as a target gene of
TGF-β1 could regulate lung cancer cell epithelial-mesenchymal
transition and silencing of SPOCK1 obviously inhibited the
proliferation and invasion of lung cancer cells (8). Previous study also found that SPOCK1,
upregulated by CHD1L, promoted proliferation and invasion of
hepatoma carcinoma cells (9).
SPOCK1 was able to promote proliferation and metastasis of
gallbladder cancer cells trough the PI3K/AKT signaling pathway
(10). The expression levels of
SPOCK1 in malignant glioma and pilocytic astrocytoma exhibited
significant differences (11),
which indicated that SPOCK1 had important effect on genesis and
progression in glioma. However, the effect of SPOCK1 on
proliferation and invasion of glioma cells and the underlying
mechanisms are far from clear.
In this study, we investigated the effect of SPOCK1
on the proliferation, apoptosis, migration and invasion through
overexpressing exogenous and RNA-interfered endogenous SPOCK1
expression in glioma cells.
Materials and methods
Cell lines and culture
The glioma cell line U251 and U87 MG cells were
obtained from Type Culture Collection of Chinese Academy of
Sciences and American Type Culture Collection, respectively. The
U251 cells were maintained in DMEM (Gibco, Carlsbad, CA, USA). The
U87 MG cells were cultured in MEM (Gibco). All the cells were
supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA),
100 µg/ml streptomycin and 100 U/ml penicillin (Hyclone),
cultured at 37°C, in a humidified environment of 5% CO2
atmosphere.
Construction of plasmids and generation
of stable cell lines
The sequences of SPOCK1 shRNA and negative control
(NC) shRNA were 5′-GUAAUGAGGAGGGCUAUUA-3′ and
5′-TTCTCCGAACGTGTCACGT-3′, respectively. The sh-SPOCK1 and sh-NC
were cloned into pGCsi-H1 vector (Genechem, Shanghai, China)
separately and transfected into U87 MG cells. The human full-length
cDNA of SPOCK1 was inserted into pEGFP-N1 vector (Clontech, San
Jose, CA, USA) and transfected into U251 cells. Control cells were
transfected with pEGFP-N1 vector. After transfection for 24 h, 400
µg/ml G418 was added into U87 MG and U251 cells for 2 weeks
to select stable SPOCK1-silencing/overexpressing clones. The mRNA
and protein expression levels of SPOCK1 were determined by qPCR and
western blotting.
Antibodies and western blotting
Rabbit anti-cleaved caspase-3 (1:1000), anti-cleaved
PARP (1:1000) antibodies were purchased from Abcam (MA, USA).
Rabbit anti-Bax (1:400), anti-Bcl-2 (1:400), anti-PI3K (1:400),
anti-β-catenin (1:400), anti-MMP2 (1:400), anti-MMP9 (1:400), mouse
anti-c-MYC (1:200), anti-cyclin D1 (1:400) were obtained from
Boster (Wuhan, China). Mouse anti-SPOCK1 (1:200), rabbit anti-p-AKT
(1:200), anti-AKT (1:200), anti-Wnt (1:200) were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-p-PI3K
(1:500) were obtained from Bioss (Beijing, China). Briefly, the
cells were lysed in RIPA and then denatured. The protein
concentration was measured using the BCA protein estimation kit
(Beyotime, Shanghai, China). Protein samples were separated on an
SDS-polyacrylamide gel and transferred to PVDF membranes. The
membranes were blocked with 5% non-fat dry milk in PBS for 2 h at
room temperature, and incubated with primary antibodies,
respectively, at 4°C overnight. After incubation with a secondary
antibody the blots were visualized by ECL detection reagent
(Beyotime).
Quantitative real-time polymerase chain
reaction (qPCR)
Quantitative PCR was used to quantify the expression
of mRNA in cultured cells. Briefly, total RNA was extracted with
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The total RNA (1
µg) was reverse transcribed by a reverse transcriptase
(Takara, Shiga, Japan). RNA expression was measured by qRT-PCR
using the SYBR-Green method (Takara) according to the
manufacturer's instructions. The primer sequences of SPOCK1 were as
follows: SPOCK1 (forward, 5′-CACTGGGTTGGACCTTCGA-3′; reverse,
5′-CTTTGGTGGCTCAGGCTCT-3′) and β-actin (forward,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′; reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′). β-actin was used as an internal
reference gene to normalize the expression of detection genes.
Relative quantification of gene was analyzed by the comparative
threshold cycle (Ct) method.
Cell proliferation assay
Cell proliferation was determined by Cell Counting
Kit-8 (CCK8) (Beyotime, Shanghai, China). Cells were seeded into
96-well plates (3×103 per well) and incubated for 24,
48, 72, 96 h, respectively. After incubation, 10 µl of CCK8
reagent was added to each well and at 450 nm the absorbance was
detected by a microplate reader (Bio-Tek, Winooski, VT, USA). The
results represent three independent experiments.
Flow cytometry for cell cycle
analysis
For the cell cycle assay, the cells in exponential
growth period were collected, washed twice with cold PBS, fixed in
cold 70% ethanol at −20°C. After washing with cold PBS the cells
were incubated with 10 mg/ml Rnase A (Beyotime) and 1 mg/ml
propidium iodide (Beyotime) at 37°C for 30 min. Cell cycle was
performed by flow cytometry (BD Biosciences, San Jose, CA,
USA).
Colony formation assay
Cells were seeded into a six-well plate (200 cells
per well). Cells were cultured at 37°C with 5% CO2 for
14 days until the clones were visible to the naked eye. Thereafter,
the medium was removed and the cells were fixed with 4%
paraformaldehyde, dyed with crystal violet. Under a microscope
(Olympus, Tokyo, Japan) stained clones with cell number >50 were
counted and digital images were taken.
Immunofluorescence (IF)
Cells grown on cover slips were fixed with 4%
paraformaldehyde for 15 min at room temperature. Then the cells
were permeabilized with 0.1% Triton-X-100 solution for 30 min.
After washing with PBS, cells were blocked with 10% goat serum for
15 min at room temperature. Then, cells were incubated with mouse
monoclonal anti-PCNA (1:50) in PBS with 10% goat serum at 4°C
overnight. After washing with PBS three times, cells were incubated
with Cy3-conjugated anti-mouse secondary antibodies for 1 h at room
temperature. The nuclei were counterstained with DAPI (Biosharp,
Seattle, WA, USA). Under a magnification of ×400, fluorescence
images of 5 different microscopic fields were captured by a
fluorescence microscope.
Wound healing assay
Cells were seeded in 12-well plates and incubated
until >80% confluence. A straight wound was created by
scratching with a 200-µl pipette tip. Floating cells were
removed by washing with serum-free medium twice. The cells were
then cultured in serum-free medium and allowed to migrate into the
wound area. Images of the migrated cells were acquired with an
inverted microscope (Olympus) at 0, 12, 24 h.
Transwell invasion assay
Transwell chambers (Corning Inc., Corning, NY,. USA)
were pre-coated with matrigel (BD Biosciences) at 37°C for 2 h.
Cells (104) in 200 µl serum-free medium were
added to the upper compartment, and to the lower chamber was added
800 µl DMEM containing 20% FBS. Then the cells were
incubated for 24 h at 37°C with 5% CO2. A cotton swab
was used to remove the non-invaded cells in the upper compartment.
The invaded cells were fixed in 4% paraformaldehyde and stained
with 0.1% crystal violet for 30 min. Under the microscope the cells
were counted in five random sights.
Gelatin zymography assay
The samples were separated in 10% SDS polyacrylamide
gel containing 0.2% gelatin in ice bath for 2.5 h. Then the gels
were washed in eluent buffer (2.5% Triton X-100, 50 mM Tris-HCl, 5
mM CaCl2, 1 µM ZnCl2, pH 7.6) for 40
min, twice; equilibrated in developing buffer (50 mM Tris-HCl, 5 mM
CaCl2, 1 µM ZnCl2, pH 7.6) for 20 min,
twice; and finally put in incubation buffer (50 mM Tris-HCl, 5 mM
CaCl2, 1 µM ZnCl2, 0.02% Brij, 0.2 M
NaCl) at 37°C for 40 h. Then, the gels were incubated with staining
buffer (0.05% Coomassie blue G-250 in 45% methanol, 10% acetic
acid, 30% methanoic acid) for 3 h and then washed with destaining
buffer (45% methanol, 10% acetic acid) until clear bands appeared.
The images were obtained by gel imaging system (Bio-Rad, Hercules,
CA, USA) and the activities of MMPs were measured by densitometric
analysis.
Flow cytometry
Annexin V-FITC/PI staining was used to measure
alive, apoptotic and necrotic cells. Briefly, cells were harvested,
washed with PBS and stained with a mixture of 100 µl Annexin
V-FITC and PI in the dark for 15 min. Then flow cytometry was used
to classify fluorescent cells. The number of apoptotic cells was
analysed by BD FACSuite software.
Hoechst 33342 staining
Hoechst 33342 staining was performed to measure
apoptotic morphology. Briefly, 5×104 cells were seeded
on cover slips and fixed with 4% paraformaldehyde for 20 min at
room temperature. After washing twice with PBS, the cells were
stained with Hoechst 33342 for 5 min. Fluorescent images were
acquired by a fluorescence microscopy (Olympus).
Statistical analysis
All results are expressed as mean ± standard
deviation (SD). Statistical analyses were performed using Student's
t-test. A p-value of <0.05 was set as the significance
level.
Results
Expression of the SPOCK1 gene in glioma
cells
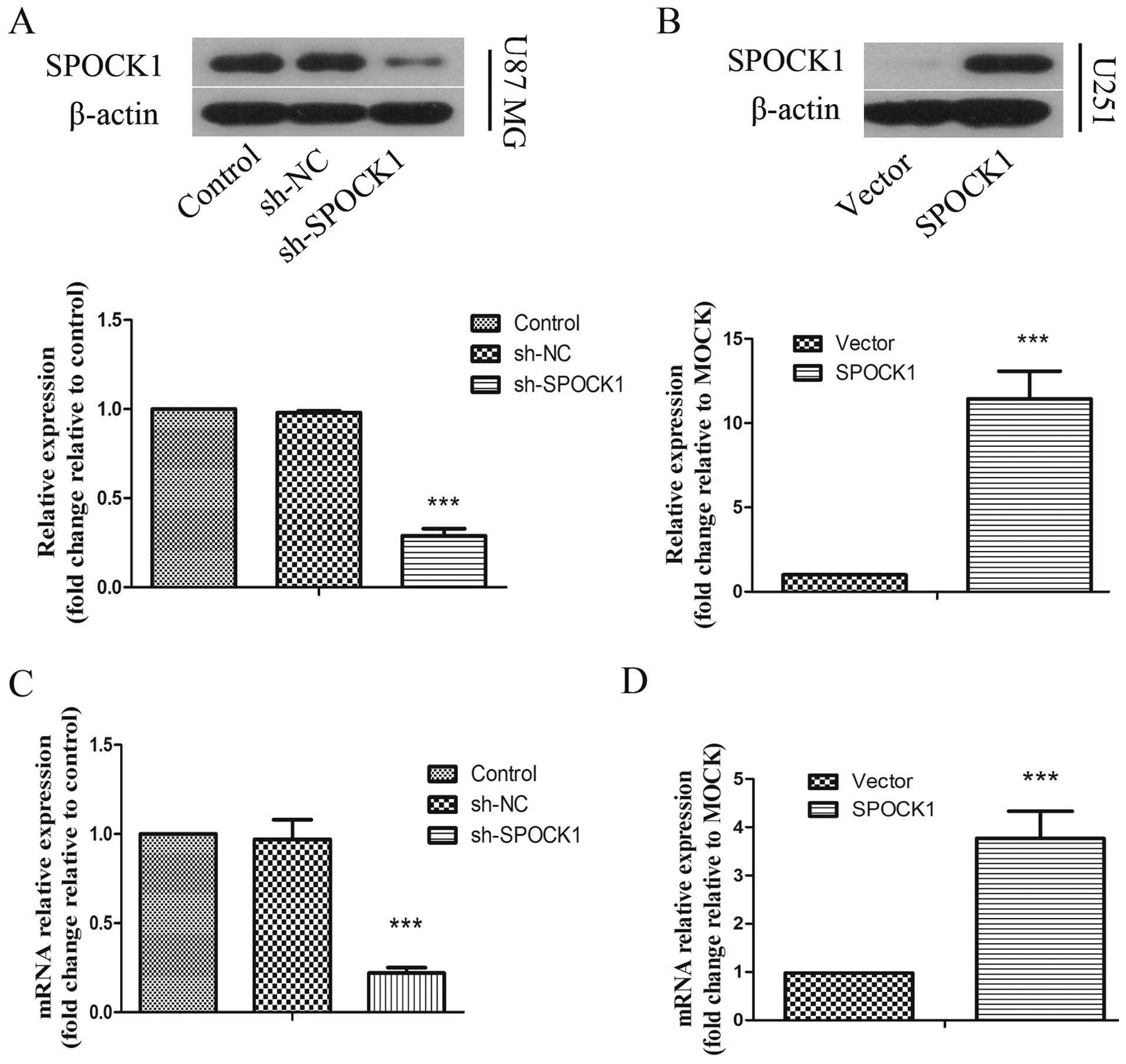
To investigate the role of SPOCK1 in glioma cells,
we chose U87 MG cells with higher SPOCK1 expression level for
stable transfection with shRNA vector toward SPOCK1 and U251 cells,
in which SPOCK1 is infrequently expressed, for stable transfection
with SPOCK1 expression vector. The expression levels of SPOCK1 was
measured by real-time PCR and western blot analysis. As shown in
Fig. 1A and C, an efficient
silencing of SPOCK1 protein and mRNA expression was shown in U87 MG
cells transfected with the SPOCK1 shRNA compared with negative
control group. An obvious high level of SPOCK1 protein and mRNA
expression was apparent in U251 cells transfected with SPOCK1
expression vector (Fig. 1B and
D).
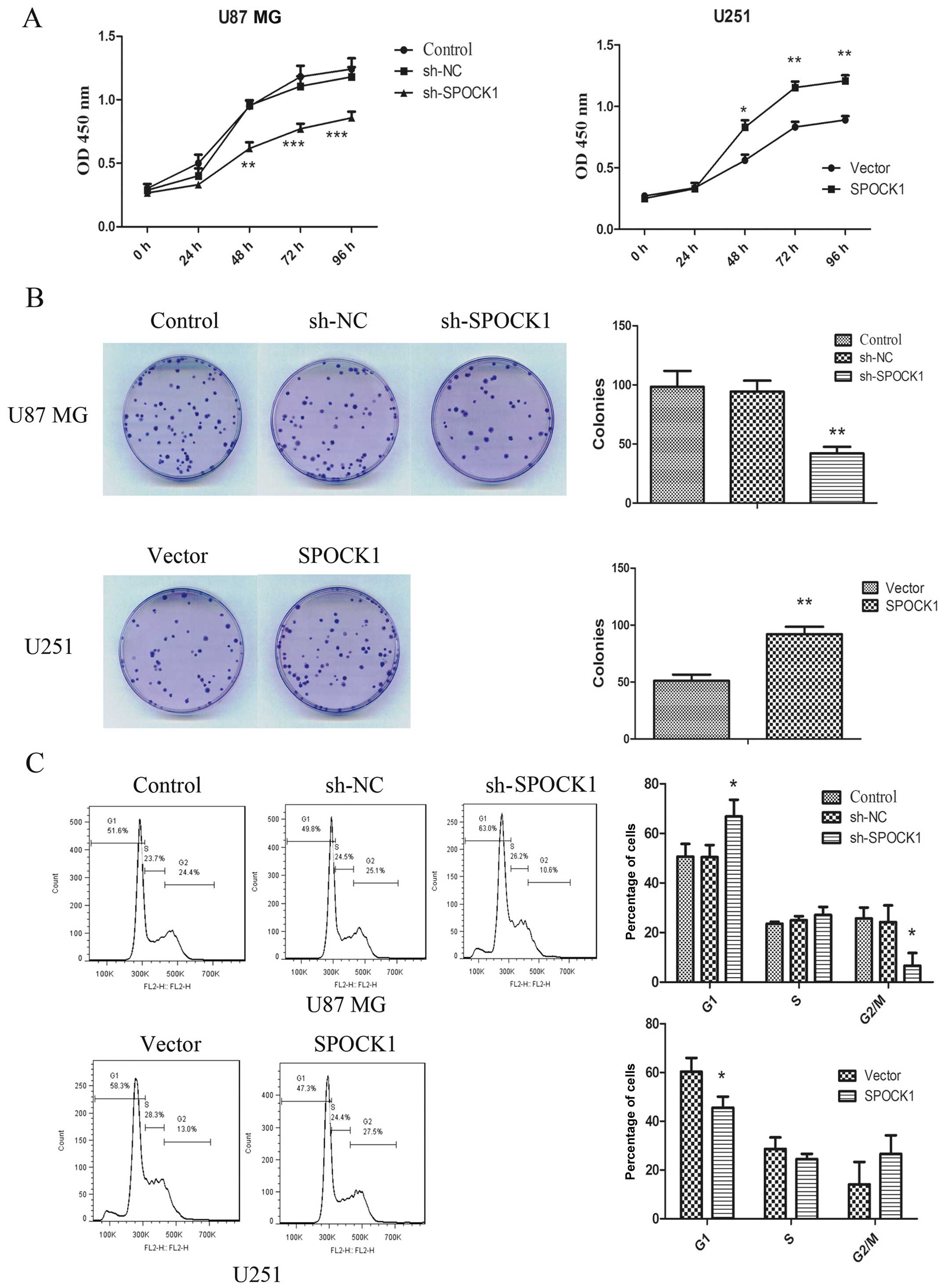
Effect of SPOCK1 on glioma cell
proliferation
The effect of SPOCK1 on the proliferation of glioma
cells were measured by CCK8 assay. As shown in Fig. 2A, silencing of SPOCK1 significantly
inhibited the proliferation of U87 MG cells. While overexpression
of SPOCK1 could promote the proliferation of U251 cells.
Additionally, the ability of glioma cells to form colonies was also
determined. As shown in Fig. 2B,
the result showed that silencing of SPOCK1 significantly decreased
the number of colonies formed by U87 MG cells. Overexpression of
SPOCK1, on the contrary, increasing colony formation in U251 cells.
Moreover, the cell cycle progression was detected by flow
cytometric analysis (Fig. 2C). The
results demonstrated that silencing of SPOCK1 resulted in a larger
fraction of the population in the G1 phase and a significant
decrease in the proportion in G2/M phase. Overexpression of SPOCK1
significantly reduced the number of cells arrested in G1 phase.
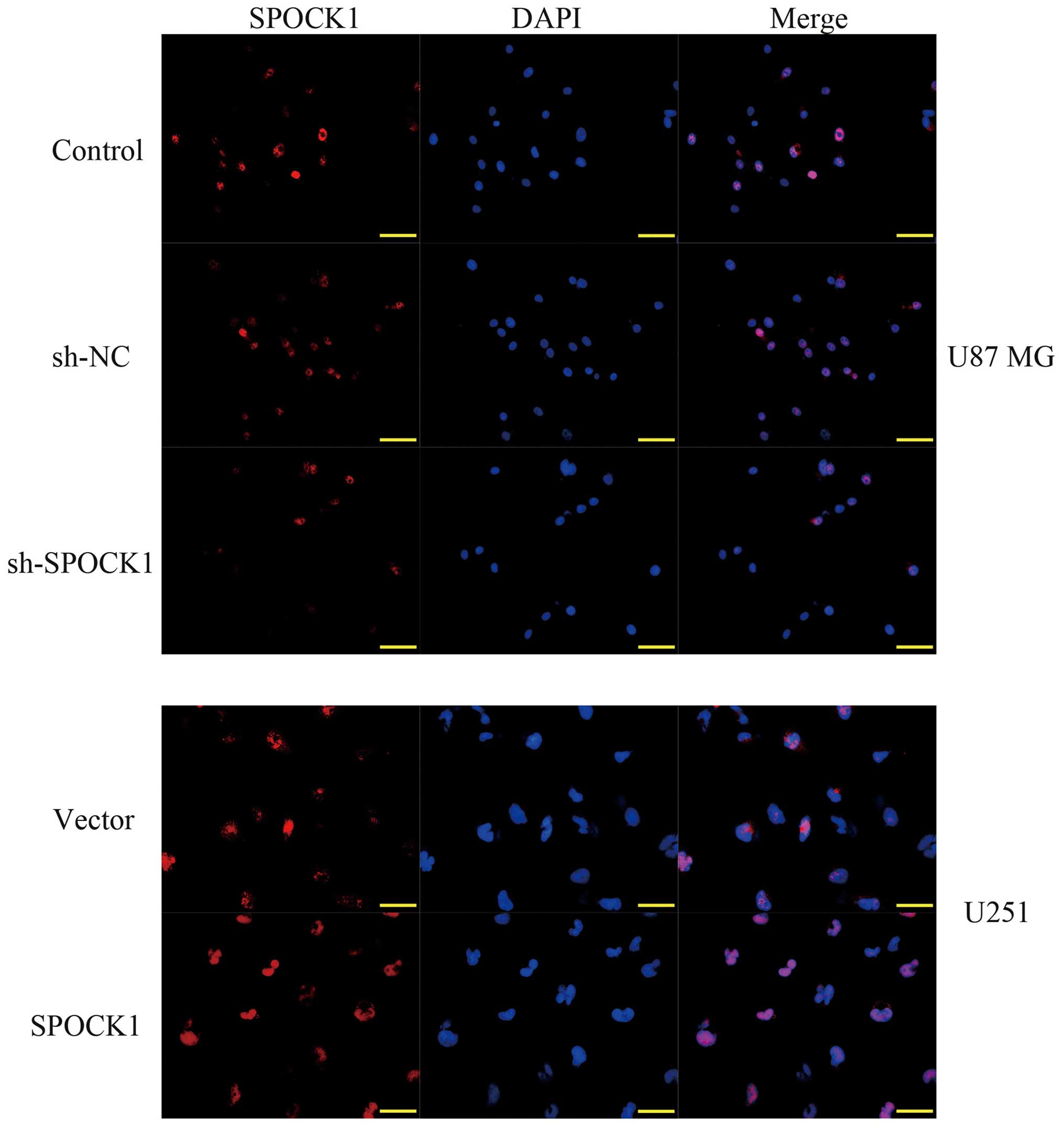
Since PCNA is an index for evaluating the ability of cell
proliferation, we subsequently detected the expression of PCNA by
immunofluorescence assay. As shown in Fig. 3, knockdown of SPOCK1 significantly
downregulated the expression of PCNA in U87 MG cells. On the
contrary, overexpression of SPOCK1 upregulated the expression of
PCNA in U251 cells. The above findings provide evidence that SPOCK1
as an oncogene may promote glioma cell growth.
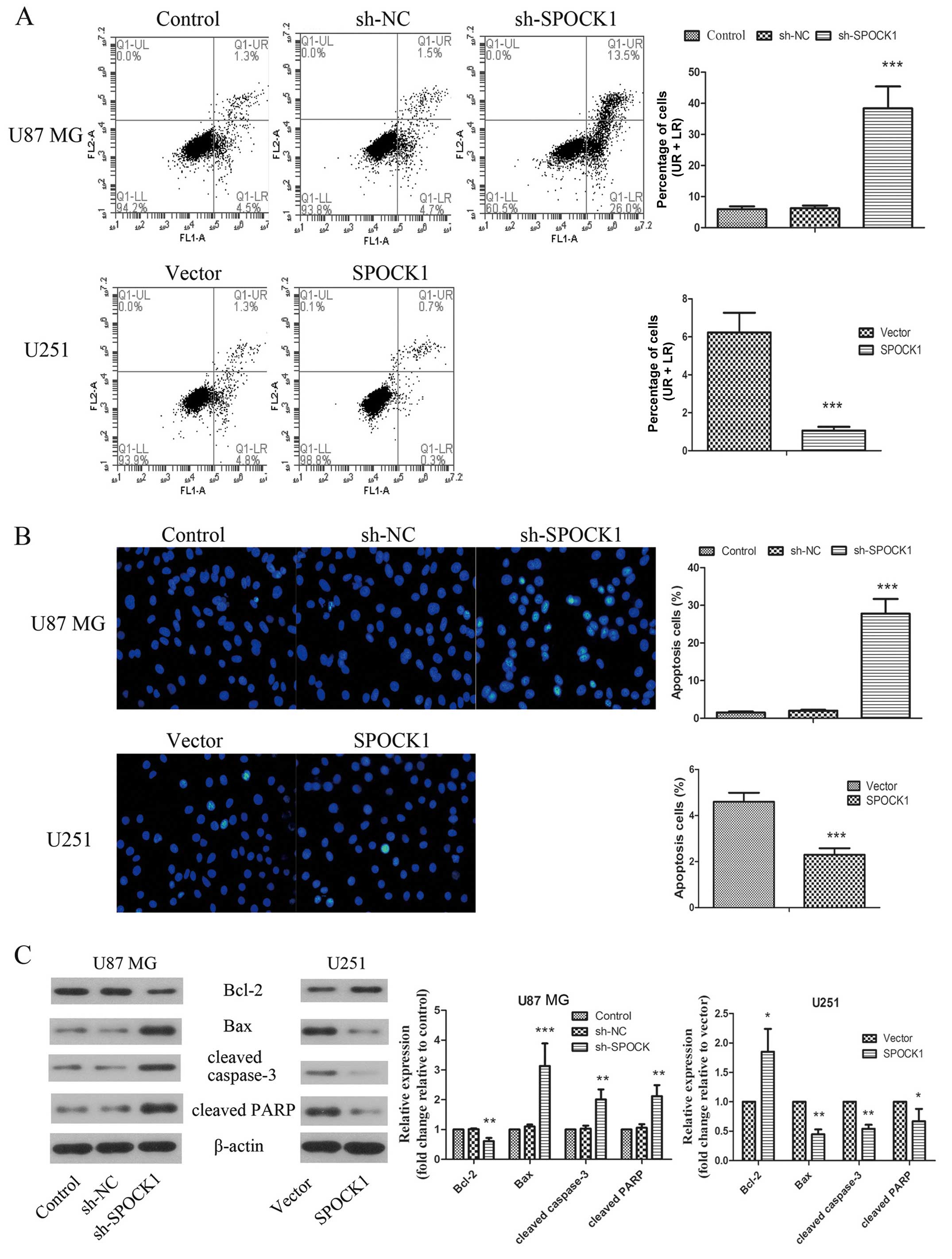
Effect of SPOCK1 on glioma cells
apoptosis
To further explore the mechanism of SPOCK1 in tumor
growth, we focused on the role of SPOCK1 in cell apoptosis. As
assessed by Annexin V-FITC and PI staining and shown in Fig. 4A, knockdown of SPOCK1 induced
obvious apoptosis in U87 MG cells. While the apoptosis was
inhibited by SPOCK1 overexpression in U251 cells. Moreover, as
shown in Fig. 4B, the nuclear
morphological changes in the apoptotic cells were revealed by the
Hoechst 33342 staining. Silencing of SPOCK1 resulted in brighter
chromatin condensation and nuclear fragmentation of the nuclei,
which was significantly suppressed by SPOCK1 overexpression. To
further confirm the effect of SPOCK1 on apoptosis, a number of
apoptosis related proteins were determined. The results showed that
knockdown of SPOCK1 induced increase in expression levels of Bax,
cleaved PARP and cleaved caspase-3 and decreased expression of
Bcl-2 in U87 MG cells. The expression changes of these apoptosis
related proteins were inverted when SPOCK1 was overexpressed in
U251 cells. These data indicated that SPOCK1 promotes glioma cell
growth by reducing cell apoptosis.
Effect of SPOCK1 on glioma cells
migration and invasion
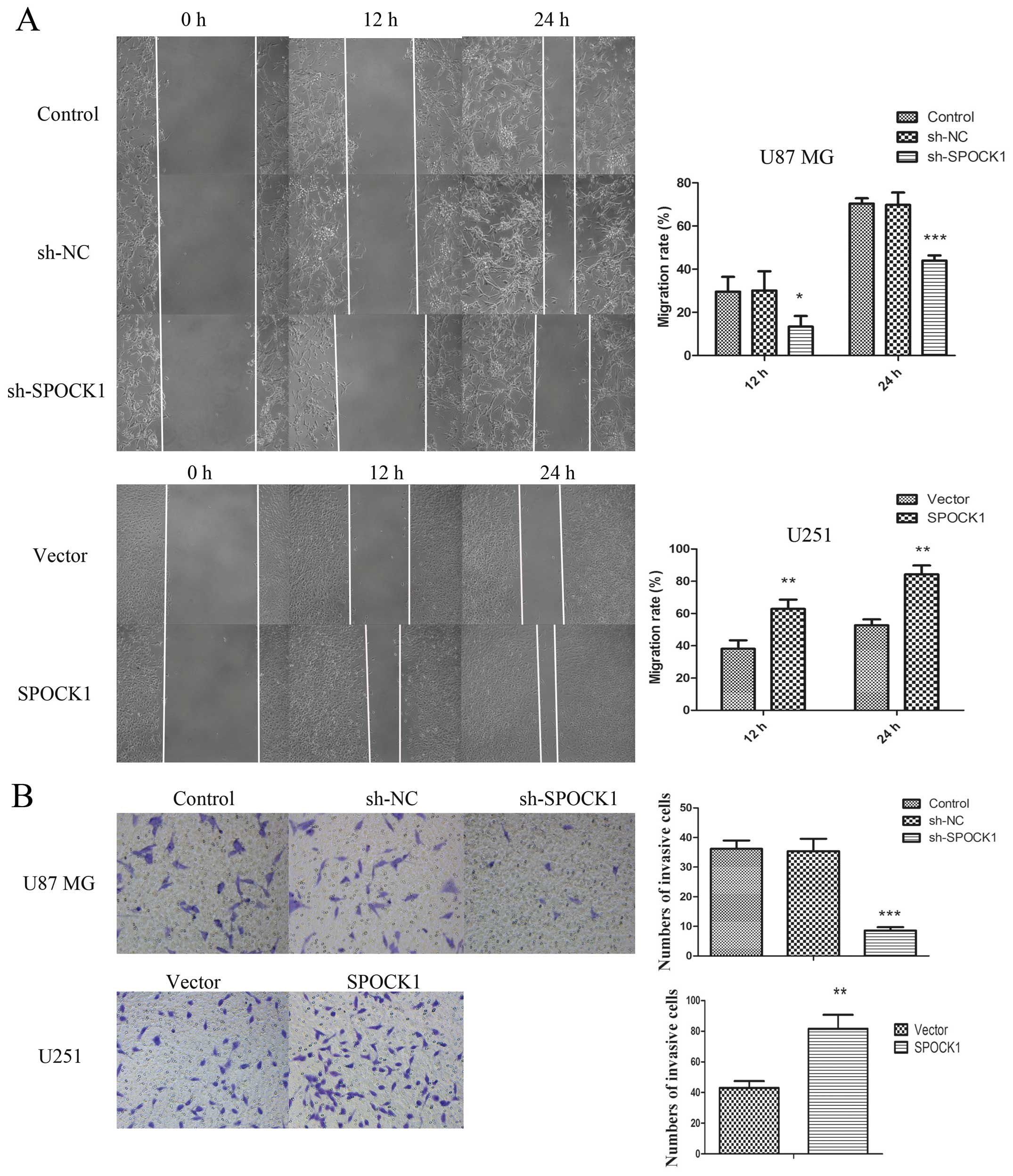
Cell migration capacity was assessed by wound
healing assay. As shown in Fig. 5A,
knockdown of SPOCK1 obviously inhibited the migration capacity of
U87 MG cells. When SPOCK1 was overexpressed in U251 cells, the
migration capacity was increased significantly. Moreover, the
invasive capacity was assessed by transwell assay and shown in
Fig. 5B. The results were similar
to the changes in migration ability that was inhibited by SPOCK1
silencing and promoted by SPOCK1 overexpression. To further confirm
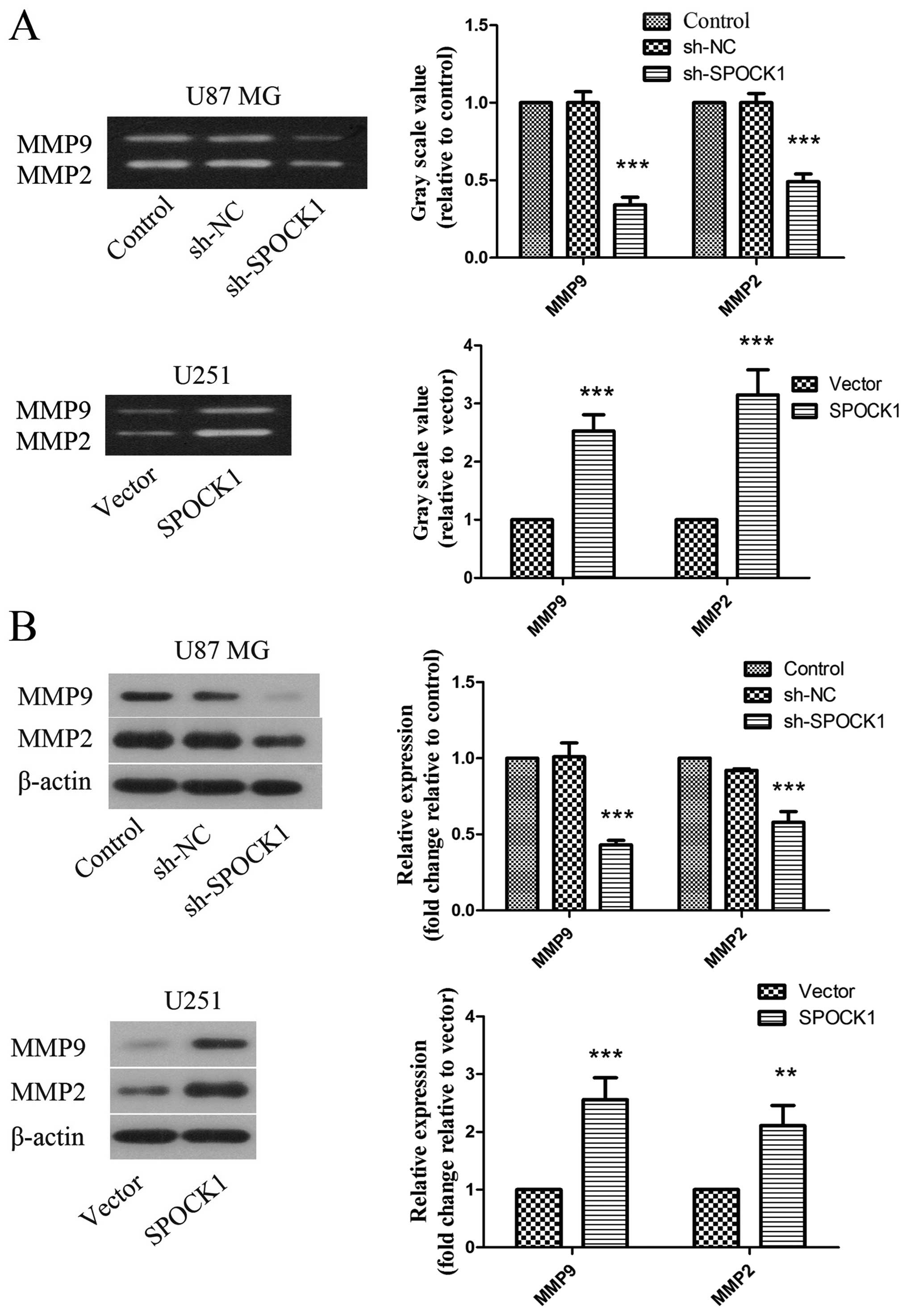
the effect of SPOCK1 on metastasis, the activity and expression of
MMP9 and MMP2 was determined by gelatin zymography and western
blotting. As shown in Fig. 6A, the
activity of MMP9 and MMP2 was restrained by SPOCK1 silencing in U87
MG cells. While overexpression of SPOCK1 could promote the activity
of MMP9 and MMP2. The changes of MMP9 and MMP2 expression were
similar to those of MMP9 and MMP2 activity (Fig. 6B).
Effect of SPOCK1 on PI3K/AKT and
Wnt/β-catenin signaling pathways
Since PI3K/AKT and Wnt/β-catenin signaling pathways
are associated intimately with proliferation and metastasis of
cancer cells, the effect of SPOCK1 on PI3K/AKT and Wnt/β-catenin
signaling pathways was evaluated. As shown in Fig. 7, knockdown of SPOCK1 significantly
suppressed the protein levels of p-PI3K, p-AKT, Wnt and β-catenin
in U87 MG cells, which could be reversed when SPOCK1 was
overexpressed in U251 cells. Moreover, the downstream targets of
Wnt/β-catenin were also assessed. The results indicated that the
protein levels of c-MYC and cyclin D1 were decreased by SPOCK1
silencing, which were increased obviously when SPOCK1 was
overexpressed.
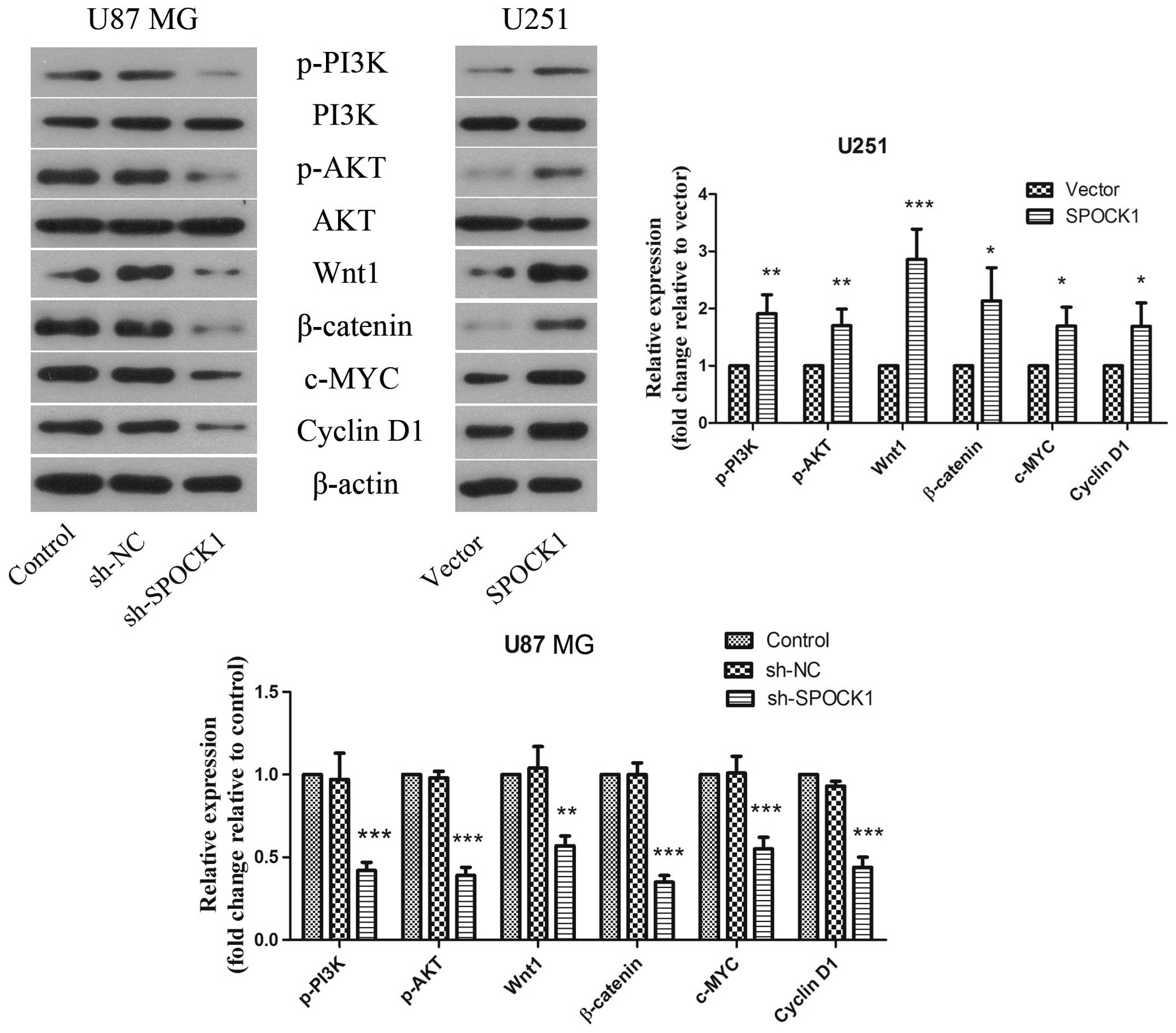 | Figure 7The effect of SPOCK1 on PI3K/AKT and
Wnt/β-catenin signaling pathways. The protein expression levels of
p-PI3K, PI3K, p-AKT, AKT, Wnt, β-catenin, c-MYC, and cyclin D1 in
glioma cells was detected by western blotting. β-actin was used as
a loading control. The results shown are representative of three
independent experiments. Each value is expressed as mean ± SD
(n=3). *P<0.05, **P<0.01,
***P<0.001, versus the NC or vector group. |
Discussion
Glioma is the most common malignancy in the central
nervous system, and is the most aggressive and progressive. Because
of the poor prognosis and high fatality, glioma severely threatens
life and health of patients. The malignant transformation of
neurogliocyte and neurone is a complicated process, involving a
large number of oncogenes and tumor suppressor genes. Increasing
evidence has demonstrated that SPOCK1 as an oncogene promoted
various cancer cell proliferation and invasion. We investigated the
effect of SPOCK1 on glioma cells proliferation, migration and
invasion, which provide a potential therapeutic target as well as a
prognostic marker for glioma.
Malignant proliferation is one of the most
significant features of tumor cells. Infinite proliferation and
anti-apoptosis are two important malignant phenotypes of glioma. In
the malignant process of glioma, the tumor cell density is
increased with significant nuclear division and atypia (12,13).
In our present study, the effect of SPOCK1 on glioma cell malignant
proliferation was investigated. As assayed by CCK8, the
proliferation of glioma cells was obviously inhibited by SPOCK1
silencing. Then clone formation ability was suppressed by SPOCK1
silencing. Finally the results of cell cycle analysis showed that
there was a larger fraction of the population in the G1 phase and a
significant decrease in the proportion in G2/M phase.
Overexpression of SPOCK1 promoted glioma cell proliferation and
clone formation, and decreased the percentage of cells in G1 phase.
These results demonstrated that SPOCK1 participated in malignant
progression of glioma via promoting glioma cell proliferation from
positive and negative aspects.
Proliferating cell nuclear antigen (PCNA) is a
nuclear protein, expressed in proliferative cells only. The
synthesis of PCNA has close relation with cellular proliferation
cycle, which is increased rapidly in late G1 phase and peaked in S
phase. Previous research found that the level of PCNA is relative
to the tumor type and clinical stage of the central nervous system
tumors (14). Another study found
that inhibition of PCNA could suppress DNA replication and prevent
cells from going into proliferation period (15). In our present study, the expression
level of PCNA was decreased by SPOCK1 silencing, while increased
when SPOCK1 was overexpressed.
Apoptosis inhibition or escape is another feature of
malignant tumor cells. We also observed SPOCK1 effects on the
apoptosis of glioma cells. Our results indicated that knockdown of
SPOCK1 induced cell apoptosis significantly, which could be
suppressed by overexpression of SPOCK1. Cell apoptosis is regulated
by a series of apoptosis-related genes, the most significant of
which are Bcl-2 and caspase gene families. Bcl-2 is an important
member of Bcl-2 gene family and is also an apoptosis suppressor
gene. Bcl-2 is expressed in brain tumors, which was significantly
positively related to the degree of tumor malignancy (16,17).
Bax is another apoptosis gene in Bcl-2 family, which and can
inhibit the anti-apoptosis effect of Bcl-2. The survival of the
cells is determined by the ratio of Bcl-2/Bax (18,19).
Caspase-3, an executioner caspase, plays a key role in the
execution phase of apoptosis by cleaving many key cellular
proteins, such as PARP (20,21).
According to our results, silencing of SPOCK1 decreased the ratio
of Bcl-2/Bax and promoted the expression of cleaved caspase-3 and
PARP. Overexpression of SPOCK1 resulted in the increased ratio of
Bcl-2/Bax and lower expression of cleaved caspase-3 and PARP.
The metastasis and recurrence are the major
prognostic factors of cancer patients. Metastasis refers to primary
malignant tumor cells invading other parts of the body and
spreading to other organs, which is one of the fundamental biologic
behaviors of tumor. Multiple steps, such as migration and invasion,
participate in the process of metastasis of tumors. Our results
demonstrated that the migration and invasion of glioma cells was
suppressed by silencing of SPOCK1 and promoted by SPOCK1
overexpression. Matrix metalloproteinases (MMPs), a family of
endopeptidases, could degrade extracellular matrix. Recent research
found that the degradation of extracellular matrix and basement
membrane played key roles in the invasion and metastasis of tumor,
which could be regulated by MMPs (22). Research also found that the levels
of MMPs were positively related to the invasive ability of glioma
cells (23). MMP2 and MMP9 are two
important MMPs, the expression levels of which are enhanced in
glioma cells (24,25). As the glioma malignancy degree
increased, the expression of MMP2 and MMP9 was increased (23,26).
Our results demonstrated that the activity and expression of MMP2
and MMP9 were restrained by SPOCK1 silencing, which could be
promoted by SPOCK1 overexpression.
Growing evidence has demonstrated that PI3K/AKT
signaling pathway played important roles in the occurrence and
development of glioblastoma (27,28).
As one of the important intracellular signal transduction pathways,
PI3K/AKT signaling pathway not only impacts on proliferation and
apoptosis of cancer cells, but also has a role in chemotherapy
reactions. Recent studies suggest that the inhibitors of PI3K/AKT
signaling pathway could fight drug resistance of various cancers
(29–31). In our present study, the protein
levels of p-PI3K, p-AKT were downregulated by knockdown of SPOCK1.
While overexpression of SPOCK1 could upregulate the protein levels
of p-PI3K, p-AKT. Wnt/β-catenin signaling pathway is a focus of
tumor biology. Increasing evidence shows that Wnt/β-catenin
signaling pathway is closely related to tumorigenesis. With the
deepening of the research, c-MYC and cyclin D1 were found to be
target genes of Wnt/β-catenin signaling pathway (32,33).
These downstream genes were closely related to proliferation and
differentiation. It was found that the activation of c-MYC played a
role in various tumors, including glioma, lymphoma, lung cancer,
and colorectal cancer (34–37). Research also found that c-MYC and
cyclin D1 was regulated by β-catenin in ovarian cancer (38). Our present study found that the
protein levels of Wnt and β-catenin were decreased by SPOCK1
silencing, and upregulated in SPOCK1 overexpression. The expression
levels of c-MYC and cyclin D1 were positively correlated with
Wnt/β-catenin.
In conclusion, our results suggest that SPOCK1 may
serve as an oncogene in glioma. Overexpression of SPOCK1 promotes
glioma cell proliferation, migration and invasion via PI3K/AKT and
Wnt/β-catenin signaling pathways, which could be reversed by SPOCK1
silencing. These observations confirm that SPOCK1 may serve as both
a treatment target and prognostic indicator for patients with
glioma.
References
|
1
|
Taylor LP: Diagnosis, treatment, and
prognosis of glioma: Five new things. Neurology. 75(Suppl 1):
S28–S32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brada M, Stenning S, Gabe R, Thompson LC,
Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K,
et al: Temozolomide versus procarbazine, lomustine, and vincristine
in recurrent high-grade glioma. J Clin Oncol. 28:4601–4608. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morris PG and Lassman AB: Medical
oncology: Optimizing chemotherapy and radiotherapy for anaplastic
glioma. Nat Rev Clin Oncol. 7:428–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabayan AJ, Green SB, Sanan A, Jenrette J,
Schultz C, Papagikos M, Tatter SP, Patel A, Amin P, Lustig R, et
al: GliaSite brachytherapy for treatment of recurrent malignant
gliomas: A retrospective multi-institutional analysis.
Neurosurgery. 58:701–709; discussion 701–709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sciumè G, Santoni A and Bernardini G:
Chemokines and glioma: Invasion and more. J Neuroimmunol. 224:8–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Butowski NA, Sneed PK and Chang SM:
Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin
Oncol. 24:1273–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song X, Han P, Liu J, Wang Y, Li D, He J,
Gong J, Li M, Tu W, Yan W, et al: Up-regulation of SPOCK1 induces
epithelial-mesenchymal transition and promotes migration and
invasion in esophageal squamous cell carcinoma. J Mol Histol.
46:347–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao L, Wang Y, Xia H, Yao C, Cai H and
Song Y: SPOCK1 is a novel transforming growth factor-β target gene
that regulates lung cancer cell epithelial-mesenchymal transition.
Biochem Biophys Res Commun. 440:792–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Chen L, Chan TH, Liu M, Kong KL, Qiu
JL, Li Y, Yuan YF and Guan XY: SPOCK1 is regulated by CHD1L and
blocks apoptosis and promotes HCC cell invasiveness and metastasis
in mice. Gastroenterology. 144:179–191.e4. 2013. View Article : Google Scholar
|
|
10
|
Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: SPOCK1 as a potential
cancer prognostic marker promotes the proliferation and metastasis
of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol
Cancer. 14:122015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colin C, Baeza N, Bartoli C, Fina F, Eudes
N, Nanni I, Martin PM, Ouafik L and Figarella-Branger D:
Identification of genes differentially expressed in glioblastoma
versus pilocytic astrocytoma using suppression subtractive
hybridization. Oncogene. 25:2818–2826. 2006. View Article : Google Scholar
|
|
12
|
Taillibert S, Pedretti M and Sanson M:
Current classification of gliomas. Presse Med. 33:1274–1277.
2004.In French. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beetz C, Bergner S, Brodoehl S, Brodhun M,
Ewald C, Kalff R, Krüger J, Patt S, Kiehntopf M and Deufel T:
Outcome-based profiling of astrocytic tumours identifies prognostic
gene expression signatures which link molecular and
morphology-based pathology. Int J Oncol. 29:1183–1191.
2006.PubMed/NCBI
|
|
14
|
Kayaselçuk F, Zorludemir S, Gümürdühü D,
Zeren H and Erman T: PCNA and Ki-67 in central nervous system
tumors: Correlation with the histological type and grade. J
Neurooncol. 57:115–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Punchihewa C, Inoue A, Hishiki A, Fujikawa
Y, Connelly M, Evison B, Shao Y, Heath R, Kuraoka I, Rodrigues P,
et al: Identification of small molecule proliferating cell nuclear
antigen (PCNA) inhibitor that disrupts interactions with PIP-box
proteins and inhibits DNA replication. J Biol Chem.
287:14289–14300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alderson LM, Castleberg RL, Harsh GR IV,
Louis DN and Henson JW: Human gliomas with wild-type p53 express
bcl-2. Cancer Res. 55:999–1001. 1995.PubMed/NCBI
|
|
17
|
Deckert-Schlüter M, Rang A and Wiestler
OD: Apoptosis and apoptosis-related gene products in primary
non-Hodgkin's lymphoma of the central nervous system. Acta
Neuropathol. 96:157–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borner C: The Bcl-2 protein family:
Sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar
|
|
20
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin C, Holland RE Jr, Donofrio JC, McCoy
MH, Tudor LR and Chambers TM: Caspase activation in equine
influenza virus induced apoptotic cell death. Vet Microbiol.
84:357–365. 2002. View Article : Google Scholar
|
|
22
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sawaya RE, Yamamoto M, Gokaslan ZL, Wang
SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG,
Nicolson GL and Rao JS: Expression and localization of 72 kDa type
IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp
Metastasis. 14:35–42. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao JS, Steck PA, Mohanam S,
Stetler-Stevenson WG, Liotta LA and Sawaya R: Elevated levels of
M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res.
53(Suppl): 2208–2211. 1993.PubMed/NCBI
|
|
25
|
Forsyth PA, Wong H, Laing TD, Rewcastle
NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM,
Sutherland G, et al: Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and
membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in
different aspects of the pathophysiology of malignant gliomas. Br J
Cancer. 79:1828–1835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sawaya R, Go Y, Kyritisis AP, Uhm J,
Venkaiah B, Mohanam S, Gokaslan ZL and Rao JS: Elevated levels of
Mr 92,000 type IV collagenase during tumor growth in vivo. Biochem
Biophys Res Commun. 251:632–636. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ströbele S, Schneider M, Schneele L,
Siegelin MD, Nonnenmacher L, Zhou S, Karpel-Massler G, Westhoff MA,
Halatsch ME and Debatin KM: A Potential role for the inhibition of
PI3K signaling in glioblastoma therapy. PLoS One. 10:e01316702015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X,
Wang S, Zhao J, Li Y and Cao Y: Resveratrol inhibits the invasion
of glioblastoma-initiating cells via down-regulation of the
PI3K/Akt/NF-κB signaling pathway. Nutrients. 7:4383–4402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi L, Fei X, Wang Z and You Y: PI3K
inhibitor combined with miR-125b inhibitor sensitize TMZ-induced
anti-glioma stem cancer effects through inactivation of
Wnt/β-catenin signaling pathway. In Vitro Cell Dev Biol Anim.
51:1047–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Geuna E, Milani A, Martinello R, Aversa C,
Valabrega G, Scaltriti M and Montemurro F: Buparlisib, an oral
pan-PI3K inhibitor for the treatment of breast cancer. Expert Opin
Investig Drugs. 24:421–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ku BM, Jho EH, Bae YH, Sun JM, Ahn JS,
Park K and Ahn MJ: BYL719, a selective inhibitor of
phosphoinositide 3-Kinase α, enhances the effect of selumetinib
(AZD6244, ARRY-142886) in KRAS-mutant non-small cell lung cancer.
Invest New Drugs. 33:12–21. 2015. View Article : Google Scholar
|
|
32
|
Brabletz T, Herrmann K, Jung A, Faller G
and Kirchner T: Expression of nuclear beta-catenin and c-myc is
correlated with tumor size but not with proliferative activity of
colorectal adenomas. Am J Pathol. 156:865–870. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang N, Wang Y, Hui L, Li X and Jiang X:
SOX 1, contrary to SOX 2, suppresses proliferation, migration, and
invasion in human laryngeal squamous cell carcinoma by inhibiting
the Wnt/β-catenin pathway. Tumour Biol. 36:8625–8635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee KS, Kwak Y, Nam KH, Kim DW, Kang SB,
Choe G, Kim WH and Lee HS: c-MYC Copy-Number Gain Is an Independent
Prognostic Factor in Patients with Colorectal Cancer. PLoS One.
10:e01397272015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leone A, Roca MS, Ciardiello C,
Terranova-Barberio M, Vitagliano C, Ciliberto G, Mancini R, Di
Gennaro E, Bruzzese F and Budillon A: Vorinostat synergizes with
EGFR inhibitors in NSCLC cells by increasing ROS via up-regulation
of the major mitochondrial porin VDAC1 and modulation of the
c-Myc-NRF2-KEAP1 pathway. Free Radic Biol Med. 89:287–299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang Q, Chen G, Zhou J, Li L, Dong Z,
Yang R, Xu L, Cui H, Xu M and Yi L: Neurotensin signaling
stimulates glioblastoma cell proliferation by upregulating c-Myc
and inhibiting miR-29b-1 and miR-129-3p. Neuro Oncol. 18:216–226.
2016. View Article : Google Scholar
|
|
37
|
Suk FM, Lin SY, Lin RJ, Hsine YH, Liao YJ,
Fang SU and Liang YC: Bortezomib inhibits Burkitt's lymphoma cell
proliferation by downregulating sumoylated hnRNP K and c-Myc
expression. Oncotarget. 6:25988–26001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu Q, Chen Z, Gong X, Cai Y, Chen Y, Ma X,
Zhu R and Jin J: β-Catenin expression is regulated by an
IRES-dependent mechanism and stimulated by paclitaxel in human
ovarian cancer cells. Biochem Biophys Res Commun. 461:21–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|